Abstract
Nematode-trapping (NT) fungi play a significant role in the biological control of plant- parasitic nematodes. NT fungi, as a predator, can differentiate into specialized structures called “traps” to capture, kill, and consume nematodes at a nutrient-deprived condition. Therefore, trap formation is also an important indicator that NT fungi transition from a saprophytic to a predacious lifestyle. With the development of gene knockout and multiple omics such as genomics, transcriptomics, and metabolomics, increasing studies have tried to investigate the regulation mechanism of trap formation in NT fungi. This review summarizes the potential regulatory mechanism of trap formation in NT fungi based on the latest findings in this field. Signaling pathways have been confirmed to play an especially vital role in trap formation based on phenotypes of various mutants and multi-omics analysis, and the involvement of small molecule compounds, woronin body, peroxisome, autophagy, and pH-sensing receptors in the formation of traps are also discussed. In addition, we also highlight the research focus for elucidating the mechanism underlying trap formation of NT fungi in the future.
1. Introduction
Plant-parasitic nematodes (PPNs) can cause direct damage to their host or act as virus vectors [1]. There are more than 4100 species of PPNs that have an impact on global agriculture and horticulture, causing an estimated annual loss of USD 173 billion [2,3]. Nematode-trapping (NT) fungi are potential biocontrol resources, which have the advantages of low toxicity, high efficiency, and environmental friendliness, so they have gradually become favored by people in recent years [3]. NT fungi can develop specialized structures called “traps”, an important indicator of the transition from saprophytic to predatory lifestyles, including constricting rings, adhesive networks, adhesive columns, and adhesive knobs, to capture, kill, and consume nematodes at a nutrient-deprived condition [4,5]. Therefore, trap formation is a crucial step in the lifestyle of NT fungi and indispensable for nematode predation. The factors involved in trap formation were variety, including multiple signal transduction pathways [6]; small molecular compounds [7,8,9,10,11,12]; intercellular communication [13]; adhesive protein [5]; nitrate assimilation [14]; woronin body [15]; peroxisome [16]; autophagy [17,18,19,20]; and pH-sensing receptors [21], according to the comprehensive research of genomics, transcriptomics, proteomics, metabolomics, and reverse genetics. Here, we review the recent progress in the regulatory mechanism of trap formation in the NT fungi based on phenotypes of various mutants and multi-omics analysis. We especially focus on the latest studies in signal transduction pathways and small molecular compounds involved in trap formation. Elucidating the molecular mechanism of trap formation will not only provide a theoretical basis for the improvement of engineering biocontrol fungi, but contribute to understanding the adaptive evolution and lifestyle transition of NT fungi.
2. Multi-Omics Analysis Promotes Research on Trap Formation of NT Fungi
Omics not only provides a macroscopic direction for the mechanism of trap formation but also provides specific targets. Comparative genomics studies have shown that many species-specific genes have expanded in the evolutionary process, and these genes may be related to the function specialization of NT fungi [5,6,22,23]. Arthrobotrys oligospora (teleomorph Orbilia auricolor), one of the typical NT fungi, was the first NT fungus whose genome and proteome were sequenced [6]. Comparative analysis showed that A. oligospora genome contains numerous pathogenicity-related genes, and a total of 398 homologous genes related to the pathogenicity of other fungi have been identified [6], while there were fewer lectin genes involved in fungus–nematode recognition in the Drechslerella stenobrocha genome [22]. This suggested that a different mechanism of trap formation may exist in various NT fungi. In addition, studies combined genome, proteome, and real-time PCR (RT-PCR) analyses to reveal that multiple signal transduction pathways have an integral part in trap formation [6]. Transcriptome sequencing and RT-PCR analysis showed that a large number of genes were significantly upregulated during the infection process of NT fungi, including genes involved in translation, amino acid metabolism, carbohydrate metabolism, cell wall and membrane biogenesis [6], secreted proteins [23], adhesion proteins [5], and the protein kinase C signal transduction pathway [22]. Comparative genomic analysis in the four representative trapping devices of NT fungi suggested that the simplification of the capture device was accompanied by the expansion of adhesion genes and the increase in adhesiveness on trap surfaces [5]. In conclusion, the above studies show that omics technologies have clarified the general direction for research on the regulation mechanism of traps formation. At the same time, the assembly of genomes in different fungi makes it possible to study the interaction between the NT fungi and nematodes at the molecular level. For instance, according to the genome sequence and annotation of Duddingtonia flagrans, a fluorescent protein system with native promoter was established, and a secretion protein PEFB was identified which was involved in the processes of infection against Caenorhabditis elegans [13]. Recently, nine species of NT fungi have been sequenced, such as adhesive-network-producing fungi A. oligospora and D. flagrans, adhesive-knob-producing fungi Monacrosporium haptotylum and Dactylellina entomopaga, constricting-ring-producing fungi D. stenobrocha and Drechslerella brochopaga, adhesive-columns-producing fungus Dactylellina cionopagum, and no trap producing fungus Dactylella cylindrospora (Table 1); these genomic information may help to elucidate the mechanism of trap formation of NT fungi.

Table 1.
Genomic features of different NT fungi.
3. Overview of Signaling Pathways Involved in Trap Formation
3.1. G-Protein Signaling Pathway Involved in Trap Formation
G-protein signaling is the most conserved signal transduction pathway in fungi, composed of heterotrimeric G-proteins (G-proteins), G-protein-coupled receptors (GPCRs), and regulators of G-protein signaling (RGSs), which plays a vital role in sensing the changes in various physical and chemical stimuli in the environment [25]. G-proteins have three subunits: Gα, Gβ, and Gγ. The latest research identified a single G-protein β-subunit gene (gpb1) in A. oligospora, and phenotypic analyses demonstrated that Δgpb1 mutants were strongly defective in the response to C. elegans and ascarosides, with the formation of few traps [24]. This means that GPCRs might be the receptor of ascarosides. In addition, G-protein signaling pathways are negatively regulated by RGSs. Recently, seven putative rgs genes were knocked out, and multi-phenotypic analyses shown that these genes affect the pathogenicity of A. oligospora to varying degrees. In particular, the ΔflbA deletion strains lost the ability to produce traps after being induced with nematodes [26]. In addition, resistance to inhibitors of cholinesterase 8 (RIC8), a conservative guanine nucleotide exchange factor, which is involved in the regulation of G-protein signaling in filamentous fungi. Recently, an orthologous RIC8 was characterized in A. oligospora, the Δric8 deletion mutants lost the ability to produce traps essential for nematode predation, accompanied by a marked reduction in cyclic adenosine monophosphate (cAMP) level. Further assay revealed that RIC8 interacted with G-protein subunit Gα1 and is involved in nematode predation through control of cell cycle, organelle, and secondary metabolism [27].
