Transcriptomic Dynamics of Active and Inactive States of Rho GTPase MoRho3 in Magnaporthe oryzae
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain Cultivation and Plant Inoculation
2.2. RNA Extraction, Sequencing Library Construction, and Illumina Sequencing
2.3. Reads Alignment and Normalization of Gene Expression Levels
2.4. Identification of DEGs and Secreted Proteins
2.5. Quantitative Real-Time PCR
2.6. Functional Annotation and Enrichment Analysis of DEGs
2.7. Identification of Rho3 Interacting Proteins
3. Results
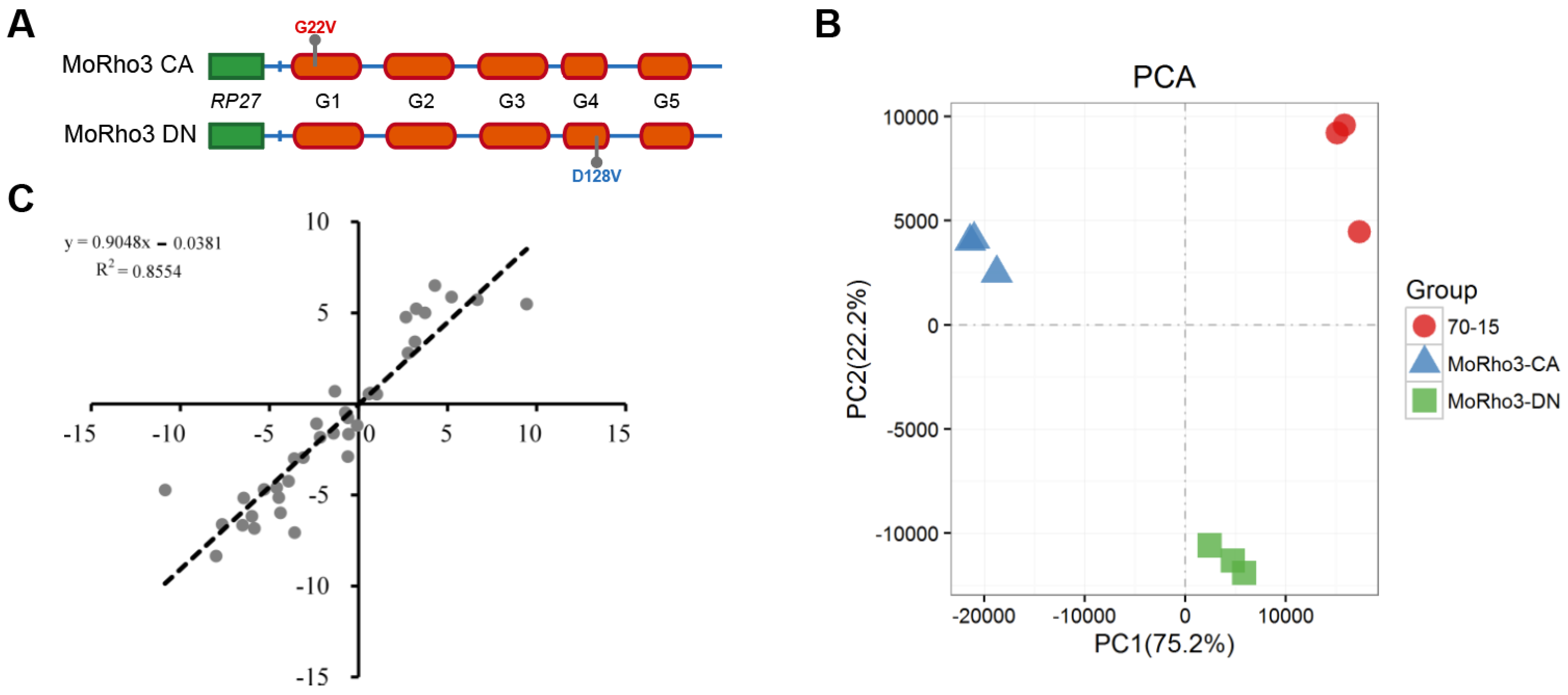
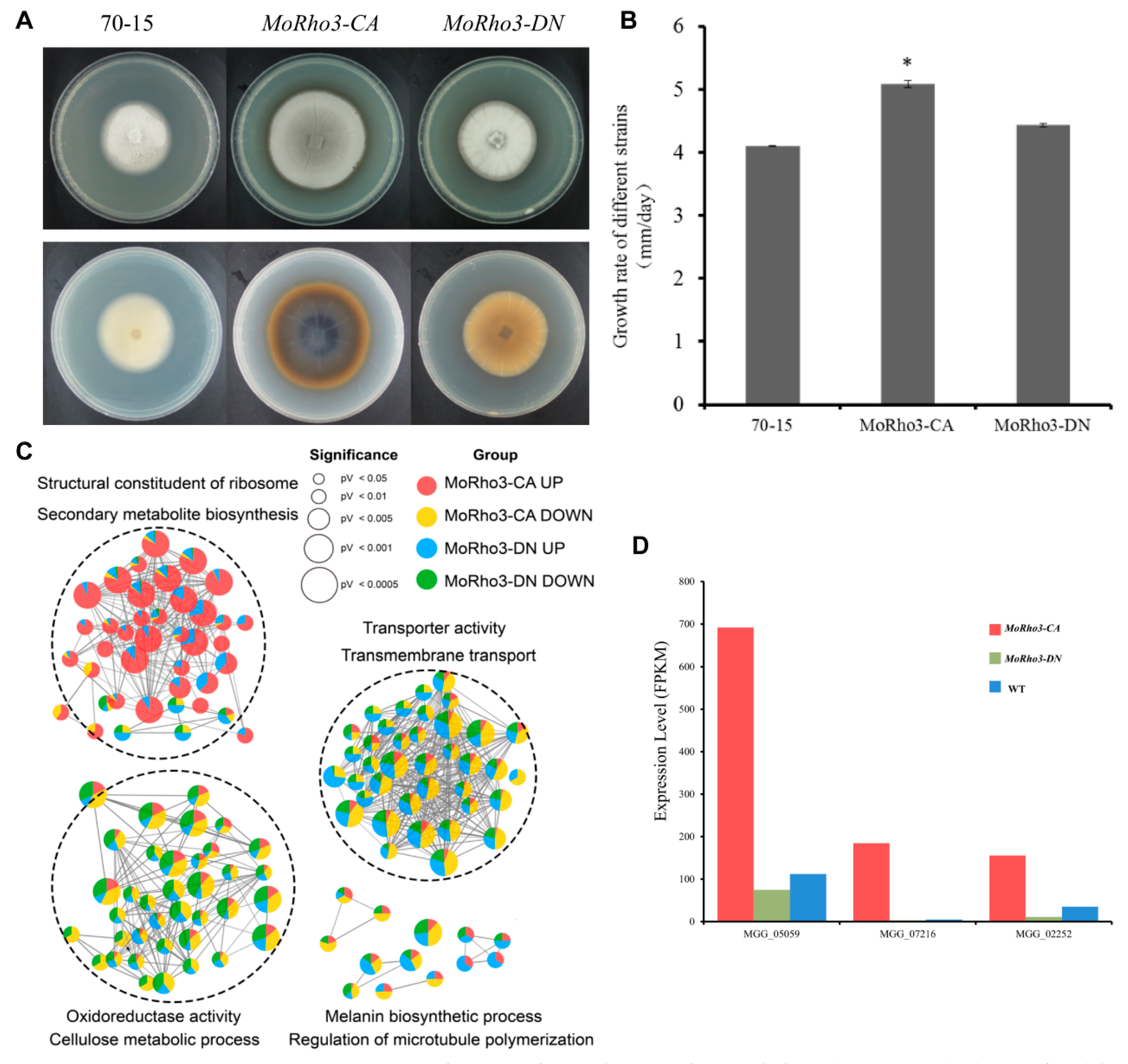
3.1. Transcriptome Sequencing of Constitutively-Active (CA) and Dominant-Negative (DN) Mutants of MoRho3
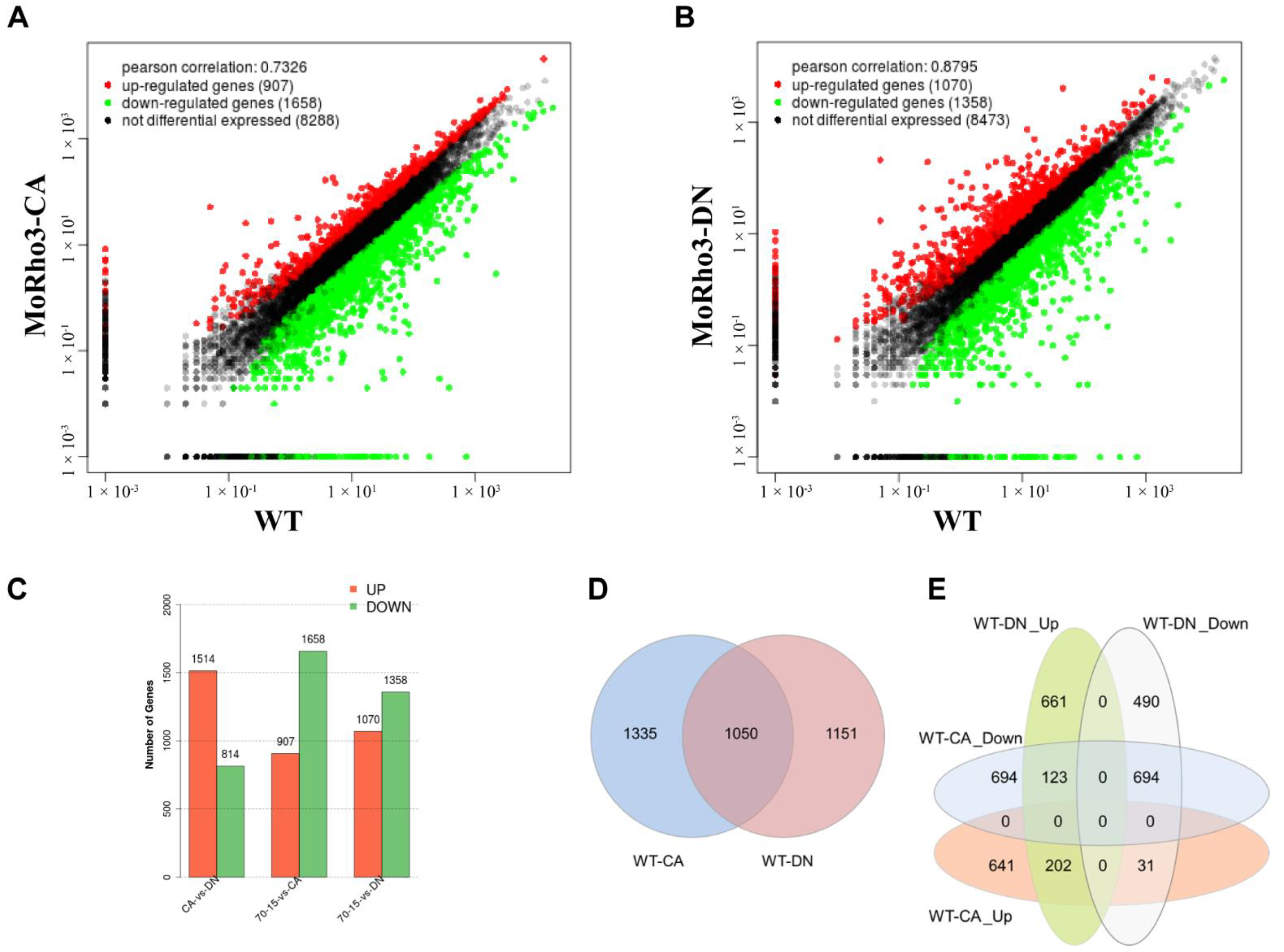
3.2. Comparison of DEGs in MoRho3 CA and DN Mutants
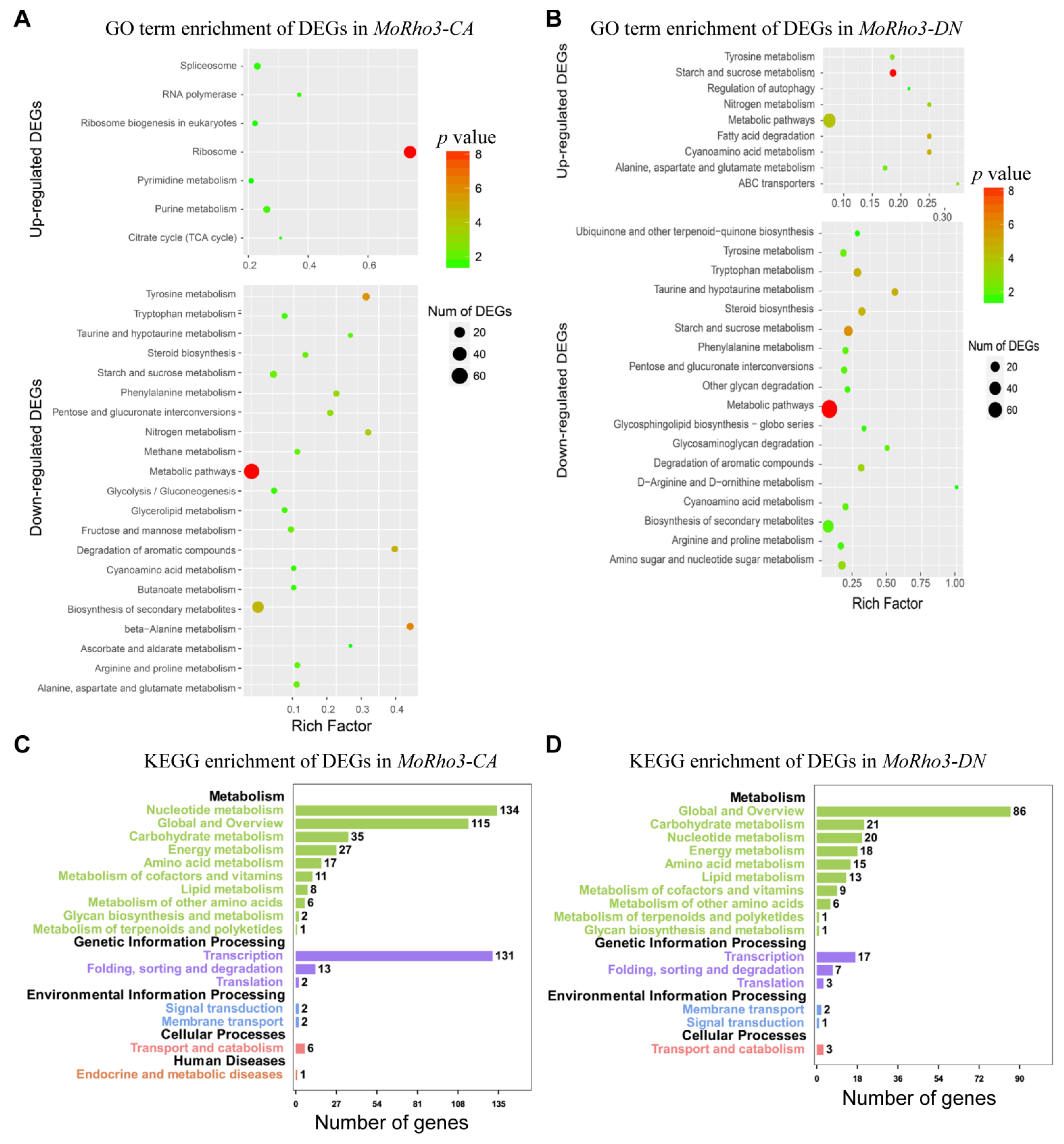
3.3. Pathways Related to CA and DN Mutation States of MoRho3
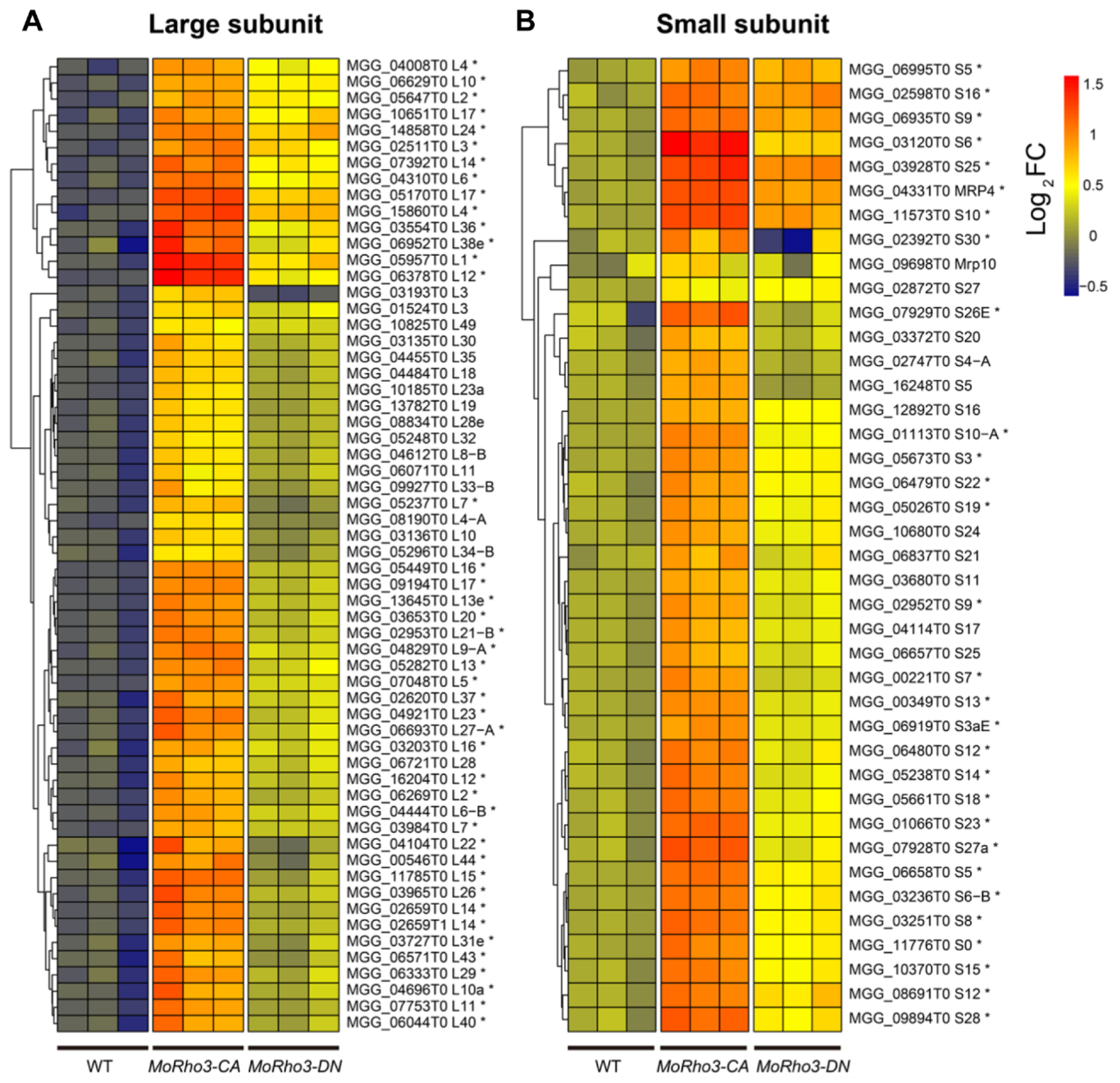
3.4. CA Mutation Enhances the Expression of Ribosome Biogenesis Genes
3.5. CA Mutation Regulates Melanin Biosynthesis
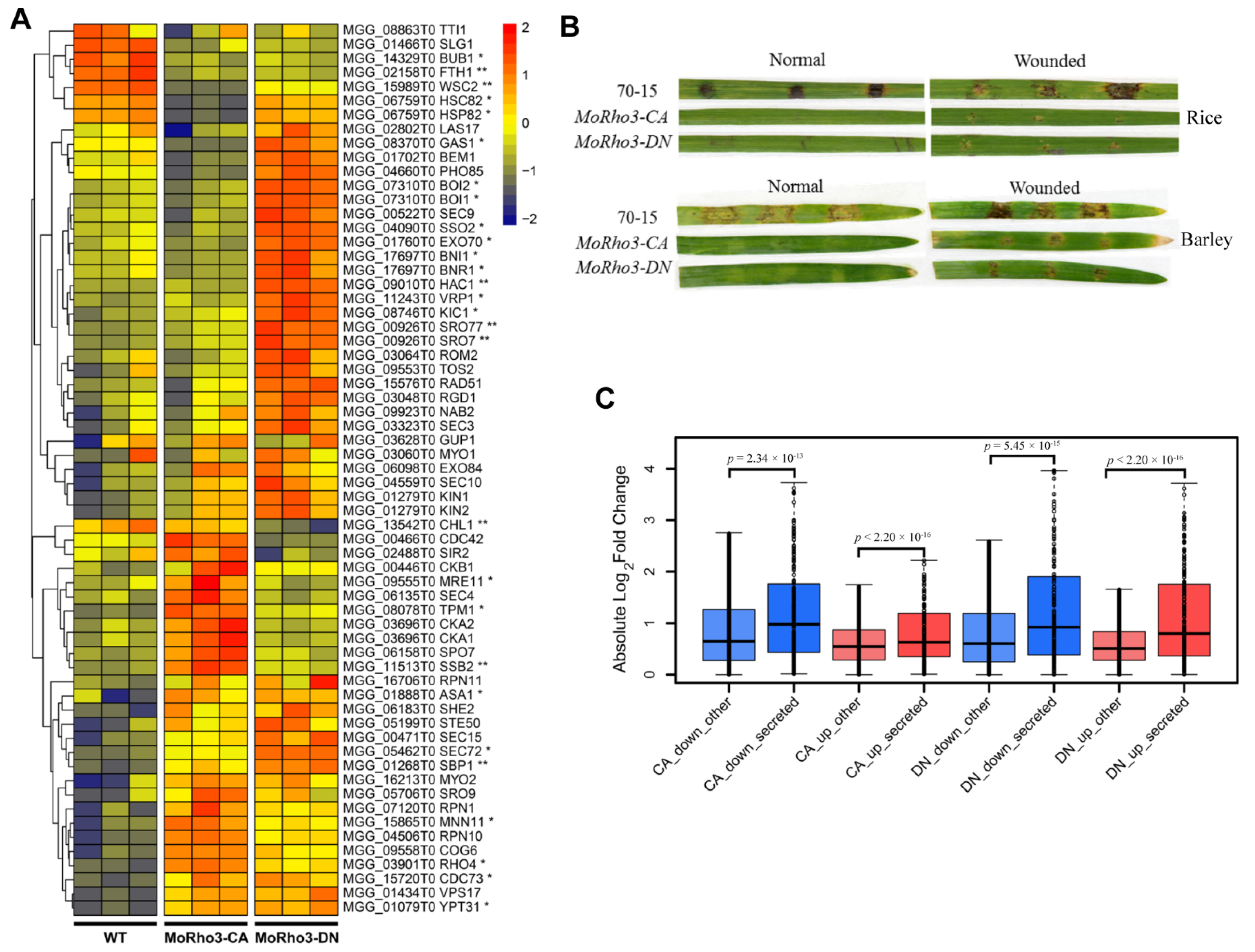
3.6. CA and DN Mutations Affect the Expression of Genes Involved in Protein Secretory Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bourne, H.R.; Sanders, D.A.; McCormick, F. The GTPase Superfamily: Conserved Structure and Molecular Mechanism. Nature 1991, 349, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Mosaddeghzadeh, N.; Ahmadian, M.R. The RHO Family GTPases: Mechanisms of Regulation and Signaling. Cells 2021, 10, 1831. [Google Scholar] [CrossRef]
- Van Aelst, L.; D’Souza-Schorey, C. Rho GTPases and Signaling Networks. Genes Dev. 1997, 11, 2295–2322. [Google Scholar] [CrossRef]
