Abstract
Our study of the secondary metabolites of coral-associated fungi produced a valuable and extra-large chemical database. Many of them exhibit strong biological activity and can be used for promising drug lead compounds. Serving as an epitome of the most promising compounds, which take the ultra-new skeletons and/or remarkable bioactivities, this review presents an overview of new compounds and bioactive compounds isolated from coral-associated fungi, covering the literature from 2010 to 2021. Its scope included 423 metabolites, focusing on the bioactivity and structure diversity of these compounds. According to structure, these compounds can be roughly classified as terpenes, alkaloids, peptides, aromatics, lactones, steroids, and other compounds. Some of them described in this review possess a wide range of bioactivities, such as anticancer, antimicrobial, antifouling, and other activities. This review aims to provide some significant chemical and/or biological enlightenment for the study of marine natural products and marine drug development in the future.
1. Introduction
Marine organisms, representing approximately 75% of all living organisms, have proven to be a rich source of inspiration for drug discovery, with success rates for marine natural products up to 4 times higher than other naturally derived compounds [1]. Research into the pharmacological properties of marine natural products (MNP) has led to the discovery of many active agents considered worthy of clinical application; to date 14 marine NPs or their derivatives are registered drugs, and another 23 are currently in clinical trials [2]. The annual reviews of marine natural products were reported by the New Zealand group in the Natural Product Reports. These reviews show that marine fungi are currently the most studied marine microorganism phyla, and over the last five years an extraordinary transformation in MNP research continued with a very significant increase in the number of new compounds reported from marine fungi. For example, in 2018, new MNPs reported from marine fungi increased by 38% relative to 2017 [3]. With up to 90% of marine species undescribed, marine fungi can inspire new discoveries and offer many novel solutions to life’s problems in the future.
Coral reefs are among the most fragile, biologically diverse and economically important ecosystems on Earth, providing ecosystem services that are vital to human societies and industries through fisheries, coastal protection, new biochemical compounds, and tourism [4,5]. Coral reefs are regarded as one of the most important shelters of microorganisms [6]. Fungi are abundant in the coral reefs and recent studies demonstrate diverse communities associated with coral. Recent advances suggest that fungi associated with marine invertebrates may play an active role in the formation of biofilms and constitute the main chemical defense mechanism of the host. It is widely accepted that small molecule natural products evolved to carry out a particular ecological function and that these finely tuned compounds can sometimes be appropriated for the treatment of disease in humans [7].
Since the 1960s, plenty of bioactive metabolites have been isolated from coral. However, the supply has become a serious obstacle to the ultimate development of these bioactive substances. This has given rise to investigations on the metabolites produced by coral-symbiotic microorganisms. Two reviews on the biological and chemical diversity of coral-associated microorganisms were published by Shao and his coworkers [8,9]. In 2018, Keller-Costa et al. studied how bioactive secondary metabolites form octocoral-associated bacteria and fungi [10]. In 2021, Seelan et al. compiled a review to summarize metabolites produced by marine fungi isolated from the soft coral genus Sarcophyton from 2010 to 2020 [11]. Similar to these prior reviews, they focused on the discovery of new compounds from natural sources and the associated biological properties of these metabolites. In this review, we provide a thorough overview of new compounds and bioactive metabolites gathered from coral-derived fungi from 2010 to 2021, which present 423 compounds of chemical structures and bioactivities.
2. Terpenes
Terpenes have been widely applied in the pharmaceutical, nutraceutical, synthetic chemistry, flavor fragrance, and possibly biofuel industries, and are essential constituents of natural products. Moreover, terpenes are a prime group of essential oils possessing a broad spectrum of antibacterial, antifungal and even antiviral activity [12,13]. Marine fungi are a significant source of terpenes, so it is necessary to carry out further investigation.
2.1. Sesquiterpenes
Previous research revealed that approximately 500 new sesquiterpenes, including about 20 new skeletons, were characterized from fungi during a five-year period (2015~2020) [14]. Soft coral-associated fungi are reported to be a rich source of sesquiterpenes. Li et al. have obtained a series of sesquiterpenoids from soft-coral associated fungi. Eleven linear triquinane sesquiterpenoids including chondrosterins A–E (1–5) [15], chondrosterin F (6) and incarnal (7) [16], hirsutanol A (8), [17] hirsutanol E (9), chondrosterin N (10) and chondrosterin O (11) [18] were isolated from the fungus Chondrostereum sp., separated from the soft coral Sarcophyton tortuosum, from the South China Sea. In addition, a new aromadendrane sesquiterpenoid, pseuboydone F (12), was extracted from the fungus Pseudallescheria boydii F44-1, derived from the soft coral Sarcophyton sp. [19]. Two new aromadendrane-type sesquiterpene diastereomers, pseuboydones A (13) and B (14), were also discovered from the marine-derived fungus Pseudallescheria boydii F19-1, collected from the soft coral Lobophytum crissum [20]. Linear triquinane sesquiterpenoids, possessing a basic skeleton 1H-cyclopenta[α]pentalene, have been isolated from different organisms including fungi, sponges and soft coral. In 2018, Li et al. published an overview covering 118 linear triquinane sesquiterpenoids [21]. From a biomedical perspective, chondrosterin A (1) with the typical α-methylene ketone group revealed significant cytotoxic activities against cancer lines A549, CNE2, and LoVo with IC50 values ranging from 2.45 to 5.47 μM, and chondrosterin B (2) exhibited antimalarial activity with an IC50 value of 3.10 μg/mL [22]; incarnal (7) had potent cytotoxic activity against cancer cell lines (CNE1, CNE2, SUNE, LoVo, KB, Be17402, MCF-7) with IC50 values ranging from 2.16 to 28.55 μg/mL; hirsutanol A (8) showed pronounced cytotoxic activities against various cancer cell lines (SW620, SW480, LoVo; Hep3B, HepG2, Bel-7402; A549; CNE1, CNE2, SUNE1; MCF7, MDA-MB-231, MDA-MB-435, MDA-MB-453 and HeLa) with IC50 values ranging from 0.58 to 8.27 μg/mL. The chemical exploration of the gorgonian-derived fungus Aspergillus sp. was carried out and afforded three known sesquiterpenoids, (R)-(-)-hydroxysydonic acid (15), (S)-(-)-5-(hydroxymethyl)-2-(2’,6’,6’-trimethyltetrahydro-2H-pyran-2-yl) phenol (16), and (S)-(+)-11-dehydrosydonic acid (17), which revealed moderate antibacterial activity against Staphylococcus aureus, Bacillus cereus, Kocuria rhizophila, Pseudomonas putida, Pseudomonas aeruginosa, Salmonella enterica, and Nocardia. brasiliensis [23] (Figure 1).
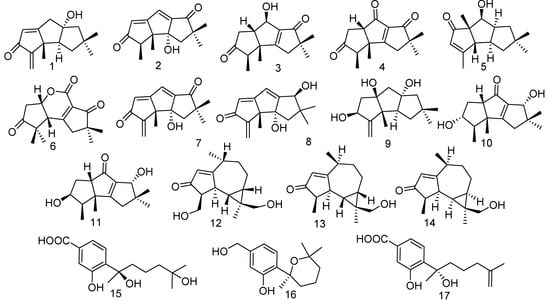
Figure 1.
The structures of compounds 1–17.
2.2. Diterpenoids
The well-known compound lovastatin (18) was extracted from a coral-derived fungus Aspergillus terreus. In vitro anti-inflammatory experiments showed that 18 has anti-inflammatory activity against NO production and was shown to have a significant inhibitory effect with an IC50 value of 17.45 μM [24]. Meanwhile, compound 18 was an inhibitor of 3-hydroxy-3-methyl-glutaryl-coenzyme as a lipid-lowering drug [25]. Furthermore, compound 18 also exhibited bioactivities of anti-cancer, prevention and treatment of neurological disorders, and antibacterial effects [26]. Two new harziane diterpene lactones, harziane lactones A and B (19 and 20) and five new harziane diterpenes, harzianones A–D (21–24) and harziane (25), were identified from the soft coral-derived fungus Trichoderma harzianum XS-20090075. Compounds 19–23 and compound 25 exhibited obvious phytotoxicity against the seedling growth of amaranth and lettuce with a concentration of 200 ppm. Moreover, at the concentration of 200 μg/mL, compounds 19, 21, 22, and 23 completely inhibited seed germination against amaranth. Compared with the positive control glyphosate, these compounds still showed phytotoxicity at a lower concentration of 50 μg/mL [27] (Figure 2).
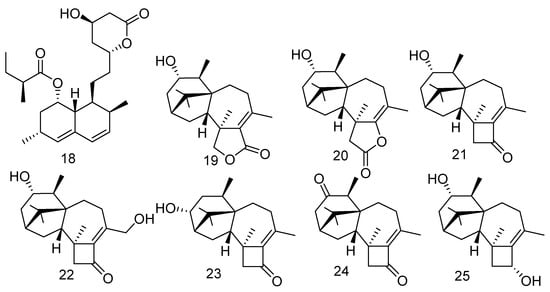
Figure 2.
The structures of compounds 18–25.
2.3. Triterpenes
A new nordammarane triterpenoid (26) was characterized from the marine strain Aspergillus fumigatus KMM 4631 collected from the soft coral Sinularia sp. [28] (Figure 3).
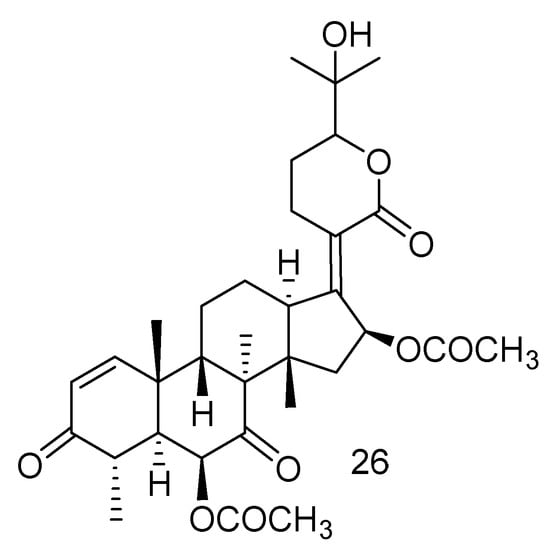
Figure 3.
The structure of compound 26.
2.4. Meroterpenoids
Fungi are the remarkable producers of meroterpenoids, which have exhibited diversified and unique structures with a wide range of bioactivities [29]. Marine-derived fungus Aspergillus sp. was obtained from the gorgonian Dichotella gemmacea collected from the South China Sea, which produced three new phenolic bisabolane-type sesquiterpenoids: (+)-methyl sydowate (27), 7-deoxy-7, 14-didehydrosydonic acid (28), and 7-deoxy-7,8-didehydrosydonic acid (29) [30]. Compound 27 showed bioactivity against S. aureus and methicillin-resistant Staphylococcus aureus (MRSA) with the same inhibition zones of 11 mm in diameter at a concentration of 100 μg/mL, while the positive control had inhibition zones of 37 and 21 mm in diameter, respectively. Moreover, compound 28 showed remarkable inhibitory activity on Gaeumannomyces graminis (MIC = 0.5 μg/mL) [31]. The chemical investigation of Aspergillus versicolor (ZJ-2008015) led to the isolation of three bisabolane sesquiterpene compounds named (+)-sydonic acid (30), expansol G (31), and (+)-sydowic acid (32). An in vitro antibacterial experiment showed that all compounds exhibited strong antibacterial activity with the MIC values of 5.3, 6.4, and 5.4 μg/mL, respectively, for Staphylococcus albus and 2.6, 6.4, and 5.4 μg/mL, respectively, for S. aureus [32]. Moreover, compound 32 also revealed cytotoxicity against murine leukemia P-388 cells (IC50 = 2.56 μg/mL) [33]. Two new phenolic bisabolane-type sesquiterpenes, namely 11,12-dihydroxysydonic acid (33) and 1-hydroxyboivinianic acid (34), were extracted from the marine-derived fungus Scopulariopsis sp. collected from the Red Sea hard coral Stylophora sp. [34]. Meanwhile, compound 34 exhibited weak activity to cancer cell lines A549, Caski, HepG 2, and MCF-7 with IC50 values of 90.6, 78.2, 75.8, and 80.4 μg/mL, respectively. Furthermore, 34 also showed antimicrobial activity against Erwinia carotovora sub sp. carotovora (MIC = 68.9 μg/mL) [35]. Craterellin D (35), a new merosesquiterpenoid, together with its known analog craterellin A (36), was isolated from a soft coral-derived Lophiostoma sp. fungus. Compound 35 was the ramification of the compound 36, whose configuration was confirmed by modified Mosher’s method and single-crystal X-ray diffraction. A primary bioassay indicated that 36 was shown to have antibacterial activity against B. cereus (MIC = 3.12 μM) [36] (Figure 4).
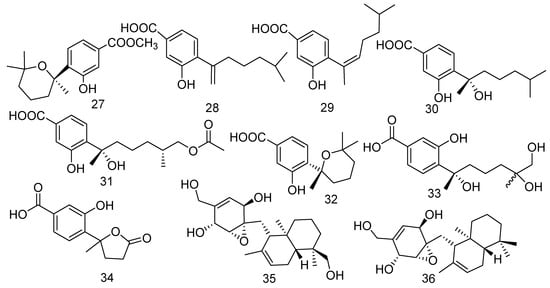
Figure 4.
The structures of compounds 27–36.
3. Alkaloids
Alkaloids have great development potential as drug scaffolds and scaffold substructures in modern antibacterial chemotherapy. There will be opportunities to accelerate the process of discovering more active alkaloids in the future [37].
3.1. Diketopiperazines
Wang et al. isolated three novel diketopiperazine alkaloids, chrysopiperazines A–C (37–39), from gorgonian-derived fungus Penicillium chrysogenum [38]. In addition, Wang’s group also discovered a strain of Nigrospora oryzae (ZJ-2008005) derived from soft coral Dendronephthya sp., which was found to produce seven isoechinulin-type alkaloids named neoechinulin A (40), preechinulin (41), isoechinulin A (42), tardioxopiperazine A (43), variecolorin L (44), dihydroxyisoechinulin A (45), and L-alanyl-L-tryptophan anhydride (46). A primary bioassay of antifouling activity against the larval settlement of barnacle Balanus amphitrite showed that compound 42 revealed significant antifouling activity with an IC50 value of 5.92 µg/mL, while 40 and 45 presented weak activity with IC50 values of 30 and 50 µg/mL, respectively [39]. Moreover, compound 40 also displayed strong cytotoxic activity against human cervical carcinoma HeLa cells and exerted anti-inflammatory, antiviral, and neuroprotective activity [40,41]. Furthermore, compound 42 revealed inhibitory activities against AChE (IC50 = 74.38 µM), and compounds 40 and 42 demonstrated the DPPH-scavenging effect at IC50 44.30 and 103 µM, respectively, while 41, 43, and 44 were inactive at IC50 > 500 µM [42,43]. In short, 40 was a bioactive compound. Compound 43 exhibited moderate immunosuppressive activity of Con A-induced and LPS-induced with IC50 values of 4.5 and 0.7µM, respectively [44] and compounds 43 and 44 showed very weak cytotoxic effects to NCI-H1975/GR cell lines with the inhibition ratio of 50.94% and 56.83%, respectively, at a concentration of 50 μM [45]. A recent study showed that compound 46 revealed inhibitory activity against TMEM16A with 65% inhibition with a concentration of 5 µg/mL) [46]. Another study found seven new isoechinulin-type alkaloids named 16α-hydroxy-17β-methoxy-deoxydihydroisoaustamide (47), 16β-hydroxy-17α-methoxy-deoxydihydroisoaustamide (48), 16α-hydroxy-17α-methoxy-deoxydihydroisoaustamide (49), 16,17-dihydroxy-deoxydihydroisoaustamide (50), 16β,17α-dihydroxy-deoxydihydroisoaustamide (51), 16α,17α-dihydroxy-deoxydihydroisoaustamide (52), and 3β-hydroxy-deoxyisoaustamide (53) isolated from the coral-derived fungus Penicillium dimorphosporum KMM 4689. On these compounds, neuroprotective activity experiments were conducted. The results indicated that compounds 50 and 52 with a concentration of 1 µM could improve the viability of PQ (paraquat)-treated (PQ = 500 µM) Neuro-2a cells by 38.6% and 30.3%, respectively, while compound 51 increased the cell survival rate by 36.5% and 39.4% at concentrations of 1 μM and 10 µM, respectively [47]. Three new indole diketopiperazine alkaloids named 11-methylneoechinulin E (54), variecolorin M (55), and (+)-variecolorin G (56), together with a known compound, (-)-variecolorin G (57), were extracted from a soft coral-associated epiphytic fungus Aspergillus sp. EGF 15-0-3. Compound 57 showed very weak cytotoxic effects to NCI-H1975/GR cell lines with the inhibition ratio of 40.65% at a concentration of 50 μM [45]. Seven notoamide-type alkaloids were obtained from a culture broth of coral-associated fungus Aspergillus ochraceus LZDX-32-15, including four new congeners, namely notoamides W-Z (58–61) and three reported alkaloids, namely notoamide G (62), avrainvillamide (63), and stephacidin B (64), which displayed inhibition against a panel of hepatocellular carcinoma (HCC) cell lines with IC50 values ranging from 0.42 to 3.39 μM. Compared with the positive control of paclitaxel with a concentration of 10 µM, these compounds also revealed notable growth inhibition against tumor cell lines (human HCC cell line SMMC-772, human colorectal carcinoma cell line HCT-8, human breast cancer cell line MCF-7, and human umbilical vein endothelial cell HUVEC) with an inhibition ratio of more than 95% [48]. In addition, compound 63 displayed considerable antiproliferative activity and reversible inhibition against human GR activity in LNCaP cell lysate (IC50 = 125 µM). But dramatically, compounds 63 and 64 were shown to be a new class of toxins with potential threat to human health in buildings [49,50]. A novel compound pseudellone D (65) was obtained from the marine-derived fungus Pseudallescheria ellipsoidea F42-3 collected from the soft coral Lobophytum crissum [51]. Two novel metabolites diketopiperazines pseuboydones C, D (66, 67) and a known compound cyclo-(Phe-Phe) (68) were produced by the marine-derived fungus associated with the soft coral Lobophytum crissum. The compound 68 showed strong activity against cell line Sf9 from the fall armyworm Spodoptera frugiperda (IC50 = 0.8 µM) [20]. A described compound named cyclo-(Pro-Val) (69) was characterized from the fungus Simplicillium sp. SCSIO 41209 gathered from soft coral and exhibited inhibition to MptpB (IC50 = 25.9 μM) [52] (Figure 5).
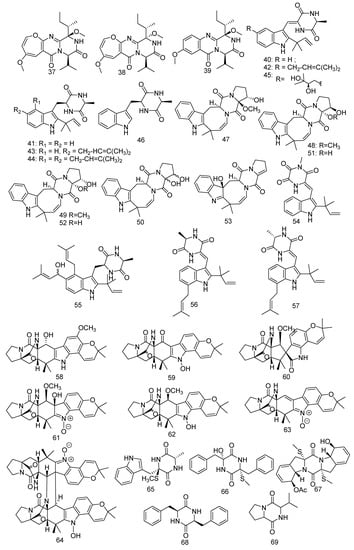
Figure 5.
The structures of compounds 37–69.
3.2. Quinazolinone Alkaloids
Gorgonian-derived fungus Aspergillus versicolor was considered to be a great potential producer of quinazolinone alkaloids. Cheng et al. conducted research on the gorgonian-derived fungus Aspergillus versicolor LZD-14-1 resulting in the identification of four described metabolites and six new polycyclic alkaloids, namely versiquinazolines A, B, G, and K (70–73) [53] and versiquinazolines L–Q (74–79) [54]. Metabolites 76–79 displayed TrxR inhibitory activity with IC50 values of 20, 12, 13, and 13 μM, respectively [53]. Bioassay results showed that 74–79 exhibited weak activity against cell line A549 at IC50 > 10 μM. Moreover, versiquinazolines P (78) and Q (79) also demonstrated significant inhibition against TrxR with IC50 values of 13.6 μM and 12.2 μM, respectively, while curcumin (positive control group) showed an IC50 value of 25 μM [54]. In addition, three new quinazolinone alkaloids, cottoquinazolines B-D (80–82), were produced from Aspergillus versicolor LCJ-5-4. The bioassay test indicated that only 82 revealed middling antifungal activity against Candida albicans (MIC = 22.6 μM) [55]. A previously unreported alkaloid, namely fumiquinazoline K (83) was discovered from a marine strain of Aspergillus fumigatus KMM 4631 [28]. Studies on gorgonian-derived fungus Scopulariopis sp. led to the isolation of fumiquinazoline L (84) which showed weak antibacterial activity against pathogenic bacteria B. subtilis, S. albus, and Vibrio parahemolyticus (MIC = 50 μM) [56]. Two new quinazolinone analogues, namely 4-(7,8-Dihydroxy-4-oxoquinazolin-3(4H)-yl), butanoic acid (85) and 4-(8-Hydroxy-4-oxoquinazolin-3(4H)-yl) butanoic acid (86), were isolated from the fungus Xylaria sp. FM1005, which was gathered from a leather coral Sinularia densa found in the area of the Big Island, Hawaii [57]. Further investigation of Neosartorya laciniosa (KUFC 7896) led to the isolation of a novel tryptoquivaline derivative, tryptoquivaline T (87) [58]. Two new alkaloid racemates, (±)-17- hydroxybrevianamide N (88&89) and (±)-N1-methyl-17-hydroxybrevia-namide (90&91), possessing a peculiar O-hydroxyphenylalanine residue and an imide subunit were obtained from a soft coral-derived Aspergillus sp. [59]. Another two new alkaloids namely pseudellones A (92) and B (93) were produced by the marine-derived fungus Pseudallescheria ellipsoidea F42−3, which was collected from a soft coral Lobophytum crissum [60] (Figure 6).
