Coccidioidomycosis in Brazil: Historical Challenges of a Neglected Disease
Abstract
:1. Coccidioidomycosis: General Characteristics
1.1. Occurrence
1.2. Mycological Features
1.3. Clinical Aspects
1.4. Diagnosis
1.5. Treatment
2. Coccidioidomycosis in Brazil
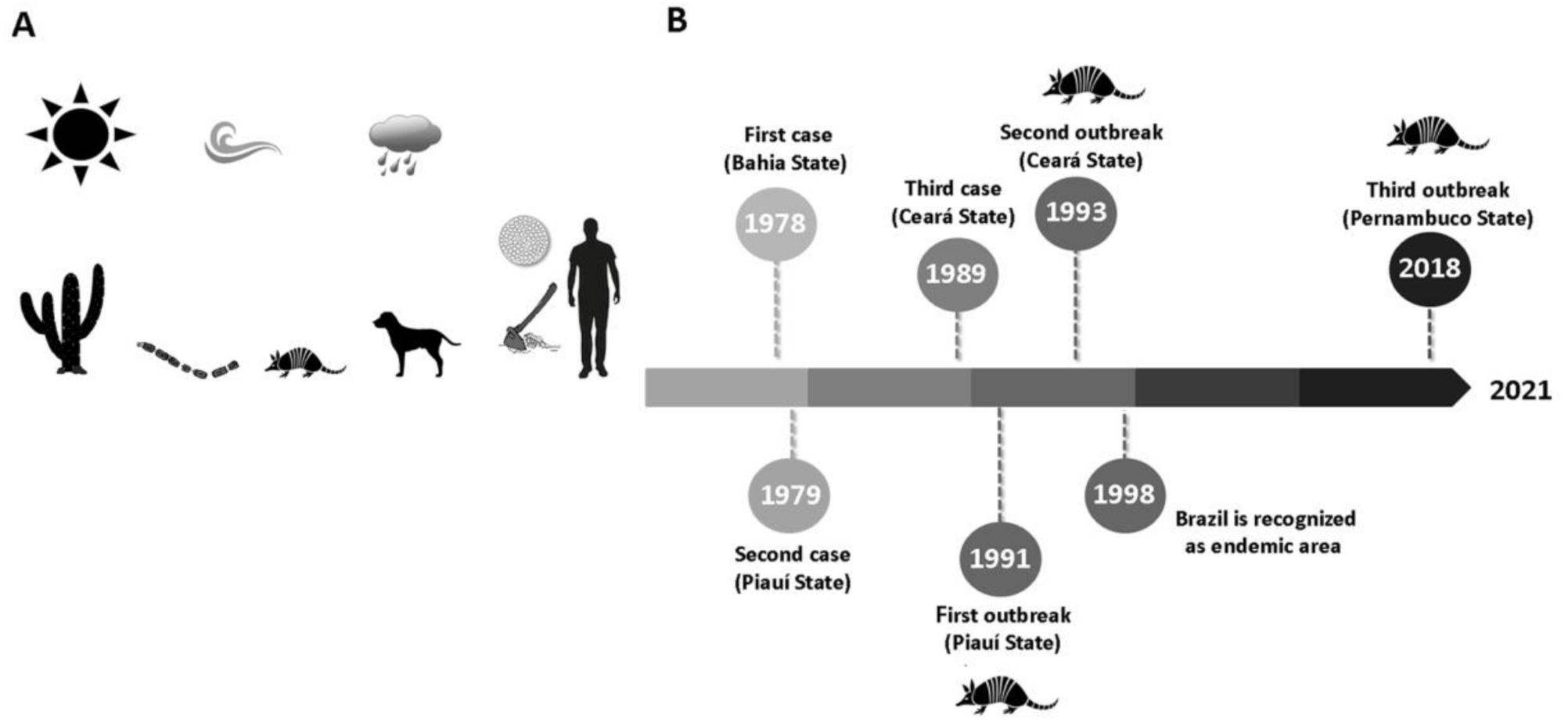
2.1. Background: A Historical Overview
2.2. Occurrence and Epidemiological Aspects: The Disease of Armadillo Hunters
2.3. Epidemiological Surveys Based on Unofficial Data: How Reliable Are These Studies?
2.4. Northeastern Brazil at a Glance: Biogeographical Aspects, Social Characteristics, and Health Issues in a Singular Region
