Parametarhizium hingganense, a Novel Ectomycorrhizal Fungal Species, Promotes the Growth of Mung Beans and Enhances Resistance to Disease Induced by Rhizoctonia solani
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal and Plant Materials
2.2. Experimental Design
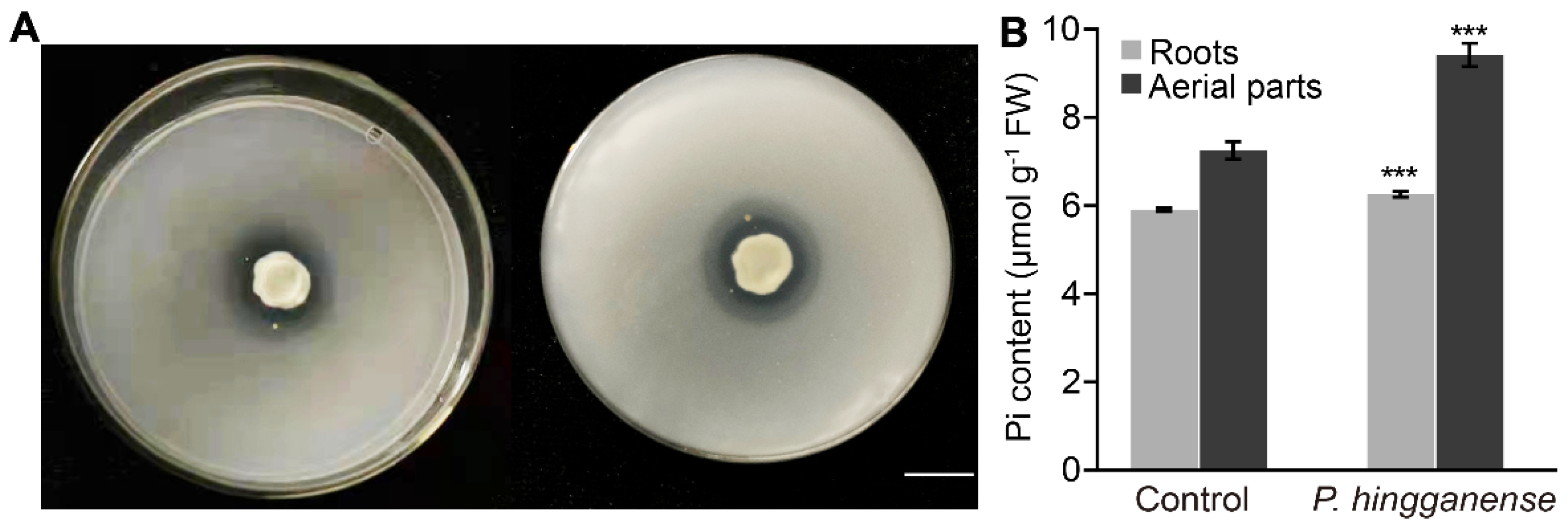
2.3. Phosphate Solubilization
2.4. Pi Content Assay
2.5. Quantification of IAA in Fungal Culture
2.6. Construction of Green Fluorescence Protein (GFP)-Tagged P. hingganense
2.7. Observation of the Colonization
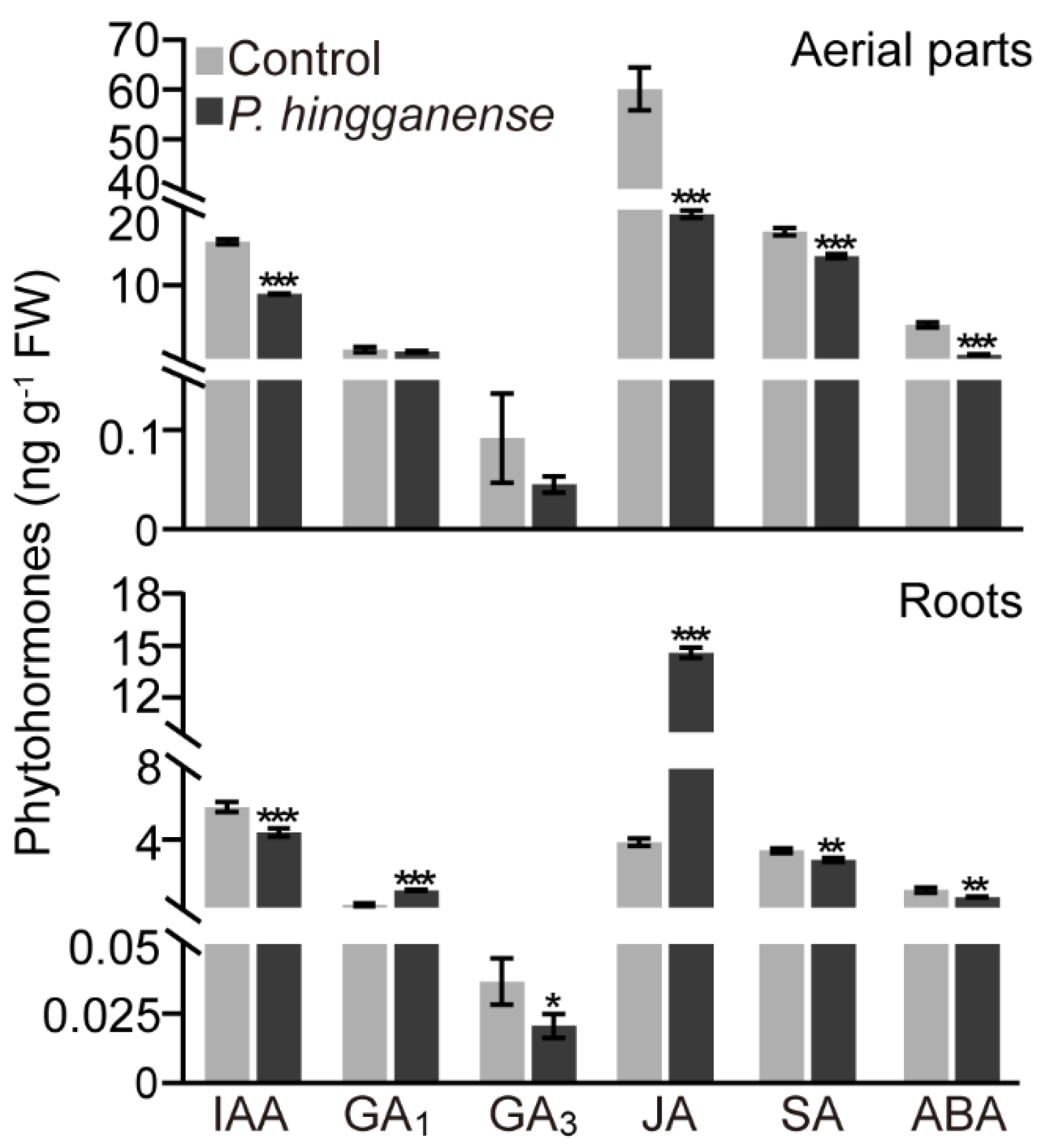
2.8. Quantification of Plant Hormones
2.9. Dual Culture of P. hingganense and R. solani
2.10. Biocontrol Effect of P. hingganense
2.11. Measurement of the Chlorophyll, MDA, and Total Phenolic and Flavonoid Content
2.12. Statistical Analysis
3. Results
3.1. P. hingganense Treatment on Seeds Promotes the Growth of Mung Bean Plants
3.2. Symbiotic Relationship between P. hingganense and Mung Bean Plants
3.3. P. hingganense Colonization Elevates the Content of JA and GA in the Roots
3.4. Symbiosis with P. hingganense Enhances Mung Bean Plant Resistance to Diseases Caused by R. solani
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Redecker, D.; Kodner, R.; Graham, L.E. Glomalean fungi from the Ordovician. Science 2000, 289, 1920–1921. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, P.; Genre, A. Mechanisms underlying beneficial plant-fungus interactions in mycorrhizal symbiosis. Nat. Commun. 2010, 1, 48. [Google Scholar] [CrossRef] [PubMed]
- Brundrett, M.C.; Tedersoo, L. Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 2018, 220, 1108–1115. [Google Scholar] [CrossRef]
- Dreischhoff, S.; Das, I.S.; Jakobi, M.; Kasper, K.; Polle, A. Local responses and systemic induced resistance mediated by ectomycorrhizal fungi. Front. Plant Sci. 2020, 11, 590063. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Poirier, Y.; Jaskolowski, A.; Clua, J. Phosphate acquisition and metabolism in plants. Curr. Biol. 2022, 32, R623–R629. [Google Scholar] [CrossRef] [PubMed]
- Landeweert, R.; Hoffland, E.; Finlay, R.D.; Kuyper, T.W.; van Breemen, N. Linking plants to rocks: Ectomycorrhizal fungi mobilize nutrients from minerals. Trends Ecol. Evol. 2001, 16, 248–254. [Google Scholar] [CrossRef]
- Becquer, A.; Trap, J.; Irshad, U.; Ali, M.A.; Claude, P. From soil to plant, the journey of P through trophic relationships and ectomycorrhizal association. Front. Plant Sci. 2014, 5, 548. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, F.; Friml, J.; Ding, Z. Auxin signaling: Research advances over the past 30 years. J. Integr. Plant Biol. 2022, 64, 371–392. [Google Scholar] [CrossRef]