The superfamily of small GTPases comprises signal transducers that regulate multiple cellular functions. RAS, RHO/RAC, RAB, ARF, and RAN are conserved groups of the small GTPases family that cycle between GTP-bound (active) and GDP-bound (inactive) conformations as a switch in signal transduction. Recently, two RAB GTPases were identified in A. oligospora: The Δrab-7A deletion strains lost the ability to produce conidia and traps; however, the Δrab-2 mutants only slightly affected the conidiation but did not affect the trap formation [28]. In another research, three RAS GTPases were characterized by gene disruption and multi-omics analysis, the traps number of Δras2 and Δrheb deletion strains were significantly decreased, but Δras3 mutants had no significant changes compared with the wild-type (WT) strain [29]. Our latest research demonstrated that three RHO GTPases (RHO2, RAC, and CDC42) played an important role in trap formation and lifestyle transition of A. oligospora. The rac was significantly upregulated, and alternative splicing events occurred in rac and rho2 during the trap formation and infection process [30]. These studies indicated that the small GTPases have pleiotropic functions in the growth and development of A. oligospora; specifically, they play a very important role in trap formation and pathogenicity. Moreover, GTPase activating proteins (GAPs) are a family of proteins that induce the hydrolysis of GTP bound to small GTPases [31]. Recently, an ARF-GAP GLO3 was identified in A. oligospora. Trap formation was delayed; no nematodes were captured at nematode induction for 12 h in the Δglo3 mutants; and captured nematodes were significantly reduced at 24, 36, and 48 h compared with the WT strain [32]. Therefore, G-proteins and small GTPases play a pleiotropic role in the growth, development, trap formation, and pathogenicity in A. oligospora and other NT fungi.
3.2. Mitogen-Activated Protein Kinase (MAPK) Signaling Pathway Is Essential for Trap Formation
Accumulating evidences implicate that G-proteins can mediate signal transfer to MAPK-signaling cascade in filamentous fungi, which plays a significant role in pathogenicity [25]. Many NT fungi require very specific abiotic and biotic stimuli to form traps, the ability to sense and respond to environmental signals is essential during the trap formation and nematode predation [33]. MAPK signaling cascades are critical for pathogenic fungi to detect surrounding organisms in the environment [4,34]. There are three major MAPK cascades that have been well-studied in yeasts and filamentous fungi, including SLT2/MPK1 (cell wall integrity pathway), FUS3/KSS1 (pheromone-response and filamentous growth pathway), and HOG1 (hyperosmolarity pathway) [35,36]. Recent research showed that slt2 was required for trap formation in two nematode-trapping fungi A. oligospora (strain ATCC24927) and M. haptotylum [34]. However, another study demonstrated that slt2 is not essential for trap development in A. oligospora (strain TWF154), and its mutant was still able to develop traps after three days of nematode exposure [4]. Meanwhile, BCK1 and MKK1, two proteins of function upstream of SLT2, were mutants unable to produce spores and mycelial traps [37]. Moreover, the trap formation and predation efficiency were reduced in Δhog1 and Δmsb2 mutants, and those mutants were highly sensitive to high osmolarity [38]. Moreover, SSK1, as an upstream regulatory protein of HOG1 signaling pathway, played a negative regulatory role in trap formation, as manifested by significantly increased trap formation and predation efficiency in Δssk1 mutants [39]. In addition, deletion of ste7 and fus3 led to complete abolishment of conidiation and traps in mutants. In addition, STE12, a conserved transcription factor acting downstream of the pheromone-response pathway, induced disruption to defects in the response to nematode pheromone in A. oligospora [4]. Similarly, in Drechslerella dactyloides, the Ddaste12 deletion strain cannot form inflationary contraction ring, which make it unable to catch nematodes [40]. In addition, inducer of meiosis 2 (IME2), a non-classical MAPK-pathway molecule, was associated with mycelial growth and development, conidiation, osmolarity, and pathogenicity in A. oligospora. The Δime2 mutant cannot form mature traps containing multi-hyphal loop, and the electron-dense bodies in trap cells were less than the WT strain [41]. Together, these observations demonstrate that MAPK cascades are essential for trap formation of NT fungi.
3.3. cAMP-Dependent Protein Kinase A (cAMP/PKA) Signaling Pathway Is Indispensable for Trap Formation
The pathogenic process is related to infection-related formation and development in many pathogenic fungi, and the cAMP/PKA pathway plays an essential role in fungal pathogenesis [25]. In our latest research, the deletion of adenylate cyclase led to the abolishment of trap formation, and the number of traps in PKA subunits mutants were reduced in varying degrees (unpublished). In addition, the stunted protein STUA functions as the downstream of cAMP/PKA signaling pathway. The sporulation capacity of ΔstuA mutants were reduced 96%, and the ability to produce mycelial traps was lost [42]. Meanwhile, the transcriptional levels of several upstream genes of cAMP/PKA pathway were significantly reduced in ΔstuA mutants, such as gpr, gα, ras2, acyA, pkaC1, and pkaC2, and some downstream genes were also significantly downregulated, including nsdc, cdc28, and cdc6 [42]. Similarly, the cAMP levels in Δras2 and Δrheb mutants were significantly lower than in the WT strain, and the downstream genes of the cAMP/PKA were significantly downregulated [29]. The cAMP level of the Δric8 mutant was reduced to 3.0%–11.3% compared to the WT strain [27]. These findings indicate that the cAMP signaling pathway is indispensable for trap formation in A. oligospora and other NT fungi.
3.4. Ca2+-Related Signaling Pathway Regulates Trap Formation
Multifunctional Ca2+/calmodulin-dependent protein kinases (CaMKs) are necessary elements in the G-protein signaling pathway. Five CaMKs were identified in A. oligospora, the number of traps in the five CaMK-encoding genes deletion strains were significantly lower than that of the WT strain, and the trap formation was delayed in ΔcamkB mutant [43]. In addition, phospholipase C (PLC), a key enzyme in the inositol phospholipid signaling pathway, hydrolyzes phospholipids to produce inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) [44]. IP3 causes the release of intracellular calcium ions (Ca2+), and DAG triggers protein kinase C activation [45]. Recent research showed that the number of traps in Δplc2 mutants was reduced and the partial hyphal loop of the traps was irregular [46]. In addition, the low-affinity calcium uptake system (LACS) also played vital role in trap formation, and the deletion of two genes for the LACS transmembrane protein resulted in a 90% trap reduction and no trap formation, respectively [47].
4. Compounds as Signal Molecules to Regulate Trap Formation
There is an evolutionary arms race between predators and prey, and just as prey evolves specific strategies to avoid being hunted, predators also evolve stronger predation strategies, such as lure compounds, to ensure adequate food [48]. Normally, NT fungi are saprophytic, but they will become predators in order to maximize the chance of survival when nutrients are deficient, and their lifestyle transitions accordingly. However, NT fungi are non-motile predators, but nematodes can move at will. Hence, NT fungi evolved an ingenious way, “VOCs”, functioning as mimic pheromone, to lure nematodes. These volatile compounds (VOCs) include dimethyl disulfide, (±)2-methyl-1-butanol, 2,4-dithiapentane, S-methyl thioacetate, and methyl 3-methyl-2-butenoate, and especially, methyl 3-methyl-2-butenoate trigger strong sex- and stage-specific attraction in several Caenorhabditis species. Correspondingly, the olfactory neuron AWCs of C. elegans sensed the odors emanating from NT fungi and responded the attraction [7,49]. Interesting is that the ascarosides, an evolutionarily highly conserved family of small molecules produced by nematodes, downregulated the expression of polyketide synthase gene (artA), which in turn promoted the formation of new traps and then resulted in trapping networks. Therefore, the concentration of ascarosides increased as the nematodes approach, which in turn downregulated arthrosporol and 6-methyl-salicylic acid (6-MSA) formation, further causing the hunting ground to be covered with traps. The ascarosides disappear when the nematodes were completely digested, the contents of arthrosporol and 6-MSA returned to normal levels, and the mycelium switched to saprotrophic growth [50].
