Recent Perspectives in the Management of Fungal Keratitis
Abstract
1. Introduction
2. Diagnosis
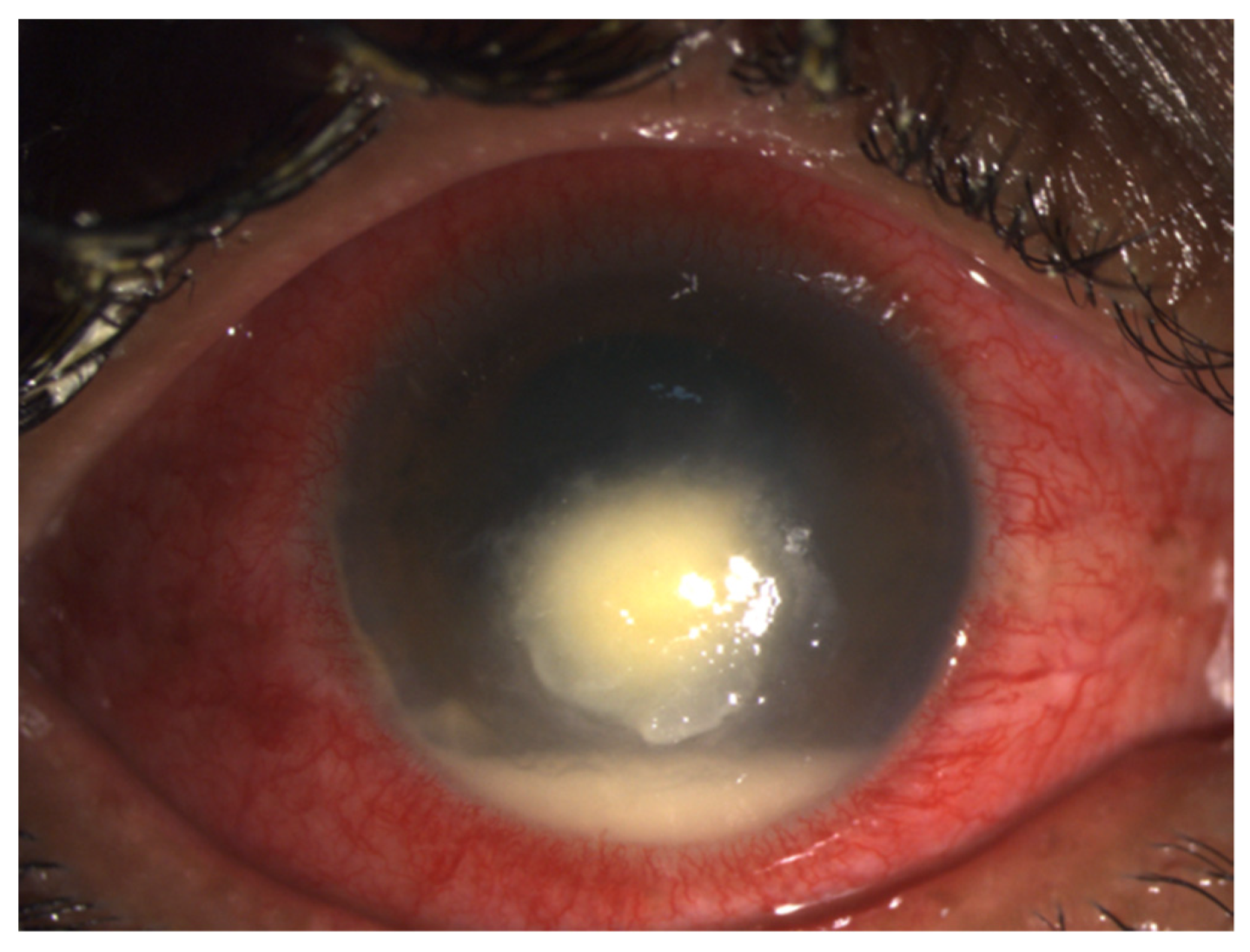
2.1. Clinical Diagnosis
2.2. Laboratory Diagnosis
2.2.1. Microscopic Examination
2.2.2. Culture
2.2.3. In Vivo Confocal Microscopy
2.2.4. Molecular Diagnostic Methods
2.2.5. Antifungal Susceptibility Testing
3. Treatment
3.1. Topical Agents
3.2. Systemic Therapy
Challenges in Azole- and Polyene-Based Therapy
3.3. Targeted Therapy
4. Antifungal Agents
4.1. Natamycin
4.2. Amphotericin B
4.3. Voriconazole
4.4. Itraconazole
4.5. Posaconazole
4.6. Ketoconazole
4.7. Luliconazole
4.8. Echinocandins
4.9. Other Therapeutic Agents
5. Nanoparticles
6. Contact-Lens-Based Drug Delivery
7. Photo Activated Chromophore for Keratitis
8. Photodynamic Therapy
9. Surgical Management
Lamellar Keratoplasty in Fungal Keratitis
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gonzales, C.A.; Srinivasan, M.; Whitcher, J.P.; Smolin, G. Incidence of corneal ulceration in Madurai district, South India. Ophthalmic Epidemiol. 1996, 3, 159–166. [Google Scholar] [CrossRef]
- Gupta, N.; Tandon, R.; Gupta, S.K.; Sreenivas, V.; Vashist, P. Burden of Corneal Blindness in India. Indian Assoc. Prev. Soc. Med. 2013, 38, 198–206. [Google Scholar]
- Ou, J.I.; Acharya, N.R. Epidemiology and treatment of fungal corneal ulcers. Int. Ophthalmol. Clin. 2007, 47, 7–16. [Google Scholar] [CrossRef]
- Gopinathan, U.; Sharma, S.; Garg, P.; Rao, G.N. Review of epidemiological features, microbiological diagnosis and treatment outcome of microbial keratitis: Experience of over a decade. Indian J. Ophthalmol. 2009, 57, 273–279. [Google Scholar]
- Bharathi, M.J.; Ramakrishnan, R.; Meenakshi, R.; Padmavathy, S.; Shivakumar, C.; Srinivasan, M. Microbial keratitis in South India: Influence of risk factors, climate, and geographical variation. Ophthalmic Epidemiol. 2007, 14, 61–69. [Google Scholar] [CrossRef]
- Thomas, P.A.; Kaliamurthy, J. Mycotic keratitis: Epidemiology, diagnosis and management. Clin. Microbiol. Infect. 2013, 19, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Satpathy, G.; Ahmed, N.H.; Nayak, N.; Tandon, R.; Sharma, N.; Agarwal, T.; Vanathi, M.; Titiyal, J.S. Spectrum of mycotic keratitis in north India: Sixteen years study from a tertiary care ophthalmic centre. J. Infect. Public Health 2019, 12, 367–371. [Google Scholar] [CrossRef]
- Chidambaram, J.; Prajna, N.; Larke, N.; Palepu, S.; Lanjewar, S.; Shah, M.; Elakkiya, S.; Lalitha, P.; Carnt, N.; Vesaluoma, M.; et al. Prospective Study of the Diagnostic Accuracy of the In Vivo Laser Scanning Confocal Microscope for Severe Microbial Keratitis. Ophthalmology 2016, 123, 2285–2293. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.L.; Cruzat, A.; Hamrah, P. Current state of in vivo confocal microscopy in management of microbial keratitis. Semin. Ophthalmol. 2010, 25, 166–170. [Google Scholar] [CrossRef]
- Goldschmidt, P.; Degorge, S.; Che Sarria, P.; Benallaoua, D.; Semoun, O.; Borderie, V.; Laroche, L.; Chaumeil, C. New strategy for rapid diagnosis and characterization of fungal infections: The example of corneal scrapings. PLoS ONE 2012, 7, e37660. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, P.; Abdel-Hadi, A.; Randhir Babu Singh, Y.; Revathi, R.; Anita, R.; Banawas, S.; Bin Dukhyil, A.A.; Alshehri, B.; Shobana, C.S.; Panneer Selvam, K.; et al. Fungal Keratitis: Epidemiology, Rapid Detection, and Antifungal Susceptibilities of Fusarium and Aspergillus Isolates from Corneal Scrapings. BioMed Res. Int. 2019, 2019, 6395840. [Google Scholar] [CrossRef]
- Niu, L.; Liu, X.; Ma, Z.; Yin, Y.; Sun, L.; Yang, L.; Zheng, Y. Fungal keratitis: Pathogenesis, diagnosis and prevention. Microb. Pathog. 2020, 138, 103802. [Google Scholar] [CrossRef] [PubMed]
- Prajna, N.V.; Krishnan, T.; Rajaraman, R.; Patel, S.; Shah, R.; Srinivasan, M.; Das, M.; Ray, K.J.; Oldenburg, C.E.; McLeod, S.D.; et al. Predictors of Corneal Perforation or Need for Therapeutic Keratoplasty in Severe Fungal Keratitis: A Secondary Analysis of the Mycotic Ulcer Treatment Trial II. JAMA Ophthalmol. 2017, 135, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Prajna, N.V.; Krishnan, T.; Rajaraman, R.; Patel, S.; Srinivasan, M.; Das, M.; Ray, K.J.; O’Brien, K.S.; Oldenburg, C.E.; McLeod, S.D.; et al. Effect of Oral Voriconazole on Fungal Keratitis in the Mycotic Ulcer Treatment Trial II (MUTT II): A Randomized Clinical Trial. JAMA Ophthalmol. 2016, 134, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Dalmon, C.; Porco, T.C.; Lietman, T.M.; Prajna, N.V.; Prajna, L.; Das, M.R.; Kumar, J.A.; Mascarenhas, J.; Margolis, T.P.; Whitcher, J.P.; et al. The clinical differentiation of bacterial and fungal keratitis: A photographic survey. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1787–1791. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, H.; Jiang, L.; Han, L.; Wang, L. Use of potassium hydroxide, Giemsa and calcofluor white staining techniques in the microscopic evaluation of corneal scrapings for diagnosis of fungal keratitis. J. Int. Med. Res. 2010, 38, 1961–1967. [Google Scholar] [CrossRef]
- Vemuganti, G.K.; Naidu, C.; Gopinathan, U. Rapid detection of fungal filaments in corneal scrapings by microwave heating-assisted Grocott’s methenamine silver staining. Indian J. Ophthalmol. 2002, 50, 326–328. [Google Scholar]
- Vengayil, S.; Panda, A.; Satpathy, G.; Nayak, N.; Ghose, S.; Patanaik, D.; Khokhar, S. Polymerase chain reaction-guided diagnosis of mycotic keratitis: A prospective evaluation of its efficacy and limitations. Investig. Ophthalmol. Vis. Sci. 2009, 50, 152–156. [Google Scholar] [CrossRef]
