Abstract
Caves can be regarded as extreme environments, and fungi are known as omnipresent and highly adaptable organisms that can easily colonize such environments. The primary objective of this study was to use the statistical analysis of sequences stored in the NCBI database, together with related metadata, to find and uncover statistically significant distribution patterns of fungi occupying different substrata inside the caves. The obtained list included a total of 1447 sequences corresponding to fungi isolated from various substrata within cave environments around the world, which corresponds to 445 fungal species, members of the 394 genera. Ascomycota was the most dominant phylum and Eurotiomycetes the dominant class of fungal dwellers in these environments. The highest species richness is detected for the genus Penicillium (57), followed by Aspergillus (51). On the other hand, the most frequently documented single species was Pseudogymnoascus destructans, isolated mostly from hibernating bats and guano, followed by Penicillium chrysogenum. Because caves have stable, nutrient-limited, low-competition microhabitats that support unusual or cryptic species, many new fungal taxa have been reported as well (such as Aspergillus, Apiotrichum, and Cephalotrichum species). Finally, cutting-edge molecular technologies and better sampling methods are revealing hitherto undiscovered fungal diversity in caves worldwide.
1. Introduction
Fungi can colonize any organic substrata within the caves; they can establish different biotic interactions with other cave organisms and are among the most dominant organisms [1]. Speleomycology is a novel scientific discipline that studies the diversity, ecology, and role of cave mycobiota [2]. The significance of cave fungi can therefore be summarized in the following points:
- Saprobe fungi are the main decomposers of organic matter in subterranean sites with a significant role in the biogeochemical cycling of elements in caves [3]. Previous speleomycological studies have suggested that fungi can colonize a variety of organic substrata within the caves, such as bat guano, moonmilk, sediments, etc. Also, cave fungi can be isolated from carcasses of cave-associated animals, which are divided into troglobites (obligate cave dwellers), troglophiles (organisms that can live and reproduce in caves but also in surface habitats with similar conditions), and trogloxenes (organisms that use caves occasionally but do not depend on them for survival) [4].
- Some fungal species are parasites and can cause diseases of cave animals. For example, Pseudogymnoascus destructans (Ascomycota, Leotiomycetes) is a causative agent of the white-nose syndrome in bats [5]. Likewise, the members of genus Arthrorhynchus (Ascomycota, Laboulbeniomycetes) are obligate parasites of bat flies and are documented in various caves [6].
- Fungi can serve as a food source for various cave-dwelling organisms. In cave ecosystems with limited external inputs, fungi may represent a crucial energy and nutrient source for certain species, including cave-adapted invertebrates [1].
- Fungi are constituents of subaerial biofilms (SAB), which are often formed on cave walls and ceilings. The metabolic activity of SAB-forming fungi could lead to weathering of stone substrata, unusual colorations on speleothems, as well as precipitation and formation of corrosion residues [7].
- Many fungal species are the main “culprits” responsible for the biodeterioration of prehistoric wall paintings in the caves with Paleolithic art [8]. Also, in the caves that are repurposed for sacral objects during the history of mankind, fungi can be biodeteriogens of murals and other artifacts deposited within [9,10]. Nevertheless, biodeterioration induced by fungi is reported for several caves from the UNESCO list of world cultural heritage sites. For example, black stains, as a biodeterioration symptom of fungal origin, are reported on walls with prehistoric paintings in the Lascaux cave [8].
- Fungal propagules, mostly spores and mycelial fragments, are always present in the cave air, and when optimal conditions are met, they could reach high concentrations expressed in CFU m−3 of air [11]. In fact, high concentrations of toxigenic and pathogenic airborne fungi in the caves are linked with impaired indoor air quality, and this issue could be regarded as a health risk factor for both visitors and cave personnel in show caves [12].
- Caves could be referred to as extreme environments, poor in nutrients, which is a limiting factor for the growth and proliferation of fungi, which are heterotrophs. Although the number of speleomycological studies constantly increases, caves are still considered biologically poorly investigated environments and a potential source for novel fungal species. For instance, Scolecobasidium lascauxense (syn. Ochroconis lascauxensis) and S. anomalum (syn. O. anomala), members of Dothideomycetes, are melanized micromycetes that were first documented, isolated, and described from the above-mentioned black stains of Paleolithic paintings in the famous Lascaux cave [13]. Furthermore, Zhang et al. [14] described 20 novel fungal species in karst caves across China. Although some fungal species, such as Acaulium caviariforme (Ascomycota, Sordariomycetes), Aspergillus baeticus, and A. thesauricus (Ascomycota, Eurotiomycetes), are only documented in cave environments, it is still discussed whether truly there exist fungi that could be named obligate troglobionts [15].
- It is a well-known fact that the presence of fungi in caves contributes to the overall biodiversity of subterranean ecosystems. Also, some fungal species reported in caves may have unique biochemical properties and be the potential source for novel organic compounds. In this sense, these fungi can be of interest for biotechnological applications, especially in the pharmaceutical industry, due to the production of metabolites with different biological activities [16].
There are few review papers regarding cave fungi. The pioneering work in this field is presented by Rutherford and Huang [17]. Furthermore, Vanderwolf et al. [18] presented the list of 1029 species of filamentous fungi, yeasts, and slime molds from a variety of substrata in caves and mines obtained after a comprehensive search of peer-reviewed literature. Kato et al. [19] highlighted multi-omics integration as a novel strategy to achieve new insights and perspectives about the biology, chemistry, and microbiology of caves. An interesting review article was presented by Cyske et al. [16]. These authors regarded the caves as “hard-to-reach environments” and, as such, a potential source of microorganisms, mostly bacteria, but also fungi, which are producers of various biologically active compounds. Also, Barbosa et al. [20] reviewed the biotechnological potential of cave fungi and reported the broad potential of compounds, antioxidants, antitumor agents, enzymes, and organic acids of cave-dwelling fungi. Additionally, Bontemps et al. [21] reviewed the microbial ecology of touristic caves with Paleolithic art, emphasizing high-throughput sequencing as a novel scientific approach in the study of cave microbiota.
To our knowledge, there are no review papers dealing with cave mycobiota that collect data from the National Center for Biotechnology (NCBI) database. The main goal of this paper is to search for and reveal statistically significant distribution patterns of fungi colonizing various substrata within the caves through the statistical analysis of sequences deposited in the NCBI database along with accompanying metadata.
2. Materials and Methods
2.1. NCBI Database Search
In order to extract nucleotide sequences from the NCBI database of all fungi related to the cave environment, advanced search options were applied on the following website: https://www.ncbi.nlm.nih.gov/ (accessed on 22 May 2023). A search query with relevant keywords separated with Boolean operators (AND and OR) took place in May 2023. To obtain sequences of fungal species present in the caves, taxonomic identifiers were used as target keywords—“Fungi” [Organism], along with relevant keywords and database fields. For the sequence type, “ITS” [All Fields] OR “rRNA” [All Fields] are used. Habitat-related terms such as “cave”, “subterranean”, “cavern”, and “guano” were used as additional keywords in the search query.
The NCBI database search was performed by all three review authors independently. The search output was extracted as a FASTA file from NCBI and sorted in an Excel file that contained accompanying metadata (accession number, isolation source, country of isolation, strain, host, etc.). Furthermore, additional searches in Web of Science (http://isiknowledge.com (accessed on 19 June 2023)), PubMed, Google Scholar, and Scopus were used to link NCBI outputs to corresponding peer-reviewed publications, while DOI and title of publication were added in the form (Supplementary Table S1, with references [1,12,14,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105]). However, the unpublished data from NCBI were also used in this study only in the cases where the isolation_source in the NCBI database refers unambiguously to cave substrata (i.e., cave sediment, cave soil, cave wall, guano…) and if they provided sufficient additional information (i.e., geo_loc_name, host…) that could be used for statistical analyses.
2.2. Statistical Anlyses
Multivariate analyses were performed in the software Canoco 5 [106]. In these analyses, individual fungal representatives, fungal classes, and phyla were used as response data. The main data set contained the list of fungal representatives that are mentioned in the NCBI database. The option “trait averages” was used in the software to group individual taxa in the corresponding higher taxonomic units—classes and phyla. When using redundance analysis (RDA), fungal phyla, as well as fungal classes, were observed in relation to the continent and substratum separately. When considering individual taxa, only those with a number of more than ten isolates were observed in relation to the continent by using canonical correspondence analysis (CCA). The substrata categorized included the terms found in the paper and are presented in Supplementary File S1. The frequencies of taxa presented in pie diagrams were composed in Microsoft Office Excel 2021.
3. Results
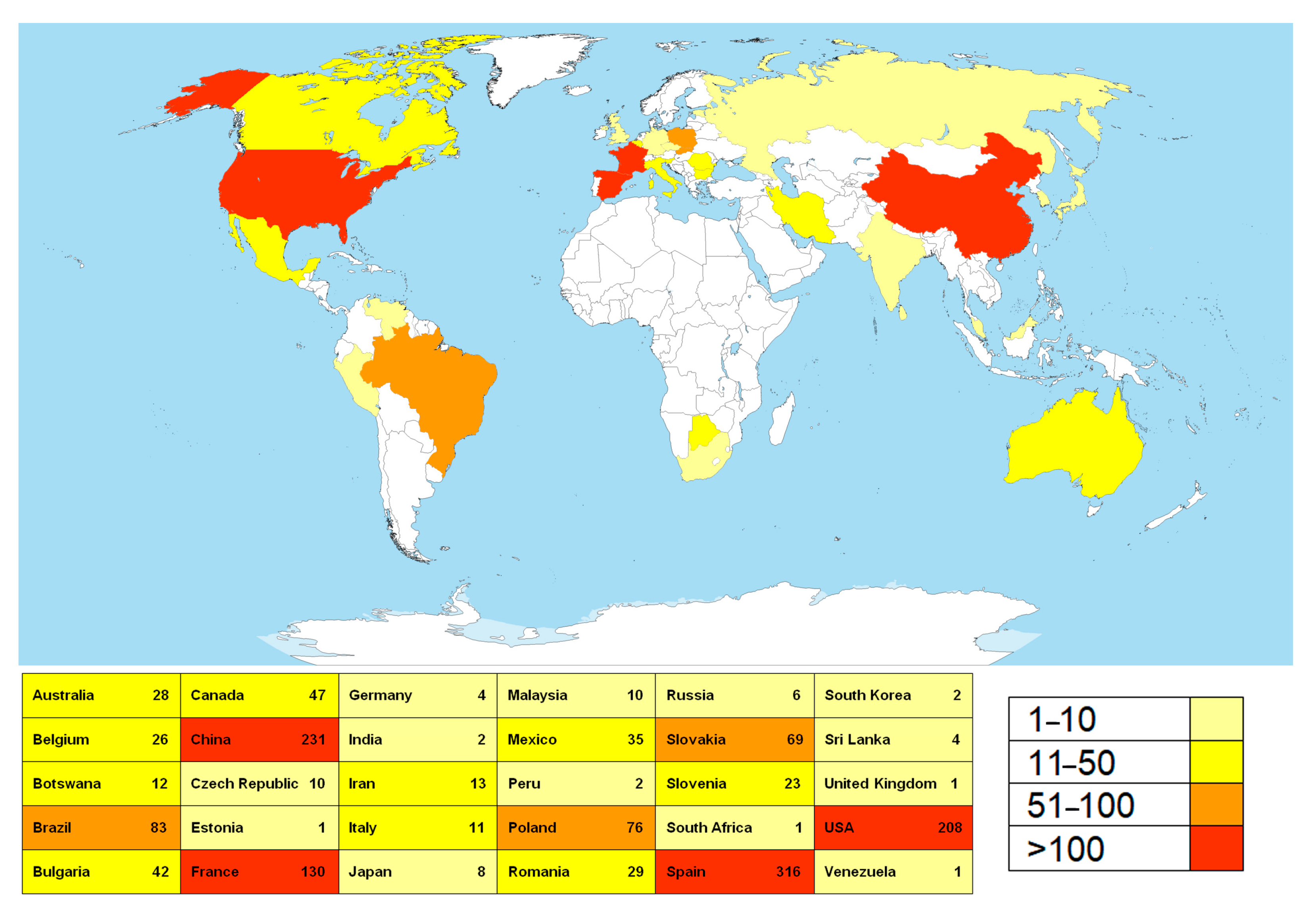
A total of 22,407 sequences were retrieved from the NCBI base. After the filtration, the list was reduced to 1447 sequences corresponding to fungi isolated from various substrata within the caves worldwide. These sequences correspond to a total of 445 fungal species, members of the 151 genera. Countries with the highest numbers of NCBI entries belonging to representatives of cave mycobiota, determined through geo_loc_name (qualifier of geographic location of the collected sample), are Spain, China, the USA, and France, followed by Brazil, Poland, and Slovakia (Figure 1).
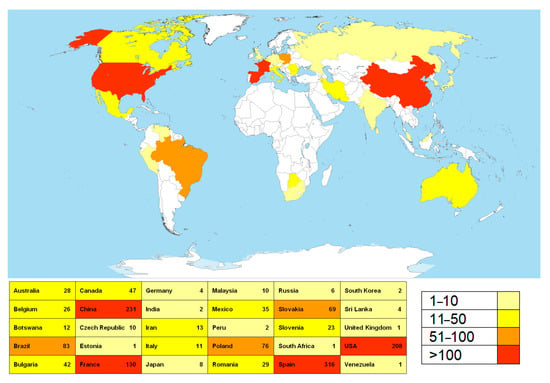
Figure 1.
The countries with the highest number of sequences of cave fungi deposited to the NCBI database. (The map was downloaded and modified from the following: https://en.wikipedia.org/wiki/File:A_large_blank_world_map_with_oceans_marked_in_blue.PNG (accessed on 28 February 2025); permission is granted to copy, distribute, and/or modify this document under the terms of the GNU Free Documentation License, Version 1.2 or any later version published by the Free Software Foundation (https://commons.wikimedia.org/wiki/Commons:GNU_Free_Documentation_License,_version_1.2 (accessed on 28 February 2025)); this file is licensed under the Creative Commons Attribution-Share Alike 3.0 Unported license (https://creativecommons.org/licenses/by-sa/3.0/deed.en (accessed on 28 February 2025)).