Trap formation is a highly energy-consuming process, and to conserve energy, NT fungi have to evolve a more effective strategy to regulate the triggering or closing of trap formation [8]: for example, triggering trap formation after sensing signals of nematodes during infection and terminating trap formation when nematodes were fully digested. Small molecular compounds played an important role in this conversion process, such as ascarosides from nematodes [8], and 6-MSA, oligosporons, arthrobotrisins, and arthrosporols isolated from A. oligospora and other NT fungi [50,51,52,53,54,55]. Recently, an increasing number of compounds and associated synthetic genes have been investigated. For instance, the latest research demonstrated that the chemical diversity of metabolites increased notably and exhibited species specificity in the process of changing lifestyle from saprophytic to predatory in NT fungi A. oligospora, A. thaumasia, and A. musiformis [56]. Volatile furanone and pyrone metabolites can help A. oligospora capture nematodes in the lifestyle transition [12], and abscisic acid was highly effective at enhancing trap formation of D. stenobrocha [57]. In addition, 6-MSA is a chemoattractant that can lure nematodes into the fungal mycelium. The artA expression can produce 6-MSA in hyphal tips, and was uncoupled from other enzymes required for the conversion of 6-MSA to arthrosporols; moreover, corresponding deletion strains produced more traps, suggesting a negative role of 6-MSA on trap formation in D. flagrans [50]. Furthermore, the gene cluster AOL_s00215 plays a key role in the production of arthrosporols in A. oligospora, and the number of traps was increased in deletion mutants of most genes in this gene cluster [10,11,58,59,60]. Arthrobotrisins were downregulated in Δric8, Δras2, and Δrheb mutants, indicating that G-proteins and small GTPases were involved in regulating the metabolism of arthrobotrisins [27,29]. Simultaneously, ammonia could function as a signaling molecule in NT fungi to trigger trap formation and kill nematodes, disrupting the gene involved in urea transport and metabolism, resulting in the abolition of urea-induced trap formation in A. oligospora [61]. Another study also demonstrated that ammonia can induce trap formation as a signal molecule in NT fungi A. oligospora, A. guizhouensis, D. phymatopaga, D. cionopaga, and D. brochopaga [62]. Furthermore, PKS−TPS hybrid pathway, for biosynthesis of sesquiterpenyl epoxy-cyclohexenoids, involved in trap formation via ammonia metabolism, deletion of most genes in the PKS−TPS hybrid pathway displayed significantly increase in trap formation [9]. Overall, the discovery of multiple compounds enriches our knowledge of the inducers in trap formation, which participate in trap formation as signaling molecules.
5. Multiple Cellular Processes Were Involved in Trap Formation
Trap formation of NT fungi was a sophisticated process and required the coordination and cooperation of diverse cellular processes, such as ubiquitin system [63,64], nitrate assimilation pathway [14], pH-sensing receptor [21], the velvet family proteins [65], scaffold proteins [66], lectins [67], actin [68], the striatin-interacting phosphatase and kinase (STRIPAK) [69], adhesin [70], reactive oxygen species [71], glycerol biosynthesis [72], milRNAs [73], woronin body [15], autophagy [17,18,19,20], and cell-to-cell communication and hyphal fusion [13]. Trap formation was affected to varying degrees by the deletion of genes associated with these cellular processes, such as reduction in number, morphological variation, and time delay in formation (Table 2). Interestingly, the organismic interaction between NT fungi and nematodes was just like a dramatic game of hunt or attack. Effectors played critical roles in regulating host cell physiology to promote virulence, biotrophic growth, or symbiosis [74,75]. In NT fungi, certain secreted proteins can be used to modulate the innate immune system of nematodes or target other intracellular processes. For instance, PEFB, a putative fungal virulence factor, was upregulated during nematodes infection and expressed in C. elegans, where it was localized to nuclei [13]. In addition, cell-to-cell communication was required for ring closure, the deletion of sofT inhibited the anastomosis of normal vegetative hyphae, resulting in spiral hyphae, while the mutant was still able to trap C. elegans [13]. The STRIPAK complex is a highly conserved signaling hub involved in the regulation of hyphal fusion. Deletion of the STRIPAK component SIPC resulted in failure to form complete loops and the formation of column-like trap structures with elongated compartments [69]. On the other hand, nitrogen plays a vital role in the growth of fungi, and autophagy was required for nitrogen homeostasis and recycling [19]. Deletion of atg8 not only abolished the autophagy induced by nematodes but also suppressed trap formation and reduces pathogenicity of A. oligospora [19]. Likewise, the formation of autophagosomes and traps were defective in Δatg1, Δatg4, and Δatg5 mutants [17,18,20].

Table 2.
A list of characterized genes contributing significantly to trap formation in NT fungi.
6. Summary and Perspectives
6.1. Multiple Signaling Pathways and Cellular Processes Co-Regulate Trap Formation
The development of multi-omics contributes to elucidating the mechanism of trap formation. Comparative genomics of nine NT fungi including two A. oligospora with different genetic backgrounds revealed commonalities and specificities of trap formation among different NT fungi [5,6,13,22,23,24]. As previously mentioned, the results of comparative genomics and multi-omics consistently showed that signal transduction pathways were closely related to the morphogenetic triggering process of traps. As a representative species of NT fungi, the formation mechanism of traps has been extensively investigated in A. oligospora, and trap formation is a complex process, which likely requires the coordination of multiple pathways. This was demonstrated by changes in the expression patterns of relevant genes in various mutant strains and interaction between some proteins (Table 2).
It is the nature of predators to sense and respond to prey. The G-protein signaling pathway was essential for the signal reception and transduction of nematodes in NT fungi. Deletion of gpb1 resulted in a remarkable reduction in trap formation [24]. Phosphorylation assay showed that phosphorylation signals were lost in both Δgpb1 and Δfus3 mutants upon nematode induction, suggesting that gpb1 may function upstream of fus3 [4]. RGSs negatively regulated G-protein signal transduction, and in the Δrgs mutants, the intracellular cAMP levels were significantly increased and the transcription levels of G-protein signaling genes were downregulated [26]. In addition, RIC8 regulated the cAMP level by interacting with Gα1, thereby participating in the fungal growth, environmental adaptation, and trap formation [27]. These findings show that G-protein regulates the cAMP/PKA and MAPK signaling pathways under the regulation of RGSs and other regulators.
In addition, the small GTPases family acts as switches in the signaling hub of molecular circuits [76]. A recent study showed that the expression levels of genes encoding regulatory subunits of PKA, MAPK, and P21-activated kinases were downregulated in Δcdc42 and Δrac mutants; thus, PKA, P21-activated kinases, and HOG1 may be downstream effectors of RHO GTPases in A. oligospora [30]. Similarly, RAS GTPases affected MAPK signaling by interacting with STE50 and directly regulating intracellular cAMP levels and the mTOR signaling pathway [29]. These indicate that small GTPases can directly or indirectly regulate MAPK, TOR, and cAMP/PKA signaling pathways. Transcription factors located downstream of these signaling pathways activate or repress the transcription of corresponding genes after receiving cascaded signals, thereby regulating the biological response of cells to external signals. STUA functions downstream of the cAMP/PKA signaling pathway, and the expression of genes involved in the G-protein signaling pathway were transcriptionally repressed in the mutants [42]. Meanwhile, reverse genetics demonstrated that STE12, which regulated the expression of “nematode-responsive genes” that trigger trap formation, was activated by the MAPK cascade in A. oligospora. GO enrichment analysis of STE12-dependent differentially expressed genes revealed that the two most significant terms were “integral component of membrane” and “oxidation–reduction process”, which were critical for hyphal growth and virulence in the NT fungi [4]. These results indicated that multiple signaling pathways co-regulate trap formation.
What can be clearly seen in recent studies is the deletion of almost all of these genes affected redox processes [26,27,28,30,34,37,42,71] and partly influence heat shock response [29], cell walls [26,27,28,32,34,41,42], and mitochondria [29,30], indicating the genes related to these processes might be involved in trap formation, which was confirmed in our latest research (unpublished). At the same time, each gene does not influence the trap formation alone: They need to cooperate with each other (Table 2).
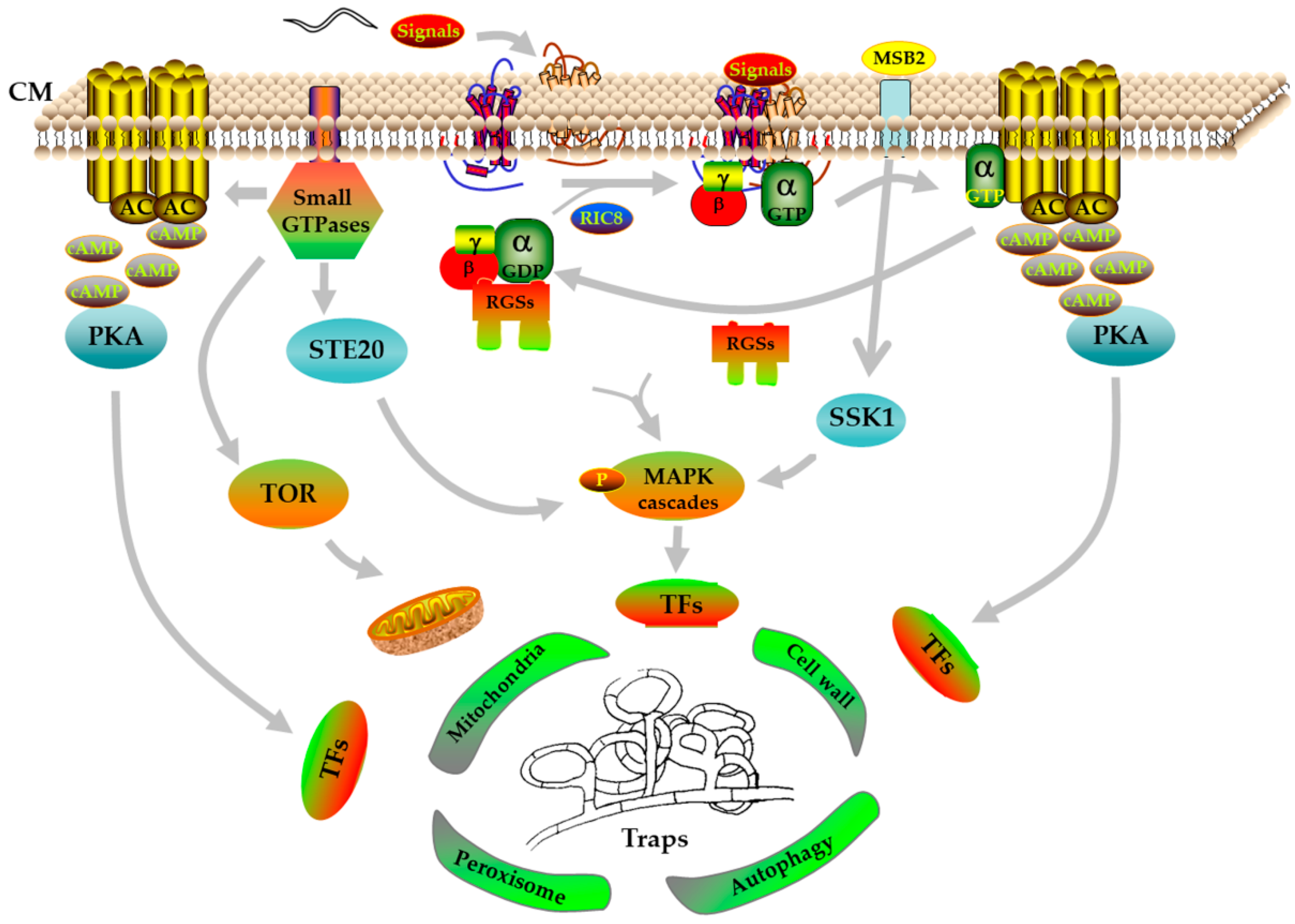
Collectively, given the recent progress in reverse genetics, knowledge obtained from different pathways based on the multi-omics and RT-PCR would contribute significantly to elucidating the specific mechanism of the trap formation, which we are only just beginning to understand. In general, it seems that GPCRs are the receptors to respond the nematode’s signals, and the G-protein signaling pathway accurately transform extracellular signals into intracellular signals with the regulation of RGSs, and then transfers to downstream cascade pathways, such as the cAMP/PKA pathway. Simultaneously, the small GTPase family also regulates the MAPK cascades by interacting with STE20 protein kinases. Subsequently, these cascade pathways further regulate a variety of cellular processes by regulating downstream transcription factors, thereby triggering trap formation (Figure 1).

Figure 1.
A proposed model for trap formation in NT fungi using A. oligospora as an example. CM, cell membrane; α, β, and γ, G-protein subunits; AC, adenylate cyclase; cAMP, cyclic adenosine monophosphate; PKA, protein kinase A; RGSs, regulators of G-protein signaling; RIC8, resistance to inhibitors of cholinesterase; MAPK, mitogen-activated protein kinase; STE20, serine/threonine protein kinase; TOR, mammalian target of rapamycin; MSB2, mucin family signaling protein; SSK1, response regulator; TFs, transcription factors; P, phosphorylation.
6.2. The Regulatory Mechanism of Trap Formation May Vary in NT Fungi
Absolutely, it is not difficult to understand that there are differences in the mechanisms of traps formation due to the differences in the morphologies of traps and predatory ways. Comparative genomics of NT fungi with different types of traps have indicated that many species-specific genes and highly expressed uncharacterized orphan genes have expanded during evolution [5,6,22,23,77]. The whole genome blast analysis and the distribution analysis of orthologous gene showed that the putative pathogen–host interaction genes included 80 specific genes in A. oligospora. Furthermore, compared with other pathogenic and non-pathogenic fungi, A. oligospora contained 53.64% abundant orphan genes [6]. Another study showed that 20% of the genes in the M. haptotylum and A. oligospora genomes were shared, but importantly, as many as 16% in each genome genes were unique. Moreover, 15 species-specific genes, unique for the M. haptotylum, were found in the cohort of the 10-fold upregulated transcripts during infection [23]. Comparative analysis found that 12 multigene families and 23 gene domains were significantly expanded in the NT fungal genomes. Notably, nine of the expanded multigene families lacked significant matches in public databases, suggesting the existence of a potential novel mechanism underlying trap formation [5]. In addition, the traps of A. oligospora and most NT fungi must be induced, but the constricting rings of D. stenobrocha can form spontaneously, and its genome was more compact than that of A. oligospora with rare repeat-induced point mutation and transposon [22]. Therefore, a more suitable interpretation is that these may be involved in the functional specialization of different NT fungi, which means that the mechanism of trap formation may differ among various NT fungi.
