Isolation of Yeast Strains with Higher Proline Uptake and Their Applications to Beer Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains and Culture Media
2.2. Measurement of Residual Proline Content
2.3. Small-Scale Screening of Proline-Utilizing Strains
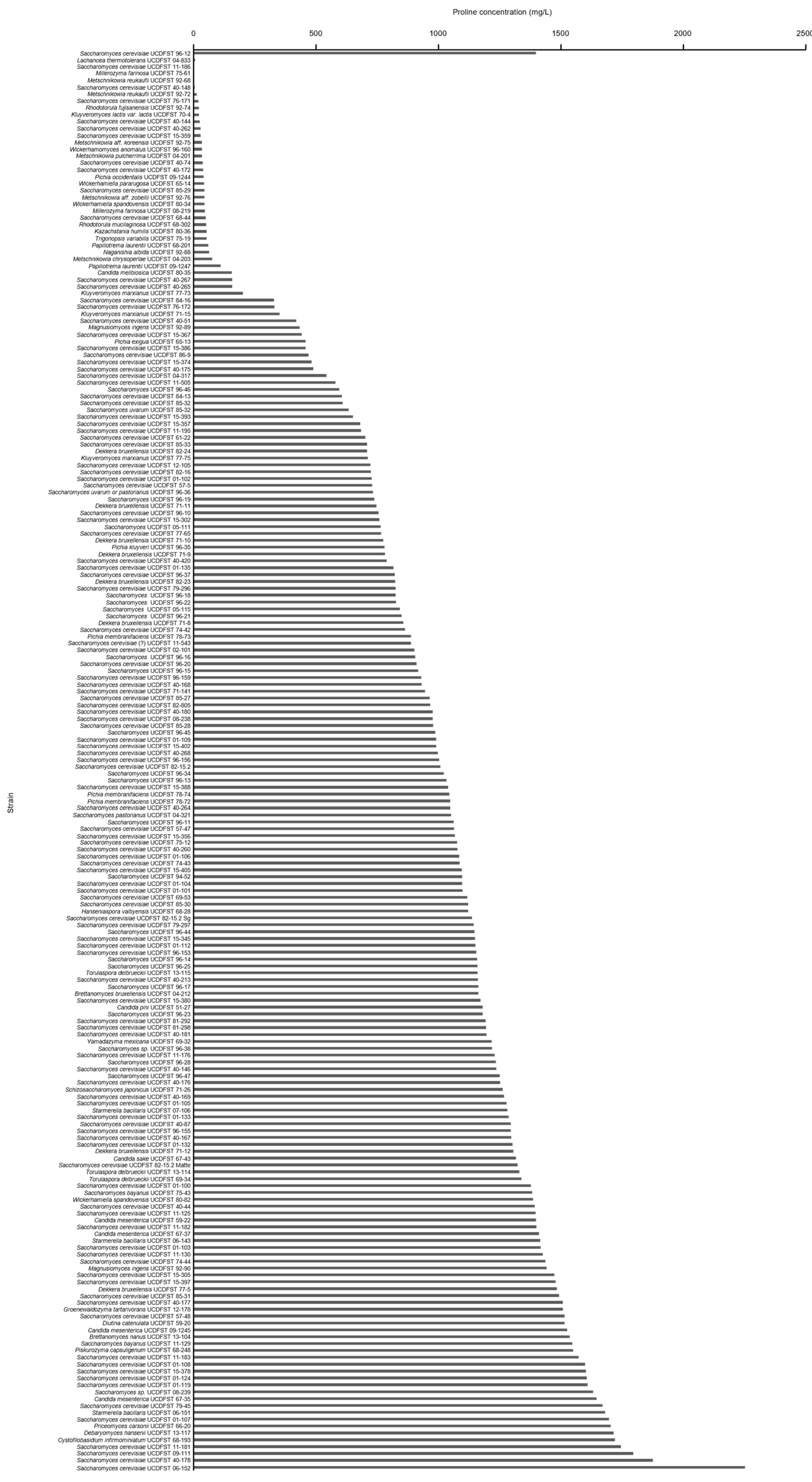
2.4. Large-Scale Screening of Proline-Utilizing Strains
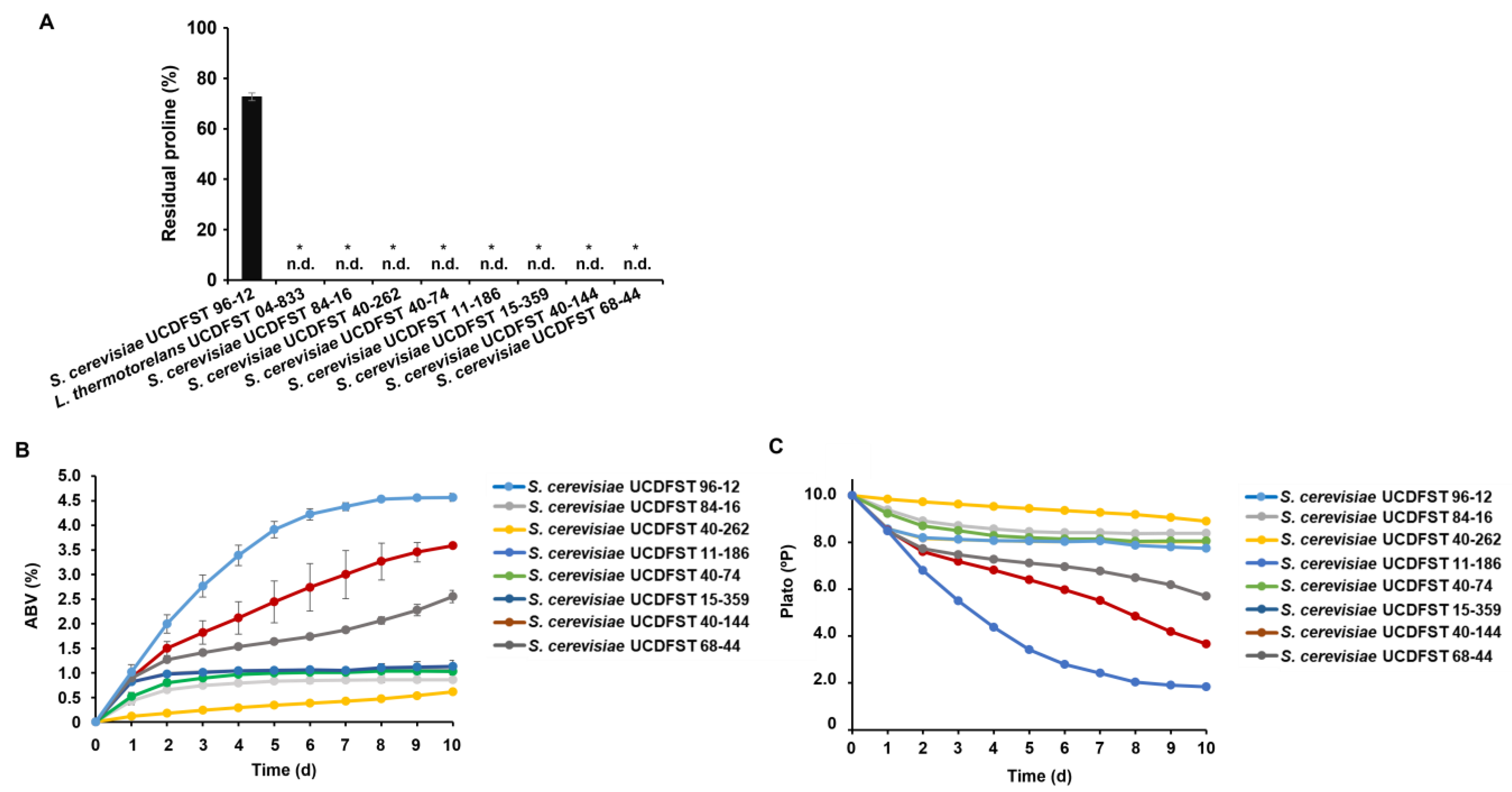
2.5. Fermentation Test
2.6. Statistical Analysis
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Connor-Cox, E.S.C.; Ingledew, W.M. Wort nitrogenous sources—Their use by brewing yeasts: A Review. J. Am. Soc. Brew. Chem. 1989, 47, 102–108. [Google Scholar]
- He, Y.; Dong, J.; Yin, H.; Zhao, Y.; Chen, R.; Wan, X.; Chen, P.; Hou, X.; Liu, J.; Chen, L. Wort composition and its impact on the flavour-active higher alcohol and ester formation of beer—A Review. J. Inst. Brew. 2014, 120, 157–163. [Google Scholar] [CrossRef]
- Procopio, S.; Krause, D.; Hofmann, T.; Becker, T. Significant amino acids in aroma compound profiling during yeast fermentation analyzed by PLS regression. LWT 2013, 51, 423–432. [Google Scholar] [CrossRef]
- Mauricio, J.C.; Valero, E.; Millán, C.; Ortega, J.M. Changes in nitrogen compounds in must and wine during fermentation and biological aging by flor yeasts. J. Agric. Food Chem. 2001, 49, 3310–3315. [Google Scholar] [CrossRef]
- Nishimura, A.; Yoshikawa, Y.; Ichikawa, K.; Takemoto, T.; Tanahashi, R.; Takagi, H. Longevity regulation by proline oxidation in yeast. Microorganisms 2021, 9, 1650. [Google Scholar] [CrossRef] [PubMed]
- Long, D.; Wilkinson, K.L.; Poole, K.; Taylor, D.K.; Warren, T.; