Fungal Pathogens of Peach Palm Leaf Spot in Thailand and Their Fungicide Sensitivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Fungal Isolation
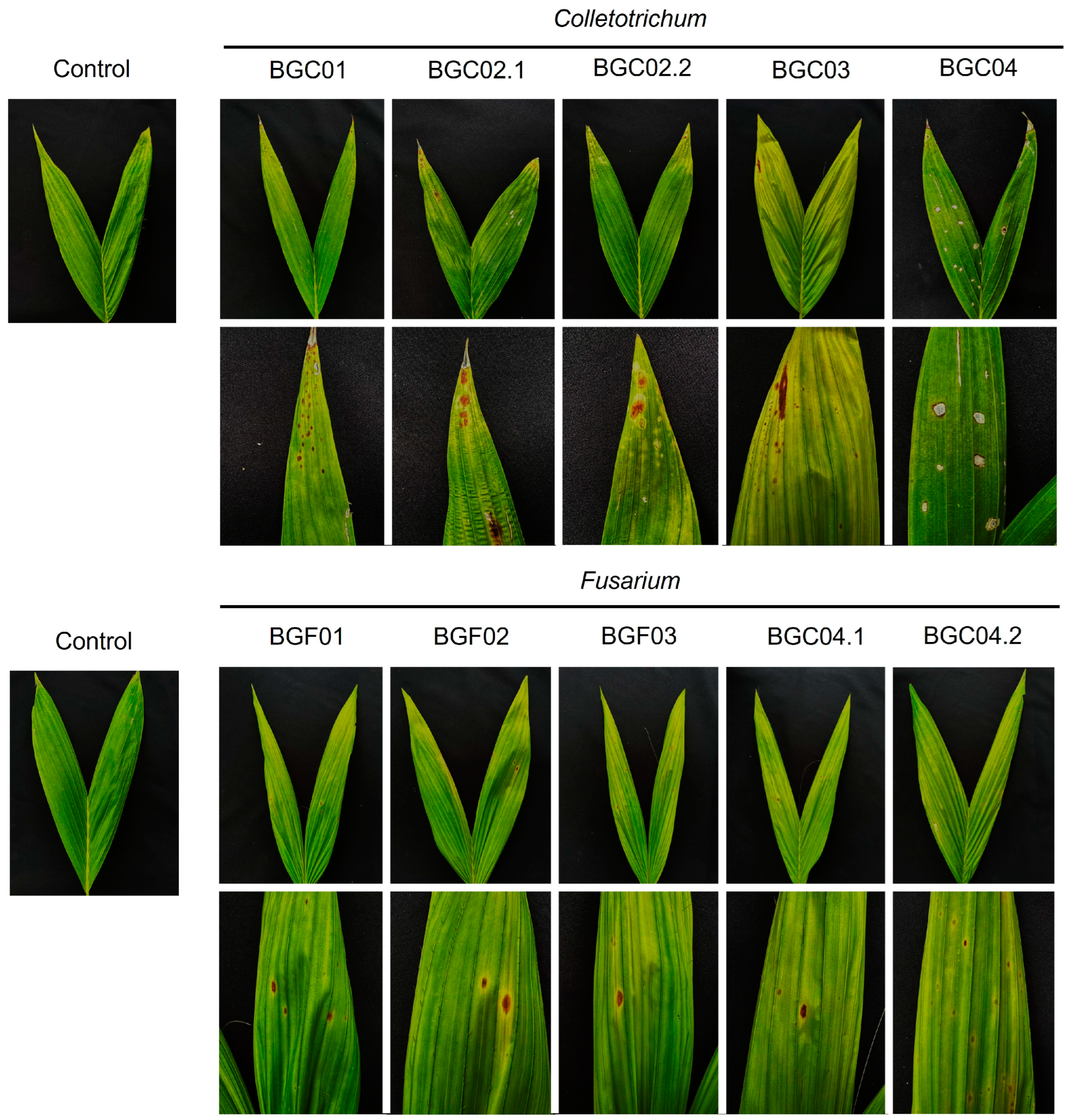
2.2. Pathogenicity Test
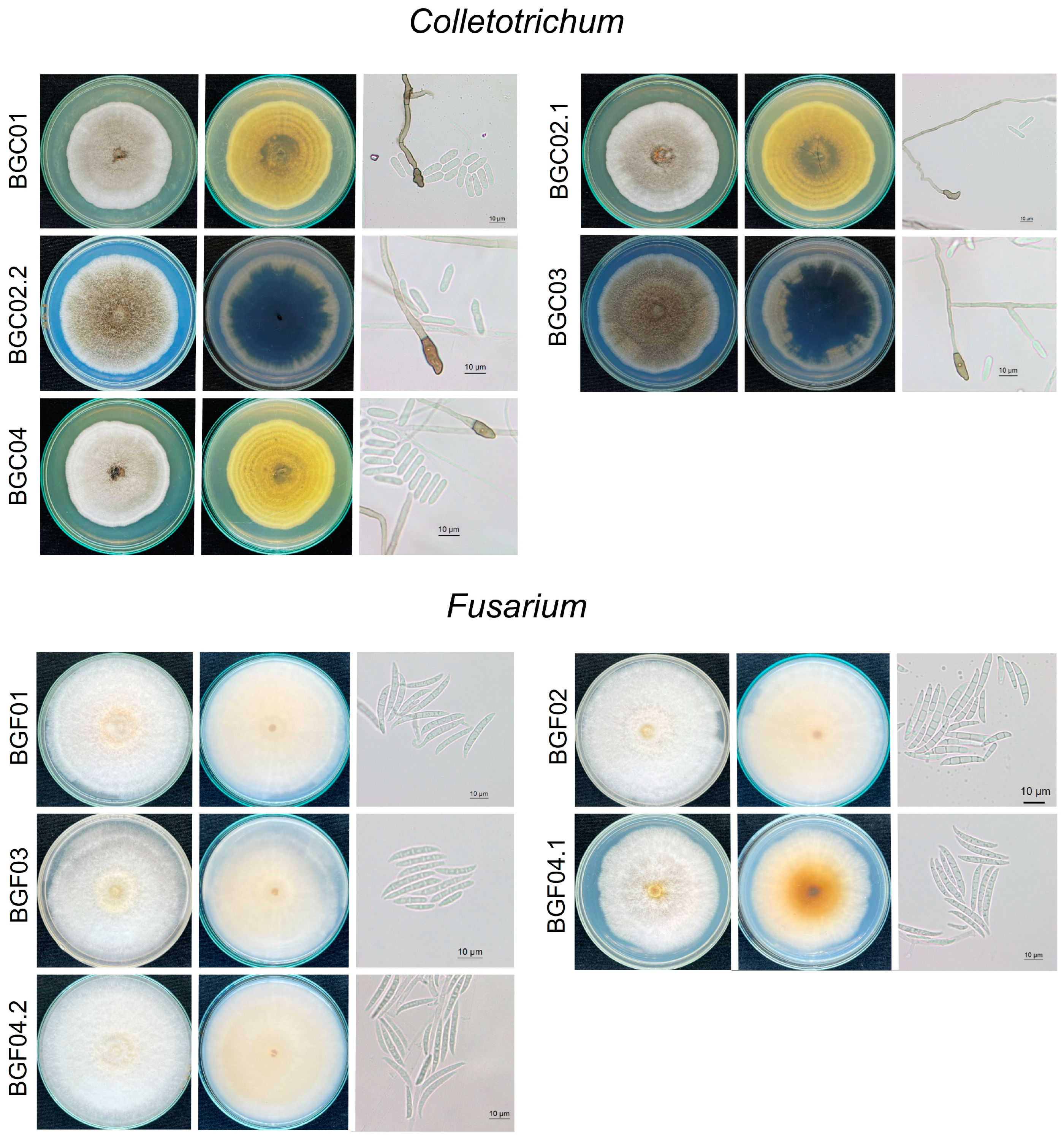
2.3. Morphological Study
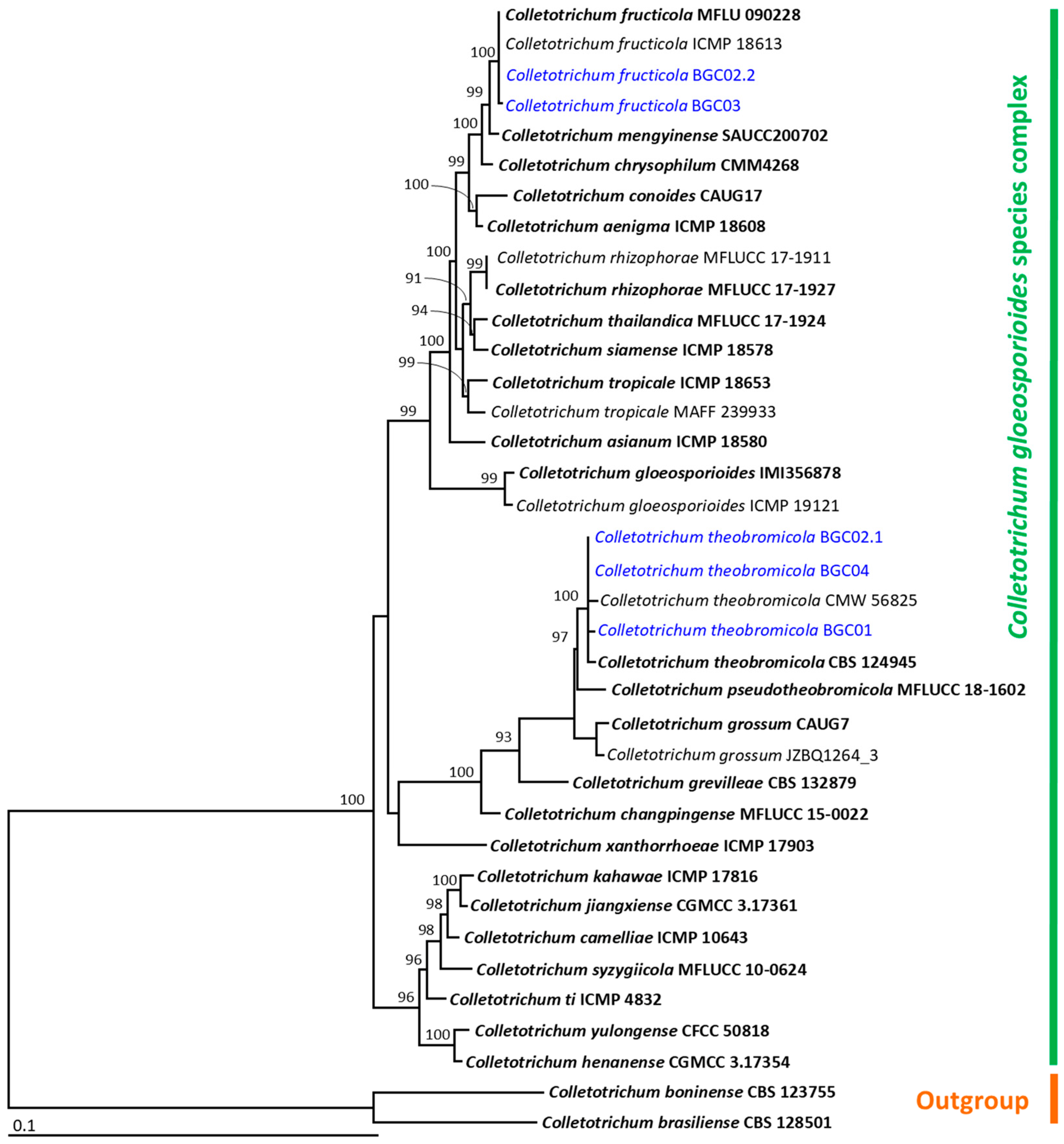
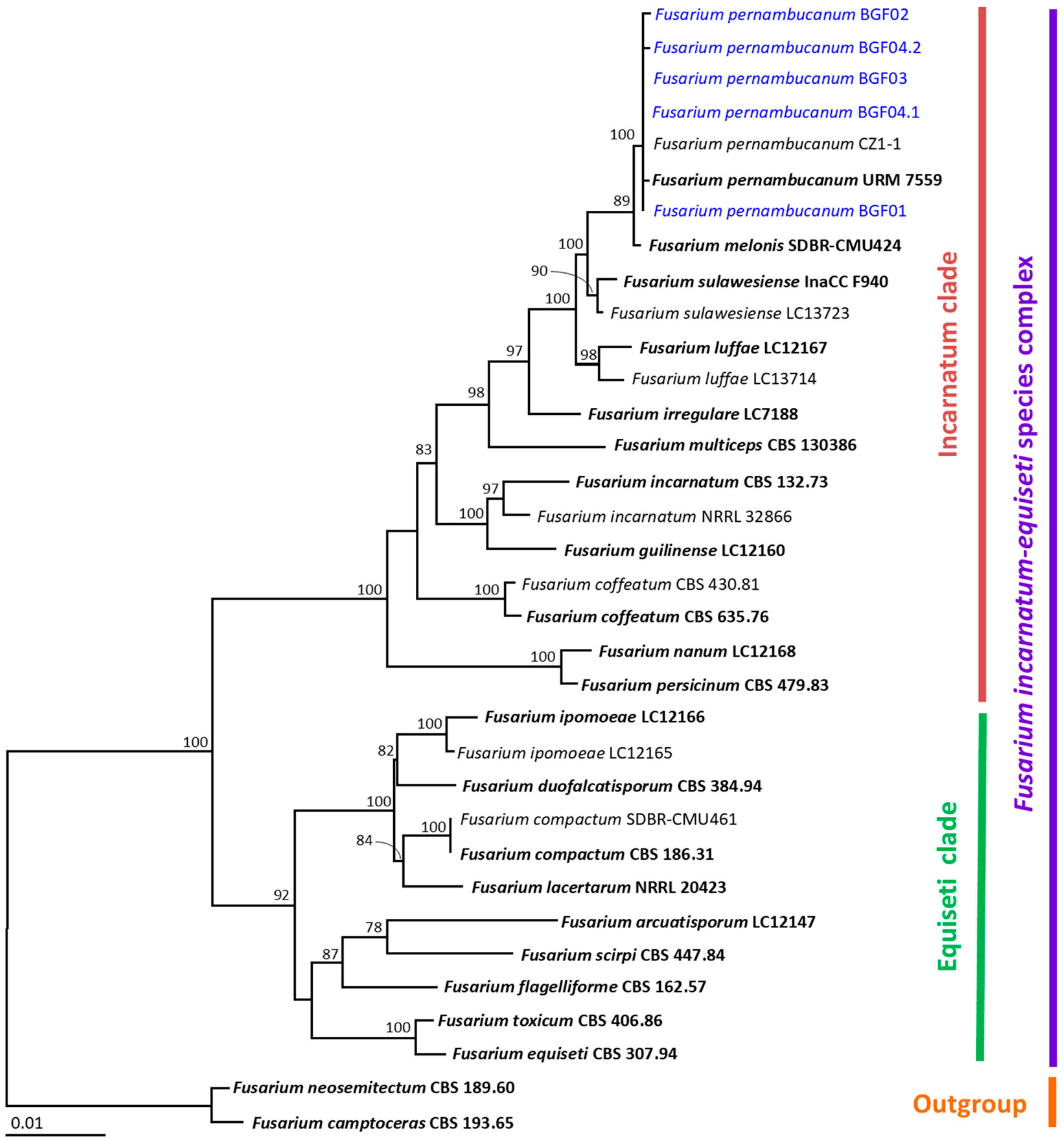
2.4. DNA Extraction, PCR Amplification, and Phylogenetic Analysis
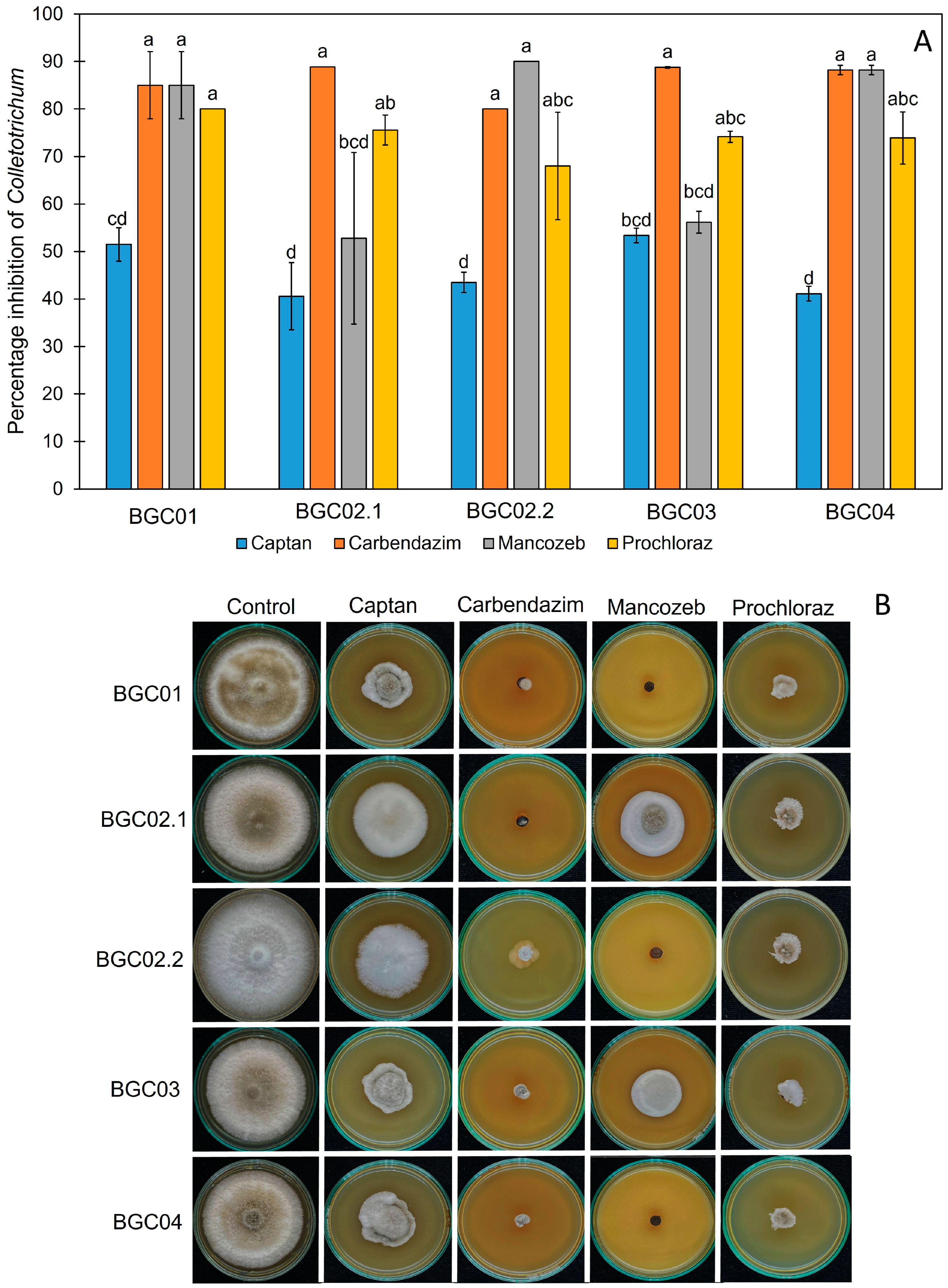
2.5. In Vitro Test of Synthetic Fungicides Against Peach Palm Leaf Spot Pathogens
2.6. Statistical Analysis
3. Results
3.1. Symptom Recognitions
3.2. Pathogenicity Test
3.3. Morphological Characteristics
3.4. Molecular Identification
3.5. The Effect of Synthetic Fungicides on the Growth of Peach Palm Leaf Spot Pathogens
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aguiar, J.P.L.; Yuyama, K.; Souza, F.d.C.d.A. Caracterização dos frutos de Pupunheira (Bactris gasipaes Kunth) cultivada na vila do Equador, RR: O que há de novo? Sci. Amazon. 2019, 8, 1–5. [Google Scholar]
- Yuyama, L.K.O.; Aguiar, J.P.L.; Yuyama, K.; Clement, C.R.; Macedo, S.H.M.; Fávaro, D.I.T.; Afonso, C.; Vasconcellos, M.B.A.; Pimentel, S.A.; Badolato, E.S.G.; et al. Chemical composition of the fruit mesocarp of three peach palm (Bactris gasipaes) populations grown in central Amazonia, Brazil. Int. J. Food Sci. Nutr. 2003, 54, 49–56. [Google Scholar] [PubMed]
- Lateme, P.; Garcia, M.-F.; Londoño, A.-M.; Rojas, M.-G.; Buldgen, A.; Souffrant, W.-B. Chemical composition and nutritive value of peach palm (Bactris gasipaes Kunth) in rats. J. Sci. Food Agric. 2005, 85, 1505–1512. [Google Scholar]
- Matos, K.A.N.; Lima, D.P.; Barbosa, A.P.P.; Mercadante, A.Z.; Chisté, R.C. Peels of tucumã (Astrocaryum vulgare) and peach palm (Bactris gasipaes) are byproducts classified as very high carotenoid sources. Food Chem. 2019, 272, e216. [Google Scholar] [CrossRef]
- Stevanato, N.; Ribeiro, T.H.; Giombelli, C.; Cardoso, T.; Wojeicchowski, J.P.; Dalva, E.; Danesi, G.; Barros, B.C.B. Effect of canning on the antioxidant activity, fiber content, and mechanical properties of different parts of peach palm Heart. J. Food Process. Preserv. 2020, 44, e14554. [Google Scholar] [CrossRef]
- Graefe, S.; Dufour, D.; van Zonneveld, M.; Rodriguez, F.; Gonzalez, A. Peach palm (Bactris gasipaes) in tropical Latin America: Implications for biodiversity conservation, natural resource management and human nutrition. Biodivers. Conserv. 2013, 22, 269–300. [Google Scholar] [CrossRef]
- Elad, Y.; Pertot, I. Climate change impacts on plant pathogens and plant diseases. J. Crop Improv. 2014, 28, 139–199. [Google Scholar] [CrossRef]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez, M.E.; Jácome, D.A.; Keil, C.B.; Montúfar, R.J.; Evans, T.A. First report of Phytophthora palmivora causing bud rot on palmito (Bactris gasipaes) in Ecuador. Plant Dis. 2016, 100, e1248. [Google Scholar] [CrossRef]
- da Rocha, F.V.R.; Severo, R.; Shibutani, L.J.S.; Vieira, D.D.S.S.; Matos, K.S.; Guimarães, S.D.S.C.; da Silva, G.F.; de França, S.M.; Sousa, E.S.; Beserra Junior, J.E.A.; et al. First report of Thielaviopsis ethacetica causing black rot in Bactris gasipaes fruit in Brazil. Plant Dis. 2023, 107, 3634. [Google Scholar] [CrossRef]
- Febriani, A.V.; Kasiamdari, R.S. Identification of Curvularia eragrostidis (Henn.) JA Mey. the leaf spot pathogen of oil palm (Elaeis guineensis Jacq.) and it’s control by false elder (Peronema canescens Jack) leaf extract. IJOP 2023, 6, 25–34. [Google Scholar]
- Sunpapao, A.; Kittimorakul, J.; Pornsuriya, C. Disease Note: Identification of Curvularia oryzae as cause of leaf spot disease on oil palm seedlings in nurseries of Thailand. Phytoparasitica 2014, 42, 529–533. [Google Scholar] [CrossRef]
- Steinberg, G.; Gurr, S.J. Fungi, fungicide discovery and global food security. Fungal Genet. Biol. 2020, 144, e103476. [Google Scholar] [CrossRef]
- Hamim, I.; Sipes, B.; Wang, Y. Editorial: Detection, characterization, and management of plant pathogens. Front. Plant Sci. 2024, 15, 1354042. [Google Scholar] [CrossRef]
- Sunpapao, A.; Suwannarach, N.; Kumla, J.; Dumhai, R.; Riangwong, K.; Sanguansub, S.; Wanchana, S.; Arikit, S. Morphological and molecular identification of plant pathogenic fungi associated with dirty panicle disease in coconuts (Cocos nucifera) in Thailand. J. Fungi 2022, 8, 335. [Google Scholar] [CrossRef]
- Cai, L.; Hyde, K.D.; Taylor, P.W.J.; Weir, B.S.; Waller, J.M.; Abang, M.M.; Zhang, Z.J.; Yang, Y.L.; Phoulivong, S.; Liu, Z.Y.; et al. A polyphasic approach for studying Colletotrichum. Fungal Divers. 2009, 39, 183–204. [Google Scholar]
- Cannon, P.F.; Damm, U.; Johnston, P.R.; Weir, B.S. Colletotrichum—Current status and future directions. Stud. Mycol. 2012, 73, 181–213. [Google Scholar] [CrossRef]
- Wang, M.M.; Chen, Q.; Diao, Y.Z.; Duan, W.J.; Cai, L. Fusarium incarnatum-equiseti complex from China. Persoonia 2019, 43, 70–89. [Google Scholar] [CrossRef]
- Bhunjun, C.S.; Phukhamsakda, C.; Jayawardena, R.S.; Jeewon, R.; Promputtha, I.; Hyde, K.D. Investigating species boundaries in Colletotrichum. Fungal Divers. 2021, 107, 107–127. [Google Scholar] [CrossRef]
