Paracoccidioidomycosis: What We Know and What Is New in Epidemiology, Diagnosis, and Treatment
Abstract
:1. Introduction
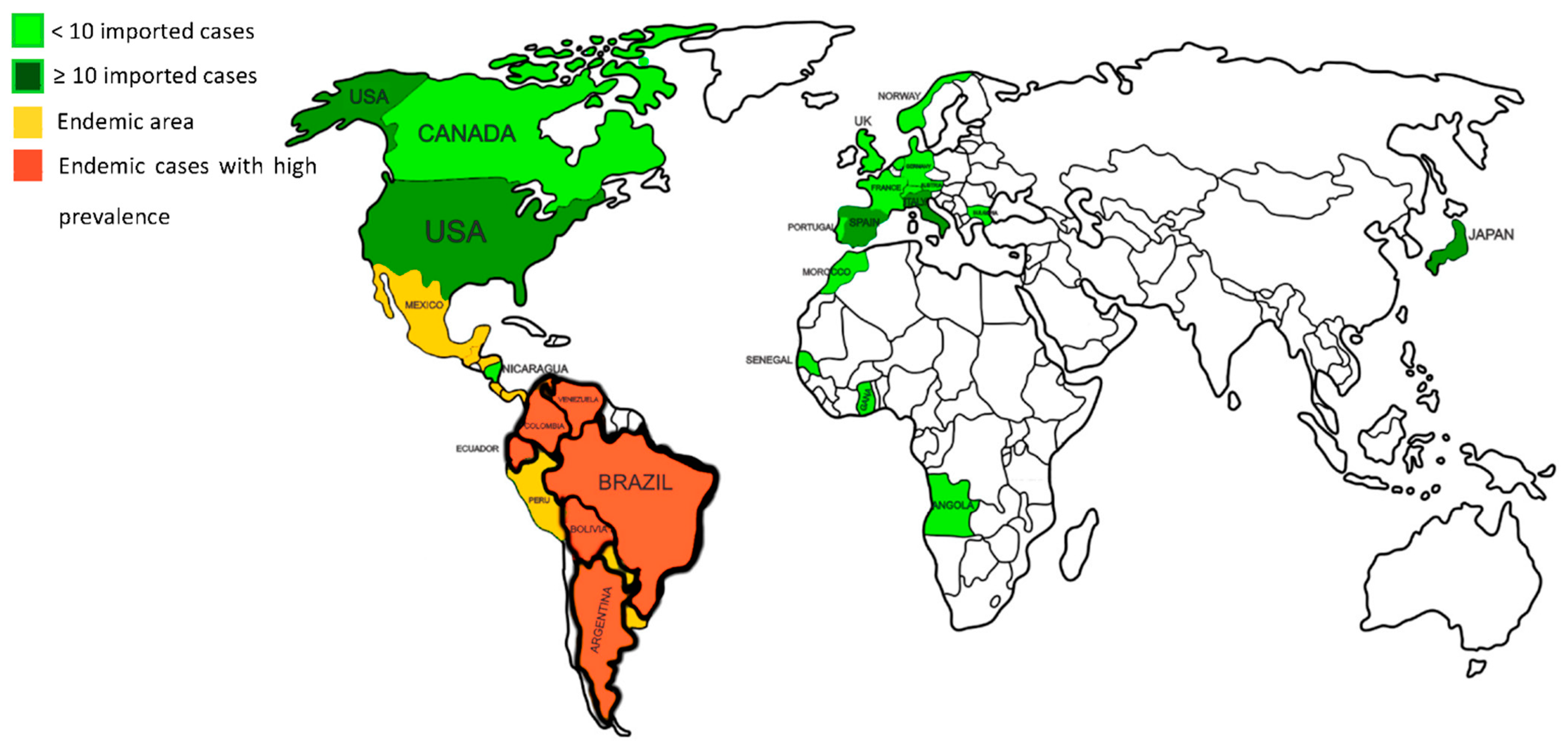
2. Epidemiology of Paracoccidioidomycosis
Species and Geographic Distribution
3. Diagnosis of Paracoccidioidomycosis
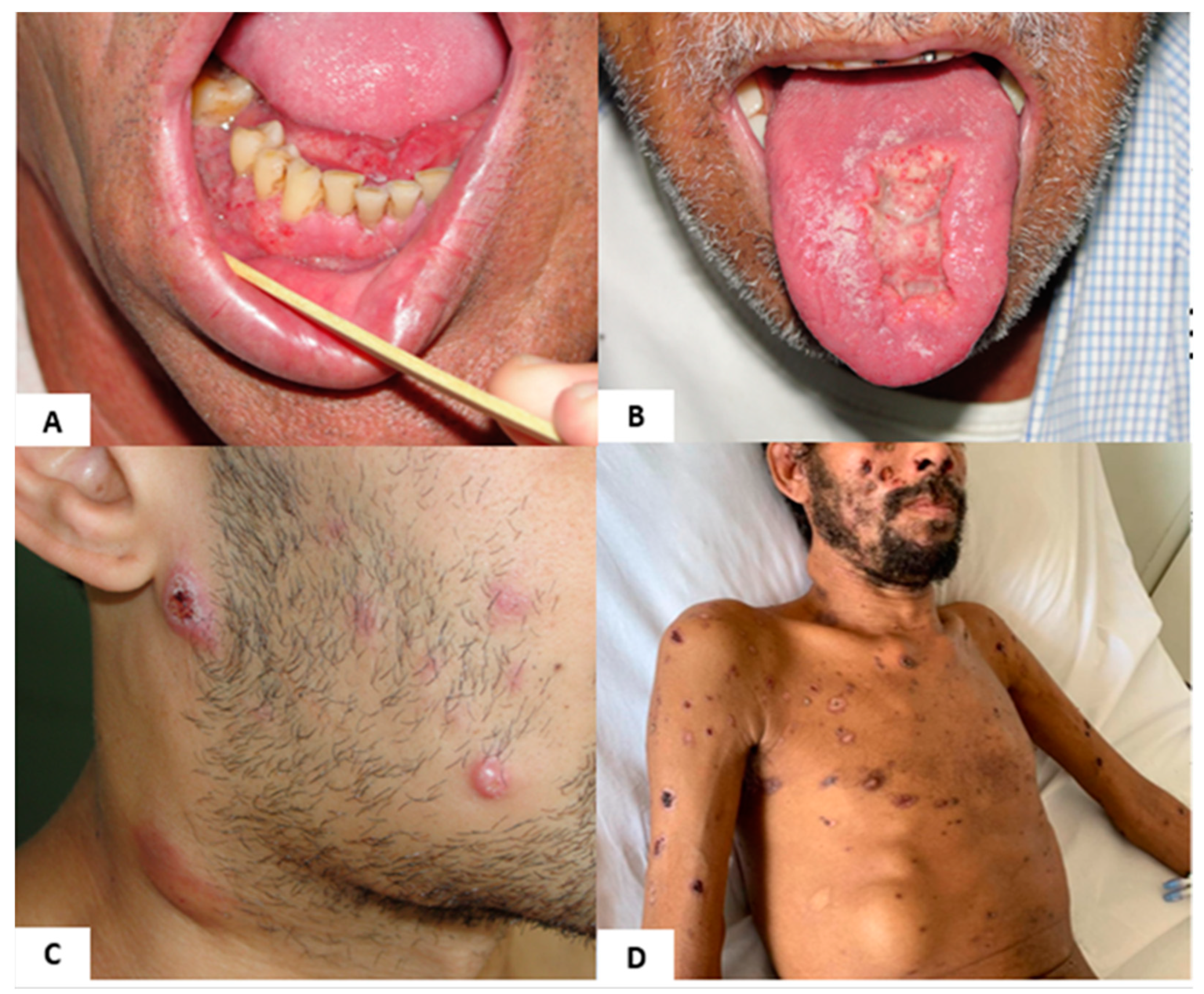
3.1. Clinical Diagnosis
3.2. Laboratory Diagnosis
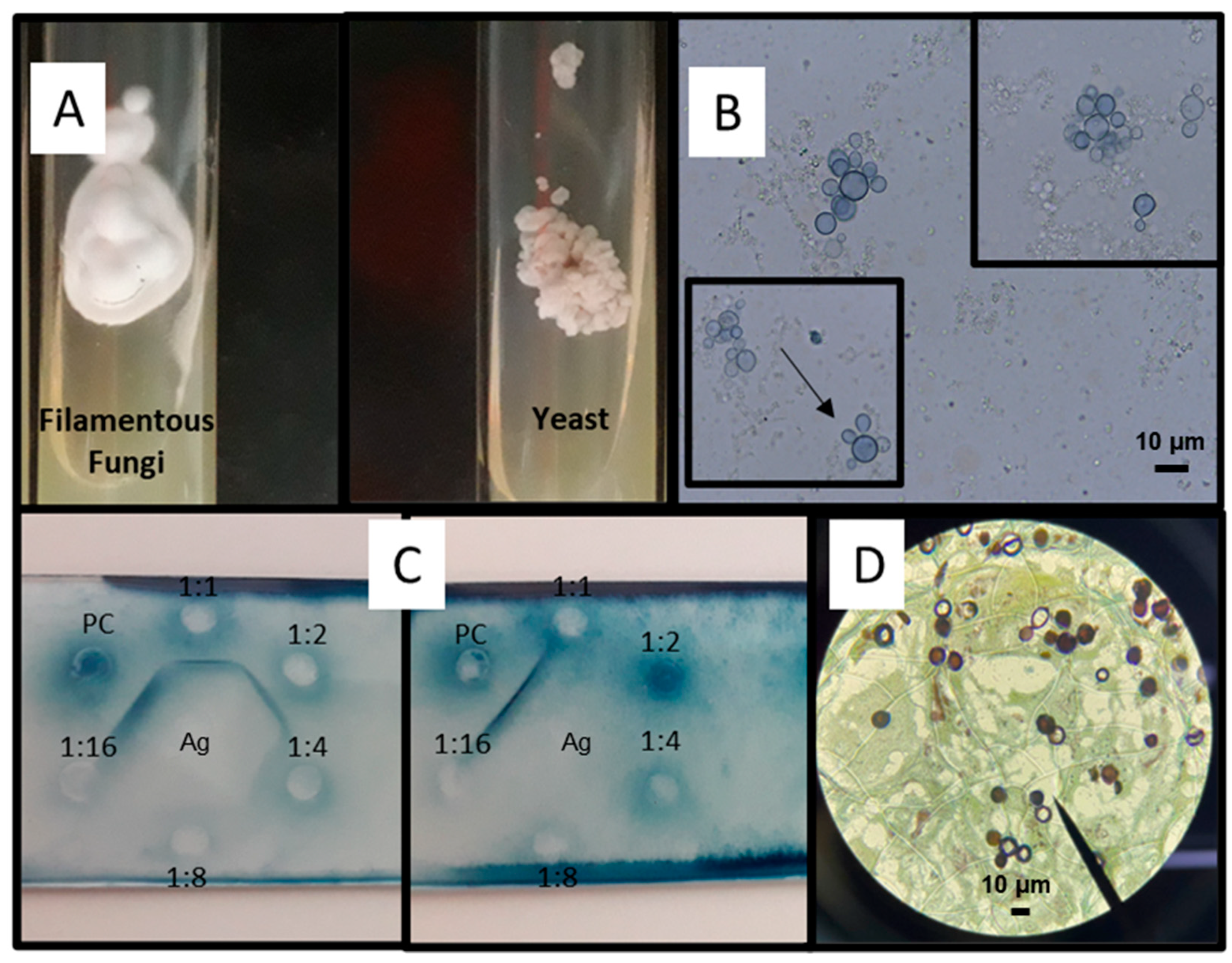
3.2.1. Mycological Diagnosis
3.2.2. Cultures
3.2.3. Histopathological Diagnosis
3.2.4. Immunological Diagnosis: Antibody Detection
3.2.5. Antigen Detection
3.2.6. Molecular Detection
4. Diagnostic Imaging
5. Treatment
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bocca, A.L.; Amaral, A.C.; Teixeira, M.M.; Sato, P.K.; Shikanai-Yasuda, M.A.; Soares Felipe, M.S. Paracoccidioidomycosis: Eco-epidemiology, taxonomy and clinical and therapeutic issues. Future Microbiol. 2013, 8, 1177–1191. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R. New Trends in Paracoccidioidomycosis Epidemiology. J. Fungi 2017, 3, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shikanai-Yasuda, M.A.; Mendes, R.P.; Colombo, A.L.; Queiroz-Telles, F.; Kono, A.S.G.; Paniago, A.M.M.; Nathan, A.; Valle, A.; Bagagli, E.; Benard, G.; et al. Brazilian guidelines for the clinical management of paracoccidioidomycosis. Rev. Soc. Bras. Med. Trop. 2017, 50, 715–740. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.; Moertl, D.; Glechner, A.; Mayr, V.; Klerings, I.; Zachariah, C.; Van den Nest, M.; Gartlehner, G.; Willinger, B. Paracoccidioidomycosis Diagnosed in Europe-A Systematic Literature Review. J. Fungi 2021, 7, 157. [Google Scholar] [CrossRef]
- Costa, A.N.; Benard, G.; Albuquerque, A.L.; Fujita, C.L.; Magri, A.S.; Salge, J.M.; Shikanai-Yasuda, M.A.; Carvalho, C.R. The lung in paracoccidioidomycosis: New insights into old problems. Clinics 2013, 68, 441–448. [Google Scholar] [CrossRef]
- Caceres, D.H.; Echeverri Tirado, L.C.; Bonifaz, A.; Adenis, A.; Gomez, B.L.; Flores, C.L.B.; Canteros, C.E.; Santos, D.W.; Arathoon, E.; Soto, E.R.; et al. Current situation of endemic mycosis in the Americas and the Caribbean: Proceedings of the first international meeting on endemic mycoses of the Americas (IMEMA). Mycoses 2022. [Google Scholar] [CrossRef]
- Moreto, T.C.; Marques, M.E.; de Oliveira, M.L.; Moris, D.V.; de Carvalho, L.R.; Mendes, R.P. Accuracy of routine diagnostic tests used in paracoccidioidomycosis patients at a university hospital. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 473–478. [Google Scholar] [CrossRef]
- da Silva, J.F.; de Oliveira, H.C.; Marcos, C.M.; Assato, P.A.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Advances and challenges in paracoccidioidomycosis serology caused by Paracoccidioides species complex: An update. Diagn. Microbiol. Infect. Dis. 2016, 84, 87–94. [Google Scholar] [CrossRef]
- Pinheiro, B.G.; Hahn, R.C.; Camargo, Z.P.; Rodrigues, A.M. Molecular Tools for Detection and Identification of Paracoccidioides Species: Current Status and Future Perspectives. J. Fungi 2020, 6, 293. [Google Scholar] [CrossRef]
- Cavalcante Rde, S.; Sylvestre, T.F.; Levorato, A.D.; de Carvalho, L.R.; Mendes, R.P. Comparison between itraconazole and cotrimoxazole in the treatment of paracoccidiodomycosis. PLoS Negl. Trop. Dis. 2014, 8, e2793. [Google Scholar] [CrossRef]
- Shikanai-Yasuda, M.A. Paracoccidioidomycosis Treatment. Rev. Inst. Med. Trop. Sao Paulo 2015, 57 (Suppl. 19), 31–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, U.V.; Oliveira, S.; Chang, M.R.; Pereira, E.F.; Marques, A.; Carvalho, L.R.; Mendes, R.P.; Paniago, A.M.M. Treatment compliance of patients with paracoccidioidomycosis in Central-West Brazil. J. Bras. Pneumol. 2019, 45, e20180167. [Google Scholar] [CrossRef] [PubMed]
- Matute, D.R.; McEwen, J.G.; Puccia, R.; Montes, B.A.; San-Blas, G.; Bagagli, E.; Rauscher, J.T.; Restrepo, A.; Morais, F.; Nino-Vega, G.; et al. Cryptic speciation and recombination in the fungus Paracoccidioides brasiliensis as revealed by gene genealogies. Mol. Biol. Evol. 2006, 23, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.M.; Theodoro, R.C.; de Carvalho, M.J.; Fernandes, L.; Paes, H.C.; Hahn, R.C.; Mendoza, L.; Bagagli, E.; San-Blas, G.; Felipe, M.S. Phylogenetic analysis reveals a high level of speciation in the Paracoccidioides genus. Mol. Phylogenet. Evol. 2009, 52, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Turissini, D.A.; Gomez, O.M.; Teixeira, M.M.; McEwen, J.G.; Matute, D.R. Species boundaries in the human pathogen Paracoccidioides. Fungal Genet. Biol. 2017, 106, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Munoz, J.F.; Farrer, R.A.; Desjardins, C.A.; Gallo, J.E.; Sykes, S.; Sakthikumar, S.; Misas, E.; Whiston, E.A.; Bagagli, E.; Soares, C.M.; et al. Genome Diversity, Recombination, and Virulence across the Major Lineages of Paracoccidioides. mSphere 2016, 1, e00213-16. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, M.M.; Cattana, M.E.; Matute, D.R.; Munoz, J.F.; Arechavala, A.; Isbell, K.; Schipper, R.; Santiso, G.; Tracogna, F.; Sosa, M.L.A.; et al. Genomic diversity of the human pathogen Paracoccidioides across the South American continent. Fungal Genet. Biol. 2020, 140, 103395. [Google Scholar] [CrossRef]