- Cherfils, J.; Zeghouf, M. Regulation of Small Gtpases by Gefs, Gaps, and Gdis. Physiol Rev. 2013, 93, 269–309. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.J.; Arentshorst, M.; Roos, E.D.; van den Hondel, C.A.; Meyer, V.; Ram, A.F.J. Functional Characterization of Rho GTPases in Aspergillus niger Uncovers Conserved and Diverged Roles of Rho Proteins within Filamentous Fungi. Mol. Microbiol. 2011, 79, 1151–1167. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Wang, J.; Zhai, Z.; Zhang, L.; Zheng, W.; Zheng, W.; Yu, W.; Zhou, J.; Lu, G. Functional Characterization of Rho Family Small GTPases in Fusarium graminearum. Fungal Genet. Biol. 2013, 61, 90–99. [Google Scholar] [CrossRef]
- Rossman, K.L.; Der, C.J.; Sondek, J. GEF Means Go: Turning on RHO GTPases with Guanine Nucleotide-Exchange Factors. Nat. Rev. Mol. Cell Biol 2005, 6, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Adamo, J.E.; Rossi, G.; Brennwald, P. The Rho GTPase Rho3 Has a Direct Role in Exocytosis That Is Distinct from Its Role in Actin Polarity. Mol. Biol Cell 1999, 10, 4121–4133. [Google Scholar] [CrossRef]
- Robinson, N.G.G.; Guo, L.; Imai, J.; Toh-e, A.; Matsui, Y.; Tamanoi, F. Rho3 of Saccharomyces cerevisiae, Which Regulates the Actin Cytoskeleton and Exocytosis, Is a GTPase Which Interacts with Myo2 and Exo70. Mol. Cell Biol 1999, 19, 3580–3587. [Google Scholar] [CrossRef] [PubMed]
- Dunkler, A.; Wendland, J. Candida albicans Rho-Type GTPase-Encoding Genes Required for Polarized Cell Growth and Cell Separation. Eukaryot Cell 2007, 6, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Wendland, J.; Philippsen, P. Cell Polarity and Hyphal Morphogenesis Are Controlled by Multiple Rho-Protein Modules in the Filamentous Ascomycete Ashbya gossypii. Genetics 2001, 157, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Vasara, T.; Salusjärvi, L.; Raudaskoski, M.; Keränen, S.; Penttilä, M.; Saloheimo, M. Interactions of the Trichoderma Reesei Rho3 with the Secretory Pathway in Yeast and T. Reesei. Mol. Microbiol 2001, 42, 1349–1361. [Google Scholar] [CrossRef] [PubMed]
- An, B.; Li, B.; Qin, G.; Tian, S. Function of Small GTPase Rho3 in Regulating Growth, Conidiation and Virulence of Botrytis cinerea. Fungal Genet. Biol. 2015, 75, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lei, M.; Zhou, Y.; Chen, F. A Comprehensive Analysis of the Small GTPases Ypt7 Involved in the Regulation of Fungal Development and Secondary Metabolism in Monascus ruber M7. Front. Microbiol. 2019, 10, 452. [Google Scholar] [CrossRef]
- Polaino, S.; Villalobos-Escobedo, J.M.; Shakya, V.P.S.; Miralles-Durán, A.; Chaudhary, S.; Sanz, C.; Shahriari, M.; Luque, E.M.; Eslava, A.P.; Corrochano, L.M. A Ras GTPase Associated Protein Is Involved in the Phototropic and Circadian Photobiology Responses in Fungi. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhao, Z.; Chen, J.; Liu, W.; Ke, H.; Zhou, J.; Lu, G.; Darvill, A.G.; Albersheim, P.; Wu, S. A Cdc42 Ortholog Is Required for Penetration and Virulence of Magnaporthe grisea. Fungal Genet. Biol. 2009, 46, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Wang, D.; Lin, X.; Li, X.; Lv, C.; Wang, D.; Zhang, L. Unveiling a Novel Role of Cdc42 in Pyruvate Metabolism Pathway to Mediate Insecticidal Activity of Beauveria bassiana. J. Fungi 2022, 8, 394. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, M.C.; Dagdas, Y.F.; Gupta, Y.K.; Mentlak, T.A.; Yi, M.; Martinez-Rocha, A.L.; Saitoh, H.; Terauchi, R.; Talbot, N.J.; Valent, B. Two Distinct Secretion Systems Facilitate Tissue Invasion by the Rice Blast Fungus Magnaporthe oryzae. Nat. Commun. 2013, 4, 1–12. [Google Scholar] [CrossRef]
- Wu, H.; Turner, C.; Gardner, J.; Temple, B.; Brennwald, P. The Exo70 Subunit of the Exocyst Is an Effector for Both Cdc42 and Rho3 Function in Polarized Exocytosis. Mol. Biol Cell 2010, 21, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Chen, J.; Liu, W.; Zheng, S.; Zhou, J.; Lu, G.; Wang, Z. A Rho3 Homolog Is Essential for Appressorium Development and Pathogenicity of Magnaporthe grisea. Eukaryot. Cell 2007, 6, 2240–2250. [Google Scholar] [CrossRef]
- Dean, R.A.; Talbot, N.J.; Ebbole, D.J.; Farman, M.L.; Mitchell, T.K.; Orbach, M.J.; Thon, M.; Kulkarni, R.; Xu, J.-R.; Pan, H. The Genome Sequence of the Rice Blast Fungus Magnaporthe grisea. Nature 2005, 434, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Pachter, L.; Salzberg, S.L. TopHat: Discovering Splice Junctions with RNA-Seq. Bioinformatics 2009, 25, 1105–1111. [Google Scholar] [CrossRef]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential Gene and Transcript Expression Analysis of RNA-Seq Experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast Gapped-Read Alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate Transcript Quantification from RNA-Seq Data with or without a Reference Genome. BMC Bioinformatics 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Sturn, A.; Quackenbush, J.; Trajanoski, Z. Genesis: Cluster Analysis of Microarray Data. Bioinformatics 2002, 18, 207–208. [Google Scholar] [CrossRef]
