Subcritical Water Extracts from Agaricus blazei Murrill’s Mycelium Inhibit the Expression of Immune Checkpoint Molecules and Axl Receptor
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of ABM Fruiting Body Extracts at Subcritical Conditions and Increasing Temperatures
2.2. Preparation of ABM Mycelium Extracts
2.3. Cell Culture
2.4. Cell Stimulation Cell Viability Assay
2.5. Gene Expression Analysis
2.6. Bone Marrow-Derived Dendritic Cells
2.7. Statistical Analysis
3. Results
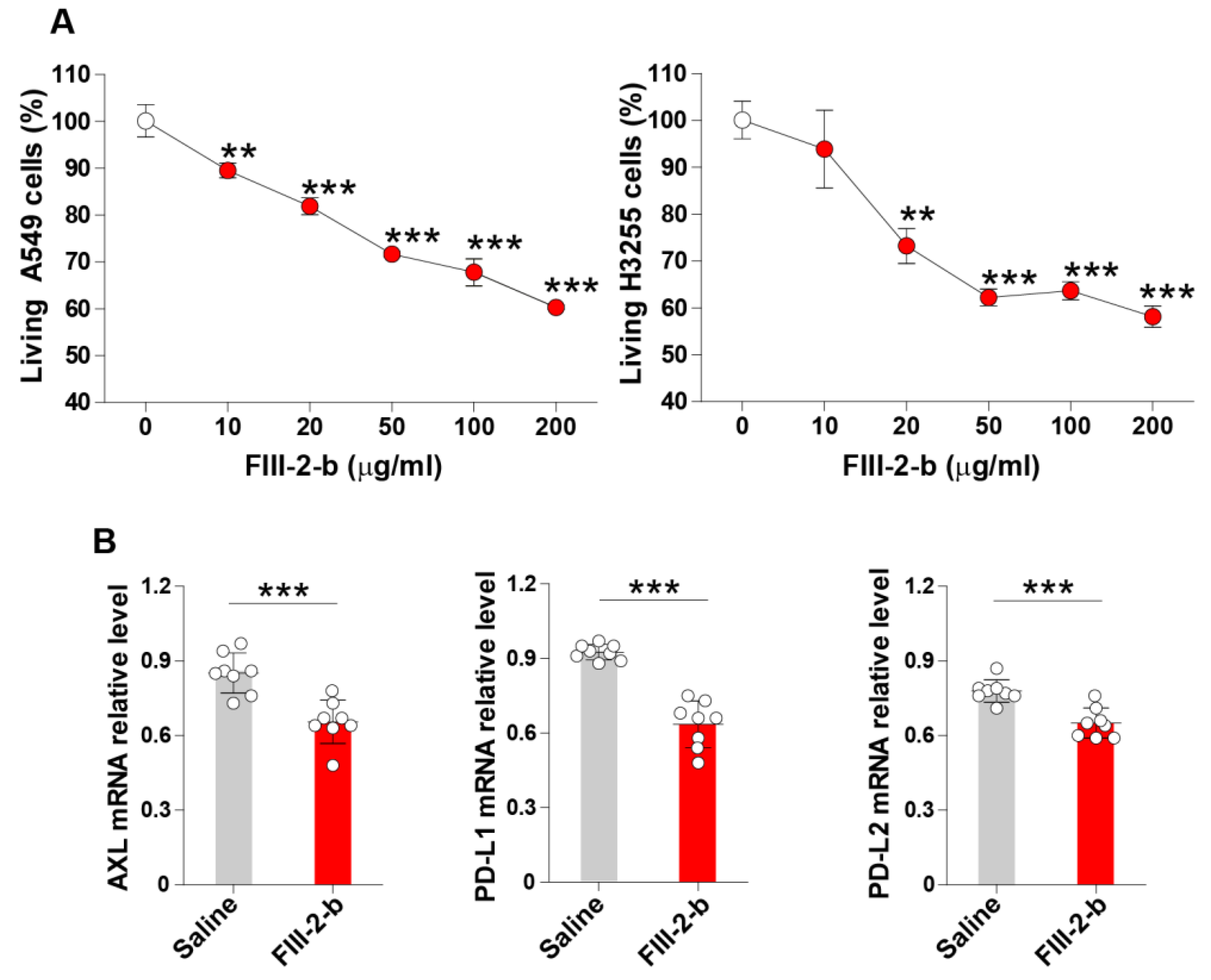
3.1. Inhibitory Activity of the FIII-2-b Polysaccharide in Lung Cancer Cells
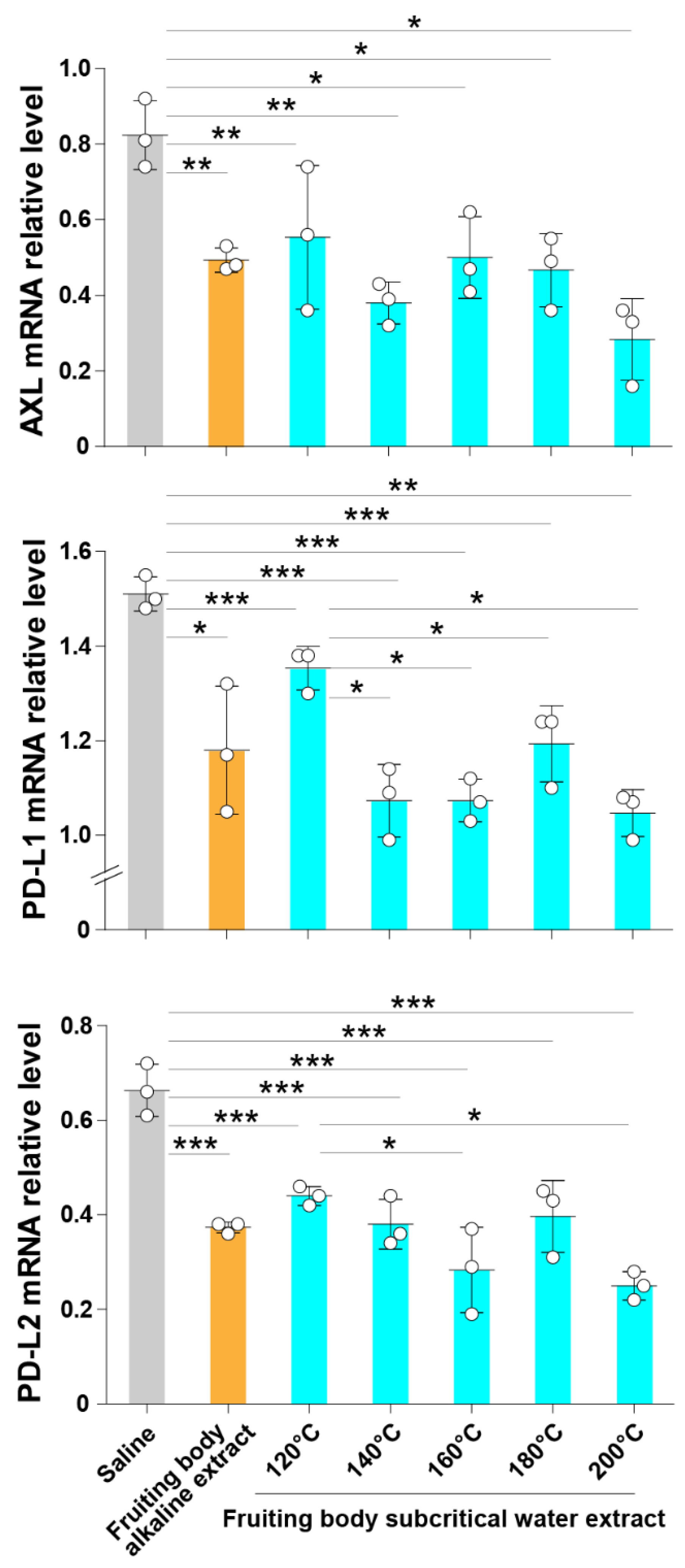
3.2. Subcritical Hot Water Extracts from the ABM’s Fruiting Body Inhibit the Expression of Axl and Immune Checkpoint Molecules
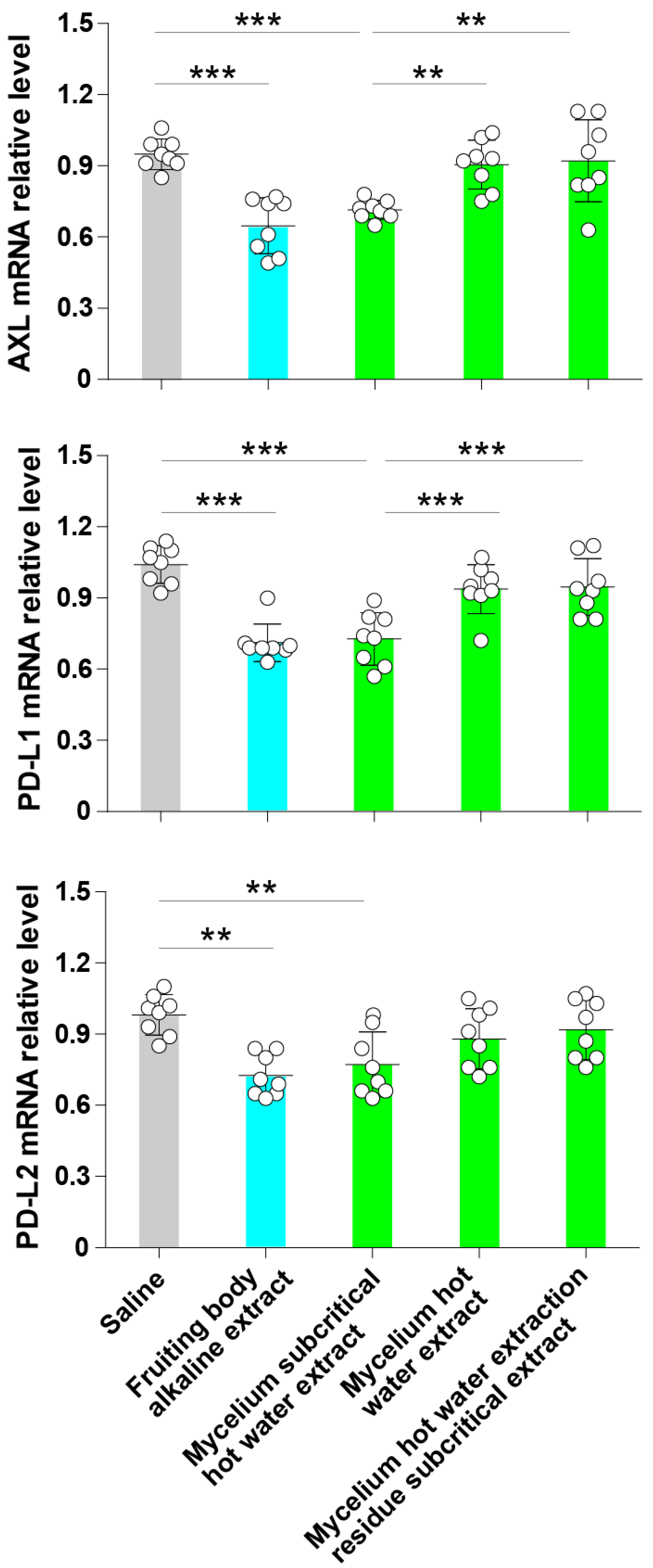
3.3. Subcritical Water Extracts from the ABM’s Mycelium Decrease the Expression of Axl, PD-L1 and PD-L2
3.4. ABM’s Mycelium Water Extracts Increase the Expression of Maturation Markers of Dendritic Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Feeney, M.J.; Dwyer, J.; Hasler-Lewis, C.M.; Milner, J.A.; Noakes, M.; Rowe, S.; Wach, M.; Beelman, R.B.; Caldwell, J.; Cantorna, M.T.; et al. Mushrooms and Health Summit proceedings. J. Nutr. 2014, 144, 1128S–1136S. [Google Scholar] [CrossRef]
- Ramoutsaki, I.A.; Papadakis, C.E.; Ramoutsakis, I.A.; Helidonis, E.S. Therapeutic methods used for otolaryngological problems during the Byzantine period. Ann. Otol. Rhinol. Laryngol. 2002, 111, 553–557. [Google Scholar] [CrossRef]
- Patel, D.K.; Dutta, S.D.; Ganguly, K.; Cho, S.J.; Lim, K.T. Mushroom-Derived Bioactive Molecules as Immunotherapeutic Agents: A Review. Molecules 2021, 26, 1359. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Nanda, P.K.; Dandapat, P.; Bandyopadhyay, S.; Gullon, P.; Sivaraman, G.K.; McClements, D.J.; Gullon, B.; Lorenzo, J.M. Edible Mushrooms as Functional Ingredients for Development of Healthier and More Sustainable Muscle Foods: A Flexitarian Approach. Molecules 2021, 26, 2463. [Google Scholar] [CrossRef] [PubMed]