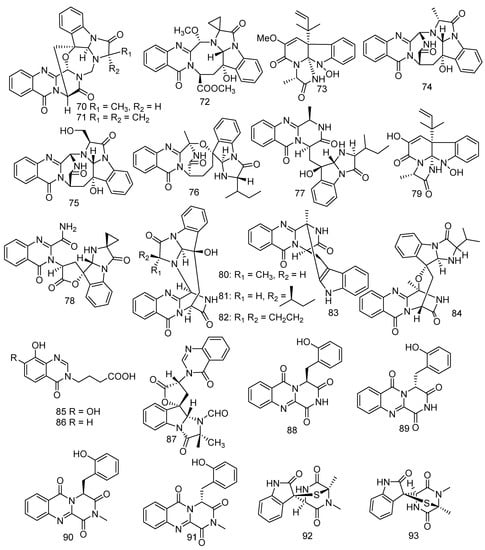
Figure 6.
The structures of compounds 70–93.
3.3. Cytochalasins
Previous chemical exploration on the Chaetomium globosum C2F17 led to the separation of 6-O-methyl-chaetoglobosin Q (94), chaetoglobosin E (95), and chaetoglobosin Fex (96). Furthermore, in an in vitro anticancer experiment, 95 exhibited strong inhibitory activity against cell lines HCT-116, K562, A549, Huh7, H1975, MCF-7, U937, BGC823, HL60, HeLa, and MOLT-4, with IC50 values of 13.5, 8.9, 5.9, 1.4, 9.2, 2.1, 1.4, 8.2, 2.5, 2.8, and 1.4 µM, respectively. Compound 96 revealed selective cytotoxic activity against cell lines Huh7, MCF-7, U937, and MOLT-4, with IC50 values of 3.0, 7.5, 4.9, and 2.9 µM, respectively, and also exhibited anti-inflammatory activity [61,62,63]. Aspergillus elegans ZJ-2008010, isolated from a soft coral Sarcophyton sp. in the South China Sea, produced seven cytochalasins, namely aspochalasin A1 (97), cytochalasin Z24 (98), aspochalasin I (99) aspochalasin J (100), aspochalasin D (101), aspochalasin H (102), and aspergillin PZ (103). According to the experimental data of biological activity assay, 99–102 showed notable antifouling activity to the larval settlement of the barnacle Balanus amphitrite with EC50 values of 34, 14, 6.2, and 37 μM, respectively. Moreover, 101 also presented antibacterial activity against S. albus, S. aureus, Escherichia coli, and B. cereus (MIC = 10 μM), while compound 99 was found to have weak activity against Staphylococcus epidermidis and S. aureus with MIC values of 20 and 10 μM, respectively; and compound 103 as well showed very weak activity against S. epidermidis (MIC = 20 μM). Furthermore, compound 99 possessed potential inhibition to melanogenesis in Mel-Ab cells (IC50 = 22.4 μM) [64]. Moreover, compound 103 displayed DPPH free radical-scavenging effects increasing with concentrations and anticancer activity against the A2780, LNCaP, and PC3 cell lines [65,66]. Another three cytochalasins, chaetoglobosins A and B (104 and 105), and cytoglobosin C (106) were obtained from the fungus Chaetomium globosum RA07-3 [67]. Compounds 104 and 105 exerted outstanding inhibition against Tetragenococcus halophilus with MIC values of 0.7 and 0.4 μM, respectively. Compound 106 also showed antibacterial activity against T. halophilus (MIC = 0.7 μM). Meanwhile, this group also obtained aspochalasin K (107) and aspochalasin E (108) from Aspergillus sp. XS-2009-0B15. In bioassays, 107 revealed antibacterial activity, while 108 showed anticancer activity against B16-F10 and HCT-116 with IC50 values of 18.5 and 6.3 μg/mL, respectively [68,69] (Figure 7).
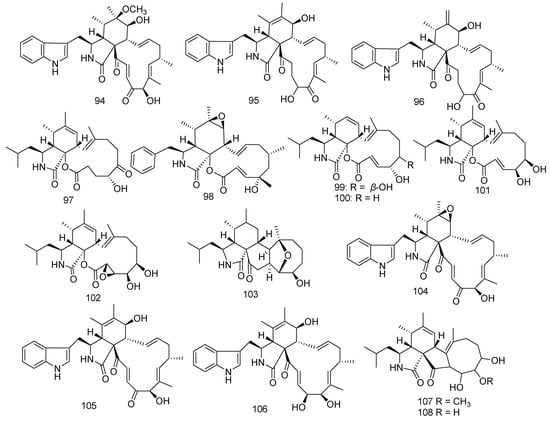
Figure 7.
The structures of compounds 94–108.
3.4. Other Alkaloids
The gorgonian coral-derived fungus Scopulariopsis sp. was regarded as a producer of five dihydroquinolin-2-one-containing alkaloids including three monoterpene alkaloids [70] and a new alkaloid. They were 4-phenyl-3, 4-dihydroquinolin-2(1H)-one named aniduquinolone A (109), aflaquinolone A (110), aflaquinolone D (111), and two 4-phenyl-3,4-dihydroquinolin-2(1H)-one named 6-deoxyaflaquinolone E (112) and aflaquinolone F (113), and a new metabolite named scopuquinolone B (114) [71]. All compounds (109–114) exhibited notable antifouling activity against the settlement of B. amphitrite cyprids with EC50 values of 17.5 pM, and MIC values of 28 nM, 2.8 nM, 1.04 μM, 0.86 μM, and 0.103 μM, respectively. Scopuquinolone B (114) possessed a high therapeutic ratio (LC50/EC50) of 222, which is stronger than the positive control Sea Nine 211 (EC50 = 4.36 μM, LC50/ EC50 = 20). Besides, no cytotoxicity was found in 109 or 110; thus, they could be considered as new nontoxic anti-larval settlement compounds, especially 109 as a potential antifouling lead compound, which possessed safety and a high therapeutic ratio (LC50/EC50 = 1200) in nature. Another study revealed that the epimers of compound 109 exhibited considerable antibacterial activity against S. aureus (ATCC700699) [72]. Compound 110 showed lethality against brine shrimp (Artemia salina, LD50 = 5.5 μM) [73]. Furthermore, 112 revealed broad antibacterial spectrum of S. aureus, B. cereus, Vibrio parahaemolyticus, N. brasiliensis, and P. putida, with MIC values of 0.78, 1.56, 6.25, 0.78, and 1.56 μM, respectively. Five new compounds were found in the associated-coral fungus Xylaria sp. FM1005, namely sinuxylamide A (115), sinuxylamide B (116), sinuxylamide C (117), sinuxylamide D (118), and assinuxylamide E (119). Compounds 115 and 116 also showed significant inhibition against the binding of fibrinogen to purified integrin IIIb/IIa in a dose-dependent manner with IC50 values of 0.89 and 0.61 μM, respectively [57]. A new compound pseuboydone E (120) and three described compounds, namely speradine C (121), 24, 25-dehydro-10,11-dihydro-20-hydro-xyaflavinin (122), and aflavinine (123), were found in a fungus associated to Lobophytum crissum. All known compounds revealed notable inhibition activity against the Sf9 cells with IC50 values of 0.9, 0.5, and 0.4 μM, respectively. Compounds 122 and 123 also showed similar significant cytotoxicity to the positive control, rotenone [20]. Ten alkaloids were produced by coral-derived fungi Aspergillus terreus including eight new alkaloids: aspergillspins A-E (124–128) [74], luteoride E (129) [24], 22-O-(N-Me-L-valyl) aflaquinolone B (130), and 22-O-(N-Me-L-valyl)-21-epi-aflaquinolone B (131) [75], and two known metabolites asperteramide A (132) and methyl 3,4,5-trimethoxy-2-(2-(nicotinamido) benzamido) benzoate (133). In addition, compound 131 revealed excellent anti-respiratory syncytial virus activity (IC50 = 42 nM) [75]. Moreover, compound 132 displayed evident antimicrobial activity against E. coli, Acinetobacter baumannii, P. aeruginosa, Klebsiella pneumonia, MRSA, Enterococcus faecalis, and C. albicans with MIC values of 8, 8, 16, 64, 64, 8, 2 μg/mL, respectively [76]. In addition, luteoride E (129) and methyl 3,4,5-trimethoxy-2-(2-(nicotinamido) benzamido) benzoate (133) exerted anti-inflammatory activity against NO production with IC50 values of 24.64 and 5.48 μM, respectively. Neoaspergillic acid (134), also derived from fungus Aspergillus sp. SCSGAF0093, exhibited bio-toxicity against the brine shrimp (Artemia salina) with an IC50 value of 90.08 µM and also demonstrated anticancer activity against SPC-A-1, BEL-7402, SGC-7901, and K562 with IC50 values of 22.2, 24.9, 8.2, and 8.0 µM, respectively. Moreover, compound 134 possessed antibacterial activity (S. aureus, S. epidermidis, B. subtilis, Bacillus dysenteriae, Bacillus proteus, E. coli) with a MIC of 1.0, 0.5, 1.9, 7.8, 7.8, and 15.6 μg/mL, respectively [77,78]. A new natural product, ethyl 2-bromo-4-chloroquinoline-3- carboxylate (135), was isolated from soft coral-associated fungus Trichoderma harzianum (XS-20090075), which was the first halogenate quinoline metabolite from the genus Trichoderma [79]. An undescribed alkaloid, namely scopulamide (136) was derived from the marine-derived fungus Scopulariopsis sp., which was found in the Red Sea hard coral Stylophora sp. [34]. Pyrophen (137) was identified from the Alternaria alternata strain D2006 associated with soft coral and was shown to be active against C. albicans with the inhibition zone 28 mm [80]. One undescribed phenylspirodrimane derivative, named arthproliferin E (138), was isolated from the soft coral-associated fungus Stachybotrys chartarum SCSIO41201 [81]. (3R,6R)-bassiatin (139) was isolated from the soft coral-derived fungus Dichotomomyces sp. L-8 and displayed significant cytotoxic activities against the human breast cancer cell line MDA-MB-435 and human lung cancer cell line Calu3 with IC50 values of 7.34 and 14.54 µM, respectively [82] (Figure 8).
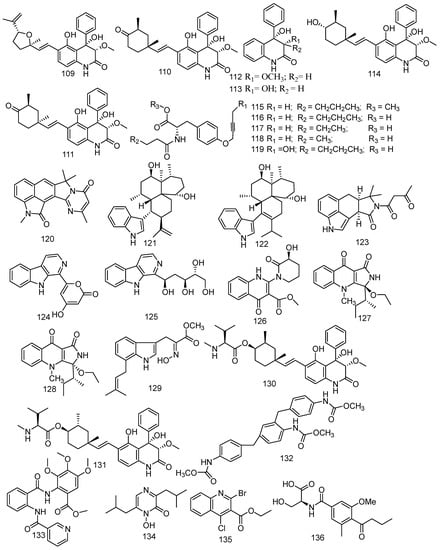
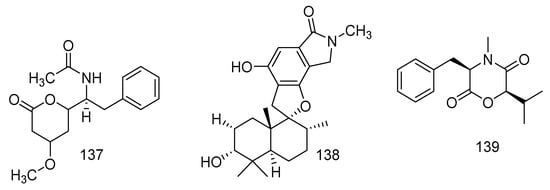
Figure 8.
The structures of compounds 109–139.
4. Peptides and Depsipeptides
4.1. Cyclopeptides
A study reported that cyclopeptides displayed high effects against various cancer cells, and some of them were even applied in clinical experiments [83]. Previous chemical exploration of Aspergillus versicolor led to the separation of 11 new cycloheptapeptides, namely, asperheptatides A−D (140−143) [84], versicoloritides A-C (144–146) [85], versicotides D–F (147–149) [86], and aspersymmetide A (150) [87]. Versicotides D–F (147–149) showed anti-atherosclerosis activity [86]. Aspersymmetide A (150) exerted weak anticancer activity against NCI-H292 and A431 with an inhibition ratio of 53.8% and 63.62% (10 μM), respectively [87]. Penicillium chrysogenum (CHNSCLM-0003), isolated from gorgonian coral, produced seven new cyclohexadepsipeptides, chrysogeamides A–G (151–157). Furthermore, compounds 151 and 152 were shown to have angiogenic activity toward zebrafish embryo (IC50 = 1.0 μg/mL) with a non-cytotoxic concentration of 100 μg/mL [88]. A new compound sinulariapeptide A (158) and three described compounds, simplicilliumtides A (159), B (160) and J (161) were derived from the soft coral-associated fungus Simplicillium sp. SCSIO 41209. In the bioassays, compounds 158–161 showed inhibition to Colletotrichum asianum with the MIC values of 4.9, 19.5, 4.9, and 9.8 μg/mL, respectively, while a positive control (actidione) with a MIC value of 10 μg/mL. Moreover, compound 160 was found to have notable inhibition to Pyricularia oryzae, while 159 and 161 exhibited weak inhibitory activities with the MIC values of 9.8, 19.5, and 78.1 μg/mL, respectively [52]. Scopularide A (162) was obtained from the fungus Scopulariopsis sp. Collected from the Red Sea hard coral Stylophora sp., which revealed remarkable cytotoxicity to the growth of the murine lymphoma cell line (L5178Y) (IC50 = 1.2 µM) [34]. Two new metabolites, asperpeptide A (163) [89] and aspergillipeptide D (164) [90], were isolated from the coral-derived fungus Aspergillus sp. Asperpeptide A (163) displayed antibacterial activity (B. cereus and S. epidermidis) with the same MIC value of 12.5 μM [89], while aspergillipeptide D (164) exhibited considerable antiviral activity against HSV-1 (IC50 = 9.5 μM) [90] (Figure 9).
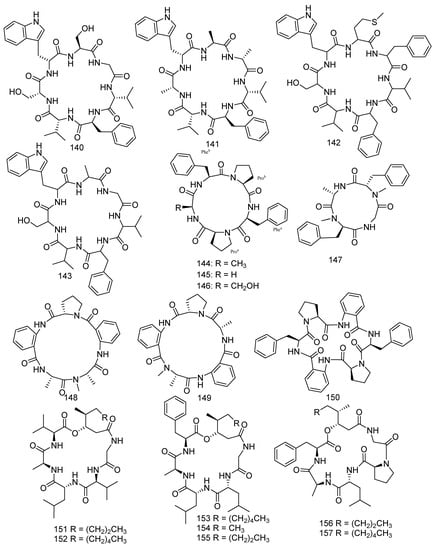
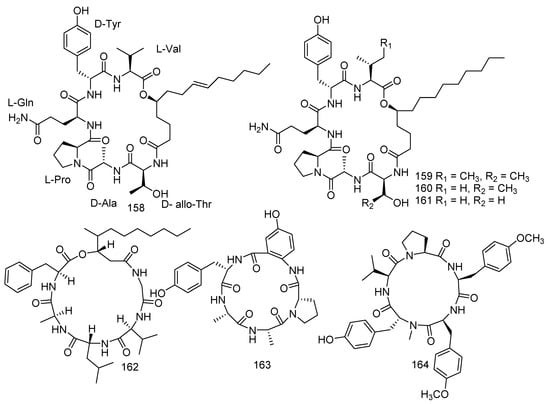
Figure 9.
The structures of compounds 140–164.
4.2. Linear Peptides
Four new compounds named sinulariapeptides B−E (165–168), together with a known metabolite hirsutellic acid A (169), were isolated from a culture broth of the soft coral-derived fungus Simplicillium sp. SCSIO 41209. Hirsutellic acid A (169) showed inhibition to MptpB (IC50 = 35.0 µM) [52]. (+) - and (−) - pestaloxazine A (170, 171), a pair of new enantiomeric alkaloid dimmers, was derived from a Pestalotiopsis sp., which have a new symmetric spiro-(oxazinane-piperazinedione) skeleton [91]. Compound 170 exhibited significant antiviral activity to EV71 (IC50 = 14.2 μM) stronger than the positive control ribavirin (IC50 = 256.1 μM). In addition, a new compound named pseudellone C (172) was found in the fungus Pseudallescheria ellipsoidea F42−3, and 172 was found for the first time in previous literature possessing a unique skeleton [60]. The genus Aspergillus was widely investigated and yielded a variety of metabolites. Thirteen compounds were isolated from coral-derived Aspergillus sp.: namely 4’-OMe-asperphenamate (173), asperphenamate (174), aspergilliamide (175), ochratoxin A n-butyl ester (176), flavacol (177), ochratoxin A (178) and ochratoxin A methyl ester (179), penilumamides B-D (180–182), and aspergillipeptides E–G (183–185). 4’-OMe-asperphenamate (173), a new phenylalanine derivative, along with a known phenylalanine derivative asperphenamate (174), was isolated from Aspergillus elegans ZJ-2008010, which was collected from a soft coral Sarcophyton sp. in the South China Sea. Both of them exhibited selective antibacterial activity against S. epidermidis, with a MIC value of 10 μM for each [65]. A novel and four known metabolites, aspergilliamide (175), ochratoxin A n-butyl ester (176), flavacol (177), ochratoxin A (188), and ochratoxin A methyl ester (189) were obtained from the strain Aspergillus sp. SCSGAF0093. Compounds 175–179 showed bio-toxicity against the brine shrimp (Artemia salina) with LC50 values of 71.09, 4.14, 205.67, 13.74, and 2.59 µM, respectively. Flavacol (177) also displayed inhibition against NADH oxidase (IC50 = 13.0 μM). Meanwhile, ochratoxin A (178) was reported as a potential nephrotoxin and latent human carcinogen [77,92,93]. Aspergillus sp. XS-20090B15 was the source of three new peptides, penilumamides B-D (180–182) [89]. Moreover, Aspergillus sp. SCSIO 41501 also yielded three novel linear peptides, aspergillipeptides E–G (183–185), and compound 183 displayed antiviral activity against HSV-1 (IC50 = 19.8 μM) [90] (Figure 10).
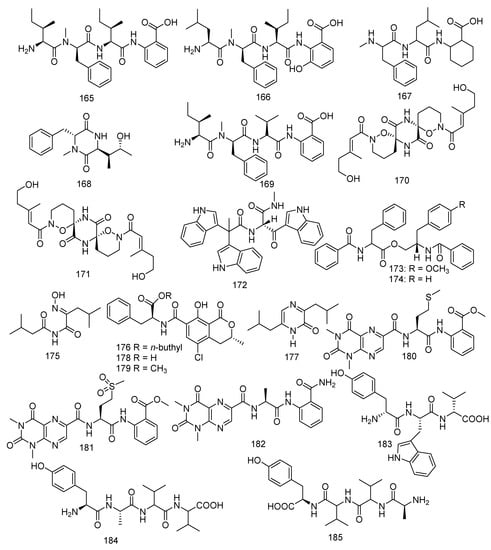
Figure 10.
The structures of compounds 165–185.
