Abstract
Although species of Absidia are known to be ubiquitous in soil, animal dung, and insect and plant debris, the species diversity of the genus and their ecological habitats have not been sufficiently investigated. In this study, we describe five new species of Absidia from forest and grassland soils in southwestern China, with support provided by phylogenetic, morphological, and physiological evidence. The species diversity and ecological habitat of Absidia are summarized. Currently, 22 species are recorded in China, which mainly occur in soil, especially in tropical and subtropical forests and mountains. An updated key to the species of Absidia in China is also provided herein. This is the first overview of the Absidia ecological habitat.
1. Introduction
The genus Absidia Tiegh., typified by A. reflexa Tiegh., was described nearly 150 years ago [1], belonging to Cunninghamellaceae, Mucorales, Mucoromycetes, Mucoromycota (http://www.indexfungorum.org/, accessed on 1 March 2022). However, it was initially placed in the family Absidiaceae [2]. With the development of molecular biology, it was grouped with Chlamydoabsidia Hesselt. and J.J. Ellis, Cunninghamella Matr., Gongronella Ribaldi, and Halteromyces Shipton and Schipper and Hesseltinella H.P. Upadhyay; this group was nominated as the family Cunninghamellaceae [3,4,5,6,7]. Nine other genera were thought to be allied with Absidia on the basis of morphological similarities, including Rhizopus Ehrenb. 1821, Phycomyces Kunze 1823, Tieghemella Berl. and De Toni 1888, Mycocladus Beauverie 1900, Lichtheimia Vuill. 1903, Proabsidia Vuill. 1903, Pseudoabsidia Bainier 1903, Protoabsidia Naumov 1935, and Gongronella Ribaldi 1952 [8]. As research progressed, Absidia s.l. has been well divided into three genera, i.e., Lichtheimia (thermotolerant, optimum growth temperature 37–45 °C), Absidia s.s. (mesophilic, optimum growth temperature 25–34 °C), and Lentamyces Kerst. Hoffm. and K. Voigt (parasitic on mucoralean fungi, optimum growth temperature 14–25 °C) [9,10,11]. Currently, species of Absidia are characterized by (1) sporangiophores single, in pairs or in groups on stolons, (2) rhizoids at both ends of stolons and never opposite the sporangiophores, (3) sporangia deliquescent-walled and apophysate, (4) columellae bearing one to several projections, (5) zygospores enclosed by appendages, and (6) optimum growth temperatures from 25 °C to 34 °C [1,9,10,11,12,13,14,15].
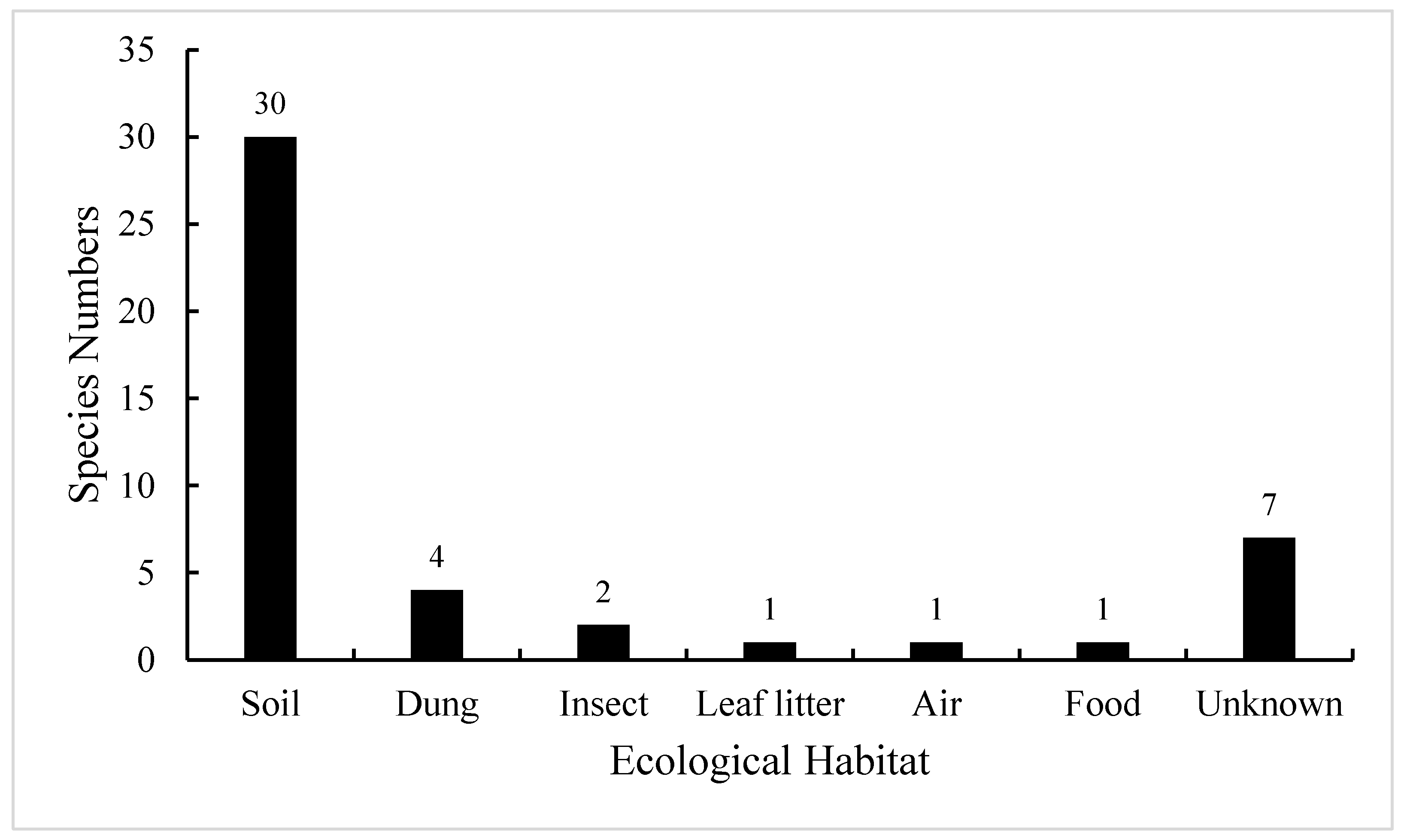
So far, a total of 46 species of Absidia have been described worldwide, and they are ubiquitous in soil, dung, insect, leaf litter, food, air, etc. (Figure 1; [16]). Among them, 30 species were initially collected from soil, including forest and rhizosphere soil, suggesting that Absidia species are mainly isolated from soil [17,18]. However, a few species are endemic to animal dung and insect remains, such as A. psychrophilia Hesselt. and J.J. Ellis from mycangia of ambrosia beetles [8,19], and A. stercoraria Hyang B. Lee et al. from rat dung [20].
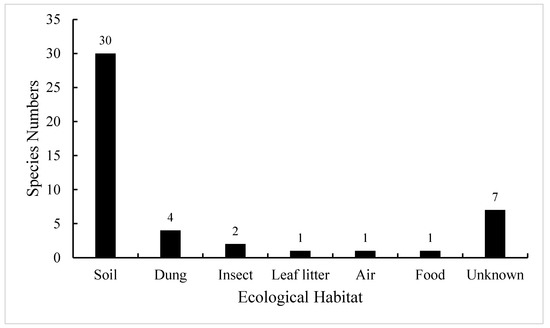
Figure 1.
Number of Absidia species in different ecological habitats when they were originally described. These data are from the Index Fungorum (http://www.indexfungorum.org/, accessed on 1 March 2022) and [16].
Absidia glauca Hagem and A. repens Tiegh. are considered as cosmopolitan species, reported in all continents except Antarctica [21,22,23]. Absidia heterospora Y. Ling was reported in China, New Zealand, and France [23,24]; A. idahoensis Hesselt. et al. and A. macrospora Váňová were reported in China, Czechia, and the USA [25,26,27]. Since 2018, 22 endemic species have been described from Korea, China, Thailand, Australia, USA, Mexico, and Brazil [14,15,16,28,29,30,31,32,33,34,35,36]. Type strains were collected from 17 countries, and the two most investigated countries are China and Brazil, with nine and eight type strains, respectively. The studies on Absidia in other countries and regions are obviously insufficient (Table 1).

Table 1.
The origin of taxonomic types in Absidia.
Therefore, there are deficiencies in the studies on species distribution and ecological habitat of Absidia [8,13,14,15,16,18,20,27]. In this paper, we propose five new species from forest and grassland soil in Sichuan, Tibet, and Yunnan in southwestern China. A key to Absidia species in China is consequently updated. Along with the taxonomical study, we also conduct a preliminary investigation on the species distribution and ecological habitat of Absidia.
2. Materials and Methods
2.1. Isolation and Strains
Strains were isolated from forest and grassland soil samples collected in September 2021, in Sichuan, Tibet, and Yunnan in southwestern China. An aliquot of soil samples (1 g) was evenly spread on 15 cm petri dishes containing potato dextrose agar medium (200 g potato, 20 g dextrose, 20 g agar, and 1000 mL distilled water) with streptomycin sulfate and ampicillin 100 mg/mL each, and then cultivated at 20 °C and 25 °C. According to morphological characteristics of Absidia, potential strains were picked out and purified. The purified living cultures (Table 2) were deposited in the China General Microbiological Culture Collection Center, Beijing, China (CGMCC) and the Shandong Normal University (XY), and dry cultures (Table 2) were deposited in the Herbarium Mycologicum Academiae Sinicae, Beijing, China (HMAS).

Table 2.
Taxa information and GenBank accession numbers used in this study.
2.2. Morphology and Maximum Growth Temperature
Pure cultures were incubated with malt extract agar medium (MEA: 30 g malt extract, 3 g peptone, 20 g agar, and 1000 mL distilled water; [37]). For morphological studies, two plates of each strain were cultured at 20 °C and 27 °C, respectively, and then examined under a stereomicroscope (SMZ1500, Nikon, Tokyo, Japan) and a light microscope (Axio Imager A2, Carl Zeiss, Oberkochen, Germany). Maximum growth temperature tests followed the methods in our previous studies [14,15,16,38,39,40,41]. For maximum growth temperature tests, three plates were incubated at 20 °C for 2 d, then incubation temperature was increased by a gradient of 1 °C until the colonies stopped growing. For morphological features, the minimum and maximum sizes based on a statistic of more than 50 measurements were adopted [16]. All cultures were triplicated.
