Volatile Fingerprint Mediates Yeast-to-Mycelial Conversion in Two Strains of Beauveria bassiana Exhibiting Varied Virulence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Growth Conditions
2.2. Caenorhabditis Elegans Killing Assays
2.3. Fungal Volatile Analyses
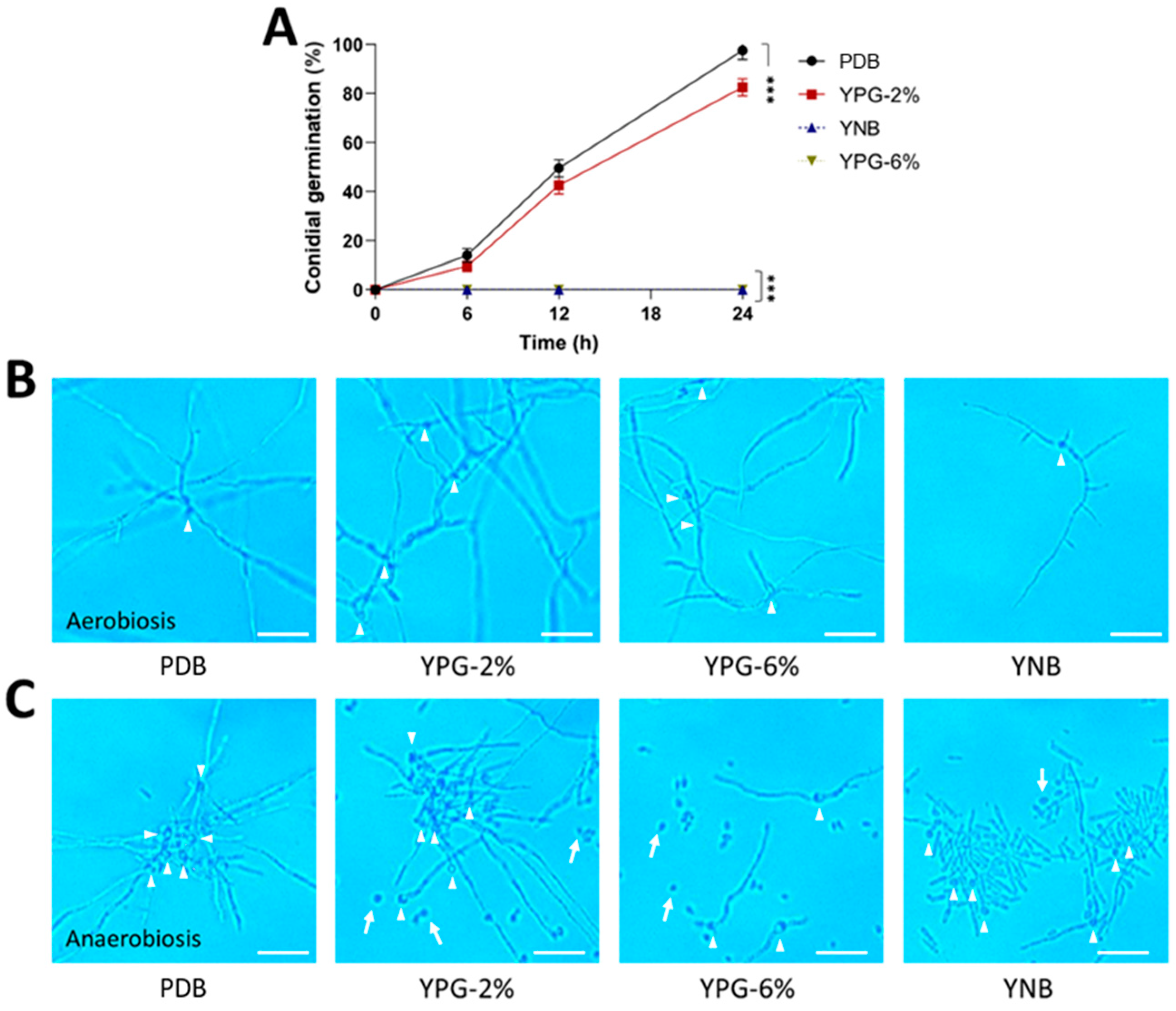
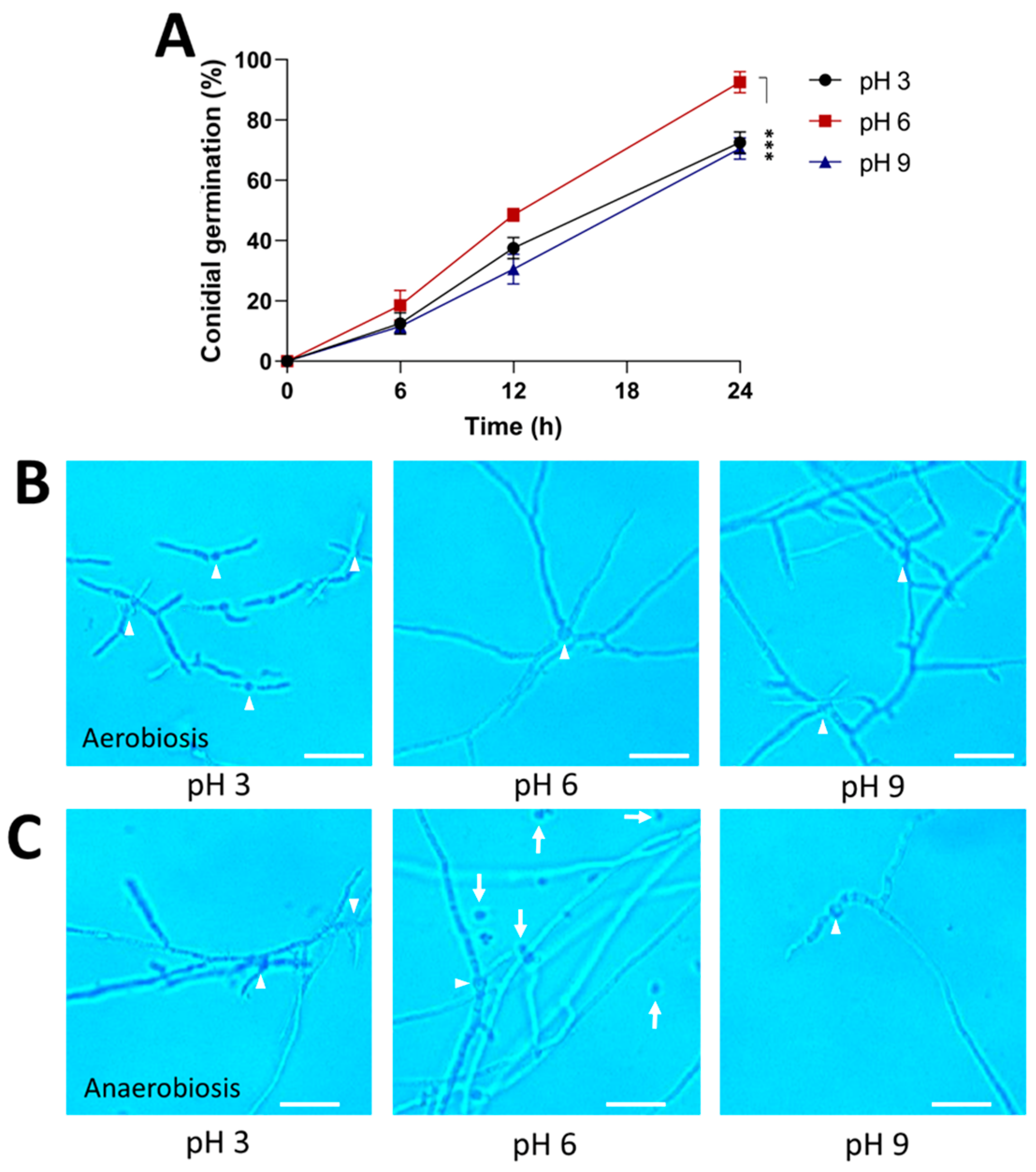
2.4. Effect of 3-Methylbutanol on Yeast-to-Mycelial Transition in B. bassiana
2.5. Statistical Analysis
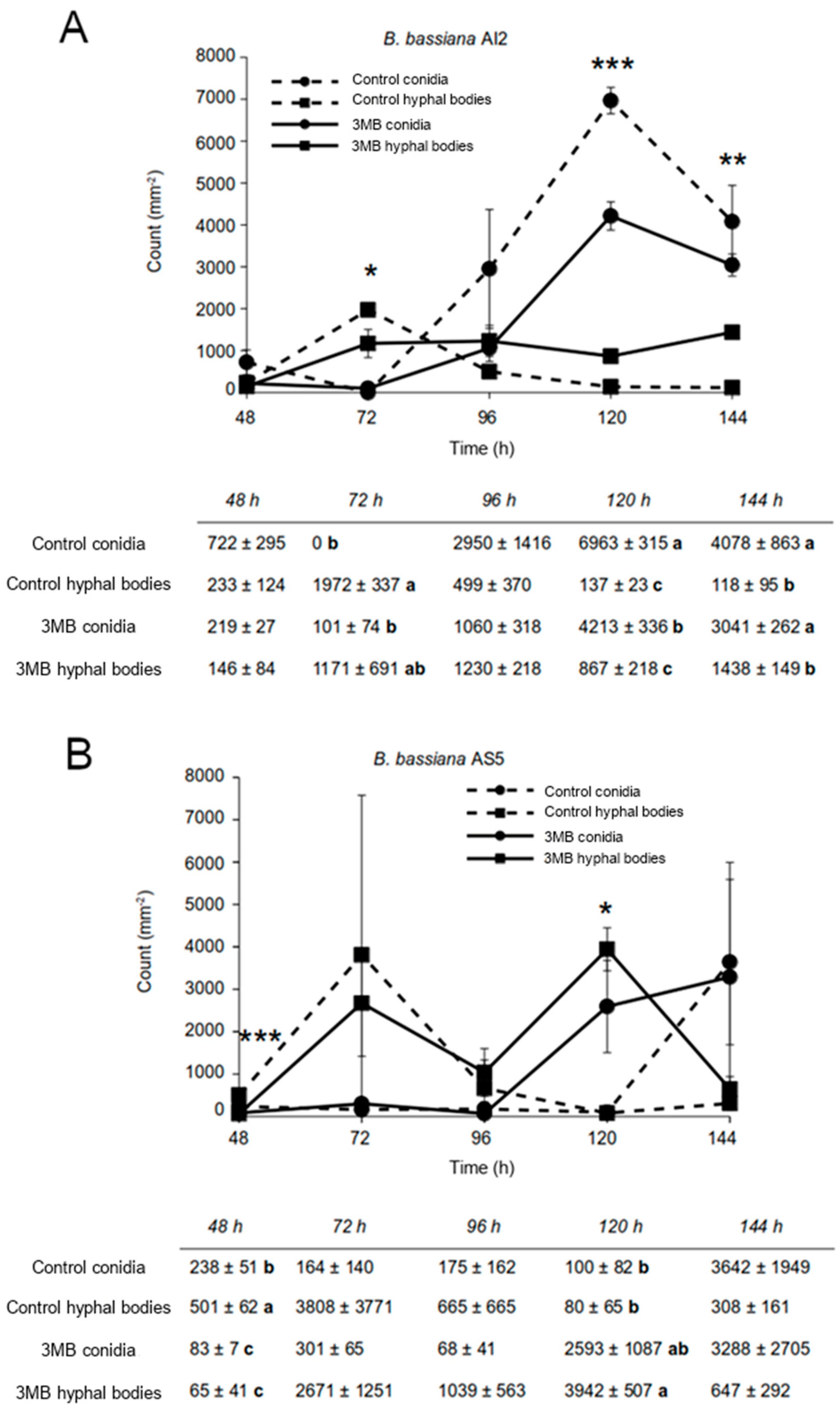
3. Results
3.1. Nematicidal Activity of B. bassiana against C. elegans
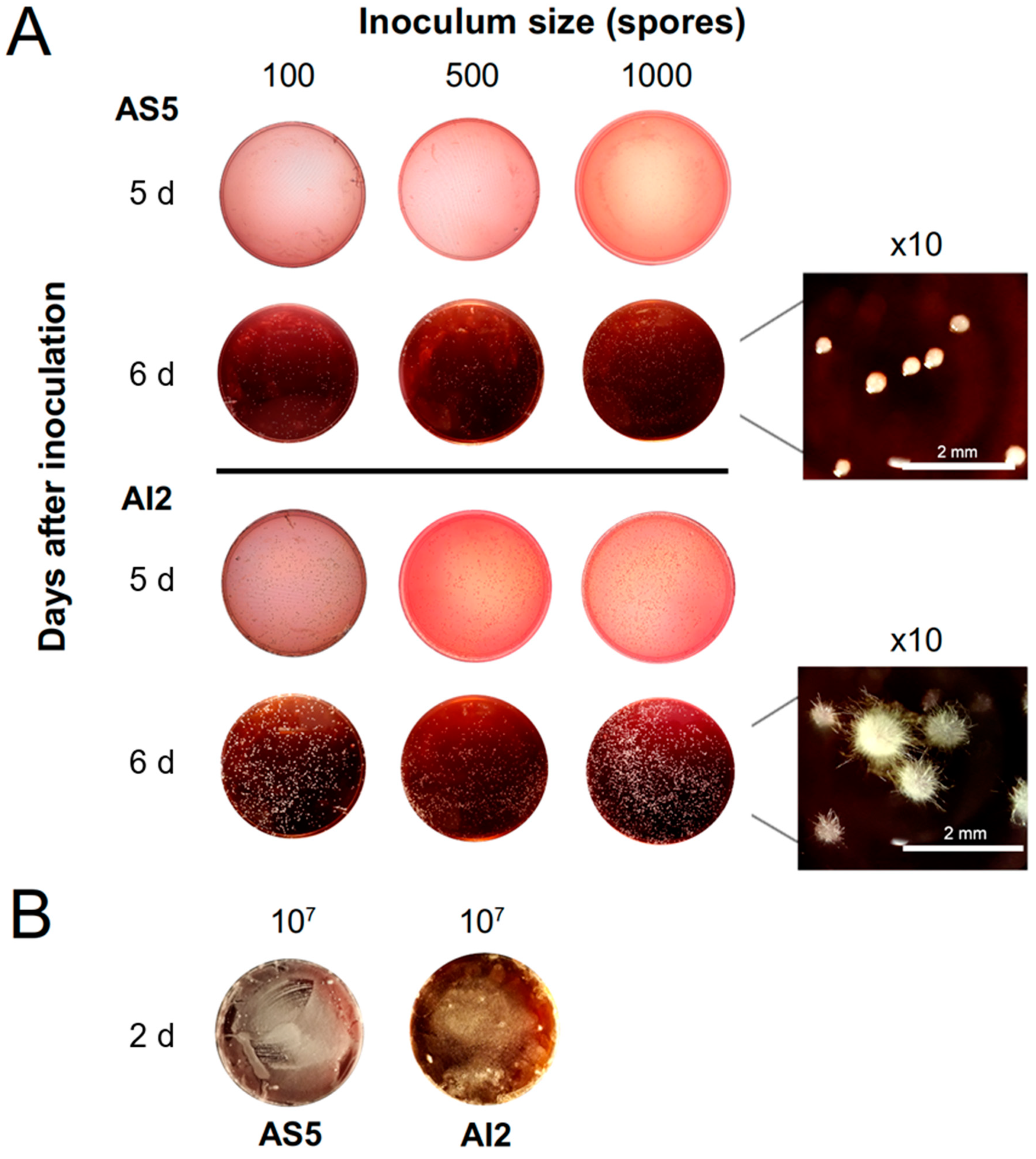
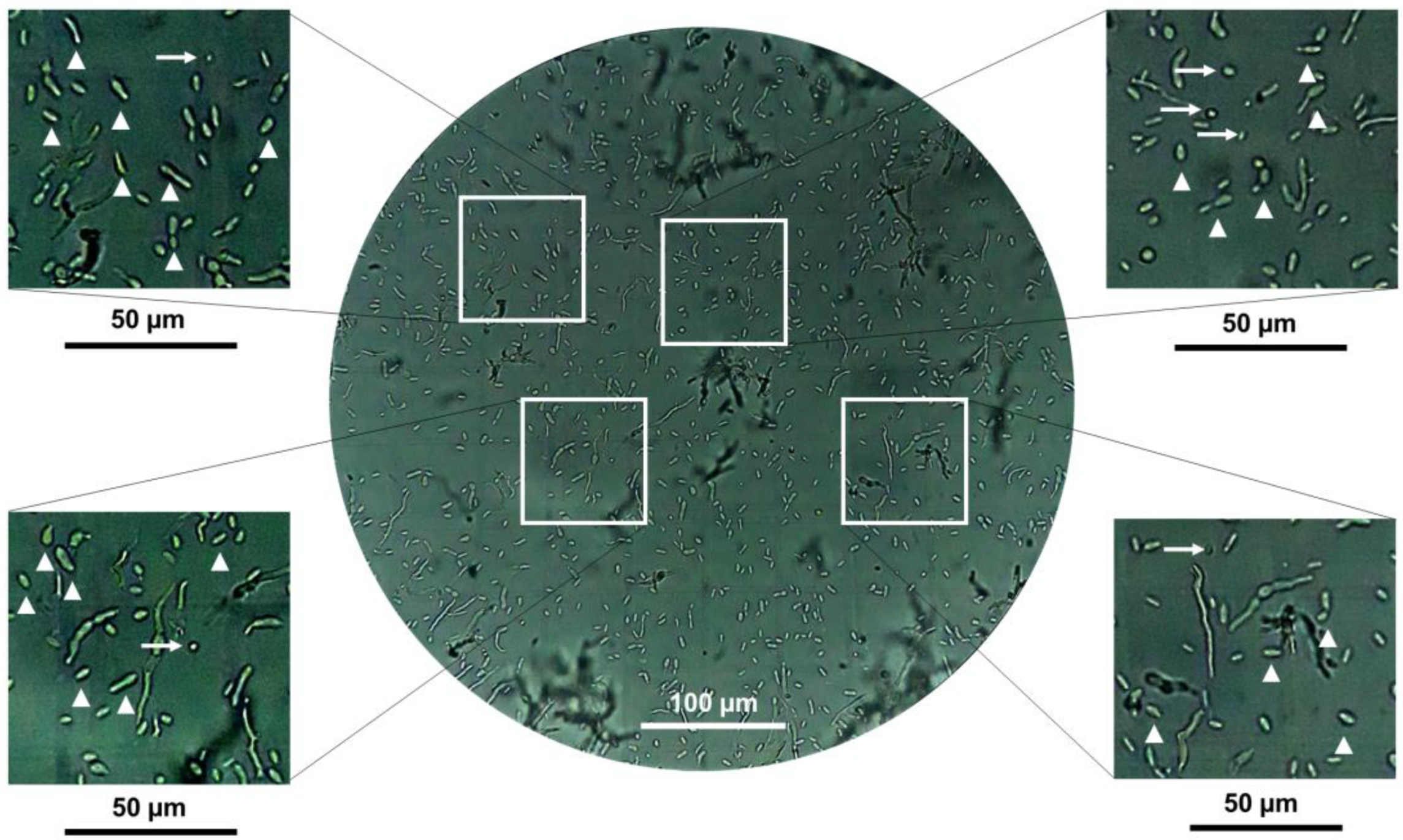
3.2. Effect of Growth Conditions on the Culture Morphology of B. bassiana Strains
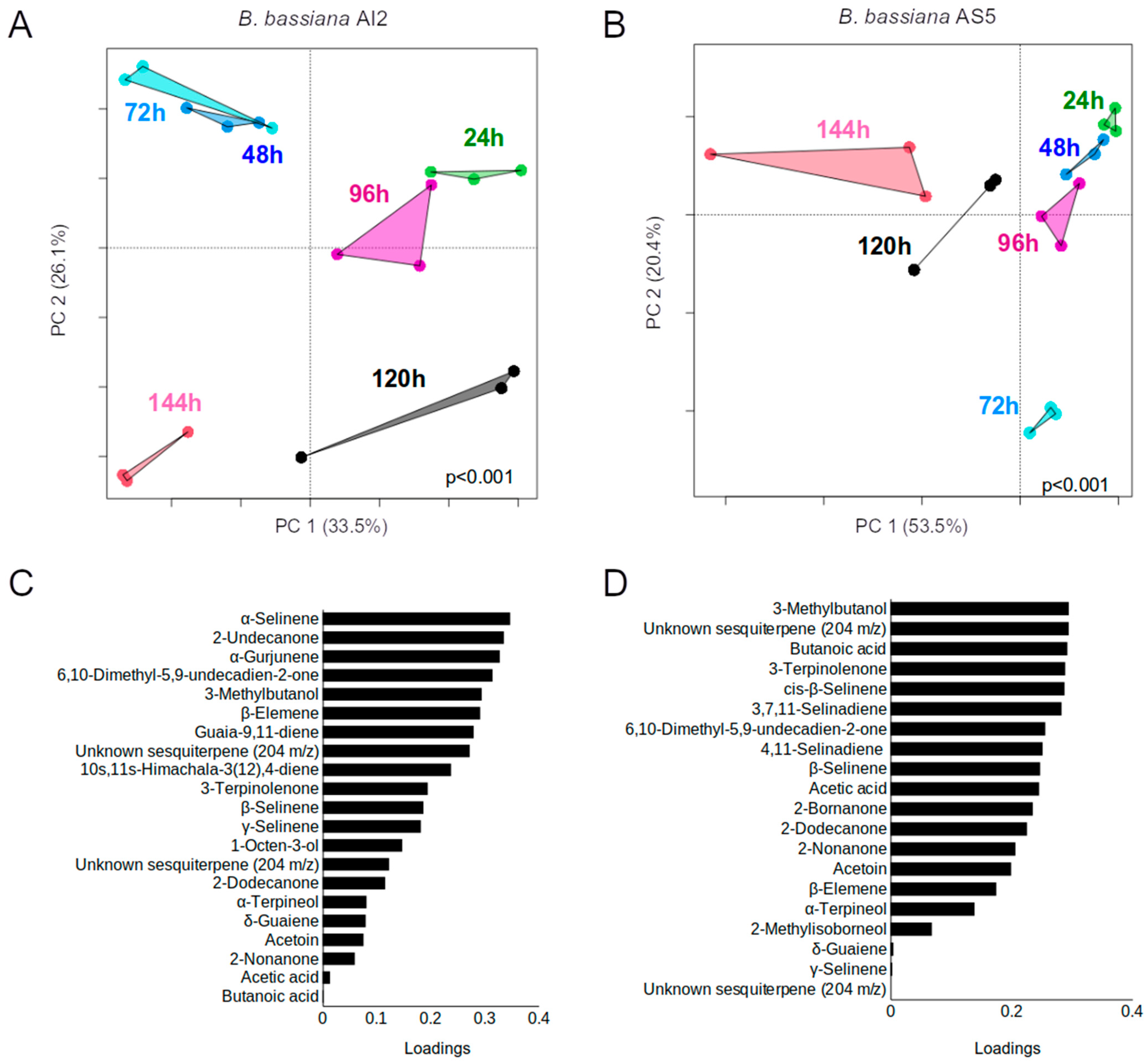
3.3. Identification of Volatile Compounds during Yeast-to-Mycelial Phases in B. bassiana
3.4. The Role of 3-Methylbutanol in the Dimorphism of B. bassiana
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naruzawa, E.S.; Bernier, L. Control of yeast-mycelium dimorphism in vitro in Dutch elm disease fungi by manipulation of external stimuli. Fungal Biol. 2014, 118, 872–884. [Google Scholar] [CrossRef]
- Berrocal, A.; Oviedo, C.; Nickerson, K.W.; Navarrete, J. Quorum sensing activity and control of yeast-mycelium dimorphism in Ophiostoma floccosum. Biotechnol. Lett. 2014, 36, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Boyce, K.J.; Andrianopoulos, A. Fungal dimorphism: The switch from hyphae to yeast is a specialized morphogenetic adaptation allowing colonization of a host. FEMS Microbiol. Rev. 2015, 39, 797–811. [Google Scholar] [CrossRef]
