Nitric Oxide Negatively Regulates the Rapid Formation of Pleurotus ostreatus Primordia by Inhibiting the Mitochondrial aco Gene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains
2.2. Mushroom Production
2.3. Determination of NO Content
2.4. Experiment with the Addition of Exogenous Sodium Nitroprusside (SNP) or 2-(4-Carboxyphenyl)-4,4,5,5-Tetramethylimidazoline-1-Oxyl-3-Oxide (cPTIO)
2.5. Determination of ACO Activity
2.6. H2O2 Content Determination
2.7. Measurement of the Activities of Isocitrate Dehydrogenase of Mitochondria (ICDHm) and α-Ketoglutarate Dehydrogenase (α-KGDH)
2.8. NADH and NAD+ Content Determination
2.9. Adenosine Triphosphate (ATP) Content Determination
2.10. Western Blot Analysis
2.11. Quantitative Real-Time PCR (qPCR)
2.12. Experiment with the Addition of Exogenous H2O2 or N,N′-Dimethylthiourea (DMTU)
2.13. Data Analysis
3. Results
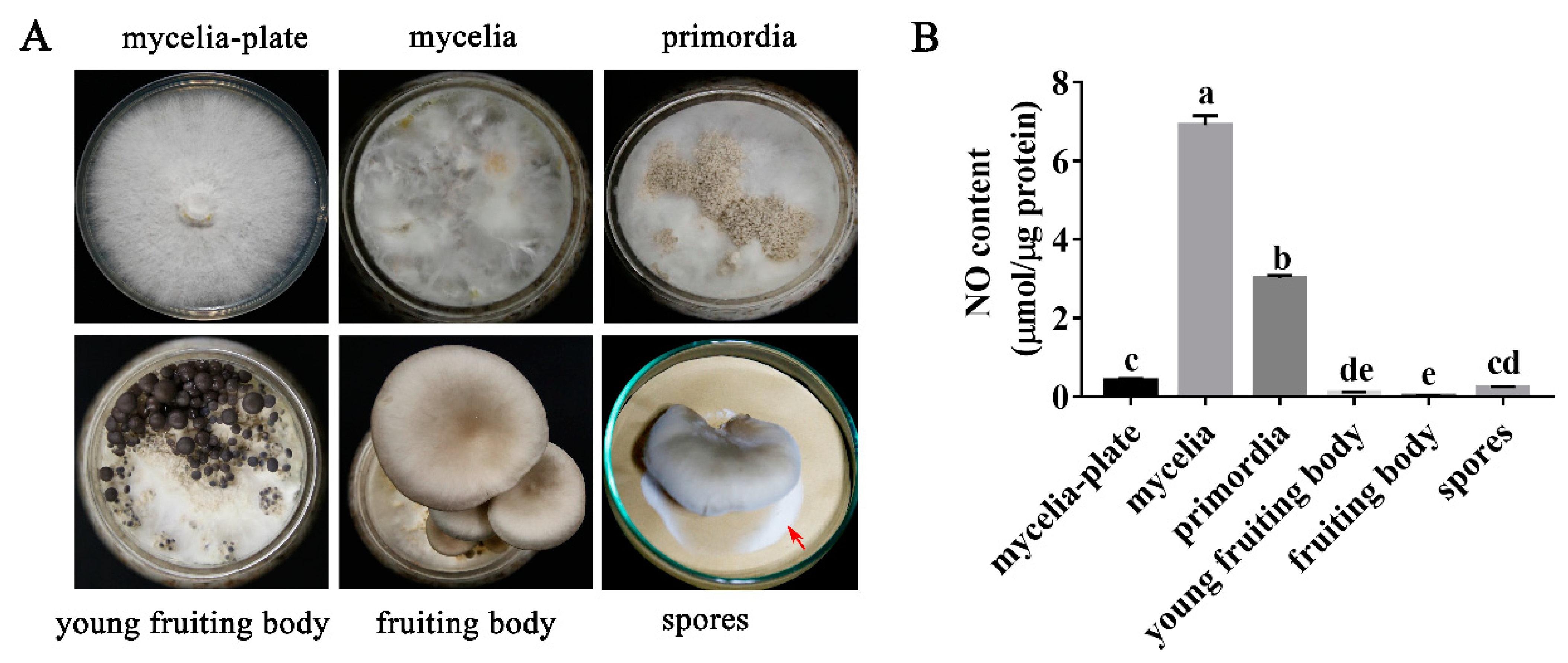
3.1. NO Content in P. ostreatus Varies in Different Developmental Stages
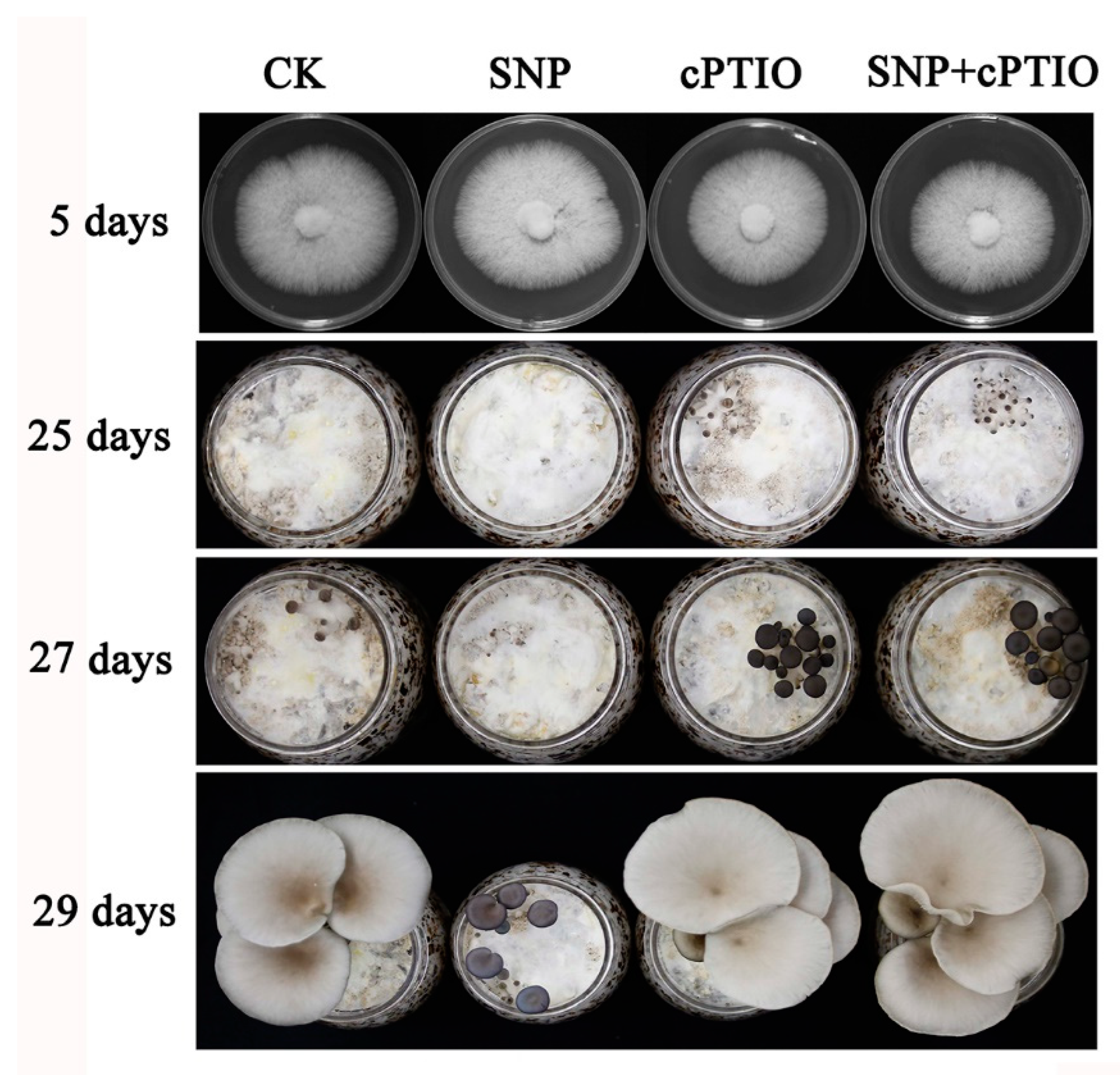
3.2. NO Plays a Negative Role in Primordia Formation
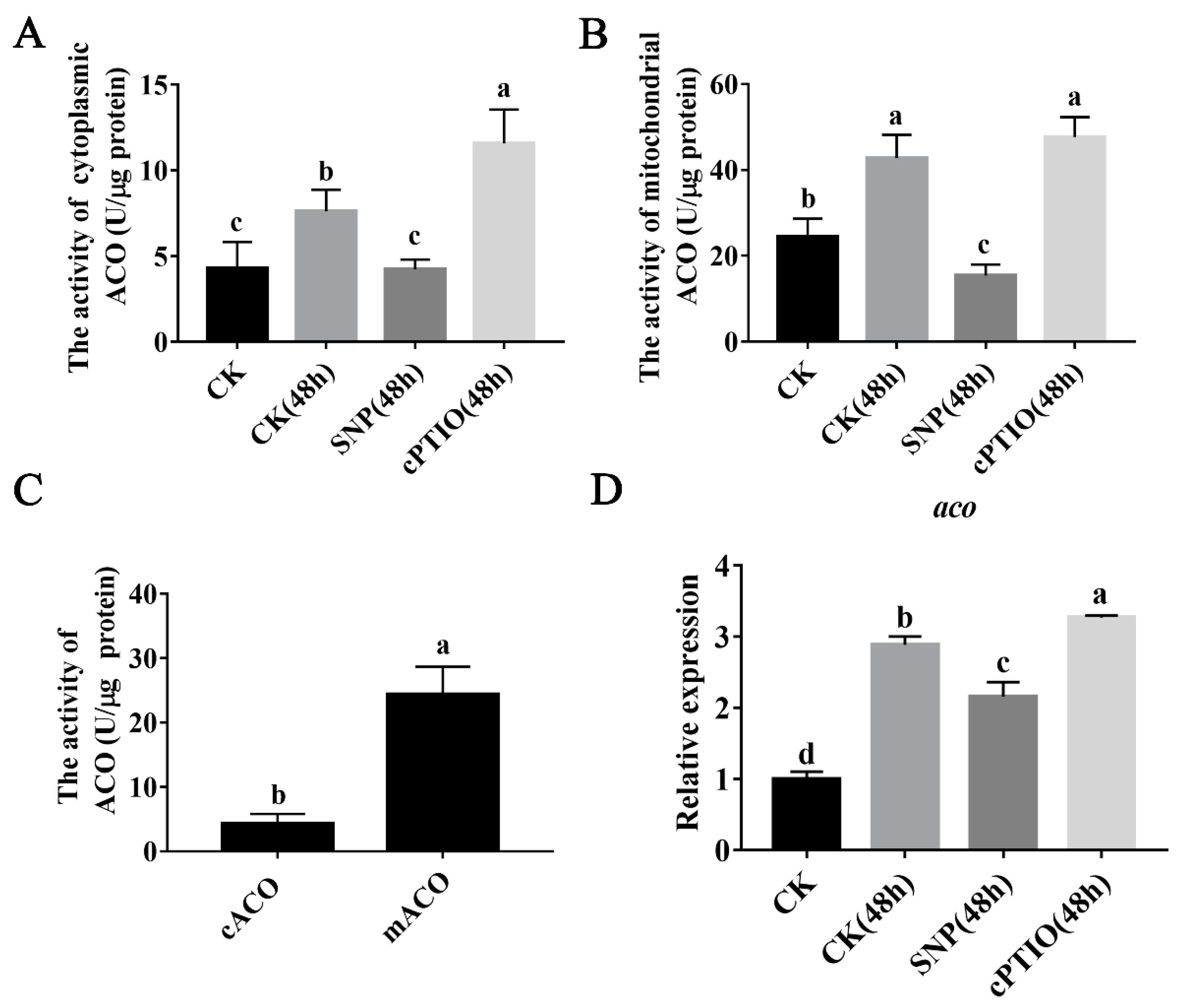
3.3. NO Inhibits ACO Enzyme Activity and Mitochondrial aco Gene Expression
3.4. The aco Gene Is Involved in Primordia Formation
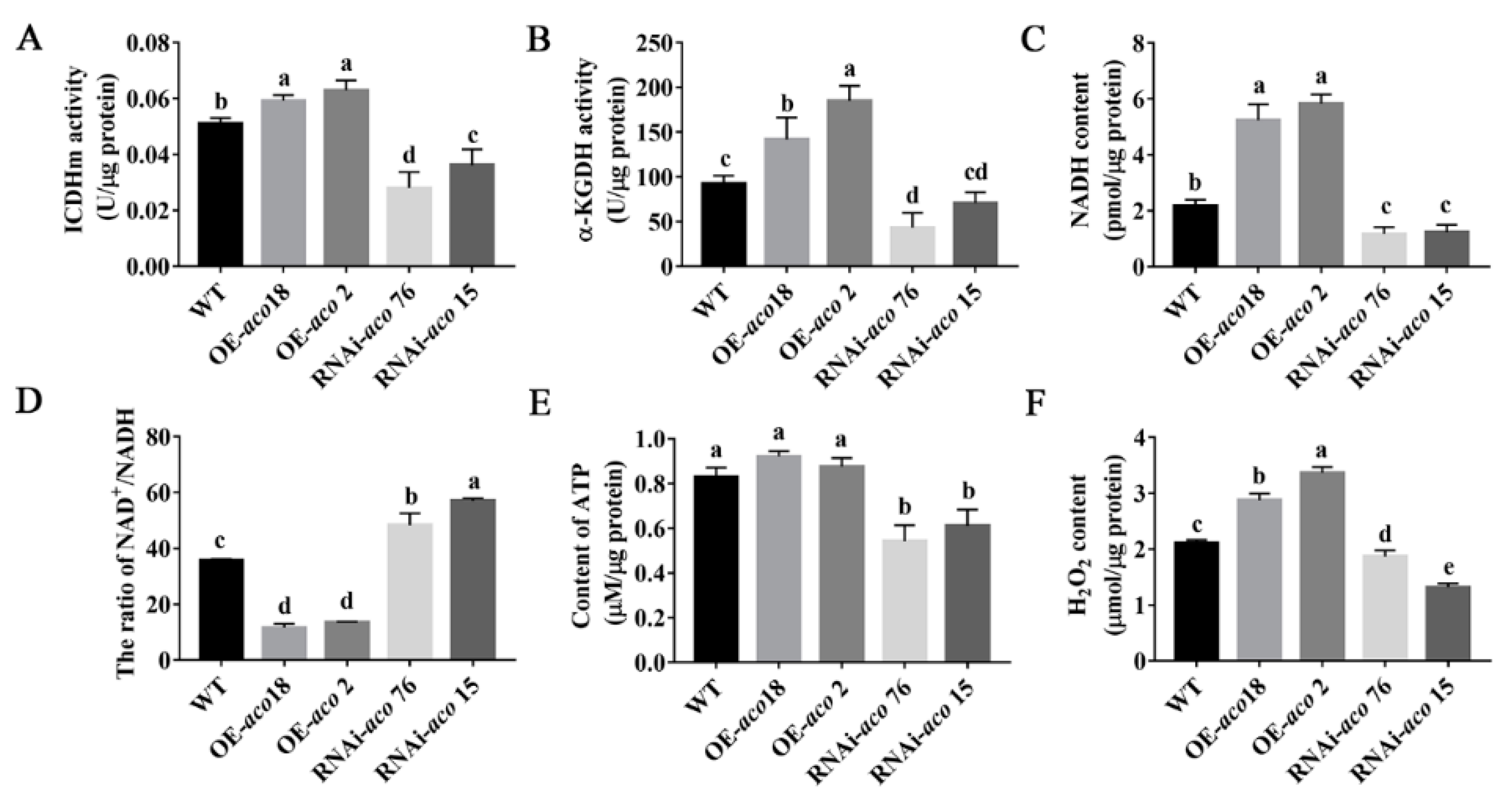
3.5. aco Gene Interference Affects Energy Metabolism and Regulates H2O2 Production and Accumulation
3.6. H2O2 Plays an Important Role in P. ostreatus Development
3.7. H2O2 Promotes the Rapid Formation of Primordia
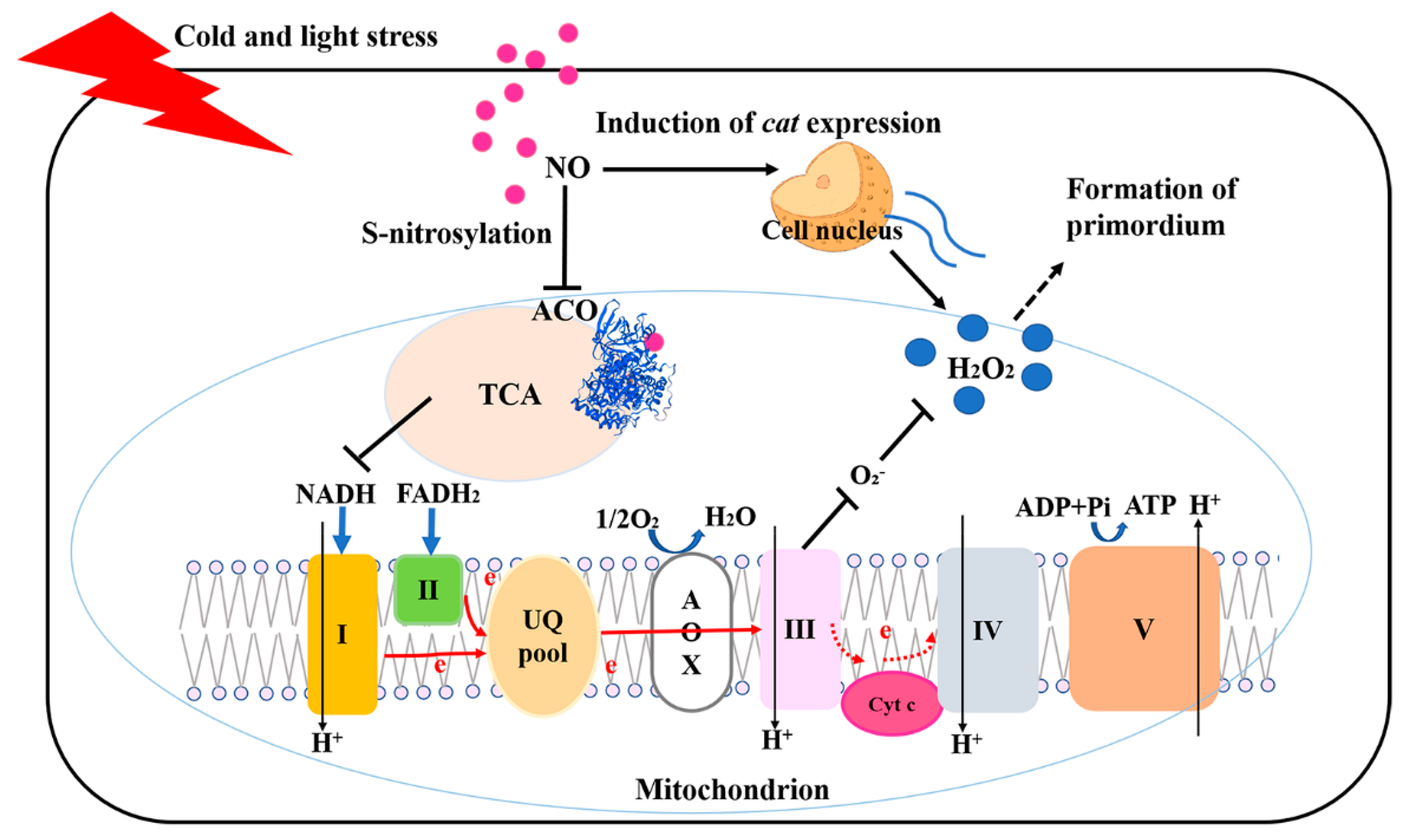
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kües, U.; Liu, Y. Fruiting body production in basidiomycetes. Appl. Microbiol. Biot. 2000, 54, 141–152. [Google Scholar] [CrossRef]
- Khan, M.A.; Tania, M. Nutritional and medicinal importance of Pleurotus mushrooms: An overview. Food Rev. Int. 2012, 28, 313–329. [Google Scholar] [CrossRef]
- Sánchez, C. Cultivation of Pleurotus ostreatus and other edible mushrooms. Appl. Microbiol. Biot. 2010, 85, 1321–1337. [Google Scholar] [CrossRef]