- Kohler, J.R.; Casadevall, A.; Perfect, J. The spectrum of fungi that infects humans. Cold Spring Harb. Perspect. Med. 2014, 5, a019273. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, H.E.; Parrent, J.L.; Jackson, J.A.; Moncalvo, J.M.; Vilgalys, R. Fungal community analysis by large-scale sequencing of environmental samples. Appl. Environ. Microbiol. 2005, 71, 5544–5550. [Google Scholar] [CrossRef] [PubMed]
- Firenzuoli, F.; Gori, L.; Lombardo, G. The Medicinal Mushroom Agaricus blazei Murrill: Review of Literature and Pharmaco-Toxicological Problems. Evid. Based Complement. Altern. Med. 2008, 5, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T. Kawariharatake, Agaricu-Blazei Murill—Medicinal-Effects and Dietary-Effects. Food Rev. Int. 1995, 11, 167–172. [Google Scholar] [CrossRef]
- Hakime-Silva, R.A.; Vellosa, J.C.; Khalil, N.M.; Khalil, O.A.; Brunetti, I.L.; Oliveira, O.M. Chemical, enzymatic and cellular antioxidant activity studies of Agaricus blazei Murrill. An. Acad. Bras. Cienc. 2013, 85, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, X.; Li, X.; Guo, X.; Sheng, Y.; Li, Y.; Xu, G.; Han, X.; An, L.; Du, P. Effects of Agaricus blazei Murrill polysaccharides on hyperlipidemic rats by regulation of intestinal microflora. Food Sci. Nutr. 2020, 8, 2758–2772. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sheng, Y.; Lu, X.; Guo, X.; Xu, G.; Han, X.; An, L.; Du, P. Isolation and purification of acidic polysaccharides from Agaricus blazei Murill and evaluation of their lipid-lowering mechanism. Int. J. Biol. Macromol. 2020, 157, 276–287. [Google Scholar] [CrossRef]
- Wei, Q.; Li, J.; Zhan, Y.; Zhong, Q.; Xie, B.; Chen, L.; Chen, B.; Jiang, Y. Enhancement of glucose homeostasis through the PI3K/Akt signaling pathway by dietary with Agaricus blazei Murrill in STZ-induced diabetic rats. Food Sci. Nutr. 2020, 8, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, F.; Jia, S.; Ren, H.; Gong, G.; Wang, Y.; Lv, Z.; Liu, Y. Drying effects on the antioxidant properties of polysaccharides obtained from Agaricus blazei Murrill. Carbohydr. Polym. 2014, 103, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Endo, M.; Matsui, T.; Katsuda, I.; Emi, N.; Kawamoto, Y.; Koike, T.; Beppu, H. Agaritine from Agaricus blazei Murrill induces apoptosis in the leukemic cell line U937. Biochim. Biophys. Acta 2011, 1810, 519–525. [Google Scholar] [CrossRef]