3. Neglected Tropical Diseases
4. Could Coccidiodomycosis Be Considered a Neglected Disease?
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Fisher, M.C.; Koenig, G.L.; White, T.J.; Taylor, J.W. Molecular and Phenotypic Description of Coccidioides posadasii sp. nov., Previously Recognized as the Non-California Population of Coccidioides immitis. Mycologia 2002, 94, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mead, H.L.; Hamm, P.S.; Shaffer, I.N.; Teixeira, M.M.; Wendel, C.; Wiederhold, N.P.; Thompson, G.R.; Muñiz-Salazar, R.; Castañón-Olivares, L.R.; Keim, P.; et al. Differential thermotolerance adaptation between species of Coccidioides. bioRxiv 2020, 12, 247635. [Google Scholar]
- McCotter, O.Z.; Benedict, K.; Engelthaler, D.M.; Komatsu, K.; Lucas, K.D.; Mohle-Boetani, J.C.; Oltean, H.; Vugia, D.; Chiller, T.M.; Cooksey, G.L.S.; et al. Update on the Epidemiology of coccidioidomycosis in the United States. Med. Mycol. 2019, 57, S30–S40. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, T.N.; Fierer, J. Coccidioides immitis and posadasii, A review of their biology, genomics, pathogenesis, and host immunity. Virulence 2018, 9, 1426–1435. [Google Scholar] [CrossRef] [Green Version]
- Kollath, D.R.; Miller, K.J.; Barker, B.M. The mysterious desert dwellers: Coccidioides immitis and Coccidioides posadasii, causative fungal agents of coccidioidomycosis. Virulence 2019, 10, 222–233. [Google Scholar] [CrossRef] [Green Version]
- Gabe, L.M.; Malo, J.; Knox, K.S. Diagnosis and Management of Coccidioidomycosis. Clin. Chest Med. 2017, 38, 417–433. [Google Scholar] [CrossRef]
- CDC. Increase in Reported Coccidioidomycosis—United States 1998–2011. Morb. Mortal. Wkly. Rep. 2013, 62, 217–221. [Google Scholar]
- Laniado-Laborin, R.; Arathoon, E.G.; Canteros, C.; Muñiz-Salazar, R.; Rendon, A. Coccidioidomycosis in Latin America. Med. Mycol. 2019, 57, S46–S55. [Google Scholar] [CrossRef]
- Baptista-Rosas, R.C.; Riquelme, M. Epidemiología de la coccidioidomicosis en México. Rev. Iberoam. Micol. 2007, 24, 100–105. [Google Scholar] [CrossRef]
- Davila, A.; Candolfi-Arballo, O.; Narvaez-Hernandez, E.; García-Arellano, A.; Lopez-Larios, A.; Cano-Rangel, A.; Castañon-Olivares, L. Coccidioidin skin test in two Mexican populations from endemic areas of coccidioidomycosis in Mexico. Int. J. Infect. Dis. 2018, 73, 167. [Google Scholar] [CrossRef]
- Davidson, A.P.; Shubitz, L.F.; Alcott, C.J.; E Sykes, J. Selected Clinical Features of Coccidioidomycosis in Dogs. Med. Mycol. 2019, 57, S67–S75. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.A.; Hidalgo, M.N.; Hodzic, E.; Diab, S.S.; Uzal, F.A. Pathology of coccidioidomycosis in llamas and alpacas. J. Vet. Diagn. Investig. 2018, 30, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Koistinen, K.; Mullaney, L.; Bell, T.; Zaki, S.; Nalca, A.; Frick, O.; Livingston, V.; Robinson, C.G.; Estep, J.S.; Batey, K.L.; et al. Coccidioidomycosis in Nonhuman Primates: Pathologic and Clinical Findings. Vet. Pathol. 2018, 55, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, R.D.A.; Silva, K.R.D.C.E.; Brilhante, R.S.N.; Moura, F.B.P.; Duarte, N.F.H.; Marques, F.J.D.F.; Filho, R.E.M.; de Araújo, R.W.B.; Bandeira, T.D.J.P.G. Coccidioides posadasiiInfection in Bats, Brazil. Emerg. Infect. Dis. 2012, 18, 668–670. [Google Scholar] [CrossRef] [PubMed]
- Van Dyke, M.C.C.; Thompson, G.R.; Galgiani, J.N.; Barker, B.M. The Rise of Coccidioides: Forces Against the Dust Devil Unleashed. Front. Immunol. 2019, 10, 2188. [Google Scholar] [CrossRef]