- Zhong, Z.; Norvienyeku, J.; Chen, M.; Bao, J.; Lin, L.; Chen, L.; Lin, Y.; Wu, X.; Cai, Z.; Zhang, Q. Directional Selection from Host Plants Is a Major Force Driving Host Specificity in Magnaporthe Species. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.-H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape Plug-in to Decipher Functionally Grouped Gene Ontology and Pathway Annotation Networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef]
- Bindea, G.; Galon, J.; Mlecnik, B. CluePedia Cytoscape Plugin: Pathway Insights Using Integrated Experimental and in Silico Data. Bioinformatics 2013, btt019, 661–663. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.M.; Adler, C.; Ball, C.; Chervitz, S.A.; Dwight, S.S.; Hester, E.T.; Jia, Y.; Juvik, G.; Roe, T.; Schroeder, M. SGD: Saccharomyces Genome Database. Nucleic Acids Res. 1998, 26, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Chaillou, T.; Kirby, T.J.; McCarthy, J.J. Ribosome Biogenesis: Emerging Evidence for a Central Role in the Regulation of Skeletal Muscle Mass. J. Cell Physiol. 2014, 229, 1584–1594. [Google Scholar] [CrossRef] [PubMed]
- Piazzi, M.; Bavelloni, A.; Gallo, A.; Faenza, I.; Blalock, W.L. Signal Transduction in Ribosome Biogenesis: A Recipe to Avoid Disaster. Int. J. Mol. Sci. 2019, 20, 2718. [Google Scholar] [CrossRef]
- Britton, R.A. Role of GTPases in Bacterial Ribosome Assembly. Annu. Rev. Microbiol. 2009, 63, 155. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Yan, Y.; Qu, Y.; Wang, J.; Feng, X.; Liu, X.; Lin, F.; Lu, J. Role Refinement of Melanin Synthesis Genes by Gene Knockout Reveals Their Functional Diversity in Pyricularia oryzae Strains. Microbiol. Res. 2021, 242, 126620. [Google Scholar] [CrossRef]