- Zhang, J.J.; Ren, A.; Chen, H.; Zhao, M.W.; Shi, L.; Chen, M.J.; Wang, H.; Feng, Z.Y. Transcriptome analysis and its application in identifying genes associated with fruiting body development in basidiomycete Hypsizygus marmoreus. PLoS ONE 2015, 10, e0123025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, D.S.; Li, C.Y.; Zhang, X.C.; Li, X.B.; Shi, L.; Ren, A.; Zhao, M.W. Functions of the nicotinamide adenine dinucleotide phosphate oxidase family in Ganoderma lucidum: An essential role in ganoderic acid biosynthesis regulation, hyphal branching, fruiting body development, oxidative-stress resistance, and ganoderic acid biosynthesis regulation. Environ. Microbiol. 2014, 16, 1709–1728. [Google Scholar] [CrossRef] [PubMed]
- Terashima, K.; Yuki, K.; Muraguchi, H.; Akiyama, M.; Kamada, T. The dst1 gene involved in mushroom photomorphogenesis of Coprinus cinereus encodes a putative photoreceptor for blue light. Genetics 2005, 171, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.J.; Guo, L.X.; Zhao, J.J.; Xie, B.G. Sequence characterization and differential expression of a glutathione S-transferase gene vv-gto1 from Volvariella volvacea. Acta Microbiol. Sin. 2014, 54, 1171–1177. (In Chinese) [Google Scholar]
- Hsu, K.H.; Lee, Y.R.; Lin, Y.L.; Chu, F.H. Cytochrome P450 genes in medicinal mushroom Antrodia cinnamomea T.T. Chang et W.N. Chou (higher Basidiomycetes) are strongly expressed during fruiting body formation. Int. J. Med. Mushrooms 2011, 13, 513–523. [Google Scholar] [CrossRef]
- Yan, J.; Lei, Z.; Wang, R.; Xie, B.; Xiao, L.; Chen, R.; Guo, L.; Xie, B. The sequence characteristics and expression models reveal superoxide dismutase involved in cold response and fruiting body development in Volvariella volvacea. Int. J. Mol. Sci. 2016, 17, 34. [Google Scholar] [CrossRef] [Green Version]
- Palmer, G.E.; Stephen, H.J. Mushrooms by magic: Making connections between signal transduction and fruiting body development in the basidiomycete fungus Schizophyllum commune. FEMS. Microbiol. Lett. 2006, 262, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Joh, J.H.; Lee, S.H.; Lee, J.S.; Kim, K.H.; Jeong, S.J.; Youn, W.H.; Kim, N.K.; Son, E.S.; Cho, Y.S.; Yoo, Y.B.; et al. Isolation of genes expressed during the developmental stages of the oyster mushroom, Pleurotus ostreatus, using expressed sequence tags. FEMS. Microbiol. Lett. 2007, 276, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Beligni, M.V.; Lamattina, L. Nitric oxide stimulates seed germination and de-etiolation, and inhibits hypocotyl elongation, three light-inducible responses in plants. Planta 2000, 210, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Neill, S.J.; Desikan, R.; Clarke, A.; Hancock, J.T. Nitric oxide is a novel component of abscisic acid signaling in stomatal guard cells. Plant Physiol. 