- Litvintseva, A.P.; Marsden-Haug, N.; Hurst, S.; Hill, H.; Gade, L.; Driebe, E.M.; Ralston, C.; Roe, C.; Barker, B.M.; Goldoft, M.; et al. Valley fever: Finding new places for an old disease: Coccidioides immitis found in Washington State soil associated with recent human infection. Clin. Infect. Dis. 2015, 60, e1–e3. [Google Scholar] [CrossRef] [Green Version]
- Cordeiro, R.A.; Brilhante, R.S.; Rocha, M.F.; Fechine, M.A.; Camara, L.M.; Camargo, Z.P.; Sidrim, J.J. Phenotypic characterization and ecological features of Coccidioides spp. from Northeast Brazil. Med. Mycol. 2006, 44, 631–639. [Google Scholar] [CrossRef]
- Wanke, B.; Lazera, M.; Monteiro, P.C.; Lima, F.C.; Leal, M.J.; Ferreira Filho, P.L.; Kaufman, L.; Pinner, R.W.; Ajello, L. Investigation of an outbreak of endemic coccidioidomycosis in Brazil’s northeastern state of Piauí with a review of the occurrence and distribution of Coccidioides immitis in three other Brazilian states. Mycopathologia 1999, 148, 57–67. [Google Scholar] [CrossRef]
- Elconin, A.F.; Egeberg, R.O.; Egeberg, M.C. Significance of soil salinity on the ecology of Coccidioides immitis. J. Bacteriol. 1964, 87, 500–503. [Google Scholar] [CrossRef] [Green Version]
- Stewart, R.A.; Meyer, K.F. Isolation of Coccidioides immitis (Stiles) from the Soil. Exp. Biol. Med. 1932, 29, 937–938. [Google Scholar] [CrossRef]
- Alvarado, P.; Teixeira, M.M.; Andrews, L.; Fernandez, A.; Santander, G.; Doyle, A.; Perez, M.; Yegres, F.; Barker, B.M. Detection of Coccidioides posadasii from xerophytic environments in Venezuela reveals risk of naturally acquired coccidioidomycosis infections. Emerg. Microbes Infect. 2018, 7, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Macedo, R.L.; Rosado, A.S.; da Mota, F.F.; Cavalcante, M.A.; Eulálio, K.D.; Filho, A.D.D.; Martins, L.M.; Lazéra, M.D.S.; Wanke, B. Molecular identification of Coccidioides spp. in soil samples from Brazil. BMC Microbiol. 2011, 11, 108–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greene, D.R.; Koenig, G.; Fisher, M.C.; Taylor, J.W. Soil isolation and molecular identification of Coccidioides immitis. Mycologia 2010, 92, 406–410. [Google Scholar] [CrossRef]
- Taylor, J.W.; Barker, B.M. The endozoan, small-mammal reservoir hypothesis and the life cycle of Coccidioides species. Med. Mycol. 2019, 57, S16–S20. [Google Scholar] [CrossRef] [PubMed]
- Stockamp, N.W.; Thompson, G.R., 3rd. Coccidioidomycosis. Infect. Dis. Clin. N. Am. 2016, 30, 229–246. [Google Scholar] [CrossRef]
- Malo, J.; Luraschi-Monjagatta, C.; Wolk, D.M.; Thompson, R.; Hage, C.A.; Knox, K.S. Update on the Diagnosis of Pulmonary Coccidioidomycosis. Ann. Am. Thorac. Soc. 2014, 11, 243–253. [Google Scholar] [CrossRef]
- Thompson, G.R., 3rd. Pulmonary coccidioidomycosis. Semin. Respir. Crit. Care Med. 2011, 32, 754–756. [Google Scholar] [CrossRef]
- Jude, C.M.; Nayak, N.B.; Patel, M.K.; Deshmukh, M.; Batra, P. Pulmonary Coccidioidomycosis: Pictorial Review of Chest Radiographic and CT Findings. Radiographics 2014, 34, 912–925. [Google Scholar] [CrossRef] [Green Version]
- Valdivia, L.; Nix, D.; Wright, M.; Lindberg, E.; Fagan, T.; Lieberman, D.; Stoffer, T.; Ampel, N.M.; Galgiani, J.N. Coccidioidomycosis as a Common Cause of Community-acquired Pneumonia. Emerg. Infect. Dis. 2006, 12, 958–962. [Google Scholar] [CrossRef]
- Twarog, M.; Thompson, G.R., 3rd. Coccidioidomycosis: Recent updates. Semin. Respir. Crit. Care Med. 2015, 36, 746–755. [Google Scholar] [CrossRef] [Green Version]
- Galgiani, J.N.; Ampel, N.M.; Blair, J.E.; Catanzaro, A.; Geertsma, F.; Hoover, S.E.; Johnson, R.H.; Kusne, S.; Lisse, J.; MacDonald, J.D.; et al. 2016 Infectious Diseases Society of America (IDSA) Clinical practice guideline for the treatment of coccidioidomycosis. Clin. Infect. Dis. 2016, 63, e112–e146. [Google Scholar] [CrossRef] [PubMed]