- Nery, A.F.; de Camargo, Z.P.; Rodrigues, A.M.; Portela, T.F.; Hoffmann-Santos, H.D.; Pinheiro, B.G.; Possa, A.P.; Cavalcante, L.; Hagen, F.; Hahn, R.C. Puzzling paracoccidioidomycosis: Factors associated with the severity of Paracoccidioides lutzii infections. Int. J. Infect. Dis. 2021, 107, 284–290. [Google Scholar] [CrossRef]
- Mavengere, H.; Mattox, K.; Teixeira, M.M.; Sepulveda, V.E.; Gomez, O.M.; Hernandez, O.; McEwen, J.; Matute, D.R. Paracoccidioides Genomes Reflect High Levels of Species Divergence and Little Interspecific Gene Flow. mBio 2020, 11, e01999-20. [Google Scholar] [CrossRef]
- Pereira, E.F.; Gegembauer, G.; Chang, M.R.; Camargo, Z.P.; Nunes, T.F.; Ribeiro, S.M.; Carvalho, L.R.; Maldonado, B.M.; Mendes, R.P.; Paniago, A.M.M. Comparison of clinico-epidemiological and radiological features in paracoccidioidomycosis patients regarding serological classification using antigens from Paracoccidioides brasiliensis complex and Paracoccidioides lutzii. PLoS Negl. Trop. Dis. 2020, 14, e0008485. [Google Scholar] [CrossRef]
- Hahn, R.C.; Rodrigues, A.M.; Della Terra, P.P.; Nery, A.F.; Hoffmann-Santos, H.D.; Gois, H.M.; Fontes, C.J.F.; de Camargo, Z.P. Clinical and epidemiological features of paracoccidioidomycosis due to Paracoccidioides lutzii. PLoS Negl. Trop. Dis. 2019, 13, e0007437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Macedo, P.M.; Teixeira, M.M.; Barker, B.M.; Zancope-Oliveira, R.M.; Almeida-Paes, R.; Francesconi do Valle, A.C. Clinical features and genetic background of the sympatric species Paracoccidioides brasiliensis and Paracoccidioides americana. PLoS Negl. Trop. Dis. 2019, 13, e0007309. [Google Scholar] [CrossRef]
- Vilela, R.; Huebner, M.; Vilela, C.; Vilela, G.; Pettersen, B.; Oliveira, C.; Mendoza, L. The taxonomy of two uncultivated fungal mammalian pathogens is revealed through phylogeny and population genetic analyses. Sci. Rep. 2021, 11, 18119. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Martinez, R.; Hernandez-Hernandez, F.; Mendez-Tovar, L.J.; Manzano-Gayosso, P.; Bonifaz, A.; Arenas, R.; Padilla-Desgarennes Mdel, C.; Estrada, R.; Chavez, G. Paracoccidioidomycosis in Mexico: Clinical and epidemiological data from 93 new cases (1972-2012). Mycoses 2014, 57, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Cordova, L.A.; Torres, J. Paracoccidioidomycosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Theodoro, R.C.; Teixeira Mde, M.; Felipe, M.S.; Paduan Kdos, S.; Ribolla, P.M.; San-Blas, G.; Bagagli, E. Genus paracoccidioides: Species recognition and biogeographic aspects. PLoS ONE 2012, 7, e37694. [Google Scholar] [CrossRef] [Green Version]
- Linares, G.; Baker, R.D.; Linares, L. Paracoccidioidomycosis in the United States (South American Blastomycosis). Arch. Otolaryngol. 1971, 93, 514–518. [Google Scholar] [CrossRef]
- Murray, H.W.; Littman, M.L.; Roberts, R.B. Disseminated paracoccidioidomycosis (South American blastomycosis) in the United States. Am. J. Med. 1974, 56, 209–220. [Google Scholar] [CrossRef]
- Rahman, R.; Davies, L.; Mohareb, A.M.; Pecanha-Pietrobom, P.M.; Patel, N.J.; Solomon, I.H.; Meredith, D.M.; Tsai, H.K.; Guenette, J.P.; Bhattacharyya, S.; et al. Delayed Relapse of Paracoccidioidomycosis in the Central Nervous System: A Case Report. Open Forum Infect. Dis. 2020, 7, ofaa077. [Google Scholar] [CrossRef]
- Chikamori, T.; Saka, S.; Nagano, H.; Saeki, S.; Lacaz Cda, S.; Rodrigues, M.C.; Cassaguerra, C.M.; Braccialli, M.L. Paracoccidioidomycosis in Japan. Report of a case. Rev. Inst. Med. Trop. Sao Paulo 1984, 26, 267–271. [Google Scholar] [CrossRef] [Green Version]
- Kawayama, T.; Sawa, A.; Sueyasu, Y.; Arikawa, K.; Shiraishi, T.; Ichikawa, Y.; Oizumi, K. [Chronic pulmonary paracoccidioidomyosis in a Japanese Adult]. Nihon Kyobu Shikkan Gakkai Zasshi 1996, 34, 911–915. [Google Scholar]
- Fujio, J.; Nishimura, K.; Miyaji, M. Epidemiological survey of the imported mycoses in Japan. Nihon Ishinkin Gakkai Zasshi 1999, 40, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Kamei, K.; Sano, A.; Kikuchi, K.; Makimura, K.; Niimi, M.; Suzuki, K.; Uehara, Y.; Okabe, N.; Nishimura, K.; Miyaji, M. The trend of imported mycoses in Japan. J. Infect. Chemother. 2003, 9, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Miyaji, M.; Kamei, K. Imported mycoses: An update. J. Infect. Chemother. 2003, 9, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, T.; Kurose, T.; Itabashi, K.; Nakano, I.; Okamoto, K.; Sano, A.; Kimura, K.; Kaji, H. [A case of chronic pulmonary paracoccidioidomycosis]. Nihon Kokyuki Gakkai Zasshi 2004, 42, 629–633. [Google Scholar] [PubMed]
- Onda, H.; Komine, M.; Murata, S.; Ohtsuki, M. Letter: Imported paracoccidioidomycosis in Japan. Dermatol. Online J. 2011, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Kurai, H.; Ohmagari, N.; Ito, K.; Kawamura, I.; Suzuki, J.; Hadano, Y.; Endo, M.; Iida, Y.; Okinaka, K.; Kamei, K. [Case of oral paracoccidioidomycosis suspected to be pharyngeal cancer]. Med. Mycol. J. 2012, 53, 49–52. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, Y.; Tachikawa, N.; Oosawa, T.; Kosuge, Y.; Kamei, K. [A case of paracoccidioidomycosis with severe adrenal insufficiency]. Kansenshogaku Zasshi 2012, 86, 291–294. [Google Scholar] [CrossRef] [Green Version]