- Silverstone, A.L.; Sun, T. Gibberellins and the Green Revolution. Trends Plant Sci. 2000, 5, 1–2. [Google Scholar] [CrossRef]
- Takeda, N.; Handa, Y.; Tsuzuki, S.; Kojima, M.; Sakakibara, H.; Kawaguchi, M. Gibberellins interfere with symbiosis signaling and gene expression and alter colonization by arbuscular mycorrhizal fungi in Lotus japonicus. Plant Physiol. 2015, 167, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.; Wang, S.; Cui, M.; Liu, J.; Chen, A.; Xu, G. Phytohormones regulate the development of arbuscular mycorrhizal symbiosis. Int. J. Mol. Sci. 2018, 19, 3146. [Google Scholar] [CrossRef] [PubMed]
- Basso, V.; Kohler, A.; Miyauchi, S.; Singan, V.; Guinet, F.; Simura, J.; Novak, O.; Barry, K.W.; Amirebrahimi, M.; Block, J.; et al. An ectomycorrhizal fungus alters sensitivity to jasmonate, salicylate, gibberellin, and ethylene in host roots. Plant Cell Environ. 2020, 43, 1047–1068. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.C.; Martinez-Medina, A.; Lopez-Raez, J.A.; Pozo, M.J. Mycorrhiza-induced resistance and priming of plant defenses. J. Chem. Ecol. 2012, 38, 651–664. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Zou, Y.N.; Liu, L.P.; Wu, Q.S. Common mycorrhizal networks activate salicylic acid defense responses of trifoliate orange (Poncirus trifoliata). J. Integr. Plant Biol. 2019, 61, 1099–1111. [Google Scholar] [CrossRef]
- Sandoval, R.F.C.; Cumagun, C.J.R. Phenotypic and molecular analyses of Rhizoctonia spp. associated with rice and other hosts. Microorganisms 2019, 7, 88. [Google Scholar] [CrossRef]
- Anderson, N.A. The genetics and pathology of Rhizoctonia solani. Annu. Rev. Phytopathol. 1982, 20, 329–347. [Google Scholar] [CrossRef]
- Tsror, L. Biology, Epidemiology and management of Rhizoctonia solani on potato. J. Phytopathol. 2010, 158, 649–658. [Google Scholar] [CrossRef]
- Kataria, H.R.; Grover, R.K. Comparison of fungicides for the control of Rhizoctonia solani causing datnping-off of mung bean (Phaseohis aureus). Ann. Appl. Biol. 1978, 88, 257–263. [Google Scholar] [CrossRef]
- Dubey, S.C.; Bhavani, R.; Singh, B. Integration of soil application and seed treatment formulations of Trichoderma species for management of wet root rot of mungbean caused by Rhizoctonia solani. Pest Manag. Sci. 2011, 67, 1163–1168. [Google Scholar] [CrossRef]