- Crous, P.W.; Lombard, L.; Sandoval-Denis, M.; Seifert, K.A.; Schroers, H.-J.; Chaverri, P.; Gené, J.; Guarro, J.; Hirooka, Y.; Bensch, K.; et al. Fusarium: More than a node or a foot-shaped basal cell. Stud. Mycol. 2021, 98, e100116. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004, 5, e113. [Google Scholar] [CrossRef] [PubMed]
- Hall, T. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the Cipres Science Gateway for Inference of Large Phylogenetic Trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; IEEE: Manhattan, NY, USA, 2010; pp. 1–8. [Google Scholar]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef] [PubMed]
- Thaochan, N.; Pornsuriya, C.; Chairin, T.; Sunpapao, A. Roles of systemic fungicide in antifungal activity and induced defense responses in rubber tree (Hevea brasiliensis) against leaf fall disease caused by Neopestalotiopsis cubana. Physiol. Molec. Plant Pathol. 2020, 111, e101511. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Chen, J.W.; Manawasinghe, I.S.; Lin, Y.H.; Jayawardena, R.S.; McKenzie, E.H.C.; Hyde, K.D.; Xiang, M.M. Identification and characterization of Colletotrichum species associated with ornamental plants in Southern China. Mycosphere 2023, 14, 262–302. [Google Scholar] [CrossRef]
- Link, J.H.F. Observationes in ordines plantarum naturales. Dissertatio Ima. Ges. Naturforsch. Freunde Berl. Mag. 1809, 3, 3–42. [Google Scholar]
- Mu, T.; Zhang, Z.; Liu, R.; Liu, S.; Li, Z.; Zhang, X.; Xia, J. Morphological and molecular phylogenetic analyses reveal three species of Colletotrichum in Shandong province, China. MycoKeys 2021, 85, 57–71. [Google Scholar] [CrossRef]