5. Aromatics
5.1. Polyketides
The following study is of great significance for some antifouling active compounds. Fourteen new polyketides named libertalides A−N (186–199), along with two known analogues aspermytin A and its acetate (200, 201) were produced by the coral-associated fungus Libertasomyces sp. In the bioassays, 187, 193, and 200 significantly induced the proliferation of CD3+ T cells with a concentration of 3 μM. Meanwhile, 190, 194, 197, and 201 obviously increased the CD4+/CD8+ ratio with 3 μM. It was the first report in the immunoregulatory activity of these metabolites [94]. In addition, aspermytin A (200) was found with activity against neurite outgrowth in rat pheochromocytoma (PC-12) cells (50 μM) [95]. Two new C12 polyketides (202, 203) with four known derivatives (204–207) were produced from a soft coral-derived fungus Cladosporium sp. TZP-29. They were named cladospolides E and F (202 and 203), secopatulolides A and C (204 and 205), 11-hydroxy-γ-dodecalactone (206), and iso-cladospolide B (207). Moreover, 202, 204–206 exerted notable lipid-lowering activity in HepG2 hepatocytes with IC50 values of 12.1, 8.4, 13.1, and 7.1 μM, respectively [96]. Four new polyketides, including an unusual naphthoquinone derivative sclerketide A (208), two azaphilone analogous sclerketides B (209) and C (210), and an α-pyrone derivative sclerketide D (211), together with two known compounds, namely isochromophilone IX (212) and sequoiatone B (213), were isolated from the gorgonian-derived fungus Penicillium sclerotiorum CHNSCLM-0013. Compounds 209–213 exhibited a potential inhibitory effect against the production of NO with the IC50 values of 3.4, 2.7, 5.5, 17.6, and 5.2 μM, respectively. As the most active compound, 210 also showed moderate cytotoxicity (IC50 = 14.8 μM) [97]. Some other new antibacterial chloro-containing polyketides were identified from alga-derived fungus [98]. Furthermore, isochromophilone IX (212) presented outstanding antibacterial activity against MRSA (MIC = 50 μg/mL) [99]. The marine strain Penicillium glabrum glmu003 yielded two novel azaphilone compounds, daldinins G (214) and H (215), and a known compound austalide V (216). Moreover, austalide V (216) exhibited weak inhibitory activity against pancreatic lipase (IC50 = 23.9 μg/mL) [100]. A new asteltoxin B (217) was obtained from the gorgonian-associated fungus Aspergillus sp. SCSGAF 0076 and revealed inhibition to human acetylcholinesterase (IC50 = 14.9 μM) [101,102]. Wang et al. found eight polyketides from gorgonian coral-derived fungus, namely aspergilone A (218), pinophilins D−F (219–221) [103], (+)-sclerotiorin (222), microketides A and B (223 and 224), and epi-pinophilin B (225). A novel compound, aspergilone A (218), was isolated from the fungus Aspergillus sp. and displayed antifouling activity against Balanus amphitrite (EC50 = 25 μg/mL) [104]. Penicillium pinophilum XS-20090E18 produced three new metabolites, pinophilins D−F (219–221). A bioactive azaphilone derivative, (+)-sclerotiorin (222) was also discovered from the Penicillium sclerotiorum fungus and possessed a wide range of bioactivities, which inhibited the larval settlement of barnacle Balanus amphitrite (EC50 = 5.6 μg/mL) and showed evident inhibition to B. subtilis, B. cereus, and Sarcina lutea with MIC values of 0.16, 0.31, and 0.31 μM, respectively [105,106]. A pair of epimeric polyketides, microketides A and B (223 and 224), were isolated from the fungus Microsphaeropsis sp. RA10-14; and 223 exhibited potential antibacterial activity against P. aeruginosa, N. brasiliensis, K. rhizophila, and Bacillus anthraci with the same MIC value of 0.19 μg/mL [107]. A novel metabolite was characterized from the fungus Aspergillus fumigatus 14–27 and identified as epi-pinophilin B (225) by spectroscopic and chemical methods [108]. From soft coral-derived fungus Stachybotrys chartarum SCSIO41201, four new polyketide derivatives, named arthproliferins A–D (226–229), were isolated; and compound 226 was found to show weak inhibitory activity against MRSA ATCC 29213 (MIC = 78 µg/mL) [81]. Polyketide pigment produced by Talaromyces spp. has been reported as a non-toxic red Monascus-like azaphilone pigment. Therefore, polyketides were not only a class of bioactive compounds, but also have been characterized as a source of red pigments [109] (Figure 11).
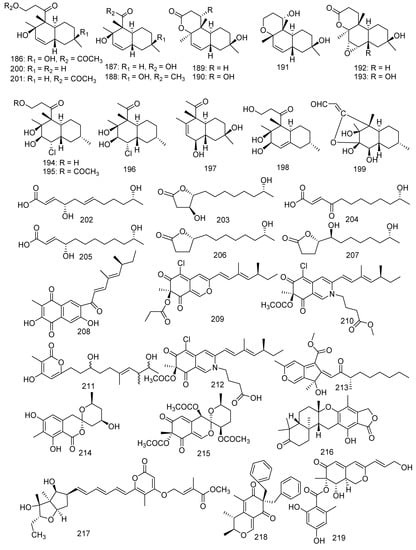
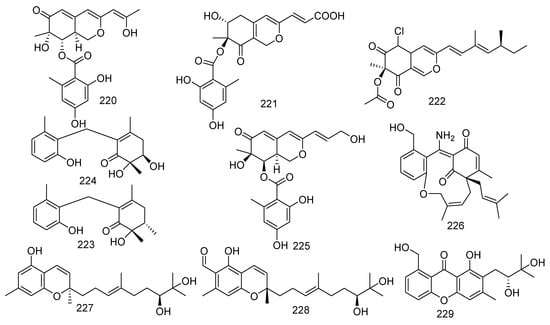
Figure 11.
The structures of compounds 186–229.
5.2. Anthraquinone
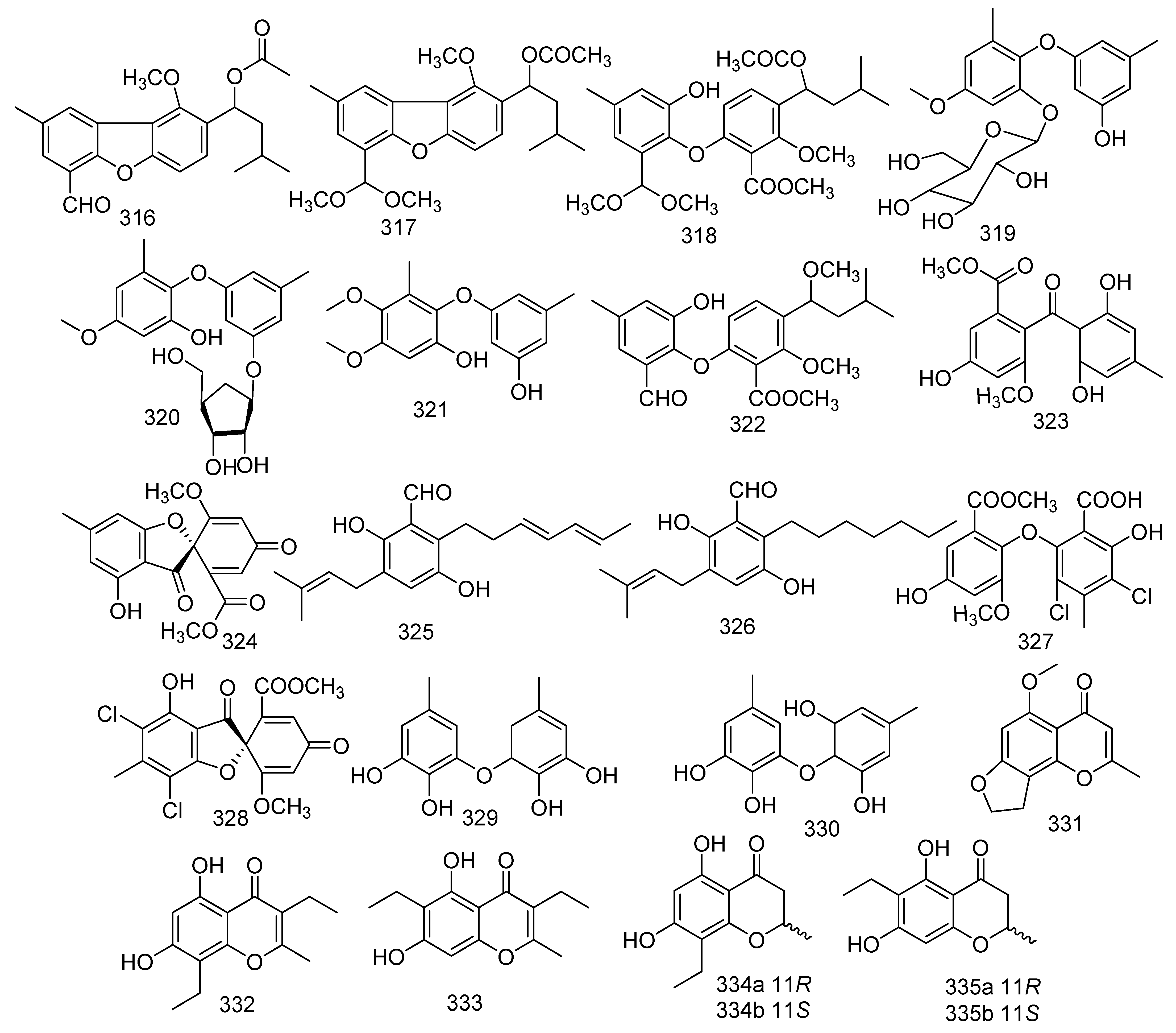
Cultivation of Penicillium sp. SCSGAF 0023 yielded a new polyketide, 6,8,5’6’-tetrahydroxy-30 methyl-flavone (230), and three known analogs emodin (231), citreorosein (232), and isorhodoptilometrin (233). Compounds 230–232 displayed significant antifouling activity against Balanus amphitrite larvae settlement with EC50 values of 6.7, 6.1, 17.9, and 13.7 μg/mL, respectively [110]. A new benzopyranone namely coniochaetone K (234), together with six known compounds, named coniochaetone A (235), 8-hydroxy-6-methylxanthone-1-carboxylic acid (236), methyl 8-hydroxy-6-methyl-9-oxo-9H-xanthene-1- carboxylate (237), methyl 8-hydroxy-6-(hydroxymethyl)-9-oxo-9H-xanthene-1-carboxylate (238), 8-(methoxycarbonyl)-1-hydroxy-9-oxo-9H-xanthene-3-carboxylic acid (239), and 3,8-dihydroxy-6-methyl-9-oxo-9H-xanthene-1-carboxylate (240), were obtained from the Beibu Gulf-derived coral symbiotic fungus Cladosporium halotolerans GXIMD 02502. Moreover, all compounds 234–240 at a concentration of 10 μM revealed outstanding cytotoxicity against two human prostatic cancer cell lines, C4-2B and 22RV1, with an inhibitory rate ranging from 55.8% to 82.1%. Among them, compound 240 was found to be the strongest with inhibitions of 82.1% and 77.7%, respectively [111]. In addition, compound 240 also showed activity against cell lines K562, HL-60, HeLa, and BGC-823 at a concentration of 100 μg/mL with an inhibition rate of 36.1%, 62.4%, 13.9%, and 11.4%, respectively [112]. Three metabolites were identified as 12-dimethoxypinselin (241), 12-O-acetyl-AGI-B4 (242), and AGI-B4 (243); from solid rice cultures of the marine-derived fungus Scopulariopsis sp., which was collected from the Red Sea hard coral Stylophora sp.. Compound 243 showed significant cytotoxicity against L5178Y mouse lymphoma cells (IC50 = 1.5 µM) [34]. A new anthraquinone derivative macrosporin 2-O-α-D-glucopyranoside (244), together with two known analogues altersolanol B (245) and altersolanol A (246), were obtained from the fungus Stemphylium lycopersici. Compound 245 exhibited evident activities against HCT-116 and MCF-7 cancer cell lines, with the IC50 values of 3.5 and 9.0 μM, respectively, while 246 showed IC50 values of 1.3 and 7.2 μM, respectively. Moreover, compound 246 also exhibited growth inhibition against Huh7 cancer stem cell-like cells (IC50 = 38.0 μM) [113]. In addition, compound 246 exhibited cytotoxic, cytostatic, anti-inflammatory, and anti-migrative activity against K562 and A549, surprisingly, while it had no effect on normal cells [114]. Wang et al. isolated 20 metabolites from coral-associated fungus. A number of new compounds were derived from the mycelia of the Alternaria sp. ZJ-2008003 strain isolated from a Sarcophyton sp. soft coral in South China Sea, including tetrahydroaltersolanols C−F (247–250) and dihydroaltersolanol A (251). The results of the antiviral activity indicated that compound 247 exhibited activity against PRRSV (IC50 = 65 μM) [115]. Investigation of the Penicillium chrysogenum derived from gorgonian resulted in the isolation of a new flavone, penimethavone A (252), and exhibited middling anticancer activity against HeLa and rhabdomyosarcoma cell lines with IC50 values of 8.41 and 8.18 μM, respectively [116]. Aspergillus candidus derived from the gorgonian coral Anthogorgia ochracea collected from the South China Sea produced a new compound, aspergivone B (253), which showed weak inhibition against α-glucosidase (IC50 = 244 μg/mL) [117]. Two new hydroxyanthraquinones, harzianumnones A (254) and B (255), together with seven known analogues (256–262), namely pachybasin (256), chrysophanol (257), frangulaemodin (258), phomarin (259), (+)-20S-isorhodoptilometrin (260), 1-hydroxy-3-hydroxymethylanthraquinone (261), and Ω-hydroxydigitoemodin (262) were discovered from the soft coral-associated fungus Trichoderma harzianum (XS-20090075). Compounds 256, 257, 258, 260, and 262 displayed moderate AChE inhibitory effects at the concentration of 100 μM. Compounds 258, 260, and 261 showed weak antibacterial activity against S. aureus with the MIC values of 6.25, 25.0, and 25.0 μM, respectively. Compounds 260 and 261 exhibited cytotoxicity against hepatoma cell line HepG2 with IC50 values of 2.10 and 9.39 μM, respectively. Compound 260 was still found to reveal cytotoxicity against cervical cancer cell line HeLa (IC50 = 8.59 μM) [118]. For use as a bio-agricultural agent with antifungal activity against phytopathogenic fungi, pachybasin (256) induced toxicity in zebrafish embryos in a dose-dependent manner. In addition, chrysophanol (257) displayed activity against human malignancy of colorectal cancer [119,120]. Three described analogues and a new anthraquinone derivative, named nidurufin (263), 8-O-methylaverufin (264), averufanin (265), and 8-O-methylnidurufin (266), were produced by fungus Aspergillus sp. derived from the gorgonian coral Dichotella gemmacea. Furthermore, nidurufin (263) exhibited excellent inhibition against K562 and HL-60 cell lines with IC50 values of 0.87 and 1.46 µM, respectively. Meanwhile, 8-O-methylnidurufin (266) and 8-O-methylaverufin (264) showed antibacterial activity against Micrococcus luteus with the same MIC values of 6.25 µM. Moreover, 264 was also active against Mucor miehei and 265 exhibited inhibitory effects on ACAT1 and ACAT2 with IC50 values of 28 and 12.1 µM, respectively [121,122,123] (Figure 12).
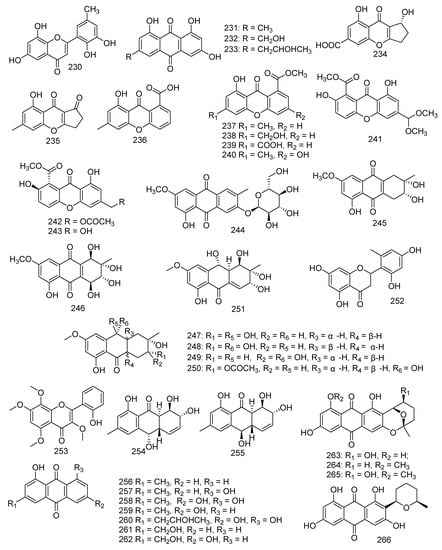
Figure 12.
The structures of compounds 230–266.
From a Penicillium sp. SCSGAF 0023, a new compound paecilin C (267) and three known compounds, secalonic acid D (268), secalonic acid B (269), and penicillixanthone A (270), were obtained; and compounds 268–270 found to show medium antibacterial activity against M. luteus, Pseudomonas nigrifaciens, E. coli, and B. subtilis with MIC values ranging from 24.4 to 390.5 µg/mL. In addition, compound 268 at a concentration of 3.125 µg/mL also displayed inhibition to the growth of Serratia onedensis MR-1 [110]. Furthermore, 268 displayed anti-tumor activity against the K562 cell line and cytotoxic activity on the human pancreatic carcinoma PANC-1 cells accustomed to glucose-starved conditions (IC50 = 0.6 µM). In addition, compounds 268 and 269 revealed inhibition against S. aureus biofilm formation at more than 90% at 6.25 µg/mL, while 268 facilitated the development of biofilm to some extent [124,125,126]. The following seven new metabolites were found from coral-associated fungus. A new anthraquinone derivative, alterporriol Y (271), was obtained from the fungus Stemphylium lycopersici [113]. Alternaria sp. ZJ-2008003 yielded five novel alterporriol-type anthranoid dimers named alterporriols N−R (272–276) [115]. Besides producing polyketides, Aspergillus sp. collected from a gorgonian Dichotella gemmacea also yielded a novel compound, aspergilone B (277) [104]. The data showed that compound 274 exhibited cytotoxic activity for PC-3 and HCT-116 cell lines with the IC50 values of 6.4 and 8.6 μM, respectively, and compound 275 also showed activity against PRRSV (IC50 =22 μM) (Figure 13).
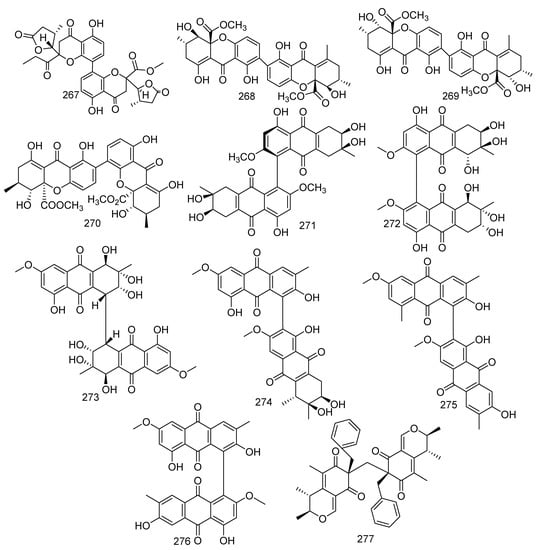
Figure 13.
The structures of compounds 267–277.
5.3. Other Aromatic Compounds
A study on fungus Pestalotiopsis sp. led to the separation of four compounds, pestalone (278) and pestalachloride B (279), (±) – pestalachloride C (280), and (±) – pestalachloride D (281). Pestalone (278) displayed activities against MRSA, 30740, 31007, 31692, 31709, 31956, Bacillus megaterium, and Micrococcus lysodeikticus with MIC values of 12.5, 6.25, 12.5, 12.5, 12.5, 0.078, and 6.25 μM, respectively, while pestalachloride B (279) displayed moderate activities against S. aureus [127]. Recent biological tests indicated that 278 exhibited moderate cytotoxicity in the National Cancer Institute’s 60 human cell line and inhibited MRSA (MIC = 37 ng/mL) [128,129]. (±) - Pestalachloride D (281), a new chlorinated benzophenone derivative, along with a related analog (±) – pestalachloride C (280), was active against E. coli, Vibrio anguillarum, and V. parahaemolyticus with the MIC values of 5, 10, and 20 μM, respectively. But in the biological activity results of zebrafish, indicated compound 281 did not display any effect on a zebrafish embryo teratogenicity assay, while 280 shows abnormal growth effects [130]. Aspergillus candidus cultured from the gorgonian coral Anthogorgia ochracea collected from the South China Sea produced a novel metabolite, aspergivone A (282) [117]. A novel skeleton structure compound represents a class of perylene derivatives with a partially perylene quinone. They were named 7-epi-8-hydroxyaltertoxin I (283), stemphytriol (284), altertoxin I (285), stemphyltoxin II (286), and stemphyperylenol (287). These five perylene derivatives were found from the marine-derived fungi Alternaria sp. ZJ-2008017 collected from a soft coral Sarcophyton sp. Compounds 283–286 were obtained from bioactive experiments of the teratogenicity and lethality of zebrafish embryo and antifouling activity. The bioassay results indicated that 285 exhibited notable teratogenicity (LC50 = 4.54 μg/mL) and lethality (EC50 = 4.21 μg/mL) of zebrafish embryo and had potent antifouling activity against the barnacle Balanus amphitrite (IC50 = 0.27 μg/mL). It might be a potential antifouling agent [131]. In addition, altertoxin I (285) could cause DNA damage, while stemphyperylenol (287) was found to be active against E. coli, S. aureus, and multi-drug-resistant P. aeruginosa [132,133,134]. Naphthalenedicarboxylic acid (288), a novel compound, was isolated from the fungus Xylaria sp. FM1005, discovered from Sinularia densa [57]. The soft-coral-derived fungus Alternaria alternata L3111′A was the source of a new perylenequinone-related compound, alternatone A (289), and a known perylenequinone named alterperylenol (290). Alternatone A (289) displayed anticancer activity against the human hepatoma carcinoma HepG-2 cell line. In addition, alterperylenol (290) exhibited cytotoxicity against A-549, HCT-116, and HeLa cell lines with IC50 values of 2.6, 2.4, and 3.1 μM, respectively [135,136]. Four new oligophenalenone dimers were yielded from the soft coral-associated fungus Talaromyces verruculosus named verruculosins A–B (291, 292), bacillisporin F (293), and xenoclauxin (294). Among which, compounds 291, 293, and 294 displayed middle inhibitory activity against CDC25B with IC50 values of 0.38, 0.40, and 0.26 µM, respectively. Furthermore, bacillisporin F (293) also exerted antibacterial activity against S. aureus with a MIC value of 15.6 µg/mL [137,138] (Figure 14).