- Badiee, P.; Nejabat, M.; Alborzi, A.; Keshavarz, F.; Shakiba, E. Comparative study of Gram stain, potassium hydroxide smear, culture and nested PCR in the diagnosis of fungal keratitis. Ophthalmic Res. 2010, 44, 251–256. [Google Scholar] [CrossRef]
- Bharathi, M.J.; Ramakrishnan, R.; Meenakshi, R.; Mittal, S.; Shivakumar, C.; Srinivasan, M. Microbiological diagnosis of infective keratitis: Comparative evaluation of direct microscopy and culture results. Br. J. Ophthalmol. 2006, 90, 1271–1276. [Google Scholar] [CrossRef]
- Sharma, S.; Kunimoto, D.Y.; Gopinathan, U.; Athmanathan, S.; Garg, P.; Rao, G.N. Evaluation of corneal scraping smear examination methods in the diagnosis of bacterial and fungal keratitis: A survey of eight years of laboratory experience. Cornea 2002, 21, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, C.; Alió, J.L. Evaluation of molecular diagnosis in fungal keratitis. Ten years of experience. J. Ophthalmic Inflamm. Infect. 2011, 1, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S. Diagnosis of infectious diseases of the eye. Eye 2012, 26, 177–184. [Google Scholar] [CrossRef]
- Ray, K.J.; Lalitha, P.; Prajna, N.V.; Rajaraman, R.; Krishnan, T.; Srinivasan, M.; Ryg, P.; McLeod, S.; Acharya, N.R.; Lietman, T.M.; et al. The Utility of Repeat Culture in Fungal Corneal Ulcer Management: A Secondary Analysis of the MUTT-I Randomized Clinical Trial. Am. J. Ophthalmol. 2017, 178, 157–162. [Google Scholar] [CrossRef]
- Ray, K.J.; Prajna, N.V.; Lalitha, P.; Rajaraman, R.; Krishnan, T.; Patel, S.; Das, M.; Shah, R.; Dhakhwa, K.; McLeod, S.D.; et al. The Significance of Repeat Cultures in the Treatment of Severe Fungal Keratitis. Am. J. Ophthalmol. 2018, 189, 41–46. [Google Scholar] [CrossRef]
- Qu, L.; Xie, L. Changing indications for lamellar keratoplasty in Shandong, 1993–2008. Chin. Med. J. 2010, 123, 3268–3271. [Google Scholar] [PubMed]
- Ansari, Z.; Miller, D.; Galor, A. Current Thoughts in Fungal Keratitis: Diagnosis and Treatment. Curr. Fungal Infect. Rep. 2013, 7, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, S.; Masoomi, A.; Ahmadikia, K.; Tabatabaei, S.A.; Soleimani, M.; Rezaie, S.; Ghahvechian, H.; Banafsheafshan, A. Fungal keratitis: An overview of clinical and laboratory aspects. Mycoses 2018, 61, 916–930. [Google Scholar] [CrossRef]
- Kanavi, M.R.; Javadi, M.; Yazdani, S.; Mirdehghanm, S. Sensitivity and specificity of confocal scan in the diagnosis of infectious keratitis. Cornea 2007, 26, 782–786. [Google Scholar] [CrossRef]
- Vaddavalli, P.K.; Garg, P.; Sharma, S.; Sangwan, V.S.; Rao, G.N.; Thomas, R. Role of confocal microscopy in the diagnosis of fungal and acanthamoeba keratitis. Ophthalmology 2011, 118, 29–35. [Google Scholar] [CrossRef]
- Brasnu, E.; Bourcier, T.; Dupas, B.; Degorge, S.; Rodallec, T.; Laroche, L.; Borderie, V.; Baudouin, C. In vivo confocal microscopy in fungal keratitis. Br. J. Ophthalmol. 2007, 91, 588–591. [Google Scholar] [CrossRef]
- Chidambaram, J.D.; Prajna, N.V.; Palepu, S.; Lanjewar, S.; Shah, M.; Elakkiya, S.; Macleod, D.; Lalitha, P.; Burton, M. In Vivo Confocal Microscopy Cellular Features of Host and Organism in Bacterial, Fungal, and Acanthamoeba Keratitis. Am. J. Ophthalmol. 2018, 190, 24–33. [Google Scholar] [CrossRef]
- He, D.; Hao, J.; Gao, S.; Wan, X.; Wang, W.; Shan, Q.; Wang, L. Etiological Analysis of Fungal Keratitis and Rapid Identification of Predominant Fungal Pathogens. Mycopathologia 2016, 181, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Itahashi, M.; Higaki, S.; Fukuda, M.; Shimomura, Y. Detection and quantification of pathogenic bacteria and fungi using real-time polymerase chain reaction by cycling probe in patients with corneal ulcer. Arch. Ophthalmol. 2010, 128, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Hopping, G.C.; Vaidyanathan, U.; Ronquillo, Y.C.; Hoopes, P.C.; Moshirfar, M. Polymerase Chain Reaction and Its Application in the Diagnosis of Infectious Keratitis. Med. Hypothesis Discov. Innov. Ophthalmol. J. 2019, 8, 152–155. [Google Scholar]
- Homa, M.; Shobana, C.S.; Singh, Y.R.B.; Manikandan, P.; Selvam, K.P.; Kredics, L.; Narendran, V.; Vágvölgyi, C.; Galgóczy, L. Fusarium keratitis in South India: Causative agents, their antifungal susceptibilities and a rapid identification method for the Fusarium solani species complex. Mycoses 2013, 56, 501–511. [Google Scholar] [CrossRef]
- Salman, A.G.; Mansour, D.E.; Radwan, A.A.; Mansour, L.E. Polymerase chain reaction in pediatric post-traumatic fungal endophthalmitis among Egyptian children. Ocul. Immunol. Inflamm. 2010, 18, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Shukla, P.K. Single-stranded conformation polymorphism of large subunit of ribosomal RNA is best suited to diagnosing fungal infections and differentiating fungi at species level. Diagn. Microbiol. Infect. Dis. 2006, 56, 45–51. [Google Scholar] [CrossRef]
- Li, Z.; Breitwieser, F.P.; Lu, J.; Jun, A.S.; Asnaghi, L.; Salzberg, S.L.; Eberhart, C.G. Identifying Corneal Infections in Formalin-Fixed Specimens Using Next Generation Sequencing. Investig. Ophthalmol. Vis. Sci. 2018, 59, 280–288. [Google Scholar] [CrossRef]
- Oechsler, R.A.; Feilmeier, M.R.; Miller, D.; Shi, W.; Hofling-Lima, A.L.; Alfonso, E.C. Fusarium keratitis: Genotyping, in vitro susceptibility and clinical outcomes. Cornea 2013, 32, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Seitzman, G.D.; Hinterwirth, A.; Zhong, L.; Cummings, S.; Chen, C.; Driver, T.H.; Lee, M.D.; Doan, T. Metagenomic Deep Sequencing for the Diagnosis of Corneal and External Disease Infections. Ophthalmology 2019, 126, 1724–1726. [Google Scholar] [CrossRef]