- Endo, M.; Beppu, H.; Akiyama, H.; Wakamatsu, K.; Ito, S.; Kawamoto, Y.; Shimpo, K.; Sumiya, T.; Koike, T.; Matsui, T. Agaritine purified from Agaricus blazei Murrill exerts anti-tumor activity against leukemic cells. Biochim. Biophys. Acta 2010, 1800, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Ito, H.; Hibasami, H. Blazein of a new steroid isolated from Agaricus blazei Murrill (himematsutake) induces cell death and morphological change indicative of apoptotic chromatin condensation in human lung cancer LU99 and stomach cancer KATO III cells. Oncol. Rep. 2008, 20, 1359–1361. [Google Scholar] [PubMed]
- Matsushita, Y.; Furutani, Y.; Matsuoka, R.; Furukawa, T. Hot water extract of Agaricus blazei Murrill specifically inhibits growth and induces apoptosis in human pancreatic cancer cells. BMC Complement. Altern. Med. 2018, 18, 319. [Google Scholar] [CrossRef]
- Jiang, L.; Yu, Z.; Lin, Y.; Cui, L.; Yao, S.; Lv, L.; Liu, J. Low-molecular-weight polysaccharides from Agaricus blazei Murrill modulate the Th1 response in cancer immunity. Oncol. Lett. 2018, 15, 3429–3436. [Google Scholar] [PubMed]
- Angeli, J.P.; Ribeiro, L.R.; Gonzaga, M.L.; Soares Sde, A.; Ricardo, M.P.; Tsuboy, M.S.; Stidl, R.; Knasmueller, S.; Linhares, R.E.; Mantovani, M.S. Protective effects of beta-glucan extracted from Agaricus brasiliensis against chemically induced DNA damage in human lymphocytes. Cell Biol. Toxicol. 2006, 22, 285–291. [Google Scholar] [CrossRef]
- Barbisan, L.F.; Scolastici, C.; Miyamoto, M.; Salvadori, D.M.; Ribeiro, L.R.; da Eira, A.F.; de Camargo, J.L. Effects of crude extracts of Agaricus blazei on DNA damage and on rat liver carcinogenesis induced by diethylnitrosamine. Genet. Mol. Res. 2003, 2, 295–308. [Google Scholar]
- Menoli, R.C.; Mantovani, M.S.; Ribeiro, L.R.; Speit, G.; Jordao, B.Q. Antimutagenic effects of the mushroom Agaricus blazei Murrill extracts on V79 cells. Mutat. Res. 2001, 496, 5–13. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Aran, V.; Omerovic, J. Current Approaches in NSCLC Targeting K-RAS and EGFR. Int. J. Mol. Sci. 2019, 20, 5701. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Camidge, D.R.; Shaw, A.T.; Gadgeel, S.; Ahn, J.S.; Kim, D.W.; Ou, S.I.; Perol, M.; Dziadziuszko, R.; Rosell, R.; et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 829–838. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Murtuza, A.; Bulbul, A.; Shen, J.P.; Keshavarzian, P.; Woodward, B.D.; Lopez-Diaz, F.J.; Lippman, S.M.; Husain, H. Novel Third-Generation EGFR Tyrosine Kinase Inhibitors and Strategies to Overcome Therapeutic Resistance in Lung Cancer. Cancer Res. 2019, 79, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, X. Study and analysis of antitumor resistance mechanism of PD1/PD-L1 immune checkpoint blocker. Cancer Med. 2020, 9, 8086–8121. [Google Scholar] [CrossRef]
- Hong, J.; Peng, D.; Chen, Z.; Sehdev, V.; Belkhiri, A. ABL regulation by AXL promotes cisplatin resistance in esophageal cancer. Cancer Res. 2013, 73, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Greger, J.; Shi, H.; Liu, Y.; Greshock, J.; Annan, R.; Halsey, W.; Sathe, G.M.; Martin, A.M.; Gilmer, T.M. Novel mechanism of lapatinib resistance in HER2-positive breast tumor cells: Activation of AXL. Cancer Res. 2009, 69, 6871–6878. [Google Scholar] [CrossRef]
- Van der Meer, J.H.; van der Poll, T.; van ‘t Veer, C. TAM receptors, Gas6, and protein S: Roles in inflammation and hemostasis. Blood 2014, 123, 2460–2469. [Google Scholar] [CrossRef] [PubMed]
- Ben-Batalla, I.; Schultze, A.; Wroblewski, M.; Erdmann, R.; Heuser, M.; Waizenegger, J.S.; Riecken, K.; Binder, M.; Schewe, D.; Sawall, S.; et al. Axl, a prognostic and therapeutic target in acute myeloid leukemia mediates paracrine crosstalk of leukemia cells with bone marrow stroma. Blood 2013, 122, 2443–2452. [Google Scholar] [CrossRef]
- Gjerdrum, C.; Tiron, C.; Hoiby, T.; Stefansson, I.; Haugen, H.; Sandal, T.; Collett, K.; Li, S.; McCormack, E.; Gjertsen, B.T.; et al. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc. Natl. Acad. Sci. USA 2010, 107, 1124–1129. [Google Scholar] [CrossRef]
- Vuoriluoto, K.; Haugen, H.; Kiviluoto, S.; Mpindi, J.P.; Nevo, J.; Gjerdrum, C.; Tiron, C.; Lorens, J.B.; Ivaska, J. Vimentin regulates EMT induction by Slug and oncogenic H-Ras and migration by governing Axl expression in breast cancer. Oncogene 2011, 30, 1436–1448. [Google Scholar] [CrossRef] [PubMed]