2.3. Molecular Phylogeny
DNA extraction, PCR amplification, and sequencing and phylogenetic analyses followed the methods in our previous studies [14,16,42,43,44]. In brief, total DNAs were extracted using a kit (GO-GPLF-400, GeneOnBio Corporation, Changchun, China) based on the instruction manual. Entire ITS and partial LSU rDNA sequences were amplified with primer pairs NS5M (5′-GGC TTA ATT TGA CTC AAC ACG G-3′) and LR5M (5′-GCT ATC CTG AGG GAA ACT TCG-3′; [14,16]). PCR procedures were as follows: an initial denaturation at 95 °C for 5 min, 30 cycles at 95 °C for 60 s, 55 °C for 45 s, and 72 °C for 60 s, and a final extra extension at 72 °C for 10 min. Sanger sequencing of PCR products were carried out by a company (SinoGenoMax, http://www.sinogenomax.com, accessed on 1 March 2022) with primers ITS1, ITS4, ITS5, and LR5M [14,16,45].
Phylogenetic analyses were conducted with maximum likelihood (ML), maximum parsimony (MP) and Bayesian inference (BI) algorithms [39,41] using RAxML (version 8. 1.12; [46]), PAUP (version 4.0b10; [47]) and MrBayes (version 3.2.7a; [48]), respectively. Maximum Likelihood analysis was performed using the GTRGAMMA substitution model with 1000 bootstrap replications. Maximum parsimony analysis was conducted with 1000 bootstrap replications using the heuristic search option with bisection and reconnection. Sequences were randomly added and max-trees were set to 5000. For BI analysis, eight cold Markov chains were run simultaneously for two million generations with the GTR + I + G model, sampling every 1000 generations and with the first 25% sampled tree being removed as burn-in. Obtained sequences and aligned dataset were deposited at GenBank (Table 2) and Supplementary File S1, respectively. Additionally, top hits of the BLAST search for ITS sequences are provided in the Supplementary Table S1.
3. Results
3.1. Phylogenetic Analyses
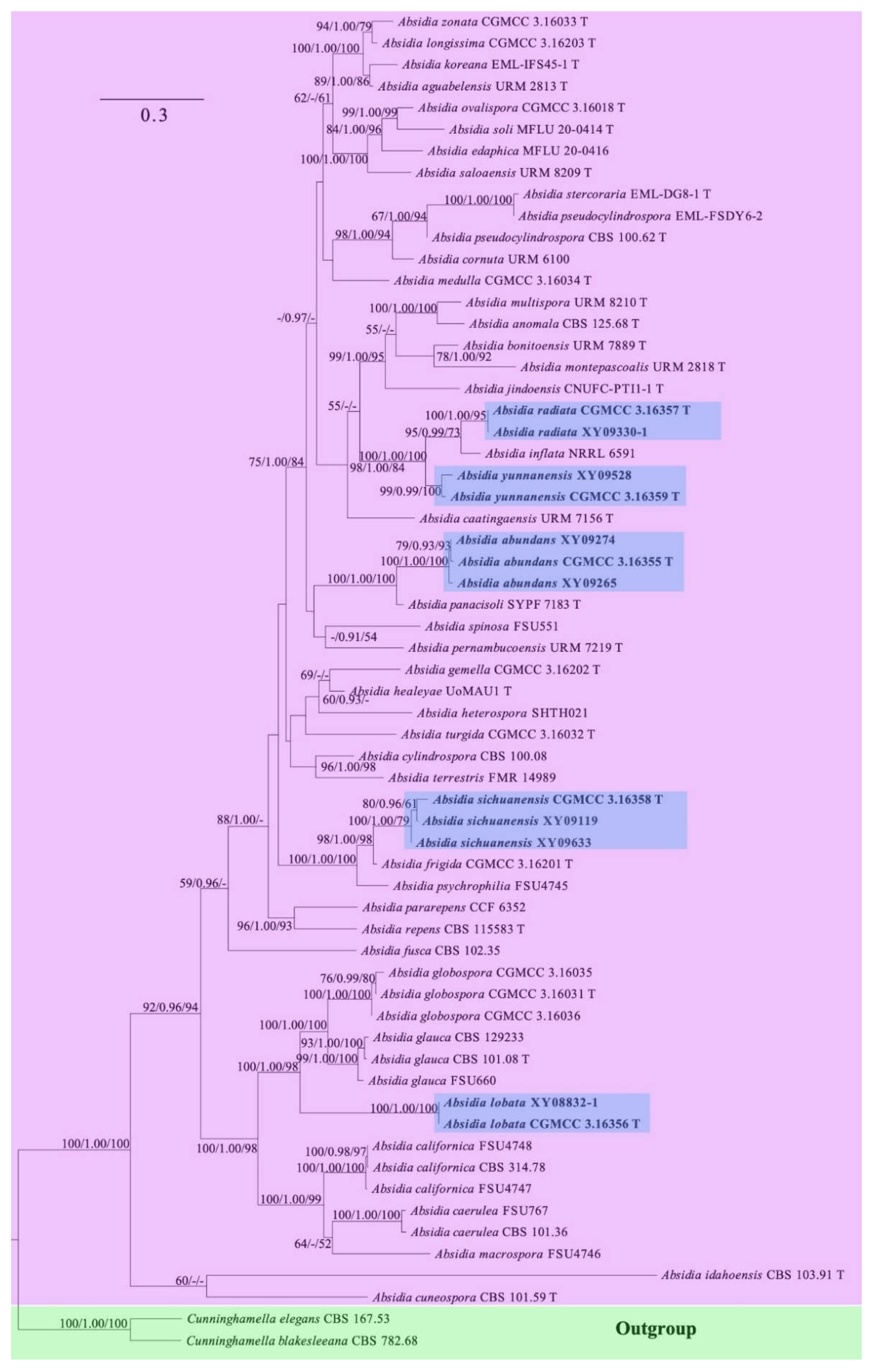
The ITS and LSU rDNA dataset for 62 strains, representing 45 species of Absidia and two species of Cunninghamella, contains 1642 characters, of which 501, 877, and 264 are constant, parsimony-informative, and parsimony-uninformative, respectively. Maximum parsimony (MP) analyses yielded two equal trees (tree length 6669, consistency index 0.3330, homoplasy index 0.6670, retention index 0.5669, and rescaled consistency index 0.1888). The most optimal model of Bayesian inference (BI) was GTR + I + G, and the average standard deviation of split frequencies was 0.07357. Topology of the ML tree was chosen to represent the phylogenetic relationship (Figure 2). In the phylogenetic tree, the five new species described herein are fully supported with Cunninghamella elegans Lendn. and Cunninghamella blakesleeana Lendn. as outgroup.
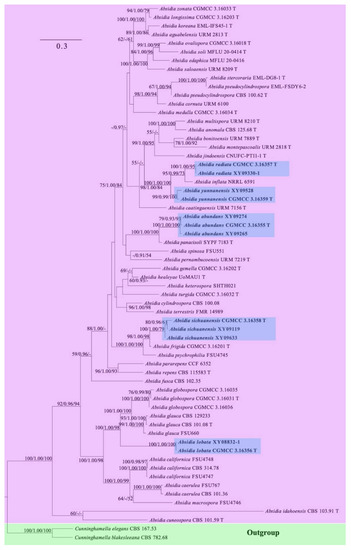
Figure 2.
Maximum likelihood (ML) phylogenetic tree of Absidia based on ITS and LSU rDNA sequences, with Cunninghamella elegans and C. blakesleeana as outgroup shaded in blue. Five new species are in shade and bold. Maximum likelihood bootstrap values (≥50%)/Bayesian inference (BI) posterior probabilities (≥0.9)/maximum parsimony (MP) bootstrap values (≥50%) of each clade are indicated along branches. “T” after strain number represents type. A scale bar in the upper left indicates substitutions per site.
3.2. Taxonomy
In this paper, five new species of Absidia are proposed from southwestern China, i.e., Sichuan, Tibet, and Yunnan. Besides the ITS and LSU rDNA sequences provided above, all the new species are illustrated along with morphological characteristics and the maximum growth temperature; a physiological trait is also presented.
3.2.1. Absidia abundans H. Zhao, Y.C. Dai and X.Y. Liu, sp. nov.
Fungal Names: FN570973. Figure 3.
