The Difference in Diversity between Endophytic Microorganisms in White and Grey Zizania latifolia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. DNA Extraction and PCR Amplification
2.3. Illumina MiSeq Sequencing
2.4. Data Processing
2.5. Statistical Analyses
3. Results
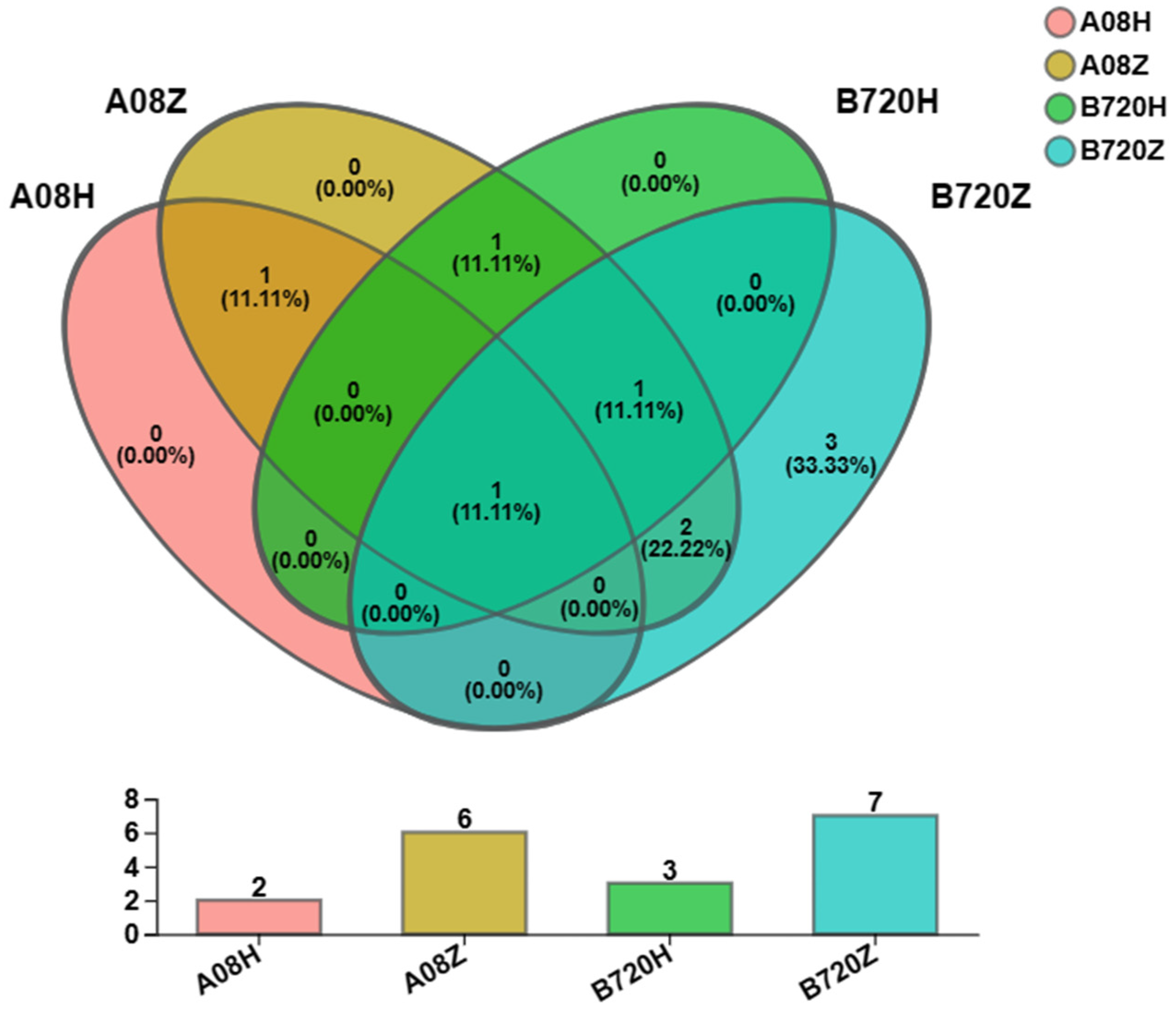
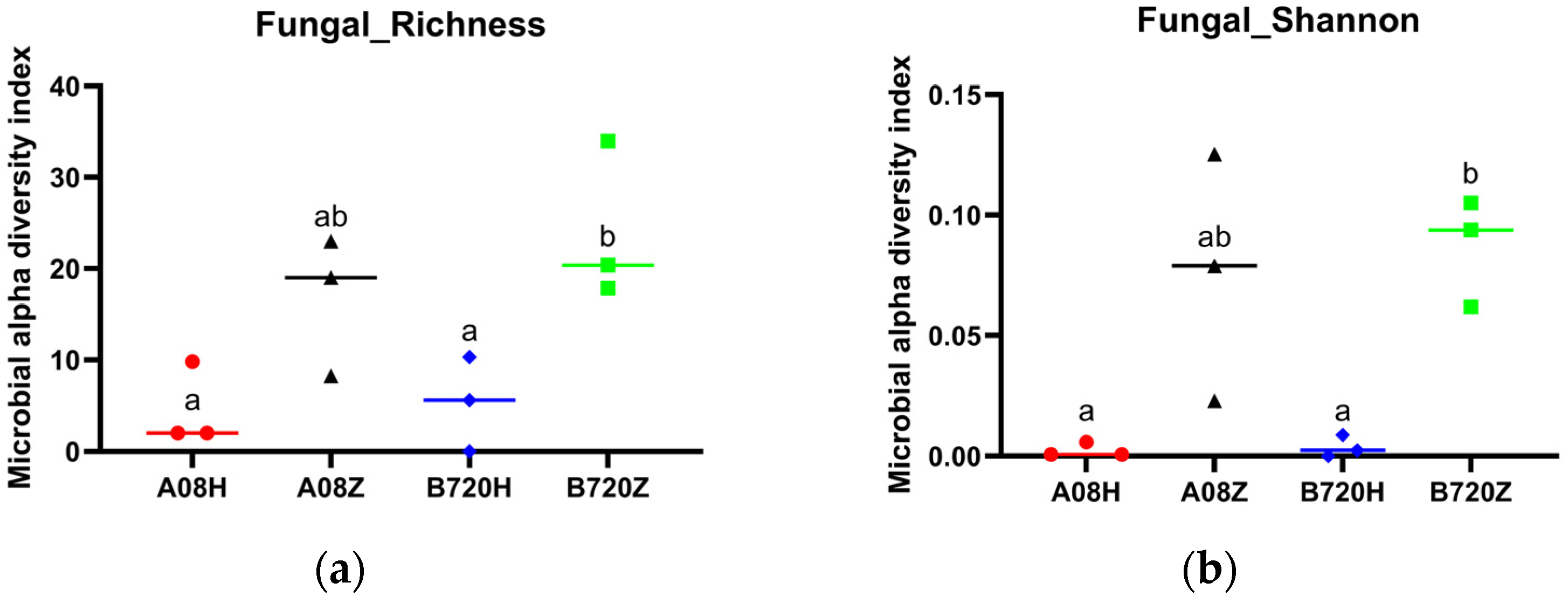
3.1. Differences in Microbial Diversity and Community Composition between White and Gray Z. latifolia
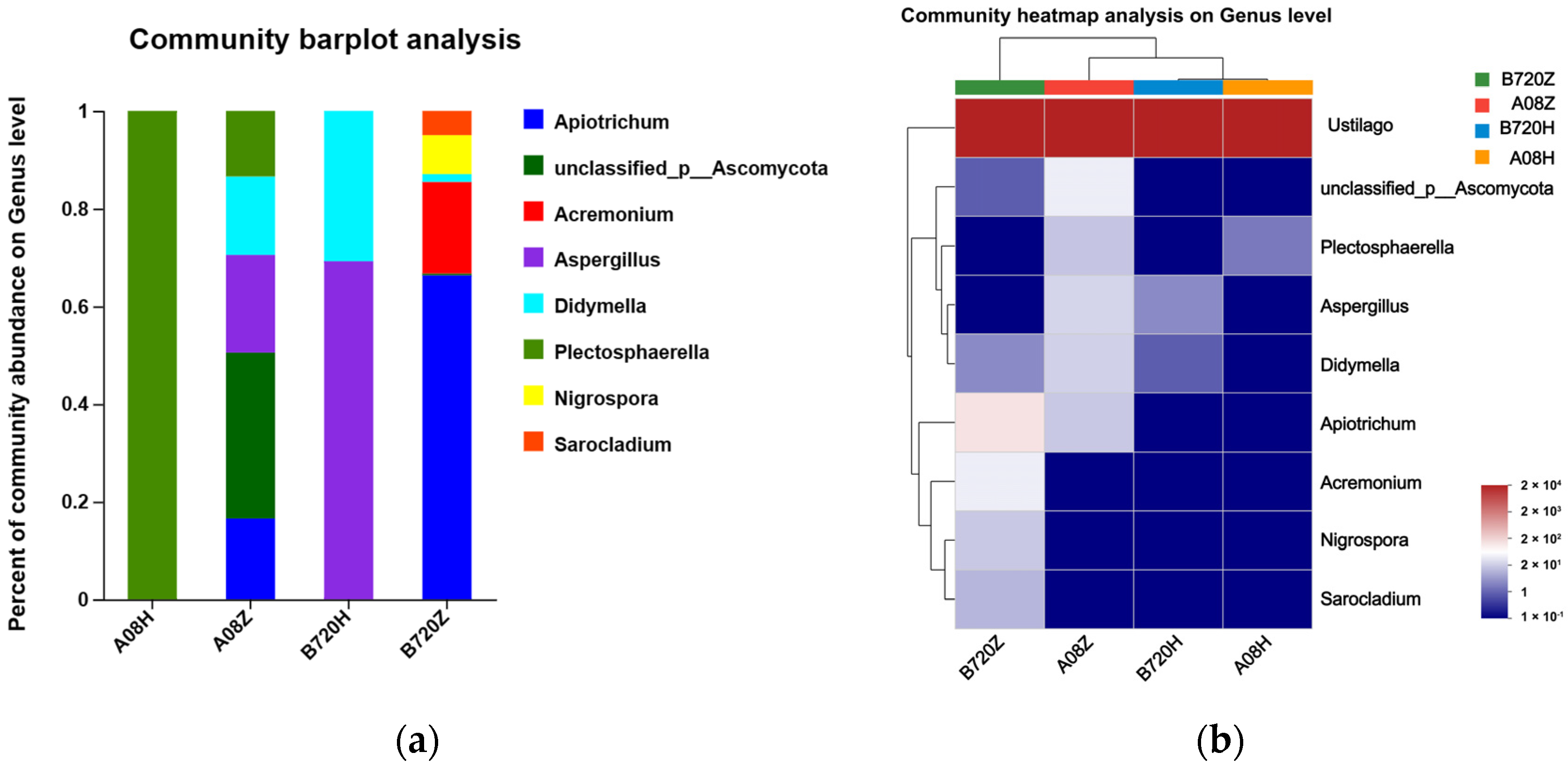
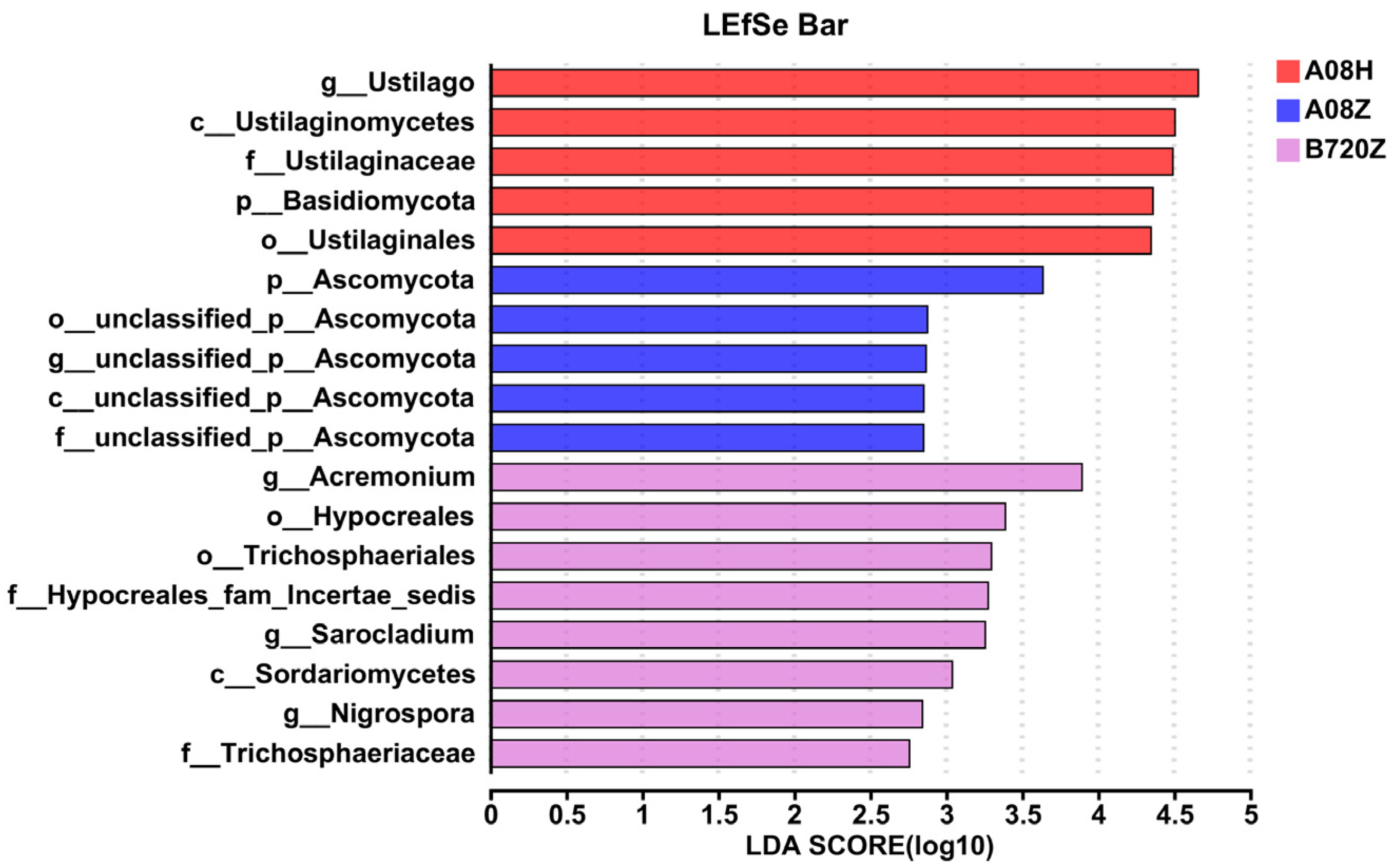
3.2. Fungal Abundance Affects Endophytic Fungi Community Composition in Z. latifolia
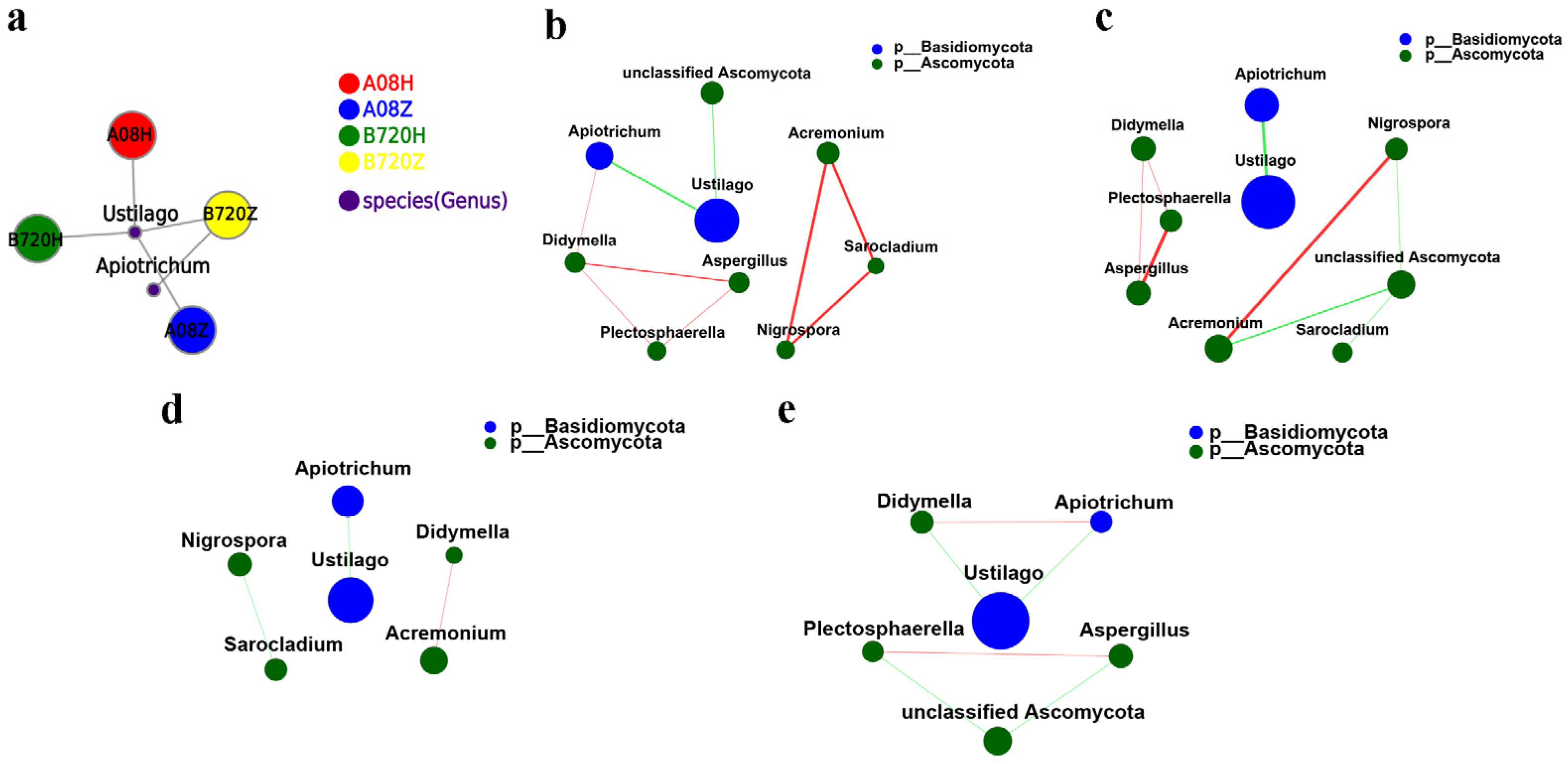
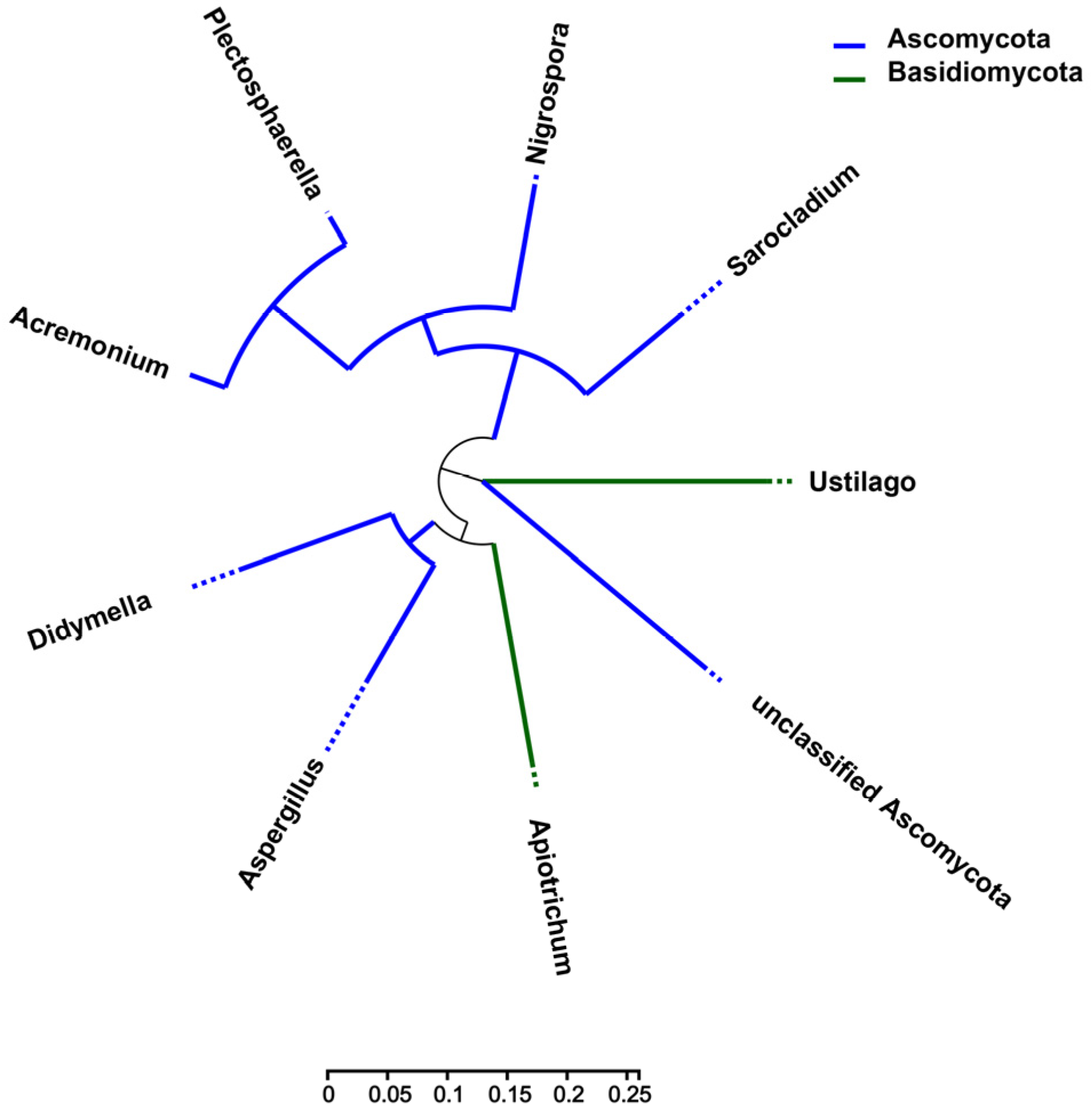
3.3. Analysis of Microbial Network Topology in Z. latifolia
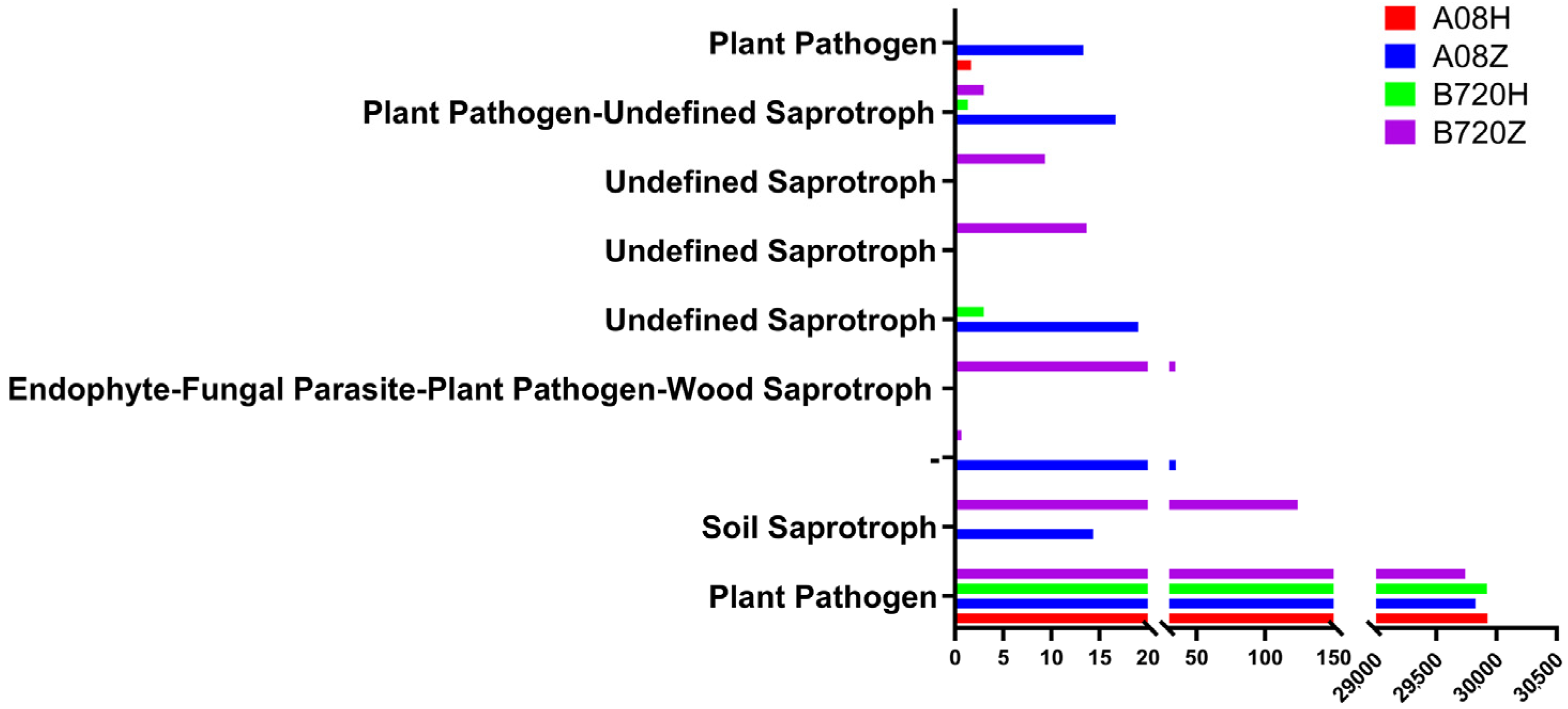