- Mascarin, G.M.; Jackson, M.A.; Kobori, N.N.; Behle, R.W.; Dunlap, C.A.; Delalibera, I. Glucose concentration alters dissolved oxygen levels in liquid cultures of Beauveria bassiana and affects formation and bioefficacy of blastospores. Appl. Microbiol. Biotechnol. 2015, 99, 6653–6665. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, G.M. Dimorphism in fungal pathogens of mammals, plants, and insects. PLoS Pathog. 2015, 11, e1004608. [Google Scholar] [CrossRef]
- Rappleye, C.A.; Goldman, W. Defining virulence genes in the dimorphic fungi. Annu. Rev. Microbiol. 2006, 60, 281–303. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, G.M. Fungal dimorphism and virulence: Molecular mechanisms for temperature adaptation, immune evasion, and in vivo survival. Mediat. Inflamm. 2017, 2017, 8491383. [Google Scholar] [CrossRef]
- Pedrini, N. The entomopathogenic fungus Beauveria bassiana shows its toxic side within insects: Expression of genes encoding secondary metabolites during pathogenesis. J. Fungi 2022, 8, 488. [Google Scholar] [CrossRef]
- Berrocal, A.; Navarrete, J.; Oviedo, C.; Nickerson, K.W. Quorum sensing activity in Ophiostoma ulmi: Effects of fusel oils and branched chain amino acids on yeast-mycelial dimorphism. J. Appl. Microbiol. 2012, 113, 126–134. [Google Scholar] [CrossRef]
- Mendoza-Mendoza, A.; Berndt, P.; Djamei, A.; Weise, C.; Linne, U.; Marahiel, M.; Vraneš, M.; Kämper, J.; Kahmann, R. Physical-chemical plant-derived signals induce differentiation in Ustilago maydis. Mol. Microbiol. 2009, 71, 895–911. [Google Scholar] [CrossRef]