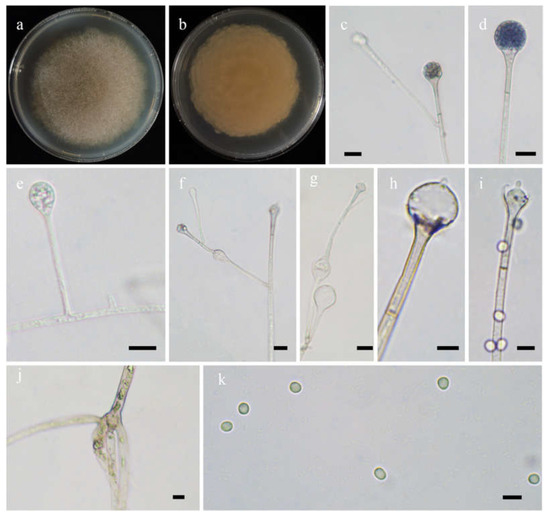
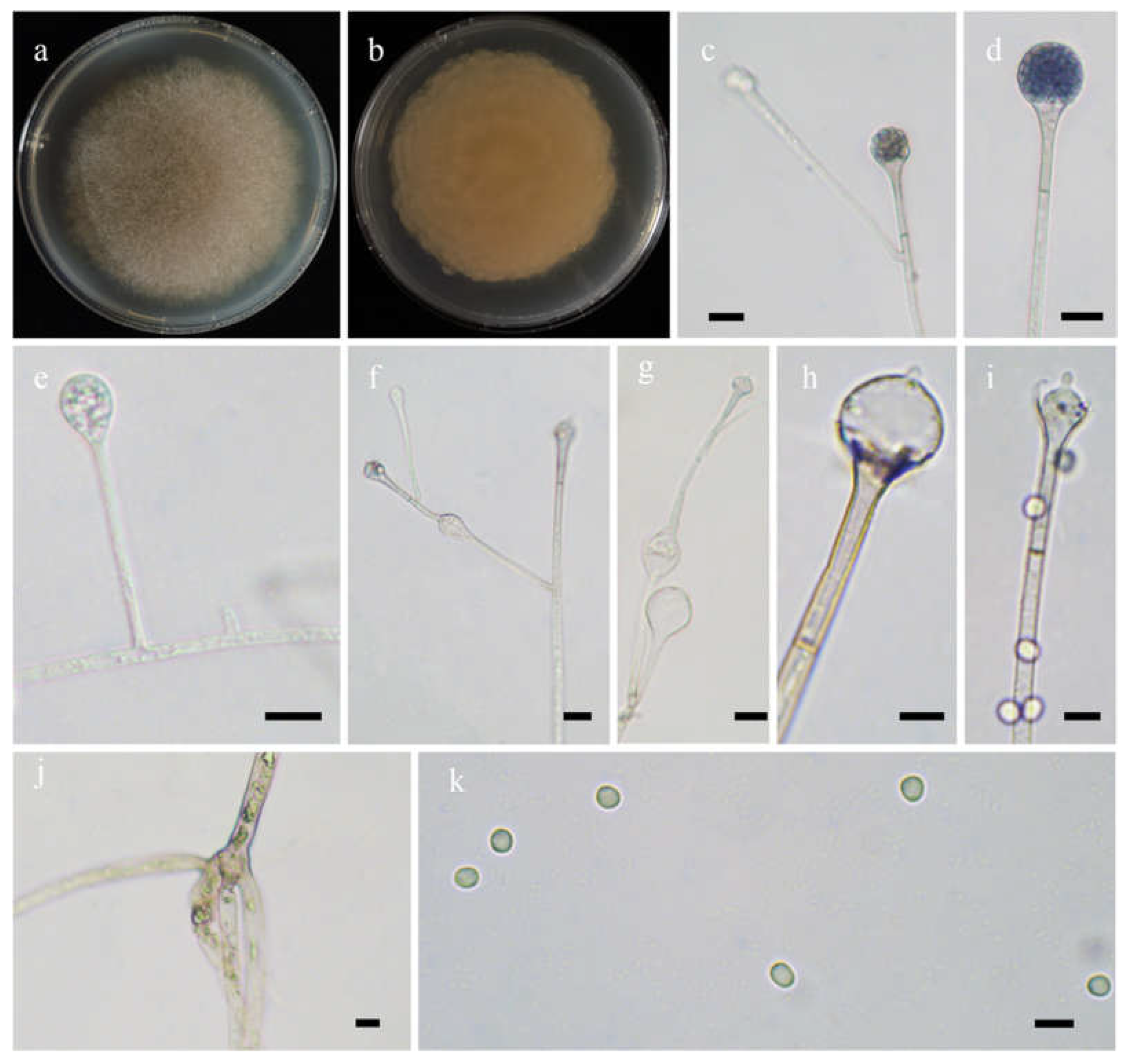
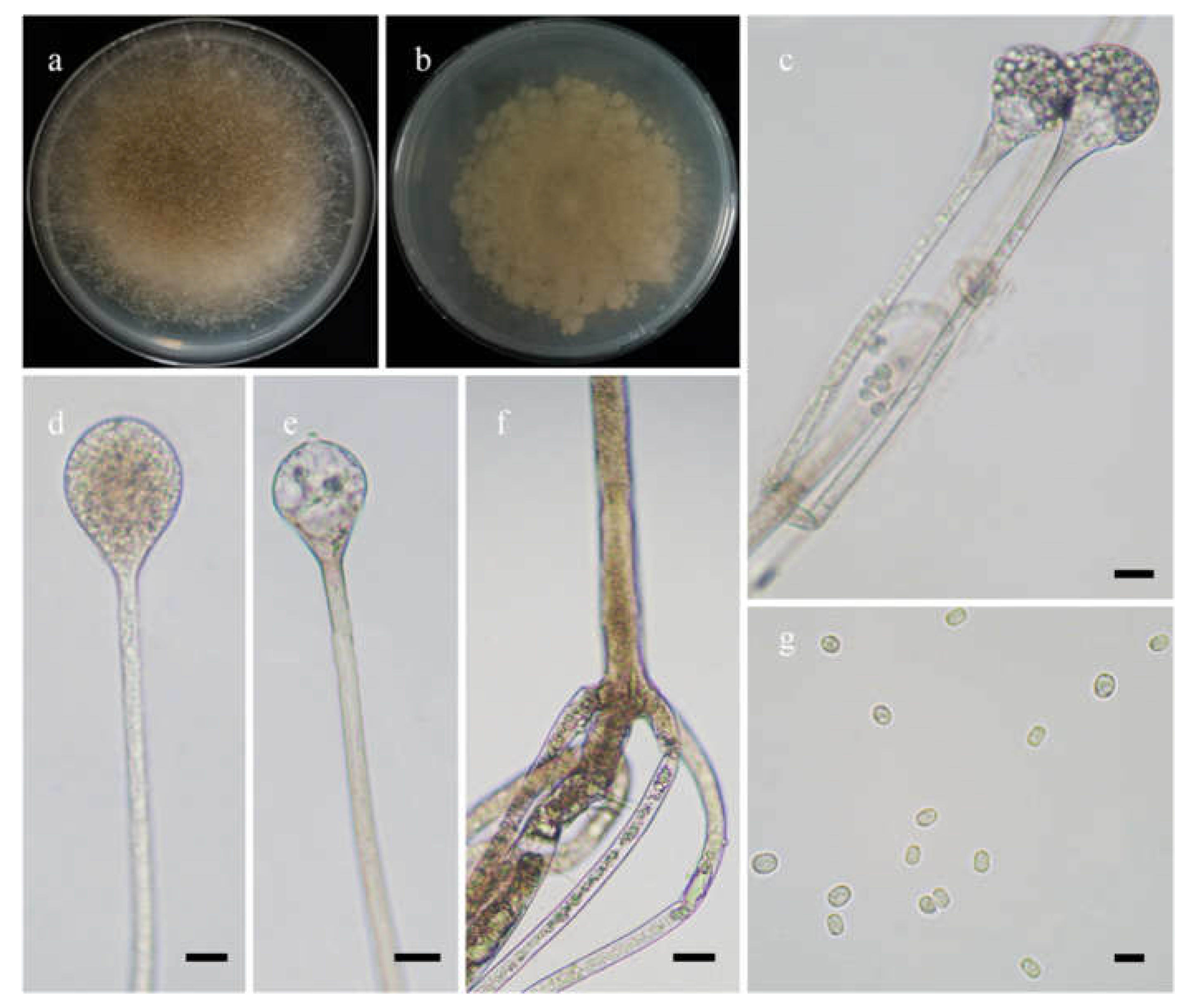
Figure 3.
Morphologies of Absidia abundans ex-holotype CGMCC 3.16255. (a,b). Colonies on MEA ((a), obverse, (b), reverse); (c–e), sporangia; (f), sympodially branched sporangiophores; (g), swellings below sporangia; (h), columellae with projections; (i), columellae with projections and collars; (j), rhizoids; (k), sporangiospores—scale bars: (c–g), (j), 10 μm, (h,i,k), 5 μm.
Etymology: abundans (Lat.) refers to the species with abundant swellings below the sporangia.
Holotype: HMAS 351932.
Description: Colonies on MEA at 27 °C for 7 days, growing moderately fast, attaining 65 mm in diameter, initially white, soon becoming gray to brown, and irregularly and concentrically zonate with ring at reverse. Hyphae are branched, hyaline at first, brownish when mature, aseptate when juvenile, septate with age, and 3.0–15.5 µm in diameter. Stolons branched, hyaline, and smooth. Rhizoids are root-like, often unbranched, and well-developed. Sporangiophores arise from stolons, always erect, unbranched, or simple if branched, monopodial, or sympodial, hyaline, swellings usually present below sporangia, oval to pyriform, hyaline, often with a septum 12.0–17.0 µm below apophyses, 35.0–170.0 µm long, and 2.0–3.5 µm wide. Sporangia are oval to subglobose, hyaline when young, dark green when old, smooth, deliquescent-walled, multi-spored, 8.0–16.5 µm long, and 8.5–16.0 µm wide. Apophyses are distinct, hyaline or subhyaline, 2.5–5.5 µm high, gradually widened upwards, 2.5–4.5 µm wide at the base, and 4.5–8.0 µm wide at the top. Collars absent or present. Columellae are subglobose or oval, hyaline, subhyaline or light brown, 4.5–10.0 µm long, and 3.5–8.0 µm wide. Projections are single if present, subhyaline, and 1.0–2.5 µm long. Sporangiospores are cylindrical, oval or subglobose, light green, smooth, 2.5–3.5 µm long, and 2.0–3.5 µm wide. Chlamydospores are absent. Zygospores are not observed.
Maximum growth temperature: 31 °C.
Materials examined: China. Yunnan, Qujing, from forest soil sample, September 2021, Heng Zhao (holotype HMAS 351932, living ex-holotype culture CGMCC 3.16255, and living cultures XY09265 and XY09274).
3.2.2. Absidia lobata H. Zhao, Y.C. Dai and X.Y. Liu, sp. nov.
Fungal Names: FN570974. Figure 4.
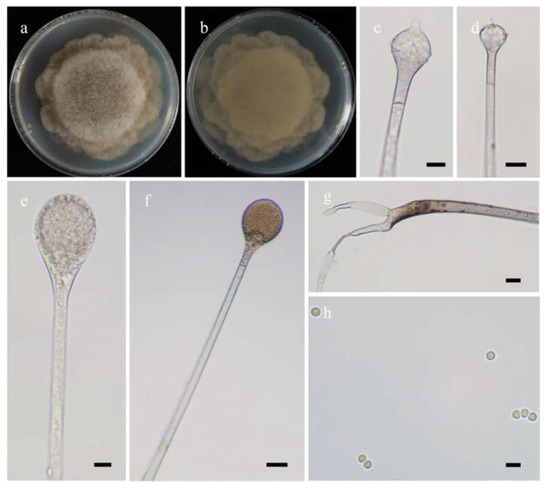
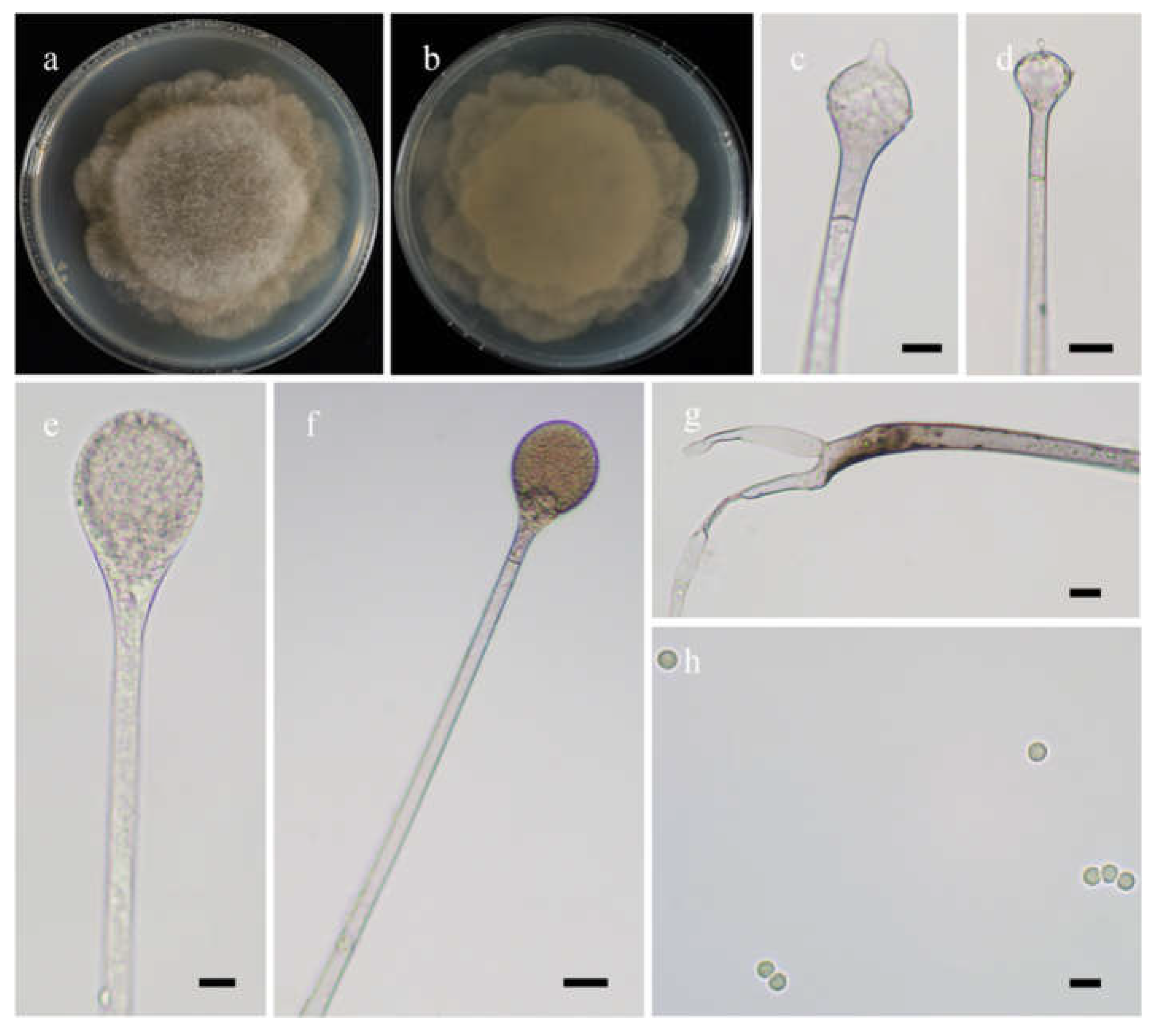
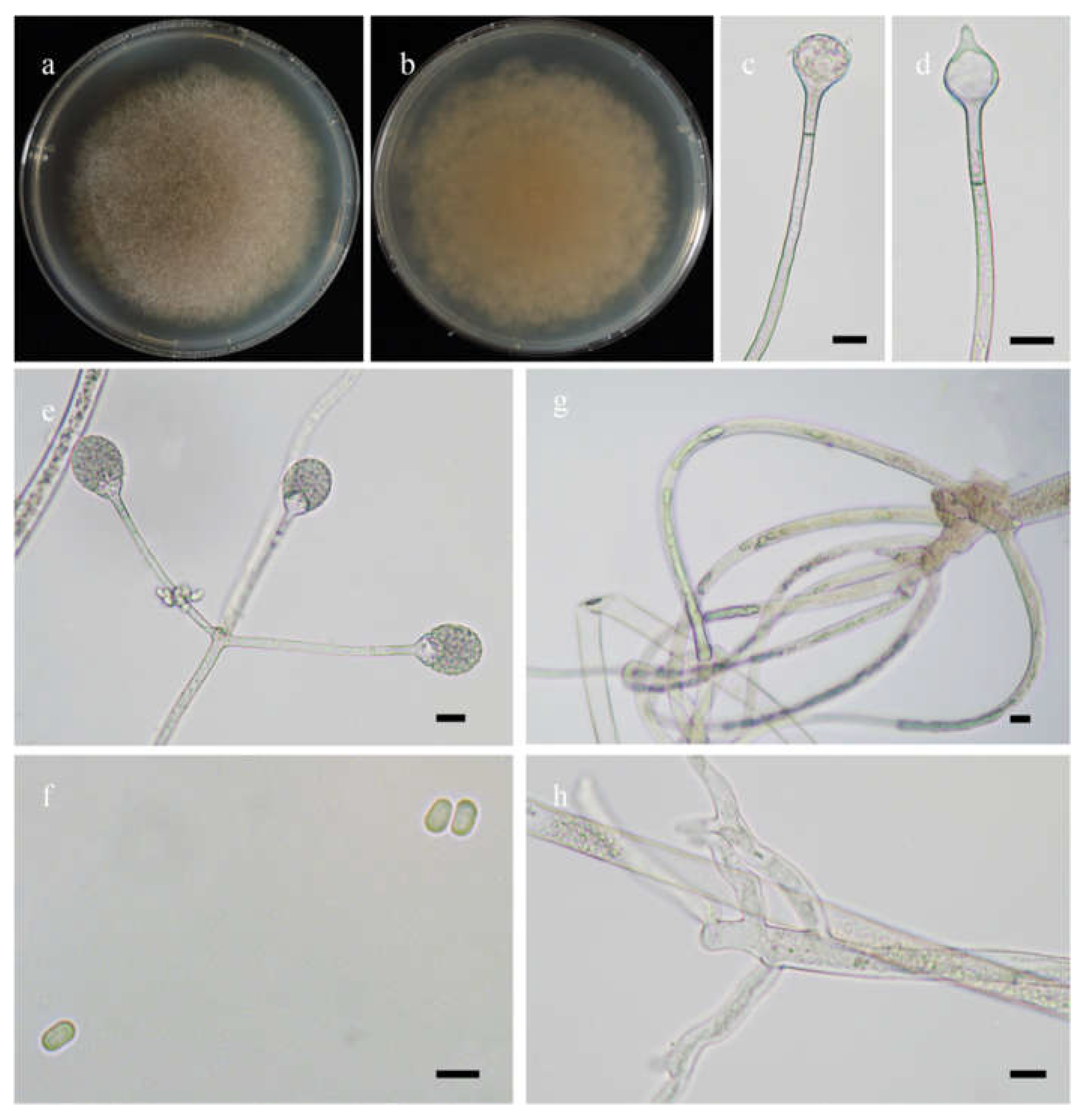
Figure 4.
Morphologies of Absidia lobata ex-holotype CGMCC 3.16256. (a,b). Colonies on MEA ((a), obverse, (b), reverse); (c,d), columellae with projections; (e,f)c sporangia; (g), rhizoids; (h), sporangiospores—scale bars: (c–e), 10 μm, (f,g), 20 μm, (h), 5 μm.
Etymology: lobata (Lat.) refers to the species with a broadly lobed edge of colonies.
Holotype: HMAS 351933.
Description: Colonies on MEA at 20 °C for 7 days, growing moderately fast, attaining 70 mm in diameter, zonate, broadly lobed at edge, white at first, gradually becoming light to dark brown and greenish, and irregular at reverse. Hyphae are branched, hyaline at first, greenish brown when mature, aseptate when juvenile, septate with age, and 4.0–21.0 µm in diameter. Stolons are branched, hyaline, brownish or light greenish, smooth, and septate. Rhizoids are sometimes unbranched and rootlike when branched. Sporangiophores arising from stolons, in pairs, unbranched, erect or slightly bent, hyaline or light brown, sometimes with a septum 13.0–22.5 µm below apophyses, 50.0–360.0 µm long, and 3.0–7.0 µm wide. Sporangia are pyriform, colorless when young, light to dark brown when old, smooth, deliquescent-walled, multi-spored, 22.0–43.5 µm long, and 18.5–31.0 µm wide. Apophyses are always distinct, hyaline to light brown, 4.0–9.0 µm high, gradually widened upwards, 4.5–8.5 µm wide at the base, and 10.0–16.0 µm wide at the top. Collars are always absent. Columellae are subglobose to depressed globose, rarely irregular, subhyaline or light brown, rough, 12.0–26.5 µm long, and 11.0–25.0 µm wide. Projections are always present, single, hyaline or subhyaline, and 2.0–6.0 µm long. Sporangiospores are mostly globose, occasionally subglobose, light green, smooth, and 2.5–3.0 µm in diameter. Chlamydospores are absent. Zygospores are not observed.
Maximum growth temperature: 26 °C.
Materials examined: China, Yunnan, Lijiang, 27°31′23″ N, 100°44′32″ E, altitude: 3153 m, from rhizosphere soil of Pinus yunnanensis Franch., September 2021, Heng Zhao (holotype HMAS 351933, living ex-holotype culture CGMCC 3.16256, and living culture XY08832-1).
3.2.3. Absidia radiata H. Zhao, Y.C. Dai and X.Y. Liu, sp. nov.
Fungal Names: FN570975. Figure 5.
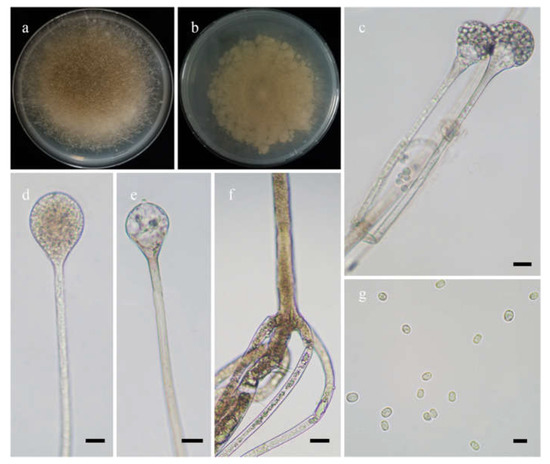
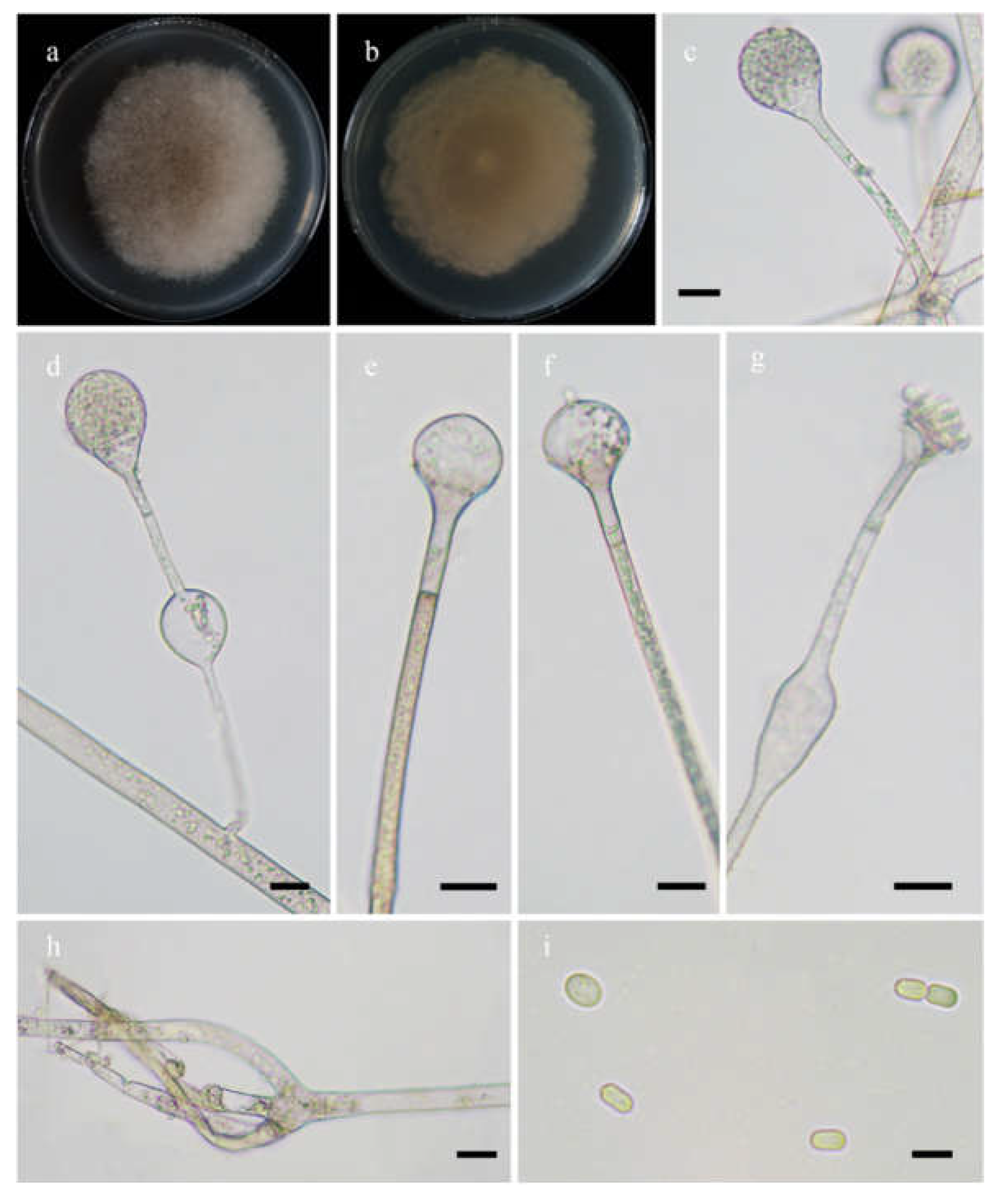
Figure 5.
Morphologies of Absidia radiata ex-holotype CGMCC 3.16257. (a,b). Colonies on MEA ((a), obverse, (b), reverse); (c,d), sporangia; (e), columellae with projections; (f), rhizoids; (g), sporangiospores—scale bars: (c–e), 10 μm, (f), 20 μm, (g), 5 μm.
Etymology: radiata (Lat.) refers to the species with a radiate shape of colonies.
Holotype: HMAS 351934.
Description: Colonies on MEA at 27 °C for 7 days, growing moderately fast, attaining 65 mm in diameter, white at first, gradually becoming light to dark brown, with adjoining satellite colonies at the edge at reverse. Hyphae are branched, hyaline when young, light brownish when old, aseptate when juvenile, septate with age, and 3.0–16.0 µm in diameter. Stolons are branched, hyaline to light brown, and smooth. Rhizoids are rootlike, unbranched, and well-developed. Sporangiophores arise from stolons, 1–5 in whorls, erect or slightly bent, unbranched, hyaline to light brown, sometimes with a septum 16.5–24.5 µm below apophyses, 45.0–273.0 µm long, and 3.0–5.0 µm wide. Sporangia are pyriform or subglobose, hyaline when young, brown when old, smooth, deliquescent-walled, multis-pored, 17.5–33.5 µm long, and 18.5–30.0 µm wide. Apophyses are distinct, hyaline to light brown, 5.5–12.5 µm high, with a turning point at the top of sporangiospores, then gradually widened upwards, 4.0–5.5 µm wide at the base, and 9.0–20.0 µm wide at the top. Collars absent. Columellae are mostly oval, depressed globose, occasionally subglobose to globose, subhyaline or hyaline, 13.5–22.5 µm long, and 14.0–24.0 µm wide. Projections are present or absent, single if present, subhyaline, and 2.0–4.5 µm long. Sporangiospores are oval, subhyaline, smooth, 3.0–5.0 µm long and 2.0–3.5 µm wide. Chlamydospores are absent. Zygospores are not observed.
Maximum growth temperature: 32 °C.
Materials examined: China, Yunnan, Yuxi, from forest soil sample, September 2021, Heng Zhao (holotype HMAS 351934, living ex-holotype culture CGMCC 3.16257, and living culture XY09330-1).
3.2.4. Absidia sichuanensis H. Zhao, Y.C. Dai and X.Y. Liu, sp. nov.
Fungal Names: FN570977. Figure 6.
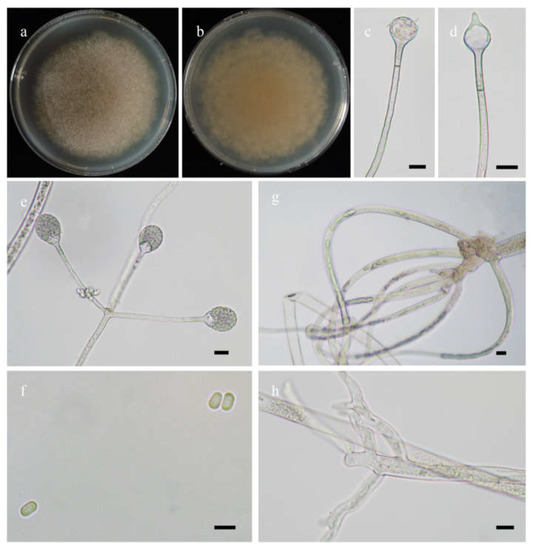
Figure 6.
Morphologies of Absidia sichuanensis ex-holotype CGMCC 3.16258. (a,b). Colonies on MEA ((a), obverse, (b), reverse); (c), columellae without projections; (d), columellae with a projection; (e), sporangia in whorls; (f), sporangiospores; (g,h). Rhizoids—scale bars: (c–e,g,h), 10 μm, (f), 5 μm.
Etymology: sichuanensis (Lat.) refers to the species found in Sichuan Province, southwest China.
Holotype: HMAS 351935.
Description: Colonies on MEA at 20 °C for 7 days, growing moderately fast, attaining 75 mm in diameter, white at first, soon becoming light brown, irregularly concentrically zonate at reverse. Hyphae are branched, hyaline at first, greenish brown when mature, aseptate when juvenile, septate with age, 5.5–15.5 µm in diameter. Stolons are branched, hyaline to brownish, smooth. Rhizoids are sometimes unbranched, fingerlike or rootlike when branched, and well-developed. Sporangiophores arise from stolons, 1–5 in whorls, unbranched, erect or slightly bent, hyaline, sometimes with a septum 14.0–16.5 µm below apophyses, 30.0–220.0 µm long, and 3.0–5.0 µm wide. Sporangia are pyriform, greenish when old, smooth, deliquescent-walled, multi-spored, 18.0–23.0 µm long and 17.0–21.5 µm wide. Apophyses distinct, hyaline or subhyaline, 2.0–6.5 µm high, gradually widened upwards but with a turning point at the top of sporangiophores, 3.0–5.0 µm wide at the base, and 5.5–12.0 µm wide at the top. Collars are usually absent and rarely present. Columellae are mostly subglobose, occasionally globose, hyaline to greenish, 7.5–13.0 µm long, and 8.0–15.5 µm wide. Projections present or absent, single if present, hyaline or subhyaline, 4.0–6.0 µm long. Sporangiospores are cylindrical, subhyaline, smooth, 3.0–4.5 µm long and 2.0–2.5 µm wide. Chlamydospores are absent. Zygospores are not observed.
Maximum growth temperature: 28 °C.
Materials examined: China. Sichuan, Ngawa, 30°42’18” N, 101°22’17” E, altitude: 3727 m, from grassland soil sample, September 2021, Heng Zhao (holotype HMAS 351935, living ex-holotype culture CGMCC 3.16258), from rhizosphere soil of Picea asperata Mast., Heng Zhao (living culture XY09119). Tibet, Bome, from rhizosphere soil of Pinus yunnanensis, September 2021, Heng Zhao (living culture XY09633).
3.2.5. Absidia yunnanensis H. Zhao, Y.C. Dai and X.Y. Liu, sp. nov.
Fungal Names: FN570978. Figure 7.
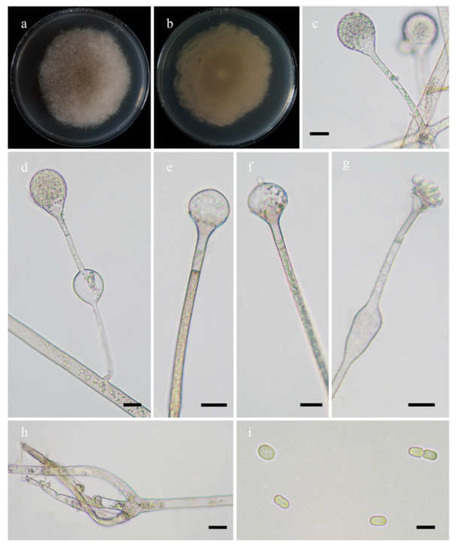
Figure 7.
Morphologies of Absidia yunnanensis ex-holotype CGMCC 3.16259. (a,b). Colonies on MEA ((a), obverse, (b), reverse); (c), sporangia; (d), swellings below sporangia; (e–g), columellae; (h), rhizoids; (i), sporangiospores—scale bars: (c–g), 10 μm, (h), 20 μm, (i), 5 μm.
Etymology: yunnanensis (Lat.) refers to the species found in Yunnan Province, southwest China.
Holotype: HMAS 351936.
Description: Colonies on MEA at 27 °C for 7 days, growing moderately fast, attaining 65 mm in diameter, initially white, soon becoming light brown, and irregularly and concentrically zonate with ring at reverse. Hyphae are branched, hyaline at first, light brown when mature, aseptate when juvenile, septate with age, and 4.0–15.5 µm in diameter. Stolons are branched, hyaline, light brown or green, smooth. Rhizoids rootlike, often unbranched, and well-developed. Sporangiophores arise from rhizoids, 2–5 in whorls, erect or slightly bent, usually unbranched, rarely monopodially branched, hyaline, light brown or green, swellings usually present below sporangia, usually oval, rarely irregular, hyaline, often with a septum 12.0–16.5 µm below apophyses, 29.0–159.0 µm long, and 2.0–5.0 µm wide. Sporangia are pyriform to subglobose, hyaline when young, light green when old, smooth, deliquescent-walled, multi-spored, 16.0–27.5 µm long, and 16.5–27.0 µm wide. Apophyses are distinct, hyaline or subhyaline, 3.0–6.5 µm high, gradually widened upwards, 3.0–7.5 µm wide at the base, and 5.5–14.0 µm wide at the top. Collars are absent or present. Columellae are oval, depressed globose, or occasionally globose, hyaline, subhyaline or light green, 6.5–18.5 µm long, and 7.5–22.0 µm wide. Projections are present or absent, single if present, subhyaline, and 1.5–3.5 µm long. Sporangiospores are cylindrical, light green, smooth, 3.5–5.0 µm long, and 2.0–4.0 µm wide. Chlamydospores are absent. Zygospores are not observed.
Maximum growth temperature: 32 °C.
Material examined: China. Yunnan, Yuxi, from forest soil sample, September 2021, Heng Zhao (holotype HMAS 351936, living ex-holotype culture CGMCC 3.16259). Chuxiong, 25°13’52” N, 101°18’24” E, altitude: 2060 m, from forest soil sample, September 2021, Heng Zhao (living culture XY09528).
3.3. Key to the Species of Absidia in China
Together with the five new species proposed in this study, a total of 51 species of Absidia have been described worldwide. Among these, 22 species are distributed in China. Consequently, we provide a key to the Chinese species of Absidia. Characteristics adopted in the key include maximum growth temperatures, hyphae, rhizoids, sporangiophores, sporangia, collars, columellae, projections, and sporangiospores.
| 1. | Maximum growth temperature ≤ 28 °C | 2 |
| 1. | Maximum growth temperature > 28 °C | 6 |
| 2. | Sporangiospores subglobose to globose | 3 |
| 2. | Sporangiospores cylindrical | 4 |
| 3. | Maximum growth temperature 26 °C; sporangiospores subglobose to globose | A. lobata |
| 3. | Maximum growth temperature 28 °C; sporangiospores globose | A. globospora |
| 4. | Maximum growth temperature 24 °C | A. frigida |
| 4. | Maximum growth temperature 28 °C | 5 |
| 5. | Sporangiophores > 5 in whorls | A. psychrophilia |
| 5. | Sporangiophores ≤ 5 in whorls | A. sichuanensis |
| 6. | Sporangiospores two or more types | 7 |
| 6. | Sporangiospores one type | 12 |
| 7. | Sporangiospores sometimes irregular in shape | 10 |
| 7. | Sporangiospores never irregular in shape | A. globospora |
| 8. | Sporangia elliptical or elongate | A. repens |
| 8. | Sporangia globose to pyriform | 9 |
| 9. | Hyphae without swellings; sporangiophores monopodial or verticillate | A. idahoensis |
| 9. | Hyphae with swellings; sporangiophores sometimes unbranched | A. turgida |
| 10. | Columellae with projections | A. abundans |
| 10. | Columellae without projections | 11 |
| 11. | Sporangiospores globose, 3.8–7.7 µm in diameter, or cylindrical to oval | A. heterospora |
| 11. | Sporangiospores globose, 2.5–3.5 µm in diameter, or cylindrical | A. gemella |
| 12. | Sporangiospores globose | A. glauca |
| 12. | Sporangiospores cylindrical, oval or ellipsoid | 13 |
| 13. | Collars absent | 14 |
| 13. | Collars present | 16 |
| 14. | Sporangiospores cylindrical | A. longissima |
| 14. | Sporangiospores oval or ellipsoid | 15 |
| 15. | Sporangiospores oval to ellipsoid; sporangiophores 2–6 in whorls with swellings | A. ovalispora |
| 15. | Sporangiospores oval; sporangiophores 2–5 in whorls without swellings | A. radiata |
| 16. | Sporangiophores neither in pairs nor in whorls | A. panacisoli |
| 16. | Sporangiophores in pairs and in whorls | 17 |
| 17. | Sporangiophores 7–11 in whorls | 18 |
| 17. | Sporangiophores ≤ 6 in whorls | 20 |
| 18. | Rhizoids aseptate | A. spinosa |
| 18. | Rhizoids septate | 19 |
| 19. | Projections < 5 µm long, tapering at top | A. zonata |
| 19. | Projections > 5 µm long, rounded at top | A. pseudocylindrospora |
| 20. | Maximum growth temperature > 34 °C | A. cylindrospora |
| 20. | Maximum growth temperature ≤ 34 °C | 21 |
| 21. | Sporangiophores always swollen | A. yunnanensis |
| 21. | Sporangiophores never swollen | A. medulla |
3.4. Species Distribution and Ecological Habitat in China
A total of 22 species of Absidia were recorded in China (Table 3), including the five new species proposed in this study [14,15,16,27,49,50,51]. All species were found in soil, such as forest soil and rhizospheric soil, whereas other habitats, including leaf litter, dung, insect remains, and plant leaves, recorded one to several species. In terms of geographical distribution, Yunnan, Xinjiang, and Taiwan top the list with twelve, six, and five records, respectively.

Table 3.
Absidia species distributions and ecological habitat in China.
4. Discussion
In this study, five new species are proposed in the genus of Absidia being supported with molecular sequences and morphological and physiological features. Phylogenetically, Absidia abundans is closely related to A. panacisoli T. Yuan Zhang et al. based on the ITS and LSU rDNA sequences (Figure 2). However, A. panacisoli is distinguished from A. abundans by a higher maximum growth temperature (33 °C vs. 31 °C), shape of sporangia (spherical or subpyriform vs. oval to subglobose), sporangiospores (short cylindrical vs. cylindrical, oval or subglobose), and azygospores (present vs. absent; [16]). Moreover, swellings below sporangia are always observed in A. abundans, while absent in A. panacisoli [16].
Absidia lobata is closely related to A. glauca and A. globospora T.K. Zong and X.Y. Liu (Figure 2), while distinguished by a lower maximum growth temperature (26 ℃ in A. lobata, 29 °C in A. glauca, 37 °C in A. globospora) [15,52]. Besides, sporangia are globose in A. globospora, while pyriform in A. lobata and A. glauca [15,52]. Moreover, zygospores and chlamydospores are produced in A. glauca, but not in A. lobata and A. globospora [15,52].
Absidia radiata is related to A. yunnanensis with full support (Figure 3). However, A. yunnanensis differs from A. radiata by colonies (light brown vs. dark brown), shape of sporangiospores (cylindrical vs. oval), and swellings below sporangia (present vs. absent).