3.1. Taxonomy of Cave Mycobiota—Dominant Phyla
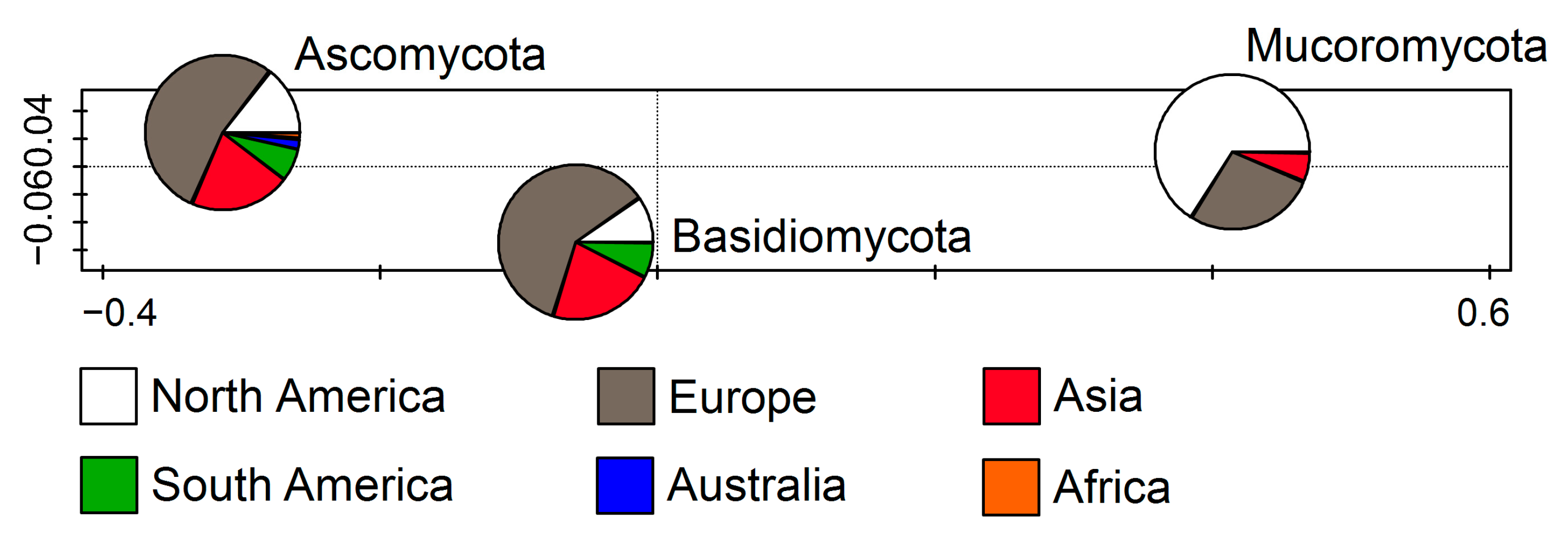
The retrieved sequences represent three fungal phyla: Ascomycota, Basidiomycota, and Mucoromycota. Up to 1074 sequences correspond to Ascomycota (83.45%), while Mucoromycota (10.33%) and Basidiomycota members (6.22%) were encountered far less frequently. According to the RDA (F = 28.7, p = 0.002), which represents the relations between the fungal phyla and the continent on which they were recorded, Ascomycota and Basidiomycota were the most frequently recorded in Europe followed by Asia, while Mucoromycota were mostly documented in North America followed by Europe (Figure 2).
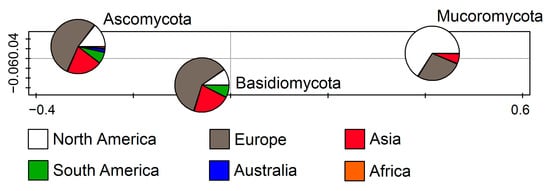
Figure 2.
Redundancy analysis (RDA) showing fungal phyla from cave environments in relation to the continent.
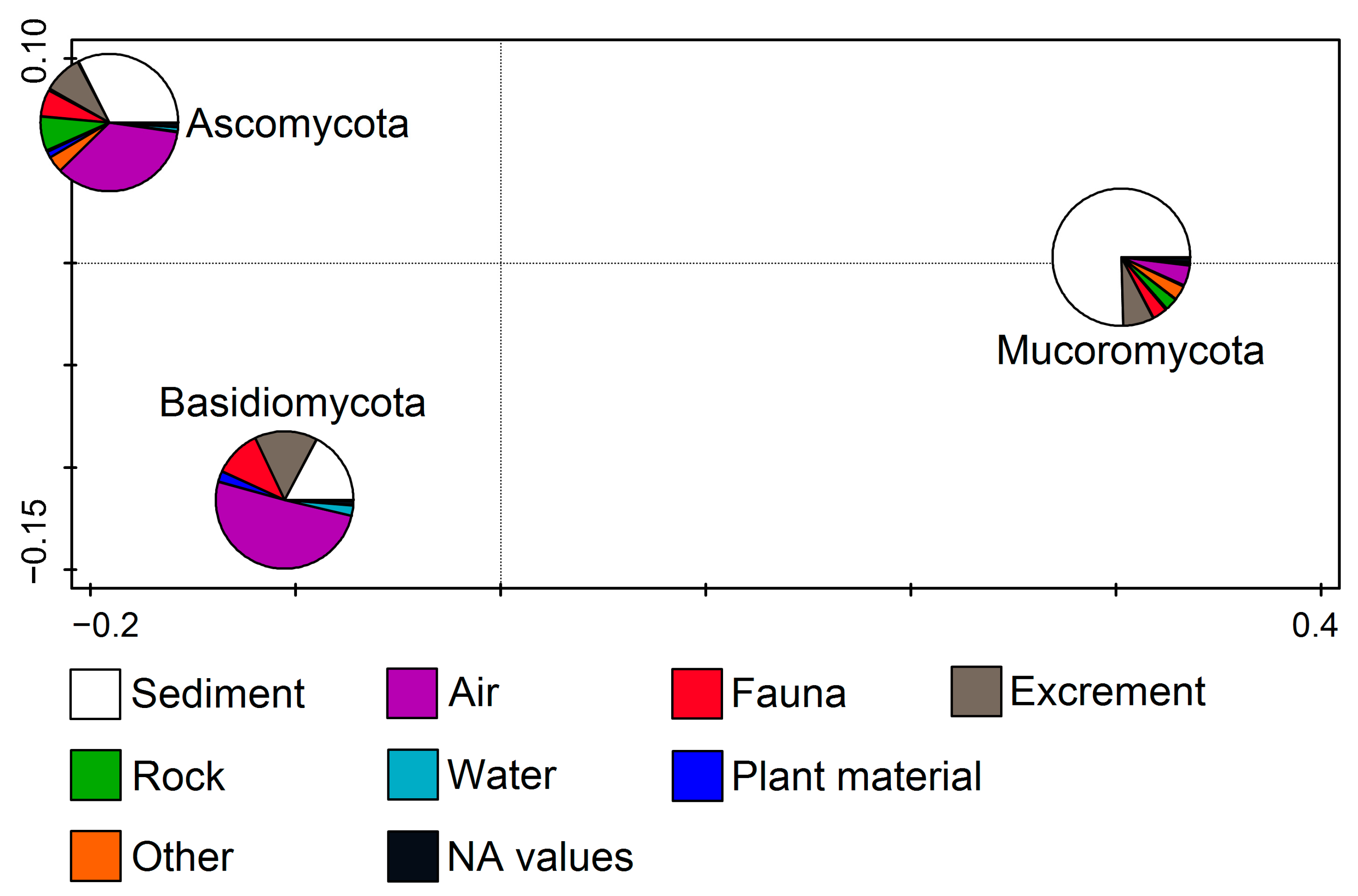
Fungal phyla in relation to the substratum (RDA, F = 11.1, p = 0.002) show that Ascomycota members were related to the air and sediment samples and the Basidiomycota mostly to the air, while the Mucoromycota isolates were mainly found in the sediment samples (Figure 3).
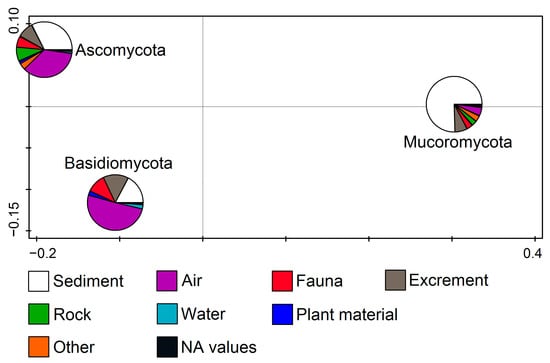
Figure 3.
Redundancy analysis (RDA) showing fungal phyla from cave environments in relation to the substratum.
3.2. Dominant Classes of Fungi in Caves
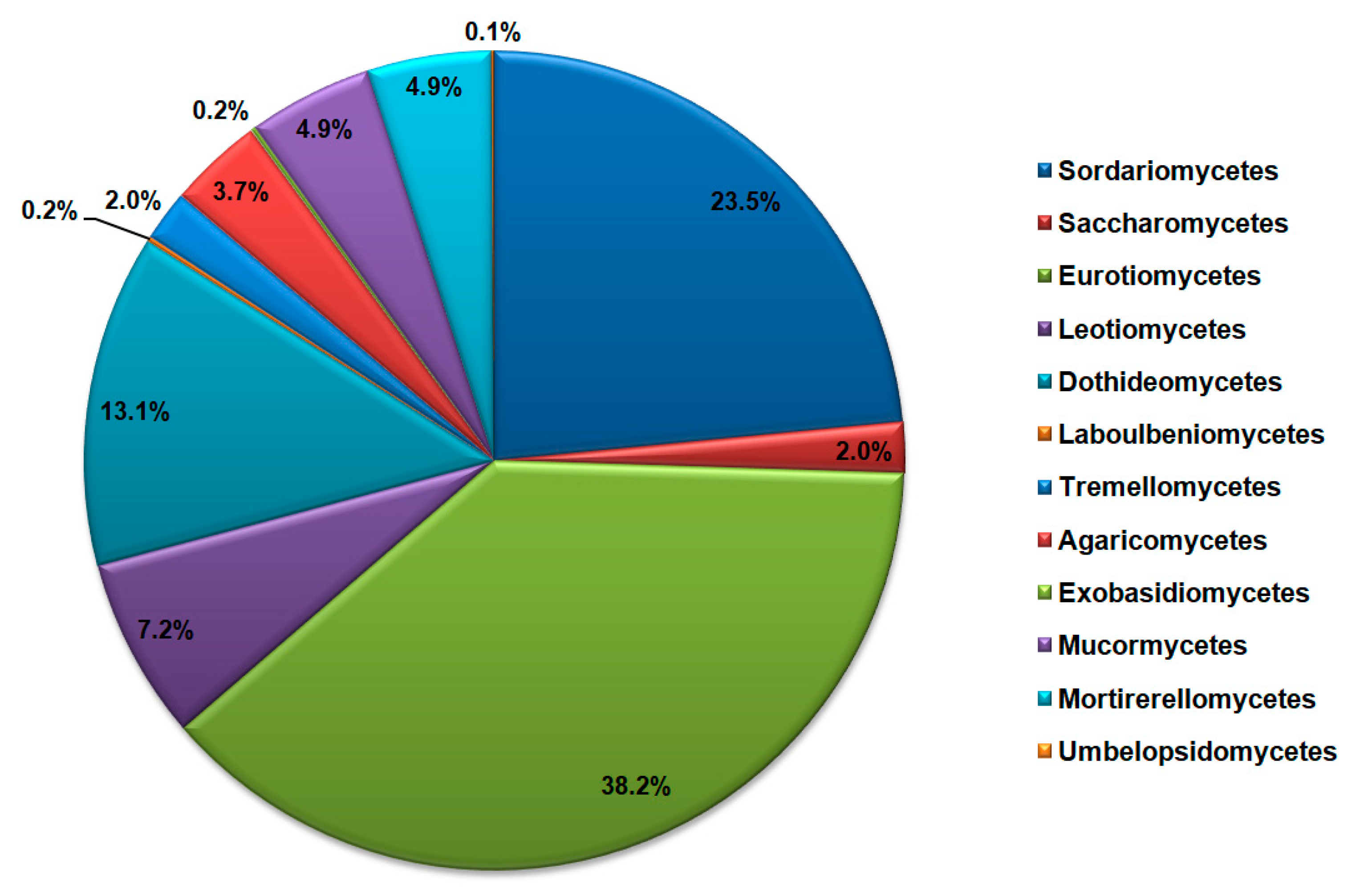
Phylum Ascomycota encompasses sequences of species belonging to classes Sordariomycetes (29.52% of all Ascomycota), Saccharomycetes (2.51%), Eurotiomycetes (47.88%), Leotiomycetes (9.03%), Dothideomycetes (16.48%), and Laboulbeniomycetes (0.19%). On the other hand, sequences of the Basidiomycota phylum were from classes Tremellomycetes (33.75% of all Basidiomycota), Agaricomycetes (62.50%), and Exobasidiomycetes (3.75%), while the phylum Mucoromycota encompasses sequences belonging to classes Mucormycetes (49.62% of all Mucoromycota), Mortirerellomycetes (49.62%) and Umbelopsidomycetes (0.01%). The most dominant classes of cave mycobiota according to the NCBI database sequences search are shown in Figure 4.
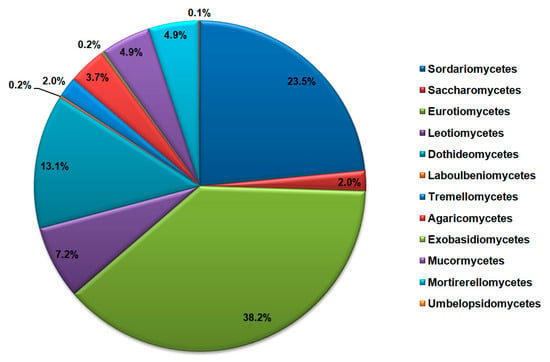
Figure 4.