- Ampel, N.M. The treatment of coccidioidomycosis. Rev. Inst. Med. Trop. São Paulo 2015, 57, 51–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parish, J.M.; Blair, J.E. Coccidioidomycosis. Mayo Clin. Proc. 2008, 83, 343–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arsura, E.L.; Bobba, R.K.; Reddy, C.M. Coccidioidal pericarditis: A case presentation and review of the literature. Int. J. Infect. Dis. 2005, 9, 104–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brilhante, R.S.N.; Cordeiro, R.A.; Rocha, M.F.G.; Fechine, M.A.B.; Furtado, F.M.; Nagao-Dias, A.T.; Camargo, Z.P.; Sidrim, J.J.C. Coccidioidal pericarditis: A rapid presumptive diagnosis by an in-house antigen confirmed by mycological and molecular methods. J. Med. Microbiol. 2008, 57, 1288–1292. [Google Scholar] [CrossRef]
- Odio, C.D.; Marciano, B.E.; Galgiani, J.N.; Holland, S.M. Risk Factors for Disseminated Coccidioidomycosis, United States. Emerg. Infect. Dis. 2017, 23, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.; Lee, K.S.; Yi, C.A.; Chung, M.J.; Kim, T.S.; Han, J. Pulmonary fungal infection: Imaging findings in immunocompetent and immunocompromised patients. Eur. J. Radiol. 2006, 59, 371–383. [Google Scholar] [CrossRef]
- Winn, R.E.; Johnson, R.; Galgiani, J.N.; Butler, C.; Pluss, J. Cavitary coccidioidomycosis with fungus ball formation. Diagnosis by fiberoptic bronchoscopy with coexistence of hyphae and spherules. Chest 1994, 105, 412–416. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, B.; Castañón, L.R.; Calderón, I.; Vázquez, M.E.; Manjarrez, M.E. Parasitic mycelial forms of Coccidioides species in Mexican patients. J. Clin. Microbiol. 2004, 42, 1247–1249. [Google Scholar] [CrossRef] [Green Version]
- Cordeiro, R.; Brilhante, R.; Rocha, M.; Sidrim, J. Coccidioides. In Molecular Detection of Human Fungal Pathogens; CRC Press: Boca Raton, FL, USA, 2011; pp. 135–144. [Google Scholar]
- Saubolle, M.A. Laboratory Aspects in the Diagnosis of Coccidioidomycosis. Ann. N. Y. Acad. Sci. 2007, 1111, 301–314. [Google Scholar] [CrossRef]
- Pappagianis, D. Serologic studies in coccidioidomycosis. Semin. Respir. Infect. 2001, 16, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Ampel, N.M. The diagnosis of coccidioidomycosis. F1000 Med. Rep. 2010, 2, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte-Escalante, E.; Frías-De-León, M.G.; Zúñiga, G.; Martinez-Herrera, E.; Acosta-Altamirano, G.; Reyes-Montes, M.D.R. Molecular markers in the epidemiology and diagnosis of coccidioidomycosis. Rev. Iberoam. Micol. 2014, 31, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Bialek, R.; Kern, J.; Herrmann, T.; Tijerina, R.; Ceceñas, L.; Reischl, U.; González, G.M. PCR Assays for Identification of Coccidioides posadasii Based on the Nucleotide Sequence of the Antigen 2/Proline-Rich Antigen. J. Clin. Microbiol. 2004, 42, 778–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binnicker, M.J.; Buckwalter, S.P.; Eisberner, J.J.; Stewart, R.A.; McCullough, A.E.; Wohlfiel, S.L.; Wengenack, N.L. Detection of Coccidioides Species in Clinical Specimens by Real-Time PCR. J. Clin. Microbiol. 2006, 45, 173–178. [Google Scholar] [CrossRef] [Green Version]
- Tintelnot, K.; de Hoog, G.S.; Antweiler, E.; Losert, H.; Seibold, M.; Brandt, M.A.; van den Ende, A.H.; Fisher, M.C. Taxonomic and diagnostic markers for identification of Coccidioides immitis and Coccidioides posadasii. Med. Mycol. 2007, 45, 385–393. [Google Scholar] [CrossRef] [Green Version]