- Lorenz, M.C.; Cutler, N.S.; Heitman, J. Characterization of alcohol-induced filamentous growth in Saccharomyces cerevisiae. Mol. Biol. Cell 2000, 11, 183–199. [Google Scholar] [CrossRef] [PubMed]
- Steyaert, J.M.; Weld, R.J.; Mendoza-Mendoza, A.; Stewart, A. Reproduction without sex: Conidiation in the filamentous fungus Trichoderma. Microbiology 2010, 156, 2887–2900. [Google Scholar] [CrossRef] [PubMed]
- Murata, W.; Kinpara, S.; Kitahara, N.; Yamaguchi, Y.; Ogita, A.; Tanaka, T.; Fujita, K. Cytoskeletal impairment during isoamyl alcohol-induced cell elongation in budding yeast. Sci. Rep. 2016, 6, 31127. [Google Scholar] [CrossRef]
- Han, T.; Cannon, R.D.; Villas-Bôas, S.G. The metabolic response of Candida albicans to farnesol under hyphae-inducing conditions. FEMS Yeast Res. 2012, 12, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Sebaa, S.; Boucherit-Otmani, Z.; Courtois, P. Effects of tyrosol and farnesol on Candida albicans biofilm. Mol. Med. Rep. 2019, 19, 3201–3209. [Google Scholar] [CrossRef]
- Chauhan, N.M.; Karuppayil, S.M. Dual identities for various alcohols in two different yeasts. Mycology 2021, 12, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.P.; Hughes, D.P. Diversity of entomopathogenic fungi: Which groups conquered the insect body? Adv. Genet. 2016, 94, 1–39. [Google Scholar] [CrossRef]
- Wang, H.; Peng, H.; Li, W.; Cheng, P.; Gong, M. The toxins of Beauveria bassiana and the strategies to improve their virulence to insects. Front. Microbiol. 2021, 12, 705343. [Google Scholar] [CrossRef]
- Wei, Q.Y.; Li, Y.Y.; Xu, C.; Wu, X.Y.; Zhang, Y.R.; Liu, H. Endophytic colonization by Beauveria bassiana increases the resistance of tomatoes against Bemisia tabaci. Arthropod-Plant Interact. 2020, 14, 289–300. [Google Scholar] [CrossRef]