- Norphanphoun, C.; Hyde, K.D. First Report of Colletotrichum fructicola, C. rhizophorae sp. nov. and C. thailandica sp. nov. on Mangrove in Thailand. Pathogens 2023, 12, 1436. [Google Scholar] [CrossRef]
- Nuangmek, W.; Kumla, J.; Khuna, S.; Lumyong, S.; Suwannarach, N. Identification and Characterization of Fusarium species causing watermelon fruit rot in northern Thailand. Plants 2023, 12, 956. [Google Scholar] [CrossRef]
- Dixit, S.; Sivalingam, P.N.; Murali-Baskaran, R.K.; Senthil-Kumar, M.; Ghosh, P.K. Plant responses to concurrent abiotic and biotic stress: Unravelling physiological and morphological mechanisms. Plant Physiol. Rep. 2023, 29, 6–17. [Google Scholar] [CrossRef]
- Hughes, S.J. Conidiophores, conidia and classification. Can. J. Bot. 1953, 31, 577–659. [Google Scholar] [CrossRef]
- Pakdeeniti, P.; Tamakaew, N.; Suwannarach, N.; Senwanna, C.; Haituk, S.; Withee, P.; Kumla, J.; Cheewangkoon, R. First report of Colletotrichum theobromicola causing centro anthracnose leaf spot in Thailand. Plant Dis. 2022, 106, e1306. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.L.; Gao, M.; Wang, L.-W.; Sun, P.N.; Xing, X.W.; Wang, J.; Zhang, X.H.; Sun, J.G. Fusarium pernambucanum causing leaf yellow spot on melon (Cucumis melo), a new disease in China. Plant Dis. 2023, 107, e3287. [Google Scholar] [CrossRef] [PubMed]
- Chaves, T.P.; Miranda, A.R.G.S.; da Paz, L.C.; Netto, M.S.B.; Lima, G.S.A.; Assunção, I.P. First report of Colletotrichum theobromicola causing anthracnose on Anthurium sp. Australasian Plant Dis. Notes 2020, 15, e27. [Google Scholar] [CrossRef]
- Marshall, J.; Whitlock, K.; Colburn, C.; Yang, X. First report of anthracnose caused by Colletotrichum theobromicola on Cyclamen persicum in South Carolina, USA. Plant Dis. 2023, 107, e3288. [Google Scholar] [CrossRef]