- Pan, S.; Cole, G.T. Molecular and biochemical characterization of a Coccidioides immitis-specific antigen. Infect. Immun. 1995, 63, 3994–4002. [Google Scholar] [CrossRef] [Green Version]
- Saubolle, M.A.; Wojack, B.R.; Wertheimer, A.M.; Fuayagem, A.Z.; Young, S.; Koeneman, B.A. Multicenter Clinical Validation of a Cartridge-Based Real-Time PCR System for Detection of Coccidioides spp. in Lower Respiratory Specimens. J. Clin. Microbiol. 2017, 56, e01277-17. [Google Scholar] [CrossRef] [Green Version]
- Patel, R.A. Moldy application of MALDI: MALDI-ToF Mass Spectrometry for fungal identification. J. Fungi 2019, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Walsh, T.J.; McCarthy, M.W. The expanding use of matrix-assisted laser desorption/ionization-time of flight mass spectroscopy in the diagnosis of patients with mycotic diseases. Expert Rev. Mol. Diagn. 2019, 19, 241–248. [Google Scholar] [CrossRef]
- Kim, M.M.; Vikram, H.R.; Kusne, S.; Seville, M.T.; Blair, J.E. Treatment of Refractory Coccidioidomycosis with Voriconazole or Posaconazole. Clin. Infect. Dis. 2011, 53, 1060–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, L.; Ting, J.; Lin, H.; Shah, R.; MacLean, M.; Peterson, M.W.; Stockamp, N.; Libke, R.; Brown, P. The Rise of Valley Fever: Prevalence and Cost Burden of Coccidioidomycosis Infection in California. Int. J. Environ. Res. Public Health 2019, 16, 1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vianna, H.; Passos, H.V.; Santana, A.V. Coccicioidomicose. Relato do primeiro caso ocorrido em nativo do Brasil. Rev. Inst. Med. Trop. S. Paulo 1979, 21, 51–55. [Google Scholar] [PubMed]
- Gomes, O.M.; Serrano, R.R.P.; Pradel, H.O.V.; Moraes, N.L.T.B.; Varella, A.L.B.; Fiorelli, A.I.; Espósito, I.; Saad, F.A.; Furlaneto, J.A.; Rothman, A.; et al. Coccidioidomicose pulmonar. Primeiro caso nacional. Rev. Assoc. Méd. Bras 1978, 24, 167–168. [Google Scholar]
- Kuhl, I.A.; Kuhl, G.; Londero, A.; Diógenes, M.J.N.; Ferreira, M.F. Coccidioidomycosis laríngea: Relato de caso. Rev. Bras Otorrinol. 1996, 62, 48–51. [Google Scholar]
- Sidrim, J.; Silva, L.C.D.; Nunes, J.; Rocha, M.; Paixão, G.C. Le Nord-Est brésilien, région d’endémie de coccidioïdomycose: A propos d’une micro-épidémie. J. Mycol. Méd. 1997, 7, 37–39. [Google Scholar]
- Pappagianis, D. Coccidioides Immitis, 9th ed.; Ajello, L., Hay, R., Eds.; Topley & Wilson’s Microbiology and Microbial Infections: London, UK, 1998; pp. 357–371. [Google Scholar]
- Araújo, P.S.R.; Souza-Junior, V.R.; Padilha, C.E.; Oliveira, M.I.; Arraes, L.C.; Vieira, R.; Antunes, A.; Lima-Neto, R.G.; Marsden, A. Coccidioidomycosis: First cases reported in Pernambuco, Brazil. Rev. Inst. Med. Trop. S Paulo 2018, 60, e75. [Google Scholar] [CrossRef]
- de Lima-Neto, R.G.; Inácio, C.P.; Costa, R.B.; Cordeiro, R.D.A.; Neves, W.W.; Neves, R.P.; Magalhães, O.M.C.; Filho, A.M.L.; de Araújo, P.S.R. Coccidioidomycosis in a Pediatric Patient. Mycopathologia 2021. [Google Scholar] [CrossRef]
- Eulálio, K.D. Echo-Epidemiological and Clinical Manifestations of Coccidioidomycosis in the States of the Piauí and the Maranhão. Ph.D. Thesis, Instituto Oswaldo Cruz, Rio de Janeiro, Brazil, 2008. [Google Scholar]
- Deus-Filho, A.D.; Deus, A.C.B.; Meneses, A.O.; Soares, A.S.; Lira, A.L.A. Manifestações cutâneo-mucosas da coccidioidomicose: Estudo de trinta casos procedentes dos estados do Piauí e Maranhão. Bras. Dermatol. 2010, 85, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Morais, J.L.D.S.; Borges, M.C.M.; Cavalcante, L.M.M.B.; Motoyama, P.V.P.; Libório, M.P.; Távora, L.G.F. Coccidioidomycosis in a reference center in Northeast Brazil: Clinical/epidemiological profile and most common radiological findings. Rev. Soc. Bras. Med. Trop. 2020, 53, 20200249. [Google Scholar] [CrossRef]
- Brilhante, R.S.; Moreira Filho, R.E.; Rocha, M.F.; Castelo-Branco, D.S.; Fechine, M.A.; Lima, R.A.; Picanço, Y.V.; Cordeiro, R.A.; Camargo, Z.P.; Queiroz, J.A.; et al. Coccidioidomycosis in armadillo hunters from the state of Ceará, Brazil. Mem. Inst. Oswaldo Cruz 2012, 107, 813–815. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, R.D.A.; Brilhante, R.S.N.; Rocha, M.F.G.; Bandeira, S.P.; Fechine, M.A.B.; de Camargo, Z.; Sidrim, J.J.C.; de Camargo, Z. Twelve years of coccidioidomycosis in Ceará State, Northeast Brazil: Epidemiologic and diagnostic aspects. Diagn. Microbiol. Infect. Dis. 2010, 66, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Togashi, R.H.; Aguiar, F.M.B.; Ferreira, D.B.; Moura, C.M.; Sales, M.T.M.; Rios, N.X. Coccidioidomicose pulmonar e extrapulmonar: Três casos em zona endêmica no interior do Ceará. J. Bras. Pneumol. 2009, 35, 275–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Costa, F.A.M.; Reis, R.C.; Benevides, F.; Tomé, G.D.S.; Holanda, M.A. Coccidioidomicose pulmonar em caçador de tatus. J. Pneumol. 2001, 27, 275–278. [Google Scholar] [CrossRef] [Green Version]
- Martins, M.A.; Araújo, E.M.P.A.; Kuwakino, M.H.; Heins-Vaccari, E.M.; del Negro, G.M.B.; Vozza, J.A.; Lacaz, C.S. Coccidioidomycosis in Brazil. A case report. Rev. Inst. Med. Trop. S Paulo 1997, 30, 299–304. [Google Scholar] [CrossRef] [Green Version]
- Veras, K.N.; Figueirêdo, B.C.S.; Martins, L.M.S.; Vasconcelos, J.T.P.; Wanke, B. Coccidioidomycosis: An unusual cause of acute respiratory distress syndrome. J. Bras Pneumol. 2003, 29, 45–48. [Google Scholar] [CrossRef]
- Eulalio, K.D.; de Macedo, R.L.; Cavalcanti, M.D.A.S.; Martins, L.M.S.; Lazéra, M.D.S.; Wanke, B. Coccidioides immitis isolated from armadillos (Dasypus novemcinctus) in the state of Piauí, northeast Brazil. Mycopathologia 2001, 149, 57–61. [Google Scholar] [CrossRef]
- Diógenes, M.J.N.; Jamacuru, W.F.; Silva, M.A.B.; Carvalho, F.F. Inquérito epidemiológico com esferulina em Jaguaribara, CE, Brasil, 1993. Bras Dermatol. 1995, 70, 525–529. [Google Scholar]
- Marengo, J.A.; Torres, R.R.; Alves, L.M. Drought in Northeast Brazil—Past, present, and future. Theor. Appl. Clim. 2017, 129, 1189–1200. [Google Scholar] [CrossRef]
- Moro, M.F.; Macedo, M.B.; Moura-Fé, M.M.; Castro, A.S.F.; CostaA, R.C. Vegetação, unidades fitoecológicas e diversidade paisagística do estado do Ceará. Rodriguésia 2015, 66, 717–743. [Google Scholar] [CrossRef]
- Fernandes, M.F.; Cardoso, D.; de Queiroz, L.P. An updated plant checklist of the Brazilian Caatinga seasonally dry forests and woodlands reveals high species richness and endemism. J. Arid. Environ. 2020, 174, 104079. [Google Scholar] [CrossRef]
- Marengo, J.A.; Bernasconi, M. Regional differences in aridity/drought conditions over Northeast Brazil: Present state and future projections. Clim. Chang. 2015, 129, 103–115. [Google Scholar] [CrossRef]
- Marengo, J.A.; Alves, L.M.; Alvala, R.C.; Cunha, A.P.; Brito, S.; Moraes, O.L. Climatic characteristics of the 2010-2016 drought in the semiarid Northeast Brazil region. An. Acad. Bras. Ciênc. 2018, 90, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Brazilian Institute of Geography and Statistics-IBGE. Censo Demográfico. 2010. Available online: https://www.ibge.gov.br/en/statistics/social/labor/18391-2010-population-census.html (accessed on 30 October 2020).