- Gana, L.P.; Etsassala, N.G.E.R.; Nchu, F. Interactive effects of water deficiency and endophytic Beauveria bassiana on plant growth, nutrient uptake, secondary metabolite contents, and antioxidant activity of Allium cepa L. J. Fungi 2022, 8, 874. [Google Scholar] [CrossRef]
- Rangel, D.E.N.; Braga, G.U.L.; Fernandes, É.K.K.; Keyser, C.A.; Hallsworth, J.E.; Roberts, D.W. Stress tolerance and virulence of insect-pathogenic fungi are determined by environmental conditions during conidial formation. Curr. Genet. 2015, 61, 383–404. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Urquiza, A.; Keyhani, N.O. Molecular genetics of Beauveria bassiana infection of insects. Adv. Genet. 2016, 94, 165–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.X.; Mouhoumed, A.Z.; Tong, S.M.; Ying, S.H.; Feng, M.G. BrlA and AbaA govern virulence-required dimorphic switch, conidiation, and pathogenicity in a fungal insect pathogen. mSystems 2019, 4, e00140-19. [Google Scholar] [CrossRef]
- Hussain, A.; Tian, M.; He, Y.; Lei, Y. Differential fluctuation in virulence and VOC profiles among different cultures of entomopathogenic fungus. J. Invertebr. Pathol. 2010, 104, 166–171. [Google Scholar] [CrossRef]
- Mburu, D.M.; Maniania, N.K.; Hassanali, A. Comparison of volatile blends and nucleotide sequences of two Beauveria bassiana isolates of different virulence and repellency towards the termite Macrotermes michealseni. J. Chem. Ecol. 2013, 39, 101–108. [Google Scholar] [CrossRef]