- Lalitha, P.; Prajna, N.V.; Sikha, M.; Gunasekaran, R.; Hinterwirth, A.; Worden, L.; Chen, C.; Zhong, L.; Liu, Z.; Lietman, T.M.; et al. Evaluation of Metagenomic Deep Sequencing as a Diagnostic Test for Infectious Keratitis. Ophthalmology 2021, 128, 473–475. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.-T.; Chen, J.-L.; Hsu, S.-L.; Chen, A.; You, H.-L. An Omics Approach to Diagnosing or Investigating Fungal Keratitis. Int. J. Mol. Sci. 2019, 20, 3631. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Wei, C.; Yang, B.-X.; Cheng, J.; Huang, Y.-S. Conjunctival microbiome changes associated with fungal keratitis: Metagenomic analysis. Int. J. Ophthalmol. 2019, 12, 194–200. [Google Scholar]
- Kalyana Chakravarthy, S.; Jayasudha, R.; Ranjith, K.; Dutta, A.; Pinna, N.K.; Mande, S.S.; Sharma, S.; Garg, P.; Murthy, S.I.; Shivaji, S. Alterations in the gut bacterial microbiome in fungal Keratitis patients. PLoS ONE 2018, 13, e0199640. [Google Scholar] [CrossRef] [PubMed]
- Embong, Z.; Hitam, W.H.W.; Yean, C.Y.; Rashid, N.H.A.; Kamarudin, B.; Abidin, S.K.Z.; Osman, S.; Zainuddin, Z.F.; Ravichandran, M. Specific detection of fungal pathogens by 18S rRNA gene PCR in microbial keratitis. BMC Ophthalmol. 2008, 8, 7. [Google Scholar] [CrossRef]
- Ghosh, A.; Basu, S.; Datta, H.; Chattopadhyay, D. Evaluation of polymerase chain reaction-based ribosomal DNA sequencing technique for the diagnosis of mycotic keratitis. Am. J. Ophthalmol. 2007, 144, 396–403. [Google Scholar] [CrossRef]
- Dingle, T.C.; Butler-Wu, S.M. Maldi-tof mass spectrometry for microorganism identification. Clin. Lab. Med. 2013, 33, 589–609. [Google Scholar] [CrossRef]
- Patel, R. A Moldy Application of MALDI: MALDI-ToF Mass Spectrometry for Fungal Identification. J. Fungi 2019, 5, 4. [Google Scholar] [CrossRef]
- Rohilla, R.; Meena, S.; Mohanty, A.; Gupta, N.; Kaistha, N.; Gupta, P.; Mangla, A.; Singh, A. Etiological spectrum of infectious keratitis in the era of MALDI-TOF-MS at a tertiary care hospital. J. Fam. Med. Prim. Care 2020, 9, 4576–4581. [Google Scholar] [CrossRef]
- Berkow, E.L.; Lockhart, S.R.; Ostrosky-Zeichner, L. Antifungal Susceptibility Testing: Current Approaches. Clin. Microbiol. Rev. 2020, 33, e00069-19. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.D.; Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 4th ed.; CLSI Document; Clinical and Laboratory Standards Institute: Pittsburgh, PA, USA, 2017; ISBN 978-1-56238-826-3. [Google Scholar]
- Alexander, B.D.; Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, 2nd ed.; CLSI Document; Clinical and Laboratory Standards Institute: Pittsburgh, PA, USA, 2017; ISBN 978-1-56238-830-0. [Google Scholar]
- Arendrup, M.C.; Cuenca-Estrella, M.; Lass-Flörl, C.; Hope, W.; EUCAST-AFST. EUCAST technical note on the EUCAST definitive document EDef 7.2: Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST). Clin. Microbiol. Infect. 2012, 18, E246–E247. [Google Scholar] [CrossRef]
- Subcommittee on Antifungal Susceptibility Testing of the ESCMID European Committee for Antimicrobial Susceptibility Testing. EUCAST Technical Note on the method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia-forming moulds. Clin. Microbiol. Infect. 2008, 14, 982–984. [Google Scholar] [CrossRef] [PubMed]
- Procop, G.W.; Clinical and Laboratory Standards Institute. Performance Standards for Antifungal Susceptibility Testing of Yeasts, 2nd ed.; CLSI Document; Clinical and Laboratory Standards Institute: Pittsburgh, PA, USA, 2020; ISBN 978-1-68440-082-9. [Google Scholar]
- Arendrup, M.C.; Meletiadis, J.; Mouton, J.W.; Guinea, J.; Cuenca-Estrella, M.; Lagrou, K.; Howard, S.J.; Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). EUCAST technical note on isavuconazole breakpoints for Aspergillus, itraconazole breakpoints for Candida and updates for the antifungal susceptibility testing method documents. Clin. Microbiol. Infect. 2016, 22, 571.e1–571.e4. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Cuenca-Estrella, M.; Lass-Flörl, C.; Hope, W.W. EUCAST technical note on Aspergillus and amphotericin B, itraconazole, and posaconazole. Clin. Microbiol. Infect. 2012, 18, E248–E250. [Google Scholar] [CrossRef] [PubMed]
- Méndez, C.C.; Serrano, M.C.; Valverde, A.; Pemán, J.; Almeida, C.; Martín-Mazuelos, E. Comparison of E-Test, disk diffusion and a modified CLSI broth microdilution (M 38-A) method for in vitro testing of itraconazole, fluconazole and voriconazole against dermatophytes. Med. Mycol. 2008, 46, 119–123. [Google Scholar] [CrossRef]
- Idelevich, E.A.; Groß, U.; Becker, K.; Bader, O. Comparative evaluation of different gradient diffusion tests for detection of azole resistance in Aspergillus fumigatus. Diagn. Microbiol. Infect. Dis. 2018, 91, 52–54. [Google Scholar] [CrossRef]
- Law, D.; Moore, C.B.; Denning, D.W. Amphotericin B resistance testing of Candida spp.: A comparison of methods. J. Antimicrob. Chemother. 1997, 40, 109–112. [Google Scholar] [CrossRef]
- Lozano-Chiu, M.; Paetznick, V.L.; Ghannoum, M.A.; Rex, J.H. Detection of resistance to amphotericin B among Cryptococcus neoformans clinical isolates: Performances of three different media assessed by using E-test and National Committee for Clinical Laboratory Standards M27-A methodologies. J. Clin. Microbiol. 1998, 36, 2817–2822. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, B.L.; Lalitha, P.; Loh, A.R.; Fothergill, A.W.; Prajna, N.V.; Srinivasan, M.; Kabra, A.; Chidambaram, J.; Acharya, N.R.; Lietman, T.M. Susceptibility testing and clinical outcome in fungal keratitis. Br. J. Ophthalmol. 2010, 94, 384–385. [Google Scholar] [CrossRef] [PubMed]