- Brazilian Institute of Geography and Statistics-IBGE. Continuous PNAD 2016: 51% of the Brazilian Population Aged 25 or Over Had Only Complete Primary Education. 2017. Available online: https://agenciadenoticias.ibge.gov.br/en/agencia-press-room/2185-news-agency/releases-en/19005-continuous-pnad-2016-51-of-the-brazilian-population-aged-25-or-over-had-only-complete-primary-education (accessed on 30 October 2020).
- Brazilian Institute of Geography and Statistics-IBGE. 2019. Extreme Poverty Affects 13.5 Million Persons and Hits Highest Level in Seven Years, Brazilian Institute of Geography and Statistics. 2019. Available online: https://agenciadenoticias.ibge.gov.br/en/agencia-news/2184-news-agency/news/25895-extreme-poverty-affects-13-5-million-persons-and-hits-highest-level-in-seven-years (accessed on 30 October 2020).
- Atlas of Human Development in Brazil. 2013. Available online: http://www.atlasbrasil.org.br (accessed on 30 October 2020).
- Massuda, A.; Hone, T.; Leles, F.A.G.; de Castro, M.C.; Atun, R. The Brazilian health system at crossroads: Progress, crisis and resilience. BMJ Glob. Health 2018, 3, e000829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- França, E.B.; Lansky, S.; Rego, M.A.S.; Malta, D.C.; França, J.S.; Teixeira, R.; Porto, D.; Almeida, M.F.; Souza, M.F.M.; Szwarcwald, C.L.; et al. Leading causes of child mortality in Brazil, in 1990 and 2015: Estimates from the Global Burden of Disease study. Rev. Bras Epidemiol. 2017, 20 (Suppl. S1), 46–60. [Google Scholar] [CrossRef] [PubMed]
- Martins-Melo, F.R.; Carneiro, M.; Heukelbach, J.; Ribeiro, A.L.P.; Werneck, G.L. The burden of Neglected Tropical Diseases in Brazil, 1990–2016: A subnational analysis from the Global Burden of Disease Study 2016. PLoS Negl. Trop. Dis. 2018, 12, e0006559. [Google Scholar] [CrossRef] [PubMed]
- WHO (World Health Organization). Investing to Overcome the Global Impact of Neglected Tropical Diseases: Third WHO Report on Neglected Tropical Diseases; World Health Organization: Geneva, Switzerland, 2015; Available online: https://www.who.int/neglected_diseases/9789241564861/en/> (accessed on 30 October 2020).
- Nii-Trebi, N.I. Emerging and Neglected Infectious Diseases: Insights, Advances, and Challenges. BioMed Res. Int. 2017, 2017, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotez, P.J. Global urbanization and the neglected tropical diseases. PLoS Negl. Trop. Dis. 2017, 11, e0005308. [Google Scholar] [CrossRef]
- Hotez, P.J.; Aksoy, S. Ten years of progress in neglected tropical disease control and Elimination. PLoS Negl. Trop. Dis. 2017, 11, e0005355. [Google Scholar] [CrossRef] [Green Version]
- WHO (World Health Organization). Recommendations for the Adoption of Additional Diseases as Neglected Tropical Diseases; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- WHO (World Health Organization). Report of the Tenth Meeting of the WHO Strategic and Technical Advisory Group for Neglected Tropical Diseases; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Martins-Melo, F.R.; Ramos, A.N.; Alencar, C.H.; Heukelbach, J. Trends and spatial patterns of mortality related to neglected tropical diseases in Brazil. Parasite Epidemiol. Control. 2016, 1, 56–65. [Google Scholar] [CrossRef] [Green Version]