- dos Santos, W.A.; da Silva, B.M.; Passos, E.D.; Zandonade, E.; Falqueto, A. [Association between smoking and paracoccidioidomycosis: A case-control study in the State of Espirito Santo, Brazil]. Cad. Saude Publica 2003, 19, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Queiroz-Telles, F.V.; Pecanha Pietrobom, P.M.; Rosa Junior, M.; Baptista, R.M.; Pecanha, P.M. New Insights on Pulmonary Paracoccidioidomycosis. Semin. Respir. Crit. Care Med. 2020, 41, 53–68. [Google Scholar] [CrossRef]
- Pecanha, P.M.; Batista Ferreira, M.E.; Massaroni Pecanha, M.A.; Schmidt, E.B.; Lamas de Araujo, M.; Zanotti, R.L.; Potratz, F.F.; Delboni Nunes, N.E.; Goncalves Ferreira, C.U.; Delmaestro, D.; et al. Paracoccidioidomycosis: Epidemiological and Clinical Aspects in 546 Cases Studied in the State of Espirito Santo, Brazil. Am. J. Trop. Med. Hyg. 2017, 97, 836–844. [Google Scholar] [CrossRef] [Green Version]
- Bellissimo-Rodrigues, F.; Bollela, V.R.; Da Fonseca, B.A.; Martinez, R. Endemic paracoccidioidomycosis: Relationship between clinical presentation and patients’ demographic features. Med. Mycol. 2013, 51, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, A.; Salazar, M.E.; Cano, L.E.; Stover, E.P.; Feldman, D.; Stevens, D.A. Estrogens inhibit mycelium-to-yeast transformation in the fungus Paracoccidioides brasiliensis: Implications for resistance of females to paracoccidioidomycosis. Infect. Immun. 1984, 46, 346–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira Gde, D.; Alves Tda, C.; Lima, S.M.; Camargo, L.M.; Sousa, C.M. Paracoccidioidomycosis in a western Brazilian Amazon State: Clinical-epidemiologic profile and spatial distribution of the disease. Rev. Soc. Bras. Med. Trop. 2014, 47, 63–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krakhecke-Teixeira, A.G.; Yamauchi, D.H.; Rossi, A.; de Sousa, H.R.; Garces, H.G.; Junior, J.L.; Junior, A.O.S.; Felipe, M.S.S.; Bagagli, E.; de Andrade, H.F., Jr.; et al. Clinical and Eco-Epidemiological Aspects of a Novel Hyperendemic Area of Paracoccidioidomycosis in the Tocantins-Araguaia Basin (Northern Brazil), Caused by Paracoccidioides sp. J. Fungi 2022, 8, 502. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.J.; Londero, A.T.; Terra, G.M.; Rozenbaum, R.; Abreu, T.F.; Nogueira, S.A. Paracoccidioidomycosis in children in the state of Rio de Janeiro (Brazil). Geographic distribution and the study of a “reservarea”. Rev. Inst. Med. Trop. Sao Paulo 1998, 40, 11–13. [Google Scholar] [CrossRef] [Green Version]
- Cadavid, D.; Restrepo, A. Factors associated with Paracoccidiodes brasiliensis infection among permanent residents of three endemic areas in Colombia. Epidemiol. Infect. 1993, 111, 121–133. [Google Scholar] [CrossRef] [Green Version]
- van Gelderen de Komaid, A.; Duran, E.; Borges de Kestelman, I. Histoplasmosis and Paracoccidioidomycosis in northwestern Argentina III. Epidemiological survey in Vipos, La Toma, and Choromoro-Trancas, Tucuman, Argentina. Eur. J. Epidemiol. 1999, 15, 383–388. [Google Scholar] [CrossRef]
- Cermeno, J.; Cermeno, J.; Godoy, G.; Hernandez, I.; Orellan, Y.; Blanco, Y.; Penna, S.; Garcia, L.; Mender, T.; Gonsalvez, M.; et al. Epidemiological study of paracoccidioidomycosis and histoplasmosis in a suburb of San Felix city, Bolivar state, Venezuela. Investig. Clin. 2009, 50, 213–220. [Google Scholar]
- Coutinho, Z.F.; Silva, D.; Lazera, M.; Petri, V.; Oliveira, R.M.; Sabroza, P.C.; Wanke, B. Paracoccidioidomycosis mortality in Brazil (1980–1995). Cad. Saude Publica 2002, 18, 1441–1454. [Google Scholar] [CrossRef] [Green Version]
- de Suguiura, I.M.S.; Ono, M.A. Compulsory notification of paracoccidioidomycosis: A 14-year retrospective study of the disease in the state of Parana, Brazil. Mycoses 2022, 65, 354–361. [Google Scholar] [CrossRef]
- Prado, M.; Silva, M.B.; Laurenti, R.; Travassos, L.R.; Taborda, C.P. Mortality due to systemic mycoses as a primary cause of death or in association with AIDS in Brazil: A review from 1996 to 2006. Mem. Inst. Oswaldo Cruz 2009, 104, 513–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paniago, A.M.; Aguiar, J.I.; Aguiar, E.S.; da Cunha, R.V.; Pereira, G.R.; Londero, A.T.; Wanke, B. [Paracoccidioidomycosis: A clinical and epidemiological study of 422 cases observed in Mato Grosso do Sul]. Rev. Soc. Bras. Med. Trop. 2003, 36, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Tobon, A.M.; Agudelo, C.A.; Osorio, M.L.; Alvarez, D.L.; Arango, M.; Cano, L.E.; Restrepo, A. Residual pulmonary abnormalities in adult patients with chronic paracoccidioidomycosis: Prolonged follow-up after itraconazole therapy. Clin. Infect. Dis. 2003, 37, 898–904. [Google Scholar] [CrossRef] [Green Version]
- Barrozo, L.V.; Benard, G.; Silva, M.E.; Bagagli, E.; Marques, S.A.; Mendes, R.P. First description of a cluster of acute/subacute paracoccidioidomycosis cases and its association with a climatic anomaly. PLoS Negl. Trop. Dis. 2010, 4, e643. [Google Scholar] [CrossRef] [Green Version]
