New Applications of Photodynamic Therapy in the Management of Candidiasis
Abstract
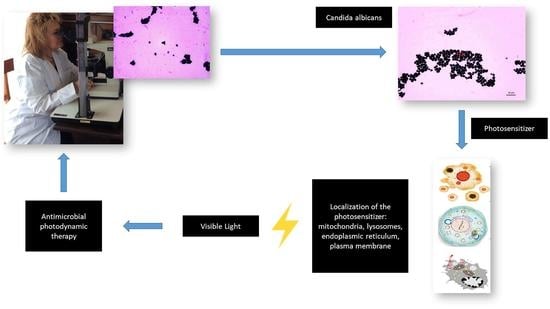
:1. Introduction
2. Materials and Methods
3. Results
3.1. Nanotechnology for Enhanced Efficiency of aPDT
3.2. Types of PSs Used in aPDT for Candida spp.
3.2.1. Cyanines
3.2.2. Chlorins
3.2.3. Porphyrins
3.2.4. Synthetic Dyes
3.2.5. Natural Dyes
3.2.6. Others
3.3. Changes in Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Junqueira, J.C. Models Hosts for the Study of Oral Candidiasis. Adv. Exp. Med. Biol. 2012, 710, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cerdeira, C.; Martínez-Herrera, E.; Carnero-Gregorio, M.; López-Barcenas, A.; Fabbrocini, G.; Fida, M.; El-Samahy, M.; González-Cespón, J.L. Pathogenesis and Clinical Relevance of Candida Biofilms in Vulvovaginal Candidiasis. Front. Microbiol. 2020, 11, 2884. [Google Scholar] [CrossRef]
- Arkowitz, R.A.; Bassilana, M. Recent advances in understanding Candida albicans hyphal growth. F1000Research 2019, 8, 700. [Google Scholar] [CrossRef] [Green Version]
- Jamiu, A.T.; Albertyn, J.; Sebolai, O.M.; Pohl, C.H. Update on Candida krusei, a potential multidrug-resistant pathogen. Med. Mycol. 2021, 59, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Černáková, L.; Light, C.; Salehi, B.; Rogel-Castillo, C.; Victoriano, M.; Martorell, M.; Sharifi-Rad, J.; Martins, N.; Rodrigues, C.F. Novel Therapies for Biofilm-Based Candida spp. Infections. Adv. Exp. Med. Biol. 2019, 1214, 93–123. [Google Scholar] [CrossRef]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy—What we know and what we don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Laguna, V.; García-Malinis, A.J.; Aspiroz, C.; Rezusta, A.; Gilaberte, Y. Antimicrobial effects of photodynamic therapy. G. Ital. Dermatol. Venereol. 2018, 153, 833–846. [Google Scholar] [CrossRef]
- Dias, L.M.; Klein, M.I.; Jordão, C.C.; Carmello, J.C.; Bellini, A.; Pavarina, A.C. Successive applications of Antimicrobial Photodynamic Therapy effects the susceptibility of Candida albicans grown in medium with or without fluconazole. Photodiagn. Photodyn. Ther. 2020, 32, 102018. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jo, Y.-U.; Na, K. Photodynamic therapy with smart nanomedicine. Arch. Pharmacal. Res. 2020, 43, 22–31. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Marioli, M.; Zhang, K. Analytical characterization of liposomes and other lipid nanoparticles for drug delivery. J. Pharm. Biomed. Anal. 2021, 192, 113642. [Google Scholar] [CrossRef]
- Sherje, A.P.; Jadhav, M.; Dravyakar, B.R.; Kadam, D. Dendrimers: A versatile nanocarrier for drug delivery and targeting. Int. J. Pharm. 2018, 548, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, S.B.; Adnan, R.; Rameez Khan, R.M.; Rashid, M. Gold, Silver, and Palladium Nanoparticles: A Chemical Tool for Biomedical Applications. Front. Chem. 2020, 8, 376. [Google Scholar] [CrossRef]
- Zafar, N.; Madni, A.; Khalid, A.; Khan, T.; Kousar, R.; Naz, S.S.; Wahid, F. Pharmaceutical and Biomedical Applications of Green Synthesized Metal and Metal Oxide Nanoparticles. Curr. Pharm. Des. 2020, 26, 5844–5865. [Google Scholar] [CrossRef]
- Niculescu, V.-C. Mesoporous silica nanoparticles for bio-applications. Front. Mater. 2020, 7, 36. [Google Scholar] [CrossRef] [Green Version]
- Calixto, G.M.F.; De Annunzio, S.R.; Victorelli, F.D.; Frade, M.L.; Ferreira, P.S.; Chorilli, M.; Fontana, C.R. Chitosan-Based Drug Delivery Systems for Optimization of Photodynamic Therapy: A Review. AAPS PharmSciTech 2019, 20, 253. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Carreiró, F.; Oliveira, A.; Neves, A.; Pires, B.; Venkatesh, D.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.-T.; Chien, H.-F.; Chang, P.-H.; Chen, Y.-C.; Jay, M.; Tsai, T.; Chen, C.-T. Photodynamic inactivation of chlorin e6-loaded CTAB-liposomes against Candida albicans. Lasers Surg. Med. 2013, 45, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Mlynarczyk, D.T.; Dlugaszewska, J.; Kaluzna-Mlynarczyk, A.; Goslinski, T. Dendrimers against fungi—A state of the art review. J. Control. Release 2021, 330, 599–617. [Google Scholar] [CrossRef]
- Hutnick, M.A.; Ahsanuddin, S.; Guan, L.; Lam, M.; Baron, E.D.; Pokorski, J.K. PEGylated Dendrimers as Drug Delivery Vehicles for the Photosensitizer Silicon Phthalocyanine Pc 4 for Candidal Infections. Biomacromolecules 2017, 18, 379–385. [Google Scholar] [CrossRef]
- Maliszewska, I.; Lisiak, B.; Popko, K.; Matczyszyn, K. Enhancement of the Efficacy of Photodynamic Inactivation of Candida albicans with the Use of Biogenic Gold Nanoparticles. Photochem. Photobiol. 2017, 93, 1081–1090. [Google Scholar] [CrossRef]
- Hsieh, Y.-H.; Chuang, W.-C.; Yu, K.-H.; Jheng, C.-P.; Lee, C.-I. Sequential Photodynamic Therapy with Phthalocyanine Encapsulated Chitosan-Tripolyphosphate Nanoparticles and Flucytosine Treatment against Candida tropicalis. Pharmaceutics 2019, 11, 16. [Google Scholar] [CrossRef] [Green Version]
- Hasanin, M.; Abdelraof, M.; Fikry, M.; Shaker, Y.; Sweed, A.; Senge, M. Development of Antimicrobial Laser-Induced Photodynamic Therapy Based on Ethylcellulose/Chitosan Nanocomposite with 5,10,15,20-Tetrakis(m-Hydroxyphenyl)porphyrin. Molecules 2021, 26, 3551. [Google Scholar] [CrossRef]
- Tang, F.; Gao, F.; Xie, W.; Li, S.; Zheng, B.; Ke, M.; Huang, J. Carboxymethyl chitosan-zinc(II) phthalocyanine conjugates: Synthesis, characterization and photodynamic antifungal therapy. Carbohydr. Polym. 2020, 235, 115949. [Google Scholar] [CrossRef] [PubMed]
- Sakima, V.T.; Barbugli, P.A.; Cerri, P.S.; Chorilli, M.; Carmello, J.C.; Pavarina, A.C.; Mima, E.G.D.O. Antimicrobial Photodynamic Therapy Mediated by Curcumin-Loaded Polymeric Nanoparticles in a Murine Model of Oral Candidiasis. Molecules 2018, 23, 2075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evangelista, R.P.; Amantino, C.F.; Pietro, R.C.; Sorrechia, R.; Piazza, R.D.; Marques, R.F.; Tedesco, A.C.; Primo, F.L. Copolymer-nanocapsules of zinc phenyl-thio-phthalocyanine and amphotericin-B in association with antimicrobial photodynamic therapy (A-PDT) applications against Candida albicans yeasts. Photodiagn. Photodyn. Ther. 2021, 34, 102273. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, M.E.; Rhodes, L. Photosensitizers for photodynamic therapy of cutaneous disease. J. Dermatol. Treat. 2003, 14, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [Green Version]
- Maliszewska, I.; Wanarska, E.; Tylus, W. Sulfonated hydroxyaluminum phthalocyanine-biogenic Au/Ag alloy nanoparticles mixtures for effective photo-eradication of Candida albicans. Photodiagn. Photodyn. Ther. 2020, 32, 102016. [Google Scholar] [CrossRef]
- Azizi, A.; Amirzadeh, Z.; Rezai, M.; Lawaf, S.; Rahimi, A. Effect of photodynamic therapy with two photosensitizers on Candida albicans. J. Photochem. Photobiol. B Biol. 2016, 158, 267–273. [Google Scholar] [CrossRef]
- Acosta, S.; Moreno-Aguilar, C.; Hernández-Sánchez, D.; Morales-Cruzado, B.; Sarmiento-Gomez, E.; Bittencourt, C.; Sánchez-Vargas, L.O.; Quintana, M. A few-layer graphene/chlorin e6 hybrid nanomaterial and its application in photodynamic therapy against Candida albicans. Beilstein J. Nanotechnol. 2020, 11, 1054–1061. [Google Scholar] [CrossRef]
- Hidalgo, K.J.R.; Carmello, J.C.; Jordão, C.C.; Barbugli, P.; Costa, C.A.D.S.; Mima, E.G.D.O.; Pavarina, A.C. Antimicrobial Photodynamic Therapy in Combination with Nystatin in the Treatment of Experimental Oral Candidiasis Induced by Candida albicans Resistant to Fluconazole. Pharmaceuticals 2019, 12, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, F.; Carmello, J.C.; Mima, E.G.D.O.; Costa, C.A.D.S.; Bagnato, V.S.; Pavarina, A.C. Photodithazine-mediated antimicrobial photodynamic therapy against fluconazole-resistant Candida albicans in vivo. Med. Mycol. 2018, 57, 609–617. [Google Scholar] [CrossRef]
- Carmello, J.C.; Alves, F.; Basso, F.G.; Costa, C.A.D.S.; Bagnato, V.S.; Mima, E.G.D.O.; Pavarina, A.C. Treatment of Oral Candidiasis Using Photodithazine®- Mediated Photodynamic Therapy In Vivo. PLoS ONE 2016, 11, e0156947. [Google Scholar] [CrossRef] [Green Version]
- Sousa, V.; Gomes, A.T.P.C.; Freitas, A.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Almeida, A. Photodynamic Inactivation of Candida albicans in Blood Plasma and Whole Blood. Antibiotics 2019, 8, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quiroga, E.D.; Cordero, P.; Mora, S.J.; Alvarez, M.G.; Durantini, E.N. Mechanistic aspects in the photodynamic inactivation of Candida albicans sensitized by a dimethylaminopropoxy porphyrin and its equivalent with cationic intrinsic charges. Photodiagnosis Photodyn. Ther. 2020, 31, 101877. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Yang, L.-J.; Chen, J.; Yang, H.; Gao, Z.-Q.; Wang, X.-L. Successful Sequential Treatment with Itraconazole and ALA-PDT for Cutaneous Granuloma by Candida albicans: A Case Report and Literature Review. Mycopathologia 2018, 183, 829–834. [Google Scholar] [CrossRef]
- Wang, Y.; Song, J.; Zhang, F.; Zeng, K.; Zhu, X. Antifungal Photodynamic Activity of Hexyl-Aminolevulinate Ethosomes Against Candida albicans Biofilm. Front. Microbiol. 2020, 11, 2052. [Google Scholar] [CrossRef]
- Aspiroz, C.; Gilaberte, Y.; Paz-Cristóbal, P.; Rezusta, A. Distal onycholysis resolved with photodynamic therapy in an elderly patient on multiple medication. Enferm. Infecc. Microbiol. Clín. 2011, 29, 626–628. [Google Scholar] [CrossRef]
- Keten, A.; Okdemir, E. Toluidine blue. Am. J. Emerg. Med. 2020, 38, 2239–2240. [Google Scholar] [CrossRef]
- Wiench, R.; Skaba, D.; Stefanik, N.; Kępa, M.; Gilowski, Ł.; Cieślar, G.; Kawczyk-Krupka, A. Assessment of sensitivity of selected Candida strains on antimicrobial photodynamic therapy using diode laser 635 nm and toluidine blue–In vitro research. Photodiagn. Photodyn. Ther. 2019, 27, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Wiench, R.; Skaba, D.; Matys, J.; Grzech-Leśniak, K. Efficacy of Toluidine Blue—Mediated Antimicrobial Photodynamic Therapy on Candida spp. A Systematic Review. Antibiotics 2021, 10, 349. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Chien, H.-F.; Lin, M.-H.; Chen, C.-P.; Shen, M.; Chen, C.-T. Chitosan Inhibits the Rehabilitation of Damaged Microbes Induced by Photodynamic Inactivation. Int. J. Mol. Sci. 2018, 19, 2598. [Google Scholar] [CrossRef]
- Merigo, E.; Chevalier, M.; Conti, S.; Ciociola, T.; Fornaini, C.; Manfredi, M.; Vescovi, P.; Doglio, A. Antimicrobial effect on Candida albicans biofilm by application of different wavelengths and dyes and the synthetic killer decapeptide KP. Laser Ther. 2019, 28, 180–186. [Google Scholar] [CrossRef]
- Garcia, B.A.; Panariello, B.H.D.; Pontes, K.M.D.F.; Duarte, S. Regimen and different surfaces interfere with photodynamic therapy on Candida albicans biofilms. J. Microbiol. Methods 2020, 178, 106080. [Google Scholar] [CrossRef] [PubMed]
- Cecatto, R.B.; de Magalhães, L.S.; Rodrigues, M.F.S.D.; Pavani, C.; Lino-Dos-Santos-Franco, A.; Gomes, M.T.; Silva, D.F.T. Methylene blue mediated antimicrobial photodynamic therapy in clinical human studies: The state of the art. Photodiagn. Photodyn. Ther. 2020, 31, 101828. [Google Scholar] [CrossRef]
- Teichert, M.; Jones, J.; Usacheva, M.; Biel, M. Treatment of oral candidiasis with methylene blue-mediated photodynamic therapy in an immunodeficient murine model. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2002, 93, 155–160. [Google Scholar] [CrossRef]
- Hosseini, N.; Yazdanpanah, S.; Saki, M.; Rezazadeh, F.; Ghapanchi, J.; Zomorodian, K. Susceptibility of Candida albicans and Candida dubliniensis to Photodynamic Therapy Using Four Dyes as the Photosensitizer. J. Dent. 2016, 17, 354–360. [Google Scholar]
- Pérez-Laguna, V.; Barrena-López, Y.; Gilaberte, Y.; Rezusta, A. Effect of photodynamic antimicrobial chemotherapy on Candida albicans in the presence of glucose. Photodiagn. Photodyn. 2019, 27, 54–58. [Google Scholar]
- Figueiredo-Godoi, L.M.A.; Menezes, R.T.; Carvalho, J.S.; Garcia, M.T.; Segundo, A.G.; Jorge, A.O.C.; Junqueira, J.C. Exploring the Galleria mellonella model to study antifungal photodynamic therapy. Photodiagn. Photodyn. Ther. 2019, 27, 66–73. [Google Scholar] [CrossRef]
- Borges Pereira Costa, A.C.; Rasteiro, V.M.C.; Pereira, C.A.; Rossoni, R.D.; Junqueira, J.C.; Cardoso Jorge, A.O. The effects of rose bengal- and erythrosine-mediated photodynamic therapy on Candida albicans. Mycoses 2012, 55, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Valkov, A.; Zinigrad, M.; Nisnevitch, M. Photodynamic Eradication of Trichophyton rubrum and Candida albicans. Pathogens 2021, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, J.; Ribeiro, M.; Rossoni, R.; Barbosa, J.; Querido, S.; Jorge, A. Antimicrobial Photodynamic Therapy: Photodynamic Antimicrobial Effects of Malachite Green on Staphylococcus, Enterobacteriaceae, and Candida. Photomed. Laser Surg. 2010, 28 (Suppl. S1), S67–S72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paz-Cristobal, M.P.; Royo, D.; Rezusta, A.; Andrés-Ciriano, E.; Alejandre, M.C.; Meis, J.; Revillo, M.J.; Aspiroz, C.; Nonell, S.; Gilaberte, Y. Photodynamic fungicidal efficacy of hypericin and dimethyl methylene blue against azole-resistant Candida albicans strains. Mycoses 2013, 57, 35–42. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, C.; Zhuge, Y.; Zhang, J.; Xu, K.; Zhang, Q.; Zhang, H.; Chen, H.; Chu, M.; Jia, C. Photodynamic Antifungal Activity of Hypocrellin A Against Candida albicans. Front. Microbiol. 2019, 10, 1810. [Google Scholar] [CrossRef] [Green Version]
- Jan, A.; Liu, C.; Deng, H.; Li, J.; Ma, W.; Zeng, X.; Ji, Y. In vitro photodynamic inactivation effects of hypocrellin B on azole-sensitive and resistant Candida albicans. Photodiagn. Photodyn. Ther. 2019, 27, 419–427. [Google Scholar] [CrossRef]
- Alshehri, A.H. Mechanical and antimicrobial effects of riboflavin-mediated photosensitization of in vitro C. albicans formed on polymethyl methacrylate resin. Photodiagn. Photodyn. Ther. 2021, 36, 102488. [Google Scholar] [CrossRef]
- Dong, S.; Shi, H.; Zhang, X.; Chen, X.; Cao, D.; Mao, C.; Gao, X.; Wang, L. Difunctional bacteriophage conjugated with photosensitizers for Candida albicans-targeting photodynamic inactivation. Int. J. Nanomed. 2018, 13, 2199–2216. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Liu, C.; Li, J.; Hao, M.; Ji, Y.; Zeng, X. The effects of aloe emodin-mediated antimicrobial photodynamic therapy on drug-sensitive and resistant Candida albicans. Photochem. Photobiol. Sci. 2020, 19, 485–494. [Google Scholar] [CrossRef]
- Yang, D.; Lv, X.; Xue, L.; Yang, N.; Hu, Y.; Weng, L.; Fu, N.; Wang, L.; Dong, X. A lipase-responsive antifungal nanoplatform for synergistic photodynamic/photothermal/pharmaco-therapy of azole-resistant Candida albicans infections. Chem. Commun. 2019, 55, 15145–15148. [Google Scholar] [CrossRef]
- Freire, F.; de Barros, P.P.; Ávila, D.D.S.; Brito, G.N.B.; Junqueira, J.; Jorge, A.O.C. Evaluation of gene expression SAP5, LIP9, and PLB2 of Candida albicans biofilms after photodynamic inactivation. Lasers Med. Sci. 2015, 30, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Freire, F.; de Barros, P.P.; Pereira, C.A.; Junqueira, J.; Jorge, A.O.C. Photodynamic inactivation in the expression of the Candida albicans genes ALS3, HWP1, BCR1, TEC1, CPH1, and EFG1 in biofilms. Lasers Med. Sci. 2018, 33, 1447–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordão, C.C.; de Sousa, T.V.; Klein, M.I.; Dias, L.M.; Pavarina, A.C.; Carmello, J.C. Antimicrobial photodynamic therapy reduces gene expression of Candida albicans in biofilms. Photodiagn. Photodyn. Ther. 2020, 31, 101825. [Google Scholar] [CrossRef] [PubMed]
- Jordão, C.C.; Klein, M.I.; Carmello, J.C.; Dias, L.M.; Pavarina, A.C. Consecutive treatments with photodynamic therapy and nystatin altered the expression of virulence and ergosterol biosynthesis genes of a fluconazole-resistant Candida albicans in vivo. Photodiagn. Photodyn. Ther. 2021, 33, 102155. [Google Scholar] [CrossRef] [PubMed]
- Alonso, G.C.; Klein, M.I.; Jordão, C.C.; Carmello, J.C.; Pavarina, A.C. Gene expression of Candida albicans strains isolates from patients with denture stomatitis submitted to treatments with photodynamic therapy and nystatin. Photodiagn. Photodyn. Ther. 2021, 35, 102292. [Google Scholar] [CrossRef]
- Stájer, A.; Kajári, S.; Gajdács, M.; Musah-Eroje, A.; Baráth, Z. Utility of Photodynamic Therapy in Dentistry: Current Concepts. Dent. J. 2020, 8, 43. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Pen, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [Green Version]
- Dias, L.D.; Blanco, K.C.; Mfouo-Tynga, I.S.; Inada, N.M.; Bagnato, V.S. Curcumin as a photosensitizer: From molecular structure to recent advances in antimicrobial photodynamic therapy. J. Photochem. Photobiol. C Photochem. Rev. 2020, 45, 100384. [Google Scholar] [CrossRef]
- Celli, J.P.; Spring, B.Q.; Rizvi, I.; Evans, C.L.; Samkoe, K.S.; Verma, S.; Pogue, B.; Hasan, T. Imaging and Photodynamic Therapy: Mechanisms, Monitoring, and Optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef] [Green Version]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Chu, D. Coherence properties of different light sources and their effect on the image sharpness and speckle of holographic displays. Sci. Rep. 2017, 7, 5893. [Google Scholar] [CrossRef]
- Rehman, S.U.; Ullah, S.; Chong, P.H.J.; Yongchareon, S.; Komosny, D. Visible Light Communication: A System Perspective—Overview and Challenges. Sensors 2019, 19, 1153. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Xu, B.; Gong, C.; Luo, J.; Zhang, Q.; Gong, Y. Fiber Optofluidic Technology Based on Optical Force and Photothermal Effects. Micromachines 2019, 10, 499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef] [PubMed]
- Atay, H.Y. Antibacterial activity of chitosan-based systems. In Functional Chitosan; Jana, S., Jana, S., Eds.; Springer: Singapore, 2019; pp. 457–489. [Google Scholar] [CrossRef]
- Laporte, A.; Nordenbrock, A.; Lenzen, S.; Elsner, M. Light-induced intracellular hydrogen peroxide generation through genetically encoded photosensitizer KillerRed-SOD1. Free Radic. Res. 2018, 52, 1170–1181. [Google Scholar] [CrossRef] [PubMed]
- Serebrovskaya, E.O.; Ryumina, A.P.; Boulina, M.E.; Shirmanova, M.V.; Zagaynova, E.V.; Bogdanova, E.A.; Lukyanov, S.A.; Lukyanov, K. Phototoxic effects of lysosome-associated genetically encoded photosensitizer KillerRed. J. Biomed. Opt. 2013, 19, 071403. [Google Scholar] [CrossRef] [Green Version]
- Liao, Z.-X.; Li, Y.-C.; Lu, H.-M.; Sung, H.-W. A genetically-encoded KillerRed protein as an intrinsically generated photosensitizer for photodynamic therapy. Biomaterials 2014, 35, 500–508. [Google Scholar] [CrossRef]
- Chabrier-Roselló, Y.; Giesselman, B.R.; De Jesús-Andino, F.J.; Foster, T.H.; Mitra, S.; Haidaris, C.G. Inhibition of electron transport chain assembly and function promotes photodynamic killing of Candida. J. Photochem. Photobiol. B Biol. 2010, 99, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Proshkina, G.; Shramova, E.; Shilova, O.; Ryabova, A.; Deyev, S. Phototoxicity of flavoprotein miniSOG induced by bioluminescence resonance energy transfer in genetically encoded system NanoLuc-miniSOG is comparable with its LED-excited phototoxicity. J. Photochem. Photobiol. B Biol. 2018, 188, 107–115. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Xiong, L.-H.; Zhao, Z.; Wang, Z.; Luo, L.; Lam, J.W.Y.; Kwok, R.T.K.; Tang, B.Z. AIE-based theranostic systems for detection and killing of pathogens. Theranostics 2019, 9, 3223–3248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yu, Q.; Li, Y.; Yang, Z.; Zhou, X.; Chen, S.; Jiang, Z.-X. Fluorinated cryptophane-A and porphyrin-based theranostics for multimodal imaging-guided photodynamic therapy. Chem. Commun. 2020, 56, 3617–3620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Bo, S.; Zeng, K.; Wang, J.; Li, Y.; Yang, Z.; Zhou, X.; Chen, S.; Jiang, Z.-X. Fluorinated porphyrin-based theranostics for dual imaging and chemo-photodynamic therapy. J. Mater. Chem. B 2020, 8, 4469–4474. [Google Scholar] [CrossRef]
- He, X.; Situ, B.; Gao, M.; Guan, S.; He, B.; Ge, X.; Li, S.; Tao, M.; Zou, H.; Tang, B.Z.; et al. Stereotactic photodynamic therapy using a two-photon AIE photosensitizer. Small 2019, 15, e1905080. [Google Scholar] [CrossRef] [PubMed]


| Nanocarriers | Characteristics | References |
|---|---|---|
| Liposomes | Liposomes are spherical vesicles bounded by a membrane bilayer composed of phospholipids. The water-soluble and fat-soluble portions of the phospholipids enable the activated release of liposome contents. The main disadvantages of liposomes are their low stability and rapid elimination from the body. | Fan et al. [11] and Sercombe et al. [18] |