- Carling, D.E.; Baird, R.E.; Gitaitis, R.D.; Brainard, K.A.; Kuninaga, S. Characterization of AG-13, a newly reported anastomosis group of Rhizoctonia solani. Phytopathology 2002, 92, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Meng, W.; Zhang, L.; Yue, Q.; Zheng, X.; Xu, L. Parametarhizium (Clavicipitaceae) gen. nov. with two new species as a potential biocontrol agent isolated from forest litters in Northeast China. Front. Microbiol. 2021, 12, 131. [Google Scholar] [CrossRef] [PubMed]
- Sung, G.H.; Sung, J.M.; Hywel-Jones, N.L.; Spatafora, J.W. A multi-gene phylogeny of Clavicipitaceae (Ascomycota, Fungi): Identification of localized incongruence using a combinational bootstrap approach. Mol. Phylogenet. Evol. 2007, 44, 1204–1223. [Google Scholar] [CrossRef] [PubMed]
- Schardl, C.L.; Young, C.A.; Hesse, U.; Amyotte, S.G.; Andreeva, K.; Calie, P.J.; Fleetwood, D.J.; Haws, D.C.; Moore, N.; Oeser, B.; et al. Plant-symbiotic fungi as chemical engineers: Multi-genome analysis of the clavicipitaceae reveals dynamics of alkaloid loci. PLoS Genet. 2013, 9, e1003323. [Google Scholar] [CrossRef]
- Mongkolsamrit, S.; Khonsanit, A.; Thanakitpipattana, D.; Tasanathai, K.; Noisripoom, W.; Lamlertthon, S.; Himaman, W.; Houbraken, J.; Samson, R.A.; Luangsa-ard, J. Revisiting Metarhizium and the description of new species from Thailand. Stud. Mycol. 2020, 95, 171–251. [Google Scholar] [CrossRef] [PubMed]
- St Leger, R.J.; Wang, J.B. Metarhizium: Jack of all trades, master of many. Open Biol. 2020, 10, 200307. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Suo, M.; Qiu, Z.; Wu, H.; Zhao, M.; Yang, H. Regulating root fungal community using Mortierella alpina for Fusarium oxysporum resistance in Panax ginseng. Front. Microbiol. 2022, 13, 850917. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Doilom, M.; Guo, J.W.; Phookamsak, R.; Mortimer, P.E.; Karunarathna, S.C.; Dong, W.; Liao, C.F.; Yan, K.; Pem, D.; Suwannarach, N.; et al. Screening of phosphate-solubilizing fungi from air and soil in Yunnan, China: Four novel species in Aspergillus, Gongronella, Penicillium, and Talaromyces. Front. Microbiol. 2020, 11, 585215. [Google Scholar] [CrossRef]
- Nautiyal, C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Chiou, T.J.; Aung, K.; Lin, S.I.; Wu, C.C.; Chiang, S.F.; Su, C.L. Regulation of phosphate homeostasis by MicroRNA in Arabidopsis. Plant Cell 2006, 18, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Bose, A.; Shah, D.; Keharia, H. Production of indole-3-acetic-acid (IAA) by the white rot fungus Pleurotus ostreatus under submerged condition of Jatropha seedcake. Mycology 2013, 4, 103–111. [Google Scholar] [CrossRef]
