Fungal Metagenome of Chernevaya Taiga Soils: Taxonomic Composition, Differential Abundance and Factors Related to Plant Gigantism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sampling
2.2. DNA Extraction and Sequencing
2.3. Plant Sample Preparation for Microscopy Visualization
2.4. Statistical and Bioinformatics Analysis
3. Results
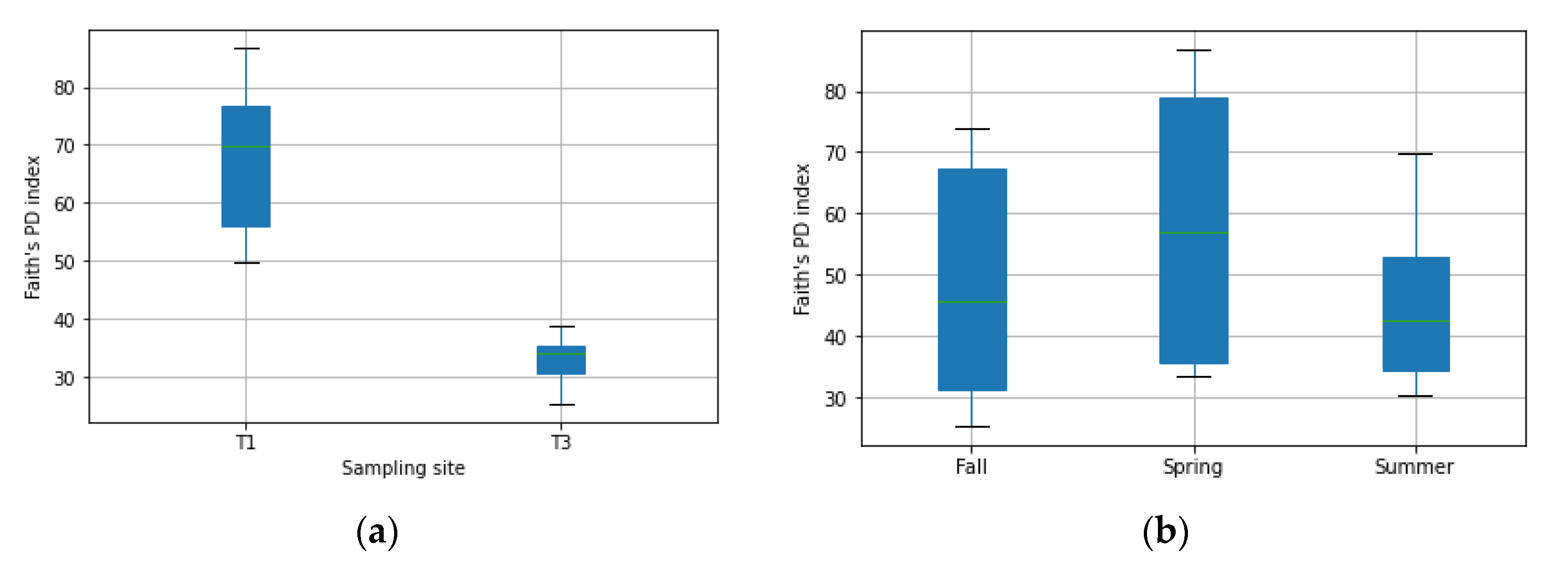
3.1. Diversity
3.2. Community Composition
3.3. Ecological Guild Analysis
3.4. Differentially Abundant Fungal Taxa
3.5. Arbuscular Mycorrhiza in the Chernevaya Taiga Soils
4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abakumov, E.; Loiko, S.; Lashchinsky, N.; Istigechev, G.; Kulemzina, A.; Smirnov, A.; Rayko, M.; Lapidus, A. Highly Productive Boreal Ecosystem Chernevaya Taiga—Unique Rainforest in Siberia. Preprints 2020. [Google Scholar] [CrossRef]
- Abakumov, E.V.; Loyko, S.V.; Istigechev, G.I.; Kulemzina, A.I.; Lashchinskiy, N.N.; Andronov, E.E.; Lapidus, A.L. Soils of Chernevaya taiga of western Siberia—Morphology, agrochemical features, microbiome. Agric. Biol. 2020, 55, 1018–1039. [Google Scholar] [CrossRef]
- Ekblad, A.; Wallander, H.; Godbold, D.L.; Cruz, C.; Johnson, D.; Baldrian, P.; Björk, R.; Epron, D.; Kieliszewska-Rokicka, B.; Kjøller, R.; et al. The production and turnover of extramatrical mycelium of ectomycorrhizal fungi in forest soils: Role in carbon cycling. Plant Soil 2013, 366, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Floudas, D.; Bentzer, J.; Ahrén, D.; Johansson, T.; Persson, P.; Tunlid, A. Uncovering the hidden diversity of litter-decomposition mechanisms in mushroom-forming fungi. ISME J. 2020, 14, 2046–2059. [Google Scholar] [CrossRef]
- Frąc, M.; Hannula, S.E.; Bełka, M.; Jędryczka, M. Fungal Biodiversity and Their Role in Soil Health. Front. Microbiol. 2018, 9, 707. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Arato, M.; Borghi, L.; Nouri, E.; Reinhardt, D. Beneficial Services of Arbuscular Mycorrhizal Fungi—From Ecology to Application. Front. Plant Sci. 2018, 9, 1270. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liang, Y.; Ghosh, A.; Song, Y.; Chen, H.; Tang, M. Assessment of arbuscular mycorrhizal fungi status and heavy metal accumulation characteristics of tree species in a lead–zinc mine area: Potential applications for phytoremediation. Environ. Sci. Pollut. Res. 2015, 22, 13179–13193. [Google Scholar] [CrossRef]
- Pfeffer, P.E.; Douds, D.D.; Bécard, G.; Shachar-Hill, Y. Carbon Uptake and the Metabolism and Transport of Lipids in an Arbuscular Mycorrhiza1. Plant Physiol. 1999, 120, 587–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nouri, E.; Breuillin-Sessoms, F.; Feller, U.; Reinhardt, D. Phosphorus and Nitrogen Regulate Arbuscular Mycorrhizal Symbiosis in Petunia hybrida. PLoS ONE 2014, 9, e90841. [Google Scholar] [CrossRef]
- Simon, L.; Bousquet, J.; Lévesque, R.C.; LaLonde, M. Origin and diversification of endomycorrhizal fungi and coincidence with vascular land plants. Nat. Cell Biol. 1993, 363, 67–69. [Google Scholar] [CrossRef]
- Rich, M.K.; Vigneron, N.; Libourel, C.; Keller, J.; Xue, L.; Hajheidari, M.; Radhakrishnan, G.V.; Le Ru, A.; Diop, S.I.; Potente, G.; et al. Lipid exchanges drove the evolution of mutualism during plant terrestrialization. Science 2021, 372, 864–868. [Google Scholar] [CrossRef]
- Saldajeno, M.G.B.; Chandanie, W.A.; Kubota, M.; Hyakumachi, M. Effects of Interactions of Arbuscular Mycorrhizal Fungi and Beneficial Saprophytic Mycoflora on Plant Growth and Disease Protection. In Mycorrhizae: Sustainable Agriculture and Forestry; Springer: Dordrecht, The Netherlands, 2008; pp. 211–226. [Google Scholar] [CrossRef]
- Hanlon, M.; Coenen, C. Genetic evidence for auxin involvement in arbuscular mycorrhiza initiation. New Phytol. 2011, 189, 701–709. [Google Scholar] [CrossRef]
- Lindahl, B.D.; E Clemmensen, K. Fungal ecology in boreal forest ecosystems. In Molecular Mycorrhizal Symbiosis; Wiley: Hoboken, NJ, USA, 2016; pp. 387–404. [Google Scholar] [CrossRef]
- Berg-Lyons, D.; Lauber, C.L.; Humphrey, G.; Thompson, L.; Gilbert, J.A.; Jansson, J.K.; Knight, R. EMP DNA Extraction Protocol v1. Protocols.io 2018. [Google Scholar] [CrossRef]
- Smith, D.P.; Peay, K.G.; Ackermann, G.; Apprill, A.; Bauer, M.; Berg-Lyons, D.; Betley, J.; Bruns, T.D.; Caporaso, J.G.; Fierer, N.; et al. EMP ITS Illumina Amplicon Protocol v1. Protocols.io 2018. [Google Scholar] [CrossRef]
- Kobae, Y.; Ohtomo, R. An improved method for bright-field imaging of arbuscular mycorrhizal fungi in plant roots. Soil Sci. Plant Nutr. 2016, 62, 27–30. [Google Scholar] [CrossRef] [Green Version]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Larsson, K.-H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE Database for Molecular Identification of Fungi: Handling Dark Taxa and Parallel Taxonomic Classifications. Nucleic Acids Res. 2018, 47, D259–D264. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Shigyo, N.; Umeki, K.; Hirao, T. Seasonal Dynamics of Soil Fungal and Bacterial Communities in Cool-Temperate Montane Forests. Front. Microbiol. 2019, 10, 1944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozupone, C.; Knight, R. UniFrac: A New Phylogenetic Method for Comparing Microbial Communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filippova, N.; Bulyonkova, T. The communities of terrestrial macrofungi in different forest types in vicinities of Khanty-Mansiysk (middle taiga zone of West Siberia). Biodivers. Data J. 2017, 5, e20732. [Google Scholar] [CrossRef] [Green Version]
- Lundell, T.K.; Mäkelä, M.R.; de Vries, R.P.; Hildén, K.S. Genomics, lifestyles and future prospects of wood-decay and litter-decomposing basidiomycota. In Advances in Botanical Research, 1st ed.; Martin, F., Ed.; Academic Press: New York, NY, USA, 2014. [Google Scholar] [CrossRef] [Green Version]
- Tamayo-Vélez, Á.; Osorio, N.W. Soil Fertility Improvement by Litter Decomposition and Inoculation with the Fungus Mortierella sp. in Avocado Plantations of Colombia. Commun. Soil Sci. Plant Anal. 2018, 49, 139–147. [Google Scholar] [CrossRef]
- Ozimek, E.; Hanaka, A. Mortierella Species as the Plant Growth-Promoting Fungi Present in the Agricultural Soils. Agriculture 2020, 11, 7. [Google Scholar] [CrossRef]
- Asplund, J.; Kauserud, H.; Bokhorst, S.; Lie, M.H.; Ohlson, M.; Nybakken, L. Fungal communities influence decomposition rates of plant litter from two dominant tree species. Fungal Ecol. 2018, 32, 1–8. [Google Scholar] [CrossRef]
- Zhan, P.; Liu, Y.; Wang, H.; Wang, C.; Xia, M.; Wang, N.; Cui, W.; Xiao, D.; Wang, H. Plant litter decomposition in wetlands is closely associated with phyllospheric fungi as revealed by microbial community dynamics and co-occurrence network. Sci. Total. Environ. 2021, 753, 142194. [Google Scholar] [CrossRef]
- Öpik, M.; Moora, M.; Zobel, M.; Saks, Ü.; Wheatley, R.; Wright, F.; Daniell, T. High diversity of arbuscular mycorrhizal fungi in a boreal herb-rich coniferous forest. New Phytol. 2008, 179, 867–876. [Google Scholar] [CrossRef]
- Lapidus, A.; Korobeynikov, A. Selected abstracts of “Bioinformatics: From Algorithms to Applications 2020” conference. BMC Bioinform. 2020, 21, 1–18. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.L.C.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef] [Green Version]
- Krishna, M.P.; Mohan, M. Litter decomposition in forest ecosystems: A review. Energy Ecol. Environ. 2017, 2, 236–249. [Google Scholar] [CrossRef]
- McGuire, K.; Allison, S.D.; Fierer, N.; Treseder, K.K. Ectomycorrhizal-Dominated Boreal and Tropical Forests Have Distinct Fungal Communities, but Analogous Spatial Patterns across Soil Horizons. PLoS ONE 2013, 8, e68278. [Google Scholar] [CrossRef] [Green Version]
- Voříšková, J.; Baldrian, P. Fungal community on decomposing leaf litter undergoes rapid successional changes. ISME J. 2012, 7, 477–486. [Google Scholar] [CrossRef]
- Burke, D.J. Shared mycorrhizal networks of forest herbs: Does the presence of conspecific and heterospecific adult plants affect seedling growth and nutrient acquisition? Botany 2012, 90, 1048–1057. [Google Scholar] [CrossRef]
- Kumar, S.; Arora, N.; Upadhyay, H. Arbuscular mycorrhizal fungi: Source of secondary metabolite production in medicinal plants. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier BV: Amsterdam, The Netherlands, 2021; pp. 155–164. [Google Scholar] [CrossRef]
- Muneer, M.; Huang, X.; Hou, W.; Zhang, Y.; Cai, Y.; Munir, M.; Wu, L.; Zheng, C. Response of Fungal Diversity, Community Composition, and Functions to Nutrients Management in Red Soil. J. Fungi 2021, 7, 554. [Google Scholar] [CrossRef] [PubMed]
- López-Mondéjar, R.; Tlaskal, V.; Větrovský, T.; Štursová, M.; Toscan, R.; da Rocha, U.N.; Baldrian, P. Metagenomics and stable isotope probing reveal the complementary contribution of fungal and bacterial communities in the recycling of dead biomass in forest soil. Soil Biol. Biochem. 2020, 148, 107875. [Google Scholar] [CrossRef]





| Sample ID | Description | Coordinates |