| Dendrimers | A dendrimer is a polyvalent polymer with a three-dimensional tree-like structure. They allow the transport of therapeutic agents physically entrapped within the dendritic scaffold or attached to end groups. To reduce its cytotoxicity, the use of macromolecules is necessary. | Sherje et al. [12] |
| Gold and silver nanoparticles | The nanoparticles consist of gold and silver particles with a typical size between 1 and 100 nm. Biodistribution and cellular absorption of the nanoparticles depend on their size and shape. There are no reliable toxicity data. | Yaqoob et al. [13] |
| Metal oxide nanoparticles | These are essential components of catalysts in electrochemical energy conversion and storage devices, including fuel cells, metal–air batteries, and water element separation systems. They are inorganic-based particles with a metal oxide core and coated with inorganic materials, such as silica or gold. They can also be coated by organic substances, such as phospholipids, polysaccharides, or peptides. They are non-toxic and highly biocompatible. | Zafar et al. [14] |
| Mesoporous silica nanoparticles | These are a mesoporous form of silica. The main characteristics of these particles are that the size of the pores and the level of porosity can be modified according to the application. They are thermally stable and biocompatible. Regarding their harmful effects, they can produce haemolysis. Prolonged retention of silica in the body can lead to carcinogenesis. | Niculescu et al. [15] |
| Chitosan | It is composed of fibres of marine origin, found naturally in the chitin of the shells of crustaceans. A linear polysaccharide is chemically composed of β-(1-4)-linked, deacetylated D-glucosamine and acetylated N-acetyl-D-glucosamine. Its biocompatibility and biodegradability are good. Chitosan acts synergistically with antimicrobial photodynamic therapy. | Calixto et al. [16] |
| Polymeric nanoparticles | They are particles of less than 1 µm in size (generally 10–500 nm). They are composed of different polymeric materials. They are stable in suspension and are readily biocompatible. As they are compounds foreign to our body, toxicity and immunogenicity can occur. | Zielińska et al. [17] |
| Nanocarrier Type | Compound | Photosensitizer | Candida spp. | Effectiveness | Reference |
|---|---|---|---|---|---|
| Liposomes | CTAB-liposomes constituted with various ratios of dimyristoyl-sn-glycero-phosphatidylcholine | Chlorine e6 | C. albicans C. krusei C. tropicalis | Improved | Yang et al. [19] |
| Dendrymers | Amino acid-based dendrimers and tetra- and octapeptides attached to poly(L-lysine) (PLL) dendrimers | NA | C. albicans C. kefyr C. tropicalis | Improved | Mlynarczyk et al. [20] |
| PEGylated dendrimers | Silicon phthalocyanine Pc 4 is | C. albicans | Increased | Baron et al. [21] | |
| Gold and silver nanoparticles | Monodispersed biogenic colloidal gold nanoparticles | Rose Bengal | C. albicans | Improved | Maliszewska et al. [22] |
| Chitosan | Cationic chitosan/tripolyphosphate nanoparticles | Phthalocyanine | C. tropicalis | Improved | Hesieh et al. [23] |
| Ethylcellulose/chitosan | 5,10,15,20-tetrakis(p-hydroxyphenyl)porphyrin (pTHPP) | C. albicans | Improved | Hasanin et al. [24] | |
| Carboxymethyl chitosan | 1-[4-(2-aminoethyl) phenoxy] zinc (II) phthalocyanine (ZnPcN) | C. albicans | Increased | Tang et al. [25] | |
| Polymeric nanoparticles | Polymeric nanocapsules | Curcumin | C. albicans | Variable | Sakima et al. [26] |
| Zinc phthalocyanine derivatives | C. albicans | Improved | Evangelista et al. [27] |
| Photosensitizer Type | Compound | Characteristics Compound Absorption Peak λ (nm) Used | Dose Range/Con-centration % | Light Dose/Power (J/cm2)/mW Used | Candida spp. | Toxicity | Outcome | References |
|---|---|---|---|---|---|---|---|---|
| Cyanines | Phthalocyanine | Cyanine dye; excited by red light: λ = 660 nm and 850 nm; penetrability 1–3 h after intravenous administration | 0.14 mg/kg intravenously | 12.6–94.5 J/cm2 | Colonies C. albicans ATCC 10231 | Minimal skin photosensitivity | To increase efficiency, nanocarriers are needed | Malisze-wska et al. [30] |