- Cai, Q.; Wang, J.; Xie, J.; Jiang, D.; Keyhani, N.M. The Spt10 GNAT superfamily protein modulates development, cell cycle progression, and virulence in the fungal insect pathogen, Beauveria bassiana. J. Fungi 2021, 7, 905. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, H.; Feng, M.; Ying, S. Sterylacetyl hydrolase 1 (BbSay1) links lipid homeostasis to conidiogenesis and virulence in the entomopathogenic fungus Beauveria bassiana. J. Fungi 2022, 8, 292. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Ordorica, A.; Contreras-Cornejo, H.A.; Orduño-Cruz, N.; Luna-Cruz, A.; Winkler, R.; Macías-Rodríguez, L. Volatiles released by Beauveria bassiana induce oviposition behavior in the fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae). FEMS Microbiol. Ecol. 2022, 98, 1–14. [Google Scholar] [CrossRef]
- Alves, S.B.; Rossi, L.S.; Lopes, R.B.; Tamai, M.A.; Pereira, R.M. Beauveria bassiana yeast phase on agar medium and its pathogenicity against Diatraea saccharalis (Lepidoptera: Crambidae) and Tetranychus urticae (Acari: Tetranychidae). J. Invertebr. Pathol. 2002, 81, 70–77. [Google Scholar] [CrossRef]
- Salcedo-Hernandez, R.; Ruiz-Herrera, J. Isolation and characterization of a mycelial cytochrome aa3-deficient mutant and the role of mitochondria in dimorphism of Mucor rouxii. Exp. Mycol. 1993, 17, 142–154. [Google Scholar] [CrossRef]
- Stiernagle, T. Maintenance of C. elegans. In WormBook; The C. elegans Research Community, Ed.; WormBook Research Community: Pasadena, CA, USA, 2006; pp. 1–11. [Google Scholar] [CrossRef]
- El-Sayed, A.M. The Pherobase: Database of Pheromones and Semiochemicals. Available online: https://www.pherobase.com (accessed on 22 June 2023).