6.3. Current Status and Future Prospects
The completion of genome sequencing is a milestone for the study of the mechanism trap formation and interaction between the NT fungi and nematodes. Although the genomes of nine NT fungi, including two A. oligospora with different genetic backgrounds, have been sequenced [5,6,13,22,23,24], research on other NT fungi is still in its infancy, except for A. oligospora and D. flagrans. Differences in trap formation between diverse NT fungi remain an interesting topic for further study. There has been considerable progress in the mechanism of trap formation with the development of multi-omics and molecular biology techniques; however, more knowledge still needs to be accumulated for parsing more comprehensive connections in multiple pathways and organelles during trap formation. According to the phenotypes of these gene mutants, it can be found that mitochondria [29,30], peroxisomes [16], nucleus [17,30,39,46], woronin bodies [15], autophagosomes [17,18,19,20], and other organelles [39,46] have changed in varying degrees. So, here, the question arises: How do they cooperate with each other in trap formation? Are they actually involved in trap formation, or are these changes simply due to defects in growth?
NT fungi are important biocontrol resources, and the traps are the key devices to capture nematodes, which will be formed after receiving external signals. Small molecule compounds act as this signal, such as ascarosides [8], abscisic acid [57], ammonia [61,62], and 6-MSA [50], and arthrosporols [50,51,52,53,54,55] were also involved in regulating trap formation. However, the metabolomic analysis showed that a large number of compounds changed during trap formation and nematode infection [27,29,39,46,56]. So, what these compounds are and how they affect trap formation require further study. Could other compounds from nematodes or the environment also affect trap formation? Moreover, signaling pathways have been identified to be involved in trap formation in multiple NT fungi [4,26,27,28,29,30,32,34,37,38,39,41,43,48,78,79], whereas there is a lack of studies about downstream transcription factors, and further research is needed on the functions of upstream receptors located in the cell wall and membrane.
In summary, increasing knowledge about the regulation mechanism of trap formation of NT fungi has been acquired from the phenotypic traits of various mutants and multi-omics analysis in recent years, whereas elucidating the molecular mechanism of trap formation is still ongoing due to the complexity of trap production by multitudinous NT fungi. Here, we reviewed the latest findings on the trap formation of NT fungi, summarized the shortcomings at present, and discussed the emphases in the future. Our review provides a solid basis for elucidating the mechanisms of trap formation and lifestyle transition of NT fungi, and will help to develop more effective anti-nematode agents by genetic modification.
Author Contributions
Conceptualization, K.-Q.Z. and J.-K.Y.; methodology, M.-C.Z.; validation and discussion, X.-M.L., N.Z. and L.Y.; data curation, M.-C.Z.; writing—original draft preparation, M.-C.Z. and J.-K.Y.; writing—review and editing, J.-K.Y.; supervision, J.-K.Y.; project administration, J.-K.Y.; funding acquisition, K.-Q.Z. and J.-K.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research described here is supported by the National Natural Science Foundation of China (no. 31960556), and the Applied Basic Research Foundation of Yunnan Province (no. 202001BB050004).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Nicol, J.M.; Turner, S.J.; Coyne, D.L.; Den Nijs, L.; Hockland, S.; Maafi, Z.T. Current nematode threats to world agriculture. In Genomics and Molecular Genetics of Plant-Nematode Interactions; Jones, J., Gheysen, G., Fenoll, C., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 21–43. [Google Scholar]
- Elling, A.A. Major emerging problems with minor Meloidogyne species. Phytopathology 2013, 103, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Phani, V.; Khan, M.R.; Dutta, T.K. Plant-parasitic nematodes as a potential threat to protected agriculture: Current status and management options. Crop Prot. 2021, 144, 105573. [Google Scholar] [CrossRef]
- Chen, S.A.; Lin, H.C.; Schroeder, F.C.; Hsueh, Y.P. Prey sensing and response in a nematode-trapping fungus is governed by the MAPK pheromone response pathway. Genetics 2021, 217, iyaa008. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Yu, Z.; Yang, J.; Xu, J.; Zhang, Y.; Liu, S.; Zou, C.; Li, J.; Liang, L.; Zhang, K.Q. Expansion of adhesion genes drives pathogenic adaptation of nematode-trapping fungi. IScience 2020, 23, 101057. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, L.; Ji, X.; Feng, Y.; Li, X.; Zou, C.; Xu, J.; Ren, Y.; Mi, Q.; Wu, J.; et al. Genomic and proteomic analyses of the fungus Arthrobotrys oligospora provide insights into nematode-trap formation. PLoS Pathog. 2011, 7, e1002179. [Google Scholar] [CrossRef]
- Hsueh, Y.P.; Gronquist, M.R.; Schwarz, E.M.; Nath, R.D.; Lee, C.H.; Gharib, S.; Schroeder, F.C.; Sternberg, P.W. Nematophagous fungus Arthrobotrys oligospora mimics olfactory cues of sex and food to lure its nematode prey. eLife 2017, 6, e20023. [Google Scholar] [CrossRef]
- Hsueh, Y.P.; Mahanti, P.; Schroeder, F.C.; Sternberg, P.W. Nematode-trapping fungi eavesdrop on nematode pheromones. Curr. Biol. 2013, 23, 83–86. [Google Scholar] [CrossRef]
- He, Z.Q.; Wang, L.J.; Wang, Y.J.; Chen, Y.H.; Wen, Y.; Zhang, K.Q.; Niu, X.M. Polyketide synthase-terpenoid synthase hybrid pathway regulation of trap formation through ammonia metabolism controls soil colonization of predominant nematode-trapping fungus. J. Agric. Food Chem. 2021, 69, 4464–4479. [Google Scholar] [CrossRef]
- Chen, Y.H.; Liu, X.; Dai, R.; Ou, X.; Xu, Z.F.; Zhang, K.Q.; Niu, X.M. Novel polyketide-terpenoid hybrid metabolites and increased fungal nematocidal ability by disruption of genes 277 and 279 in nematode-trapping fungus Arthrobotrys oligospora. J. Agric. Food Chem. 2020, 68, 7870–7879. [Google Scholar] [CrossRef]