3.4. Functional Analysis of Microorganisms in Z. latifolia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ye, C.-W.; He, C.; Zhang, B.-W.; Wang, L.-X.; Wang, L.-F. Inhibition of lignification of Zizania latifolia with radio frequency treatments during postharvest. BMC Chem. 2020, 14, 4. [Google Scholar] [CrossRef]
- Yan, N.; Du, Y.-M.; Liu, X.-M.; Cheng, C.; John, S.; Zhang, H.-B.; Liu, Y.-H.; Zhang, Z.-F. Morphological characteristics, nutrients, and bioactive compounds of Zizania latifolia, and health benefits of its seeds. Molecules 2018, 23, 1561. [Google Scholar] [CrossRef]
- Huang, J.; Wu, W.-J.; Fang, X.-J.; Chen, H.-J.; Han, Y.-C.; Niu, B.; Gao, H.-Y. Zizania latifolia Cell Wall Polysaccharide Metabolism and Changes of Related Enzyme Activities during Postharvest Storage. Foods 2022, 11, 392. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Liu, Y.; Fan, X.-R.; Li, W.; Liu, Y.-L. Landscape-scale genetic structure of wild rice Zizania latifolia: The roles of rivers, mountains and fragmentation. Front. Ecol. Evol. 2017, 5, 17. [Google Scholar] [CrossRef]
- Qian, B.-J.; Luo, Y.-L.; Deng, Y.; Cao, L.-K.; Yang, H.-S.; Shen, Y.-P.; Ping, J. Chemical composition, angiotensin-converting enzyme-inhibitory activity and antioxidant activities of few-flower wild rice (Zizania latifolia Turcz.). J. Sci. Food Agric. 2012, 92, 159–164. [Google Scholar] [CrossRef]
- Wu, W.-J.; Han, Y.-C.; Niu, B.; Yang, B.-Q.; Liu, R.-L.; Fang, X.-J.; Chen, H.-Z.; Xiao, S.-Y.; Mohamed, A.F.; Zheng, S.-Q.; et al. Recent advances in Zizania latifolia: A comprehensive review on phytochemical, health benefits and applications that maximize its value. Crit. Rev. Food Sci. 2023, 11–15. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Cao, Q.-C.; Hu, P.; Cui, H.