- De Bekker, C.; Smith, P.B.; Patterson, A.D.; Hughes, D.P. Metabolomics reveals the heterogeneous secretome of two entomopathogenic fungi to ex vivo cultured insect tissues. PLoS ONE 2013, 8, e70609. [Google Scholar] [CrossRef]
- Santi, L.; Coutinho-Rodrigues, C.J.B.; Berger, M.; Klein, L.A.S.; De Souza, E.M.; Rosa, R.L.; Guimarães, J.A.; Yates III, J.R.; Perinotto, W.M.S.; Bittencourt, V.R.E.P.; et al. Secretomic analysis of Beauveria bassiana related to cattle tick, Rhipicephalus microplus, infection. Folia Microbiol. 2019, 64, 361–372. [Google Scholar] [CrossRef]
- Sánchez-Gómez, T.; Harte, S.J.; Zamora, P.; Bareyre, M.; Díez, J.J.; Herrero, B.; Niño-Sánchez, J.; Martín-García, J. Nematicidal effect of Beauveria species and the mycotoxin beauvericin against pinewood nematode Bursaphelenchus xylophilus. Front. For. Glob. Chang. 2023, 6, 1229456. [Google Scholar] [CrossRef]
- Hu, R.; Bai, P.; Liu, B.; Yu, J. On the virulence of two Beauveria bassiana strains against the fall webworm, Hyphantria cunea (Durry) (Lepidoptera: Erebidae), larvae and their biological properties in relation to different abiotic factors. Egypt. J. Biol. Pest Control. 2021, 31, 107. [Google Scholar] [CrossRef]
- Mascarin, G.M.; Iwanicki, N.S.A.; Ramirez, J.L.; Delalibera, I.; Dunlap, C.A. Transcriptional responses of Beauveria bassiana blastospores cultured under varying glucose concentrations. Front. Cell. Infect. Microbiol. 2021, 11, 644372. [Google Scholar] [CrossRef] [PubMed]
- Padmavathi, J.; Uma Devi, K.; Uma Maheswara Rao, C. The optimum and tolerance pH range is correlated to colonial morphology in isolates of the entomophatogenic fungus Beauveria bassiana-a potential biopesticide. World J. Microbiol. Biothecnol. 2003, 19, 469–477. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Yao, J.; Wang, B.; Huang, B.; Li, Z.; Fan, M.; Sun, J. Evaluation of Beauveria bassiana (Hyphomycetes) isolates as potential agents for control of Dendroctonus valens. Insect Sci. 2011, 18, 209–216. [Google Scholar] [CrossRef]
- Valero-Jiménez, C.A.; Debets, A.J.M.; van Kan, J.A.; Schoustra, S.E.; Takken, W.; Zwaan, B.J.; Koenraddt, C.J.M. Natural variation in virulence of the entomophatogenic fungus Beauveria bassiana against malaria mosquitoes. Malar. J. 2014, 13, 479. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Zhang, T.; Liu, P.; Bai, Y.; Chen, Q.; Zhang, Y.; Keyhani, N.O. The Beauveria bassiana Gas3 β-glucanosyltransferase contributes to fungal adaptation to extreme alkaline conditions. Appl. Environ. Microbiol. 2018, 84, e01086-18. [Google Scholar] [CrossRef]
- Lagomarsino Oneto, D.; Golan, J.; Mazzino, A.; Pringle, A.; Seminara, A. Timing of fungal spore release dictates survival during atmospheric transport. Proc. Natl. Acad. Sci. USA 2020, 117, 5134–5143. [Google Scholar] [CrossRef]
- Talaei-Hassanloui, R.; Kharazi-Pakdel, A.; Goettel, M.; Mozaffari, J. Variation in virulence of Beauveria bassiana isolates and its relatedness to some morphological characteristics. Biocontrol Sci. Technol. 2006, 16, 525–534. [Google Scholar] [CrossRef]
- Romón, P.; Hatting, H.; Goldarazena, A.; Iturrondobeitia, J.C. Variation in virulence of Beauveria bassiana and B. pseudobassiana to the pine weevil Pissodes nemorensis in relation to mycelium characteristics and virulence genes. Fungal Biol. 2017, 121, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Nickerson, K.W.; Atkin, A.L.; Hornby, J.M. Quorum sensing in dimorphic fungi: Farnesol and beyond. Appl. Environ. Microbiol. 2006, 72, 3805–3813. [Google Scholar] [CrossRef]
- Boucias, D.; Liu, S.; Meagher, R.; Baniszewski, J. Fungal dimorphism in the entomopathogenic fungus Metarhizium rileyi: Detection of an in vivo quorum-sensing system. J. Invertebr. Pathol. 2016, 136, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Cao, L.; Qiu, X.; Han, R. Quorum sensing activity and hyphal growth by external stimuli in the entomophatogenic fungus Ophiocordyceps sinensis. Insects 2020, 11, 205. [Google Scholar] [CrossRef]
- Nemčovič, M.; Jakubíková, L.; Víden, I.; Farkaš, V. Induction of conidiation by endogenous volatile compounds in Trichoderma spp. FEMS Microbiol. Lett. 2008, 284, 231–236. [Google Scholar] [CrossRef]
- Xu, P.; Zhu, F.; Buss, G.K.; Leal, W.S. 1-Octen-3-ol-the attractant that repels [version 1; referees:4 approved]. F1000Research 2015, 4, 156. [Google Scholar] [CrossRef]
- Cui, K.; Zhang, L.; He, L.; Zhang, Z.; Zhang, T.; Mu, W.; Lin, J.; Liu, F. Toxicological effects of the fungal volatile compound 1-octen-3-ol against the red flour beetle, Tribolium castaneum (Herbst). Ecotoxicol. Environ. Saf. 2021, 208, 111597. [Google Scholar] [CrossRef]