- Xu, Z.F.; Chen, Y.H.; Song, T.Y.; Zeng, Z.J.; Yan, N.; Zhang, K.Q.; Niu, X.M. Nematicidal key precursors for the biosynthesis of morphological regulatory arthrosporols in the nematode-trapping fungus Arthrobotrys oligospora. J. Agric. Food Chem. 2016, 64, 7949–7956. [Google Scholar] [CrossRef]
- Wang, B.L.; Chen, Y.H.; He, J.N.; Xue, H.X.; Yan, N.; Zeng, Z.J.; Bennett, J.W.; Zhang, K.Q.; Niu, X.M. Integrated metabolomics and morphogenesis reveal volatile signaling of the nematode-trapping fungus Arthrobotrys oligospora. Appl. Environ. Microbiol. 2018, 84, e02749-17. [Google Scholar] [CrossRef] [PubMed]
- Youssar, L.; Wernet, V.; Hensel, N.; Yu, X.; Hildebrand, H.G.; Schreckenberger, B.; Kriegler, M.; Hetzer, B.; Frankino, P.; Dillin, A.; et al. Intercellular communication is required for trap formation in the nematode-trapping fungus Duddingtonia flagrans. PLoS Genet. 2019, 15, e1008029. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Liu, Z.; Liu, L.; Li, J.; Gao, H.; Yang, J.; Zhang, K.Q. The nitrate assimilation pathway is involved in the trap formation of Arthrobotrys oligospora, a nematode-trapping fungus. Fungal Genet. Biol. 2016, 92, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Gao, H.; Li, J.; Liu, L.; Liu, Z.; Zhang, K.Q. The Woronin body in the nematophagous fungus Arthrobotrys oligospora is essential for trap formation and efficient pathogenesis. Fungal Biol. 2017, 121, 11–20. [Google Scholar] [CrossRef]
- Liu, Q.; Li, D.; Jiang, K.; Zhang, K.Q.; Yang, J. AoPEX1 and AoPEX6 are required for mycelial growth, conidiation, stress response, fatty acid utilization, and trap formation in Arthrobotrys oligospora. Microbiol. Spectr. 2022; in press. [Google Scholar]
- Zhou, D.; Zhu, Y.; Bai, N.; Yang, L.; Xie, M.; Yang, J.; Zhu, M.; Zhang, K.Q.; Yang, J. AoATG5 plays pleiotropic roles in vegetative growth, cell nucleus development, conidiation, and virulence in the nematode-trapping fungus Arthrobotrys oligospora. Sci. China Life Sci. 2021, 65, 412–425. [Google Scholar] [CrossRef]
- Zhou, D.; Xie, M.; Bai, N.; Yang, L.; Zhang, K.Q.; Yang, J. The autophagy-related gene Aolatg4 regulates hyphal growth, sporulation, autophagosome formation, and pathogenicity in Arthrobotrys oligospora. Front. Microbiol. 2020, 11, 592524. [Google Scholar] [CrossRef]
- Chen, Y.L.; Gao, Y.; Zhang, K.Q.; Zou, C.G. Autophagy is required for trap formation in the nematode-trapping fungus Arthrobotrys oligospora. Environ. Microbiol. Rep. 2013, 5, 511–517. [Google Scholar] [CrossRef]
- Zhou, D.; Zhu, Y.; Bai, N.; Xie, M.; Zhang, K.Q.; Yang, J. Aolatg1 and Aolatg13 regulate autophagy and play different roles in conidiation, trap formation, and pathogenicity in the nematode-trapping fungus Arthrobotrys oligospora. Front. Cell Infect. Microbiol. 2022, 11, 824407. [Google Scholar] [CrossRef]
- Li, J.; Wu, R.; Wang, M.; Borneman, J.; Yang, J.; Zhang, K.Q. The pH sensing receptor AopalH plays important roles in the nematophagous fungus Arthrobotrys oligospora. Fungal Biol. 2019, 123, 547–554. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, W.; Lai, Y.; Xiang, M.; Wang, X.; Zhang, X.; Liu, X. Drechslerella stenobrocha genome illustrates the mechanism of constricting rings and the origin of nematode predation in fungi. BMC Genom. 2014, 15, 114. [Google Scholar] [CrossRef]
- Meerupati, T.; Andersson, K.M.; Friman, E.; Kumar, D.; Tunlid, A.; Ahren, D. Genomic mechanisms accounting for the adaptation to parasitism in nematode-trapping fungi. PLoS Genet. 2013, 9, e1003909. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.T.; de Ulzurrun, G.V.D.; Goncalves, A.P.; Lin, H.C.; Chang, C.W.; Huang, T.Y.; Chen, S.A.; Lai, C.K.; Tsai, I.J.; Schroeder, F.C.; et al. Natural diversity in the predatory behavior facilitates the establishment of a robust model strain for nematode-trapping fungi. Proc. Natl. Acad. Sci. USA 2020, 117, 6762–6770. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wright, S.J.; Krystofova, S.; Park, G.; Borkovich, K.A. Heterotrimeric G protein signaling in filamentous fungi. Annu. Rev. Microbiol. 2007, 61, 423–452. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Zhao, Y.; Wang, Y.; Yang, L.; Li, D.; Yang, J.; Jiang, K.; Zhang, K.Q.; Yang, J. Functional analysis of seven regulators of G protein signaling (RGSs) in the nematode-trapping fungus Arthrobotrys oligospora. Virulence 2021, 12, 1825–1840. [Google Scholar] [CrossRef] [PubMed]
- Bai, N.; Zhang, G.; Wang, W.; Feng, H.; Yang, X.; Zheng, Y.; Yang, L.; Xie, M.; Zhang, K.Q.; Yang, J. Ric8 acts as a regulator of G-protein signalling required for nematode-trapping lifecycle of Arthrobotrys oligospora. Environ. Microbiol. 2021; in press. [Google Scholar]
- Yang, X.; Ma, N.; Yang, L.; Zheng, Y.; Zhen, Z.; Li, Q.; Xie, M.; Li, J.; Zhang, K.Q.; Yang, J. Two Rab GTPases play different roles in conidiation, trap formation, stress resistance, and virulence in the nematode-trapping fungus Arthrobotrys oligospora. Appl. Microbiol. Biotechnol. 2018, 102, 4601–4613. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, X.M.; Xie, M.H.; Bai, N.; Yang, J.L.; Jiang, K.X.; Zhang, K.Q.; Yang, J.K. Pleiotropic roles of Ras GTPases in the nematode-trapping fungus Arthrobotrys oligospora identified through multi-omics analyses. IScience 2021, 24, 102820. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, X.; Bai, N.; Yang, X.; Zhang, K.Q.; Yang, J. Transcriptomic analysis reveals that Rho GTPases regulate trap development and lifestyle transition of the nematode-trapping fungus Arthrobotrys oligospora. Microbiol. Spectr. 2022, 10, e0175921. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.Z.; Randazzo, P.A. Arf GAPs and membrane traffic. J. Cell Sci. 2006, 119, 1203–1211. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, X.; Xie, M.; Zhang, G.; Yang, L.; Bai, N.; Zhao, Y.; Li, D.; Zhang, K.Q.; Yang, J. The Arf-GAP AoGlo3 regulates conidiation, endocytosis, and pathogenicity in the nematode-trapping fungus Arthrobotrys oligospora. Fungal Genet. Biol. 2020, 138, 103352. [Google Scholar] [CrossRef]
- De Ulzurrun, G.V.D.; Hsueh, Y.P. Predator-prey interactions of nematode-trapping fungi and nematodes: Both sides of the coin. Appl. Microbiol. Biotechnol. 2018, 102, 3939–3949. [Google Scholar] [CrossRef]