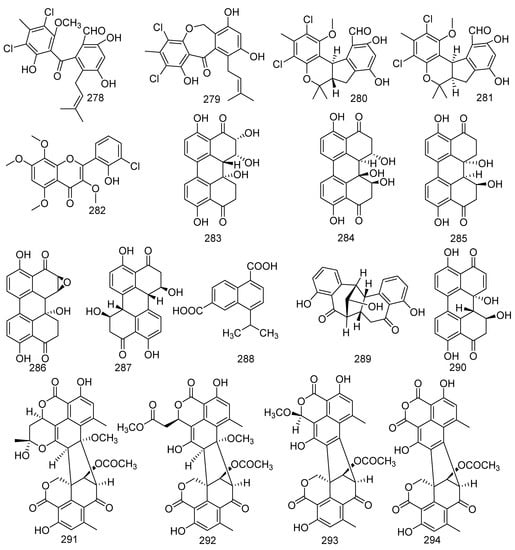
Figure 14.
The structures of compounds 278–294.
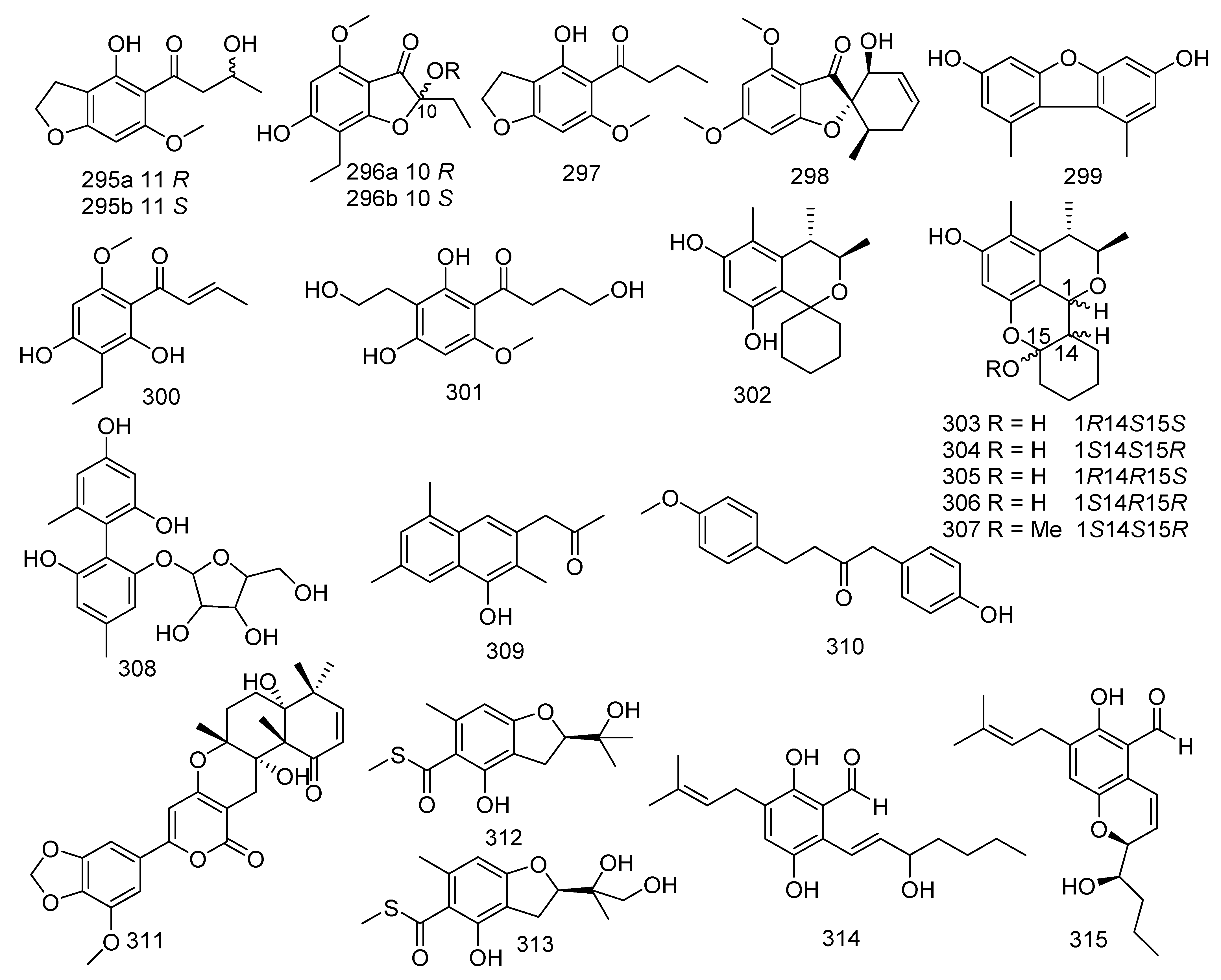
Two new chromanones named phomalichenones H, J (295, 296) and a described compound phomalichenone D (297) were obtained from the coral-associated fungus Parengyodontium album SCSIO 40430. Compound 297 revealed inhibition against MRSA shhs-A1, with MIC values of 32–64 μg/mL [139]. A new griseofulvin derivative, eupenigriseofulvin (298), was yielded from the EtOAc extract of Eupenicillium sp. SCSIO41208 [140]. 3,7-dihydroxy-1,9-dimethyl dibenzofuran (299) was obtained from a soft coral-derived fungus Talaromyces sp. SCSIO 041201. The biological assay indicated that compound 299 displayed antifouling activity against Bugula neritina larva with a LC50 value of 3.06 μg/mL; and it showed moderate antibacterial activities against E. coli, MRSA, S. aureus, and E. faecalis, with MIC values ranging from 0.45 to 15.6 μg/mL, respectively [141]. Two known analogues named phomalichenone A (300) and phomalichenone B (301) were isolated from the coral-derived fungus Parengyodontium album sp. SCSIO 40430, and both of them showed inhibitory activity against MRSA shhs-A1 and Mycobacterium tuberculosis H37Ra with MIC values of 64, 64, 16, and 64 μg/mL, respectively [139]. Six new citrinin analogues penicitols A (302), E−I (303–307), were isolated from a coral-associated fungus Penicillium citrinum, of which 302 exhibited cytotoxic activity against K562, A549, HL-60, BEL-7402, MCE-7, HCT-116, and MDA-MB-231 tumor cells, with IC50 values of 8.8, 28, 20, 12, 15, 24, and 26 μM, respectively. Moreover, 307 showed cytotoxic activity against A549 and BEL-7402, with IC50 values of 19 and 17 μM, respectively. Compounds 302 and 307 displayed antibacterial activity against S. aureus, B. subtilis, and Vancomycin-resistant E. faecalis 1010798 (VRE), with MIC values of 16, 32, 32 and 64, 32, 32 μM, respectively [142]. A new polyphenol, talaversatili A (308), was isolated from a soft coral-derived fungus Talaromyces sp. SCSIO 041201 [141]. The strain Dichotomomyces sp. L-8 associated with the soft coral Lobophytum crissum produced two new compounds, dichotones A (309) and B (310) [82]. Territrem A (311) was isolated and identified from a coral-derived fungus Aspergillus terreus. Compound 311 was active against NO production with significant inhibitory potency (IC50 =29.34 μM) [24]. Two new sulfur-containing benzofuran derivatives, eurothiocins A (312) and B (313), were produced by the fungus Eurotium rubrum SH-823, which was gathered from a Sarcophyton sp. soft coral collected in the South China Sea. Compounds 312 and 313 showed strong inhibitory effects against α-glucosidase with IC50 values of 17.1 and 42.6 μM, respectively. A recent study showed that 312 displayed anti-neuroinflammatory effects [143,144]. Shao et al. found eight novel metabolites and seven known compounds from coral-derived fungi, which were identified as 3′-OH-tetrahydro-auroglaucin (314) and (3′S*,4′R*)-6-(3′,5-epoxy-4′-hydroxy-1′-heptenyl)-2-hydroxy-3-(3′′-methyl-2′′-butenyl) benzaldehyde (315), talaromycins A–C (316–318), phomaethers A–C (319–321); and tenellic acid A methyl ester (322), sulochrin (323), (–)-bis-dechlorogeodin (324), isodihydroauroglaucin (325), flavoglaucin (326), 2,4-diphenyldichloroasterric acid (327), and geodin (328), respectively. Two novel metabolites, 3′-OH-tetrahydro-auroglaucin (314) and (3′S*,4′R*)-6-(3′,5-epoxy-4′-hydroxy-1′-heptenyl)-2-hydroxy-3-(3′′-methyl-2′′-butenyl) benzaldehyde (315) were gathered from a fungus Eurotium sp. [145]. Three novel diphenyl ether derivatives, talaromycins A–C (316–318), together with a known metabolite tenellic acid A methyl ester (322), were obtained from fungus Talaromyces sp. In addition, compound 318 exerted inhibition against the larval settlement of the barnacle Balanus amphitrite with an EC50 value of 2.8 μg/mL. Furthermore, compound 322 showed outstanding anticancer activity against HepG2, Hep3B, MCF-7/ADR, PC-3, and HCT-116 with IC50 values of 4.3, 9, 8.2, and 9.8 μM, respectively. [146]. Another three new compounds, phomaethers A–C (319–321) were obtained from a gorgonian-derived fungus Phoma sp. (TA07-1), while compounds 319 and 321 exhibited evident antibacterial activity against five pathogenic bacteria (S. albus, S. aureus, E. coli, V. parahaemolyticus, V. anguillarum) with MIC values ranging from 0.312 to 10 μM [147]. Examination of the fungus Aspergillus sp. associated with coral led to the separation of six known compounds. Both sulochrin (323) and (–)-bis-dechlorogeodin (324) isolated from Aspergillus sp. derived from soft coral were active against V. anguillarum, Aeromonas salmonicida, and P. aeruginosa with MICs of 15.06, 15.15, 7.53 μM and 30.12, 30.3, and 3.78 μM, respectively. Moreover, 324 also revealed moderate activity against Jurkat, A549, and HeLa cells with IC50 values of 10.69, 10.69, and 3.56 μM, respectively [148]. Two known benzaldehyde compounds, isodihydroauroglaucin (325) and flavoglaucin (326), exhibited evident antiviral activity against HSV-1 with EC50 values of 4.73 and 6.95 μM, respectively. In addition, flavoglaucin (326) displayed considerable activity against DPPH (IC50 = 11.3 μM) [149,150]. Two known derivatives were isolated from soft coral Sinularia sp. derived fungus Aspergillus sp., namely 2,4-diphenyldichloroasterric acid (327) and geodin (328). In the bioassays, compound 327 displayed inhibition to S. aureus (MIC = 12.5 μM) and 328 exhibited cytotoxic activity against six human cancer cell lines, including BT474, NCI-H460, H-1975, K562, DU145, and A549, with IC50 values of 8.88, 9.22, 9.96, 11.14, 14.44, and 11.05 μM, respectively. Meanwhile, geodin (328) was also considered as a potential semisynthesized compound for the purpose of producing derivatives with better insecticidal activities [151,152]. The fungus Scopulariopsis sp. obtained from the Red Sea hard coral Stylophora sp. resulted in the discovery of two phenyl ethers violaceols I and II (329 and 330). Moreover, compounds 329 and 330 showed remarkable cytotoxicity toward the growth of L5178Y with IC50 values of 9.5 and 9.2 µM, respectively, while the positive control was 4.5 µM (kahalalide F). In addition, both of them displayed antimicrobial activities against Staphylococcus saprophyticus, S. aureus, MRSA, B. subtilis, B. cereus, Salmonella typhimurium, Shigella sonnei, and C. albicans (MIC < 9.765–312.5 μg/mL); and they were also found to act as actin inhibitors inducing cell shape elongation in fibroblast cells [34,153,154]. The chemical investigation of Parengyodontium album SCSIO 40430 led to three new chromanones, phomalichenones K-M (331–333) and two known metabolites, 8-ethyl-5,7-dihydroxy-2-methylchroman-4-one (334), and (±)-trieusol D (335). Compounds 334 and 335 showed inhibition against MRSA shhs-A1, with the same MIC value of 64 μg/mL, and they were also active against M. tuberculosis H37Ra with MIC values of 32 and 64 μg/mL, respectively [139] (Figure 15).
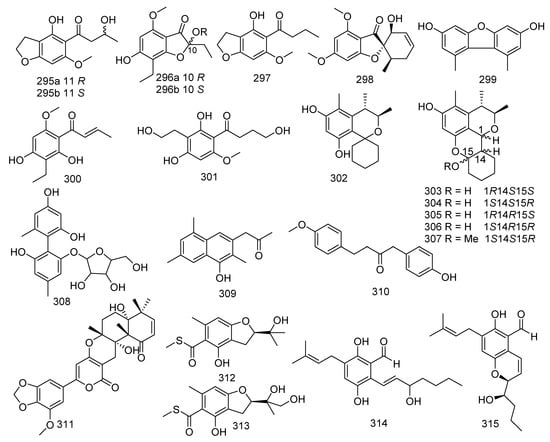
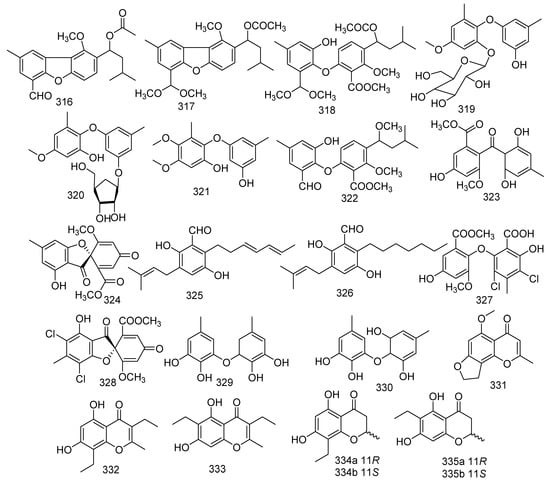
Figure 15.
The structures of compounds 295–335.
6. Lactones
A known ten-membered macrolide named (3Z,5S,6E,8S,9S,10R)-8-chloro-5,8,9,10-tetrahydro-5,9-dihydroxy-10-methyl-2H-oxecin-2-one (336) was obtained from a soft coral-derived fungus Lophiostoma sp. ZJ-2008011 [36]. The bioassay test showed that 336 exhibited antibacterial activities against B. cereus (MIC = 3.12 μM). Aspergillus versicolor LCJ-5-4 yielded a new lactone, marked as versicolactone A (337) [85]. A recent study indicated that sorbicillinoid derivatives from marine-derived fungus also possessed radical scavenging activities. In the last four years, 69 new sorbicillinoids were identified from fungi [155,156]. Dimethyl incisterol A3 (338) and (l7R)-17-methylincisterol (339) were obtained from a soft coral-derived fungus Talaromyces sp. SCSIO 041201. Both 338 and 339 showed antifouling activities to Bugula neritina larva, with LC50 values of 3.13 and 4.15 μg/mL, respectively [141]. Four new butanolide derivatives 8’’R,9’’-diolversicolactone B (340), 8’’S,9’’-diolversicolactone (341), 3’-isoamylene butyrolactone IV (342), and 4’-dehydroxy aspernolide A (343), together with two described compounds named butyrolactone I (344) and versicolactone B (345), were produced by a coral-associated fungus Aspergillus terreus. They were tested for the inhibition of NO production in RAW264.7 mouse macrophages induced by LPS at a concentration of 20 μM (positive control group indomethacin of 50 μM). The inhibitory effect of compound 345 was significantly stronger than that of indomethacin. In addition, compounds 342 and 344 also displayed a modest inhibitory effect on NO production with inhibition ratios of nearly 25.1% and 25.3%, respectively [157]. A new compound named versicolactone G (346) was isolated from a coral-associated fungus Aspergillus terreus, which showed remarkable anti-inflammatory activity against NO production (IC50 = 15.72 μM). Other studies indicated that butyrolactone I (344) presented with antitumor activity against HL-60 with an IC50 value of 13.22 μM and ameliorated AlCl3-induced cognitive deficits in zebrafish in a dose-dependent manner [24,158,159]. (5S,6S)-dihydroxylasiodiplodin (347) was produced by the fungus Pseudallescheria ellipsoidea F42-3 derived from the soft coral Lobophytum crissum [51]. Four described metabolites, 2-O-Methylbutyrolactone I (348), 2-O-Methylbutyrolactone II (349), demethoxycarbonylbutyrolactone II (350), and butyrolactone III (351), were obtained from fungus Aspergillus sp. Compounds 349–351 showed inhibition against the settlement of barnacle Balanus amphitrite with EC50 values of 2.10, 4.25, and 2.89 µg/mL, respectively. Furthermore, compounds 348 and 349 as well exhibited outstanding antibacterial activities toward S. aureus, S. epidermidis, and B. cereus, V. parahaemolyticus, and V. anguillarum with MIC values ranging from 1.56 to 12.5 µM [160]. Three novel metabolites, cochliomycins A-C (352–354), along with six known analogues, diacetyl derivative (355), monoacetyl derivative (356), zeaenol (357), LL-Z1640-1 (358), LL-Z1640-2 (359), and paecilomycin F (360), were isolated from the fungus Cochliobolus lunatus from the gorgonian Dichotella gemmacea. In addition, compounds 353, 355–358, and 360 exhibited antifouling activity against the larval settlement of the barnacle Balanus amphitrite with EC50 values of 1.2, 15.4, 12.5, 5.0, 5.3, and 17.9 µg/mL, respectively. Moreover, compound 359 showed inhibition to HgCl2-induced JNK phosphorylation at 5–100 ng/mL [161,162]. The chemical exploration of Aspergillus sp. SCSGAF 0076 led to the isolation of three novel compounds, aspergillides A-C (361–363). Furthermore, compound 363 exerted considerable antifouling activity against Bugula neritina larvae settlement with LC50/EC50 > 25 [101,163,164]. A known metabolite satratoxin F (364) was discovered from the soft coral-derived fungus Stachybotrys chartarum SCSIO41201, and 364 showed weak inhibitory activity against MRSA ATCC 29213 with the MIC value of 39 µg/mL. Furthermore, compound 364 displayed excellent cytotoxic activities against five human cancer cell lines (MDA-MB-231, C4-2B, MGC803, MDA-MB-468, and A549) with IC50 values less than 39 nM [81]. Five new mycophenolic acid derivatives, 6-(5-carboxy-3-methylpent-2-enyl)-7-hydroxy-3,5-dimethoxy-4-methylphthalan-1-one (365), 6-(5-methoxycarbonyl-3-methylpent-2-enyl)-3,7-dihydroxy-5-methoxy-4-methylphthalan-1-one (366), 6-(3-carboxybutyl)-7-hydroxy-5-methoxy-4-methylphthalan-1-one (367), 6-(5-(2,3-dihydroxy-1-carboxyglyceride)-3-methylpent-2-enyl)-7-hydroxy-5-methoxy-4-methylphthalan-1-one (368), and 6-(5-(1-carboxy-4-N-carboxylate)-3-methylpent-2-enyl)-7-hydroxy-5-methoxy-4-methylphthalan-1-one (369); and five already reported metabolites 8-O-methyl mycophenolic acid (370), 3-hydroxymycophenolic acid (371), N-mycophenoyl-L-valine (372), N-mycophenoyl-L-phenyloalanine (373), and N-mycophenoyl-L-alanine (374) were obtained from the coral-derived fungus Penicillium bialowiezense. Moreover, compounds 365–374 were tested for immune inhibitory activity; and compounds 372, 372 and 366 showed significant inhibition to IMPDH2 with IC50 values ranging from 0.84 to 0.95 µM; while 366–369 and 372–374 revealed notable inhibitory potency with IC50 values ranging from 3.27 to 24.68 µM [165]. A new azaphthalide derivative, (S)-3-hydroxy-2,7-dimethylfuro [3,4-b] pyridin-5(7H)-one (375), and a new phthalide derivative, (S)-7-hydroxy-3-((S)-1-hydroxyethyl) isobenzofuran-1-(3H)-one (376), together with two known compounds (R)-3-hydroxymellein (377) [166] and (3R,4S)-trans-4-hydroxymellein (378) [167], were obtained from the coral-associated fungus Aspergillus sp. SCSIO41405. The isolated compounds were tested for antibacterial and enzyme inhibition activities. Compound 377 revealed weak antibacterial activity against methicillin-resistant Staphylococcus epidermidis, and 378 also exhibited antibacterial activity against E. faecalis, with the same MIC value of 100 μg/mL [168]. Ten novel enantiomers (±)-eurotiumides A-E (379–383) were isolated from a gorgonian-derived fungus Eurotium sp. XS-200900E6. In the bioassays, (+)- and (-)-eurotiumides B (380) and D (381) displayed antifouling activities against the larval settlement of the barnacle Balanus amphitrite with the EC50 values ranging from 0.7 to 2.3 μg/mL, and compounds 379–382 also showed potent antibacterial activities [169]. A novel scopupyrone (384) was produced by the solid rice cultures of the marine-derived fungus Scopulariopsis sp. collected from the Red Sea hard coral Stylophora sp. [34] (Figure 16).