|---|---|---|
| T1 | Chernevaya taiga, Kemerovo oblast, 38 km of railway Tomsk—Taiga station. Fir stands with an admixture of aspen. | 56.18399 85.28246 |
| T3 | Control point, Tomsk oblast, block 86, 1 km left of Shigarsky tract. Aeolian-fluvial plain underlain by loamy deposits, poorer community—birch-aspen-pine forest, fern-broadgrass. | 56.28826 84.48016 |
| Tomsk T1-T3 Spring | |||||
| # | baseMean | log2FC | lfcSE | Rank1 | Rank2 |
| 1 | 477.04 | −11.47 | 1.17 | k__Fungi | p__Glomeromycota |
| 2 | 60.64 | 5.81 | 1.12 | k__Fungi | p__Calcarisporiellomycota |
| Tomsk T1-T3 Summer | |||||
| # | baseMean | log2FC | lfcSE | Rank1 | Rank2 |
| 1 | 29339.96 | 2.59 | 0.47 | k__Fungi | p__Basidiomycota |
| 2 | 636.93 | 4.00 | 1.24 | k__Fungi | p__Mucoromycota |
| 3 | 109.29 | −3.28 | 0.49 | k__Fungi | p__Rozellomycota |
| Tomsk T1-T3 Fall | |||||
| # | baseMean | log2FC | lfcSE | Rank1 | Rank2 |
| 1 | 81.89 | 2.98 | 0.66 | k__Fungi | p__unidentified |
| 2 | 107.28 | 3.80 | 0.80 | k__Fungi | p__Mucoromycota |
| 3 | 13544.93 | −2.44 | 0.39 | k__Fungi | p__Mortierellomycota |
| 4 | 42.61 | −3.39 | 0.74 | k__Fungi | p__Rozellomycota |
| 5 | 100.61 | −4.11 | 1.56 | k__Fungi | p__Glomeromycota |
| baseMean | log2FC | padj | Kingdom | Family | Genus | Species | Trophic Mode | Guild |
|---|---|---|---|---|---|---|---|---|
| 14.72 | 7.21 | 0.03 | Fungi | Glomeraceae | unidentified | unidentified | Symbiotroph | Arbuscular mycorrhizal |
| 48.45 | −9.15 | 0.00 | Fungi | Claroideoglomeraceae | unidentified | unidentified | Symbiotroph | Arbuscular mycorrhizal |
| 26.54 | −8.28 | 0.00 | Fungi | Claroideoglomeraceae | unidentified | unidentified | Symbiotroph | Arbuscular mycorrhizal |
| 26.18 | −8.27 | 0.00 | Fungi | Claroideoglomeraceae | unidentified | unidentified | Symbiotroph | Arbuscular mycorrhizal |
| 13.77 | −7.34 | 0.00 | Fungi | Glomeraceae | Glomus | unidentified | Symbiotroph | Arbuscular mycorrhizal |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rayko, M.; Sokornova, S.; Lapidus, A. Fungal Metagenome of Chernevaya Taiga Soils: Taxonomic Composition, Differential Abundance and Factors Related to Plant Gigantism. J. Fungi 2021, 7, 908. https://doi.org/10.3390/jof7110908
Rayko M, Sokornova S, Lapidus A. Fungal Metagenome of Chernevaya Taiga Soils: Taxonomic Composition, Differential Abundance and Factors Related to Plant Gigantism. Journal of Fungi. 2021; 7(11):908. https://doi.org/10.3390/jof7110908
Chicago/Turabian StyleRayko, Mikhail, Sophie Sokornova, and Alla Lapidus. 2021. "Fungal Metagenome of Chernevaya Taiga Soils: Taxonomic Composition, Differential Abundance and Factors Related to Plant Gigantism" Journal of Fungi 7, no. 11: 908. https://doi.org/10.3390/jof7110908
APA StyleRayko, M., Sokornova, S., & Lapidus, A. (2021). Fungal Metagenome of Chernevaya Taiga Soils: Taxonomic Composition, Differential Abundance and Factors Related to Plant Gigantism. Journal of Fungi, 7(11), 908. https://doi.org/10.3390/jof7110908