| Indomethacin green | λ = 606 and 808 nm | 0.1 mL | 10 J/cm2 | C. albicans suspensions ATCC 10231 | NA | Effective | Azizi et al. [31] | |
| Silicon phthalocyanine Pc 4 | λ = 670–675 | 5 µM of Pc4 encapsulated in PEGylated PAMAM nanoparticles | 10 J/cm2 | C. albicans strains SC 5314 | NA | Effective | Hutnick et al. [21] | |
| Chlorins | Chlorin e6 plus graphene | λ = 632 nm during 15 min | 2 mL | 150 mW | Cells of C. albicans ATCC 90028 | NA | Effective | Acosta et al. [32] |
| Photoditazine® (PDZ) consists of a N-methyl-D-glucosamine | λ = 660 nm during 2–8 min | 200 mg/L | 50 J/cm2 690 mW during 19 min | Tongues of mice with oral candidiasis: C. albicans ATCC 96901 | NA | Efficacy of therapies evaluated by microbiological, macroscopic, histopathological and confocal scanning laser microscopy | Hidalgo et al. [33] | |
| λ = 650–680 nm | 100 mg/L | 37.2 J/cm2 during 14 min | Tongues of mice with oral candidiasis: C. albicans ATCC 10231 | NA | Effective, but in vivo studies needed | Alves et al. [34] | ||
| λ = 660 nm | 100 mg/L | 37.5 J/cm2 22.3 mW | Tongues of mice with oral candidiasis: C. albicans ATCC 90028 | Safe | Effective | Carmello et al. [35] | ||
| Porphyrins | Hematoporphyrin and porfimer sodium | Tetrapyrrole structure; excited by red light: λ = 660- nm-635 nm; penetrability time a 20 min | 0.5–5 mg/kg Intravenous-ly | 75–250 J/cm2 | C. albicans cells in suspension ATCC 10231 | Great photosensitivity for 6–10 weeks after treatment | Effective | Sousa et al. [36] |
| (ALA 5,10,15,20-tetrakis[4-(3 N,Ndimethylaminopropoxy)phenyl]porphyrin (TAPP) and 5,10,15,20-tetrakis[4-(3-N,N,N-trimethylaminepropoxy)phenyl] porphyrin | λ = 350–800 nm during 30 min | 5 μM | 90 mW/cm2 | C. albicans strain PC31 | NA | Effective | Quiroga et al. [37] | |
| Tetrapyrrole structure 630–760 nm ALA and MAL are protoporphyrin IX precursors and, therefore, inactive drugs | 10–60 mg/kg orally 10–30% topically 3–6% Intravesicularly | 50–150 J/cm2 | C. albicans (in vivo) NA | Neurotoxicity | Effective | Cai et al. [38] | ||
| MAL) Hexyl-aminolevulinate and ethosomes | λ = 633 ± 10 nm | 100 µL of 0.5% hexyl-aminolevulinate and ethosomes | 60 mW/cm2, distance 10 cm | C. albicans strain SC 5314 | NA | Effective | Wang et al. [39] | |
| 5-Methyl-aminolevulinate | λ= 630 nm | 16–20% | 37 J/cm2 | C. albicans (in vivo) NA | NA | Effective | Aspiroz et al. [40] | |
| Synthetic Dyes | Toluidine blue | λ = 635 nm | NA | 400 mW 24 J/cm2 300 mW 18 J/cm2 20,000 mW 22 J/cm2 | Strains of C. albicans, ATCC 10231 C. glabrata ATCC 15126 and C. krusei ATCC 14243 | NA | Variable, depends on yeast type | Keten et al. [41] and Wiench et al. [42] |
| Toluidine ortho blue | λ = 630–660 nm | 0.1 μM | 50 J/cm2 | Strains of C. albicans, ATCC 10231 C. glabrata ATCC 15126 and C. krusei ATCC 14243, C. tropicalis ATCC 750 and C. parapsilosis ATCC 22019. | NA | Efficacy reaches 80% | Wiench et al. [43] and Lin et al. [44] | |
| Toluidine blue | λ = 405–650 nm | 20 μg/mL | 10 J/cm2 | C. albicans SC5314 | NA | Effective | Merigo et al. [45] | |
| λ = 635 nm | 44 μM | 175.2 J/cm2, 2 min | C. albicans biofilms SN425 | NA | Variable | Garcia et al. [46] | ||
| Methylene blue | λ = 635 nm | 0.0003 to 0.06 molar | 6- 18 J/cm2 50–750 Mw, from 8 s to 10 min | C. albicans in vivo NA | NA | Variable | Boltes Cecatto et al. [47] | |
| λ = 664 nm | 25–500 μg/mL | 275 J/cm2 and 400 mW during 687.5 s | Candida albicans NA | NA | Effective | Teichert et al. [48] | ||
| λ = 660 nm | 0.01–0.001 mg/mL | 76.8 J/cm2 and 25 mW, 5–30 min | C. albicans ATCC 5314 and C. dubliniensis ATCC 6144 | NA | Partially effective | Hosseini et al. [49] | ||
| Methylene blue and glucose | ʎ = 660 nm at a radiant power of 473 mW and 50 mM glucose | 0 mM glucose 100 μM MB | 10, 30 and 60 J/cm2 473 mW | Cells and biofilm C. albicans ATCC 10231 | NA | Partial effect | Oliveira-Silva et al. [50] | |
| Methylene blue and G. mellonella | λ = 660 nm | 10 µL | 10–15 J/cm2 | C. albicans strain ATCC 18804 | NA | Effective | Figueiredo-Godoi et al. [51] | |