- Zhang, A.; Lu, P.; Dahl-Roshak, A.M.; Paress, P.S.; Kennedy, S.; Tkacz, J.S.; An, Z. Efficient disruption of a polyketide synthase gene (pks1) required for melanin synthesis through Agrobacterium-mediated transformation of Glarea lozoyensis. Mol. Genet. Genom. 2003, 268, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Welti, R.; Wang, X. Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography-mass spectrometry. Nat. Protoc. 2010, 5, 986–992. [Google Scholar] [CrossRef]
- Meng, W.; Xu, L.; Du, Z.Y.; Wang, F.; Zhang, R.; Song, X.; Lam, S.M.; Shui, G.; Li, Y.; Chye, M.L. RICE ACYL-COA-BINDING PROTEIN6 affects acyl-coa homeostasis and growth in rice. Rice 2020, 13, 75. [Google Scholar] [CrossRef]
- Hao, Q.Y.; Albaghdady, D.M.D.; Xiao, Y.N.; Xiao, X.Q.; Mo, C.M.; Tian, T.; Wang, G.F. Endophytic Metarhizium anisopliae is a potential biocontrol agent against wheat Fusarium head blight caused by Fusarium graminearum. J. Plant Pathol. 2021, 103, 875–885. [Google Scholar] [CrossRef]
- Lisker, N.; Katan, J.; Henis, Y. Lesion formation on bean seedling hypocotyls by Rhizoctonia solani as affected by size and nutrition of propagules. Ann. Bot. 1980, 45, 365–368. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, Q.; He, M.; Zhong, X.; Tang, G. Wilting index and root morphological characteristics used as drought-tolerance variety selection at the seedling stage in soybean (Glycine max L.). Plant Growth Regul. 2020, 92, 29–42. [Google Scholar] [CrossRef]
- Guo, Z.H.; Pogancev, G.; Meng, W.; Du, Z.Y.; Liao, P.; Zhang, R.; Chye, M.L. The overexpression of rice ACYL-COA-BINDING PROTEIN4 improves salinity tolerance in transgenic rice. Environ. Exp. Bot. 2021, 183, 104349. [Google Scholar] [CrossRef]
- Martinez-Arias, C.; Sobrino-Plata, J.; Ormeno-Moncalvillo, S.; Gil, L.; Rodriguez-Calcerrada, J.; Martin, J.A. Endophyte inoculation enhances Ulmus minor resistance to Dutch elm disease. Fungal Ecol. 2021, 50, 101024. [Google Scholar] [CrossRef]
- Kurepin, L.V.; Zaman, M.; Pharis, R.P. Phytohormonal basis for the plant growth promoting action of naturally occurring biostimulators. J. Sci. Food Agric. 2014, 94, 1715–1722. [Google Scholar] [CrossRef] [PubMed]
- Rich, M.K.; Vigneron, N.; Libourel, C.; Keller, J.; Xue, L.; Hajheidari, M.; Radhakrishnan, G.V.; Le Ru, A.; Diop, S.I.; Potente, G.; et al. Lipid exchanges drove the evolution of mutualism during plant terrestrialization. Science 2021, 372, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Policelli, N.; Horton, T.R.; Hudon, A.T.; Patterson, T.R.; Bhatnagar, J.M. Back to Roots: The role of ectomycorrhizal fungi in boreal and temperate forest restoration. Front. Glob. Chang. 2020, 3, 97. [Google Scholar] [CrossRef]