2002, 128, 13–16. [Google Scholar] [CrossRef]
- Graziano, M.; Beligni, M.V.; Lamattina, L. Nitric oxide improves internal iron availability in plants. Plant Physiol. 2002, 130, 1852–1859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leshem, Y.A.Y.; Wills, R.B.H.; Ku, V.V. Evidence for the function of the free radical gas—Nitric oxide (NO)—As an endogenous maturation and senescence regulating factor in higher plants. Plant Physiol. Bioch. 1998, 36, 825–833. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Lim, J.Y.; Xu, J.Y.; Yu, J.H.; Zheng, W.F. Nitric oxide as a developmental and metabolic signal in filamentous fungi. Mol. Microbiol. 2020, 113, 872–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcos, A.T.; Ramos, M.S.; Schinko, T.; Strauss, J.; Canovas, D. Nitric oxide homeostasis is required for light-dependent regulation of conidiation in Aspergillus. Fungal. Genet. Biol. 2020, 137, 103337. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.X.; Yuan, W.W.; Sun, M.N.; Zhang, X.G.; Zheng, W.F. Regulatory effects of nitric oxide on reproduction and melanin biosynthesis in onion pathogenic fungus Stemphylium eturmiunum. Fungal Biol. 2021, 125, 519–531. [Google Scholar] [CrossRef]
- Hou, L.D.; Zhao, M.R.; Huang, C.Y.; Wu, X.L.; Zhang, J.X. Nitric oxide improves the tolerance of Pleurotus ostreatus to heat stress by inhibiting mitochondrial aconitase. Appl. Environ. Microbiol. 2020, 86, e02303–e02319. [Google Scholar] [CrossRef] [Green Version]
- Makoto, H.; Luigi, D.B.; Amedeo, A.; Mikio, N. Cytosolic aconitase participates in the glyoxylate cycle in etiolated pumpkin cotyledons. Plant Cell Physiol. 1995, 36, 669–680. [Google Scholar] [CrossRef]
- Beinert, H.; Kennedy, M.C.; Stout, C.D. Aconitase as ironminus signsulfur protein, enzyme, and iron-regulatory protein. Chem. Rev. 1996, 96, 2335. [Google Scholar] [CrossRef]