- Bolanos-Garcia, V.M.; Blundell, T.L. BUB1 and BUBR1: Multifaceted Kinases of the Cell Cycle. Trends Biochem. Sci. 2011, 36, 141–150. [Google Scholar] [CrossRef]
- Masgrau, A.; Battola, A.; Sanmartin, T.; Pryszcz, L.P.; Gabaldón, T.; Mendoza, M. Distinct Roles of the Polarity Factors Boi1 and Boi2 in the Control of Exocytosis and Abscission in Budding Yeast. Mol. Biol. Cell 2017, 28, 3082–3094. [Google Scholar] [CrossRef]
- Zheng, H.; Zhong, Z.; Shi, M.; Zhang, L.; Lin, L.; Hong, Y.; Fang, T.; Zhu, Y.; Guo, J.; Zhang, L. Comparative Genomic Analysis Revealed Rapid Differentiation in the Pathogenicity-Related Gene Repertoires between Pyricularia oryzae and Pyricularia penniseti Isolated from a Pennisetum Grass. BMC Genomics 2018, 19, 927. [Google Scholar] [CrossRef]
- Ceresini, P.C.; Castroagudin, V.L.; Rodrigues, F.A.; Rios, J.A.; Eduardo Aucique-Pérez, C.; Moreira, S.I.; Alves, E.; Croll, D.; Maciel, J.L.N. Wheat Blast: Past, Present, and Future. Annu Rev Phytopathol 2018, 56, 427–456. [Google Scholar] [CrossRef] [PubMed]
- Devanna, B.N.; Jain, P.; Solanke, A.U.; Das, A.; Thakur, S.; Singh, P.K.; Kumari, M.; Dubey, H.; Jaswal, R.; Pawar, D. Understanding the Dynamics of Blast Resistance in Rice-Magnaporthe oryzae Interactions. J. Fungi 2022, 8, 584. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Y.; Jenkinson, J.M.; Holcombe, L.J.; Soanes, D.M.; Veneault-Fourrey, C.; Bhambra, G.K.; Talbot, N.J. The Molecular Biology of Appressorium Turgor Generation by the Rice Blast Fungus Magnaporthe grisea. Biochem. Soc. Trans. 2005, 33, 384–388. [Google Scholar] [CrossRef]