- Arantes, T.D.; Theodoro, R.C.; Teixeira Mde, M.; Bosco Sde, M.; Bagagli, E. Environmental Mapping of Paracoccidioides spp. in Brazil Reveals New Clues into Genetic Diversity, Biogeography and Wild Host Association. PLoS Negl. Trop. Dis. 2016, 10, e0004606. [Google Scholar] [CrossRef] [Green Version]
- Mangiaterra, M.L.; Giusiano, G.E.; Alonso, J.M.; Gorodner, J.O. [Paracoccidioides brasiliensis infection in a subtropical region with important environmental changes]. Bull. Soc. Pathol. Exot. 1999, 92, 173–176. [Google Scholar]
- Giusiano, G.; Aguirre, C.; Vratnica, C.; Rojas, F.; Corallo, T.; Cattana, M.E.; Fernandez, M.; Mussin, J.; de Los Angeles Sosa, M. Emergence of acute/subacute infant-juvenile paracoccidioidomycosis in Northeast Argentina: Effect of climatic and anthropogenic changes? Med. Mycol. 2019, 57, 30–37. [Google Scholar] [CrossRef] [PubMed]
- do Valle, A.C.F.; Marques de Macedo, P.; Almeida-Paes, R.; Romao, A.R.; Lazera, M.D.S.; Wanke, B. Paracoccidioidomycosis after Highway Construction, Rio de Janeiro, Brazil. Emerg. Infect. Dis. 2017, 23, 1917–1919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benard, G.; Romano, C.C.; Cacere, C.R.; Juvenale, M.; Mendes-Giannini, M.J.; Duarte, A.J. Imbalance of IL-2, IFN-gamma and IL-10 secretion in the immunosuppression associated with human paracoccidioidomycosis. Cytokine 2001, 13, 248–252. [Google Scholar] [CrossRef]
- Juvenale, M.; Negro, G.; Duarte, A.J.S.; Benard, G. Antibody isotypes to a Paracoccidioides brasiliensis somatic antigen in sub-acute and chronic form paracoccidioidomycosis. J. Med. Microbiol. 2001, 50, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Dutra, L.M.; Silva, T.H.M.; Falqueto, A.; Pecanha, P.M.; Souza, L.R.M.; Goncalves, S.S.; Velloso, T.R.G. Oral paracoccidioidomycosis in a single-center retrospective analysis from a Brazilian southeastern population. J. Infect. Public Health 2018, 11, 530–533. [Google Scholar] [CrossRef]
- Almeida, F.A.; Neves, F.F.; Mora, D.J.; Reis, T.A.; Sotini, D.M.; Ribeiro, B.M.; Andrade-Silva, L.E.; Nascentes, G.N.; Ferreira-Paim, K.; Silva-Vergara, M.L. Paracoccidioidomycosis in Brazilian Patients With and Without Human Immunodeficiency Virus Infection. Am. J. Trop. Med. Hyg. 2017, 96, 368–372. [Google Scholar] [CrossRef] [Green Version]
- Covre, L.C.P.; Hombre, P.M.; Falqueto, A.; Pecanha, P.M.; Valim, V. Pulmonary paracoccidioidomycosis: A case report of reactivation in a patient receiving biological therapy. Rev. Soc. Bras. Med. Trop. 2018, 51, 249–252. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, J.N., Jr.; Pecanha-Pietrobom, P.M.; Colombo, A.L. Paracoccidioidomycosis in Immunocompromised Patients: A Literature Review. J. Fungi 2018, 5, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falcao, E.M.; de Macedo, P.M.; Freitas, D.F.S.; Freitas, A.D.; Grinsztejn, B.; Veloso, V.G.; Almeida-Paes, R.; do Valle, A.C.F. Paracoccidioidomycosis in people living with HIV/AIDS: A historical retrospective cohort study in a national reference center for infectious diseases, Rio de Janeiro, Brazil. PLoS Negl. Trop. Dis. 2022, 16, e0010529. [Google Scholar] [CrossRef]
- Benard, G. An overview of the immunopathology of human paracoccidioidomycosis. Mycopathologia 2008, 165, 209–221. [Google Scholar] [CrossRef] [Green Version]
- Jasenosky, L.D.; Scriba, T.J.; Hanekom, W.A.; Goldfeld, A.E. T cells and adaptive immunity to Mycobacterium tuberculosis in humans. Immunol. Rev. 2015, 264, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Shikanai-Yasuda, M.A.; Conceicao, Y.M.; Kono, A.; Rivitti, E.; Campos, A.F.; Campos, S.V. Neoplasia and paracoccidioidomycosis. Mycopathologia 2008, 165, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Baldo, M.E. A diagnostic challenge in an individual with Paracoccidioidomycosis during hospitalization in times of COVID-19. Int. J. Innov. Educ. Res. 2022, 10, 149–158. [Google Scholar] [CrossRef]
- de Macedo, P.M.; Freitas, D.F.S.; Varon, A.G.; Lamas, C.D.C.; Ferreira, L.C.F.; Freitas, A.D.; Ferreira, M.T.; Nunes, E.P.; Siqueira, M.M.; Veloso, V.G.; et al. COVID-19 and acute juvenile paracoccidioidomycosis coinfection. PLoS Negl. Trop. Dis. 2020, 14, e0008559. [Google Scholar] [CrossRef]
- Messina, F.A.; Giusiano, G.; Santiso, G. Endemic Mycoses and COVID-19: A Review. Curr. Fungal Infect. Rep. 2022, 16, 98–106. [Google Scholar] [CrossRef]
- Thompson, G.R., 3rd; Le, T.; Chindamporn, A.; Kauffman, C.A.; Alastruey-Izquierdo, A.; Ampel, N.M.; Andes, D.R.; Armstrong-James, D.; Ayanlowo, O.; Baddley, J.W.; et al. Global guideline for the diagnosis and management of the endemic mycoses: An initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology. Lancet Infect. Dis. 2021, 21, e364–e374. [Google Scholar] [CrossRef]
- Lauand, F. [Contribution to the study of the morphology of Paracoccidioides brasiliensis in oral tissues]. Rev. Inst. Med. Trop. Sao Paulo 1966, 8, 69–78. [Google Scholar] [PubMed]
- Teles, F.R.; Martins, M.L. Laboratorial diagnosis of paracoccidioidomycosis and new insights for the future of fungal diagnosis. Talanta 2011, 85, 2254–2264. [Google Scholar] [CrossRef]