- Tang, Y.; Guest, J.R. Direct evidence for mRNA binding and post-transcriptional regulation by Escherichia coli aconitases. Microbiology 1999, 145, 3069–3079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Commichau, F.M.; Stülke, J. Trigger enzymes: Bifunctional proteins active in metabolism and in controlling gene expression. Mol. Microbiol. 2008, 67, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Moeder, W.; Pozo, O.D.; Navarre, D.A.; Martin, G.B.; Klessig, D.F. Aconitase plays a role in regulating resistance to oxidative stress and cell death in Arabidopsis and Nicotiana benthamiana. Plant Mol. Biol. 2007, 63, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Carrari, F.; Nunes-Nesi, A.; Gibon, Y.; Lytovchenko, A.; Loureiro, M.E.; Fernie, A.R. Reduced expression of aconitase results in an enhanced rate of photosynthesis and marked shifts in carbon partitioning in illuminated leaves of wild species tomato. Plant Physiol. 2003, 133, 1322–1335. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Guest, J.R.; Artymiuk, P.J.; Read, R.C.; Green, J. Post-transcriptional regulation of bacterial motility by aconitase proteins. Mol. Microbiol. 2004, 51, 1817–1826. [Google Scholar] [CrossRef] [Green Version]
- Michta, E.; Schad, K.; Kai, B.; Ort-Winklbauer, R.; Mast, Y. The bifunctional role of aconitase in Streptomyces viridochromogenes Tü494. Environ. Microbiol. 2012, 14, 3203–3219. [Google Scholar] [CrossRef]
- Qu, J.B.; Zhao, M.R.; Tom, H.; Feng, X.X.; Zhang, J.X.; Huang, C.Y. Identification and characterization of small noncoding RNAs in genome sequences of the edible fungus Pleurotus ostreatus. Biomed. Res. Int. 2016, 2016, 2503023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.N.; Wu, X.L.; Gao, W.; Zhao, M.R.; Zhang, J.X.; Huang, C.Y. Differential expression patterns of Pleurotus ostreatus catalase genes during developmental stages and under heat stress. Genes 2017, 8, 335. [Google Scholar] [CrossRef] [Green Version]
- Buchanan, B.B.; Gruissem, W.; Jones, R.L. Biochemistry and Molecular Biology of Plants, 2nd ed.; Wiley: Hoboken, NJ, USA, 2015; pp. 585–588. [Google Scholar]
- Hu, W.Y.; Zhang, X.Y.; Godana, E.A.; Gu, X.Y.; Zhao, L.N.; Zhang, H.Y. Yarrowia lipolytica reduces the disease incidence of asparagus infected by Fusarium proliferatum by affecting respiratory metabolism and energy status. Biol. Control 2021, 159, 104625. [Google Scholar] [CrossRef]