- Dagdas, Y.F.; Yoshino, K.; Dagdas, G.; Ryder, L.S.; Bielska, E.; Steinberg, G.; Talbot, N.J. Septin-Mediated Plant Cell Invasion by the Rice Blast Fungus, Magnaporthe oryzae. Science 2012, 336, 1590–1595. [Google Scholar] [CrossRef]
- Saunders, D.G.O.; Aves, S.J.; Talbot, N.J. Cell Cycle–Mediated Regulation of Plant Infection by the Rice Blast Fungus. Plant. Cell 2010, 22, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.J.; Ferrari, M.A. Role of Melanin in Appressorium Function. Exp. Mycol. 1989, 13, 403–418. [Google Scholar] [CrossRef]
- Fu, T.; Kim, J.-O.; Han, J.-H.; Gumilang, A.; Lee, Y.-H.; Kim, K.S. A Small GTPase RHO2 Plays an Important Role in Pre-Infection Development in the Rice Blast Pathogen Magnaporthe oryzae. Plant. Pathol. J. 2018, 34, 470. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, W.; Zheng, S.; Zhang, D.; Sang, W.; Chen, X.; Li, G.; Lu, G.; Wang, Z. Rac1 Is Required for Pathogenicity and Chm1-Dependent Conidiogenesis in Rice Fungal Pathogen Magnaporthe grisea. PLoS Pathog. 2008, 4, e1000202. [Google Scholar] [CrossRef]
- Ridley, A. Rho GTPases: Integrating Integrin Signaling. J. Cell Biol. 2000, 150, F107–F109. [Google Scholar] [CrossRef]
- Carlson, M.A.; Haddad, B.G.; Weis, A.J.; Blackwood, C.S.; Shelton, C.D.; Wuerth, M.E.; Walter, J.D.; Spiegel, P.C., Jr. Ribosomal Protein L7/L12 Is Required for GTP Ase Translation Factors EF-G, RF 3, and IF 2 to Bind in Their GTP State to 70S Ribosomes. FEBS J. 2017, 284, 1631–1643. [Google Scholar] [CrossRef]