Most dominant classes of fungi in the caves according to the NCBI database search, represented as the total number of DNA sequences.
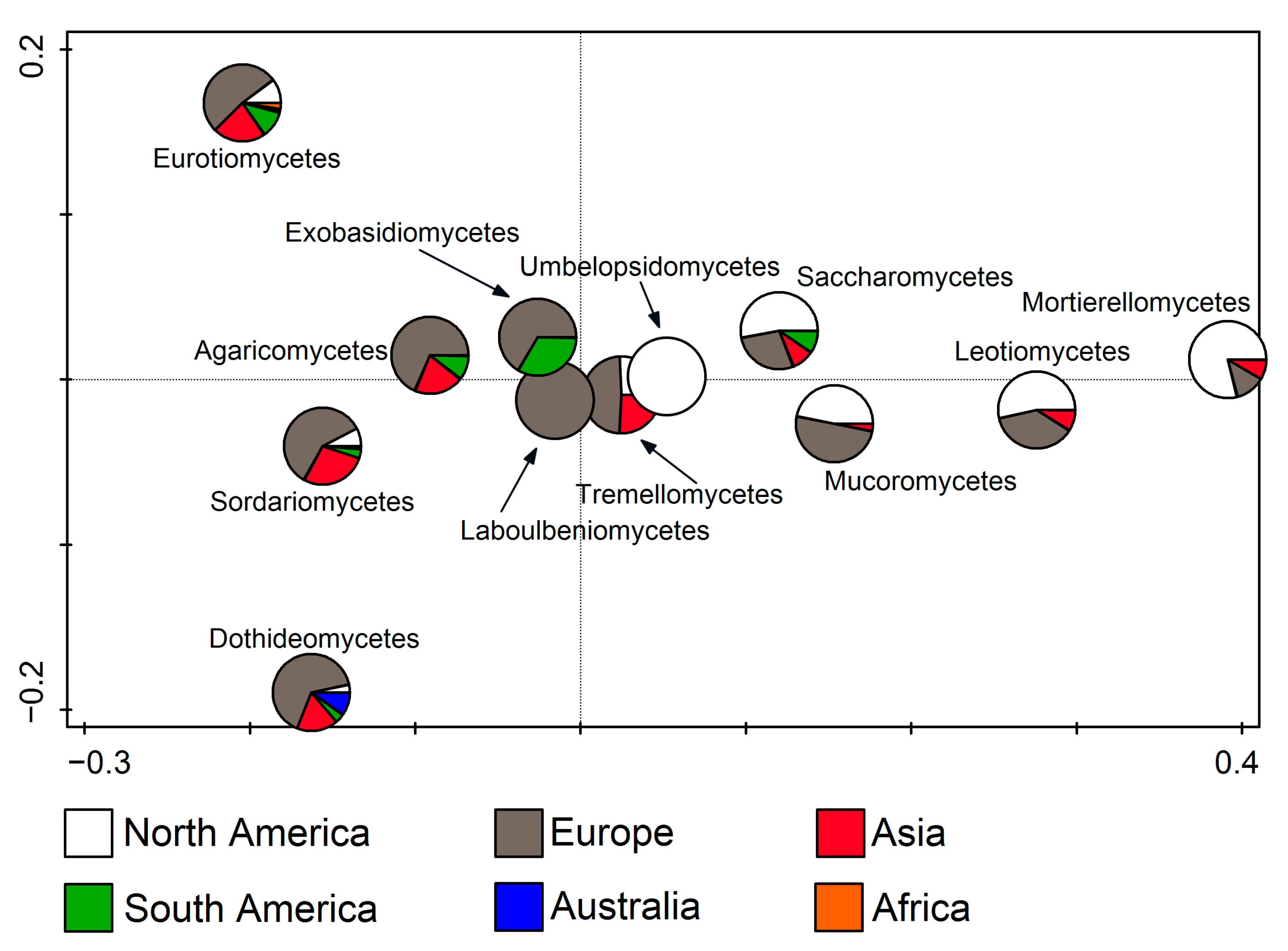
RDA was also used to relate fungal classes to the continents (F = 11.9, p = 0.002). It was noted that Laboulbeniomycetes members were found exclusively in Europe, and Umbelopsidomycetes in North America. Furthermore, it was clearly recognizable that Eurotiomycetes, Agaricomycetes, Sordariomycetes, Dothideomycetes, Tremellomycetes, Mucoromycetes, and Exobasidiomycetes entries dominate in Europe, while Saccharomycetes, Leotiomycetes, and Mortierellomycetes mostly occur in North America. In addition to these two continents, Asia stands out with Eurotiomycetes, Agaricomycetes, Sordariomycetes, Dothideomycetes, Tremellomycetes entries, and South America with Exobasidiomycetes. (Figure 5).
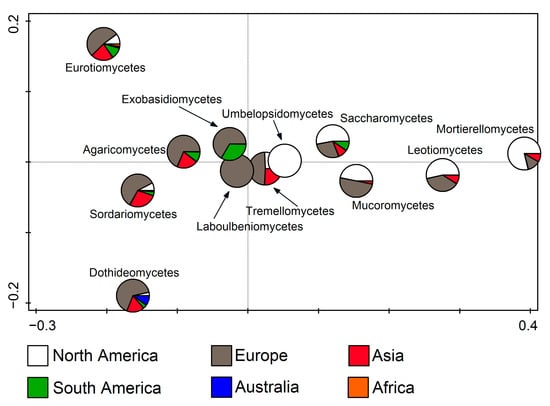
Figure 5.
Redundancy analysis (RDA) showing fungal classes from cave environments in relation to the continent.
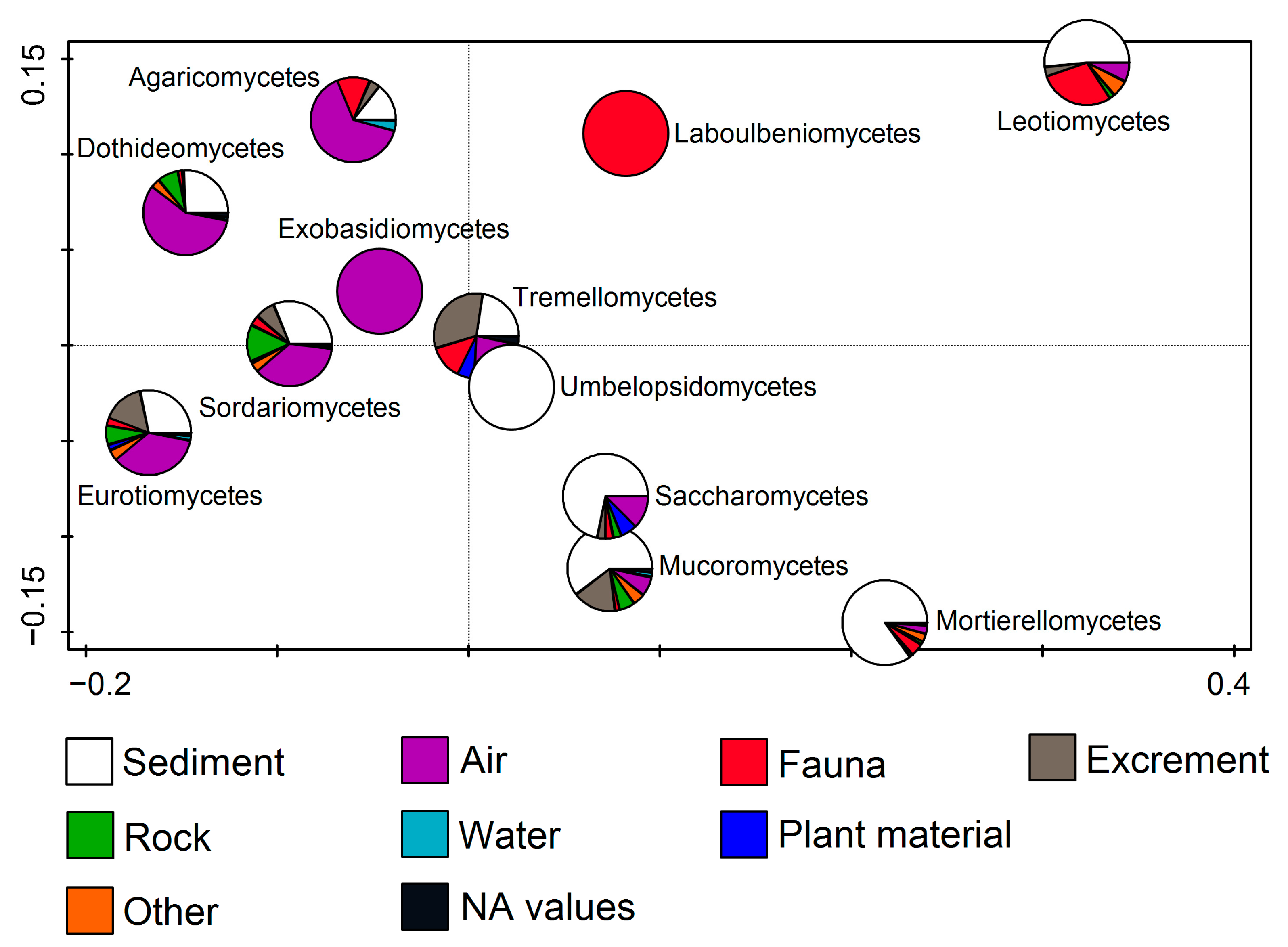
By observing fungal classes in relation to the substratum, RDA (F = 7.8, p = 0.002) showed that members of some classes were connected to only one substratum, while others dominated on multiple substrata. Exobasidiomycetes were related only to air, while Agaricomycetes, Dothideomycetes, Sordariomycetes, and Eurotiomycetes were mostly related to air, followed by the sediment. Members of Umbelopsidomycetes were only found on sediment. Saccharomycetes, Mucoromycetes, and Mortierellomycetes members dominated on sediments, while Tremellomycetes were mostly related to sediments and excrements (including guano). Finally, Leotiomycetes dominated on sediment, followed by fauna (including many bats), while Laboulbeniomycetes representatives were only found on fauna (Figure 6).
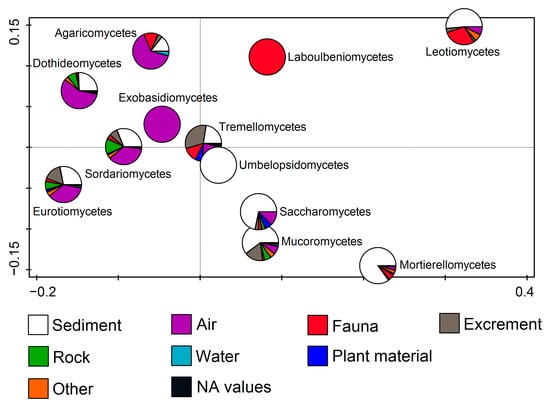
Figure 6.
Redundancy analysis (RDA) showing fungal classes from cave environments in relation to the substratum.
3.3. Dominant Fungal Genera in Caves
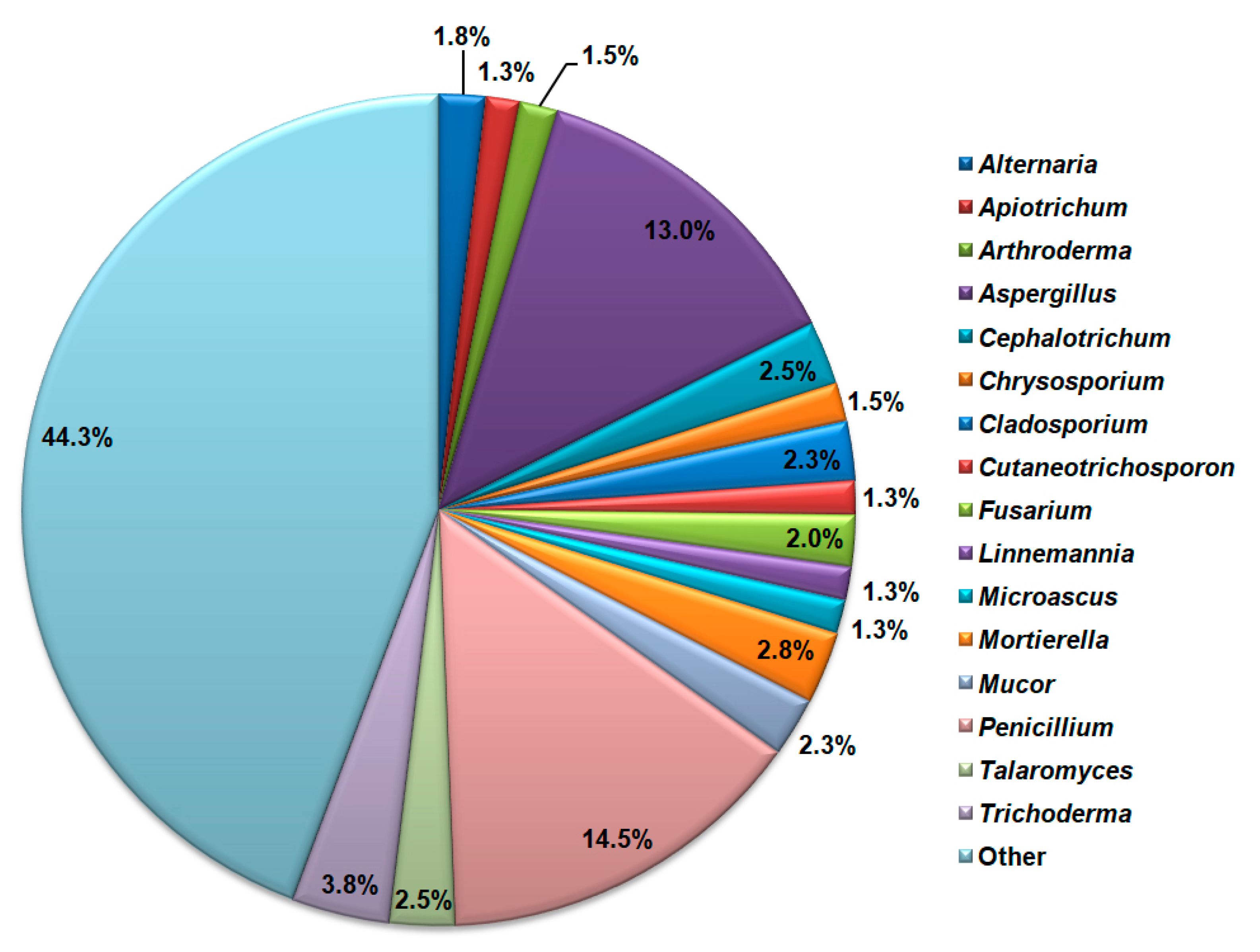
All NCBI-retrieved sequences belong to 151 fungal genera. The highest species richness is detected for the genus Penicillium with 57 detected species, followed by the genus Aspergillus with 51 detected species of cave mycobiota (Figure 7). Trichoderma, Mortierella, Talaromyces, Mucor, and Fusarium genera also had a significant number of cave mycobiota species.