- Hou, L.D.; Wang, L.N.; Wu, X.L.; Gao, W.; Zhang, J.X.; Huang, C.Y. Expression patterns of two pal genes of Pleurotus ostreatus across developmental stages and under heat stress. BMC Microbiol. 2019, 19, 231. [Google Scholar] [CrossRef] [PubMed]
- Mora-Lugo, R.; Zimmermann, J.; Rizk, A.M.; Fernandez-Lahore, M. Development of a transformation system for Aspergillus sojae based on the Agrobacterium tumefaciens-mediated approach. BMC Microbiol. 2014, 14, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, M.; Wu, X.L.; Huang, C.Y.; Qiu, Z.H.; Zhang, J.X. Trehalose induced by reactive oxygen species relieved the radial growth defects of Pleurotus ostreatus under heat stress. Appl. Microbiol. Biotechnol. 2019, 103, 5379–5390. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Z.; Wang, A.; Zou, Y.J.; Su, N.; Loscalzo, J.; Yang, Y. In vivo monitoring of cellular energy metabolism using SoNar, a highly responsive sensor for NAD+/NADH redox state. Nat. Protoc. 2016, 11, 1345–1359. [Google Scholar] [CrossRef]
- Ying, W.H. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: Regulation and biological consequences. Antioxid Redox Signal. 2008, 10, 179–206. [Google Scholar] [CrossRef] [Green Version]
- Niu, L.J.; Liao, W.B. Hydrogen peroxide signaling in plant development and abiotic responses: Crosstalk with nitric oxide and calcium. Front. Plant Sci. 2016, 7, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, L.D.; Liu, Z.Q.; Yan, K.X.; Xu, L.J.; Chang, M.C.; Meng, J.L. Mnsod1 promotes the development of Pleurotus ostreatus and enhances the tolerance of mycelia to heat stress. Microb. Cell Fact. 2022, 21, 155. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.B.; Huang, G.B.; Yu, J.H.; Zhang, M.L. Nitric oxide and hydrogen peroxide alleviate drought stress in marigold explants and promote its adventitious root development. Plant Physiol. Biochem. 2012, 58, 6–15. [Google Scholar] [CrossRef]