- Berclaz, G.; Altermatt, H.J.; Rohrbach, V.; Kieffer, I.; Dreher, E.; Andres, A.C. Estrogen dependent expression of the receptor tyrosine kinase axl in normal and malignant human breast. Ann. Oncol. 2001, 12, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Davra, V.; Kimani, S.G.; Calianese, D.; Birge, R.B. Ligand Activation of TAM Family Receptors-Implications for Tumor Biology and Therapeutic Response. Cancers 2016, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Holland, S.J.; Pan, A.; Franci, C.; Hu, Y.; Chang, B.; Li, W.; Duan, M.; Torneros, A.; Yu, J.; Heckrodt, T.J.; et al. R428, a selective small molecule inhibitor of Axl kinase, blocks tumor spread and prolongs survival in models of metastatic breast cancer. Cancer Res. 2010, 70, 1544–1554. [Google Scholar] [CrossRef] [PubMed]
- Tsukita, Y.; Fujino, N.; Miyauchi, E.; Saito, R.; Fujishima, F.; Itakura, K.; Kyogoku, Y.; Okutomo, K.; Yamada, M.; Okazaki, T.; et al. Axl kinase drives immune checkpoint and chemokine signalling pathways in lung adenocarcinomas. Mol. Cancer 2019, 18, 24. [Google Scholar] [CrossRef]
- Cheng, Y.; Fumin Xue, F.; Yu, S.; Du, S.; Yangm, Y. Subcritical water extraction of natural products. Molecules 2021, 26, 4004. [Google Scholar] [CrossRef]
- Leong, Y.K.; Yang, F.C.; Chang, J.S. Extraction of polysaccharides from edible mushrooms: Emerging technologies and recent advances. Carbohydr. Polym. 2021, 251, 117006. [Google Scholar] [CrossRef] [PubMed]
- Kawagishi, H.; Inagaki, R.; Kanao, T.; Mizuno, T.; Shimura, K.; Ito, H.; Hagiwara, T.; Nakamura, T. Fractionation and antitumor activity of the water-insoluble residue of Agaricus blazei fruiting bodies. Carbohydr. Res. 1989, 186, 267–273. [Google Scholar] [CrossRef]
- Bouike, G.; Nishitani, Y.; Shiomi, H.; Yoshida, M.; Azuma, T.; Hashimoto, T.; Kanazawa, K.; Mizuno, M. Oral Treatment with Extract of Agaricus blazei Murill Enhanced Th1 Response through Intestinal Epithelial Cells and Suppressed OVA-Sensitized Allergy in Mice. Evid. Based Complement. Altern. Med. 2011, 2011, 532180. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, M.; Minato, K.; Ito, H.; Kawade, M.; Terai, H.; Tsuchida, H. Anti-tumor polysaccharide from the mycelium of liquid-cultured Agaricus blazei mill. Biochem. Mol. Biol. Int. 1999, 47, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, M.; Morimoto, M.; Minato, K.; Tsuchida, H. Polysaccharides from Agaricus blazei stimulate lymphocyte T-cell subsets in mice. Biosci. Biotechnol. Biochem. 1998, 62, 434–437. [Google Scholar] [CrossRef][Green Version]