-F.; Yu, X.-P.; Ye, Z.-H. Investigation on the differentiation of two Ustilago esculenta strains-implications of a relationship with the host phenotypes appearing in the fields. BMC Microbiol. 2017, 17, 228. [Google Scholar] [CrossRef]
- Wang, S.-Q.; Gao, L.-D.; Yin, Y.-M.; Zhang, Y.-F.; Tang, J.-T.; Cui, H.-F.; Li, S.-Y.; Zhang, Z.-J.; Ye, Z.-H.; Xia, W.-Q. Transcriptome Comparison between Two Strains of Ustilago esculenta during the Mating. J. Fungi 2023, 9, 32. [Google Scholar] [CrossRef]
- Jose, R.C.; Bengyella, L.; Handique, P.J.; Talukdar, N.C. Cellular and proteomic events associated with the localized formation of smut-gall during Zizania latifolia-Ustilago esculenta interaction. Microb. Pathog. 2019, 12, 79–84. [Google Scholar] [CrossRef]
- You, W.-Y.; Liu, Q.; Zou, K.-Q.; Yu, X.-P.; Cui, H.-F.; Ye, Z.-H. Morphological and molecular differences in two strains of Ustilago esculenta. Curr. Microbiol 2011, 62, 44–54. [Google Scholar] [CrossRef]