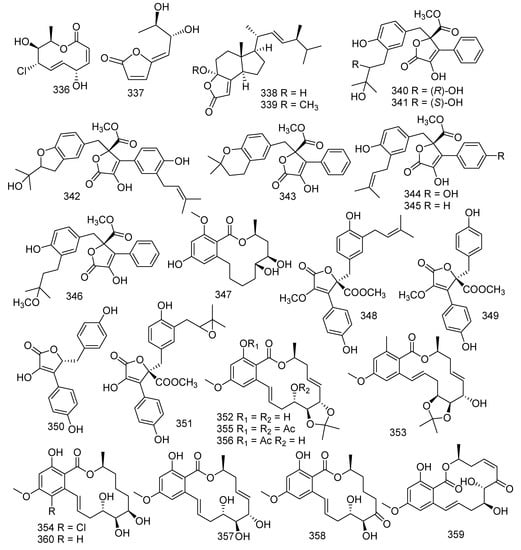
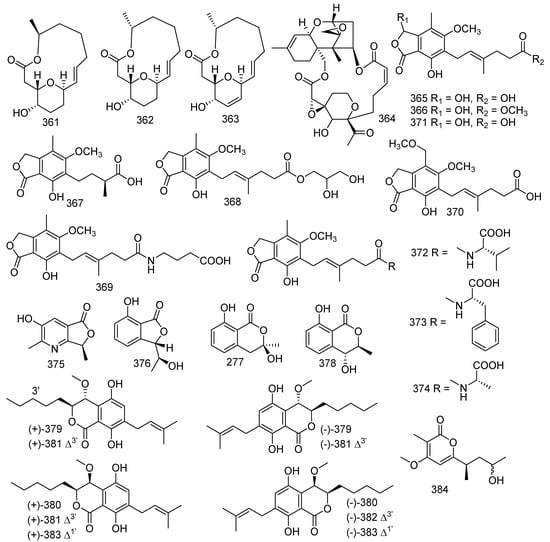
Figure 16.
The structures of compounds 336–384.
7. Steroids
Four new compounds, arthriniumsteroids A−D (385–388), together with a known compound named eoaspergillic acid (389), were obtained from the soft coral-derived fungus Simplicillium lanosoniveum SCSIO41212. All compounds displayed weak inhibitory activities against LPS-induced NO production in RAW 264.7 cells with inhibitory rates ranging from 21.4%−44.6% [170]. Four new steroid derivatives, penicildiones A−D (390–393), together with a described compound, stachybotrylactone B (394), were given by soft coral-derived fungus Penicillium sp. SCSIO41201. Moreover, compound 394 showed evident cytotoxic activity against HL-60, K562, MOLT-4, ACHN, 786-O, and OS-RC-2 cell lines with IC50 values of 5.23, 4.12, 4.31, 23.55, 7.65, and 10.81 μM, respectively [171]. Further investigation of coral-derived fungus Aspergillus sp. led to separation of three known compounds, melilotigenin C (395) [168], 14α-hydroxyergosta-4,7,22-triene-3,6-dione (396) [24], chaxine C (397), and a novel compound, 24,28-didehydro-2 (398) [160]. Melilotigenin C (395) revealed moderate inhibition to pancreatic lipase (IC50 = 15.6 μg/mL) [168]. 396 exhibited outstanding anti-inflammatory activity against NO production (IC50 = 26.83 μM) [24]. In addition, both 397 and 398 showed activities against the larval settlement of barnacle Balanus amphitrite with EC50 values of 2.50 and 18.40 μg/mL, respectively [160]. 5α,8α-epidioxy-ergosta-6,22E-dien-3β-ol (399) was yielded by the gorgonian-derived fungus Xylaria sp. C-2 and displayed bioactivity against E. coli, P. putida, and K. rhizophila with MIC values of 3.13, 1.56, and 6.25 μM, respectively [172]. A novel pregnane, 3α-hydroxy-7-ene-6,20-dione (400) was found from a fungus Cladosporium sp. WZ-2008-0042 and showed significant antiviral activity against RSV (IC50 = 0.12 μM) [173] (Figure 17).
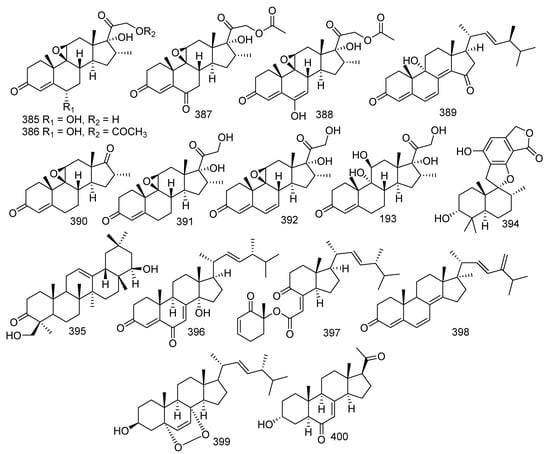
Figure 17.
The structures of compounds 385–400.
8. Other Compounds
Research on the soft coral-derived fungus Trichoderma harzianum (XS-20090075) led to the isolation of two new natural products, methyl-trichoharzin (401) and trichoharzin B (402), and two known compounds, eujavanicol A (403) and nafuredin (404). Compounds 401, 403, and 404 displayed antifouling activity against larval settlement of Bugula neritina with EC50 values of 29.8, 35.6, and 21.4 μg/mL respectively. Another study showed that eujavanicol A (403) revealed bioactivity against E. coli with a MIC value of 5.0 μg/mL [79,174]. A polysaccharide named AW1 (405) was isolated from the Aspergillus ochraceus collected from coral Dichotella gemmacea. AW1(405) was relatively rarely found in the marine metabolites. AW1 (405) is an extracellular polysaccharide that possesses a novel galactomannan and the molecular weight was 29 kDa [175]. Three new compounds (406–408), together with four known analogues named pseurotin A (409), AM6898B (410), and (−)-ovalicin derivative (411), chlovalicin (412), were obtained from the fungus Pseudallescheria boydii collected from the South China Sea soft coral Sinularia sandensis. Compound 409 increased the number of osteoclasts at 0.1 μM, while compound 407 decreased the numbers of osteoclasts at 1 μM; and compounds 410 and 411 increased the number of osteoclasts at both 0.1 and 1 μM. In contrast, compound 412 significantly decreased the number and reduced the area of the osteoclasts at both 0.1 and 1 μM. Pseurotin A (409) was active against the phytopathogenic bacteria Erwinia carotovora and Pseudomonas syringae with IC50 values of 220 and 112 μg/mL, respectively. It was still active against B. cereus and Shigella shiga with an MIC of 64 μg/mL. Meanwhile, it also inhibited the production of IgE with an IC50 value of 3.6 μM [176,177,178,179]. Penicillium pinophilum XS-20090E18 was the source of a new metabolite, along with three previously described compounds, hydroxypenicillide (413), penicillide (414), isopenicillide (415), and purpactin A (416). Meanwhile, all of them displayed antifouling activity against the settlement of the barnacle Balanus amphitrite with EC50 values of 6.0, 2.6, 20, and 10 μg/mL, respectively. Penicillide (414) was also regarded as a calcium-activated papain-like protease with an IC50 value of 7.1 μM. Purpactin A (416) was considered as an inhibitor of TMEM16A chloride channels and mucin secretion in airway epithelial cells with an approximate IC50 value of 2 μM; and displayed moderate inhibition against MCF7, H460, and SF268 with IC50 values of 20.5, 17.6, and 21.9 μM, respectively [103,180,181,182]. A novel hexahydrobenzopyran derivative, cytosporin L (417) and a bioactive compound, cytosporin D (418), were isolated from a fungus Eutypella sp.; and both of them exerted considerable inhibition against RSV with the IC50 values of 72.01 and 30.25 μM, respectively. Furthermore, compound 417 revealed bioactivity to Micrococcus lysodeikticus and Enterobacter aerogenes with the same MIC value of 3.12 μM. Cytosporin D (418) presented antimicrobial activity against E. coli and S. aureus. [183,184]. Two new metabolites together with a known toxin, aluminiumneoaspergillin (419), zirconiumneoaspergillin (420) and ferrineoaspergillin (421), were isolated from Aspergillus sp. SCSGAF0093. All of them exhibited lethality against brine shrimp (Artemia salina) with LC50 values of 6.61, 10.76, and 29.62 μM, respectively [77]. A new fatty acid, (2E,4E,6S)-6-hydroxydeca-2,4-dienoic acid (422), was produced by the gorgonian-associated fungus Xylaria sp. C-2 [172]. Cladosporilactam A (423), a new bicyclic lactam, was isolated from a gorgonian-derived Cladosporium sp. fungus and exhibited potent anticancer activity against HeLa with an IC50 value of 0.76 µM [185] (Figure 18).
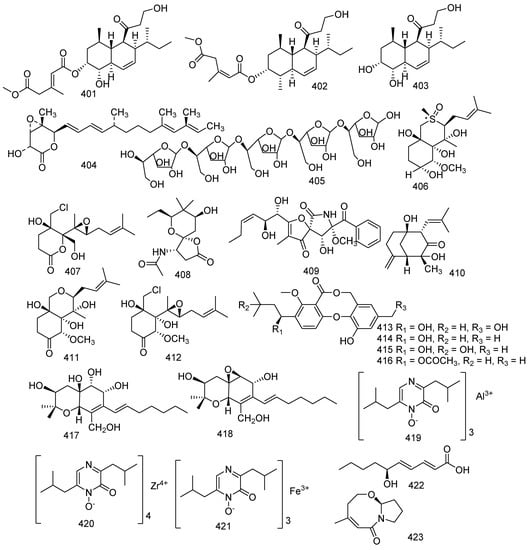
Figure 18.
The structures of compounds 401–423.
9. Comprehensive Overview and Conclusions
This review concluded that coral-associated fungi are a productive source of structurally diversified secondary metabolites with various bioactivities. In particular, the present review includes 423 metabolites of newly discovered compounds and bioactive compounds with a wide range of biological activities of anticancer, antimicrobial, anti-inflammatory, anti-fouling, and other bioactivities. In addition to the current in vitro bioassays, further clinical studies of these bioactive compounds are required to determine their potential therapeutic applications.
Generally, marine natural products isolated from coral-associated fungi are dominated by aromatics (35%) and alkaloids (24%), followed by lactones, peptides, terpenes, steroids, and other compounds (Figure 19, Table S6).
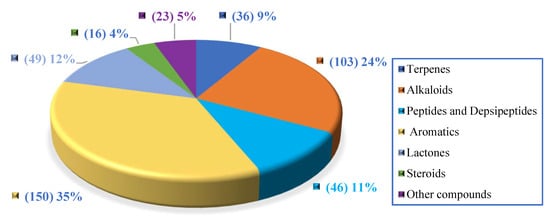
Figure 19.
Distribution of the compounds according to chemical structure.
These natural compounds were isolated from several coral-derived fungi covered soft coral, gorgonian coral, hard coral, leather coral, stony coral, and some unknown ones. Among them, soft coral-derived fungi are the dominant producers of natural products, comprising more than 53% of total molecules. And the second, gorgonian-derived fungi, accounts for 37% (Figure 20, Table S7). Generally, the epizoic relationship between symbiotic or epiphytic fungi and their host animals or environment induces the production of metabolites.
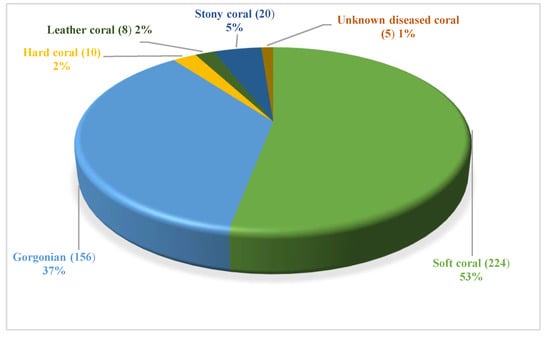
Figure 20.
The strain source of the natural products from coral-derived fungi.
Bioactive compounds are comprised of anticancer (22%), antimicrobial (28%), anti-fouling (17%), anti-inflammatory (7%), and other biological activities (26%) (Figure 21, Table S1–S5). Among them, other activities include neuroprotective activity, AChE inhibitory activity, α-glucosidase activity etc. It follows that compound produced by coral-associated fungi reveal great potential bioactivities.
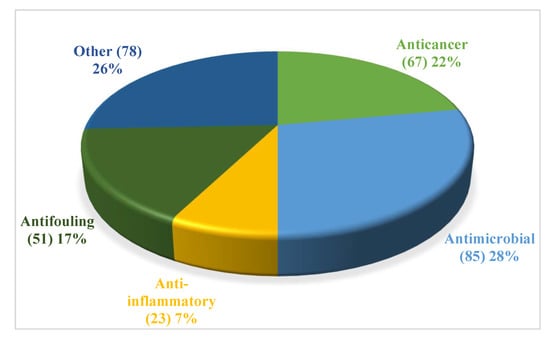
Figure 21.
Distribution of the compounds according to bioactivities.
Structurally, aromatics (36%) and alkaloids (27%) included the major proportions of bioactive compounds, followed by lactones (11%) (Figure 22, Table S1–S5). What is noteworthy is that satratoxin F (364) as a known lactone displayed excellent cytotoxic activities against five human cancer cell lines (MDA-MB-231, C4-2B, MGC803, MDA-MB-468, and A549) with EC50 values less than 39 nM. A previous review indicated that fungi was regarded as the best candidate as a source of anticancer agents [186]. In addition, another alkaloid, aflaquinolone D (111), exhibited notable antifouling activity with EC50 values of 2.8 nM. Obviously, compounds containing heteroatoms exhibited more potential bioactivities.
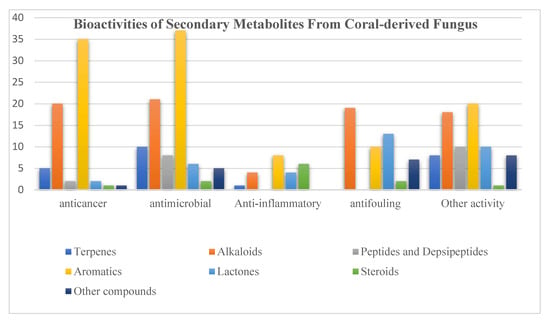
Figure 22.
Distribution of the bioactive compounds according to structures.
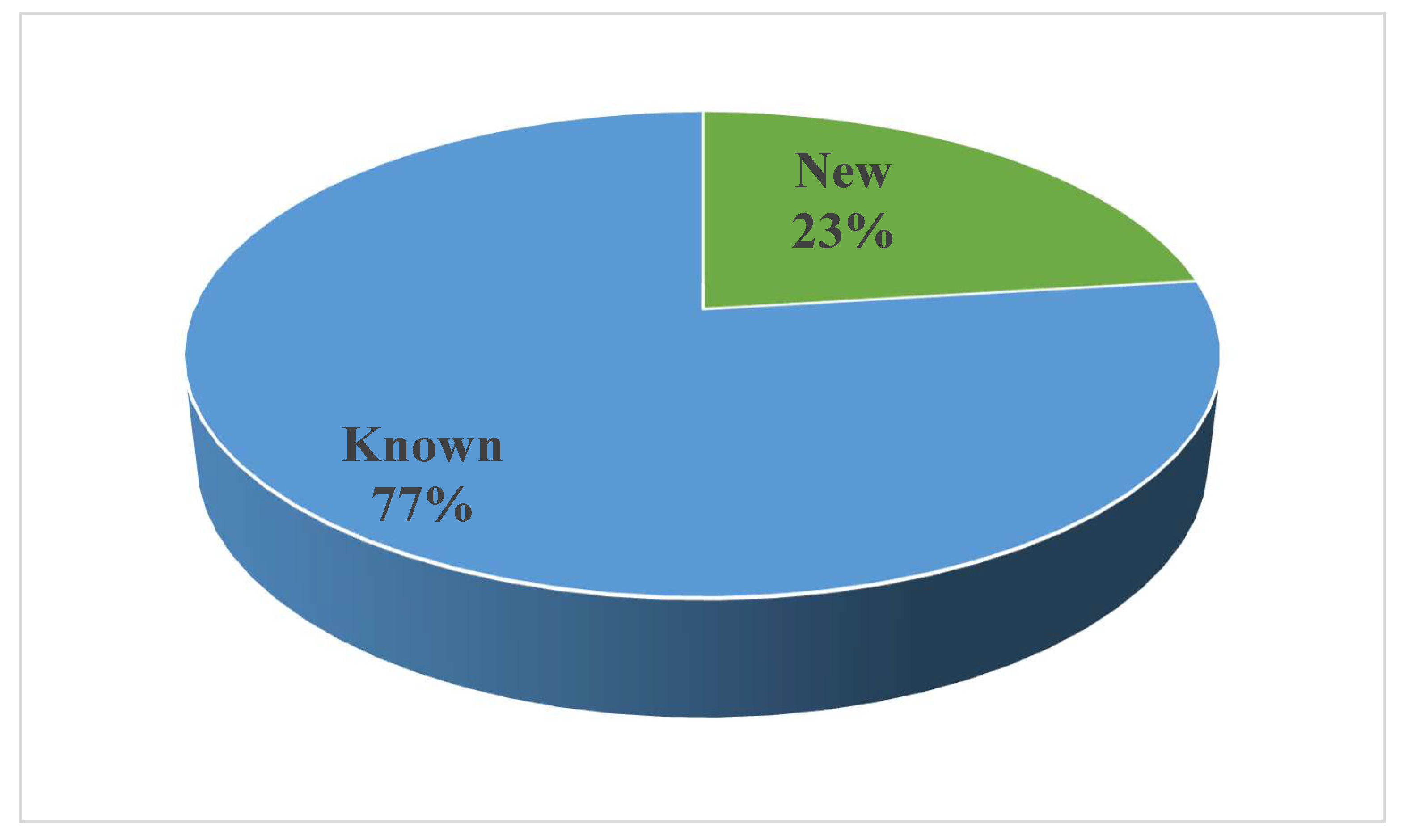
Noteworthy is that several compounds exhibit different bioactivities. As seen in Figure 21, the biological activities mainly focus on anticancer and antimicrobial activity. Furthermore, new compounds accounted for 23%, whereas known compounds made up 77% (Figure 23). New technologies and methods should be applied to improve the discovery of new compounds and it is necessary to explore the bioactivities of known metabolites through effective biological screening methods.
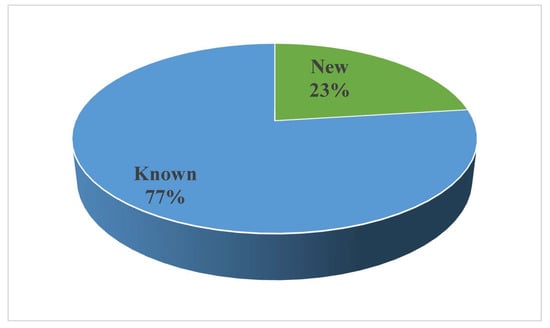
Figure 23.
Distribution of the bioactive compounds between new compounds and known compounds.
In the last few decades, secondary metabolites gathered from coral-associated fungi have shown noteworthy levels in a number of clinical targets, and many of them are structurally unique and possess remarkable biological and pharmacological properties, such as anticancer and antimicrobial activity. Some of them are lead compounds and potential clinical drug candidates, such as satratoxin F with antitumor activity and aurasperone B with radical-scavenging activity against 2,2-diphenyl-1-picryl-hydrazyl. Meanwhile, marine natural products exhibit diversified bioactivity due to their special chemical structures and potential interactions with proteins.
Drug discovery from coral-associated fungi has been a sustainable and intelligent methodology that can surmount supply issues through the large-scale fermentation of fungi. Furthermore, some key biosynthetic gene clusters in the regulation of fungi expressing unique skeleton metabolites with high bioactivity remain silent [187]. Therefore, it is necessary to apply novel methods and technologies with the purpose of activating the expression of unique secondary metabolites. On the basis of simulating natural conditions, it is appropriate to add some stimulus.