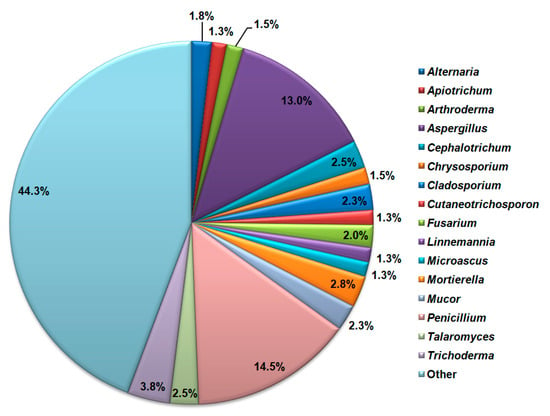
Figure 7.
Cave mycobiota genera with the highest species richness according to the NCBI database search.
3.4. Dominant Fungal Species in Caves
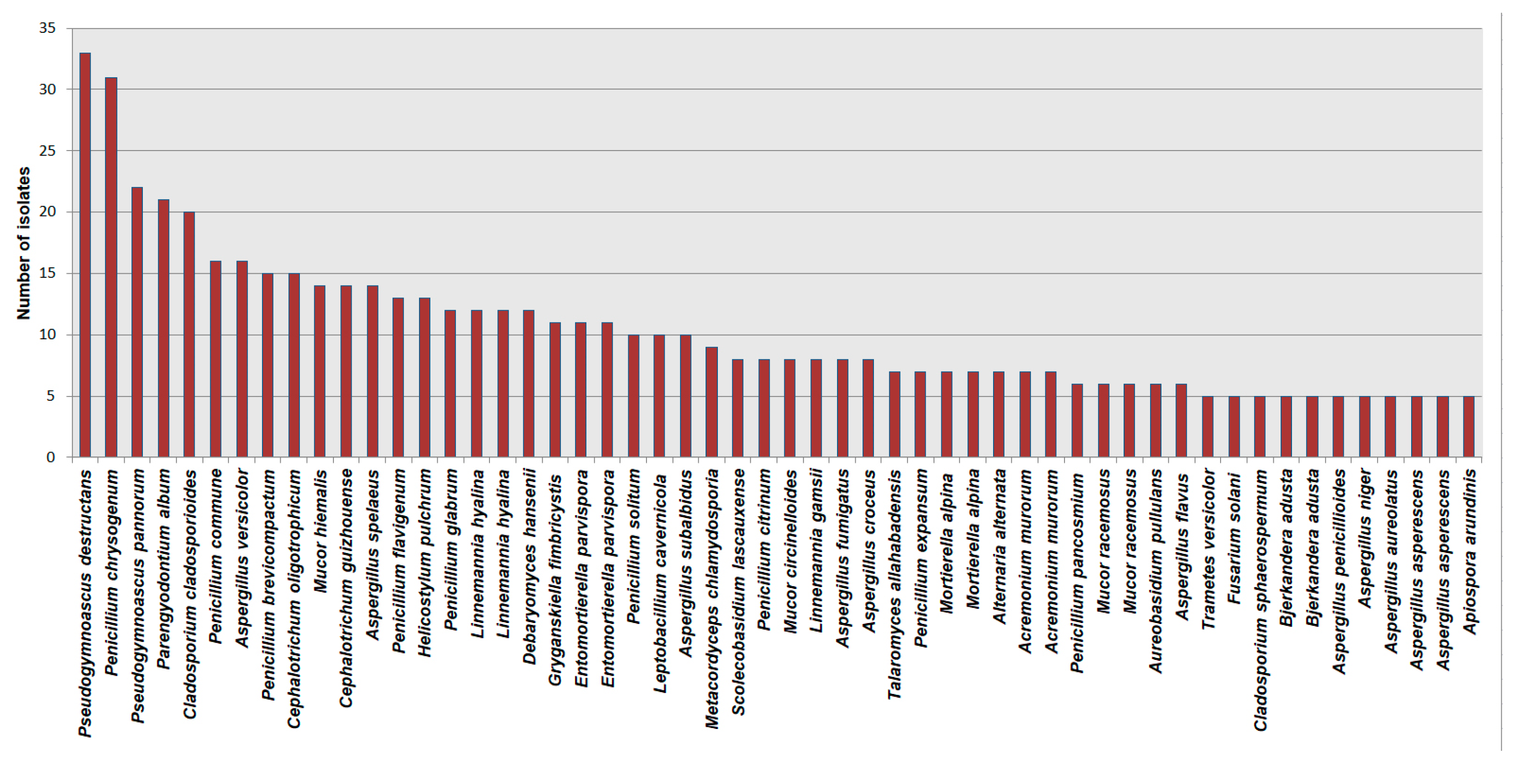
The most frequently encountered species is Pseudogymnoascus destructans, isolated mostly from hibernating bats and guano (33 hits), followed by Penicillium chrysogenum (31 hits). The list of the most dominant cave mycobiota species worldwide is presented in Figure 8.
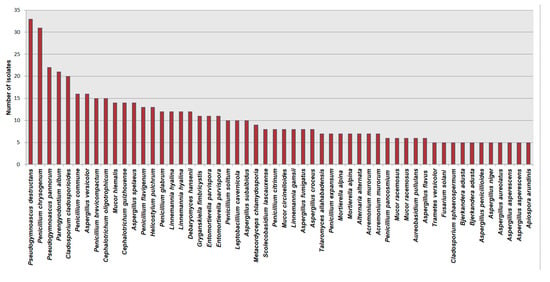
Figure 8.
The list of dominant cave mycobiota species according to the NCBI database.
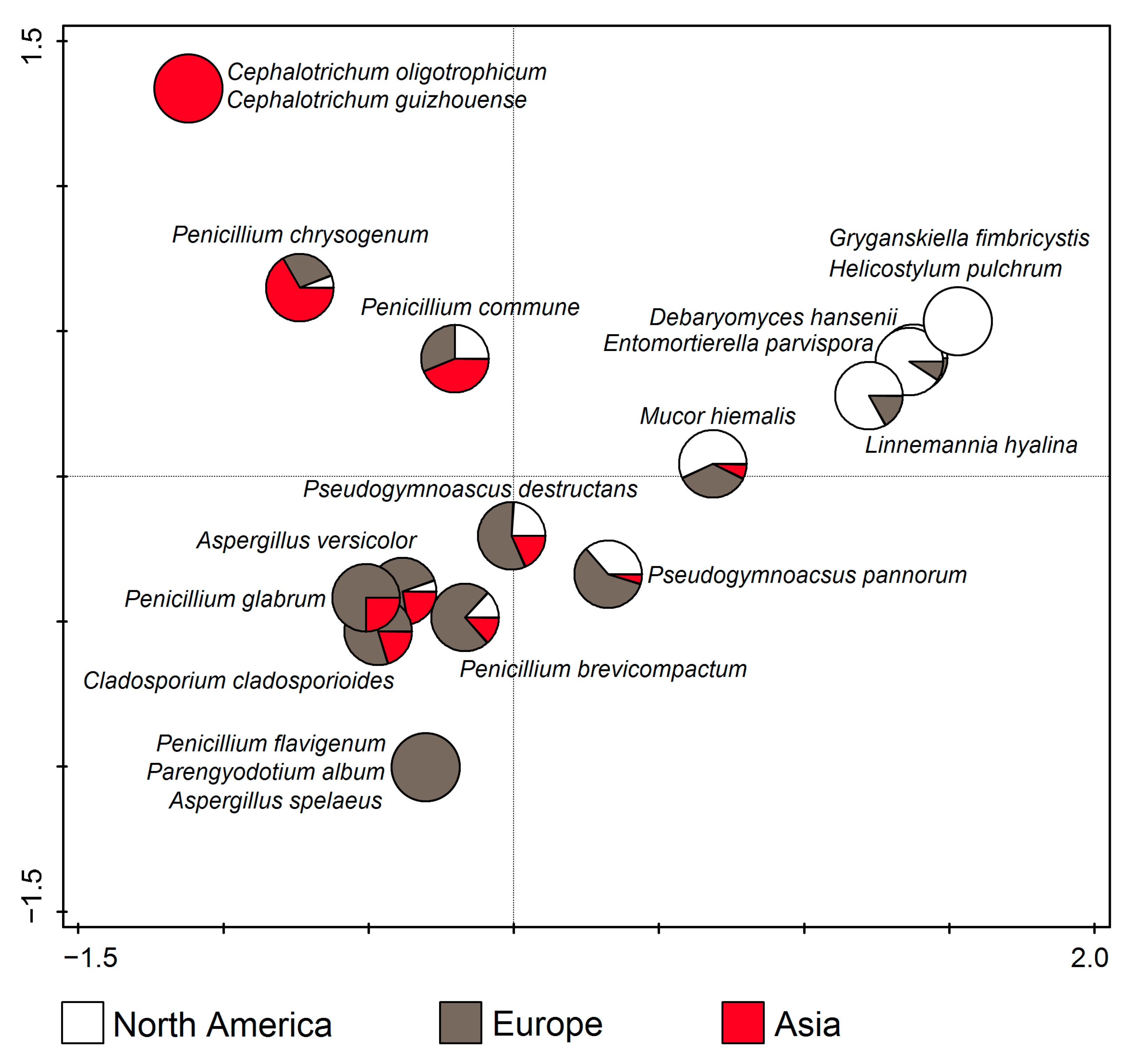
According to RDA (F = 10.0, p = 0.002), Cephalotrichum oligotrophicum and C. guizhouense were only found in Asia, Aspergillus spelaeus, Parengyodotium album, and Penicillium flavigenum in Europe, and Gryganskiella fimbricystis and Helicostylum pulchrum in North America. Apart from that, the taxa in the upper left part of the ordination diagram dominated in Asia, those in the upper right in North America, while those in the lower half of the diagram dominated in Europe. (Figure 9).
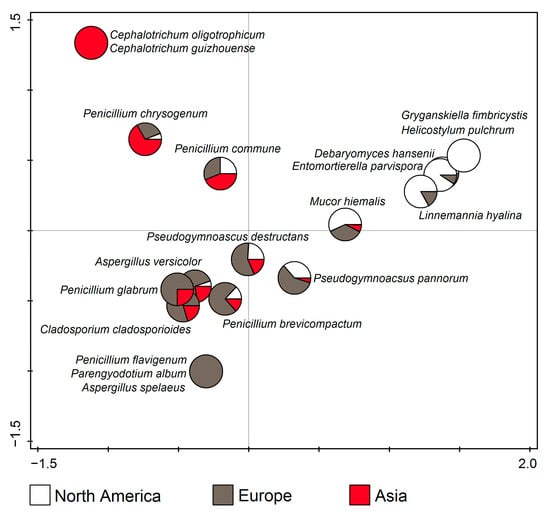
Figure 9.
Canonical correspondence analysis (CCA) showing dominant fungal species from cave environments in relation to the continent.
3.5. New Fungal Species Described in Caves
The NCBI database search revealed the presence of 43 fungal species detected for the first time in caves (Table 1). The genus with the most described cave species was Aspergillius, with seven species first reported from various cave substrata (cave, air, sediment, guano) in Spain, Romania, Brazil, China, and Botswana. Also, cave species richness was documented for the genera Apiotrichum and Cephalotrichum, with both having four species described for the first time in cave environments. All newly discovered Apiotrichum species are from bat guano in Akiyoshi-do cave in Japan. On the other hand, three new species of genus Cephalotrichum were recorded for stone substratum in China, while the C. lignatile is isolated from timber in a cave in Belgium. The substrata with the most reported new fungal species are guano and stone.

Table 1.
Novel fungal species isolated from various cave substrata according to NCBI search.
4. Discussion
This study represents the first attempt to create a review regarding cave mycobiota via NCBI data search accompanied by a statistical approach. These types of papers, with catalogs of DNA sequences, serve as comprehensive compilations of genetic information about a group of organisms that are of interest. In fact, the significance of these studies lies in the fact that they organize data from various manuscripts and databases, making it easier for researchers to access, analyze, and utilize the information. Additionally, since it is very well known that NCBI and other biotechnology repositories contain vast amounts of genomic data, review papers that catalog this information provide a centralized resource, making it more accessible even for those researchers who may not have the time or expertise to navigate complex databases. It should be noted that analyzing a large set of sequences collectively can reveal insights that may not be apparent when examining individual sequences in a sole study. Finally, reviews of this type provide a resource for those working on computational analysis, allowing them to test algorithms, develop models, and draw conclusions based on a well-curated set of genetic data.