- Barelli, L.; Moreira, C.C.; Bidochka, M.J. Initial stages of endophytic colonization by Metarhizium involves rhizoplane colonization. Microbiology 2018, 164, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Fang, W.; Tong, J.; Liu, S.; Wu, H.; Shi, J. Metarhizium robertsii as a promising microbial agent for rice in situ cadmium reduction and plant growth promotion. Chemosphere 2022, 305, 135427. [Google Scholar] [CrossRef]
- Khan, A.L.; Hamayun, M.; Khan, S.A.; Kang, S.M.; Shinwari, Z.K.; Kamran, M.; Ur Rehman, S.; Kim, J.G.; Lee, I.J. Pure culture of Metarhizium anisopliae LHL07 reprograms soybean to higher growth and mitigates salt stress. World J. Microbiol. Biotechnol. 2012, 28, 1483–1494. [Google Scholar] [CrossRef]
- Liao, X.; Lovett, B.; Fang, W.; St Leger, R.J. Metarhizium robertsii produces indole-3-acetic acid, which promotes root growth in Arabidopsis and enhances virulence to insects. Microbiology 2017, 163, 980–991. [Google Scholar] [CrossRef]
- Hiruma, K.; Gerlach, N.; Sacristan, S.; Nakano, R.T.; Hacquard, S.; Kracher, B.; Neumann, U.; Ramirez, D.; Bucher, M.; O’Connell, R.J.; et al. Root endophyte Colletotrichum tofieldiae confers plant fitness benefits that are phosphate status dependent. Cell 2016, 165, 464–474. [Google Scholar] [CrossRef]
- Qi, S.; Wang, J.; Wan, L.; Dai, Z.; da Silva Matos, D.M.; Du, D.; Egan, S.; Bonser, S.P.; Thomas, T.; Moles, A.T. Arbuscular mycorrhizal fungi contribute to phosphorous uptake and allocation strategies of Solidago canadensis in a phosphorous-deficient environment. Front. Plant Sci. 2022, 13, 831654. [Google Scholar] [CrossRef]
- Eissenstat, D.M.; Kucharski, J.M.; Zadworny, M.; Adams, T.S.; Koide, R.T. Linking root traits to nutrient foraging in arbuscular mycorrhizal trees in a temperate forest. New Phytol. 2015, 208, 114–124. [Google Scholar] [CrossRef]
- Ho-Plagaro, T.; Garcia-Garrido, J.M. Molecular regulation of arbuscular mycorrhizal symbiosis. Int. J. Mol. Sci. 2022, 23, 5960. [Google Scholar] [CrossRef] [PubMed]
- Cope, K.R.; Bascaules, A.; Irving, T.B.; Venkateshwaran, M.; Maeda, J.; Garcia, K.; Rush, T.A.; Ma, C.; Labbe, J.; Jawdy, S.; et al. The ectomycorrhizal fungus Laccaria bicolor produces lipochitooligosaccharides and uses the common symbiosis pathway to colonize populus roots. Plant Cell 2019, 31, 2386–2410. [Google Scholar] [CrossRef] [PubMed]