- Liu, F.; Weir, B.S.; Damm, U.; Crous, P.W.; Wang, Y.; Zhang, M. Unraveling the Colletotrichum gloeosporioides species complex. Stud. Mycol. 2022, 101, 1–41. [Google Scholar] [CrossRef]
- Phoulivong, S.; Cai, L.; Chen, H.; McKenzie, E.H.; Abdelsalam, K.; Chukeatirote, E.; Hyde, K.D. Colletotrichum species from tropical orchid plants in Thailand. Fungal Divers. 2010, 42, 47–99. [Google Scholar]
- Hyde, K.D.; Cai, L.; Cannon, P.F.; Crouch, J.A.; Peres, N.A. Colletotrichum—Names in current use. Fungal Divers. 2009, 39, 147–182. [Google Scholar]
- Li, S.; Zhang, W. Occurrence of postharvest fruit rot of mango caused by Fusarium pernambucanum in China. Plant Dis. 2023, 107, e2526. [Google Scholar] [CrossRef]
- Perez, P.M.; Alberto, R.T. Chemical management of anthra2cnose-twister (Colletotrichum gloeosporioides and Fusarium fujikuroi) disease of onion (Allium cepa). Plant Pathol. Quar. 2020, 10, 198–216. [Google Scholar]
- Mondani, L.; Chiusa, G.; Battilani, P. Chemical and biological control of Fusarium species involved in garlic dry rot at early crop stages. Eur. J. Plant Pathol. 2021, 160, 575–587. [Google Scholar] [CrossRef]
- Poromarto, S.H.; Hadiwiyono; Supyani; Supriyadi; Permatasari, F.I. Fungicide resistance of Fusarium oxysporum f. sp. cepae isolated from shallot in Brebes. IOP Conf. Ser. Earth Environ. Sci. 2023, 1180, e01203. [Google Scholar]


| Fungal Isolate | Gene | GenBank Accession Number | The Closely Related Type Fungal Species/Similarity Value (%) |
|---|---|---|---|
| BGC01 | ITS | PQ764973 | Colletotrichum theobromicola CBS 124945/100.00 |
| gapdh | PQ772077 | Colletotrichum theobromicola CBS 124945/98.88 | |
| cal | PQ772078 | Colletotrichum theobromicola CBS 124945/99.59 | |