The majority of NCBI entries of cave mycobiota are sequences belonging to the members of Ascomycota (83.45%). This is an expected result since this is the fungal phylum with the highest species richness, dominating in both described and estimated global fungal diversity. It contains over 64,000 described species, which is more than 75% of all known fungi [108]. The actual number is likely much higher, with estimates exceeding 2 million species [109]. Namely, the isolation of fungi from various substrata, especially in extreme environments, such as caves, deserts, deep sea habitats, and even Antarctic soils, always results in a high diversity of Ascomycota compared to other fungal phyla [110,111,112]. Unlike Basidiomycota taxa, which need substrata rich in nutrients (such as wood and dung) and often rely on symbiotic relationships with plants, or are plant pathogens, ascomycetes do not depend on photosynthetic organisms and can thrive in complete darkness [48]. Furthermore, many ascomycetes produce antifungal or antibacterial compounds that help them outcompete other microorganisms, giving them a selective advantage in caves where microbial competition is high [113]. Also, many ascomycetes can form SAB [114] and live as endolithic or epilithic fungi [115], contributing to the microbial communities on various stone substrata within the caves, such as cave walls, stalagmites, and stalactites. Due to all these traits (protective secondary metabolites and ability to form SAB), ascomycetes can not only outcompete Basidiomycota members but can also outcompete species of Mucoromycota, which are less competitive in oligotrophic conditions.
According to the RDA analyses, members of the phylum Mucoromycota are predominantly documented in North America. On the other hand, Histoplasma capsulatum, the causative agent of Darling’s disease, is also often documented in Central and Eastern United States caves with high organic matter, particularly in bat and bird guano deposits [116]. It appears that Mucoromycota members are often documented in North American caves where H. capsulatum is reported, and while both thrive in caves with high nitrogen organic content matter, such as guano deposits [117], benefiting from similar environmental conditions; their ecological roles and frequencies differ [118]. It should also be noted that in studies of cave mycobiota conducted in North America, Mucoromycota species could be detected due to sampling bias in caves with potential risks of histoplasmosis outbursts.
RDA analyses additionally showed that propagules of Ascomycota and Basidiomycota (conidia or basidiospores) are more frequently encountered in cave air than the sporangiospores of Mucoromycota. Members of Ascomycota and Basidiomycota phyla actively discharge their spores into the air either by producing dry, hydrophobic conidia that easily become airborne or by ballistic discharge [119]. On the other hand, sporangiospores of Mucoromycota species are often passively released when sporangia ruptures, which often requires mechanical disturbance (e.g., wind, animal movement, or water flow). Furthermore, Mucoromycota species sporangiospores tend to be sticky, larger, and settle quickly, reducing their presence in airborne cave samples [120]. On the contrary, many spores of Ascomycota and Basidiomycota are smaller (~2–10 µm), lightweight, and can remain suspended in the air for long periods of time [121], unlike Mucoromycota sporangiospores, which are larger (often >10 µm), heavier, settle more quickly, tend to aggregate, and are less likely to be widely dispersed in still cave air. This in mind, Mucoromycota spores tend to stay near their source (e.g., soil, guano, decaying material) unless disturbed by human activity or animal movement [122]. Also, many cave mycological studies use airborne spore sampling with sedimentation plates or air samplers, which favor fungi that release lightweight spores.
According to the NCBI database search, the most dominant fungal classes within the caves are Eurotiomycetes and Sordariomycetes. This is mostly due to their adaptations to oligotrophic and extreme environments. The most frequently encountered genera in caves belonging to the class Eurotiomycetes are Aspergillus and Penicillium, which are characterized by thick-walled spores (conidia) that can survive harsh conditions, including desiccation and low nutrient availability [122]. Some Eurotiomycetes are commonly found in soil, dust, and on animals, allowing them to be introduced into caves through human visitation or animal migration [123]. On the other hand, many Sordariomycetes, such as Fusarium, Trichoderma, and Chaetomium, are decomposers that break down organic matter like guano, plant debris [124], or even keratinous material from animal remains [125]. Therefore, their ability to degrade complex organic compounds allows them to persist in caves where nutrient sources are scarce. Similarly, Sordariomycetes produce resistant spores (ascospores and conidia) that can remain dormant for long periods and germinate when conditions become favorable. Air currents, water, animals, and even human visitors can introduce and disperse these spores. It is known that bat and bird guano often serve as hotspots for fungal activity, including many Sordariomycetes. Species like Fusarium and Beauveria have been found in guano deposits, where they contribute to nutrient cycling and sometimes even act as opportunistic pathogens of insects [48,126]. According to RDA analyses, only two fungal classes are linked with specific substratum. Namely, Laboulbeniomycetes were recorded only on fauna elements in Europe, while Exobasidiomycetes were exclusively airborne. The NCBI database search revealed the presence of only two entries of Laboulbeniomycetes in caves, both members of the genus Arthrorhynchus (A. eucampsipodae MT241715 and A. nycteribiae MT235715) recorded on bat flies Nycteribia vexata and Penicillidia conspicua parasitizing chiropterans Myotis blythii and M. schreibersii in Mandrata Cave in Bulgaria [88]. Representatives of the class Exobasidiomycetes documented in this study (members of genera Sympodiomycopsis, Tilletiopsis, and Golubevia) are exclusively associated with plants, as saprobes or pathogens—causative agents of galls (abnormal outgrowths of plant tissues) [127]—and as such cannot colonize any substratum within the caves. Therefore, these species could be regarded as trogloxenic. Furthermore, these fungi produce a variety of fungal spores, among them basidiospores and balistospores, which could be dispersed by air currents and as such detected via air sampling devices. Finally, there is only one NCBI entry of Umbelopsidomycetes, exclusively found in the cave of North America (Umbelopsis isabellina KC009121) from bat sediments [61].
Genera Penicillium and Aspergillus showed the highest species richness in caves worldwide. Members of both genera can break down a wide range of organic materials, including guano, plant debris, and even mineral substrata. Some species can utilize nitrogen sources from bat guano effectively and were found to be dominant in this substratum [128]. It is well known that Aspergilli and Penicillia produce airborne conidia that are highly resistant and easily dispersed by air currents, water, and animal movements, allowing them to colonize different cave microhabitats. Many Penicillium species can tolerate low temperatures, high humidity, and nutrient-poor environments, which are common in caves. Some also exhibit resistance to desiccation. Conversely, Aspergillus species, particularly xerophilic ones (e.g., A. niger, A. flavus), are well adapted to extreme cave conditions, including low water availability and oligotrophic environments [122]. Species belonging to both genera produce antifungal, antibacterial, and mycotoxic compounds, which allow them to compete with other cave microorganisms [122,129]. Furthermore, both genera benefit from the relatively stable temperature and humidity levels found in caves, which support their slow but steady growth. While Penicillium species tend to dominate in cooler and more humid cave environments, Aspergillus species (especially A. niger, A. flavus, and A. fumigatus) are more frequently found in drier, warmer conditions and are more tolerant to low water activity [130]. Some Aspergillus species such as A. fumigatus (eight NCBI entries in research presented here) are opportunistic pathogens that can affect birds and humans, especially in enclosed cave environments with high spore concentrations [11,61]. On the contrary, Penicillium species generally pose less of a health risk, although their spores can be allergenic [131]. The Penicillium species with the most cave mycobiota NCBI hits is P. chrysogenum. The most entries in the NCBI database of this species are from China and Spain, and their sources are, in the majority of cases, sediment, air, and rock substratum. The presence of this species on rock substratum must not be neglected since this species has the potential to deteriorate limestone substrata, including cave walls [132,133].
Pseudogymnoascus destructans is the most frequently encountered fungal species documented during the NCBI database search with 33 hits. Most of the entries are from the USA, Italy, and Russia, and bat species Myotis lucifugus, Myotis emarginatus, Perimyotis subflavus, and Myotis dasycneme are listed as hosts. The majority of outbreaks of P. destructans occur primarily in temperate regions of North America and Europe, where cave-dwelling bats hibernate [134]. Detected for the first time in a cave in Albany (New York) in 2006, the fungus has since spread to over 35 US states and 7 Canadian provinces [135]. Although detected in various countries in Central and Eastern Europe, European bats seem to have coevolved with P. destructans, experiencing fewer mortality events compared to those inhabiting North America [136]. Although P. destructans is commonly found in caves and mines, where it infects hibernating bats, it has also been detected in non-cave environments, such as soil and bat roosts outside caves, and as such can be regarded as troglophilic or trogloxenic.
Many novel fungal species were documented in caves because these environments harbor low-competition, nutrient-limited, and stable microhabitats that favor unique or cryptic species. Additionally, improved sampling techniques and state-of-the-art molecular tools are uncovering previously unknown fungal diversity in caves around the world [14,104]. Additionally, caves contain microhabitats that are rarely sampled in surface ecosystems, increasing the likelihood of finding new species, e.g., bat guano piles, which are rich in nitrogen (therefore “attracting” unique fungal communities) [116]. However, it should be emphasized that some fungi isolated from caves may be cryptic species—closely related to known taxa but genetically distinct. Likewise, caves can act as refugia, preserving ancient fungal lineages that are extinct or rare in surface habitats [137]. Modern culture-independent techniques (e.g., metabarcoding, environmental DNA studies) allow researchers to detect fungi that are difficult to cultivate in laboratory conditions [138]. In fact, many previously undescribed fungal species are detected only after the molecular analysis of cave samples [14,24]. Finally, novel fungal species are often isolated because researchers are specifically looking for organisms with biotechnological potential (e.g., antibiotic production, the biodegradation of pollutants) [15,20].
To overcome the restrictions of the low availability of nutrients in cave ecosystems, microbial populations create collective structures (such as various kinds of biofilms) where they collaborate and establish mutualistic connections rather than fighting for resources. This is the reason why the majority of environmental microorganisms (including fungi) cannot grow in laboratory settings due to the fact that their growth is dependent on certain interactions with other species and cannot occur on its own [139]. Furthermore, fungi and other microorganisms in caves often rely on alternative metabolic pathways, such as breaking down unusual organic compounds [140]. Many psychrotolerant and xerotolerant fungi thrive in caves, including species that may go unnoticed in drier surface environments [110]. Some cave fungi exhibit adaptations similar to extremophiles, tolerating high salinity, heavy metals, or radiation [141]. Therefore, it could be stated that caves act as “natural laboratories for extremophiles”.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/jof11040286/s1: Supplementary Table S1 (List of NCBI entries of fungi from cave environments); Supplementary File S1.