- Talhari, C.; de Souza, J.V.; Parreira, V.J.; Reinel, D.; Talhari, S. Oral exfoliative cytology as a rapid diagnostic tool for paracoccidioidomycosis. Mycoses 2008, 51, 177–178. [Google Scholar] [CrossRef]
- Pinheiro, B.G.; Possa, A.P.; Della Terra, P.P.; de Carvalho, J.A.; Ricci, G.; Nishikaku, A.S.; Hahn, R.C.; Camargo, Z.P.; Rodrigues, A.M. A New Duplex PCR-Assay for the Detection and Identification of Paracoccidioides Species. J. Fungi 2021, 7, 169. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.C.; Hagen, F.; Mendes, R.P.; Burger, E.; Nery, A.F.; Siqueira, N.P.; Guevara, A.; Rodrigues, A.M.; de Camargo, Z.P. Paracoccidioidomycosis: Current Status and Future Trends. Clin. Microbiol. Rev. 2022, e0023321. [Google Scholar] [CrossRef]
- Queiroz-Telles, F.; Escuissato, D.L. Pulmonary paracoccidioidomycosis. Semin. Respir. Crit. Care Med. 2011, 32, 764–774. [Google Scholar] [CrossRef] [Green Version]
- Brummer, E.; Castaneda, E.; Restrepo, A. Paracoccidioidomycosis: An update. Clin. Microbiol. Rev. 1993, 6, 89–117. [Google Scholar] [CrossRef]
- Negro, G.; Pereira, C.N.; Andrade, H.F.; Palacios, S.A.; Vidal, M.M.S.; Charbel, C.E.; Benard, G. Evaluation of tests for antibody response in the follow-up of patients with acute and chronic forms of paracoccidioidomycosis. J. Med. Microbiol. 2000, 49, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Colombo, A.; Queiroz-Telles, F. Paracoccidioidomycosis. In Atlas of Infections Diseases, 2nd ed.; Mandell, G.D., Kauffman, C.A., Eds.; Current Medicine: Philadelphia, PA, USA, 2007; pp. 53–70. [Google Scholar]
- Ameen, M.; Talhari, C.; Talhari, S. Advances in paracoccidioidomycosis. Clin. Exp. Dermatol. 2010, 35, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Gaviria, M.; Rivera, V.; Munoz-Cadavid, C.; Cano, L.E.; Naranjo, T.W. Validation and clinical application of a nested PCR for paracoccidioidomycosis diagnosis in clinical samples from Colombian patients. Braz. J. Infect. Dis. 2015, 19, 376–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Londero, A.T.; Severo, L.C.; Ramos, C.D. Small forms and hyphae of paracoccidioides brasiliensis in human tissue. Mycopathologia 1980, 72, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.P.; Cavalcante, R.S.; Marques, S.A.; Marques, M.E.A.; Venturini, J.; Sylvestre, T.F.; Paniago, A.M.M.; Pereira, A.C.; da Silva, J.F.; Fabro, A.T.; et al. Paracoccidioidomycosis: Current Perspectives from Brazil. Open Microbiol. J. 2017, 11, 224–282. [Google Scholar] [CrossRef] [Green Version]
- Zancopé-Oliveira, R.M.; Pizzini, C.V.; Muniz, M.d.M.; Valle, A.C.F.d.; Almeida-Paes, R. Diagnostic aspects of Paracoccidioidomycosis. Curr. Trop. Med. Rep. 2014, 1, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Restrepo, A.; Robledo, M.; Giraldo, R.; Hernandez, H.; Sierra, F.; Gutierrez, F.; Londono, F.; Lopez, R.; Calle, G. The gamut of paracoccidioidomycosis. Am. J. Med. 1976, 61, 33–42. [Google Scholar] [CrossRef]
- Montenegro, M.R.; Miyaji, M.; Franco, M.; Nishimura, K.; Coelho, K.I.; Horie, Y.; Mendes, R.P.; Sano, A.; Fukushima, K.; Fecchio, D. Isolation of fungi from nature in the region of Botucatu, state of Sao Paulo, Brazil, an endemic area of paracoccidioidomycosis. Mem. Inst. Oswaldo Cruz 1996, 91, 665–670. [Google Scholar] [CrossRef] [Green Version]
- Restrepo, A.; Tobón, A.M.; Cano, L.E. Paracoccidioides brasiliensis. In Principles and Practice of Infectious Diseases, 7th ed.; Mendell, G.D.B., Bennett, J.E., Dolin, R., Eds.; Elsevier: Philadelphia, PA, USA, 2010; pp. 3336–3357. [Google Scholar]
- Restrepo, A.; Tobón, A.M.; Agudelo, C.A. Paracoccidioidomycosis. In Diagnosis and Treatment of Human Mycoses. Infectious Disease; Hospenthal, D.R., Rinaldi, M.G., Eds.; Humana Press: Totowa, NJ, USA, 2008. [Google Scholar]
- Alvarado, P.; Teixeira, M.M.; Cavallera, E.; Paes, H.C.; Guerra, G.; Santander, G.; Merino-Alado, R. Epidemiology of paracoccidioidomycosis in Venezuela: A retrospective study from 1954 to 2019. Mem. Inst. Oswaldo Cruz 2021, 116, e210203. [Google Scholar] [CrossRef]
- Sifuentes-Osornio, J.; Corzo-Leon, D.E.; Ponce-de-Leon, L.A. Epidemiology of Invasive Fungal Infections in Latin America. Curr. Fungal Infect. Rep. 2012, 6, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Gomez, B.L.; Figueroa, J.I.; Hamilton, A.J.; Diez, S.; Rojas, M.; Tobon, A.M.; Hay, R.J.; Restrepo, A. Antigenemia in patients with paracoccidioidomycosis: Detection of the 87-kilodalton determinant during and after antifungal therapy. J. Clin. Microbiol. 1998, 36, 3309–3316. [Google Scholar] [CrossRef] [Green Version]
- da Silva, S.H.; Colombo, A.L.; Blotta, M.H.; Queiroz-Telles, F.; Lopes, J.D.; de Camargo, Z.P. Diagnosis of neuroparacoccidioidomycosis by detection of circulating antigen and antibody in cerebrospinal fluid. J. Clin. Microbiol. 2005, 43, 4680–4683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques da Silva, S.H.; Colombo, A.L.; Blotta, M.H.; Lopes, J.D.; Queiroz-Telles, F.; Pires de Camargo, Z. Detection of circulating gp43 antigen in serum, cerebrospinal fluid, and bronchoalveolar lavage fluid of patients with paracoccidioidomycosis. J. Clin. Microbiol. 2003, 41, 3675–3680. [Google Scholar] [CrossRef] [Green Version]