- Mizuno, T.; Hagiwara, T.; Nakamura, T.; Ito, H.; Shimura, K.; Sumiya, T.; Asakura, A. Antitumor activity and some properties of water-soluble polysaccharides from “Himematsutake”, the fruiting body of Agaricus blazei Murrill. Agric. Biol. Chem. 1990, 54, 2889–2896. [Google Scholar]
- Kaneno, R.; Fontanari, L.M.; Santos, S.A.; Di Stasi, L.C.; Rodrigues Filho, E.; Eira, A.F. Effects of extracts from Brazilian sun-mushroom (Agaricus blazei) on the NK activity and lymphoproliferative responsiveness of Ehrlich tumor-bearing mice. Food Chem. Toxicol. 2004, 42, 909–916. [Google Scholar] [CrossRef]
- Gonzaga, M.L.; Bezerra, D.P.; Alves, A.P.; de Alencar, N.M.; Mesquita Rde, O.; Lima, M.W.; Soares Sde, A.; Pessoa, C.; de Moraes, M.O.; Costa-Lotufo, L.V. In vivo growth-inhibition of Sarcoma 180 by an alpha-(1-->4)-glucan-beta-(1-->6)-glucan-protein complex polysaccharide obtained from Agaricus blazei Murill. J. Nat. Med. 2009, 63, 32–40. [Google Scholar] [CrossRef]
- Paolino, M.; Penninger, J.M. The Role of TAM Family Receptors in Immune Cell Function: Implications for Cancer Therapy. Cancers 2016, 8, 97. [Google Scholar] [CrossRef]
- Malya, I.Y.; Wu, J.; Harada, E.; Toda, M.; D’Alessandro-Gabazza, C.N.; Yasuma, T.; Gabazza, E.C.; Choi, J.H.; Hirai, H.; Kawagishi, H. Plant growth regulators and Axl and immune checkpoint inhibitors from the edible mushroom Leucopaxillus giganteus. Biosci. Biotechnol. Biochem. 2020, 84, 1332–1338. [Google Scholar] [CrossRef]
- Ridwan, A.Y.; Wu, J.; Harada, E.; CN, D.A.-G.; Toda, M.; Yasuma, T.; Gabazza, E.C.; Choi, J.H.; Hirai, H.; Kawagishi, H. Axl and immune checkpoints inhibitors from fruiting bodies of Pleurocybella porrigens. J. Antibiot. 2020, 73, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Hafizi, S.; Dahlback, B. Gas6 and protein S. Vitamin K-dependent ligands for the Axl receptor tyrosine kinase subfamily. FEBS J. 2006, 273, 5231–5244. [Google Scholar] [CrossRef]