- Lalitha, P.; Prajna, N.V.; Oldenburg, C.E.; Srinivasan, M.; Krishnan, T.; Mascarenhas, J.; Vaitilingam, C.M.; McLeod, S.D.; Zegans, M.E.; Porco, T.C.; et al. Organism, MIC, and Outcome in a Fungal Corneal Ulcer Clinical Trial. Cornea 2012, 31, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Lass-Flörl, C.; Kofler, G.; Kropshofer, G.; Hermans, J.; Kreczy, A.; Dierich, M.P.; Niederwieser, D. In-vitro testing of susceptibility to amphotericin B is a reliable predictor of clinical outcome in invasive aspergillosis. J. Antimicrob. Chemother. 1998, 42, 497–502. [Google Scholar] [CrossRef]
- Sun, C.Q.; Lalitha, P.; Prajna, N.V.; Karpagam, R.; Geetha, M.; O’Brien, K.S.; Oldenburg, C.E.; Ray, K.J.; McLeod, S.D.; Acharya, N.R.; et al. Association between in vitro susceptibility to natamycin and voriconazole and clinical outcomes in fungal keratitis. Ophthalmology 2014, 121, 1495–1500.e1. [Google Scholar] [CrossRef]
- Saha, S.; Sengupta, J.; Banerjee, D.; Saha, S.; Khetan, A.; Mandal, S.M. Systemic Evaluation on Antifungal Susceptibility of Keratitis Associated Fungal Pathogens in Eastern India. Med. Microbiol. Diagn. 2014, 3, 1. [Google Scholar] [CrossRef]
- Prajna, N.V.; Krishnan, T.; Rajaraman, R.; Patel, S.; Shah, R.; Srinivasan, M.; Devi, L.; Das, M.; Ray, K.J.; O’Brien, K.S.; et al. Adjunctive Oral Voriconazole Treatment of Fusarium Keratitis. JAMA Ophthalmol. 2017, 135, 520–525. [Google Scholar] [CrossRef]
- Maharana, P.K.; Sharma, N.; Nagpal, R.; Jhanji, V.; Das, S.; Vajpayee, R.B. Recent advances in diagnosis and management of Mycotic Keratitis. Indian J. Ophthalmol. 2016, 64, 346–357. [Google Scholar] [PubMed]
- Sharma, N.; Sahay, P.; Maharana, P.K.; Singhal, D.; Saluja, G.; Bandivadekar, P.; Chako, J.; Agarwal, T.; Sinha, R.; Titiyal, J.S.; et al. Management Algorithm for Fungal Keratitis: The TST (Topical, Systemic, and Targeted Therapy) Protocol. Cornea 2019, 38, 141–145. [Google Scholar] [CrossRef]
- Bunya, V.Y.; Hammersmith, K.M.; Rapuano, C.J.; Ayres, B.D.; Cohen, E.J. Topical and oral voriconazole in the treatment of fungal keratitis. Am. J. Ophthalmol. 2007, 143, 151–153. [Google Scholar] [CrossRef]
- Dick, J.D.; Merz, W.G.; Saral, R. Incidence of polyene-resistant yeasts recovered from clinical specimens. Antimicrob. Agents Chemother. 1980, 18, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Kanafani, Z.A.; Perfect, J.R. Antimicrobial resistance: Resistance to antifungal agents: Mechanisms and clinical impact. Clin. Infect. Dis. 2008, 46, 120–128. [Google Scholar] [CrossRef]
- Hamilton-Miller, J.M. Chemistry and biology of the polyene macrolide antibiotics. Bacteriol. Rev. 1973, 37, 166–196. [Google Scholar] [CrossRef]
- Kelly, S.L.; Lamb, D.C.; Kelly, D.E.; Manning, N.J.; Loeffler, J.; Hebart, H.; Schumacher, U.; Einsele, H. Resistance to fluconazole and cross-resistance to amphotericin B in Candida albicans from AIDS patients caused by defective sterol delta5,6-desaturation. FEBS Lett. 1997, 400, 80–82. [Google Scholar] [CrossRef]
- Kaur, I.P.; Rana, C.; Singh, H. Development of effective ocular preparations of antifungal agents. J. Ocul. Pharmacol. Ther. 2008, 24, 481–493. [Google Scholar] [CrossRef]
- Thomas, P.A. Current perspectives on ophthalmic mycoses. Clin. Microbiol. Rev. 2003, 16, 730–797. [Google Scholar] [CrossRef] [PubMed]
- Sahay, P.; Singhal, D.; Nagpal, R.; Maharana, P.K.; Farid, M.; Gelman, R.; Sinha, R.; Agarwal, T.; Titiyal, J.S.; Sharma, N. Pharmacologic therapy of mycotic keratitis. Surv. Ophthalmol. 2019, 64, 380–400. [Google Scholar] [CrossRef]
- Sharma, N.; Agarwal, P.; Sinha, R.; Titiyal, J.S.; Velpandian, T.; Vajpayee, R.B. Evaluation of intrastromal voriconazole injection in recalcitrant deep fungal keratitis: Case series. Br. J. Ophthalmol. 2011, 95, 1735–1737. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, J.; Li, Y.; Han, X.; Zheng, W.; Yang, J.; Xu, G. A Combination of Intrastromal and Intracameral Injections of Amphotericin B in the Treatment of Severe Fungal Keratitis. J. Ophthalmol. 2016, 2016, 3436415. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Valenzuela, E.; Song, C.D. Intracorneal injection of amphothericin B for recurrent fungal keratitis and endophthalmitis. Arch. Ophthalmol. 2005, 123, 1721–1723. [Google Scholar] [CrossRef]
- Cavallini, G.M.; Ducange, P.; Volante, V.; Benatti, C. Successful treatment of Fusarium keratitis after photo refractive keratectomy. Indian J. Ophthalmol. 2013, 61, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Eleiwa, T.; Ozcan, E.; Abdelrahman, S.; Solyman, O.; Elhusseiny, A.; Youssef, G.; Bayoumy, A. Case Series of Perforated Keratomycosis after Laser-Assisted In Situ Keratomileusis. Case Rep. Ophthalmol. Med. 2020, 2020, 7237903. [Google Scholar] [CrossRef]
- Yoon, K.-C.; Jeong, I.-Y.; Im, S.-K.; Chae, H.-J.; Yang, S.-Y. Therapeutic effect of intracameral amphotericin B injection in the treatment of fungal keratitis. Cornea 2007, 26, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Kalaiselvi, G.; Narayana, S.; Krishnan, T.; Sengupta, S. Intrastromal voriconazole for deep recalcitrant fungal keratitis: A case series. Br. J. Ophthalmol. 2015, 99, 195–198. [Google Scholar] [CrossRef]
- Prakash, G.; Sharma, N.; Goel, M.; Titiyal, J.S.; Vajpayee, R.B. Evaluation of intrastromal injection of voriconazole as a therapeutic adjunctive for the management of deep recalcitrant fungal keratitis. Am. J. Ophthalmol. 2008, 146, 56–59. [Google Scholar] [CrossRef]
- Narayana, S.; Krishnan, T.; Ramakrishnan, S.; Samantaray, P.P.; Austin, A.; Pickel, J.; Porco, T.; Lietman, T.; Rose-Nussbaumer, J. Mycotic Antimicrobial Localized Injection (MALIN): A Randomized Clinical Trial Evaluating Intrastromal Injection of Voriconazole. Ophthalmology 2019, 126, 1084–1089. [Google Scholar] [CrossRef]