- Blotta, M.H.; Mamoni, R.L.; Oliveira, S.J.; Nouer, S.A.; Papaiordanou, P.M.; Goveia, A.; Camargo, Z.P. Endemic regions of paracoccidioidomycosis in Brazil: A clinical and epidemiologic study of 584 cases in the southeast region. Am. J. Trop. Med. Hyg. 1999, 61, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Wanke, B.; Londero, A.T. Epidemiology and paracoccidioidomycosis infection. In Paracoccidioidomycosis; Franco, M.F., Lacaz, C.S., Restrepo-Moreno, A., Del Negro, G., Eds.; CRC Press: Boca Raton, FL, USA, 1993; pp. 109–120. [Google Scholar]
- de Camargo, Z.P. Serology of paracoccidioidomycosis. Mycopathologia 2008, 165, 289–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Santos, P.O.; Rodrigues, A.M.; Fernandes, G.F.; da Silva, S.H.; Burger, E.; de Camargo, Z.P. Immunodiagnosis of paracoccidioidomycosis due to Paracoccidioides brasiliensis using a latex test: Detection of specific antibody anti-gp43 and specific antigen gp43. PLoS Negl. Trop. Dis. 2015, 9, e0003516. [Google Scholar] [CrossRef]
- Restrepo, A.; Moncada, L.H. [A slide latex test for the diagnosis of paracoccidiodomycosis]. Bol. Oficina Sanit. Panam. 1978, 84, 520–532. [Google Scholar]
- Mendes-Giannini, M.J.; Bueno, J.P.; Shikanai-Yasuda, M.A.; Ferreira, A.W.; Masuda, A. Detection of the 43,000-molecular-weight glycoprotein in sera of patients with paracoccidioidomycosis. J. Clin. Microbiol. 1989, 27, 2842–2845. [Google Scholar] [CrossRef] [Green Version]
- Blotta, M.H.; Altemani, A.M.; Amaral, E.; Silva, L.J.; Camargo, Z.P. Placental involvement in paracoccidioidomycosis. J. Med. Vet. Mycol. 1993, 31, 249–257. [Google Scholar] [CrossRef]
- Taborda, C.P.; Camargo, Z.P. Diagnosis of paracoccidioidomycosis by dot immunobinding assay for antibody detection using the purified and specific antigen gp43. J. Clin. Microbiol. 1994, 32, 554–556. [Google Scholar] [CrossRef] [Green Version]
- Camargo, Z.P.; Berzaghi, R.; Amaral, C.C.; Silva, S.H. Simplified method for producing Paracoccidioides brasiliensis exoantigens for use in immunodiffusion tests. Med. Mycol. 2003, 41, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Xavier, M.O.; Pasqualotto, A.C.; Cardoso, I.C.; Severo, L.C. Cross-reactivity of Paracoccidioides brasiliensis, Histoplasma capsulatum, and Cryptococcus species in the commercial Platelia Aspergillus enzyme immunoassay. Clin. Vaccine Immunol. 2009, 16, 132–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silveira-Gomes, F.; Sarmento, D.N.; Pinto, T.M.; Pimentel, R.F.; Nepomuceno, L.B.; Espirito Santo, E.P.; Mesquita-da-Costa, M.; Camargo, Z.P.; Marques-da-Silva, S.H. Development and evaluation of a latex agglutination test for the serodiagnosis of paracoccidioidomycosis. Clin. Vaccine Immunol. 2011, 18, 604–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo, A.S.A.; Santos, D.; Lima, S.L.; Rodrigues, A.M.; de Camargo, Z.P.; Finkelman, M.; Colombo, A.L. Evaluation of (1 --> 3)-beta-D-glucan assay for diagnosing paracoccidioidomycosis. Mycoses 2020, 63, 38–42. [Google Scholar] [CrossRef]
- Maifrede, S.B.; Kruschewsky, W.L.L.; Patricio, S.A.; Falqueto, A.; Pecanha, P.M.; Malaquias, L.C.C.; Possa, A.P.; de Camargo, Z.P.; Rodrigues, A.M.; Goncalves, S.S.; et al. Screening paracoccidioidomycosis by double immunodiffusion test in a referred diagnostic center in Brazilian southeastern: An accessible tool. Infection 2021, 49, 1257–1264. [Google Scholar] [CrossRef]
- Cocio, T.A.; Martinez, R. Serological diagnosis of paracoccidioidomycosis using a Paracoccidioides spp. comercial antigen and the counterimmunoelectrophoresis method. Braz. J. Infect. Dis. 2021, 25, 101607. [Google Scholar] [CrossRef] [PubMed]
- Silva Ferreira, C.; de Castro Ribeiro, E.M.; Miranda Goes, A.; Mello Silva, B. Current strategies for diagnosis of paracoccidioidomycosis and prospects of methods based on gold nanoparticles. Future Microbiol. 2016, 11, 973–985. [Google Scholar] [CrossRef]
- Gegembauer, G.; Araujo, L.M.; Pereira, E.F.; Rodrigues, A.M.; Paniago, A.M.; Hahn, R.C.; de Camargo, Z.P. Serology of paracoccidioidomycosis due to Paracoccidioides lutzii. PLoS Negl. Trop. Dis. 2014, 8, e2986. [Google Scholar] [CrossRef]
- Mendes-Giannini, M.J.; Ricci, L.C.; Uemura, M.A.; Toscano, E.; Arns, C.W. Infection and apparent invasion of Vero cells by Paracoccidioides brasiliensis. J. Med. Vet. Mycol. 1994, 32, 189–197. [Google Scholar] [CrossRef]
- Cano, L.E.; Restrepo, A. Predictive value of serologic tests in the diagnosis and follow-up of patients with paracoccidioidomycosis. Rev. Inst. Med. Trop. Sao Paulo 1987, 29, 276–283. [Google Scholar] [CrossRef] [Green Version]
- Camargo, Z.P.; Unterkircher, C.; Travassos, L.R. Identification of antigenic polypeptides of Paracoccidioides brasiliensis by immunoblotting. J. Med. Vet. Mycol. 1989, 27, 407–412. [Google Scholar] [CrossRef]