- Zhen, Z.; Xing, X.; Xie, M.; Yang, L.; Yang, X.; Zheng, Y.; Chen, Y.; Ma, N.; Li, Q.; Zhang, K.Q.; et al. MAP kinase Slt2 orthologs play similar roles in conidiation, trap formation, and pathogenicity in two nematode-trapping fungi. Fungal Genet. Biol. 2018, 116, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.E.; Thorner, J. Function and regulation in MAPK signaling pathways: Lessons learned from the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta Mol. Cell Res. 2007, 1773, 1311–1340. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Zhang, X.; Liu, H.; Xu, J.R. Mitogen-activated protein kinase signaling in plant pathogenic fungi. PLoS Pathog. 2018, 14, e1006875. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.H.; Yang, J.L.; Jiang, K.X.; Bai, N.; Zhu, M.C.; Zhu, Y.M.; Zhang, K.Q.; Yang, J.K. AoBck1 and AoMkk1 are necessary to maintain cell wall integrity, vegetative growth, conidiation, stress resistance, and pathogenicity in the nematode-trapping fungus Arthrobotrys oligospora. Front. Microbiol. 2021, 12, 649582. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.Y.; Chen, S.A.; Hsueh, Y.P. The high osmolarity glycerol (HOG) pathway functions in osmosensing, trap morphogenesis and conidiation of the nematode-trapping fungus Arthrobotrys oligospora. J. Fungi 2020, 6, 191. [Google Scholar] [CrossRef]
- Jiang, K.X.; Liu, Q.Q.; Bai, N.; Zhu, M.C.; Zhang, K.Q.; Yang, J.K. AoSsk1, a response regulator required for mycelial growth and development, stress responses, trap formation, and the secondary metabolism in Arthrobotrys oligospora. J. Fungi 2022, 8, 260. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, W.; Chen, Y.; Xiang, M.; Liu, X. DdaSTE12 is involved in trap formation, ring inflation, conidiation, and vegetative growth in the nematode-trapping fungus Drechslerella dactyloides. Appl. Microbiol. Biotechnol. 2021, 105, 7379–7393. [Google Scholar] [CrossRef]
- Xie, M.; Bai, N.; Yang, J.; Jiang, K.; Zhou, D.; Zhao, Y.; Li, D.; Niu, X.; Zhang, K.Q.; Yang, J. Protein kinase Ime2 is required for mycelial growth, conidiation, osmoregulation, and pathogenicity in nematode-trapping fungus Arthrobotrys oligospora. Front. Microbiol. 2020, 10, 3065. [Google Scholar] [CrossRef]
- Xie, M.; Wang, Y.; Tang, L.; Yang, L.; Zhou, D.; Li, Q.; Niu, X.; Zhang, K.Q.; Yang, J. AoStuA, an APSES transcription factor, regulates the conidiation, trap formation, stress resistance and pathogenicity of the nematode-trapping fungus Arthrobotrys oligospora. Environ. Microbiol. 2019, 21, 4648–4661. [Google Scholar] [CrossRef]
- Zhen, Z.; Zhang, G.; Yang, L.; Ma, N.; Li, Q.; Ma, Y.; Niu, X.; Zhang, K.Q.; Yang, J. Characterization and functional analysis of calcium/calmodulin-dependent protein kinases (CaMKs) in the nematode-trapping fungus Arthrobotrys oligospora. Appl. Microbiol. Biotechnol. 2019, 103, 819–832. [Google Scholar] [CrossRef]
- Khalil, H.B.; Wang, Z.; Wright, J.A.; Ralevski, A.; Donayo, A.O.; Gulick, P.J. Heterotrimeric Gα subunit from wheat (Triticum aestivum), GA3, interacts with the calcium-binding protein, Clo3, and the phosphoinositide-specific phospholipase C, PI-PLC1. Plant Mol. Biol. 2011, 77, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Meijer, H.J.G.; Munnik, T. Phospholipid-based signaling in plants. Annu. Rev. Plant Biol. 2003, 54, 265–306. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Ma, N.; Bai, N.; Zhu, M.; Zhang, K.Q.; Yang, J. Phospholipase C (AoPLC2) regulates mycelial development, trap morphogenesis, and pathogenicity of the nematode-trapping fungus Arthrobotrys oligospora. J. Appl. Microbiol. 2022, 132, 2144–2156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hu, C.; Hussain, M.; Chen, J.; Xiang, M.; Liu, X. Role of low-affinity calcium system member Fig1 homologous proteins in conidiation and trap-formation of nematode-trapping fungus Arthrobotrys oligospora. Sci. Rep. 2019, 9, 4440. [Google Scholar] [CrossRef] [PubMed]
- Dawkins, R.; Krebs, J.R. Arms races between and within species. Proc. R. Soc. B 1979, 205, 489–511. [Google Scholar]
- Zhu, M.; Chen, Y.; Zhao, N.; Bai, H.; Zhang, K.; Huang, X. Multiple olfactory pathways contribute to the lure process of Caenorhabditis elegans by pathogenic bacteria. Sci. China Life Sci. 2021, 64, 1346–1354. [Google Scholar] [CrossRef]
- Yu, X.; Hu, X.; Pop, M.; Wernet, N.; Kirschhofer, F.; Brenner-Weiss, G.; Keller, J.; Bunzel, M.; Fischer, R. Fatal attraction of Caenorhabditis elegans to predatory fungi through 6-methyl-salicylic acid. Nat. Commun. 2021, 12, 5462. [Google Scholar] [CrossRef]
- Stadler, M.; Sterner, O.; Anke, H. New biologically active compounds from the nematode-trapping fungus Arthrobotrys oligospora Fresen. Z. Naturforsch. C 1993, 48, 843–850. [Google Scholar] [CrossRef]
- Anderson, M.G.; Jarman, T.B.; Rickards, R.W. Structures and absolute configurations of antibiotics of the oligosporon group from the nematode-trapping fungus Arthrobotrys oligospora. J. Antibiot. 1995, 48, 391–398. [Google Scholar] [CrossRef][Green Version]
- Wei, L.X.; Zhang, H.X.; Tan, J.L.; Chu, Y.S.; Li, N.; Xue, H.X.; Wang, Y.L.; Niu, X.M.; Zhang, Y.; Zhang, K.Q. Arthrobotrisins A-C, oligosporons from the nematode-trapping fungus Arthrobotrys oligospora. J. Nat. Prod. 2011, 74, 1526–1530. [Google Scholar] [CrossRef]
- Zhang, H.X.; Tan, J.L.; Wei, L.X.; Wang, Y.L.; Zhang, C.P.; Wu, D.K.; Zhu, C.Y.; Zhang, Y.; Zhang, K.Q.; Niu, X.M. Morphology regulatory metabolites from Arthrobotrys oligospora. J. Nat. Prod. 2012, 75, 1419–1423. [Google Scholar] [CrossRef] [PubMed]
- He, Z.Q.; Tan, J.L.; Li, N.; Zhang, H.X.; Chen, Y.H.; Wang, L.J.; Zhang, K.Q.; Niu, X.M. Sesquiterpenyl epoxy-cyclohexenoids and their signaling functions in nematode-trapping fungus Arthrobotrys oligospora. J. Agric. Food Chem. 2019, 67, 13061–13072. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.H.; Yang, C.T.; Chang, H.Y.; Hsueh, Y.P.; Hsu, C.C. Nematode-trapping fungi produce diverse metabolites during predator-prey interaction. Metabolites 2020, 10, 117. [Google Scholar] [CrossRef]