| Rose Bengal | Rose Bengal excited by green light at λ = 450–600 nm | Cultures 10 µmol/L; biofilms 40 µmol/L | 36 J/cm2 for 180 s | Planktonic cultures and biofilms of C. albicans ATCC 18804 | NA | Reduction in number of hyphae | Costa et al. [52] | |
| Crystal violet | N-tetra-penta-hexametil p-rosanilinas; λ =660 nm | 1 g/10 mL | 76.8 J/cm2, 25 mW for 5–30 min | C. albicans ATCC 5314 and C. dubliniensis ATCC 6144 | NA | Effective | Hosseine et al. [49] | |
| Malachite green | 400–700 nm | 50–500 µM | 1.9 ± 0.1 mW/cm2 for 30 min | Planktonic cultures of C. albicans ATCC 90028 | NA | Effective | Valkov et al. [53] | |
| Malachite green | λ = 660 nm | 0.1 mL | 26 J/cm 35 mW during 4.45 min | Microbial suspensions containing 106 cells/mL C. albicans C. tropicalis, C. parapsilosis, C. krusei and C. glabrata In vivo NA | NA | Low cost, highly efficient, and short application time | Junqueira et al. [54] | |
| Natural dyes | Hyperecin | λ = 570 nm | 0.62 μmol/L | 18–37 J/cm2 | C. albicans strains AZN 9635,45632Hand AMO7/0267 | NA | Effective | Paz-Cristobal et al. [55] |
| Hipocrellin A and B | Peak at λ = 600–900 nm; λ = 470 nm | 1 mg/mL to 10 μM | 72 J/cm2 30 min | C. albicans strains SC 53114, ATCC 18804 and 07318 | NA | Effective | Yang et al. [56] and Jan et al. [57] | |
| Riboflavin | Λ = 360 and 440 nm | 0,1% | blue LED light (light dose) | Culture of C. albicans ATCC 18804 | NA | Effective | Alshehri et al. [58] | |
| Chlorophyll | Pheophorbide A conjugated with JM-phage by EDC/NHS crosslinking | Tetrapyrrole structure λ = 670 nm | 5 μM | 50 mW/cm2 for 10 min | C albicans cells ATCC 10231 | NA | Effective | Dong et al. [59] |
| Others | Aloe-emodin (1,8-dihydrox 3-(hydroxymethyl)-9,10-anthracenedione) | λ = 400–780 nm | 0.1–100 μM for 30 s to 5 min | 4.8 J/cm2 during 1 min | C. albicans cell suspension ATCC 10231 | NA | Effective | Ma et al. [60] |
| Candida spp. | Photosensitizer | Genes | Results | Origin | References |
|---|---|---|---|---|---|
| C. albicans ATCC 18804 | Methylene blue | SAP5, LIP9 and PLB2 | 60% SAP5/50% LIP9 and PLB2 | Biofilms | Freire et al. [62] |
| C. albicans ATCC 18804 | Methylene blue | ALS3, HWP1, BCR1, TEC1, CPH1 y EFG1 | ALS3, HWP1, BCR1, TEC1, CPH1 y EFG1 | Strains C. albicans from patients with a control strain (ATCC 18804) | Freire et al. [63] |
| C. albicans ATCC 90028 | Photodithazine (PDZ and Curcumin | ALS1, HWP1, CAP1, CAT1, SOD1 | ALS1, HWP1, CAP1, CAT1, SOD1 | Biofilms | Jordão et al. [64] |
| C. albicans ATCC 96901 | Photodithazine® (PDZ) | ALS1, HWP1, EFG1, CAT1, CAP1, SOD1, SAP1, PLB1 and LIP3 | Special decrease in SOD1 | Colonies from tongues of mice | Jordão et al. [65] |
| C. albicans ATCC 90028 | Photodithazine® (PDZ) plus Nystatine | ALS1, HWP1, EFG1, CAP1, CAT1, SOD1, SAP1, PLB1 y LIP3 | PLB1 y ACT1 | Dental patient samples | Alonso et al. [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Cerdeira, C.; Martínez-Herrera, E.; Fabbrocini, G.; Sanchez-Blanco, B.; López-Barcenas, A.; EL-Samahy, M.; Juárez-Durán, E.R.; González-Cespón, J.L. New Applications of Photodynamic Therapy in the Management of Candidiasis. J. Fungi 2021, 7, 1025. https://doi.org/10.3390/jof7121025
Rodríguez-Cerdeira C, Martínez-Herrera E, Fabbrocini G, Sanchez-Blanco B, López-Barcenas A, EL-Samahy M, Juárez-Durán ER, González-Cespón JL. New Applications of Photodynamic Therapy in the Management of Candidiasis. Journal of Fungi. 2021; 7(12):1025. https://doi.org/10.3390/jof7121025
Chicago/Turabian StyleRodríguez-Cerdeira, Carmen, Erick Martínez-Herrera, Gabriella Fabbrocini, Beatriz Sanchez-Blanco, Adriana López-Barcenas, May EL-Samahy, Eder R. Juárez-Durán, and José Luís González-Cespón. 2021. "New Applications of Photodynamic Therapy in the Management of Candidiasis" Journal of Fungi 7, no. 12: 1025. https://doi.org/10.3390/jof7121025
APA StyleRodríguez-Cerdeira, C., Martínez-Herrera, E., Fabbrocini, G., Sanchez-Blanco, B., López-Barcenas, A., EL-Samahy, M., Juárez-Durán, E. R., & González-Cespón, J. L. (2021). New Applications of Photodynamic Therapy in the Management of Candidiasis. Journal of Fungi, 7(12), 1025. https://doi.org/10.3390/jof7121025