- Hung, R.; Lee, S.; Bennet, J.W. The effects of low concentration of the enantiomers of mushroom alcohol (1-octen-3-ol) on Arabidopsis thaliana. Mycology 2014, 5, 73–80. [Google Scholar] [CrossRef]
- Gershenzon, J.; Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef]
- Hornby, J.M.; Jensen, E.C.; Lisec, A.D.; Tasto, J.J.; Jahnke, B.; Shoemaker, R.; Dussault, P.; Nickerson, K.W. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 2001, 67, 2982–2992. [Google Scholar] [CrossRef] [PubMed]
- Rodilla, J.M.; Silva, L.A.; Martinez, N.; Lorenzo, D.; Davyt, D.; Castillo, L.; Giménez, C.; Cabrera, R.; González-Coloma, A.; Zrostlíková, J.; et al. Advances in the identification and agrochemical importance of sesquiterpenoids from Bulnesia sarmientoi essential oil. Ind. Crops Prod. 2011, 33, 497–503. [Google Scholar] [CrossRef]
- Benelli, G.; Govindarajan, M.; AlSalhi, M.S.; Devanesan, S.; Maggi, F. High toxicity of camphene and γ-elemene from Wedelia prostrata essential oil against larvae of Spodoptera litura (Lepidoptera: Noctuidae). Environ. Sci. Pollut. Res. 2018, 25, 10383–10391. [Google Scholar] [CrossRef] [PubMed]
- Murcia-Meseguer, A.; Alves, T.J.S.; Budia, F.; Ortiz, A.; Medina, P. Insecticidal toxicity of thirteen comercial plant essential oils against Spodoptera exigua (Lepidoptera:Noctuidae). Phytoparasitica 2018, 46, 233–245. [Google Scholar] [CrossRef]
- Liu, J.; Hua, J.; Qu, B.; Guo, X.; Wang, Y.; Shao, M.; Luo, S. Insecticidal terpenes from the essential oils of Artemisia nakaii and their inhibitory effects on acetylcholinesterase. Front. Plant Sci. 2021, 12, 720816. [Google Scholar] [CrossRef]
- Bittas, V.; MacCartney, N.; Li, N.; Demers, J.; Kim, J.; Kim, H.; Brown, K.; Kang, S. Fusarium oxysporum volatiles enhance plant growth via affecting auxin transport and signaling. Front. Microbiol. 2015, 6, 1248. [Google Scholar] [CrossRef]
- Lee, S.; Behringer, G.; Hung, R.; Bennett, J. Effects on fungal volatile organic compounds on Arabidopsis thaliana growth and gene expression. Fungal Ecol. 2019, 37, 1–9. [Google Scholar] [CrossRef]
- Hauser, M.; Horn, P.; Tournu, H.; Hauser, N.C.; Hoheisel, J.D.; Alistair, J.P.; Brown, A.J.P.; Dickinson, J.R. A transcriptome analysis of isoamyl alcohol-induced filamentation in yeast reveals a novel role forGre2p as isovaleraldehyde reductase. FEMS Yeast Res. 2007, 7, 84–92. [Google Scholar] [CrossRef]
- Stevens, T.M. Free amino acids in the hemolymph of the American cockroach, Periplaneta americana L. Comp. Biochem. Physiol. 1961, 3, 304–309. [Google Scholar] [CrossRef]
- Wyatt, G.R.; Loughheed, T.C.; Wyatt, S.S. The chemistry of insect hemolymph. J. Gen. Physiol. 1956, 39, 853–868. [Google Scholar] [CrossRef]

| Duration of Fungal Growth (h) | |||||||
|---|---|---|---|---|---|---|---|
| Compounds | Ix | 24 | 48 | 72 | 96 | 120 | 144 |
| 3-Methylbutanol | 1186 | 70.57 ± 9.89 ab | 91.56 ± 0.91 a | 62.52 ± 4.01 b | 61.03 ± 6.29 b | 59.42 ± 5.66 b | 78.99 ± 0.21 ab |
| 1-Octen-3-ol | 1451 | 2.97 ± 1.03 ns | 0.91 ± 0.18 ns | nd | nd | nd | nd |
| Total alcohols | 73.55 (8.86) ab | 92.47 (0.72) a | 62.52 (4.01) b | 61.03 (6.29) b | 59.42 (5.66) b | 78.99 (0.21) ab | |
| Acetoin | 1285 | nd | 0.65 ± 0.04 b | 0.32 ± 0.02 b | nd | 0.35 ± 0.01 b | 0.86 ± 0.03 ab |
| 2-Nonanone | 1389 | 9.31 ± 5.38 bc | 0.90 ± 0.11 c | 0.61 ± 0.06 c | 4.59 ± 0.83 bc | 6.01 ± 0.27 bc | 4.99 ± 0.29 bc |
| 2-Undecanone | 1557 | nd | nd | nd | nd | 0.53 ± 0.26 | nd |
| 2-Dodecanone | 1599 | nd | nd | nd | 1.60 ± 1.05 c | 14.04 ± 1.23 ab | 6.99 ± 0.95 bc |
| 6,10-Dimethyl-5,9-undecadien-2-one | 1850 | nd | nd | nd | nd | 0.18 ± 0.03 ns | nd |
| Total ketones | 9.30 (5.38) de | 1.55 (0.07) de | 0.94 (0.091) e | 6.19 (0.22) de | 21.13 (1.20) bc | 12.85 (0.71) cd | |
| Acetic acid | 1456 | 8.68 ± 1.55 a | 1.50 ± 0.34 b | 0.88 ± 0.19 b | nd | nd | nd |
| Butanoic acid | 1633 | 3.79 ± 0.62 ns | 1.01 ± 0.20 ns | 0.43 ± 0.07 ns | nd | nd | nd |
| Total acids | 12.47 (1.89) a | 2.52 (0.36) b | 1.31 (0.25) b | nd | nd | nd | |
| β-Elemene | 1508 | nd | nd | nd | 0.92 ± 0.08 ns | 4.04 ± 3.14 ns | nd |
| Unknown sesquiterpene (m.w. 204) | 1575 | nd | nd | 0.88 ± 0.10 b | 0.71 ± 0.12 b | 0.45 ± 0.08 b | nd |
| Unknown sesquiterpene (m.w. 204) | 1586 | nd | 1.63 ± 0.29 e | 13.88 ± 2.12 bc | 13.03 ± 2.42 bc | 5.00 ± 0.39 cde | 3.54 ± 0.24 de |
| α-Selinene | 1673 | nd | nd | nd | 0.78 ± 0.27 ns | 1.45 ± 1.17 ns | nd |
| Guaia-9,11-diene | 1678 | nd | nd | 1.24 ± 0.20 ns | 0.39 ± 0.01 ns | 1.68 ± 1.46 ns | nd |