- Zavala-Gonzalez, E.A.; Rodriguez-Cazorla, E.; Escudero, N.; Aranda-Martinez, A.; Martinez-Laborda, A.; Ramirez-Lepe, M.; Vera, A.; Lopez-Llorca, L.V. Arabidopsis thaliana root colonization by the nematophagous fungus Pochonia chlamydosporia is modulated by jasmonate signaling and leads to accelerated flowering and improved yield. New Phytol. 2017, 213, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Foo, E.; Ross, J.J.; Jones, W.T.; Reid, J.B. Plant hormones in arbuscular mycorrhizal symbioses: An emerging role for gibberellins. Ann. Bot. 2013, 111, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Shaul-Keinan, O.; Gadkar, V.; Ginzberg, I.; Grunzweig, J.M.; Chet, I.; Elad, Y.; Wininger, S.; Belausov, E.; Eshed, Y.; Atzmon, N.; et al. Hormone concentrations in tobacco roots change during arbuscular mycorrhizal colonization with Glomus intraradices. New Phytol. 2002, 154, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Hamayun, M.; Hussain, A.; Khan, S.A.; Kim, H.Y.; Khan, A.L.; Waqas, M.; Irshad, M.; Iqbal, A.; Rehman, G.; Jan, S.; et al. Gibberellins producing endophytic fungus Porostereum spadiceum AGH786 rescues growth of salt affected soybean. Front. Microbiol. 2017, 8, 686. [Google Scholar] [CrossRef] [PubMed]
- Kaling, M.; Schmidt, A.; Moritz, F.; Rosenkranz, M.; Witting, M.; Kasper, K.; Janz, D.; Schmitt-Kopplin, P.; Schnitzler, J.P.; Polle, A. Mycorrhiza-triggered transcriptomic and metabolomic networks impinge on herbivore fitness. Plant Physiol. 2018, 176, 2639–2656. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef] [Green Version]



| Wilting Index | Hypocotyl Lesion Index | Lodging Index | |
|---|---|---|---|
| Control | 66.25% | 71% | 66.25% |
| P. hingganense | 48.75% | 57% | 42.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Gao, S.; Bai, Y.; Meng, W.; Xu, L. Parametarhizium hingganense, a Novel Ectomycorrhizal Fungal Species, Promotes the Growth of Mung Beans and Enhances Resistance to Disease Induced by Rhizoctonia solani. J. Fungi 2022, 8, 934. https://doi.org/10.3390/jof8090934
Gao Y, Gao S, Bai Y, Meng W, Xu L. Parametarhizium hingganense, a Novel Ectomycorrhizal Fungal Species, Promotes the Growth of Mung Beans and Enhances Resistance to Disease Induced by Rhizoctonia solani. Journal of Fungi. 2022; 8(9):934. https://doi.org/10.3390/jof8090934
Chicago/Turabian StyleGao, Ying, Siyu Gao, Yang Bai, Wei Meng, and Lijian Xu. 2022. "Parametarhizium hingganense, a Novel Ectomycorrhizal Fungal Species, Promotes the Growth of Mung Beans and Enhances Resistance to Disease Induced by Rhizoctonia solani" Journal of Fungi 8, no. 9: 934. https://doi.org/10.3390/jof8090934
APA StyleGao, Y., Gao, S., Bai, Y., Meng, W., & Xu, L. (2022). Parametarhizium hingganense, a Novel Ectomycorrhizal Fungal Species, Promotes the Growth of Mung Beans and Enhances Resistance to Disease Induced by Rhizoctonia solani. Journal of Fungi, 8(9), 934. https://doi.org/10.3390/jof8090934