- Saluja, G.; Sharma, N.; Agarwal, R.; Sharma, H.P.; Singhal, D.; Kumar Maharana, P.; Sinha, R.; Agarwal, T.; Velpandian, T.; Titiyal, J.S.; et al. Comparison of Safety and Efficacy of Intrastromal Injections of Voriconazole, Amphotericin B and Natamycin in Cases of Recalcitrant Fungal Keratitis: A Randomized Controlled Trial. Clin. Ophthalmol. 2021, 15, 2437–2446. [Google Scholar] [CrossRef]
- Lalitha, P.; Vijaykumar, R.; Prajna, N.V.; Fothergill, A.W. In Vitro Natamycin Susceptibility of Ocular Isolates of Fusarium and Aspergillus Species: Comparison of Commercially Formulated Natamycin Eye Drops to Pharmaceutical-Grade Powder. J. Clin. Microbiol. 2008, 46, 3477–3478. [Google Scholar] [CrossRef] [PubMed]
- Prajna, N.V.; Krishnan, T.; Mascarenhas, J.; Rajaraman, R.; Prajna, L.; Srinivasan, M.; Raghavan, A.; Oldenburg, C.E.; Ray, K.J.; Zegans, M.E.; et al. The mycotic ulcer treatment trial: A randomized trial comparing natamycin vs. voriconazole. JAMA Ophthalmol. 2013, 131, 422–429. [Google Scholar] [CrossRef]
- Sharma, S.; Das, S.; Virdi, A.; Fernandes, M.; Sahu, S.K.; Kumar Koday, N.; Ali, M.H.; Garg, P.; Motukupally, S.R. Re-appraisal of topical 1% voriconazole and 5% natamycin in the treatment of fungal keratitis in a randomised trial. Br. J. Ophthalmol. 2015, 99, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- FlorCruz, N.V.; Evans, J.R. Medical interventions for fungal keratitis. Cochrane Database Syst. Rev. 2015, CD004241. [Google Scholar] [CrossRef]
- Mimouni, M.; Tam, G.; Paitan, Y.; Kidron, D.; Segev, F. Safety and efficacy of intrastromal injection of 5% natamycin in experimental fusarium keratitis. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2014, 30, 543–547. [Google Scholar] [CrossRef]
- Velpandian, T.; Nirmal, J.; Sharma, H.; Sharma, S.K.; Sharma, N.; Halder, N. Novel Water Soluble Sterile Natamycin Formulation (Natasol) for Fungal Keratitis. Eur. J. Pharm. Sci. 2021, 163, 105857. [Google Scholar] [CrossRef]
- Nett, J.E.; Andes, D.R. Antifungal Agents: Spectrum of Activity, Pharmacology, and Clinical Indications. Infect. Dis. Clin. N. Am. 2016, 30, 51–83. [Google Scholar] [CrossRef]
- Ostrosky-Zeichner, L.; Marr, K.A.; Rex, J.H.; Cohen, S.H. Amphotericin B: Time for a new “gold standard”. Clin. Infect. Dis. 2003, 37, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Li, L.; Xie, H. Corneal and aqueous humor concentrations of amphotericin B using three different routes of administration in a rabbit model. Ophthalmic Res. 2010, 43, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Rudramurthy, S.M.; Gupta, A.; Choudhary, H.; Singh, S.; Thakur, A.; Jatana, M. Evaluation of Liposomal and Conventional Amphotericin B in Experimental Fungal Keratitis Rabbit Model. Transl. Vis. Sci. Technol. 2019, 8, 35. [Google Scholar] [CrossRef]
- Aydin, B.; Cubuk, M.O.; Ucgul, A.; Ertop, M.; Ozmen, M.C.; Atalay, T.; Akata, F. Combined Intrastromal Voriconazole and Amphotericin B Treatment for Persistent Fungal Keratitis. Eye Contact Lens 2020, 46, 269–273. [Google Scholar] [CrossRef]
- Deswal, J.; Arya, S.K. Intracorneal Amphotericin B Injection in a Case of Indolent Candidal Keratitis. J. Clin. Diagn. Res. JCDR 2017, 11, ND01–ND02. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Sankaran, P.; Agarwal, T.; Arora, T.; Chawla, B.; Titiyal, J.S.; Tandon, R.; Satapathy, G.; Vajpayee, R.B. Evaluation of Intracameral Amphotericin B in the Management of Fungal Keratitis: Randomized Controlled Trial. Ocul. Immunol. Inflamm. 2016, 24, 493–497. [Google Scholar] [CrossRef]
- Bell, R.W.; Ritchey, J.P. Subconjunctival nodules after amphotericin B injection. Medical therapy for Aspergillus corneal ulcer. Arch. Ophthalmol. 1973, 90, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Han Shu, T.; Hussein, A.; Kursiah, M.R. Conjunctiva Necrosis Following Subconjunctival Amphotericin B Injection in Fungal Keratitis. Cureus 2019, 11, e5580. [Google Scholar] [CrossRef]
- Nada, W.M.; Al Aswad, M.A.; El-Haig, W.M. Combined intrastromal injection of amphotericin B and topical fluconazole in the treatment of resistant cases of keratomycosis: A retrospective study. Clin. Ophthalmol. 2017, 11, 871–874. [Google Scholar] [CrossRef]
- Carrasco, M.A.; Genesoni, G. Treatment of severe fungal keratitis with subconjunctival amphotericin B. Cornea 2011, 30, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Roy, G.; Galigama, R.D.; Thorat, V.S.; Mallela, L.S.; Roy, S.; Garg, P.; Venuganti, V.V.K. Amphotericin B containing microneedle ocular patch for effective treatment of fungal keratitis. Int. J. Pharm. 2019, 572, 118808. [Google Scholar] [CrossRef]
- Jones, A.; Muhtaseb, M. Use of voriconazole in fungal keratitis. J. Cataract Refract. Surg. 2008, 34, 183–184. [Google Scholar] [CrossRef] [PubMed]
- Freda, R. Use of oral voriconazole as adjunctive treatment of severe cornea fungal infection: Case report. Arq. Bras. Oftalmol. 2006, 69, 431–434. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hariprasad, S.M.; Mieler, W.F.; Lin, T.K.; Sponsel, W.E.; Graybill, J.R. Voriconazole in the treatment of fungal eye infections: A review of current literature. Br. J. Ophthalmol. 2008, 92, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Singhal, D.; Maharana, P.K.; Sinha, R.; Agarwal, T.; Upadhyay, A.D.; Velpandian, T.; Satpathy, G.; Titiyal, J.S. Comparison of Oral Voriconazole Versus Oral Ketoconazole as an Adjunct to Topical Natamycin in Severe Fungal Keratitis: A Randomized Controlled Trial. Cornea 2017, 36, 1521–1527. [Google Scholar] [CrossRef]