- Stuart, H.C.; Jia, Z.; Messenberg, A.; Joshi, B.; Underhill, T.M.; Moukhles, H.; Nabi, I.R. Localized Rho GTPase Activation Regulates RNA Dynamics and Compartmentalization in Tumor Cell Protrusions. J. Biol. Chem. 2008, 283, 34785–34795. [Google Scholar] [CrossRef] [PubMed]
- Bloom, G.S.; Richards, B.W.; Leopold, P.L.; Ritchey, D.M.; Brady, S.T. GTP Gamma S Inhibits Organelle Transport along Axonal Microtubules. J. Cell Biol. 1993, 120, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Wang, J.; Huang, P.; Liu, X.; Lu, J.; Lin, F.-C. PoRal2 Is Involved in Appressorium Formation and Virulence via Pmk1 MAPK Pathways in the Rice Blast Fungus Pyricularia oryzae. Front. Plant. Sci. 2021, 12, 1946. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Chen, X.; Lin, L.; Zhang, L.; Wang, L.; Bao, J.; Zhang, D. Transcriptomic Dynamics of Active and Inactive States of Rho GTPase MoRho3 in Magnaporthe oryzae. J. Fungi 2022, 8, 1060. https://doi.org/10.3390/jof8101060
Li Q, Chen X, Lin L, Zhang L, Wang L, Bao J, Zhang D. Transcriptomic Dynamics of Active and Inactive States of Rho GTPase MoRho3 in Magnaporthe oryzae. Journal of Fungi. 2022; 8(10):1060. https://doi.org/10.3390/jof8101060
Chicago/Turabian StyleLi, Qian, Xi Chen, Lianyu Lin, Lianhu Zhang, Li Wang, Jiandong Bao, and Dongmei Zhang. 2022. "Transcriptomic Dynamics of Active and Inactive States of Rho GTPase MoRho3 in Magnaporthe oryzae" Journal of Fungi 8, no. 10: 1060. https://doi.org/10.3390/jof8101060
APA StyleLi, Q., Chen, X., Lin, L., Zhang, L., Wang, L., Bao, J., & Zhang, D. (2022). Transcriptomic Dynamics of Active and Inactive States of Rho GTPase MoRho3 in Magnaporthe oryzae. Journal of Fungi, 8(10), 1060. https://doi.org/10.3390/jof8101060