Author Contributions
Conceptualization, M.S. and Ž.S.; methodology, M.S. and S.P.; software, S.P.; validation, M.S.; formal analysis, Ž.S. and S.P.; investigation, M.S., Ž.S. and S.P.; resources, M.S.; data curation, M.S. and S.P.; writing—original draft preparation, M.S. and Ž.S.; writing—review and editing, M.S., S.P. and Ž.S.; visualization, S.P. and Ž.S.; supervision, M.S.; project administration, M.S.; funding acquisition, M.S., S.P. and Ž.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science, Technological Development and Innovation of the Republic of Serbia, grant number 451-03-136/2025-03/200178.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wasti, I.G.; Fui, F.S.; Zhi, T.Q.; Mun, C.W.; Kassim, M.H.S.; Dawood, M.M.; Hasan, N.H.; Subbiah, V.K.; Khan, F.A.A.; Seelan, J.S.S. Fungi from Dead Arthropods and Bats of Gomantong Cave, Northern Borneo, Sabah (Malaysia). J. Cave Karst Stud. 2020, 82, 4. [Google Scholar] [CrossRef]
- Pusz, W.; Ogórek, R.; Uklańska-Pusz, C.M.; Zagożdżon, P. Speleomycological Research in Underground Osówka Complex in Sowie Mountains (Lower Silesia, Poland). Int. J. Speleol. 2014, 43, 3. [Google Scholar] [CrossRef]
- Zhu, H.Z.; Jiang, C.Y.; Liu, S.J. Microbial Roles in Cave Biogeochemical Cycling. Front. Microbiol. 2022, 13, 950005. [Google Scholar] [CrossRef]
- Trajano, E. Ecological classification of subterranean organisms. Encycl. Caves 2012, 2, 275–277. [Google Scholar]
- Hoyt, J.R.; Kilpatrick, A.M.; Langwig, K.E. Ecology and Impacts of White-Nose Syndrome on Bats. Nat. Rev. Microbiol. 2021, 19, 196–210. [Google Scholar] [CrossRef]
- Haelewaters, D.; Page, R.A.; Pfister, D.H. Laboulbeniales Hyperparasites (Fungi, Ascomycota) of Bat Flies: Independent Origins and Host Associations. Ecol. Evol. 2018, 8, 8396–8418. [Google Scholar] [CrossRef]
- Popović, S.; Simić, G.; Stupar, M.; Unković, N.; Krunić, O.; Savić, N.; Grbić, M. Cave Biofilms: Characterization of Phototrophic Cyanobacteria and Algae and Chemotrophic Fungi from Three Caves in Serbia. J. Cave Karst Stud. 2017, 79, 10–23. [Google Scholar] [CrossRef]
- Bastian, F.; Jurado, V.; Nováková, A.; Alabouvette, C.; Sáiz-Jiménez, C. The Microbiology of Lascaux Cave. Microbiology 2010, 156, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Wu, F.; He, D.; Xu, R.; Feng, H.; Chen, T.; Wang, W. Seasonal Variation of Airborne Fungi of the Tiantishan Grottoes and Western Xia Museum, Wuwei, China. Sci. Cold Arid Reg. 2021, 13, 522–532. [Google Scholar] [CrossRef]
- Ljaljević Grbić, M.; Dimkić, I.; Savković, Ž.; Stupar, M.; Knežević, A.; Jelikić, A.; Unković, N. Mycobiome Diversity of the Cave Church of Sts. Peter and Paul in Serbia—Risk Assessment Implication for the Conservation of Rare Cavern Habitat Housing a Peculiar Fresco Painting. J. Fungi 2022, 8, 1263. [Google Scholar] [CrossRef]
- Stupar, M.; Savković, Ž.; Popović, S.; Simić, G.S.; Ljaljević Grbić, M. Speleomycology of Air in Stopića Cave (Serbia). Microb. Ecol. 2023, 86, 2021–2031. [Google Scholar] [CrossRef]
- Ogórek, R. Speleomycology of Air in Demänovská Cave of Liberty (Slovakia) and New Airborne Species for Fungal Sites. J. Cave Karst Stud. 2018, 80, 3, 153–160. [Google Scholar] [CrossRef]
- Martin-Sanchez, P.M.; Nováková, A.; Bastian, F.; Alabouvette, C.; Sáiz-Jiménez, C. Use of Biocides for the Control of Fungal Outbreaks in Subterranean Environments: The Case of the Lascaux Cave in France. Environ. Sci. Technol. 2012, 46, 3762–3770. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Liu, F.; Zhou, X.; Liu, X.Z.; Liu, S.J.; Cai, L. Culturable Mycobiota from Karst Caves in China, with Descriptions of 20 New Species. Persoonia 2017, 39, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.F.; Zhao, P.; Cai, L. Origin of Cave Fungi. Front. Microbiol. 2018, 9, 1407. [Google Scholar] [CrossRef] [PubMed]
- Cyske, Z.; Jaroszewicz, W.; Żabińska, M.; Lorenc, P.; Sochocka, M.; Bielańska, P.; Węgrzyn, G. Unexplored Potential: Biologically Active Compounds Produced by Microorganisms from Hard-to-Reach Environments and Their Applications. Acta Biochim. Pol. 2021, 68, 565–574. [Google Scholar] [CrossRef]
- Rutherford, J.M.; Huang, L.M. A study of fungi of remote sediments in West Virginia caves and a comparison with reported species in the literature. Natl. Speleol. Soc. Bull. 1994, 56, 38–45. [Google Scholar]
- Vanderwolf, K.J.; Malloch, D.; McAlpine, D.F. A world review of fungi, yeasts, and slime molds in caves. Int. J. Speleol. 2013, 42, 77–96. [Google Scholar] [CrossRef]
- Kato, N.N.; Arini, G.S.; Silva, R.R.; Bichuette, M.E.; Bitencourt, J.A.P.; Lopes, N.P. The world of cave microbiomes: Biodiversity, ecological interactions, chemistry, and the multi-omics integration. J. Braz. Chem. Soc. 2024, 35, e20230148. [Google Scholar] [CrossRef]
- Barbosa, R.N.; Felipe, M.T.C.; Silva, L.F.; Silva, E.A.; Silva, S.A.; Herculano, P.N.; Prazeres, J.F.S.A.; Lima, J.M.S.; Bezerra, J.D.P.; Moreira, K.A.; et al. A Review of the Biotechnological Potential of Cave Fungi: A Toolbox for the Future. J. Fungi 2025, 11, 145. [Google Scholar] [CrossRef]
- Bontemps, Z.; Alonso, L.; Pommier, T.; Hugoni, M.; Moënne-Loccoz, Y. Microbial ecology of tourist Paleolithic caves. Sci. Total Environ. 2022, 816, 151492. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, H.T.; Barton, H.A. Comparison of the White-Nose Syndrome Agent Pseudogymnoascus to Cave-Dwelling Relatives Suggests Reduced Saprotrophic Enzyme Activity. PLoS ONE 2014, 9, e86437. [Google Scholar] [CrossRef]
- Del Valle, A.; Martínez, V.; Figueroa, M.; Raja, H.A.; Mata, R. Alkaloids from the Fungus Penicillium spathulatum as α-Glucosidase Inhibitors. Planta Med. 2016, 82, 1345–1351. [Google Scholar] [CrossRef]
- Visagie, C.M.; Yilmaz, N.; Vanderwolf, K.; Renaud, J.B.; Sumarah, M.W.; Houbraken, J.; Assebgui, R.; Seifert, K.A.; Malloch, D. Penicillium Diversity in Canadian Bat Caves, Including a New Species, P. speluncae. Fungal Syst. Evol. 2020, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Woudenberg, J.H.C.; Sandoval-Denis, M.; Houbraken, J.; Seifert, K.A.; Samson, R.A. Cephalotrichum and related synnematous fungi with notes on species from the built environment. Stud. Mycol. 2017, 88, 137–159. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.J.; Frisvad, J.C.; Sun, B.D.; Varga, J.; Kocsubé, S.; Dijksterhuis, J.; Kim, D.H.; Hong, S.B.; Houbraken, J.; Samson, R.A. Aspergillus Section Nidulantes (Formerly Emericella): Polyphasic Taxonomy, Chemistry and Biology. Stud. Mycol. 2016, 84, 1–118. [Google Scholar] [CrossRef]
- Carmichael, S.K.; Zorn, B.T.; Santelli, C.M.; Roble, L.A.; Carmichael, M.J.; Bräuer, S.L. Nutrient Input Influences Fungal Community Composition and Size and Can Stimulate Manganese (II) Oxidation in Caves. Environ. Microbiol. Rep. 2015, 7, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Houbraken, J.; Frisvad, J.C.; Seifert, K.A.; Overy, D.P.; Tuthill, D.M.; Valdez, J.G.; Samson, R.A. New penicillin-producing Penicillium species and an overview of section Chrysogena. Persoonia Mol. Phylogeny Evol. Fungi 2012, 29, 78–100. [Google Scholar] [CrossRef] [PubMed]
- Puechmaille, S.J.; Wibbelt, G.; Korn, V.; Fuller, H.; Forget, F.; Mühldorfer, K.; Kurth, A.; Bogdanowicz, W.; Borel, C.; Bosch, T.; et al. Pan-European Distribution of White-Nose Syndrome Fungus (Geomyces destructans) Not Associated with Mass Mortality. PLoS ONE 2011, 6, e19167. [Google Scholar] [CrossRef]
- Martínková, N.; Bačkor, P.; Bartonička, T.; Blažková, P.; Červený, J.; Falteisek, L.; Gaisler, J.; Hanzal, V.; Horáček, D.; Hulva, P.; et al. Increasing Incidence of Geomyces destructans Fungus in Bats from the Czech Republic and Slovakia. PLoS ONE 2010, 5, e13853. [Google Scholar] [CrossRef]
- Gargas, A.; Trest, M.T.; Christensen, M.; Volk, T.J.; Blehert, D.S. Geomyces destructans sp. nov. Associated with Bat White-Nose Syndrome. Mycotaxon 2009, 108, 147–154. [Google Scholar] [CrossRef]
- Silva, E.I.P.; Jayasingha, P.; Senanayake, S.; Dandeniya, A.; Munasinghe, D.H. Microbiological Study in a Gneissic Cave from Sri Lanka, with Special Focus on Potential Antimicrobial Activities. Int. J. Speleol. 2021, 50, 4. [Google Scholar] [CrossRef]
- Dlauchy, D.; Tornai-Lehoczki, J.; Sedláček, I.; Audy, M.; Péter, G. Debaryomyces psychrosporus sp. nov., a yeast species from a Venezuelan cave. Antonie Van Leeuwenhoek 2011, 99, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Pusz, W.; Ogórek, R.; Knapik, R.; Kozak, B.; Bujak, H. The Occurrence of Fungi in the Recently Discovered Jarkowicka Cave in the Karkonosze Mts. (Poland). Geomicrobiol. J. 2015, 32, 59–67. [Google Scholar] [CrossRef]
- Alves, V.C.S.; Lira, R.A.; Lima, J.M.S.; Barbosa, R.N.; Bento, D.M.; Barbier, E.; Bezerra, J.D.P. Unravelling the Fungal Darkness in a Tropical Cave: Richness and the Description of One New Genus and Six New Species. Fungal Syst. Evol. 2022, 10, 139–167. [Google Scholar] [CrossRef]
- Singh, S.K.; Thapliyal, M.; Guleri, S.; Singh, K.; Bajpayee, A.B.; Saklani, K.; Kumar, A.; Sahni, S.; Kumar, R. First report on occurrence of Clonostachys in cave ecosystem from India. J. Mycopathol. Res. 2022, 60, 267–271. [Google Scholar]
- Ogórek, R.; Suchodolski, J.; Piecuch, A.; Przywara, K.; Višňovská, Z. Keratinophilic and Keratinolytic Fungi in Cave Ecosystems: A Culture-Based Study of Brestovská Cave and Demänovská Ľadová and Slobody Caves (Slovakia). Appl. Sci. 2022, 12, 1455. [Google Scholar] [CrossRef]
- Jurado, V.; Del Rosal, Y.; Liñan, C.; Martin-Pozas, T.; Gonzalez-Pimentel, J.L.; Saiz-Jimenez, C. Diversity and Seasonal Dynamics of Airborne Fungi in Nerja Cave, Spain. Appl. Sci. 2021, 11, 6236. [Google Scholar] [CrossRef]
- Liu, X.; Tibpromma, S.; Zhang, F.; Xu, J.; Chethana, K.W.T.; Karunarathna, S.C.; Mortimer, P.E. Neopestalotiopsis cavernicola sp. nov. from Gem Cave in Yunnan Province, China. Phytotaxa 2021, 512, 1–27. [Google Scholar] [CrossRef]
- Ogórek, R.; Speruda, M.; Borzęcka, J.; Piecuch, A.; Cal, M. First Speleomycological Study on the Occurrence of Psychrophilic and Psychrotolerant Aeromycota in the Brestovská Cave (Western Tatras Mts., Slovakia) and First Reports for Some Species at Underground Sites. Biology 2021, 10, 497. [Google Scholar] [CrossRef]
- Habibi, A.; Safaiefarahani, B. Identification of Fungi from Soil and Sediment in Jefriz Cave; the First Survey in a Cave from Iran. J. Cave Karst Stud. 2021, 83, 71–77. [Google Scholar] [CrossRef]
- Torres-Cruz, T.J.; Porras-Alfaro, A.; Caimi, N.A.; Nwabologu, O.; Strach, E.W.; Read, K.J.; Northup, D.E. Are Microclimate Conditions in El Malpais National Monument Caves in New Mexico, USA Suitable for Pseudogymnoascus Growth? Int. J. Speleol. 2019, 48, 7. [Google Scholar] [CrossRef]
- Man, B.; Wang, H.; Xiang, X.; Wang, R.; Yun, Y.; Gong, L. Phylogenetic Diversity of Culturable Fungi in the Heshang Cave, Central China. Front. Microbiol. 2015, 6, 1158. [Google Scholar] [CrossRef] [PubMed]
- Leplat, J.; Francois, A.; Bousta, F. Leptobacillium cavernicola, a newly discovered fungal species isolated from several Paleolithic-decorated caves in France. Phytotaxa 2022, 571, 186–196. [Google Scholar] [CrossRef]
- de Sá, A.S.F.; Leonardo-Silva, L.; Xavier-Santos, S. Expanding the Geographical Distribution of Blastobotrys malaysiensis (Saccharomycetales) Beyond the Asian Continent—A Cave Fungus First Reported in the Americas. Biodivers. Data J. 2022, 10, e80226. [Google Scholar] [CrossRef]
- Leplat, J.; François, A.; Touron, S.; Frouin, M.; Portais, J.-C.; Bousta, F. Aerobiological Behavior of Paleolithic Rock Art Sites in Dordogne (France): A Comparative Study in Protected Sites Ranging from Rock Shelters to Caves, with and without Public Access. Aerobiologia 2020, 36, 355–374. [Google Scholar] [CrossRef]