- Corpas, F.J.; Leterrier, M.; Valderrama, R.; Airaki, M.; Chaki, M.; Palma, J.M.; Barroso, J.B. Nitric oxide imbalance provokes a nitrosative response in plants under abiotic stress. Plant Sci. 2011, 181, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Seligman, K.; Saviani, E.E.; Oliveira, H.C.; Pinto-Maglio, C.A.; Salgado, I. Floral transition and nitric oxide emission during flower development in Arabidopsis thaliana is affected in nitrate reductase-deficient plants. Plant Cell Physiol. 2008, 49, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.W.; Luo, S.; Zhang, G.C.; Feng, L.Y.; Zheng, C.; Zhou, Y.H.; Du, J.B.; Yuan, M.; Chen, Y.E.; Wang, C.Q.; et al. Nitric oxide induces monosaccharide accumulation through enzyme S-nitrosylation. Plant Cell Environ. 2017, 40, 1834–1848. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Geisler, M.; Johansson, H.; Harholt, J.; Scheller, H.V.; Mellerowicz, E.J.; Kleczkowski, L.A. UDP-glucose pyrophosphorylase is not rate limiting, but is essential in Arabidopsis. Plant Cell Physiol. 2009, 50, 998–1011. [Google Scholar] [CrossRef] [Green Version]
- Lushchak, O.V.; Piroddi, M.; Galli, F.; Lushchak, V.I. Aconitase post-translational modification as a key in linkage between Krebs cycle, iron homeostasis, redox signaling, and metabolism of reactive oxygen species. Redox. Rep. 2014, 19, 8–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarre, D.A.; Wendehenne, D.; Durner, J.; Noad, R.; Klessig, D.F. Nitric oxide modulates the activity of tobacco aconitase. Plant Physiol. 2000, 122, 573–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Zhu, T.; Yang, T.; Yang, Z.Y.; Ren, A.; Shi, L.; Zhu, J.; Yu, H.S.; Zhao, M.W. Nitric oxide regulates ganoderic acid biosynthesis by the S-nitrosylation of aconitase under heat stress in Ganoderma lucidum. Environ. Microbiol. 2021, 23, 682–695. [Google Scholar] [CrossRef]
- Peyret, P.; Perez, P.; Alric, M. Structure, genomic organization, and expression of the Arabidopsis thaliana aconitase gene: Plant aconitase show significant homology with mammalian iron-responsive element-binding protein. J. Biol. Chem. 1995, 270, 8131–8137. [Google Scholar] [CrossRef] [Green Version]
- Gruer, M.J.; Artymiuk, P.J.; Guest, J.R. The aconitase family: Three structural variations on a common theme. Trends. Biochem. Sci. 1997, 22, 3–6. [Google Scholar] [CrossRef]