| α-Terpineol | 1697 | 4.67 ± 2.26 ab | 1.17 ± 0.18 b | 0.72 ± 0.15 b | 0.52 ± 0.14 b | 0.31 ± 0.09 b | 0.28 ± 0.01 b |
| 10s,11s-Himachala-3(12),4-diene | 1704 | nd | nd | 0.39 ± 0.04 ns | 0.32 ± 0.06 ns | 0.24 ± 0.06 ns | nd |
| β-Selinene | 1713 | nd | nd | 3.97 ± 0.52 ns | 3.33 ± 0.71 ns | 1.15 ± 0.12 ns | 0.82 ± 0.02 ns |
| γ-Selinene | 1718 | nd | nd | 5.46 ± 0.61 b | 4.46 ± 0.93 b | 1.62 ± 0.29 cd | 1.09 ± 0.07 cd |
| α-Gurjunene | 1733 | nd | nd | nd | nd | 0.46 ± 0.08 | nd |
| δ-Guaiene | 1757 | nd | 0.62 ± 0.02 d | 7.33 ± 1.00 b | 7.42 ± 1.51 b | 2.20 ± 0.19 cd | 1.48 ± 0.14 d |
| 3-Terpinolenone | 1923 | nd | nd | 1.29 ± 0.36 ns | 0.82 ± 0.05 ns | 0.78 ± 0.12 ns | 0.90 ± 0.08 ns |
| Total terpenes | 4.67 (2.26) d | 3.43 (0.45) d | 35.21 (4.20) b | 32.76 (6.30) b | 19.44 (5.66) bcd | 8.14 (0.51) d | |
| Duration of Fungal Growth (h) | |||||||
|---|---|---|---|---|---|---|---|
| Compounds | Ix | 24 | 48 | 72 | 96 | 120 | 144 |
| 3-Methylbutanol | 1186 | 70.41 ± 8.06 ab | 59.86 ± 5.15 b | 16.63 ± 0.22 c | 28.86 ± 3.22 c | 64.96 ± 5.26 b | 79.07 ± 1.75 ab |
| Total alcohols | 70.41 (8.06) ab | 59.86 (5.15) b | 16.63 (0.22) c | 28.86 (3.22) c | 64.96 (5.26) b | 79.07 (1.75) ab | |
| Acetoin | 1285 | 4.26 ± 1.64 a | 2.46 ± 0.88 ab | 0.45 ± 0.18 b | 0.61 ± 0.02 b | 2.64 ± 0.04 ab | 2.50 ± 1.15 ab |
| 2-Nonanone | 1389 | 0.47 ± 0.08 c | 2.64 ± 0.51 bc | 0.41 ± 0.05 c | 20.81 ± 4.44 a | 12.34 ± 0.66 ab | nd |
| 2-Dodecanone | 1599 | nd | nd | nd | 18.54 ± 2.75 a | 9.93 ± 2.70 abc | nd |
| 6,10-Dimethyl-5,9-undecadien-2-one | 1850 | nd | nd | nd | 0.51 ± 0.05 ns | 1.76 ± 1.40 ns | nd |
| Total ketones | 4.74 (1.61) de | 5.10 (1.39) de | 0.86 (0.15) e | 40.49 (3.30) a | 26.68 (3.41) b | 2.50 (1.15) de | |
| Acetic acid | 1456 | 8.04 ± 2.13 a | 5.03 ± 0.71 ab | 0.66 ± 0.23 b | 0.67 ± 0.17 b | 0.39 ± 0.04 b | nd |
| Butanoic acid | 1633 | 5.96 ± 2.96 ns | nd | nd | nd | nd | nd |
| Total acids | 14.00 (3.90) a | 5.03 (0.71) b | 0.66 (0.23) b | 0.67 (0.17) b | 0.39 (0.04) b | nd | |
| (+)-2-Bornanone | 1383 | nd | nd | nd | nd | 0.57 ± 0.26 | nd |
| β-Elemene | 1508 | nd | nd | nd | 1.38 ± 0.37 ns | nd | 2.12 ± 0.83 ns |
| Unknown sesquiterpene (m.w. 204) | 1575 | nd | nd | 2.00 ± 0.20 a | 1.08 ± 0.16 b | nd | nd |
| Unknown sesquiterpene (m.w. 204) | 1586 | nd | 14.17 ± 2.52 b | 43.86 ± 0.51 a | 12.46 ± 3.65 bcd | 3.02 ± 0.31 e | 3.49 ± 1.58 de |
| 2-Methylisoborneol | 1592 | nd | nd | nd | nd | nd | 0.77 ± 0.19 |
| 2-Isopropenyl-4a,8-dimethyl-1,2,3,4,4a,5,6,7-octahydronaphthalene | 1625 | nd | nd | 0.89 ± 0.26 ns | nd | 0.66 ± 0.27 ns | nd |
| α-Terpineol | 1697 | 10.83 ± 5.25 a | 4.24 ± 0.23 ab | 1.24 ± 0.18 b | 1.87 ± 0.72 b | 0.82 ± 0.12 b | 0.66 ± 0.10 b |
| β-Selinene | 1713 | nd | 2.84 ± 0.03 ns | 6.08 ± 0.11 ns | 2.02 ± 0.48 ns | 0.77 ± 0.25 ns | 9.52 ± 5.62 ns |
| γ-Selinene | 1718 | nd | 3.53 ± 0.39 bc | 8.42 ± 0.25 a | 3.33 ± 0.91 bc | 0.88 ± 0.45 cd | 0.74 ± 0.33 d |
| 3,7(11)-Selinadiene | 1726 | nd | nd | 0.47 ± 0.08 ns | 1.14 ± 0.62 ns | nd | nd |
| 2,4-Diisopropenyl-1-methyl-1-vinylcyclohexane | 1752 | nd | nd | 0.69 ± 0.12 b | 3.83 ± 0.47 a | nd | nd |
| δ-Guaiene | 1757 | nd | 5.19 ± 0.86 bc | 18.13 ± 0.26 a | nd | 1.20 ± 0.30 d | 1.09 ± 0.40 d |
| 3-Terpinolenone | 1923 | nd | nd | nd | 2.82 ± 1.72 ns | nd | nd |
| Total terpenes | 10.83 (5.52) cd | 29.99 (3.07) bc | 81.83 (0.57) a | 29.96 (6.17) bc | 7.95 (1.92) d | 18.42 (2.62) bcd | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Ordorica, A.; Patiño-Medina, J.A.; Meza-Carmen, V.; Macías-Rodríguez, L. Volatile Fingerprint Mediates Yeast-to-Mycelial Conversion in Two Strains of Beauveria bassiana Exhibiting Varied Virulence. J. Fungi 2023, 9, 1135. https://doi.org/10.3390/jof9121135
Ramírez-Ordorica A, Patiño-Medina JA, Meza-Carmen V, Macías-Rodríguez L. Volatile Fingerprint Mediates Yeast-to-Mycelial Conversion in Two Strains of Beauveria bassiana Exhibiting Varied Virulence. Journal of Fungi. 2023; 9(12):1135. https://doi.org/10.3390/jof9121135
Chicago/Turabian StyleRamírez-Ordorica, Arturo, José Alberto Patiño-Medina, Víctor Meza-Carmen, and Lourdes Macías-Rodríguez. 2023. "Volatile Fingerprint Mediates Yeast-to-Mycelial Conversion in Two Strains of Beauveria bassiana Exhibiting Varied Virulence" Journal of Fungi 9, no. 12: 1135. https://doi.org/10.3390/jof9121135
APA StyleRamírez-Ordorica, A., Patiño-Medina, J. A., Meza-Carmen, V., & Macías-Rodríguez, L. (2023). Volatile Fingerprint Mediates Yeast-to-Mycelial Conversion in Two Strains of Beauveria bassiana Exhibiting Varied Virulence. Journal of Fungi, 9(12), 1135. https://doi.org/10.3390/jof9121135