- Abrashev, R.; Krumova, E.; Petrova, P.; Eneva, R.; Kostadinova, N.; Miteva-Staleva, J.; Engibarov, S.; Stoyancheva, G.; Gocheva, Y.; Kolyovska, V.; et al. Distribution of a Novel Enzyme of Sialidase Family Among Native Filamentous Fungi. Fungal Biol. 2021, 125, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.O.; Bezerra, J.D.; Oliveira, T.G.; Barbier, E.; Bernard, E.; Machado, A.R.; Souza-Motta, C.M. Living in the Dark: Bat Caves as Hotspots of Fungal Diversity. PLoS ONE 2020, 15, e0243494. [Google Scholar] [CrossRef]
- Crous, P.W.; Cowan, D.A.; Maggs-Kölling, G.; Yilmaz, N.; Larsson, E.; Angelini, C.; Brandrud, T.E.; Dearnaley, J.D.W.; Dima, B.; Dovana, F.; et al. Fungal Planet description sheets: 1112–1181. Persoonia Mol. Phylogeny Evol. Fungi 2020, 45, 251. [Google Scholar] [CrossRef]
- Liu, F.; Wang, J.; Li, H.; Wang, W.; Cai, L. Setophoma spp. on Camellia sinensis. Fungal Syst. Evol. 2019, 4, 43–57. [Google Scholar] [CrossRef]
- Garzoli, L.; Riccucci, M.; Patriarca, E.; Debernardi, P.; Boggero, A.; Pecoraro, L.; Picco, A.M. First Isolation of Pseudogymnoascus destructans, the Fungal Causative Agent of White-Nose Disease, in Bats from Italy. Mycopathologia 2019, 184, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Nováková, A.; Hubka, V.; Valinová, Š.; Kolařík, M.; Hillebrand-Voiculescu, A.M. Cultivable Microscopic Fungi from an Underground Chemosynthesis-Based Ecosystem: A Preliminary Study. Folia Microbiol. 2018, 63, 43–55. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Hubka, V.; Ezekiel, C.N.; Hong, S.-B.; Nováková, A.; Chen, A.J.; Arzanlou, M.; Larsen, T.O.; Sklenář, F.; Mahakarnchanakul, W.; et al. Taxonomy of Aspergillus Section Flavi and Their Production of Aflatoxins, Ochratoxins and Other Mycotoxins. Stud. Mycol. 2019, 93, 1–63. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Lasek-Nesselquist, E.; Chaturvedi, V.; Chaturvedi, S. Trichoderma polysporum Selectively Inhibits White-Nose Syndrome Fungal Pathogen Pseudogymnoascus destructans Amidst Soil Microbes. Microbiome 2018, 6, 139. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Ma, Y.; Mao, L.; Wu, F.; Ma, X.; Feng, H. Molecular Characterization of Airborne Fungi in Caves of the Mogao Grottoes, Dunhuang, China. Int. Biodeterior. Biodegrad. 2011, 65, 726–731. [Google Scholar] [CrossRef]
- Tanney, J.B.; Nguyen, H.D.; Pinzari, F.; Seifert, K.A. A Century Later: Rediscovery, Culturing and Phylogenetic Analysis of Diploöspora rosea, a Rare Onygenalean Hyphomycete. Antonie van Leeuwenhoek 2015, 108, 1023–1035. [Google Scholar] [CrossRef]
- Out, B.; Boyle, S.; Cheeptham, N. Identification of Fungi from Soil in the Nakimu Caves of Glacier National Park. J. Exp. Microbiol. Immunol. 2016, 2, 26–32. [Google Scholar]
- Lueschow, S.R.; Johnson, L.J.A.N.; Williams, T.F.; McCleery, R.; Porras-Alfaro, A. Opportunistic Pseudogymnoascus and Geomyces Species Isolated from Humans and Bats. Sydowia 2019, 71, 25–33. [Google Scholar] [CrossRef]
- Ogórek, R.; Pusz, W.; Matkowski, K.; Pląskowska, E. Assessment of Abundance and Species Composition of Filamentous Fungi in the Underground Rzeczka Complex in Sowie Mountains (Lower Silesia, Poland). Geomicrobiol. J. 2014, 31, 900–906. [Google Scholar] [CrossRef]
- Ogórek, R.; Pusz, W.; Zagożdżon, P.P.; Kozak, B.; Bujak, H. Abundance and Diversity of Psychrotolerant Cultivable Mycobiota in Winter of a Former Aluminous Shale Mine. Geomicrobiol. J. 2017, 34, 823–833. [Google Scholar] [CrossRef]
- Zhang, T.; Victor, T.R.; Rajkumar, S.S.; Li, X.; Okoniewski, J.C.; Hicks, A.C.; Davis, A.D.; Broussard, K.; LaDeau, S.L.; Chaturvedi, S.; et al. Mycobiome of the Bat White Nose Syndrome Affected Caves and Mines Reveals Diversity of Fungi and Local Adaptation by the Fungal Pathogen Pseudogymnoascus (Geomyces) destructans. PLoS ONE 2014, 9, e108714. [Google Scholar] [CrossRef] [PubMed]
- Adetutu, E.M.; Thorpe, K.; Bourne, S.; Cao, X.; Shahsavari, E.; Kirby, G.; Ball, A.S. Phylogenetic Diversity of Fungal Communities in Areas Accessible and Not Accessible to Tourists in Naracoorte Caves. Mycologia 2011, 103, 959–968. [Google Scholar] [CrossRef]
- Wibbelt, G.; Kurth, A.; Hellmann, D.; Weishaar, M.; Barlow, A.; Veith, M.; Prüger, J.; Görföl, T.; Grosche, L.; Bontadina, F.; et al. White-Nose Syndrome Fungus (Geomyces destructans) in Bats, Europe. Emerg. Infect. Dis. 2010, 16, 1237. [Google Scholar] [CrossRef] [PubMed]
- Karunarathna, S.C.; Dong, Y.; Karasaki, S.; Tibpromma, S.; Hyde, K.D.; Lumyong, S.; Xu, J.; Sheng, J.; Mortimer, P.E. Discovery of novel fungal species and pathogens on bat carcasses in a cave in Yunnan Province, China. Emerg. Microbes Infect. 2020, 9, 1554–1566. [Google Scholar] [CrossRef]
- Dyląg, M.; Sawicki, A.; Ogórek, R. Diversity of Species and Susceptibility Phenotypes Toward Commercially Available Fungicides of Cultivable Fungi Colonizing Bones of Ursus spelaeus on Display in Niedźwiedzia Cave (Kletno, Poland). Diversity 2019, 11, 224. [Google Scholar] [CrossRef]
- Carvalho, J.L.; Lima, J.M.; Barbier, E.; Bernard, E.; Bezerra, J.D.; Souza-Motta, C.M. Ticket to Ride: Fungi from Bat Ectoparasites in a Tropical Cave and the Description of Two New Species. Brazilian J. Microbiol. 2022, 53, 2077–2091. [Google Scholar] [CrossRef] [PubMed]
- Zalar, P.; Gubenšek, A.; Gostincar, C.; Kostanjšek, R.; Bizjak-Mali, L.; Gunde-Cimerman, N. Cultivable Skin Mycobiota of Healthy and Diseased Blind Cave Salamander (Proteus anguinus). Front. Microbiol. 2022, 13, 926558. [Google Scholar] [CrossRef] [PubMed]
- Leplat, J.; François, A.; Bousta, F. Simplicillium pech-merlensis, a new fungal species isolated from the Pech-Merle show cave. Phytotaxa 2021, 521, 80–94. [Google Scholar] [CrossRef]
- Sanchez-Moral, S.; Jurado, V.; Fernandez-Cortes, A.; Cuezva, S.; Martin-Pozas, T.; Gonzalez-Pimentel, J.L.; Ontañon, R.; Saiz-Jimenez, C. Environment-Driven Control of Fungi in Subterranean Ecosystems: The Case of La Garma Cave (Northern Spain). Int. Microbiol. 2021, 24, 573–591. [Google Scholar] [CrossRef] [PubMed]
- Zareshahi, F.; Abolmaali, S.H.; Astaneh, S.D.A.; Asghari, A. Isolating Sorbicilin-Producing Fungi from Darband Cave and Evaluating the Sorbicilin Biomedical Applications. Stud. Fungi 2020, 5, 103–112. [Google Scholar] [CrossRef]
- Kuzmina, L.Y.; Gilvanova, E.A.; Galimzianova, N.F.; Chervyatsova, O.Y.; Ryabova, A.S.; Dzhabrailov, S.E.M.; Melentiev, A.I.; Aktuganov, G.E. The Novel Strain Acidomyces acidophilum Isolated from Acidophilic Biofilms (Snottites) Located in the Sheki-Heh Cave (North Caucasus). Curr. Microbiol. 2022, 79, 63. [Google Scholar] [CrossRef]
- Hubka, V.; Nováková, A.; Kolařík, M.; Jurjević, Ž.; Peterson, S.W. Revision of Aspergillus section Flavipedes: Seven new species and proposal of section Jani sect. nov. Mycologia 2015, 107, 169–208. [Google Scholar] [CrossRef] [PubMed]
- Hubka, V.; Nováková, A.; Peterson, S.W.; Frisvad, J.C.; Sklenář, F.; Matsuzawa, T.; Kolařík, M. A Reappraisal of Aspergillus Section Nidulantes with Descriptions of Two New Sterigmatocystin-Producing Species. Plant Syst. Evol. 2016, 302, 1267–1299. [Google Scholar] [CrossRef]
- Visagie, C.M.; Goodwell, M.; Nkwe, D.O. Aspergillus Diversity from the Gcwihaba Cave in Botswana and Description of One New Species. Fungal Syst. Evol. 2021, 8, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Nováková, A.; Kolařík, M. Chrysosporium speluncarum, a new species resembling Ajellomyces capsulatus, obtained from bat guano in caves of temperate Europe. Mycol. Prog. 2010, 9, 253–260. [Google Scholar] [CrossRef]
- Jurado, V.; Porca, E.; Cuezva, S.; Fernandez-Cortes, A.; Sánchez-Moral, S.; Sáiz-Jiménez, C. Fungal outbreak in a show cave. Sci. Total Environ. 2010, 408, 3632–3638. [Google Scholar] [CrossRef] [PubMed]
- Jurado, V.; Fernandez-Cortes, A.; Cuezva, S.; Laiz, L.; Cañaveras, J.C.; Sanchez-Moral, S.; Saiz-Jimenez, C. The fungal colonisation of rock-art caves: Experimental evidence. Naturwissenschaften 2009, 96, 1027–1034. [Google Scholar] [CrossRef]
- Mitova, M.M.; Iliev, M.; Nováková, A.; Gorbushina, A.A.; Groudeva, V.I.; Martin-Sanchez, P.M. Diversity and biocide susceptibility of fungal assemblages dwelling in the Art Gallery of Magura Cave, Bulgaria. Int. J. Speleol. 2017, 46, 8. [Google Scholar] [CrossRef]
- Kiyuna, T.; An, K.D.; Kigawa, R.; Sano, C.; Sugiyama, J. Noteworthy anamorphic fungi, Cephalotrichum verrucisporum, Sagenomella striatispora, and Sagenomella griseoviridis, isolated from biodeteriorated samples in the Takamatsuzuka and Kitora Tumuli, Nara, Japan. Mycoscience 2017, 58, 320–327. [Google Scholar] [CrossRef]
- Camino, L.P.; Idnurm, A.; Cerda-Olmedo, E. Diversity, ecology, and evolution in Phycomyces. Fungal Biol. 2015, 119, 1007–1021. [Google Scholar] [CrossRef] [PubMed]
- Martin-Sanchez, P.M.; Nováková, A.; Bastian, F.; Alabouvette, C.; Saiz-Jimenez, C. Two new species of the genus Ochroconis, O. lascauxensis and O. anomala isolated from black stains in Lascaux Cave, France. Fungal Biol. 2012, 116, 574–589. [Google Scholar] [CrossRef]
- Tsuneda, A.; Davey, M.L.; Tsuneda, I.; Hudgins, A.; Currah, R.S. Endophoma, a new didymellaceous endoconidial genus from bat-cave soil. Mycologia 2011, 103, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.R.; Cai, L.; Liu, F. Oligotrophic fungi from a carbonate cave, with three new species of Cephalotrichum. Mycology 2017, 8, 164–177. [Google Scholar] [CrossRef]
- Busquets, A.; Fornós, J.J.; Zafra, F.; Lalucat, J.; Merino, A. Microbial communities in a coastal cave: Cova des Pas de Vallgornera (Mallorca, Western Mediterranean). Int. J. Speleol. 2014, 43, 8. [Google Scholar] [CrossRef]
- Garcia-Anton, E.; Cuezva, S.; Jurado, V.; Porca, E.; Miller, A.Z.; Fernandez-Cortes, A.; Saiz-Jimenez, C.; Sánchez-Moral, S. Combining stable isotope (δ 13 C) of trace gases and aerobiological data to monitor the entry and dispersion of microorganisms in caves. Environ. Sci. Pollut. Res. 2014, 21, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Martín Sánchez, P.M. Las manchas negras de la cueva de Lascaux: Origen, evolución y caracterización de un brote fúngico. Doctoral Dissertation, Instituto de Recursos Naturales y Agrobiología de Sevilla, Sevilla, Spain, 18 April 2012. [Google Scholar]
- Becker, P.; van den Eynde, C.; Baert, F.; D’hooge, E.; De Pauw, R.; Normand, A.C.; Piarroux, R.; Stubbe, D. Remarkable fungal biodiversity on northern Belgium bats and hibernacula. Mycologia 2023, 115, 484–498. [Google Scholar] [CrossRef]
- Haelewaters, D.; Dima, B.; Abdel-Hafiz, A.I.I.; Abdel-Wahab, M.A.; Abul-Ezz, S.R.; Acar, I.; Aguirre-Acosta, E.; Aime, M.C.; Aldemir, S.; Ali, M.; et al. Fungal Systematics and Evolution: FUSE 6. Sydowia 2020, 72, 231–356. [Google Scholar] [CrossRef]
- Jiang, J.R.; Chen, Q.; Cai, L. Polyphasic characterisation of three novel species of Paraboeremia. Mycol. Prog. 2017, 16, 285–295. [Google Scholar] [CrossRef]
- Nováková, A.L.E.N.A.; Savická, D.; Kolařík, M. Two novel species of the genus Trichosporon isolated from a cave environment. Czech Mycol. 2015, 67, 233–239. [Google Scholar] [CrossRef]
- Martin-Sanchez, P.M.; Jurado, V.; Porca, E.; Bastian, F.; Lacanette, D.; Alabouvette, C.; Saiz-Jimenez, C. Airborne Microorganisms in Lascaux Cave (France). Int. J. Speleol. 2014, 43, 295–303. [Google Scholar] [CrossRef]