| act | PQ772079 | Colletotrichum theobromicola CBS 124945/97.43 | |
| tub | PQ772080 | Colletotrichum theobromicola CBS 124945/100.00 | |
| BGC02.1 | ITS | PV298543 | Colletotrichum theobromicola CBS 124945/100.00 |
| gapdh | PV334903 | Colletotrichum theobromicola CBS 124945/98.87 | |
| cal | PV334907 | Colletotrichum theobromicola CBS 124945/99.59 | |
| act | PV334911 | Colletotrichum theobromicola CBS 124945/97.43 | |
| tub | PV334915 | Colletotrichum theobromicola CBS 124945/100.00 | |
| BGC02.2 | ITS | PV290482 | Colletotrichum fructicola MFU 090228/100.00 |
| gapdh | PV334904 | Colletotrichum fructicola MFU 090228/100.00 | |
| cal | PV334908 | Colletotrichum fructicola MFU 090228/100.00 | |
| act | PV334912 | Colletotrichum mengyinense SAUCC 200702/100.00 | |
| tub | PV334916 | Colletotrichum theobromicola CBS 124945/100.00 | |
| BGC03 | ITS | PV298544 | Colletotrichum fructicola MFU 090228/100.00 |
| gapdh | PV334905 | Colletotrichum fructicola MFU 090228/100.00 | |
| cal | PV334909 | Colletotrichum fructicola MFU 090228/100.00 | |
| act | PV334913 | Colletotrichum mengyinense SAUCC 200702/100.00 | |
| tub | PV334917 | Colletotrichum fructicola MFU 090228/100.00 | |
| BGC04 | ITS | PV290486 | Colletotrichum theobromicola CBS 124945/100 |
| gapdh | PV334906 | Colletotrichum theobromicola CBS 124945/98.87 | |
| cal | PV334910 | Colletotrichum theobromicola CBS 124945/99.60 | |
| act | PV334914 | Colletotrichum theobromicola CBS 124945/97.47 | |
| tub | PV334918 | Colletotrichum theobromicola CBS 124945/100.00 | |
| BGF01 | cal | PQ772082 | Fusarium melonis SDBR-CMU424/100.00 |
| tef1-α | PQ772081 | Fusarium pernambucanum URM 7559/99.56 | |
| rpb2 | PQ772083 | Fusarium pernambucanum URM 7559/100.00 | |