- Wu, X.; Liu, X.; Koul, S.; Lee, C.Y.; Zhang, Z.; Halmos, B. AXL kinase as a novel target for cancer therapy. Oncotarget 2014, 5, 9546–9563. [Google Scholar] [CrossRef] [PubMed]

| Gene | Direction | Sequence (5′ > 3′) | Length | Tm (°C) | Ref | Position | Product |
|---|---|---|---|---|---|---|---|
| GAPDH | Forward | GGAGCGAGATCCCTCCAAAAT | 21 | 61.6 | NM_001256799 | 108–128 | 197 bp |
| Reverse | GGCTGTTGTCATACTTCTCATGG | 23 | 60.9 | 304–282 | |||
| AXL | Forward | TGCCATTGAGAGTCTAGCTGAC | 22 | 63.4 | NM_001699 | 2311–2322 | 218 bp |
| Reverse | TTAGCTCCCAGCACCGCGAC | 20 | 71.7 | 2528–2509 | |||
| PD-L1 | Forward | GGACAAGCAGTGACCATCAAG | 21 | 60.9 | NM_014143 | 500–520 | 235 bp |
| Reverse | CCCAGAATTACCAAGTGAGTCCT | 23 | 61.3 | 734–712 | |||
| PD-L2 | Forward | ACCGTGAAAGAGCCACTTTG | 20 | 60.5 | NM_025239 | 206–225 | 121 bp |
| Reverse | GCGACCCCATAGATGATTATGC | 22 | 60.7 | 326–305 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasuma, T.; Toda, M.; Kobori, H.; Tada, N.; D’Alessandro-Gabazza, C.N.; Gabazza, E.C. Subcritical Water Extracts from Agaricus blazei Murrill’s Mycelium Inhibit the Expression of Immune Checkpoint Molecules and Axl Receptor. J. Fungi 2021, 7, 590. https://doi.org/10.3390/jof7080590
Yasuma T, Toda M, Kobori H, Tada N, D’Alessandro-Gabazza CN, Gabazza EC. Subcritical Water Extracts from Agaricus blazei Murrill’s Mycelium Inhibit the Expression of Immune Checkpoint Molecules and Axl Receptor. Journal of Fungi. 2021; 7(8):590. https://doi.org/10.3390/jof7080590
Chicago/Turabian StyleYasuma, Taro, Masaaki Toda, Hajime Kobori, Naoto Tada, Corina N. D’Alessandro-Gabazza, and Esteban C. Gabazza. 2021. "Subcritical Water Extracts from Agaricus blazei Murrill’s Mycelium Inhibit the Expression of Immune Checkpoint Molecules and Axl Receptor" Journal of Fungi 7, no. 8: 590. https://doi.org/10.3390/jof7080590
APA StyleYasuma, T., Toda, M., Kobori, H., Tada, N., D’Alessandro-Gabazza, C. N., & Gabazza, E. C. (2021). Subcritical Water Extracts from Agaricus blazei Murrill’s Mycelium Inhibit the Expression of Immune Checkpoint Molecules and Axl Receptor. Journal of Fungi, 7(8), 590. https://doi.org/10.3390/jof7080590