- Xu, L.L.; Lai, Y.L.; Wang, L.; Liu, X.Z. Effects of abscisic acid ad nitric oxide on trap formation and trapping of nematodes by the fungus Drechslerella stenobrocha AS6.1. Fungal Biol. 2011, 115, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Song, T.Y.; Xu, Z.F.; Chen, Y.H.; Ding, Q.Y.; Sun, Y.R.; Miao, Y.; Zhang, K.Q.; Niu, X.M. Potent nematicidal activity and new hybrid metabolite production by disruption of a cytochrome P450 gene involved in the biosynthesis of morphological regulatory arthrosporols in nematode-trapping fungus Arthrobotrys oligospora. J. Agric. Food Chem. 2017, 65, 4111–4120. [Google Scholar] [CrossRef]
- Xu, Z.F.; Wang, B.L.; Sun, H.K.; Yan, N.; Zeng, Z.J.; Zhang, K.Q.; Niu, X.M. High trap formation and low metabolite production by disruption of the polyketide synthase gene involved in the biosynthesis of arthrosporols from nematode-trapping fungus Arthrobotrys oligospora. J. Agric. Food Chem. 2015, 63, 9076–9082. [Google Scholar] [CrossRef]
- Teng, L.L.; Song, T.Y.; Chen, Y.H.; Chen, Y.G.; Zhang, K.Q.; Li, S.H.; Niu, X.M. Novel polyketide-terpenoid hybrid metabolites from a potent nematicidal Arthrobotrys oligospora mutant delta AOL_s00215g278. J. Agric. Food Chem. 2020, 68, 11449–11458. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.H.; Zou, C.G.; Ji, X.L.; Liu, T.; Zhao, P.J.; Liang, L.M.; Xu, J.P.; An, Z.Q.; Zheng, X.; et al. Bacteria can mobilize nematode-trapping fungi to kill nematodes. Nat. Commun. 2014, 5, 5776. [Google Scholar] [CrossRef]
- Su, H.N.; Xu, Y.Y.; Wang, X.; Zhang, K.Q.; Li, G.H. Induction of trap formation in nematode-trapping fungi by bacteria-released ammonia. Lett. Appl. Microbiol. 2016, 62, 349–353. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, J.; Fan, Y.; Hussain, M.; Liu, X.; Xiang, M. The E3-ligase AoUBR1 in N-end rule pathway is involved in the vegetative growth, secretome, and trap formation in Arthrobotrys oligospora. Fungal Biol. 2021, 125, 532–540. [Google Scholar] [CrossRef]
- Peng, H.; Dong, X.; Lu, H.; Kong, X.; Zha, X.; Wang, Y. A putative F-box-domain-encoding gene AOL_s00076g207 regulates the development and pathogenicity of Arthrobotrys oligospora. J. Basic Microbiol. 2022, 62, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zheng, Y.; Ma, Y.; Yang, L.; Xie, M.; Zhou, D.; Niu, X.; Zhang, K.Q.; Yang, J. The velvet proteins VosA and VelB play different roles in conidiation, trap formation, and pathogenicity in the nematode-trapping fungus Arthrobotrys oligospora. Front. Microbiol. 2019, 10, 1917. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, M.; Cui, P.; Tian, M.; Xu, Y.; Zheng, X.; Zhang, K.; Li, G.; Wang, X. Arrestin-coding genes regulate endocytosis, sporulation, pathogenicity, and stress resistance in Arthrobotrys oligospora. Front. Cell Infect. Microbiol. 2022, 12, 754333. [Google Scholar] [CrossRef] [PubMed]
- Si, J.; Dong, X.; Zhang, G.; Lu, H.; Tang, K.; Zhang, L.; Kong, X.; Sheng, K.; Wang, J.; Zha, X.; et al. The fucose-specific lectin gene AOL_s00054g276 affects trap formation and nematocidal activity of the nematophagous fungus Arthrobotrys oligospora. FEMS Microbiol. Lett. 2022, 369, fnac013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhu, X.; Sun, F.; Zhang, K.; Niu, S.; Huang, X. The roles of actin cytoskeleton and actin-associated protein Crn1p in trap formation of Arthrobotrys oligospora. Res. Microbiol. 2017, 168, 655–663. [Google Scholar] [CrossRef]
- Wernet, V.; Waeckerle, J.; Fischer, R. The STRIPAK component SipC is involved in morphology and cell-fate determination in the nematode-trapping fungus Duddingtonia flagrans. Genetics 2022, 220, iyab153. [Google Scholar] [CrossRef]
- Liang, L.; Shen, R.; Mo, Y.; Yang, J.; Ji, X.; Zhang, K.Q. A proposed adhesin AoMad1 helps nematode-trapping fungus Arthrobotrys oligospora recognizing host signals for life-style switching. Fungal Genet. Biol. 2015, 81, 172–181. [Google Scholar] [CrossRef]
- Li, X.; Kang, Y.Q.; Luo, Y.L.; Zhang, K.Q.; Zou, C.G.; Liang, L.M. The NADPH oxidase AoNoxA in Arthrobotrys oligospora functions as an initial factor in the infection of Caenorhabditis elegans. J. Microbiol. 2017, 55, 885–891. [Google Scholar] [CrossRef]
- Wu, Q.Y.; Zhu, Y.Y.; Zou, C.G.; Kang, Y.Q.; Liang, L.M. GPH1 is involved in glycerol accumulation in the three-dimensional networks of the nematode-trapping fungus Arthrobotrys oligospora. J. Microbiol. 2016, 54, 768–773. [Google Scholar] [CrossRef]
- Ji, X.; Li, H.; Zhang, W.; Wang, J.; Liang, L.; Zou, C.; Yu, Z.; Liu, S.; Zhang, K.Q. The lifestyle transition of Arthrobotrys oligospora is mediated by microRNA-like RNAs. Sci. China Life Sci. 2020, 63, 543–551. [Google Scholar] [CrossRef]
- Han, X.; Altegoer, F.; Steinchen, W.; Binnebesel, L.; Schuhmacher, J.; Glatter, T.; Giammarinaro, P.I.; Djamei, A.; Rensing, S.A.; Reissmann, S.; et al. A kiwellin disarms the metabolic activity of a secreted fungal virulence factor. Nature 2019, 565, 650. [Google Scholar] [CrossRef] [PubMed]
- Lo Presti, L.; Kahmann, R. How filamentous plant pathogen effectors are translocated to host cells. Curr. Opin. Plant Biol. 2017, 38, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Takai, Y.; Kaibuchi, K.; Kikuchi, A.; Kawata, M. Small GTP-Binding Proteins. In International Review of Cytology; Jeon, K.W., Friedlander, M., Eds.; Academic Press: Cambridge, MA, USA, 1992; Volume 133, pp. 187–230. [Google Scholar]
- Andersson, K.M.; Kumar, D.; Bentzer, J.; Friman, E.; Ahren, D.; Tunlid, A. Interspecific and host-related gene expression patterns in nematode-trapping fungi. BMC Genom. 2014, 15, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.M.; Zou, C.G.; Xu, J.; Zhang, K.Q. Signal pathways involved in microbe-nematode interactions provide new insights into the biocontrol of plant-parasitic nematodes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20180317. [Google Scholar] [CrossRef]
- Ma, N.; Jiang, K.-X.; Bai, N.; Li, D.-N.; Zhang, K.-Q.; Yang, J.-K. Functional analysis of two affinity cAMP phosphodiesterases in the nematode-trapping fungus Arthrobotrys oligospora. Pathogens 2022, 11, 405. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).