| BGF02 | cal | PV334919 | Fusarium melonis SDBR-CMU424/100.00 |
| tef1-α | PV334923 | Fusarium pernambucanum URM 7559/99.57 | |
| rpb2 | PV334927 | Fusarium pernambucanum URM 7559/100.00 | |
| BGF03 | cal | PV334920 | Fusarium melonis SDBR-CMU424/100.00 |
| tef1-α | PV334924 | Fusarium pernambucanum URM 7559/99.56 | |
| rpb2 | PV334928 | Fusarium pernambucanum URM 7559/100.00 | |
| BGF04.1 | cal | PV334921 | Fusarium melonis SDBR-CMU424/100.00 |
| tef1-α | PV334925 | Fusarium pernambucanum URM 7559/99.56 | |
| rpb2 | PV334929 | Fusarium pernambucanum URM 7559/100.00 | |
| BGF04.2 | cal | PV334922 | Fusarium melonis SDBR-CMU424/100.00 |
| tef1-α | PV334926 | Fusarium pernambucanum URM 7559/99.56 | |
| rpb2 | PV334930 | Fusarium pernambucanum URM 7559/100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wonglom, P.; Suwannarach, N.; Kumla, J.; Sunpapao, A. Fungal Pathogens of Peach Palm Leaf Spot in Thailand and Their Fungicide Sensitivity. J. Fungi 2025, 11, 318. https://doi.org/10.3390/jof11040318
Wonglom P, Suwannarach N, Kumla J, Sunpapao A. Fungal Pathogens of Peach Palm Leaf Spot in Thailand and Their Fungicide Sensitivity. Journal of Fungi. 2025; 11(4):318. https://doi.org/10.3390/jof11040318
Chicago/Turabian StyleWonglom, Prisana, Nakarin Suwannarach, Jaturong Kumla, and Anurag Sunpapao. 2025. "Fungal Pathogens of Peach Palm Leaf Spot in Thailand and Their Fungicide Sensitivity" Journal of Fungi 11, no. 4: 318. https://doi.org/10.3390/jof11040318
APA StyleWonglom, P., Suwannarach, N., Kumla, J., & Sunpapao, A. (2025). Fungal Pathogens of Peach Palm Leaf Spot in Thailand and Their Fungicide Sensitivity. Journal of Fungi, 11(4), 318. https://doi.org/10.3390/jof11040318