- Esposito, G.; Vos, M.; Vilain, S.; Swerts, J.; Jorge, D.S.V.; Meensel, S.V.; Onno, S.; Verstreken, P. Aconitase causes iron toxicity in Drosophila pink1 mutants. PLoS Genet. 2013, 9, e1003478. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.J.; Hu, C.C.; Xie, B.G.; Zhang, L.; Yan, S.J.; Wang, W.; Tao, Y.X.; Li, S.J. A single transcription factor PDD1 determines development and yield of Winter Mushroom Flammulina velutipes. Appl. Environ. Microbiol. 2019, 85, e01735-19. [Google Scholar] [CrossRef]
- Somerville, G.A.; Chaussee, M.S.; Morgan, C.I.; Fitzgerald, J.R.; Dorward, D.W.; Reitzer, L.J.; Musser, J.M. Staphylococcus aureus aconitase inactivation unexpectedly inhibits post-exponential-phase growth and enhances stationary-phase survival. Infect. Immun. 2002, 70, 6373–6382. [Google Scholar] [CrossRef] [Green Version]
- Viollier, P.H.; Nguyen, K.T.; Minas, W.; Folcher, M.; Dale, G.E.; Thompson, C.J. Roles of Aconitase in growth, metabolism, and morphological differentiation of Streptomyces coelicolor. J. Bacteriol. 2001, 183, 3193–3203. [Google Scholar] [CrossRef]
- Wilson, T.J.G.; Bertrand, N.; Tang, J.L.; Feng, J.X.; Pan, M.Q.; Barber, C.E.; Dow, M.J.; Daniels, M.J. The rpfA gene of Xanthomonas campestris pathovar campestris, which is involved in the regulation of pathogenicity factor production, encodes an aconitase. Mol. Microbiol. 1998, 28, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qin, R.Y.; Li, H.; Xu, R.F.; Qiu, C.H.; Sun, Y.C.; Ma, H.; Yang, Y.C.; Ni, D.H.; Li, L.; et al. Identification and analysis of the mechanism underlying heat-inducible expression of rice aconitase 1. Plant Sci. 2015, 233, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Sweetlove, L.J.; Heazlewood, J.L.; Herald, V.; Holtzapffel, R.; Day, D.A.; Millar, C.J. The impact of oxidative stress on Arabidopsis mitochondria. Plant J. 2003, 32, 891–904. [Google Scholar] [CrossRef] [PubMed]
- Fayssal, S.A.; Alsanad, M.A.; Yordanova, M.H.; Sebaaly, Z.E.; Najjar, R.; Sassine, Y.N. Effect of olive pruning residues on substrate temperature and production of oyster mushroom (Pleurotus ostreatus). Acta Hortic. 2021, 1327, 245–252. [Google Scholar] [CrossRef]