Absidia sichuanensis is most closely related to A. frigida H. Zhao et al. and A. psychrophilia (Figure 4), but differs by a higher maximum growth temperature (28 °C in A. sichuanensis, 24 °C in A. frigida, and 25 °C in A. psychrophilia), sporangia (17.0–21.5 µm wide in A. sichuanensis, 12.5–32.0 µm wide in A. frigida, and 20.0–50.0 µm wide in A. psychrophilia), columellae (8.0–15.5 µm wide in A. sichuanensis, 15.5–18.0 µm wide in A. frigida, and 6.5–30.0 µm wide in A. psychrophilia), and number of whorls (1–5 in A. sichuanensis, 1–4 in A. frigida, and 1–8 in A. psychrophilia [8,16]. In addition, collars are always absent in A. sichuanensis and A. frigida but present in A. psychrophilia [8,16]. Moreover, a morphological feature table including six species of Absidia without DNA sequences is listed for distinguishing between the five new species proposed in this study (Table 4).

Table 4.
Morphological features of the five new Absidia species and other six related species without DNA evidence.
Species of Absidia synthesized important metabolites, such as α-galactosidase, laccase, chitosan, and fatty acids [57,58,59,60], and our results provide a basis for their applications. In addition to the five new species proposed in this paper, 51 species of Absidia have previously been described from all around the world. A total of 22 species are recorded in China (https://nmdc.cn/fungarium/fungi/chinadirectories, accessed on 1 March 2022), accounting for 43% of worldwide species of the genus Absidia [14,15,16,18,27,39,40,41,42,43,44,45,46,47,48,49,50,51]. This result suggests that Absidia, a genus of Mucoromycota, is diverse, needing in-depth investigations to discover and describe more potential new species, as in Ascomycota and Basidiomycota [13,61,62,63,64].
The species of Absidia are ubiquitous in soil, dung, and decaying plants, as well as insect remains [1,8,15,16,51]. Most species, including the five new species described herein, are reported from soil and, hence, soil is their main habitat. Some species may be associated with plants (Table 3). For example, A. lobata and A. panacisoli were described in rhizosphere soil with Pinus yunnanensis and Panax notoginseng (Burkill) F. H. Chen ex C. Chow and W. G. Huang, respectively [16].
In China, most species of Absidia are recorded in Yunnan, Xinjiang, and Taiwan (Table 3), located in tropical, subtropical, and temperate zones. At the same time, a number of Absidia are described from Brazil and Thailand, which have a similar climate [17,28,29,30,33,34]. However, species of Absidia are rarely adapted to high temperatures, so that strains in tropical areas are usually described from forest soil or mountains [16,17,28,29,33,34]. Consequently, species diversity of Absidia in tropical and subtropical forest soil needs to be further explored.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof8050471/s1, Supplementary File S1: The aligned dataset for 62 strains, representing 45 species of Absidia and two species of Cunninghamella; Supplementary Table S1: Top hits for the new species based on BLAST search for ITS sequences from type materials.
Author Contributions
H.Z., experiment, validation, and writing—original draft preparation; Y.N., methodology; T.-K.Z., Y.-J.W. and M.W., resources and visualization; Y.-C.D. and X.-Y.L., funding acquisition, projection administration, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Second Tibetan Plateau Scientific Expedition and Research Program (STEP), Grant No. 2019QZKK0503, and the National Natural Science Foundation of China, Grant Nos. 31970009 and 32170012.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Sequences have been deposited in GenBank (Table 2).
Acknowledgments
Many scholars are thanked for their help with sampling and depositing. They are Hong-Min Zhou, Meng Zhou (Beijing Forestry University), Ze-Fen Yu, Min Qiao (Yunnan University), Ke Wang, Zhuo Du, and You-Zhi Wang (Institute of Microbiology, Chinese Academy of Sciences).
Conflicts of Interest
All authors declare no conflict of interest.
References
- Van Tieghem, P. Troisième mémoire sur les Mucorinées. Ann. Sci. Nat. Bot. Ser. 1876, 4, 312–399. [Google Scholar]
- Von Arx, J. On Mucoraceae s. str. and other families of the Mucorales. Sydowia 1984, 35, 10–26. [Google Scholar]
- Voigt, K.; Cigelnik, E.; O’donnell, K. Phylogeny and PCR identification of clinically important Zygomycetes based on nuclear ribosomal-DNA sequence data. J. Clin. Microbiol. 1999, 37, 3957–3964. [Google Scholar] [CrossRef] [Green Version]
- Voigt, K.; Wöstemeyer, J. Phylogeny and origin of 82 zygomycetes from all 54 genera of the Mucorales and Mortierellales based on combined analysis of actin and translation elongation factor EF-1α genes. Gene 2001, 270, 113–120. [Google Scholar] [CrossRef]
- O’donnell, K.; Lutzoni, F.M.; Ward, T.J.; Benny, G.L. Evolutionary relationships among mucoralean fungi (Zygomycota): Evidence for family polyphyly on a large scale. Mycologia 2001, 93, 286–297. [Google Scholar] [CrossRef]
- Cannon, P.F.; Kirk, P.M. Fungal Families of the World; Cabi: Wallingford, UK, 2007. [Google Scholar]
- Kirk, P.M.; Cannon, P.F.; Minter, D.W.; Stalpers, J.A. Dictionary of the Fungi, 10th ed.; CAB International: Wallingford, UK, 2008. [Google Scholar]
- Hesseltine, C.W.; Ellis, J.J. The genus Absidia: Gongronella and cylindrical-spored species of Absidia. Mycologia 1964, 56, 568–601. [Google Scholar] [CrossRef]
- Hoffmann, K. Identification of the genus Absidia (Mucorales, Zygomycetes): A comprehensive taxonomic revision. In Molecular Identification of Fungi; Gherbawy, Y., Voigt, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 439–460. [Google Scholar] [CrossRef]
- Hoffmann, K.; Discher, S.; Voigt, K. Revision of the genus Absidia (Mucorales, Zygomycetes) based on physiological, phylogenetic, and morphological characters; thermotolerant Absidia spp. form a coherent group, Mycocladiaceae fam. nov. Mycol. Res. 2007, 111, 1169–1183. [Google Scholar] [CrossRef]
- Hoffmann, K.; Voigt, K. Absidia parricida plays a dominant role in biotrophic fusion parasitism among mucoralean fungi (Zygomycetes): Lentamyces, a new genus for A. parricida and A. zychae. Plant Biol. 2009, 11, 537–554. [Google Scholar] [CrossRef]
- Benny, G.L.; Humber, R.A.; Morton, J.B. Zygomycota: Zygomycetes. In Systematics and Evolution. The Mycota (A Comprehensive Treatise on Fungi as Experimental Systems for Basic and Applied Research); McLaughlin, D.J., McLaughlin, E.G., Lemke, P.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; Volume 7A, pp. 113–146. [Google Scholar] [CrossRef]
- Liu, X.Y. Taxonomy and Phylogeny of the Absidia (Cunninghamellaceae, Mucorales) Introducing Nine New Species and Two New Combinations from China; Research Square: Durham, NC, USA, 2021. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, J.; Zong, T.K.; Liu, X.L.; Ren, L.Y.; Lin, Q.; Qiao, M.; Nie, Y.; Zhang, Z.D.; Liu, X.Y. Two new species in the family Cunninghamellaceae from China. Mycobiology. 2021, 49, 142–150. [Google Scholar] [CrossRef]
- Zong, T.K.; Zhao, H.; Liu, X.L.; Ren, L.Y.; Zhao, C.L.; Liu, X.Y. Taxonomy and phylogeny of four new species in Absidia (Cunninghamellaceae, Mucorales) from China. Front. Microbiol. 2021, 12, 2181. [Google Scholar] [CrossRef]
- Zhao, H.; Nie, Y.; Zong, T.K.; Dai, Y.C.; Liu, X.Y. Three new species of Absidia (Mucoromycota) from China based on phylogeny, morphology and physiology. Diversity. 2022, 14, 132. [Google Scholar] [CrossRef]
- Hurdeal, V.G.; Gentekaki, E.; Lee, H.B.; Jeewon, R.; Hyde, K.D.; Tibpromma, S.; Mortimer, P.E.; Xu, J. Mucoralean fungi in Thailand: Novel species of Absidia from tropical forest soil. Cryptogam. Mycol. 2021, 42, 39–61. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Yu, Y.; Zhu, H.; Yang, S.Z.; Yang, T.M.; Zhang, M.Y.; Zhang, Y.X. Absidia panacisoli sp. nov., isolated from rhizosphere of Panax notoginseng. Int. J. Syst. Evol. Microbiol. 2018, 68, 2468–2472. [Google Scholar] [CrossRef] [PubMed]
- Kaitera, J.; Henttonen, H.M.; Müller, M.M. Fungal species associated with butt rot of mature Scots pine and Norway spruce in northern boreal forests of Northern Ostrobothnia and Kainuu in Finland. Eur. J. Plant Pathol. 2019, 154, 541–554. [Google Scholar] [CrossRef]
- Li, G.J.; Hyde, K.D.; Zhao, R.L.; Hongsanan, S.; Abdel-Aziz, F.A.; Abdel-Wahab, M.A.; Alvarado, P.; Alves-Silva, G.; Ammirati, J.F.; Ariyawansa, H.A.; et al. Fungal diversity notes 253–366: Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2016, 78, 1–237. [Google Scholar] [CrossRef]
- Absidia glauca Hagem in GBIF Secretariat. GBIF Backbone Taxonomy. Checklist Dataset. 2021. Available online: https://doi.org/10.15468/39omei (accessed on 22 April 2022).
- Absidia repens Tiegh. in GBIF Secretariat. GBIF Backbone Taxonomy. Checklist Dataset. 2021. Available online: https://doi.org/10.15468/39omei (accessed on 22 April 2022).
- Větrovský, T.; Morais, D.; Kohout, P.; Lepinay, C.; Algora, G.C.; Awokunle, H.S.; Baldrian, P. GlobalFungi, a global database of fungal occurrences from high-throughput-sequencing metabarcoding studies. Sci. Data 2020, 7, 228. [Google Scholar] [CrossRef] [PubMed]
- Absidia heterospora Y. Ling in GBIF Secretariat. GBIF Backbone Taxonomy. Checklist Dataset. 2021. Available online: https://doi.org/10.15468/39omei (accessed on 22 April 2022).