- Carter, M. After the trap snaps in the plant immune response. Cell Host Microbe 2023, 31, 323–324. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.-B.; Qiu, J.; Han, Z.-J.; Ye, Z.-H.; Chen, C.; Liu, C.-J.; Xin, X.-F.; Ye, C.-Y.; Wang, Y.-Y.; Xie, H.-Q.; et al. A host plant genome (Zizania latifolia) after a century-long endophyte infection. Plant J. Cell Mol. Biol. 2015, 83, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, J.F.; Zhong, H.Y.; Chen, J.M. Germicide Fenaminosulf Promots Gall Formation of Zizania latifolia without directly affecting the growth of endophytic fungus Ustilago esculenta. BMC Plant Biol. 2022, 22, 418. [Google Scholar] [CrossRef] [PubMed]
- Collinge, D.B.; Jensen, B.; Jørgensen, H.J. Fungal endophytes in plants and their relationship to plant disease. Curr. Opin. Microbiol. 2022, 69, 102177. [Google Scholar] [CrossRef] [PubMed]
- Carrión Víctor, J.; Perez Jaramillo, J.; Cordovez, V.; Tracanna, V.; de Hollander, M.; RuizBuck, D.; Mendes, L.W.; van Ijcken, W.F.J.; GomezExposito, R.; Elsayed, S.S.; et al. Pathogen-induced activation of disease-suppressive functions in the endophytic root microbiome. Science 2019, 6465, 606–612. [Google Scholar] [CrossRef]
- Wang, M.-Z.; Zhu, P.-L.; Zhao, S.-W.; Nie, C.-Z.-P.; Wang, N.-F.; Du, X.-F.; Zhou, Y.-B. Characterization, antioxidant activity and immunomodulatory activity of polysaccharides from the swollen culms of Zizania latifolia. Int. J. Biol. Macromol. 2017, 95, 809–817. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Garrido-Oter, R.; Münch, P.C.; Weiman, A.; Dröge, J.; Pan, Y.; McHardy, A.C.; Schulze-Lefert, P. Structure and function of the bacterial root microbiota in wild and domesticated barley. Cell Host Microbe 2015, 17, 392–403. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Rott, M.; Schlaeppi, K.; Ver Loren van Themaat, E.; Ahmadinejad, N.; Assenza, F.; Rauf, P.; Huettel, B.; Reinhardt, R.; Schmelzer, E.; et al. Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 2012, 488, 91–95. [Google Scholar] [CrossRef]
- Adams, R.I.; Miletto, M.; Taylor, J.W.; Bruns, T.D. Dispersal in microbes: Fungi in indoor air are dominated by outdoor air and show dispersal limitation at short distances. ISME J. 2013, 7, 1262–1273. [Google Scholar] [CrossRef]
- Illumina. 16S Metagenomic Sequencing Library Preparation. 2013. Available online: https://support.illumina.com/downloads/16s_metagenomic_sequencing_library_preparation.html (accessed on 28 December 2022).