- Fernandez-Cortes, A.; Cuezva, S.; Sanchez-Moral, S.; Cañaveras, J.C.; Porca, E.; Jurado, V.; Martin-Sanchez, P.M.; Saiz-Jimenez, C. Detection of human-induced environmental disturbances in a show cave. Environ. Sci. Pollut. Res. 2011, 18, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Nouri, H.; Moghimi, H.; Geranpayeh Vaghei, M.; Nasr, S. Blastobotrys persicus sp. nov., an Ascomycetous Yeast Species Isolated from Cave Soil. Antonie Van Leeuwenhoek 2018, 111, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Rehner, S.A.; Minnis, A.M.; Sung, G.H.; Luangsa-ard, J.J.; Devotto, L.; Humber, R.A. Phylogeny and systematics of the anamorphic, entomopathogenic genus Beauveria. Mycologia 2011, 103, 1055–1073. [Google Scholar] [CrossRef]
- Flieger, M.; Bandouchova, H.; Cerny, J.; Chudíčková, M.; Kolarik, M.; Kovacova, V.; Martínková, N.; Novák, P.; Šebesta, O.; Stodůlková, E.; et al. Vitamin B2 as a virulence factor in Pseudogymnoascus destructans skin infection. Sci. Rep. 2016, 6, 33200. [Google Scholar] [CrossRef]
- Moñino, I.D. Evaluación y Control de Comunidades Microbianas en Cuevas Turísticas. Doctoral Dissertation, Universidad de Sevilla, Sevilla, Spain, 2014. [Google Scholar]
- Pikula, J.; Bandouchova, H.; Novotný, L.; Meteyer, C.U.; Zukal, J.; Irwin, N.R.; Zima, J.; Martínková, N. Histopathology confirms white-nose syndrome in bats in Europe. J. Wildl. Dis. 2012, 48, 207–211. [Google Scholar] [CrossRef]
- Porca, E.; Jurado, V.; Martin-Sanchez, P.M.; Hermosín, B.; Bastian, F.; Alabouvette, C.; Saiz-Jimenez, C. Aerobiology: An ecological indicator for early detection and control of fungal outbreaks in caves. Ecol. Indic. 2011, 11, 1594–1598. [Google Scholar] [CrossRef]
- Hubka, V.; Nováková, A.; Jurjević, Ž.; Sklenář, F.; Frisvad, J.C.; Houbraken, J.; Kolařík, M. Polyphasic Data Support the Splitting of Aspergillus candidus into Two Species; Proposal of Aspergillus dobrogensis sp. nov. Int. J. Syst. Evol. Microbiol. 2018, 68, 995–1011. [Google Scholar] [CrossRef]
- Glässnerová, K.; Sklenář, F.; Jurjević, Ž.; Houbraken, J.; Yaguchi, T.; Visagie, C.M.; Gené, J.; Siqueira, J.P.Z.; Kubátová, A.; Kolařík, M.; et al. A monograph of Aspergillus section Candidi. Stud. Mycol. 2022, 102, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Bian, C.; Kusuya, Y.; Sklenář, F.; D’hooge, E.; Yaguchi, T.; Ban, S.; Visagie, C.M.; Houbraken, J.; Takahashi, H.; Hubka, V. Reducing the number of accepted species in Aspergillus series Nigri. Stud. Mycol. 2022, 102, 95–132. [Google Scholar] [CrossRef] [PubMed]
- Mulec, J.; Covington, E.; Walochnik, J. Is bat guano a reservoir of Geomyces destructans? Open J. Vet. Med. 2013, 3, 161–167. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, S.Y.; Chung, C.U.; Park, J.S.; Kim, Y.J.; Oem, J.K. Fungal diversity in Korean caves and cave-inhabiting bats with attention to Pseudogymnoascus species. Diversity 2023, 15, 198. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Zhou, S.Y.; Eurwilaichitr, L.; Ingsriswang, S.; Raza, M.; Chen, Q.; Cai, L. Culturable Mycobiota from Karst Caves in China II, with Descriptions of 33 New Species. Fungal Divers. 2021, 106, 29–136. [Google Scholar] [CrossRef]
- Takashima, M.; Kurakado, S.; Cho, O.; Kikuchi, K.; Sugiyama, J.; Sugita, T. Description of Four Apiotrichum and Two Cutaneotrichosporon Species Isolated from Guano Samples from Bat-Inhabited Caves in Japan. Int. J. Syst. Evol. Microbiol. 2020, 70, 4458–4469. [Google Scholar] [CrossRef]
- ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012; p. 496. [Google Scholar]
- Sun, B.D.; Houbraken, J.; Frisvad, J.C.; Jiang, X.Z.; Chen, A.J.; Samson, R.A. New species in Aspergillus section Usti and an overview of Aspergillus section Cavernicolarum. Int. J. Syst. Evol. Microbiol. 2020, 70, 004425. [Google Scholar] [CrossRef]
- Beimforde, C.; Feldberg, K.; Nylinder, S.; Rikkinen, J.; Tuovila, H.; Dörfelt, H.; Schmidt, A.R. Estimating the Phanerozoic History of the Ascomycota Lineages: Combining Fossil and Molecular Data. Mol. Phylogenet. Evol. 2014, 78, 386–398. [Google Scholar] [CrossRef]
- Senanayake, I.C.; Pem, D.; Rathnayaka, A.R.; Wijesinghe, S.N.; Tibpromma, S.; Wanasinghe, D.N.; Phookamsak, R.; Kularathnage, N.D.; Gomdola, D.; Harishchandra, D.; et al. Predicting Global Numbers of Teleomorphic Ascomycetes. Fungal Divers. 2022, 114, 237–278. [Google Scholar] [CrossRef]
- Durán, P.; Barra, P.J.; Jorquera, M.A.; Viscardi, S.; Fernandez, C.; Paz, C.; Bol, R. Occurrence of Soil Fungi in Antarctic Pristine Environments. Front. Bioeng. Biotechnol. 2019, 7, 28. [Google Scholar] [CrossRef]
- Challacombe, J.F.; Hesse, C.N.; Bramer, L.M.; McCue, L.A.; Lipton, M.; Purvine, S.; Nicora, C.; Gallegos-Graves, L.V.; Porras-Alfaro, A.; Kuske, C.R. Genomes and Secretomes of Ascomycota Fungi Reveal Diverse Functions in Plant Biomass Decomposition and Pathogenesis. BMC Genom. 2019, 20, 976. [Google Scholar] [CrossRef]
- Feng, L.; Song, Q.; Jiang, Q.; Li, Z. The Horizontal and Vertical Distribution of Deep-Sea Sediments Fungal Community in the South China Sea. Front. Mar. Sci. 2021, 8, 592784. [Google Scholar] [CrossRef]
- Segundo, W.O.P.F.; de Oliveira, R.S.; Lima, R.M.; Santiago, P.A.L.; de Oliveira, L.A.; Cortez, A.C.A.; Lima, E.S.; de Souza, É.S.; Frickmann, H.; de Souza, J.V.B. Antimicrobial Potential of Metabolites in Fungal Strains Isolated from a Polluted Stream: Annulohypoxylon stygium WL1B5 Produces Metabolites against Extended-Spectrum Beta-Lactamase-Positive Escherichia coli. Antibiotics 2022, 12, 27. [Google Scholar] [CrossRef]
- Villa, F.; Cappitelli, F. The Ecology of Subaerial Biofilms in Dry and Inhospitable Terrestrial Environments. Microorganisms 2019, 7, 380. [Google Scholar] [CrossRef] [PubMed]
- Stoppiello, G.A.; Muggia, L.; De Carolis, R.; Coleine, C.; Selbmann, L. Ecological Niche Drives Fungal and Bacterial Diversity in Endolithic and Epilithic Communities Inhabiting Granites in Victoria Land, Antarctica. Polar Biol. 2025, 48, 16. [Google Scholar] [CrossRef]
- Dimkić, I.; Fira, D.; Janakiev, T.; Kabić, J.; Stupar, M.; Nenadić, M.; Grbić, M.L. The Microbiome of Bat Guano: For What Is This Knowledge Important? Appl. Microbiol. Biotechnol. 2021, 105, 1407–1419. [Google Scholar] [CrossRef]
- Ogórek, R.; Dyląg, M.; Kozak, B.; Višňovská, Z.; Tančinová, D.; Lejman, A. Fungi Isolated and Quantified from Bat Guano and Air in Harmanecka and Driny Caves (Slovakia). J. Cave Karst Stud. 2016, 78, 41–49. [Google Scholar] [CrossRef]
- Jofre, G.I.; Singh, A.; Mavengere, H.; Sundar, G.; D’Agostino, E.; Chowdhary, A.; Matute, D.R. An Indian lineage of Histoplasma with strong signatures of differentiation and selection. Fungal Genet. Biol. 2022, 158, 103654. [Google Scholar] [CrossRef]
- Money, N.P. Spore production, discharge, and dispersal. In The Fungi; Watkinson, S.C., Boddy, L., Money, N.P., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 67–97. [Google Scholar]
- Ingold, C.T.; Hudson, H.J. The Biology of Fungi; Springer: Berlin/Heidelberg, Germany, 1993. [Google Scholar]
- Elbert, W.; Taylor, P.E.; Andreae, M.O.; Pöschl, U. Contribution of fungi to primary biogenic aerosols in the atmosphere: Wet and dry discharged spores. Atmos. Chem. Phys. 2007, 7, 4569–4588. [Google Scholar] [CrossRef]
- Samson, R.A.; Houbraken, J.; Thrane, U.; Frisvad, J.C.; Andersen, B. Food and Indoor Fungi; Westerdijk Fungal Biodiversity Institute: Utrecht, The Netherlands, 2019. [Google Scholar]
- Poli, A.; Zanellati, A.; Piano, E.; Biagioli, F.; Coleine, C.; Nicolosi, G.; Varese, G.C. Cultivable fungal diversity in two karstic caves in Italy: Under-investigated habitats as source of putative novel taxa. Sci. Rep. 2024, 14, 4164. [Google Scholar] [CrossRef]
- Zhang, N.; Castlebury, L.A.; Miller, A.N.; Huhndorf, S.M.; Schoch, C.L.; Seifert, K.A.; Samuels, G.J.; Rossman, A.Y.; Rogers, J.D.; Sung, G.H. An overview of the systematics of the Sordariomycetes based on a four-gene phylogeny. Mycologia 2006, 98, 1076–1087. [Google Scholar] [CrossRef]
- Wang, H.Y.; Li, X.; Dong, C.B.; Zhang, Y.W.; Chen, W.H.; Liang, J.D.; Han, Y.F. Two new species of Sordariomycetes (Chaetomiaceae and Nectriaceae) from China. MycoKeys 2024, 102, 301. [Google Scholar] [CrossRef]
- Vega, F.E.; Goettel, M.S.; Blackwell, M.; Chandler, D.; Jackson, M.A.; Keller, S.; Koike, M.; Maniania, N.K.; Monzón, A.; Ownley, B.H.; et al. Fungal entomopathogens: New insights on their ecology. Fungal Ecol. 2009, 2, 149–159. [Google Scholar] [CrossRef]
- Begerow, D.; Stoll, M.; Bauer, R. A phylogenetic hypothesis of Ustilaginomycotina based on multiple gene analyses and morphological data. Mycologia 2006, 98, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.L.S.; Resende Stoianoff, M.A.D.; Lopes Ferreira, R. Mycological Study for a Management Plan of a Neotropical Show Cave (Brazil). Int. J. Speleol. 2013, 42, 10. [Google Scholar] [CrossRef]
- Bladt, T.T.; Frisvad, J.C.; Knudsen, P.B.; Larsen, T.O. Anticancer and Antifungal Compounds from Aspergillus, Penicillium, and Other Filamentous Fungi. Molecules 2013, 18, 11338–11376. [Google Scholar] [CrossRef] [PubMed]
- Júnior, D.P.L.; Yamamoto, A.C.A.; de Souza Amadio, J.V.R.; Martins, E.R.; do Santos, F.A.L.; Simoes, S.D.A.A.; Hahn, R.C. Trichocomaceae: Biodiversity of Aspergillus spp. and Penicillium spp. Residing in Libraries. J. Infect. Dev. Ctries. 2012, 6, 734–743. [Google Scholar] [CrossRef]
- Shen, H.D.; Tam, M.F.; Tang, R.B.; Chou, H. Aspergillus and Penicillium Allergens: Focus on Proteases. Curr. Allergy Asthma Rep. 2007, 7, 351–356. [Google Scholar] [CrossRef]
- Suchy, H.; Zalar, P.; Macedo, M.F. Microbial Diversity of Biodeteriorated Limestone Cultural Heritage Assets Identified Using Molecular Approaches—A Literature Review. Appl. Sci. 2024, 14, 7429. [Google Scholar] [CrossRef]
- Ahmed, E.A.E.; Mohamed, R.M. Bacterial Deterioration in the Limestone Minaret of Prince Muhammad and Suggested Treatment Methods, Akhmim, Egypt. Geomaterials 2022, 12, 37–58. [Google Scholar] [CrossRef]
- Whiting-Fawcett, F.; Blomberg, A.S.; Troitsky, T.; Meierhofer, M.B.; Field, K.A.; Puechmaille, S.J.; Lilley, T.M. A Palearctic View of a Bat Fungal Disease. Conserv. Biol. 2025, 39, e14265. [Google Scholar] [CrossRef]
- Gómez-Rodríguez, R.A.; Sánchez-Cordero, V.; Boyer, D.; Schondube, J.E.; Rodríguez-Moreno, Á.; Gutiérrez-Granados, G. Risk of Infection of White-Nose Syndrome in North American Vespertilionid Bats in Mexico. Ecol. Inform. 2022, 72, 101869. [Google Scholar] [CrossRef]
- Zukal, J.; Bandouchova, H.; Brichta, J.; Cmokova, A.; Jaron, K.S.; Kolarik, M.; Martínková, N. White-Nose Syndrome without Borders: Pseudogymnoascus destructans Infection Tolerated in Europe and Palearctic Asia but Not in North America. Sci. Rep. 2016, 6, 19829. [Google Scholar] [CrossRef]
- Mammola, S.; Piano, E.; Cardoso, P.; Vernon, P.; Domínguez-Villar, D.; Culver, D.C.; Pipan, T.; Isaia, M. Climate Change Going Deep: The Effects of Global Climatic Alterations on Cave Ecosystems. Anthr. Rev. 2019, 6, 98–116. [Google Scholar] [CrossRef]
- Pfendler, S.; Karimi, B.; Maron, P.-A.; Ciadamidaro, L.; Valot, B.; Bousta, F.; Alaoui-Sosse, L.; Alaoui-Sosse, B.; Aleya, L. Biofilm Biodiversity in French and Swiss Show Caves Using the Metabarcoding Approach: First Data. Sci. Total Environ. 2018, 615, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk-Żak, K.; Zielenkiewicz, U. Microbial Diversity in Caves. Geomicrobiol. J. 2016, 33, 20–38. [Google Scholar] [CrossRef]
- Barton, H.A.; Northup, D.E. Geomicrobiology in Cave Environments: Past, Current, and Future Perspectives. J. Cave Karst Stud. 2007, 69, 163–178. [Google Scholar]
- Das, T.; Al-Tawaha, A.R.; Pandey, D.K.; Nongdam, P.; Shekhawat, M.S.; Dey, A.; Choudhary, K.; Sahay, S. Halophilic, Acidophilic, Alkaliphilic, Metallophilic, and Radioresistant Fungi: Habitats and Their Living Strategies. In Extremophilic Fungi: Ecology, Physiology and Applications; Springer Nature: Singapore, 2022; pp. 171–193. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).