In conclusion, the present review elucidated chemical structures of 423 compounds obtained from coral-associated fungi, many of them with bioactivities as promising drug lead compounds. These metabolites exhibit diversified structures and various bioactivities. In particular, some compounds revealed remarkable activity, even stronger than the positive controls. These findings indicate that these compounds have great potential in the treatment of diseases. But the bioactivity of these metabolites was mainly tested in vitro; thus, more attention should be paid to the molecular mechanism and further in vivo and preclinical studies. In conclusion, there are many coral-associated secondary metabolites with notable biological activity, and numerous drug lead compounds and new metabolites are still waiting to be discovered.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jof8101043/s1, Table S1. Anticancer activity; Table S2. Antimicrobial activities; Table S3. Anti-inflammatory activities; Table S4. Antifouling activities; Table S5. Other activities; Table S6. Distribution of the compounds according to chemical structure; Table S7. The strain source of the natural products from coral-derived fungi.
Author Contributions
B.Y. and Y.L. conceived and revised this article, Y.C., X.P., Y.H., X.L., and X.Z. conducted the literature analysis. Y.C. performed analyses on the available data. B.Y. and Y.C. wrote the first draft of the manuscript, and all authors participated in the writing and revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from the National Natural Science Foundation of China (21977102, 81860626, 81973235, 82073762, 42276128), Guangdong Basic and Applied Basic Research Foundation (2021B1515120046, 2019B151502042, 2020A1515011045).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to the analytical facilities (Z. Xiao, A. Sun, X. Zheng, Y. Zhang) in SCSIO.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sigwart, J.D.; Blasiak, R.; Jaspars, M.; Jouffray, J.-B.; Tasdemir, D. Unlocking the potential of marine biodiscovery. Nat. Prod. Rep. 2021, 38, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.-N.; Liu, H.-W.; Keller, N.P.; Yin, W.-B. Harnessing diverse transcriptional regulators for natural product discovery in fungi. Nat. Prod. Rep. 2020, 37, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2020, 37, 175–223. [Google Scholar] [CrossRef] [PubMed]
- Hoegh-Guldberg, O.; Mumby, P.J.; Hooten, A.J.; Steneck, R.S.; Greenfield, P.; Gomez, E.; Harvell, C.D.; Sale, P.F.; Edwards, A.J.; Caldeira, K.; et al. Coral reefs under rapid climate change and ocean acidification. Science 2007, 318, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Connell, J.H. Diversity in tropical rain forests and coral reefs—high diversity of trees and corals is maintained only in a non-equilibrium state. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef]
- Fouillaud, M.; Venkatachalam, M.; Llorente, M.; Magalon, H.; Cuet, P.; Dufosse, L. Biodiversity of Pigmented Fungi Isolated from Marine Environment in La Reunion Island, Indian Ocean: New Resources for Colored Metabolites. J. Fungi 2017, 3, 36. [Google Scholar] [CrossRef]
- Hughes, C.C. Chemical labeling strategies for small molecule natural product detection and isolation. Nat. Prod. Rep. 2021, 38, 1684–1705. [Google Scholar] [CrossRef]
- Hou, X.-M.; Hai, Y.; Gu, Y.-C.; Wang, C.-Y.; Shao, C.-L. Chemical and Bioactive Marine Natural Products of Coral-Derived Microorganisms (2015–2017). Curr. Med. Chem. 2019, 26, 6930–6941. [Google Scholar] [CrossRef]
- Hou, X.-M.; Xu, R.-F.; Gu, Y.-C.; Wang, C.-Y.; Shao, C.-L. Biological and Chemical Diversity of Coral-Derived Microorganisms. Curr.Med. Chem. 2015, 22, 3707–3762. [Google Scholar] [CrossRef]
- Raimundo, I.; Silva, S.G.; Costa, R.; Keller-Costa, T. Bioactive Secondary Metabolites from Octocoral-Associated MicrobesNew Chances for Blue Growth. Mar. Drugs 2018, 16, 485. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Palaniveloo, K.; Alias, S.A.; Seelan, J.S.S. Species Diversity and Secondary Metabolites of Sarcophyton-Associated Marine Fungi. Molecules 2021, 26, 3227. [Google Scholar] [CrossRef] [PubMed]
- Tetali, S.D. Terpenes and isoprenoids: A wealth of compounds for global use. Planta 2019, 249, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef]
- Dai, Q.; Zhang, F.-L.; Feng, T. Sesquiterpenoids Specially Produced by Fungi: Structures, Biological Activities, Chemical and Biosynthesis (2015–2020). J. Fungi 2021, 7, 1026. [Google Scholar] [CrossRef]
- Li, H.-J.; Xie, Y.-L.; Xie, Z.-L.; Chen, Y.; Lam, C.-K.; Lan, W.-J. Chondrosterins A-E, Triquinane-Type Sesquiterpenoids from Soft Coral-Associated Fungus Chondrostereum sp. Mar. Drugs 2012, 10, 627–638. [Google Scholar] [CrossRef]
- Li, H.-J.; Chen, T.; Xie, Y.-L.; Chen, W.-D.; Zhu, X.-F.; Lan, W.-J. Isolation and Structural Elucidation of Chondrosterins F-H from the Marine Fungus Chondrostereum sp. Mar. Drugs 2013, 11, 551–558. [Google Scholar] [CrossRef]
- Li, H.-J.; Lan, W.-J.; Lam, C.-K.; Yang, F.; Zhu, X.-F. Hirsutane Sesquiterpenoids from the Marine-Derived Fungus Chondrostereum sp. Chem. Biodivers. 2011, 8, 317–324. [Google Scholar] [CrossRef]
- Huang, L.; Lan, W.-J.; Li, H.-J. Two new hirsutane-type sesquiterpenoids chondrosterins N and O from the marine fungus Chondrostereum sp. Nat. Prod. Res. 2018, 32, 1578–1582. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.-X.; Guo, Q.; Ran, Y.-Q.; Qiu, Y.; Lan, W.-J.; Li, H.-J. New Aromadendrane Sesquiterpenoid Pseuboydone F from the Marine-derived Fungus Pseudallescheria Boydii F44-1. Rec. Nat. Prod. 2020, 14, 166–170. [Google Scholar] [CrossRef]
- Lan, W.-J.; Wang, K.-T.; Xu, M.-Y.; Zhang, J.-J.; Lam, C.-K.; Zhong, G.-H.; Xu, J.; Yang, D.-P.; Li, H.-J.; Wang, L.-Y. Secondary metabolites with chemical diversity from the marine-derived fungus Pseudallescheria boydii F19-1 and their cytotoxic activity. Rsc. Adv. 2016, 6, 76206–76213. [Google Scholar] [CrossRef]
- Qiu, Y.; Lan, W.-J.; Li, H.-J.; Chen, L.-P. Linear Triquinane Sesquiterpenoids: Their Isolation, Structures, Biological Activities, and Chemical Synthesis. Molecules 2018, 23, 2095. [Google Scholar] [CrossRef]
- Bunbamrung, N.; Intaraudom, C.; Dramae, A.; Boonyuen, N.; Veeranondha, S.; Rachtawee, P.; Pittayakhajonwut, P. Antimicrobial activity of illudalane and alliacane sesquiterpenes from the mushroom Gloeostereum incarnatum BCC41461. Phytochem. Lett. 2017, 20, 274–281. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Liu, Y.-F.; Cao, F.; Wang, C.-Y. Bisabolane-Type Sesquiterpenoids from a Gorgonian-Derived Aspergillus sp. Fungus Induced by DNA Methyltransferase Inhibitor. Chem. Nat. Compd. 2016, 52, 1129–1132. [Google Scholar] [CrossRef]
- Liu, M.; Sun, W.; Wang, J.; He, Y.; Zhang, J.; Li, F.; Qi, C.; Zhu, H.; Xue, Y.; Hu, Z.; et al. Bioactive secondary metabolites from the marine-associated fungus Aspergillus terreus. Bioorg. Chem. 2018, 80, 525–530. [Google Scholar] [CrossRef]
- Pandey, V.V.; Varshney, V.K.; Pandey, A. Lovastatin: A Journey from Ascomycetes to Basidiomycetes Fungi. J. Biolog. Act. Prod. Nat. 2019, 9, 162–178. [Google Scholar] [CrossRef]
- Xiong, Z.; Cao, X.; Wen, Q.; Chen, Z.; Cheng, Z.; Huang, X.; Zhang, Y.; Long, C.; Zhang, Y.; Huang, Z. An overview of the bioactivity of monacolin K / lovastatin. Food Chem. Toxicol. 2019, 131, 110585. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.-L.; Yang, L.-J.; Shi, T.; Wang, C.-Y.; Shao, C.-L.; Wang, C.-Y. Potent Phytotoxic Harziane Diterpenes from a Soft Coral-Derived Strain of the Fungus Trichoderma harzianum XS-20090075. Sci. Rep. 2019, 9, 13345. [Google Scholar] [CrossRef]
- Afiyatullov, S.S.; Zhuravleva, O.I.; Antonov, A.S.; Kalinovsky, A.I.; Pivkin, M.V.; Menchinskaya, E.S.; Aminin, D.L. New Metabolites from the Marine-derived Fungus Aspergillus fumigatus. Nat. Prod. Commun. 2012, 7, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.H.; Wu, Z.E.; Liu, L.; Chen, S.H. The chemistry and biology of fungal meroterpenoids (2009–2019). Org. Biomol. Chem. 2021, 19, 1644–1704. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.-Y.; Wang, C.-Y.; Liu, Q.-A.; Shao, C.-L.; She, Z.-G.; Lin, Y.-C. Five Sesquiterpenoids from a Marine-Derived Fungus Aspergillus sp. Isolated from a Gorgonian Dichotella gemmacea. Mar. Drugs 2010, 8, 941–949. [Google Scholar] [CrossRef]
- Li, X.D.; Li, X.M.; Xu, G.M.; Zhang, P.; Wang, B.G. Antimicrobial Phenolic Bisabolanes and Related Derivatives from Penicillium aculeatum SD-321, a Deep Sea Sediment-Derived Fungus. J. Nat. Prod. 2015, 78, 844–849. [Google Scholar] [CrossRef]
- Zheng, C.; Shao, C.; Wang, K.; Zhao, D.; Wang, Y.; Wang, C. Secondary metabolites and their bioactivities of a soft coral-derived fungus Aspergillus versicolor (ZJ-2008015). Chin. J. Mar. Drugs 2012, 31, 7–13. [Google Scholar]
- Riga, R.; Happyana, N.; Hakim, E.H. Sesquiterpenes produced by Pestalotiopsis microspora HF 12440 isolated from Artocarpus heterophyllus. Nat. Prod. Res. 2020, 34, 2229–2231. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, M.S.; Ebada, S.S.; Ashour, M.L.; Ebrahim, W.; Mueller, W.E.G.; Mandi, A.; Kurtan, T.; Singab, A.; Lin, W.; Liu, Z.; et al. Xanthones and sesquiterpene derivatives from a marine-derived fungus Scopulariopsis sp. Tetrahedron 2016, 72, 2411–2419. [Google Scholar] [CrossRef]
- Guo, Z.Y.; Tan, M.H.; Liu, C.X.; Lv, M.M.; Deng, Z.S.; Cao, F.; Zou, K.; Proksch, P. Aspergoterpenins A(-)D: Four New Antimicrobial Bisabolane Sesquiterpenoid Derivatives from an Endophytic Fungus Aspergillus versicolor. Molecules 2018, 23, 1291. [Google Scholar] [CrossRef]
- Zheng, C.-J.; Shao, C.-L.; Chen, M.; Niu, Z.-G.; Zhao, D.-L.; Wang, C.-Y. Merosesquiterpenoids and Ten-Membered Macrolides from a Soft Coral-Derived Lophiostoma sp Fungus. Chem. Biodivers. 2015, 12, 1407–1414. [Google Scholar] [CrossRef]
- Cushnie, T.P.; Cushnie, B.; Lamb, A.J. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int. J. Antimicrob. Ag. 2014, 44, 377–386. [Google Scholar] [CrossRef]
- Xu, W.F.; Mao, N.; Xue, X.J.; Qi, Y.X.; Wei, M.Y.; Wang, C.Y.; Shao, C.L. Structures and Absolute Configurations of Diketopiperazine Alkaloids Chrysopiperazines A(-)C from the Gorgonian-Derived Penicillium chrysogenum Fungus. Mar. Drugs 2019, 17, 250. [Google Scholar] [CrossRef]
- Sun, X.-P.; Xu, Y.; Cao, F.; Xu, R.-F.; Zhang, X.-L.; Wang, C.-Y. Isoechinulin-Type Secondary metabolites with chemical diversity from the marine-derived. Chem. Nat. Compd. 2014, 50, 1153–1155. [Google Scholar]
- Wijesekara, I.; Li, Y.-X.; Vo, T.-S.; van Ta, Q.; Ngo, D.-H.; Kim, S.-K. Induction of apoptosis in human cervical carcinoma HeLa cells by neoechinulin A from marine-derived fungus Microsporum sp. Process Biochem. 2013, 48, 68–72. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Bahukhandi, A.; Dhyani, P.; Sati, P.; Capanoglu, E.; Docea, A.O.; Al-Harrasi, A.; Dey, A.; Calina, D. Therapeutic Potential of Neoechinulins and Their Derivatives: An Overview of the Molecular Mechanisms Behind Pharmacological Activities. Front. Nutr. 2021, 8, 664197. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, C.; He, F.; Zhang, W.; Leng, A.; Ying, X. Two new alkaloids from Portulaca oleracea L. and their bioactivities. Fitoterapia 2019, 136, 104166. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.L.; Lu, Z.Y.; Tao, H.W.; Zhu, T.J.; Fang, Y.C.; Gu, Q.Q.; Zhu, W.M. Isoechinulin-type alkaloids, variecolorins A-L, from halotolerant Aspergillus variecolor. J. Nat. Prod. 2007, 70, 1558–1564. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, H.; Fujimaki, T.; Okuyama, E.; Yamazaki, M. Immunomodulatory constituents from an ascomycete, Microascus tardifaciens. Chem. Pharm. Bull. 1999, 47, 1426–1432. [Google Scholar] [CrossRef]
- Wei, X.; Feng, C.; Wang, S.-Y.; Zhang, D.-M.; Li, X.-H.; Zhang, C.-X. New Indole Diketopiperazine Alkaloids from Soft Coral-Associated Epiphytic Fungus Aspergillus sp. EGF 15-0-3. Chem. Biodivers. 2020, 17, e2000106. [Google Scholar] [CrossRef]
- Ningsih, B.N.S.; Rukachaisirikul, V.; Phongpaichit, S.; Preedanon, S.; Sakayaroj, J.; Muanprasat, C. A nonadride derivative from the marine-derived fungus Aspergillus chevalieri PSU-AMF79. Nat. Prod. Res. 2022, 16, 1–8. [Google Scholar] [CrossRef]
- Zhuravleva, O.I.; Antonov, A.S.; Trang, V.T.D.; Pivkin, M.V.; Khudyakova, Y.V.; Denisenko, V.A.; Popov, R.S.; Kim, N.Y.; Yurchenko, E.A.; Gerasimenko, A.V.; et al. New Deoxyisoaustamide Derivatives from the Coral-Derived Fungus Penicillium dimorphosporum KMM 4689. Mar. Drugs 2021, 19, 32. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, T.; Liu, D.; Guan, G.; Huang, J.; Proksch, P.; Chen, X.; Lin, W. Notoamide-type alkaloid induced apoptosis and autophagy via a P38/JNK signaling pathway in hepatocellular carcinoma cells. Rsc. Adv. 2019, 9, 19855–19868. [Google Scholar] [CrossRef] [PubMed]
- Wulff, J.E.; Herzon, S.B.; Siegrist, R.; Myers, A.G. Evidence for the rapid conversion of stephacidin B into the electrophilic monomer avrainvillamide in cell culture. J. Am. Chem. Soc. 2007, 129, 4898–4899. [Google Scholar] [CrossRef]
- Mikkola, R.; Andersson, M.A.; Hautaniemi, M.; Salkinoja-Salonen, M.S. Toxic indole alkaloids avrainvillamide and stephacidin B produced by a biocide tolerant indoor mold Aspergillus westerdijkiae. Toxicon 2015, 99, 58–67. [Google Scholar] [CrossRef]
- Wang, K.-T.; Xu, M.-Y.; Liu, W.; Li, H.-J.; Xu, J.; Yang, D.-P.; Lan, W.-J.; Wang, L.-Y. Two Additional New Compounds from the Marine-Derived Fungus Pseudallescheria ellipsoidea F42-3. Molecules 2016, 21, 442. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Lin, Y.; Pang, X.; Luo, X.; Salendra, L.; Wang, J.; Zhou, X.; Lu, Y.; Yang, B.; Liu, Y. Peptides from the Soft Coral-associated Fungus Simplicillium sp. SCSIO41209. Phytochemistry 2018, 154, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Lou, L.; Liu, D.; Li, X.; Proksch, P.; Yin, S.; Lin, W. Versiquinazolines A-K, Fumiquinazoline-Type Alkaloids from the Gorgonian-Derived Fungus Aspergillus Versicolor LZD-14-1. J. Nat. Prod. 2016, 79, 2941–2952. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Liu, D.; Cheng, W.; Proksch, P.; Lin, W. Versiquinazolines L-Q, new polycyclic alkaloids from the marine-derived fungus Aspergillus versicolor. Rsc. Advances 2018, 8, 31427–31439. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Teng, X.; Wang, Y.; Liu, P.; Li, G.; Zhu, W. New Quinazolinone Alkaloids within Rare Amino Acid Residue from Coral-Associated Fungus, Aspergillus Versicolor LCJ-5-4. Org. Lett. 2011, 13, 1130–1133. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.-L.; Xu, R.-F.; Wei, M.-Y.; She, Z.-G.; Wang, C.-Y. Structure and Absolute Alkaloids within Rare Amino Acid Residue from a Gorgonian-Derived Scopulariopsis sp. Fungus. J. Nat.l Prod. 2013, 76, 779–782. [Google Scholar] [CrossRef]
- Zaman, K.A.U.; Park, J.H.; DeVine, L.; Hu, Z.; Wu, X.; Kim, H.S.; Cao, S. Secondary Metabolites from the Leather Coral-Derived Fungal Strain Xylaria sp. FM1005 and Their Glycoprotein IIb/IIIa Inhibitory Activity. J. Nat. Prod. 2021, 84, 466–473. [Google Scholar] [CrossRef]
- Gomes, N.M.; Bessa, L.J.; Buttachon, S.; Costa, P.M.; Buaruang, J.; Dethoup, T.; Silva, A.M.S.; Kijjoa, A. Antibacterial and Antibiofilm Activities of Tryptoquivalines and Meroditerpenes Isolated from the Marine-Derived Fungi Neosartorya paulistensis, N. laciniosa, N. tsunodae, and the Soil Fungi N. fischeri and N. siamensis. Mar. Drugs 2014, 12, 822–839. [Google Scholar] [CrossRef]
- Xu, W.-F.; Chao, R.; Hai, Y.; Guo, Y.-Y.; Wei, M.-Y.; Wang, C.-Y.; Shao, C.-L. 17-Hydroxybrevianamide N and Its N1-Methyl Derivative, Quinazolinones from a Soft-Coral-Derived Aspergillus sp. Fungus: 13S Enantiomers as the True Natural Products. J. Nat. Prod. 2021, 84, 1353–1358. [Google Scholar] [CrossRef]
- Liu, W.; Li, H.-J.; Xu, M.-Y.; Ju, Y.-C.; Wang, L.-Y.; Xu, J.; Yang, D.-P.; Lan, W.-J. Pseudellones A-C, Three Alkaloids from the Marine-Derived Fungus Pseudallescheria ellipsoidea F42-3. Org. Lett. 2015, 17, 5156–5159. [Google Scholar] [CrossRef]