- Chen, D.; Tan, S.; Zou, W. Treating Fungal Keratitis with Oral Voriconazole Only: A Case Series. Klin. Mon. Augenheilkd. 2021, 238, 55–59. [Google Scholar] [CrossRef]
- Diekema, D.J.; Messer, S.A.; Hollis, R.J.; Jones, R.N.; Pfaller, M.A. Activities of Caspofungin, Itraconazole, Posaconazole, Ravuconazole, Voriconazole, and Amphotericin B against 448 Recent Clinical Isolates of Filamentous Fungi. J. Clin. Microbiol. 2003, 41, 3623–3626. [Google Scholar] [CrossRef]
- Al-Hatmi, A.M.S.; Meletiadis, J.; Curfs-Breuker, I.; Bonifaz, A.; Meis, J.F.; De Hoog, G.S. In vitro combinations of natamycin with voriconazole, itraconazole and micafungin against clinical Fusarium strains causing keratitis. J. Antimicrob. Chemother. 2016, 71, 953–955. [Google Scholar] [CrossRef]
- Miller, D.; Alfonso, E.C. Management of fungal keratitis: Topical or Systemic therapy? Vis. Pan-Am. 2014, 13, 73. [Google Scholar]
- Lestner, J.; Hope, W.W. Itraconazole: An update on pharmacology and clinical use for treatment of invasive and allergic fungal infections. Expert Opin. Drug Metab. Toxicol. 2013, 9, 911–926. [Google Scholar] [CrossRef] [PubMed]
- Wagh, V.D.; Deshmukh, O.J. Itraconazole Niosomes Drug Delivery System and Its Antimycotic Activity against Candida albicans. ISRN Pharm. 2012, 2012, 653465. [Google Scholar] [CrossRef]
- Lee, E.A.; Balakrishnan, P.; Song, C.K.; Choi, J.H.; Noh, G.Y.; Park, C.G.; Choi, A.J.; Chung, S.J.; Shim, C.K.; Kim, D.D. Microemulsion-based Hydrogel Formulation of Itraconazole for Topical Delivery. J. Pharm. Investig. 2010, 40, 305–311. [Google Scholar] [CrossRef]
- Nakarani, M.; Misra, A.K.; Patel, J.K.; Vaghani, S.S. Itraconazole nanosuspension for oral delivery: Formulation, characterization and in vitro comparison with marketed formulation. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2010, 18, 84–90. [Google Scholar]
- Mukherjee, S.; Ray, S.; Thakur, R.S. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J. Pharm. Sci. 2009, 71, 349–358. [Google Scholar] [CrossRef]
- ElMeshad, A.N.; Mohsen, A.M. Enhanced corneal permeation and antimycotic activity of itraconazole against Candida albicans via a novel nanosystem vesicle. Drug Deliv. 2016, 23, 2115–2123. [Google Scholar] [CrossRef]
- Badawi, A.A.; El-Nabarawi, M.A.; El-Setouhy, D.A.; Alsammit, S.A. Formulation and stability testing of itraconazole crystalline nanoparticles. AAPS PharmSciTech 2011, 12, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Permana, A.D.; Utami, R.N.; Layadi, P.; Himawan, A.; Juniarti, N.; Anjani, Q.K.; Utomo, E.; Mardikasari, S.A.; Arjuna, A.; Donnelly, R.F. Thermosensitive and mucoadhesive in situ ocular gel for effective local delivery and antifungal activity of itraconazole nanocrystal in the treatment of fungal keratitis. Int. J. Pharm. 2021, 602, 120623. [Google Scholar] [CrossRef]
- Nagappan, V.; Deresinski, S. Posaconazole: A Broad-Spectrum Triazole Antifungal Agent. Clin. Infect. Dis. 2007, 45, 1610–1617. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Cuenca-Estrella, M.; Donnelly, J.P.; Hope, W.; Lass-Flörl, C.; Rodriguez-Tudela, J.-L.; European Committee on Antimicrobial Susceptibility Testing. Subcommittee on antifungal susceptibility testing (EUCAST-AFST) EUCAST technical note on posaconazole. Clin. Microbiol. Infect. 2011, 17, E16–E17. [Google Scholar] [CrossRef][Green Version]
- Sponsel, W.E.; Graybill, J.R.; Nevarez, H.L.; Dang, D. Ocular and systemic posaconazole(SCH-56592) treatment of invasive Fusarium solani keratitis and endophthalmitis. Br. J. Ophthalmol. 2002, 86, 829–830. [Google Scholar] [CrossRef] [PubMed]
- Guarascio, A.J.; Slain, D. Review of the new delayed-release oral tablet and intravenous dosage forms of posaconazole. Pharmacotherapy 2015, 35, 208–219. [Google Scholar] [CrossRef]
- Arnoldner, M.A.; Kheirkhah, A.; Jakobiec, F.A.; Durand, M.L.; Hamrah, P. Successful treatment of Paecilomyces lilacinus keratitis with oral posaconazole. Cornea 2014, 33, 747–749. [Google Scholar] [CrossRef] [PubMed]
- Tu, E.Y.; McCartney, D.L.; Beatty, R.F.; Springer, K.L.; Levy, J.; Edward, D. Successful treatment of resistant ocular fusariosis with posaconazole (SCH-56592). Am. J. Ophthalmol. 2007, 143, 222–227. [Google Scholar] [CrossRef]
- Altun, A.; Kurna, S.A.; Sengor, T.; Altun, G.; Olcaysu, O.O.; Aki, S.F.; Simsek, M.H. Effectiveness of posaconazole in recalcitrant fungal keratitis resistant to conventional antifungal drugs. Case Rep. Ophthalmol. Med. 2014, 2014, 701653. [Google Scholar] [CrossRef] [PubMed]
- Tu, E.Y.; Park, A.J. Recalcitrant Beauveria bassiana keratitis: Confocal microscopy findings and treatment with posaconazole (Noxafil). Cornea 2007, 26, 1008–1010. [Google Scholar] [CrossRef]
- Xiao, L.; Madison, V.; Chau, A.S.; Loebenberg, D.; Palermo, R.E.; McNicholas, P.M. Three-dimensional models of wild-type and mutated forms of cytochrome P450 14alpha-sterol demethylases from Aspergillus fumigatus and Candida albicans provide insights into posaconazole binding. Antimicrob. Agents Chemother. 2004, 48, 568–574. [Google Scholar] [CrossRef]
- Ferguson, T.J.; Downes, R.A.; Isada, C.M.; Goshe, J.M. High-Dose Oral Posaconazole for the Treatment of Recalcitrant Fungal Keratitis. Cornea 2021. [Google Scholar] [CrossRef]
- Durgun, M.E.; Kahraman, E.; Hacıoğlu, M.; Güngör, S.; Özsoy, Y. Posaconazole micelles for ocular delivery: In vitro permeation, ocular irritation and antifungal activity studies. Drug Deliv. Transl. Res. 2021. [Google Scholar] [CrossRef]
- Thomas, P.A.; Kalavathy, C.M.; Abraham, D.J.; Rajasekaran, J. Oral ketoconazole in Keratomycosis. Indian J. Ophthalmol. 1987, 35, 197–203. [Google Scholar] [PubMed]
- Gupta, A.K.; Lyons, D.C.A. The Rise and Fall of Oral Ketoconazole. J. Cutan. Med. Surg. 2015, 19, 352–357. [Google Scholar] [CrossRef]