- Du, D.F.; Gao, X.; Geng, J.; Li, Q.Y.; Li, L.Q.; Lv, Q.; Li, X.J. Identification of key proteins and networks related to grain development in wheat (Triticum aestivum L.) by comparative transcription and proteomic analysis of allelic variants in TaGW2-6A. Front. Plant Sci. 2016, 7, 922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweetlove, L.J.; Katherine, F.M.B.; Nunes-Nesi, A.; Fernie, A.; Ratcliffe, R.G. Not just a circle: Flux modes in the plant TCA cycle. Trends Plant Sci. 2010, 15, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.M.; Qu, J.T.; Zhang, L.; Xu, X.Y.; Wei, G.; Zhao, Z.F.; Ren, M.Z.; Cao, M. Identification and characterization of the TCA cycle genes in maize. BMC Plant Biol. 2019, 19, 592. [Google Scholar] [CrossRef] [Green Version]
- Nazir, F.; Hussain, A.; Fariduddin, Q. Hydrogen peroxide modulate photosynthesis and antioxidant systems in tomato (Solanum lycopersicum L.) plants under copper stress. Chemosphere 2019, 230, 544–558. [Google Scholar] [CrossRef]
- Bagheri, M.; Gholami, M.; Baninasab, B. Hydrogen peroxide-induced salt tolerance in relation to antioxidant systems in pistachio seedlings. Sci. Hortic-Amsterdam. 2019, 243, 207–213. [Google Scholar] [CrossRef]
- Zhou, B.; Li, N.; Zhang, Z.; Huang, X.; Chen, H.; Hu, Z.; Pang, X.; Liu, W.; Lu, Y. Hydrogen peroxide and nitric oxide promote reproductive growth in Litchi chinensis. Bio. Plant. 2012, 56, 321–329. [Google Scholar] [CrossRef]



| Primer | Sequence (5′→3′) | Note |
|---|---|---|
| qPCR_β-actin-F | AGTCGGTGCCTTGGTTAT | qPCR |
| qPCR_β-actin-R | ATACCGACCATCACACCT | |
| qPCR_aco-F | CCTCACCGTTCTCAATGTT | |
| qPCR_aco-R | CGACGAAGGCATGAGTAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, L.; Huang, C.; Wu, X.; Zhang, J.; Zhao, M. Nitric Oxide Negatively Regulates the Rapid Formation of Pleurotus ostreatus Primordia by Inhibiting the Mitochondrial aco Gene. J. Fungi 2022, 8, 1055. https://doi.org/10.3390/jof8101055
Hou L, Huang C, Wu X, Zhang J, Zhao M. Nitric Oxide Negatively Regulates the Rapid Formation of Pleurotus ostreatus Primordia by Inhibiting the Mitochondrial aco Gene. Journal of Fungi. 2022; 8(10):1055. https://doi.org/10.3390/jof8101055
Chicago/Turabian StyleHou, Ludan, Chenyang Huang, Xiangli Wu, Jinxia Zhang, and Mengran Zhao. 2022. "Nitric Oxide Negatively Regulates the Rapid Formation of Pleurotus ostreatus Primordia by Inhibiting the Mitochondrial aco Gene" Journal of Fungi 8, no. 10: 1055. https://doi.org/10.3390/jof8101055