- Puccia, R.; Schenkman, S.; Gorin, P.A.; Travassos, L.R. Exocellular components of Paracoccidioides brasiliensis: Identification of a specific antigen. Infect. Immun. 1986, 53, 199–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batista, J., Jr.; de Camargo, Z.P.; Fernandes, G.F.; Vicentini, A.P.; Fontes, C.J.; Hahn, R.C. Is the geographical origin of a Paracoccidioides brasiliensis isolate important for antigen production for regional diagnosis of paracoccidioidomycosis? Mycoses 2010, 53, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, C.F.; da Silva, S.H.; Camargo, Z.P. Improvement of the specificity of an enzyme-linked immunosorbent assay for diagnosis of paracoccidioidomycosis. J. Clin. Microbiol. 2005, 43, 1944–1946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, A.M.; Kubitschek-Barreira, P.H.; Pinheiro, B.G.; Teixeira-Ferreira, A.; Hahn, R.C.; de Camargo, Z.P. Immunoproteomic Analysis Reveals Novel Candidate Antigens for the Diagnosis of Paracoccidioidomycosis Due to Paracoccidioides lutzii. J. Fungi 2020, 6, 357. [Google Scholar] [CrossRef] [PubMed]
- Freitas-da-Silva, G.; Roque-Barreira, M.C. Antigenemia in paracoccidioidomycosis. J. Clin. Microbiol. 1992, 30, 381–385. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.F.; Bocca, A.L.; Ferracini, R., Jr.; Figueiredo, F.; Silva, C.L. Cellular requirements for immunomodulatory effects caused by cell wall components of Paracoccidioides brasiliensis on antibody production. Clin. Exp. Immunol. 1997, 109, 261–271. [Google Scholar] [CrossRef]
- Gomez, B.L.; Figueroa, J.I.; Hamilton, A.J.; Ortiz, B.; Robledo, M.A.; Hay, R.J.; Restrepo, A. Use of monoclonal antibodies in diagnosis of paracoccidioidomycosis: New strategies for detection of circulating antigens. J. Clin. Microbiol. 1997, 35, 3278–3283. [Google Scholar] [CrossRef] [Green Version]
- Xavier, M.O.; Araujo, J.S.; Aquino, V.R.; Severo, C.B.; Guazzelli, L.S.; Severo, L.C.; Pasqualotto, A.C. Variability in Galactomannan detection by Platelia Aspergillus EIA according to the Aspergillus species. Rev. Inst. Med. Trop. Sao Paulo 2013, 55. [Google Scholar] [CrossRef]
- Gomes, G.M.; Cisalpino, P.S.; Taborda, C.P.; de Camargo, Z.P. PCR for diagnosis of paracoccidioidomycosis. J. Clin. Microbiol. 2000, 38, 3478–3480. [Google Scholar] [CrossRef]
- Endo, S.; Komori, T.; Ricci, G.; Sano, A.; Yokoyama, K.; Ohori, A.; Kamei, K.; Franco, M.; Miyaji, M.; Nishimura, K. Detection of gp43 of Paracoccidioides brasiliensis by the loop-mediated isothermal amplification (LAMP) method. FEMS Microbiol. Lett. 2004, 234, 93–97. [Google Scholar] [CrossRef]
- Buitrago, M.J.; Merino, P.; Puente, S.; Gomez-Lopez, A.; Arribi, A.; Zancope-Oliveira, R.M.; Gutierrez, M.C.; Rodriguez-Tudela, J.L.; Cuenca-Estrella, M. Utility of real-time PCR for the detection of Paracoccidioides brasiliensis DNA in the diagnosis of imported paracoccidioidomycosis. Med. Mycol. 2009, 47, 879–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koishi, A.C.; Vituri, D.F.; Dionizio Filho, P.S.; Sasaki, A.A.; Felipe, M.S.; Venancio, E.J. A semi-nested PCR assay for molecular detection of Paracoccidioides brasiliensis in tissue samples. Rev. Soc. Bras. Med. Trop. 2010, 43, 728–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, L.; de Carvalho, L.F.; Romano, C.C. Application of PCR in serum samples for diagnosis of paracoccidioidomycosis in the southern Bahia-Brazil. PLoS Negl. Trop. Dis. 2012, 6, e1909. [Google Scholar] [CrossRef] [Green Version]
- Cunha, P.G.e.a. Annual Congress of the European Association of Nuclear Medicine October 13–17, 2018 Dusseldorf, Germany. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1–844. [Google Scholar] [CrossRef]
- Rosa Junior, M.; Baldon, I.V.; Amorim, A.F.C.; Fonseca, A.P.A.; Volpato, R.; Lourenco, R.B.; Baptista, R.M.; de Mello, R.A.F.; Pecanha, P.; Falqueto, A. Imaging paracoccidioidomycosis: A pictorial review from head to toe. Eur. J. Radiol. 2018, 103, 147–162. [Google Scholar] [CrossRef]
- Vermelho, M.B.; Correia, A.S.; Michailowsky, T.C.; Suzart, E.K.; Ibanes, A.S.; Almeida, L.A.; Khoury, Z.; Barba, M.F. Abdominal alterations in disseminated paracoccidioidomycosis: Computed tomography findings. Radiol. Bras. 2015, 48, 81–85. [Google Scholar] [CrossRef] [Green Version]
- Rosa Junior, M.; Amorim, A.C.; Baldon, I.V.; Martins, L.A.; Pereira, R.M.; Campos, R.P.; Goncalves, S.S.; Velloso, T.R.G.; Pecanha, P.; Falqueto, A. Paracoccidioidomycosis of the Central Nervous System: CT and MR Imaging Findings. AJNR Am. J. Neuroradiol. 2019, 40, 1681–1688. [Google Scholar] [CrossRef] [Green Version]
- Rosa, J.M.; Grenfell, M.L.R.; PeCanha, P.M. Hippocampal sclerosis in paracoccidioidomycosis. Arq. Neuropsiquiatr. 2020, 78, 384. [Google Scholar] [CrossRef]
- Colli, B.O.; Assirati Junior, J.A.; Machado, H.R.; Figueiredo, J.F.; Chimelli, L.; Salvarani, C.P.; Dos Santos, F. Intramedullary spinal cord paracoccidioidomycosis. Report of two cases. Arq. Neuropsiquiatr. 1996, 54, 466–473. [Google Scholar] [CrossRef] [Green Version]
- Savarese, L.G.; Monsignore, L.M.; de Andrade Hernandes, M.; Martinez, R.; Nogueira-Barbosa, M.H. Magnetic resonance imaging findings of paracoccidioidomycosis in the musculoskeletal system. Trop. Med. Int. Health 2015, 20, 1346–1354. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, J.; Lopes Colombo, A.; Denning, D.W. The case for paracoccidioidomycosis to be accepted as a neglected tropical (fungal) disease. PLoS Negl. Trop. Dis. 2019, 13, e0007195. [Google Scholar] [CrossRef] [PubMed]