- Giacomazzi, J.; Baethgen, L.; Carneiro, L.C.; Millington, M.A.; Denning, D.W.; Colombo, A.L.; Pasqualotto, A.C. In association with the LIFE program the burden of serious human fungal infections in Brazil. Mycoses 2016, 59, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Borba, H.H.L.; Steimbach, L.M.; Riveros, B.S.; Tonin, F.S.; Ferreira, V.L.; Bagatim, B.A.Q.; Balan, G.; Pontarolo, R.; Wiens, A. Cost-effectiveness of amphotericin B formulations in the treatment of systemic fungal infections. Mycoses 2018, 61, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Molloy, S.F.; Chiller, T.; Greene, G.S.; Burry, J.; Govender, N.P.; Kanyama, C.; Mfinanga, S.; Lesikari, S.; Mapoure, Y.N.; Kouanfack, C.; et al. Cryptococcal meningitis: A neglected NTD? PLoS Negl. Trop. Dis. 2017, 11, e0005575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Queiroz-Telles, F.; Fahal, A.H.; Falci, D.R.; Caceres, D.H.; Chiller, T.; Pasqualotto, A.C. Neglected endemic mycoses. Lancet Infect. Dis. 2017, 17, e367–e377. [Google Scholar] [CrossRef]
- Rodrigues, M.L. Neglected disease, neglected populations: The fight against Cryptococcus and cryptococcosis. Mem. Inst. Oswaldo Cruz 2018, 113, e180111. [Google Scholar] [CrossRef]
- Griffiths, J.; Colombo, A.L.; Denning, D.W. The case for paracoccidioidomycosis to be accepted as a neglected tropical (fungal) disease. PLoS Negl. Trop. Dis. 2019, 13, e0007195. [Google Scholar] [CrossRef] [PubMed]
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef]
- Rodriguez-Morales, A.J.; Gallego, V.; Escalera-Antezana, J.P.; Méndez, C.A.; Zambrano, L.I.; Franco-Paredes, C.; Suárez, J.A.; Rodriguez-Enciso, H.D.; Balbin-Ramon, G.J.; Savio-Larriera, E.; et al. COVID-19 in Latin America: The implications of the first confirmed case in Brazil. Travel Med. Infect. Dis. 2020, 35, 101613. [Google Scholar] [CrossRef]
- ECLAC. Cómo evitar que la crisis del COVID-19 se transforme en una crisis alimentaria: Acciones urgentes contra el hambre en América Latina y el Caribe. Econ. Comm. Latin Am. Caribb. 2020. [Google Scholar]
- Hotez, P.J. Ten global “hotspots” for the neglected tropical diseases. PLoS Negl. Trop. Dis. 2014, 8, e2496. [Google Scholar] [CrossRef]
- Brazil. Boletim Epidemiológico. Secretaria de Vigilância em Saúde. Ministério da Saúde. 2020. Available online: http://www.aids.gov.br/pt-br/pub/2020/boletim-epidemiologico-de-turbeculose-2020 (accessed on 10 October 2020).
- Kirkland, T.N. The Quest for a Vaccine Against Coccidioidomycosis: A Neglected Disease of the Americas. J. Fungi 2016, 2, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnato, A.E.; Sanders, G.D.; Owens, D.K. Cost-effectiveness of a potential vaccine for Coccidioides immitis. Emerg. Infect. Dis. 2001, 7, 797–806. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordeiro, R.; Moura, S.; Castelo-Branco, D.; Rocha, M.F.; Lima-Neto, R.; Sidrim, J.J. Coccidioidomycosis in Brazil: Historical Challenges of a Neglected Disease. J. Fungi 2021, 7, 85. https://doi.org/10.3390/jof7020085
Cordeiro R, Moura S, Castelo-Branco D, Rocha MF, Lima-Neto R, Sidrim JJ. Coccidioidomycosis in Brazil: Historical Challenges of a Neglected Disease. Journal of Fungi. 2021; 7(2):85. https://doi.org/10.3390/jof7020085
Chicago/Turabian StyleCordeiro, Rossana, Santiago Moura, Débora Castelo-Branco, Marcos Fábio Rocha, Reginaldo Lima-Neto, and José Júlio Sidrim. 2021. "Coccidioidomycosis in Brazil: Historical Challenges of a Neglected Disease" Journal of Fungi 7, no. 2: 85. https://doi.org/10.3390/jof7020085
APA StyleCordeiro, R., Moura, S., Castelo-Branco, D., Rocha, M. F., Lima-Neto, R., & Sidrim, J. J. (2021). Coccidioidomycosis in Brazil: Historical Challenges of a Neglected Disease. Journal of Fungi, 7(2), 85. https://doi.org/10.3390/jof7020085