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Mo, P.; Xu, L.; Chen, D.; Chen, Z.; Li, B. Proposal of Streptomyces sporoverrucosus Gause et al. 1983 as a later heterotypic synonym of Streptomyces goshikiensis Niida et al. 1966 and an emended description of Streptomyces goshikiensis. Antonie Van Leeuwenhoek 2023, 116, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, R.H.; Larsson, K.-H.; Taylor, A.F.S.; Bengtsson, P.J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef]
- Langille, M.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Thurber, R.L.V.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Jiang, X.T.; Peng, X.; Deng, G.H.; Sheng, H.F.; Wang, Y.; Zhou, H.W.; Tam, N.F. Illumina sequencing of 16S rRNA tag revealed spatial variations of bacterial communities in a mangrove wetland. Microb. Ecol. 2013, 66, 96–104. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Barberan, A.; Bates, S.T.; Casamayor, E.O.; Fierer, N. Using network analysis to explore co-occurrence patterns in soil microbial communitie. ISME J. 2012, 6, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-P.; Xu, S.-Y.; Kong, M.-Q.; Dai, H.-B.; Liu, Y.-C.; Miao, M.-M. Isolation, identification and artificial inoculation of Ustilago esculenta on Zizania latifolia. Hortic. Plant J. 2021, 7, 347–358. [Google Scholar] [CrossRef]
- Yang, H.; Hou, Y.-X.; Zhang, S.; Kong, Q.-L.; Fan, Z.-J.; Li, S.-R.; Meng, M.; Li, H.-Y.; Zhang, Y.; Qiao, J.-L. Study on the role of wild soybean endophytic fungi on crop growth promotion. J. Agron. 2020, 10, 31–34. (In Chinese) [Google Scholar]
- Zhang, J.-Z.; Chu, F.-Q.; Guo, D.-P.; Hyde, K.D.; Xie, G.-L. Cytology and ultrastructure of interactions between Ustilago esculenta and Zizania latifolia. Mycol. Prog. 2012, 11, 499–508. [Google Scholar] [CrossRef]
- Michalska-Smith, M.; Song, Z.-W.; Spawn-Lee, S.A.; Hansen, Z.A.; Johnson, M.; May, G.; Borer, E.T.; Seabloom, E.W.; Kinkel, L.L. Network structure of resource use and niche overlap within the endophytic microbiome. ISME J. 2022, 16, 435–446. [Google Scholar] [CrossRef]
- Wijesooriya, W.A.D.K.; Deshappriya, N. An inoculum of endophytic fungi for improved growth of a traditional rice variety in Sri Lanka. Trop. Plant Res. 2016, 3, 470–480. [Google Scholar] [CrossRef]
- Yan, N.; Wang, X.Q.; Xu, X.F.; Guo, D.P.; Wang, Z.D.; Zhang, J.Z.; Hyde, K.D.; Liu, H.L. Plant growth and Photosynth eticperformance of Zizania latifolia are altered by endophytic Ustilago esculenta infection. Physiol. Mol. Plant Pathol. 2013, 83, 75–83. [Google Scholar] [CrossRef]
- Zhang, S.P.; Huang, R.; Li, F.-F.; Wei, H.-X.; Fang, X.-W.; Xie, X.-S.; Lin, D.-G.; Wu, S.-H.; He, J. Antiviral anthraquinones and azaphilones produced by an endophytic fungus Nigrospora sp. from Aconitum carmichaeli. Fitoterapia 2016, 112, 85–89. [Google Scholar] [CrossRef]
- Terrell, E.E.; Batra, L.R. Zizania latifolia and Ustilago esculenta, a Grass-Fungus Association. Econ. Bot. 1982, 36, 274–285. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Hu, C.; Song, R.; Yin, Z.; Wang, L.; Shi, L.; Li, W.; Zheng, Z.; Yang, M. The Difference in Diversity between Endophytic Microorganisms in White and Grey Zizania latifolia. J. Fungi 2023, 9, 1067. https://doi.org/10.3390/jof9111067
Li Y, Hu C, Song R, Yin Z, Wang L, Shi L, Li W, Zheng Z, Yang M. The Difference in Diversity between Endophytic Microorganisms in White and Grey Zizania latifolia. Journal of Fungi. 2023; 9(11):1067. https://doi.org/10.3390/jof9111067
Chicago/Turabian StyleLi, Yipeng, Cailin Hu, Ruiqi Song, Zhihui Yin, Lingyun Wang, Lin Shi, Wei Li, Zhaisheng Zheng, and Mengfei Yang. 2023. "The Difference in Diversity between Endophytic Microorganisms in White and Grey Zizania latifolia" Journal of Fungi 9, no. 11: 1067. https://doi.org/10.3390/jof9111067
APA StyleLi, Y., Hu, C., Song, R., Yin, Z., Wang, L., Shi, L., Li, W., Zheng, Z., & Yang, M. (2023). The Difference in Diversity between Endophytic Microorganisms in White and Grey Zizania latifolia. Journal of Fungi, 9(11), 1067. https://doi.org/10.3390/jof9111067