- Ishibashi, Y. Oral ketoconazole therapy for keratomycosis. Am. J. Ophthalmol. 1983, 95, 342–345. [Google Scholar] [CrossRef]
- Rajaraman, R.; Bhat, P.; Vaidee, V.; Maskibail, S.; Raghavan, A.; Sivasubramaniam, S.; Namperumalsamy, V.P. Topical 5% Natamycin With Oral Ketoconazole in Filamentous Fungal Keratitis: A Randomized Controlled Trial. Asia-Pac. J. Ophthalmol. 2015, 4, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Foley, K.A.; Versteeg, S.G. New Antifungal Agents and New Formulations Against Dermatophytes. Mycopathologia 2017, 182, 127–141. [Google Scholar] [CrossRef]
- Todokoro, D.; Suzuki, T.; Tamura, T.; Makimura, K.; Yamaguchi, H.; Inagaki, K.; Akiyama, H. Efficacy of Luliconazole Against Broad-Range Filamentous Fungi Including Fusarium solani Species Complex Causing Fungal Keratitis. Cornea 2019, 38, 238–242. [Google Scholar] [CrossRef]
- Patil, A.; Majumdar, S. Echinocandins in Ocular Therapeutics. J. Ocul. Pharmacol. Ther. 2017, 33, 340–352. [Google Scholar] [CrossRef]
- Gil-Lamaignere, C.; Salvenmoser, S.; Hess, R.; Müller, F.-M.C. Micafungin Enhances Neutrophil Fungicidal Functions against Candida Pseudohyphae. Antimicrob. Agents Chemother. 2004, 48, 2730–2732. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Denning, D.W. Echinocandins: A new class of antifungal. J. Antimicrob. Chemother. 2002, 49, 889–891. [Google Scholar] [CrossRef]
- Revankar, S.; Sobel, J. Are Echinocandins Better Than Azoles for Invasive Candidiasis? Curr. Fungal Infect. Rep. 2012, 7, 79–82. [Google Scholar] [CrossRef]
- Neoh, C.F.; Daniell, M.; Chen, S.C.-A.; Stewart, K.; Kong, D.C.M. Clinical utility of caspofungin eye drops in fungal keratitis. Int. J. Antimicrob. Agents 2014, 44, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Vorwerk, C.K.; Tuchen, S.; Streit, F.; Binder, L.; Hofmüller, W.; Behrens-Baumann, W. Aqueous humor concentrations of topically administered caspofungin in rabbits. Ophthalmic Res. 2009, 41, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Neoh, C.F.; Leung, L.; Misra, A.; Vajpayee, R.B.; Davies, G.E.; Fullinfaw, R.O.; Stewart, K.; Kong, D.C.M. Penetration of topically administered 0.5-percent caspofungin eye drops into human aqueous humor. Antimicrob. Agents Chemother. 2011, 55, 1761–1763. [Google Scholar] [CrossRef][Green Version]
- Neoh, C.F.; Leung, L.; Vajpayee, R.B.; Stewart, K.; Kong, D.C.M. Treatment of Alternaria keratitis with intrastromal and topical caspofungin in combination with intrastromal, topical, and oral voriconazole. Ann. Pharmacother. 2011, 45, e24. [Google Scholar] [CrossRef] [PubMed]
- Tu, E.Y. Alternaria keratitis: Clinical presentation and resolution with topical fluconazole or intrastromal voriconazole and topical caspofungin. Cornea 2009, 28, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Sarrió, M.; Duch-Samper, A.; Cisneros-Lanuza, A.; Díaz-Llopis, M.; Peman-Garcíia, J.; Vazquez-Polo, A. Successful topical application of caspofungin in the treatment of fungal keratitis refractory to voriconazole. Arch. Ophthalmol. 2010, 128, 941–942. [Google Scholar] [CrossRef]
- Gregory, M.E.; Macdonald, E.C.A.; Lockington, D.; Ramaesh, K. Recurrent fungal keratitis following penetrating keratoplasty: An unusual source of infection. Arch. Ophthalmol. 2010, 128, 1490–1491. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Spriet, I.; Delaere, L.; Lagrou, K.; Peetermans, W.E.; Maertens, J.; Willems, L. Intraocular penetration of voriconazole and caspofungin in a patient with fungal endophthalmitis. J. Antimicrob. Chemother. 2009, 64, 877–878. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Dogru, M.; Goto, E.; Fujishima, H.; Tsubota, K. Successful topical application of a new antifungal agent, micafungin, in the treatment of refractory fungal corneal ulcers: Report of three cases and literature review. Cornea 2005, 24, 748–753. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Murat, D.; Kojima, T.; Shimazaki, J.; Tsubota, K. The comparison of solitary topical micafungin or fluconazole application in the treatment of Candida fungal keratitis. Br. J. Ophthalmol. 2011, 95, 1406–1409. [Google Scholar] [CrossRef] [PubMed]
- Pinna, A.; Donadu, M.G.; Usai, D.; Dore, S.; Boscia, F.; Zanetti, S. In Vitro Antimicrobial Activity of a New Ophthalmic Solution Containing Hexamidine Diisethionate 0.05% (Keratosept). Cornea 2020, 39, 1415–1418. [Google Scholar] [CrossRef] [PubMed]
- Pinna, A.; Donadu, M.G.; Usai, D.; Dore, S.; D’Amico-Ricci, G.; Boscia, F.; Zanetti, S. In vitro antimicrobial activity of a new ophthalmic solution containing povidone-iodine 0.6% (IODIM®). Acta Ophthalmol. 2020, 98, e178–e180. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Yi, J.; Lv, S.; Zhang, B. Ocular amphotericin B delivery by chitosan-modified nanostructured lipid carriers for fungal keratitis-targeted therapy. J. Liposome Res. 2017, 27, 228–233. [Google Scholar] [CrossRef]
- Ciolino, J.B.; Hudson, S.P.; Mobbs, A.N.; Hoare, T.R.; Iwata, N.G.; Fink, G.R.; Kohane, D.S. A prototype antifungal contact lens. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6286–6291. [Google Scholar] [CrossRef]
- Schnitzler, E.; Spörl, E.; Seiler, T. Irradiation of cornea with ultraviolet light and riboflavin administration as a new treatment for erosive corneal processes, preliminary results in four patients. Klin. Mon. Augenheilkd. 2000, 217, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Prajna, N.V.; Radhakrishnan, N.; Lalitha, P.; Austin, A.; Ray, K.J.; Keenan, J.D.; Porco, T.C.; Lietman, T.M.; Rose-Nussbaumer, J. Cross-Linking–Assisted Infection Reduction. Ophthalmology 2020, 127, 159–166. [Google Scholar] [CrossRef]