- Hesseltine, C.W.; Mahoney, M.K.; Peterson, S.W. A new species of Absidia from an alkali bee brood chamber. Mycologia 1990, 82, 523–526. [Google Scholar] [CrossRef]
- Vánová, M. Contribution to the taxonomy of the genus Absidia (Mucorales) I. Absidia macrospora sp. nov. Ceská Mykologie 1968, 22, 296–300. [Google Scholar]
- Zheng, R.Y.; Liu, X.Y. Species Catalogue of China, Volume 3. Fungi: Chtrid, Zygomycotan, Glomeromycotan Fungi; Science Press: Beijing, China, 2018. [Google Scholar]
- Cordeiro, L.; Lee, H.B.; Nguyen, T.T.T.; Gurgel, L.M.S.; de Azevedo, A.L.C.M. Absidia bonitoensis (Mucorales, Mucoromycota), a new species isolated from the soil of an upland Atlantic forest in Northeastern Brazil. Nova Hedwig. 2021, 112, 241–251. [Google Scholar] [CrossRef]
- Cordeiro, T.R.L.; Nguyen, T.T.T.; Lima, D.X.; da Silva, S.B.G.; de Lima, C.F.; Leitão, J.D.; Gurgel, L.M.S.; Lee, H.B.; de Santiago, A.L.M.A. Two new species of the industrially relevant genus Absidia (Mucorales) from soil of the Brazilian Atlantic Forest. Acta Bot. Bras. 2020, 34, 549–558. [Google Scholar] [CrossRef]
- Crous, P.W.; Cowan, D.A.; Maggs-Kölling, G.; Yilmaz, N.; Thangavel, R.; Wingfield, M.J.; Noordeloos, M.E.; Dima, B.; Brandrud, T.E.; Jansen, G.M.; et al. Fungal planet description sheets: 1182–1283. Pers. Mol. Phylogeny Evol. Fungi 2021, 46, 313–528. [Google Scholar] [CrossRef]
- Crous, P.W.; Luangsa-Ard, J.J.; Wingfield, M.J.; Carnegie, A.J.; Hernández-Restrepo, M.; Lombard, L.; Roux, J.; Barreto, R.W.; Baseia, I.G.; Cano-Lira, J.F.; et al. Fungal Planet description sheets: 785–867. Pers. Mol. Phylogeny Evol. Fungi 2018, 41, 238–417. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Wingfield, M.J.; Chooi, Y.H.; Gilchrist, C.L.; Lacey, E.; Pitt, J.I.; Roets, F.; Swart, W.J.; Cano-Lira, J.F.; Valenzuela-Lopez, N.; et al. Fungal planet description sheets: 1042–1111. Pers. Mol. Phylogeny Evol. Fungi 2020, 44, 301–459. [Google Scholar] [CrossRef] [PubMed]
- Leitao, J.D.; Cordeiro, T.R.; Nguyen, T.T.T.; Lee, H.B.; Gurgel, L.M.; de Santiago, A.L.D.A. Absidia aguabelensis sp. nov.: A new mucoralean fungi isolated from a semiarid region in Brazil. Phytotaxa 2021, 516, 83–91. [Google Scholar] [CrossRef]
- Lima, D.X.; Cordeiro, T.R.; de Souza, C.A.; de Oliveira, R.J.; Lee, H.B.; Souza-Motta, C.M.; de Santiago, A.L.A. Morphological and molecular evidence for two new species of Absidia from Neotropic soil. Phytotaxa 2020, 446, 61–71. [Google Scholar] [CrossRef]
- Urquhart, A.S.; Idnurm, A. Absidia healeyae: A new species of Absidia (Mucorales) isolated from Victoria, Australia. Mycoscience 2021, 62, 331–335. [Google Scholar] [CrossRef]
- Wanasinghe, D.N.; Phukhamsakda, C.; Hyde, K.D.; Jeewon, R.; Lee, H.B.; Jones, E.G.; Tibpromma, S.; Tennakoon, D.S.; Dissanayake, A.J.; Jayasiri, S.C.; et al. Fungal diversity notes 709–839: Taxonomic and phylogenetic contributions to fungal taxa with an emphasis on fungi on Rosaceae. Fungal Divers. 2018, 89, 1–236. [Google Scholar] [CrossRef]
- Benny, G.L. Methods used by Dr. RK Benjamin, and other mycologists, to isolate zygomycetes. Aliso 2008, 26, 37–61. [Google Scholar] [CrossRef]
- Zheng, R.Y.; Chen, G.Q.; Hu, F.M. Monosporus varieties of Syncephalastrum. Mycosystema 1988, 1, 35–52. [Google Scholar]
- Zheng, R.Y.; Liu, X.Y. Taxa of Pilaira (Mucorales, Zygomycota) from China. Nova Hedwig. 2009, 88, 255–267. [Google Scholar] [CrossRef]
- Zheng, R.Y.; Chen, G.Q.; Huang, H.; Liu, X.Y. A monograph of Rhizopus. Sydowia 2007, 59, 273–372. [Google Scholar]
- Zheng, R.Y.; Liu, X.Y.; Li, R.Y. More Rhizomucor causing human mucormycosis from China: R. chlamydosporus sp. nov. Sydowia 2009, 61, 135–147. [Google Scholar]
- Nie, Y.; Cai, Y.; Gao, Y.; Yu, D.S.; Wang, Z.M.; Liu, X.Y.; Huang, B. Three new species of Conidiobolus sensu stricto from plant debris in eastern China. MycoKeys 2020, 73, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Wang, Z.M.; Zhao, H.; Liu, X.Y.; Huang, B. Complete mitochondrial genome of Neoconidiobolus thromboides (Entomophthorales: Ancylistaceae). Mitochondrial DNA Part B. 2021, 6, 1840–1841. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Yu, D.S.; Wang, C.F.; Liu, X.Y.; Huang, B. A taxonomic revision of the genus Conidiobolus (Ancylistaceae, Entomophthorales): Four clades including three new genera. MycoKeys 2020, 66, 55–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press, Inc.: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Swofford, D.L. PAUP*: Phylogenetic analysis using parsimony (* and Other Methods); Version 4.0b10; Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Ho, H.M.; Chuang, S.C.; Chen, S.J. Notes on zygomycetes of Taiwan (IV): Three Absidia species (Mucoraceae). Fungal Sci. 2004, 19, 125–131. [Google Scholar]
- Hsu, T.H.; Ho, H.M. Notes on Zygomycetes of Taiwan VIII: Three new records of Absidia in Taiwan. Fungal Sci. 2010, 25, 5–11. [Google Scholar] [CrossRef]
- Hsu, T.H.; Ho, H.M.; Chien, C.Y. Taxonomic Study of Absidia sensu lato in Taiwan. Asian Mycological Congress and 11th International Marine and Freshwater Mycology Symposium Abstract Book; Walter de Gruyter: Berlin, Germany, 2009; p. 42. [Google Scholar]
- Ellis, J.J.; Hesseltine, C.W. The genus Absidia: Globose-spored species. Mycologia 1965, 57, 222–235. [Google Scholar] [CrossRef]
- Mehrotra, B.S.; Nand, K. An interesting new species of Absidia. Can. J. Bot. 1967, 45, 2223–2224. [Google Scholar] [CrossRef]
- Ellis, J.J.; Hesseltine, C.W. Species of Absidia with ovoid sporangiospores. II. Sabouraudia 1967, 5, 59–77. [Google Scholar] [CrossRef]
- Vanova, M. Contribution to the taxonomy of the genus Absidia (Mucorales). III. Absidia fassatiae spec. nov. Ceska Mykol. 1971, 25, 173–176. [Google Scholar]
- Subrahmanyam, A. Bat guano fungi. India J. Bot. 1990, 13, 154–158. [Google Scholar]
- Davoust, N.; Persson, A. Effects of growth morphology and time of harvesting on the chitosan yield of Absidia repens. Appl. Microbiol. Biotechnol. 1992, 37, 572–575. [Google Scholar] [CrossRef]
- Kitahata, S.; Ishikawa, H.; Miyata, T.; Tanaka, O. Production of rubusoside derivatives by transgalactosylation of various α-galactosidases. Agric. Biol. Chem. 1989, 53, 2929–2934. [Google Scholar] [CrossRef]
- Kristanti, R.A.; Zubir, M.M.F.A.; Hadibarata, T. Biotransformation studies of cresol red by Absidia spinosa M15. J. Environ. Manag. 2016, 172, 107–111. [Google Scholar] [CrossRef]
- Zhao, H.; Lv, M.L.; Liu, Z.; Zhang, M.Z.; Wang, Y.N.; Ju, X.; Song, Z.; Ren, L.Y.; Jia, B.S.; Qiao, M.; et al. High-yield oleaginous fungi and high-value microbial lipid resources from Mucoromycota. BioEnerg Res. 2021, 14, 1196–1206. [Google Scholar] [CrossRef]
- Wu, F.; Yuan, H.S.; Zhou, L.W.; Yuan, Y.; Cui, B.K.; Dai, Y.C. Polypore diversity in South China. Mycosystema 2020, 39, 653–682. [Google Scholar] [CrossRef]
- Zheng, H.D.; Zhuang, W.Y.; Wang, X.C.; Zeng, Z.Q.; Wei, S.L. Ascomycetes from the Qilian Mountains, China—Pezizomycetes and Leotiomycetes. Mycosystema 2020, 39, 1823–1845. [Google Scholar] [CrossRef]
- Dai, Y.C.; Yang, Z.L.; Cui, B.K.; Wu, G.; Yuan, H.S.; Zhou, L.W.; He, S.H.; Ge, Z.W.; Wu, F.; Wei, Y.L.; et al. Diversity and systematics of the important macrofungi in Chinese forests. Mycosystema 2021, 40, 770–805. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, L.W.; Vlasák, J.; Dai, Y.C. Global diversity and systematics of Hymenochaetaceae with poroid hymenophore. Fungal Divers. 2022, 113, 1–192. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).