- Zheng, Q.C.; Kong, M.Z.; Zhao, Q.; Chen, G.D.; Tian, H.Y.; Li, X.X.; Guo, L.D.; Li, J.; Zheng, Y.Z.; Gao, H. Chaetoglobosin Y, a new cytochalasan from Chaetomium globosum. Fitoterapia 2014, 93, 126–131. [Google Scholar] [CrossRef]
- Luo, X.-W.; Gao, C.-H.; Lu, H.-M.; Wang, J.-M.; Su, Z.-Q.; Tao, H.-M.; Zhou, X.-F.; Yang, B.; Liu, Y.-H. HPLC-DAD-Guided Isolation of Diversified Chaetoglobosins from the Coral-Associated Fungus Chaetomium globosum C2F17. Molecules 2020, 25, 1237. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Song, Y.X.; Liu, X.Q.; Gong, W.; Li, E.G.; Tan, R.X.; Hou, Y.Y. Chaetoglobosin Fex from the Marine-Derived Endophytic Fungus Inhibits Induction of Inflammatory Mediators via Toll-Like Receptor 4 Signaling in Macrophages. Biol. Pharm. Bull. 2011, 34, 1864–1873. [Google Scholar] [CrossRef] [PubMed]
- Choo, S.J.; Yun, B.S.; Ryoo, I.J.; Kim, Y.H.; Bae, K.H.; Yoo, I.D. Aspochalasin I, a Melanogenesis Inhibitor from Aspergillus sp. J. Microbiol. Biotechnol. 2009, 19, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.-J.; Shao, C.-L.; Wu, L.-Y.; Chen, M.; Wang, K.-L.; Zhao, D.-L.; Sun, X.-P.; Chen, G.-Y.; Wang, C.-Y. Bioactive Phenylalanine Derivatives and Cytochalasins from the Soft Coral-Derived Fungus, Aspergillus elegans. Mar. Drugs 2013, 11, 2054–2068. [Google Scholar] [CrossRef]
- Erden, Y.; Tekin, S.; Ceylan, K.B.; Tekin, C.; Kirbag, S. Antioxidant, Antimicrobial and Anticancer Activities of the Aspergillin PZ and Terphenyllin Secondary Metabolites: An in vitro Study. Gazi University J. Sci. 2019, 32, 792–800. [Google Scholar] [CrossRef]
- Guo, Z.-L.; Zheng, J.-J.; Cao, F.; Wang, C.; Wang, C.-Y. Chemical Constituents of the Gorgonian-Derived Fungus Chaetomium globosum. Chem. Nat. Compd. 2017, 53, 199–202. [Google Scholar] [CrossRef]
- Naruse, N.; Yamamoto, H.; Murata, S.; Sawada, Y.; Fukagawa, Y.; Oki, T. Aspochalasin-E, A New Antibiotic Isolated from A Fungus. J. Antibiot. 1993, 46, 679–681. [Google Scholar] [CrossRef]
- Hao, J.-D.; Zheng, J.-J.; Chen, M.; Wang, C.-Y. Cytochalasins from the Gorgonian-Derived Fungus Aspergillus sp. XS-2009-0B15. Chem. Nat. Compd. 2017, 53, 732–735. [Google Scholar] [CrossRef]
- Shao, C.-L.; Xu, R.-F.; Wang, C.-Y.; Qian, P.-Y.; Wang, K.-L.; Wei, M.-Y. Potent Antifouling Marine Dihydroquinolin-2(1H)-one-Containing Alkaloids from the Gorgonian Coral-Derived Fungus Scopulariopsis sp. Mar. Biotechnol. 2015, 17, 408–415. [Google Scholar] [CrossRef]
- Mou, X.-F.; Liu, X.; Xu, R.-F.; Wei, M.-Y.; Fang, Y.-W.; Shao, C.-L. Scopuquinolone B, a new monoterpenoid dihydroquinolin-2(1H)-one isolated from the coral-derived Scopulariopsis sp fungus. Nat. Prod. Res. 2018, 32, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Ebada, S.S.; Ebrahim, W. A new antibacterial quinolone derivative from the endophytic fungus Aspergillus versicolor strain Eich.5.2.2. S. Afr. J. Bot. 2020, 134, 151–155. [Google Scholar] [CrossRef]
- An, C.Y.; Li, X.M.; Luo, H.; Li, C.S.; Wang, M.H.; Xu, G.M.; Wang, B.G. 4-Phenyl-3,4-dihydroquinolone derivatives from Aspergillus nidulans MA-143, an endophytic fungus isolated from the mangrove plant Rhizophora stylosa. J. Nat. Prod. 2013, 76, 1896–1901. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liang, X.; Huang, Z.H.; Qi, S.H. New alkaloids and isocoumarins from the marine gorgonian-derived fungus Aspergillus sp. SCSIO 41501. Nat. Prod. Res. 2020, 34, 1992–2000. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Shao, C.L.; Meng, H.; She, Z.G.; Wang, C.Y. Anti-respiratory syncytial virus prenylated dihydroquinolone derivatives from the gorgonian-derived fungus Aspergillus sp. XS-20090B15. J. Nat. Prod. 2014, 77, 2720–2724. [Google Scholar] [CrossRef]
- Liu, M.; He, Y.; Shen, L.; al Anbari, W.H.; Li, H.; Wang, J.; Qi, C.; Hu, Z.; Zhang, Y. Asperteramide A, an Unusual N-Phenyl-Carbamic Acid Methyl Ester Trimer Isolated from the Coral-Derived Fungus Aspergillus Terreus. Eur. J. Org. Chem. 2019, 2019, 2928–2932. [Google Scholar] [CrossRef]
- Xu, X.; He, F.; Zhang, X.; Bao, J.; Qi, S. New mycotoxins from marine-derived fungus Aspergillus sp. SCSGAF0093. Food Chem. Toxicol. 2013, 53, 46–51. [Google Scholar] [CrossRef]
- Zhu, F.; Wu, J.S.; Chen, G.Y.; Lu, W.H.; Pan, J.H. Biosynthesis, Characterization and Biological Evalutation of Fe(III) and Cu(II) Complexes of Neoaspergillic Acid, a Hydroxamate Siderophore Produced by Co-cultures of two Marine-derived Mangrove Epiphytic Fungi. Nat. Prod. Commun. 2011, 6, 1137–1140. [Google Scholar] [CrossRef]
- Yu, J.-Y.; Shi, T.; Zhou, Y.; Xu, Y.; Zhao, D.-L.; Wang, C.-Y. Naphthalene derivatives and halogenate quinoline from the coral-derived fungus Trichoderma harzianum (XS-20090075) through OSMAC approach. J. Asian Nat. Prod. Res. 2021, 23, 250–257. [Google Scholar] [CrossRef]
- Shaaban, M.; Shaaban, K.A.; Abdel-Aziz, M.S. Seven naphtho-γ-pyrones from the marine-derived fungus Alternaria alternata: Structure elucidation and biological properties. Org. Med. Chem. Lett. 2012, 2, 6. [Google Scholar] [CrossRef]
- Yang, B.; Long, J.; Pang, X.; Lin, X.; Liao, S.; Wang, J.; Zhou, X.; Li, Y.; Liu, Y. Structurally diverse polyketides and phenylspirodrimanes from the soft coral-associated fungus Stachybotrys chartarum SCSIO41201. J. Antibiot. 2021, 74, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-H.; Chen, Y.-X.; Yu, J.-C.; Yuan, J.; Li, H.-J.; Ma, W.-Z.; Watanapokasin, R.; Hu, K.-C.; Niaz, S.I.; Yang, D.-P.; et al. Secondary Metabolites from the Marine-Derived Fungus Dichotomomyces sp. L-8 and Their Cytotoxic Activity. Molecules 2017, 22, 444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.N.; Xia, Y.X.; Zhang, H.J. Natural Cyclopeptides as Anticancer Agents in the Last 20 Years. Int. J. Mol. Sci. 2021, 22, 3973. [Google Scholar] [CrossRef]
- Chao, R.; Hou, X.-M.; Xu, W.-F.; Hai, Y.; Wei, M.-Y.; Wang, C.-Y.; Gu, Y.-C.; Shao, C.-L. Targeted Isolation of Asperheptatides from a Coral-Derived Fungus Using LC-MS/MS-Based Molecular Networking and Antitubercular Activities of Modified Cinnamate Derivatives. J. Nat. Prod. 2021, 84, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Teng, X.; Wang, Y.; Liu, P.; Wang, H.; Li, J.; Li, G.; Zhu, W. Cyclopeptides and polyketides from coral-associated fungus, Aspergillus versicolor LCJ-5-4. Tetrahedron 2011, 67, 7085–7089. [Google Scholar] [CrossRef]
- Chen, R.; Cheng, Z.; Huang, J.; Liu, D.; Wu, C.; Guo, P.; Lin, W. Versicotides D–F, new cyclopeptides with lipid-lowering activities. Rsc. Adv. 2017, 7, 49235–49243. [Google Scholar] [CrossRef]
- Hou, X.M.; Zhang, Y.H.; Hai, Y.; Zheng, J.Y.; Gu, Y.C.; Wang, C.Y.; Shao, C.L. Aspersymmetide A, a New Centrosymmetric Cyclohexapeptide from the Marine-Derived Fungus Aspergillus versicolor. Mar. Drugs 2017, 15, 363. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.-M.; Li, Y.-Y.; Shi, Y.-W.; Fang, Y.-W.; Chao, R.; Gu, Y.-C.; Wang, C.-Y.; Shao, C.-L. Integrating Molecular Networking and H-1 NMR To Target the Isolation of Chrysogeamides from a Library of Marine-Derived Penicillium Fungi. J. Org. Chem. 2019, 84, 1228–1237. [Google Scholar]
- Chen, M.; Shao, C.L.; Fu, X.M.; Kong, C.J.; She, Z.G.; Wang, C.Y. Lumazine peptides penilumamides B-D and the cyclic pentapeptide asperpeptide A from a gorgonian-derived Aspergillus sp. fungus. J. Nat. Prod. 2014, 77, 1601–1606. [Google Scholar] [CrossRef]
- Ma, X.; Nong, X.-H.; Ren, Z.; Wang, J.; Liang, X.; Wang, L.; Qi, S.-H. Antiviral peptides from marine gorgonian-derived fungus Aspergillus sp. SCSIO 41501. Tetrahedron Lett. 2017, 58, 1151–1155. [Google Scholar] [CrossRef]
- Jia, Y.-L.; Wei, M.-Y.; Chen, H.-Y.; Guan, F.-F.; Wang, C.-Y.; Shao, C.-L. (+)- and ()-Pestaloxazine A, a Pair of Antiviral Enantiomeric Alkaloid Dimers with a Symmetric Spiro oxazinane-piperazinedione Skeleton from Pestalotiopsis sp. Org. Lett. 2015, 17, 4216–4219. [Google Scholar] [CrossRef] [PubMed]
- Battilani, P.; Pietri, A. Ochratoxin A in grapes and wine. Eur. J. Plant Pathol. 2002, 108, 639–643. [Google Scholar] [CrossRef]
- Lopez-Gresa, M.P.; Gonzalez, M.C.; Primo, J.; Moya, P.; Romero, V.; Estornell, E. Circumdatin H, a new inhibitor of mitochondrial NADH oxidase, from Aspergillus ochraceus. J. Antibiot. 2005, 58, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-Z.; Kurtan, T.; Mandi, A.; Tang, H.; Chou, Y.; Soong, K.; Su, L.; Sun, P.; Zhuang, C.-L.; Zhang, W. Immunomodulatory Polyketides from a Phoma-like Fungus Isolated from a Soft Coral. J. Nat. Prod. 2017, 80, 2930–2940. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Miura, S.; Yamashita, Y.; Ohta, T. Aspermytin A: A new neurotrophic polyketide isolated from a marine-derived fungus of the genus Aspergillus. Bioorg. Med. Chem. Lett. 2004, 14, 417–420. [Google Scholar] [CrossRef]
- Zhu, M.; Gao, H.; Wu, C.; Zhu, T.; Che, Q.; Gu, Q.; Guo, P.; Li, D. Lipid-lowering polyketides from a soft coral-derived fungus Cladosporium sp. TZP29. Bioorg. Med. Chem. Lett. 2015, 25, 3606–3609. [Google Scholar] [CrossRef]
- Liu, Z.; Qiu, P.; Liu, H.; Li, J.; Shao, C.; Yan, T.; Cao, W.; She, Z. Identification of anti-inflammatory polyketides from the coral-derived fungus Penicillium sclerotiorin: In vitro approaches and molecular-modeling. Bioorg. Chem. 2019, 88, 102973. [Google Scholar] [CrossRef]
- Zhuravleva, O.I.; Oleinikova, G.K.; Antonov, A.S.; Kirichuk, N.N.; Pelageev, D.N.; Rasin, A.B.; Menshov, A.S.; Popov, R.S.; Kim, N.Y.; Chingizova, E.A.; et al. New Antibacterial Chloro-Containing Polyketides from the Alga-Derived Fungus Asteromyces cruciatus KMM 4696. J. Fungi 2022, 8, 454. [Google Scholar] [CrossRef]
- Michael, A.P.; Grace, E.J.; Kotiw, M.; Barrow, R.A. Isochromophilone IX, a novel GABA-containing metabolite isolated from a cultured fungus, Penicillium sp. Aust. J. Chem. 2003, 56, 13–15. [Google Scholar] [CrossRef]
- Zhang, H.; Lei, X.-X.; Shao, S.; Zhou, X.; Li, Y.; Yang, B. Azaphilones and Meroterpenoids from the Soft Coral-Derived Fungus Penicillium glabrum glmu003. Chem. Biodivers. 2021, 18, 1–6. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, X.-Y.; Xu, X.-Y.; He, F.; Nong, X.-H.; Qi, S.-H. New cyclic tetrapeptides and asteltoxins from gorgonian-derived fungus Aspergillus sp. SCSGAF 0076. Tetrahedron 2013, 69, 2113–2117. [Google Scholar] [CrossRef]
- Wang, M.; Sun, M.; Hao, H.; Lu, C. Avertoxins A-D, Prenyl Asteltoxin Derivatives from Aspergillus versicolor Y10, an Endophytic Fungus of Huperzia serrata. J. Nat. Prod. 2015, 78, 3067–3070. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.L.; Shao, C.L.; Zhang, Q.; Wang, K.L.; Guan, F.F.; Shi, T.; Wang, C.Y. Azaphilone and Diphenyl Ether Derivatives from a Gorgonian-Derived Strain of the Fungus Penicillium pinophilum. J. Nat. Prod. 2015, 78, 2310–2314. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.L.; Wang, C.Y.; Wei, M.Y.; Gu, Y.C.; She, Z.G.; Qian, P.Y.; Lin, Y.C. Aspergilones A and B, two benzylazaphilones with an unprecedented carbon skeleton from the gorgonian-derived fungus Aspergillus sp. Bioorg. Med. Chem. Lett. 2011, 21, 690–693. [Google Scholar] [CrossRef]
- Wu, N.-N.; Hou, X.-M.; Wei, M.-Y.; Zheng, J.-Y.; Shao, C.-L. Antifungal and Antibacterial Activities of Azaphilones from the Gorgonian-Derived Penicillium sclerotiorum Fungus. Chem. Nat. Compd. 2019, 55, 549–551. [Google Scholar] [CrossRef]
- Wei, M.Y.; Wang, C.F.; Wang, K.L.; Qian, P.Y.; Wang, C.Y.; Shao, C.L. Preparation, Structure, and Potent Antifouling Activity of Sclerotioramine Derivatives. Mar. Biotechnol. 2017, 19, 372–378. [Google Scholar] [CrossRef]
- Liu, Y.F.; Zhang, Y.H.; Shao, C.L.; Cao, F.; Wang, C.Y. Microketides A and B, Polyketides from a Gorgonian-Derived Microsphaeropsis sp. Fungus. J. Nat. Prod. 2020, 83, 1300–1304. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Peng, X.Y.; Feng, L.X.; Zhu, H.J.; Cao, F.; Wang, C.Y. A new epimer of azaphilone derivative pinophilin B from the gorgonian-derived fungus Aspergillus fumigatus 14–27. Nat. Prod. Res. 2021, 35, 2232–2238. [Google Scholar] [CrossRef]
- Lebeau, J.; Venkatachalam, M.; Fouillaud, M.; Petit, T.; Vinale, F.; Dufosse, L.; Caro, Y. Production and New Extraction Method of Polyketide Red Pigments Produced by Ascomycetous Fungi from Terrestrial and Marine Habitats. J. Fungi 2017, 3, 34. [Google Scholar] [CrossRef]
- Bao, J.; Sun, Y.-L.; Zhang, X.-Y.; Han, Z.; Gao, H.-C.; He, F.; Qian, P.-Y.; Qi, S.-H. Antifouling and antibacterial polyketides from marine gorgonian coral-associated fungus Penicillium sp. SCSGAF 0023. J. Antibiot. 2013, 66, 219–223. [Google Scholar] [CrossRef]
- Wang, C.-N.; Lu, H.-M.; Gao, C.-H.; Guo, L.; Zhan, Z.-Y.; Wang, J.-J.; Liu, Y.-H.; Xiang, S.-T.; Wang, J.; Luo, X.-W. Cytotoxic benzopyranone and xanthone derivatives from a coral symbiotic fungus Cladosporium halotolerans GXIMD 02502. Nat. Prod. Res. 2020, 35, 5596–5603. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.W.; Cui, C.B.; Li, C.W.; Wu, C.J.; Peng, J.X.; Li, D.H. Rare Chromones from a Fungal Mutant of the Marine-Derived Penicillium purpurogenum G59. Mar. Drugs 2015, 13, 5219–5236. [Google Scholar] [CrossRef]
- Li, J.; Zheng, Y.-B.; Kurtan, T.; Liu, M.-X.; Tang, H.; Zhuang, C.-L.; Zhang, W. Anthraquinone derivatives from a coral associated fungus Stemphylium lycopersici. Nat. Prod. Res. 2020, 34, 2116–2123. [Google Scholar] [CrossRef] [PubMed]
- Teiten, M.H.; Mack, F.; Debbab, A.; Aly, A.H.; Dicato, M.; Proksch, P.; Diederich, M. Anticancer effect of altersolanol A, a metabolite produced by the endophytic fungus Stemphylium globuliferum, mediated by its pro-apoptotic and anti-invasive potential via the inhibition of NF-kappaB activity. Bioorg. Med. Chem. 2013, 21, 3850–3858. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.-J.; Shao, C.-L.; Guo, Z.-Y.; Chen, J.-F.; Deng, D.-S.; Yang, K.-L.; Chen, Y.-Y.; Fu, X.-M.; She, Z.-G.; Lin, Y.-C.; et al. Bioactive Hydroanthraquinones and Anthraquinone Dimers from a Soft Coral-Derived Alternaria sp. Fungus. J. Nat.Prod. 2012, 75, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.M.; Wang, C.Y.; Gu, Y.C.; Shao, C.L. Penimethavone A, a flavone from a gorgonian-derived fungus Penicillium chrysogenum. Nat. Prod. Res. 2016, 30, 2274–2277. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, X.-L.; Wang, Y.; Zheng, J.-Y.; Wang, C.-Y.; Shao, C.-L. Aspergivones A and B, two new flavones isolated from a gorgonian-derived Aspergillus candidus fungus. Nat. Prod. Res. 2017, 31, 32–36. [Google Scholar] [CrossRef]
- Shi, T.; Hou, X.-M.; Li, Z.-Y.; Cao, F.; Zhang, Y.-H.; Yu, J.-Y.; Zhao, D.-L.; Shao, C.-L.; Wang, C.-Y. Harzianumnones A and B: Two hydroxyanthraquinones from the coral-derived fungus Trichoderma harzianum. Rsc. Adv. 2018, 8, 27596–27601. [Google Scholar] [CrossRef]
- Lin, Y.R.; Peng, K.C.; Chan, M.H.; Peng, H.L.; Liu, S.Y. Effect of Pachybasin on General Toxicity and Developmental Toxicity in Vivo. J Agric Food Chem. 2017, 65, 10489–10494. [Google Scholar] [CrossRef]
- Deng, M.; Xue, Y.; Xu, L.; Wang, Q.; Wei, J.; Ke, X.; Wang, J.; Chen, X. Chrysophanol exhibits inhibitory activities against colorectal cancer by targeting decorin. Cell Biochem. Funct. 2020, 38, 47–57. [Google Scholar] [CrossRef]
- Maskey, R.P.; Grun-Wollny, I.; Laatsch, H. Isolation, structure elucidation and biological activity of 8-O-methylaverufin and 1,8-O-dimethylaverantin as new antifungal agents from Penicillium chrysogenum. J. Antibiot. 2003, 56, 459–463. [Google Scholar] [CrossRef]
- Sakai, K.; Ohte, S.; Ohshiro, T.; Matsuda, D.; Masuma, R.; Rudel, L.L.; Tomoda, H. Selective inhibition of acyl-CoA: Cholesterol acyltransferase 2 isozyme by flavasperone and sterigmatocystin from Aspergillus species. J. Antibiot. 2008, 61, 568–572. [Google Scholar] [CrossRef]
- Chen, M.; Shao, C.L.; Kong, C.J.; She, Z.G.; Wang, C.Y. A New Anyhraquinone Derivative from A Gorgonian-derived Fungus Aspergillus sp. Chem. Nat. Compd. 2014, 50, 617–620. [Google Scholar] [CrossRef]
- Tang, R.; Kimishima, A.; Setiawan, A.; Arai, M. Secalonic acid D as a selective cytotoxic substance on the cancer cells adapted to nutrient starvation. J. Nat. Med. 2020, 74, 495–500. [Google Scholar] [CrossRef]