- Wei, A.; Wang, K.; Wang, Y.; Gong, L.; Xu, J.; Shao, T. Evaluation of corneal cross-linking as adjuvant therapy for the management of fungal keratitis. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, J.M.; Caballero-Lima, D.; Hillarby, M.C.; Shawcross, S.G.; Brahma, A.; Carley, F.; Read, N.D.; Radhakrishnan, H. Evaluation of Corneal Cross-Linking for Treatment of Fungal Keratitis: Using Confocal Laser Scanning Microscopy on an Ex Vivo Human Corneal Model. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6367–6373. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, H.B.; Kalkancı, A.; Bilgihan, K.; Göçün, P.U.; Öğüt, B.; Karakurt, F.; Erdoğan, M. Comparison of corneal collagen cross-linking (PACK-CXL) and voriconazole treatments in experimental fungal keratitis. Acta Ophthalmol. 2019, 97, e91–e96. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, H.; Yue, J.; Liu, S.; Li, Z.; Wang, L. Antimicrobial efficacy of corneal cross-linking in vitro and in vivo for Fusarium solani: A potential new treatment for fungal keratitis. BMC Ophthalmol. 2018, 18, 65. [Google Scholar] [CrossRef]
- González Castellanos, J.C.; Osaba, M.; Reviglio, V.; Canchi, M.T.; Arrigone, M.C.; Reviglio, V.E. Early treatment of bilateral fungal keratitis with corneal cross-linking as adjuvant therapy. Oxf. Med. Case Rep. 2020, 2020, omaa032. [Google Scholar] [CrossRef]
- Mikropoulos, D.G.; Kymionis, G.D.; Voulgari, N.; Kaisari, E.; Nikolakopoulos, K.A.; Katsanos, A.; Konstas, A.G. Intraoperative Photoactivated Chromophore for Infectious Keratitis–Corneal Cross-Linking (PACK-CXL) during Penetrating Keratoplasty for the Management of Fungal Keratitis in an Immunocompromised Patient. Ophthalmol. Ther. 2019, 8, 491–495. [Google Scholar] [CrossRef]
- Jeyalatha Mani, V.; Parthasarathy, D.; Padmanabhan, P.; Narayanan, N.; Lakshmipathy, M.; Pachayappan, S.K.; Jayavel, P.; Therese, K.L.; Rao Madhavan, H.N.; Jambulingam, M. Therapeutic effect of corneal crosslinking on fungal keratitis: Efficacy of corneal collagen crosslinking as an adjuvant therapy for fungal keratitis in a tertiary eye hospital in south India. Ocul. Immunol. Inflamm. 2020. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yu, T.; Gao, X.; Wu, X.-Y. Accelerated corneal collagen cross-linking in clinical management of infectious keratitis. J. Int. Med. Res. 2020, 48, 0300060520926411. [Google Scholar] [CrossRef] [PubMed]
- Amescua, G.; Arboleda, A.; Nikpoor, N.; Durkee, H.; Relhan, N.; Aguilar, M.C.; Flynn, H.W., Jr.; Miller, D.; Parel, J.M. Rose Bengal Photodynamic Antimicrobial Therapy: A Novel Treatment for Resistant Fusarium Keratitis. Cornea 2017, 36, 1141–1144. [Google Scholar] [CrossRef] [PubMed]
- Arboleda, A.; Miller, D.; Cabot, F.; Taneja, M.; Aguilar, M.C.; Alawa, K.; Amescua, G.; Yoo, S.H.; Parel, J.-M. Assessment of rose bengal versus riboflavin photodynamic therapy for inhibition of fungal keratitis isolates. Am. J. Ophthalmol. 2014, 158, 64–70.e2. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Peng, C.; Shi, C.; Jiang, M.; Chau, J.H.C.; Liu, Z.; Bai, H.; Kwok, R.T.K.; Lam, J.W.Y.; Shi, Y.; et al. Mitochondria-Specific Aggregation-Induced Emission Luminogens for Selective Photodynamic Killing of Fungi and Efficacious Treatment of Keratitis. ACS Nano 2021, 15, 12129–12139. [Google Scholar] [CrossRef]
- Mundra, J.; Dhakal, R.; Mohamed, A.; Jha, G.; Joseph, J.; Chaurasia, S.; Murthy, S. Outcomes of therapeutic penetrating keratoplasty in 198 eyes with fungal keratitis. Indian J. Ophthalmol. 2019, 67, 1599–1605. [Google Scholar] [CrossRef]
- Rautaraya, B.; Sharma, S.; Kar, S.; Das, S.; Sahu, S.K. Diagnosis and treatment outcome of mycotic keratitis at a tertiary eye care center in eastern India. BMC Ophthalmol. 2011, 11, 39. [Google Scholar] [CrossRef]
- Xie, L.; Zhai, H.; Shi, W. Penetrating keratoplasty for corneal perforations in fungal keratitis. Cornea 2007, 26, 158–162. [Google Scholar] [CrossRef]
- Xie, L.; Dong, X.; Shi, W. Treatment of fungal keratitis by penetrating keratoplasty. Br. J. Ophthalmol. 2001, 85, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Shi, W.; Liu, Z.; Li, S. Lamellar keratoplasty for the treatment of fungal keratitis. Cornea 2002, 21, 33–37. [Google Scholar] [CrossRef]
- Sabatino, F.; Sarnicola, E.; Sarnicola, C.; Tosi, G.M.; Perri, P.; Sarnicola, V. Early deep anterior lamellar keratoplasty for fungal keratitis poorly responsive to medical treatment. Eye 2017, 31, 1639–1646. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Zhang, Y.; Ren, Y.; Zhao, Z.; Hua, S.; Li, J.; Wang, H.; Ye, C.; Kim, A.D.; Wang, L.; et al. Deep anterior lamellar keratoplasty with cross-linked acellular porcine corneal stroma to manage fungal keratitis. Xenotransplantation 2021, 28, e12655. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raj, N.; Vanathi, M.; Ahmed, N.H.; Gupta, N.; Lomi, N.; Tandon, R. Recent Perspectives in the Management of Fungal Keratitis. J. Fungi 2021, 7, 907. https://doi.org/10.3390/jof7110907
Raj N, Vanathi M, Ahmed NH, Gupta N, Lomi N, Tandon R. Recent Perspectives in the Management of Fungal Keratitis. Journal of Fungi. 2021; 7(11):907. https://doi.org/10.3390/jof7110907
Chicago/Turabian StyleRaj, Nimmy, Murugesan Vanathi, Nishat Hussain Ahmed, Noopur Gupta, Neiwete Lomi, and Radhika Tandon. 2021. "Recent Perspectives in the Management of Fungal Keratitis" Journal of Fungi 7, no. 11: 907. https://doi.org/10.3390/jof7110907
APA StyleRaj, N., Vanathi, M., Ahmed, N. H., Gupta, N., Lomi, N., & Tandon, R. (2021). Recent Perspectives in the Management of Fungal Keratitis. Journal of Fungi, 7(11), 907. https://doi.org/10.3390/jof7110907