- Rauseo, A.M.; Mazi, P.; Lewis, P.; Burnett, B.; Mudge, S.; Spec, A. Bioavailability of Single-Dose SUBA-Itraconazole Compared to Conventional Itraconazole under Fasted and Fed Conditions. Antimicrob. Agents Chemother. 2021, 65, e0013421. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Spec, A.; Miceli, M.; McGwin, G.; McMullen, R.; Thompson, G.R.R. An open-label comparative trial of SUBA-itraconazole (SUBA) versus conventional itraconazole (c-itra) for treatment of proven and probable endemic mycoses (MSG-15): A pharmacokinetic (PK) and adverse Event (AE) analysis. Open Forum Infect. Dis. 2021, 8, S72. [Google Scholar] [CrossRef]
- Pecanha, P.M.; de Souza, S.; Falqueto, A.; Grao-Veloso, T.R.; Lirio, L.V.; Ferreira, C.U., Jr.; Santos, A.R.; Costa, H.G.; de Souza, L.R.; Tuon, F.F. Amphotericin B lipid complex in the treatment of severe paracoccidioidomycosis: A case series. Int. J. Antimicrob. Agents 2016, 48, 428–430. [Google Scholar] [CrossRef]
- Queiroz-Telles, F.; Goldani, L.Z.; Schlamm, H.T.; Goodrich, J.M.; Espinel-Ingroff, A.; Shikanai-Yasuda, M.A. An open-label comparative pilot study of oral voriconazole and itraconazole for long-term treatment of paracoccidioidomycosis. Clin. Infect. Dis. 2007, 45, 1462–1469. [Google Scholar] [CrossRef] [Green Version]
- Thompson, G.R., 3rd; Rendon, A.; Ribeiro Dos Santos, R.; Queiroz-Telles, F.; Ostrosky-Zeichner, L.; Azie, N.; Maher, R.; Lee, M.; Kovanda, L.; Engelhardt, M.; et al. Isavuconazole Treatment of Cryptococcosis and Dimorphic Mycoses. Clin. Infect. Dis. 2016, 63, 356–362. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, A. The Therapy of Pulmonary Fibrosis in Paracoccidioidomycosis: What Are the New Experimental Approaches? J. Fungi 2020, 6, 217. [Google Scholar] [CrossRef]
- Puerta-Arias, J.D.; Pino-Tamayo, P.A.; Arango, J.C.; Gonzalez, A. Depletion of Neutrophils Promotes the Resolution of Pulmonary Inflammation and Fibrosis in Mice Infected with Paracoccidioides brasiliensis. PLoS ONE 2016, 11, e0163985. [Google Scholar] [CrossRef] [Green Version]
- Magalhaes, A.; Ferreira, K.S.; Almeida, S.R.; Nosanchuk, J.D.; Travassos, L.R.; Taborda, C.P. Prophylactic and therapeutic vaccination using dendritic cells primed with peptide 10 derived from the 43-kilodalton glycoprotein of Paracoccidioides brasiliensis. Clin. Vaccine Immunol. 2012, 19, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Taborda, C.P.; Uran, M.E.; Nosanchuk, J.D.; Travassos, L.R. Paracoccidioidomycosis: Challenges in the Development of a Vaccine against an Endemic Mycosis in the Americas. Rev. Inst. Med. Trop. Sao Paulo 2015, 57 (Suppl. 19), 21–24. [Google Scholar] [CrossRef]
- Naranjo, T.W.; Lopera, D.E.; Diaz-Granados, L.R.; Duque, J.J.; Restrepo, A.M.; Cano, L.E. Combined itraconazole-pentoxifylline treatment promptly reduces lung fibrosis induced by chronic pulmonary paracoccidioidomycosis in mice. Pulm. Pharmacol. Ther. 2011, 24, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Finato, A.C.; Almeida, D.F.; Dos Santos, A.R.; Nascimento, D.C.; Cavalcante, R.S.; Mendes, R.P.; Soares, C.T.; Paniago, A.M.M.; Venturini, J. Evaluation of antifibrotic and antifungal combined therapies in experimental pulmonary paracoccidioidomycosis. Med. Mycol. 2020, 58, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Puerta-Arias, J.D.; Pino-Tamayo, P.A.; Arango, J.C.; Salazar-Pelaez, L.M.; Gonzalez, A. Itraconazole in combination with neutrophil depletion reduces the expression of genes related to pulmonary fibrosis in an experimental model of paracoccidioidomycosis. Med. Mycol. 2018, 56, 579–590. [Google Scholar] [CrossRef] [Green Version]
- Boniche-Alfaro, C.; Kischkel, B.; Thomaz, L.; Carvalho-Gomes, M.M.; Lopes-Bezerra, L.M.; Nosanchuk, J.D.; Taborda, C.P. Antibody- Based Immunotherapy Combined With Antimycotic Drug TMP-SMX to Treat Infection With Paracoccidioides brasiliensis. Front. Immunol. 2021, 12, 725882. [Google Scholar] [CrossRef] [PubMed]
- Arango, J.C.; Puerta-Arias, J.D.; Pino-Tamayo, P.A.; Salazar-Pelaez, L.M.; Rojas, M.; Gonzalez, A. Impaired anti-fibrotic effect of bone marrow-derived mesenchymal stem cell in a mouse model of pulmonary paracoccidioidomycosis. PLoS Negl. Trop. Dis. 2017, 11, e0006006. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peçanha, P.M.; Peçanha-Pietrobom, P.M.; Grão-Velloso, T.R.; Rosa Júnior, M.; Falqueto, A.; Gonçalves, S.S. Paracoccidioidomycosis: What We Know and What Is New in Epidemiology, Diagnosis, and Treatment. J. Fungi 2022, 8, 1098. https://doi.org/10.3390/jof8101098
Peçanha PM, Peçanha-Pietrobom PM, Grão-Velloso TR, Rosa Júnior M, Falqueto A, Gonçalves SS. Paracoccidioidomycosis: What We Know and What Is New in Epidemiology, Diagnosis, and Treatment. Journal of Fungi. 2022; 8(10):1098. https://doi.org/10.3390/jof8101098
Chicago/Turabian StylePeçanha, Paulo Mendes, Paula Massaroni Peçanha-Pietrobom, Tânia Regina Grão-Velloso, Marcos Rosa Júnior, Aloísio Falqueto, and Sarah Santos Gonçalves. 2022. "Paracoccidioidomycosis: What We Know and What Is New in Epidemiology, Diagnosis, and Treatment" Journal of Fungi 8, no. 10: 1098. https://doi.org/10.3390/jof8101098