- Ren, H.; Tian, L.; Gu, Q.Q.; Zhu, W.M. Secalonic acid D.; A cytotoxic constituent from marine lichen-derived fungus Gliocladium sp. T31. Arch. Pharm. Res. 2006, 29, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Nong, X.H.; Zhang, X.Y.; Xu, X.Y.; Amin, M.; Qi, S.H. Screening of Anti-Biofilm Compounds from Marine-Derived Fungi and the Effects of Secalonic Acid D on Staphylococcus aureus Biofilm. J Microbiol. Biotech. 2017, 27, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-F.; Wang, Y.; Zhang, X.-L.; Wei, M.-Y.; Wang, C.-Y.; Shao, C.-L. Two Dichlorinated Benzophenone Derivatives from the Soft Coral-Derived Pestalotiopsis sp Fungus and Their Antibacterial Activity. Chem. Nat. Compd. 2017, 53, 1174–1176. [Google Scholar] [CrossRef]
- Cueto, M.; Jensen, P.R.; Kauffman, C.; Fenical, W.; Lobkovsky, E.; Clardy, J. Pestalone, a new antibiotic produced by a marine fungus in response to bacterial challenge. J. Nat. Prod. 2001, 64, 1444–1446. [Google Scholar] [CrossRef]
- Augner, D.; Krut, O.; Slavov, N.; Gerbino, D.C.; Sahl, H.G.; Benting, J.; Nising, C.F.; Hillebrand, S.; Kronke, M.; Schmalz, H.G. On the antibiotic and antifungal activity of pestalone, pestalachloride A, and structurally related compounds. J. Nat. Prod. 2013, 76, 1519–1522. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.-Y.; Li, D.; Shao, C.-L.; Deng, D.-S.; Wang, C.-Y. (+/-)-Pestalachloride D, an Antibacterial Racemate of Chlorinated Benzophenone Derivative from a Soft Coral-Derived Fungus Pestalotiopsis sp. Mar. Drugs 2013, 11, 1050–1060. [Google Scholar] [CrossRef]
- Zheng, C.-J.; Fu, X.-M.; Zhang, X.-L.; Kong, W.-W.; Wang, C.-Y. Bioactive Perylene Derivatives from a Soft Coral-Derived Fungus Alternaria sp. (ZJ-2008017). Chem. Nat. Compd. 2015, 51, 766–768. [Google Scholar] [CrossRef]
- Pace, N.R. A molecular view of microbial diversity and the biosphere. Science 1997, 276, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Anyanwu, C.S.; Sorensen, J.L. Secondary Metabolites from a Strain of Alternaria tenuissima Isolated from Northern Manitoba Soil. Nat. Prod. Commun. 2015, 10, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chen, Y.; Tang, X.; Wang, D.; Miao, Y.; Zhang, J.; Li, R.; Zhang, L.; Chen, J. General toxicity and genotoxicity of altertoxin I: A novel 28-day multiendpoint assessment in male Sprague-Dawley rats. J. Appl. Toxicol. 2022, 42, 1310–1322. [Google Scholar] [CrossRef]
- Zhao, D.-L.; Cao, F.; Wang, C.-Y.; Yang, L.-J.; Shi, T.; Wang, K.-L.; Shao, C.-L.; Wang, C.-Y. Alternatone A, an Unusual Perylenequinone-Related Compound from a Soft-Coral-Derived Strain of the Fungus Alternaria alternata. J. Nat. Prod. 2019, 82, 3201–3204. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.X.; Xing, Y.N.; Zhang, M.; Zhao, Y.; Wei, Y.Y.; Zhang, B.; Jiao, R.H. Alternatones A and B, two polyketides possessing novel skeletons from entophyte Alternaria alternate L-10. J. Asian Nat. Prod. Res. 2022, 24, 353–360. [Google Scholar] [CrossRef]
- Wang, M.; Yang, L.; Feng, L.; Hu, F.; Zhang, F.; Ren, J.; Qiu, Y.; Wang, Z. Verruculosins A-B, New Oligophenalenone Dimers from the Soft Coral-Derived Fungus Talaromyces verruculosus. Mar. Drugs 2019, 17, 516. [Google Scholar] [CrossRef]
- Zang, Y.; Genta-Jouve, G.; Escargueil, A.E.; Larsen, A.K.; Guedon, L.; Nay, B.; Prado, S. Antimicrobial Oligophenalenone Dimers from the Soil Fungus Talaromyces stipitatus. J. Nat. Prod. 2016, 79, 2991–2996. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, Y.; Zhang, L.; Liu, Z.; Liu, W.; Xu, H.; Zhang, Q.; Zhang, H.; Yan, Y.; Liu, Z.; et al. Structures and absolute configurations of phomalones from the coral-associated fungus Parengyodontium album sp. SCSIO 40430. Org. Biomol. Chem. 2021, 19, 6030–6037. [Google Scholar] [CrossRef]
- Li, Y.; Tan, Y.; Liu, J.; Zhou, X.; Zeng, S.; Dong, J.; Liu, Y.; Yang, B. A new griseofulvin derivative from a soft coral-derived fungus Eupenicillium sp. SCSIO41208. Nat. Prod. Res. 2020, 34, 2971–2975. [Google Scholar] [CrossRef]
- Li, J.X.; Lei, X.X.; Tan, Y.H.; Liu, Y.H.; Yang, B.; Li, Y.Q. Two new bioactive polyphenols from the soft coral-derived fungus Talaromyces sp. SCSIO 041201. Nat. Prod. Res. 2021, 35, 5778–5785. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Li, P.; Jiang, Y.; Tang, X.; Zhang, W.; Wang, Q.; Li, G. Penitol A and Penicitols E-I: Citrinin Derivatives from Penicillium citrinum and the Structure Revision of Previously Proposed Analogues. J. Nat. Prod. 2021, 84, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xia, G.; Chen, S.; Liu, Y.; Li, H.; She, Z. Eurothiocin A and B, Sulfur-Containing Benzofurans from a Soft Coral-Derived Fungus Eurotium rubrum SH-823. Mar. Drugs 2014, 12, 3669–3680. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Anh, C.V.; Cho, D.Y.; Choi, D.K.; Kang, J.S.; Trinh, P.T.H.; Choi, B.K.; Lee, H.S. New Polyenes from the Marine-Derived Fungus Talaromyces cyanescens with Anti-Neuroinflammatory and Cytotoxic Activities. Molecules 2021, 26, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhao, Q.; Hao, J.D.; Wang, C.Y. Two benzaldehyde derivatives and their artefacts from a gorgonian-derived Eurotium sp. fungus. Nat. Prod. Res. 2017, 31, 268–274. [Google Scholar] [CrossRef]
- Chen, M.; Han, L.; Shao, C.L.; She, Z.G.; Wang, C.Y. Bioactive Diphenyl Ether Derivatives from a Gorgonian-Derived Fungus Talaromyces sp. Chem. Biodivers. 2015, 12, 443–450. [Google Scholar] [CrossRef]
- Shi, T.; Qi, J.; Shao, C.L.; Zhao, D.L.; Hou, X.M.; Wang, C.Y. Bioactive Diphenyl Ethers and Isocoumarin Derivatives from a Gorgonian-Derived Fungus Phoma sp. (TA07-1). Mar. Drugs 2017, 15, 145–151. [Google Scholar] [CrossRef]
- Said, G.; Hou, X.-M.; Liu, X.; Chao, R.; Jiang, Y.-Y.; Zheng, J.-Y.; Shao, C.-L. Antimicrobial and Cytotoxic Activities of Secondary Metabolites from the Soft Coral Derived Fungus Aspergillus sp. Chem. Nat. Compd. 2019, 55, 531–533. [Google Scholar] [CrossRef]
- Liang, T.-M.; Fang, Y.-W.; Zheng, J.-Y.; Shao, C.-L. Secondary Metabolites Isolated from the Gorgonian-Derived Fungus Aspergillus ruber and Their Antiviral Activity. Chem. Nat. Compd. 2018, 54, 559–561. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Lee, U.; Kang, J.S.; Choi, H.D.; Son, B.W. A new radical scavenging anthracene glycoside, asperflavin ribofuranoside, and polyketides from a marine isolate of the fungus Microsporum. Chem. Pharm. Bull. 2006, 54, 882–883. [Google Scholar] [CrossRef]
- Said, G.; Mou, X.-F.; Fang, Y.-W.; Liang, T.-M.; Wei, M.-Y.; Chen, G.-Y.; Shao, C.-L. Secondary Metabolites Isolated from the Soft Coral-Derived Fungus Aspergillus sp. from the South China Sea. Chem. Nat. Compd. 2018, 54, 547–549. [Google Scholar] [CrossRef]
- Chao, R.; Said, G.; Zhang, Q.; Qi, Y.X.; Hu, J.; Zheng, C.J.; Zheng, J.Y.; Shao, C.L.; Chen, G.Y.; Wei, M.Y. Design, Semisynthesis, Insecticidal and Antibacterial Activities of a Series of Marine-Derived Geodin Derivatives and Their Preliminary Structure-Activity Relationships. Mar. Drugs 2022, 20, 82. [Google Scholar] [CrossRef] [PubMed]
- Nuankeaw, K.; Chaiyosang, B.; Suebrasri, T.; Kanokmedhakul, S.; Lumyong, S.; Boonlue, S. First report of secondary metabolites, Violaceol I and Violaceol II produced by endophytic fungus, Trichoderma polyalthiae and their antimicrobial activity. Mycoscience 2020, 61, 16–21. [Google Scholar] [CrossRef]
- Asami, Y.; Jang, J.H.; Oh, H.; Sohn, J.H.; Kim, J.W.; Moon, D.O.; Kwon, O.; Kawatani, M.; Osada, H.; Kim, B.Y.; et al. Violaceols Function as Actin Inhibitors Inducing Cell Shape Elongation in Fibroblast Cells. Biosci. Biotechnol. Biochem. 2012, 76, 1431–1437. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, X.; Xue, M.; Zhao, Z.; Zhang, H.; Xu, D.; Lai, D.; Zhou, L. Recent Advances in Sorbicillinoids from Fungi and Their Bioactivities (Covering 2016-2021). J. Fungi 2022, 8, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Wang, S.; Huo, R.; Li, E.; Wang, M.; Ren, J.; Pan, Y.; Liu, L.; Liu, G. Sorbicillinoid Derivatives with the Radical Scavenging Activities from the Marine-Derived Fungus Acremonium chrysogenum C10. J. Fungi 2022, 8, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhou, Q.; Wang, J.; Liu, J.; Qi, C.; Lai, Y.; Zhu, H.; Xue, Y.; Hu, Z.; Zhang, Y. Anti-inflammatory butenolide derivatives from the coral-derived fungus Aspergillus terreus and structure revisions of aspernolides D and G, butyrolactone VI and 4,8-diacetoxy butyrolactone VI. Rsc. Adv. 2018, 8, 13040–13047. [Google Scholar] [CrossRef]
- Liu, B.; Chen, N.; Xu, Y.; Zhang, J.W.; Sun, Y.; Zhao, L.Z.; Ji, Y.B. A new benzophenone with biological activities from metabolites of butyrolactone I in rat faeces. Nat. Prod. Res. 2021, 35, 2489–2497. [Google Scholar] [CrossRef]
- Nie, Y.; Yang, J.; Zhou, L.; Yang, Z.; Liang, J.; Liu, Y.; Ma, X.; Qian, Z.; Hong, P.; Kalueff, A.V.; et al. Marine fungal metabolite butyrolactone I prevents cognitive deficits by relieving inflammation and intestinal microbiota imbalance on aluminum trichloride-injured zebrafish. J. Neuroinflammation 2022, 19, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, K.L.; Liu, M.; She, Z.G.; Wang, C.Y. Bioactive Steroid Derivatives and Butyrolactone Derivatives from a Gorgonian-Derived Aspergillus sp. Fungus. Chem. Biodivers. 2015, 12, 1398–1406. [Google Scholar] [CrossRef]
- Matsuoka, M.; Wispriyono, B.; Iryo, Y.; Igisu, H.; Sugiura, T. Inhibition of HgCl2-induced mitogen-activated protein kinase activation by LL-Z1640-2 in CCRF-CEM cells. Eur. J. Pharmacol. 2000, 409, 155–158. [Google Scholar] [CrossRef]
- Shao, C.L.; Wu, H.X.; Wang, C.Y.; Liu, Q.A.; Xu, Y.; Wei, M.Y.; Qian, P.Y.; Gu, Y.C.; Zheng, C.J.; She, Z.G.; et al. Potent antifouling resorcylic acid lactones from the gorgonian-derived fungus Cochliobolus lunatus. J. Nat. Prod. 2011, 74, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Hande, S.M.; Uenishi, J.i. Total synthesis of aspergillide B and structural discrepancy of aspergillide A. Tetrahedron Lett. 2009, 50, 189–192. [Google Scholar] [CrossRef]
- Nagasawa, T.; Kuwahara, S. Enantioselective synthesis of aspergillide B. Biosci. Biotechnol. Biochem. 2009, 73, 1893–1894. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, B.; Li, F.; Liu, M.; Lin, S.; Wang, J.; Xue, Y.; Zhu, H.; Sun, W.; Hu, Z.; et al. Mycophenolic Acid Derivatives with Immunosuppressive Activity from the Coral-Derived Fungus Penicillium bialowiezense. Mar. Drugs 2018, 16, 230–239. [Google Scholar] [CrossRef]
- Abou-Mansour, E.; Debieux, J.L.; Ramirez-Suero, M.; Benard-Gellon, M.; Magnin-Robert, M.; Spagnolo, A.; Chong, J.; Farine, S.; Bertsch, C.; L‘Haridon, F.; et al. Phytotoxic metabolites from Neofusicoccum parvum, a pathogen of Botryosphaeria dieback of grapevine. Phytochemistry 2015, 115, 207–215. [Google Scholar] [CrossRef]
- Tian, Y.Q.; Lin, X.P.; Liu, J.; Kaliyaperumal, K.; Ai, W.; Ju, Z.R.; Yang, B.; Wang, J.; Yang, X.W.; Liu, Y. Ascomycotin A, a new citromycetin analogue produced by Ascomycota sp. Ind19F07 isolated from deep sea sediment. Nat. Prod. Res. 2015, 29, 820–826. [Google Scholar] [CrossRef]
- Peng, Q.; Cai, J.; Long, J.; Yang, B.; Lin, X.; Wang, J.; Xiao, J.; Liu, Y.; Zhou, X. New azaphthalide and phthalide derivatives from the marine coral-derived fungus Aspergillus sp. SCSIO41405. Phytochemistry Lett. 2021, 43, 94–97. [Google Scholar] [CrossRef]
- Chen, M.; Shao, C.-L.; Wang, K.-L.; Xu, Y.; She, Z.-G.; Wang, C.-Y. Dihydroisocoumarin derivatives with antifouling activities from a gorgonian-derived Eurotium sp. fungus. Tetrahedron 2014, 70, 9132–9138. [Google Scholar] [CrossRef]
- Li, J.; Tao, H.; Lei, X.-x.; Zhang, H.; Zhou, X.; Liu, Y.; Li, Y.; Yang, B. Arthriniumsteroids A-D, four new steroids from the soft coral-derived fungus Simplicillium lanosoniveum SCSIO41212. Steroids 2021, 171, 108831. [Google Scholar] [CrossRef]
- Long, J.-Y.; Wang, J.-F.; Liao, S.-R.; Lin, X.-P.; Zhou, X.-F.; Li, Y.-Q.; Yang, B.; Liu, Y.-H. Four new steroids from the marine soft coral-derived fungus Penicillium sp. SCSIO41201. Chinese J. Nat. Med. 2020, 18, 250–255. [Google Scholar] [CrossRef]
- Sun, D.-W.; Cao, F.; Liu, M.; Guan, F.-F.; Wang, C.-Y. New Fatty Acid From a Gorgonian-Derived Xylaria sp. Fungus. Chem. Nat. Compd. 2017, 53, 227–230. [Google Scholar] [CrossRef]
- Yu, M.L.; Guan, F.F.; Cao, F.; Jia, Y.L.; Wang, C.Y. A new antiviral pregnane from a gorgonian-derived Cladosporium sp. fungus. Nat. Prod. Res. 2018, 32, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Kusari, S.; Golz, C.; Laatsch, H.; Strohmann, C.; Spiteller, M. Epigenetic Modulation of Endophytic Eupenicillium sp. LG41 by a Histone Deacetylase Inhibitor for Production of Decalin-Containing Compounds. J. Nat. Prod. 2017, 80, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Mao, W.; Yan, M.; Zhao, C.; Li, N.; Shan, J.; Lin, C.; Liu, X.; Guo, T.; Guo, T.; et al. Galactomannan with novel structure produced by the coral endophytic fungus Aspergillus ochraceus. Carbohyd. Polym. 2014, 105, 325–333. [Google Scholar] [CrossRef]
- Liu, D.-H.; Sun, Y.-Z.; Kurtan, T.; Mandi, A.; Tang, H.; Li, J.; Su, L.; Zhuang, C.-L.; Liu, Z.-Y.; Zhang, W. Osteoclastogenesis Regulation Metabolites from the Coral-Associated Fungus Pseudallescheria boydii TW-1024-3. J. Nat. Prod. 2019, 82, 1274–1282. [Google Scholar] [CrossRef]
- Mehedi, M.A.U.; Molla, A.H.; Khondkar, P.; Sultana, S.; Islam, M.A.; Rashid, M.A.; Chowdhury, R. Pseurotin A: An Antibacterial Secondary Metabolite from Aspergillus fumigatus. Asian J. Chem. 2010, 22, 2611–2614. [Google Scholar]
- Schmeda-Hirschmann, G.; Hormazabal, E.; Rodriguez, J.A.; Theoduloz, C. Cycloaspeptide A and pseurotin A from the endophytic fungus Penicillium janczewskii. J. Biosci. 2008, 63, 383–388. [Google Scholar] [CrossRef]
- Ishikawa, M.; Ninomiya, T.; Akabane, H.; Kushida, N.; Tsujiuchi, G.; Ohyama, M.; Gomi, S.; Shito, K.; Murata, T. Pseurotin A and its analogues as inhibitors of immunoglobuline E production. Bioorg. Med. Chem. Lett. 2009, 19, 1457–1460. [Google Scholar] [CrossRef]
- Chung, M.C.; Lee, H.J.; Chun, H.K.; Kho, Y.H. Penicillide, a nonpeptide calpain inhibitor, produced by Penicillium sp. F60760. J. Microbiol. Biotechnol. 1998, 8, 188–190. [Google Scholar]
- Yimnual, C.; Satitsri, S.; Ningsih, B.N.S.; Rukachaisirikul, V.; Muanprasat, C. A fungus-derived purpactin A as an inhibitor of TMEM16A chloride channels and mucin secretion in airway epithelial cells. Biomed. Pharmacother. 2021, 139, 111583. [Google Scholar] [CrossRef] [PubMed]
- Sy-Cordero, A.A.; Figueroa, M.; Raja, H.A.; Avina, M.E.M.; Croatt, M.P.; Adcock, A.F.; Kroll, D.J.; Wani, M.C.; Pearce, C.J.; Oberlies, N.H. Spiroscytalin, a new tetramic acid and other metabolites of mixed biogenesis from Scytalidium cuboideum. Tetrahedron 2015, 71, 8899–8904. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.X.; Sun, D.W.; Zheng, C.J.; Wang, C.Y. A new hexahydrobenzopyran derivative from the gorgonian-derived Fungus Eutypella sp. Nat. Prod. Res. 2017, 31, 1640–1646. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.X.; Tan, H.B.; Chen, K.; Zhao, L.Y.; Chen, Y.C.; Li, S.N.; Li, H.H.; Zhang, W.M. Cytosporins A-D, novel benzophenone derivatives from the endophytic fungus Cytospora rhizophorae A761. Org. Biomol. Chem. 2019, 17, 2346–2350. [Google Scholar] [CrossRef]
- Cao, F.; Yang, Q.; Shao, C.L.; Kong, C.J.; Zheng, J.J.; Liu, Y.F.; Wang, C.Y. Bioactive 7-Oxabicyclic [6.3.0] lactam and 12-Membered Macrolides from a Gorgonian-Derived Cladosporium sp. Fungus. Mar. Drugs 2015, 13, 4171–4178. [Google Scholar] [CrossRef]
- Noman, E.; Al-Shaibani, M.M.; Bakhrebah, M.A.; Almoheer, R.; Al-Sahari, M.; Al-Gheethi, A.; Mohamed, R.M.S.R.; Almulaiky, Y.Q.; Abdulaal, W.H. Potential of Anti-Cancer Activity of Secondary Metabolic Products from Marine Fungi. J. Fungi 2021, 7, 436. [Google Scholar] [CrossRef]
- Knox, B.P.; Keller, N.P. Key Players in the Regulation of Fungal Secondary Metabolism, Biosynthesis and Molecular Genetics of Fungal Secondary Metabolites; Springer: New York, NY, USA, 2015; pp. 13–28. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).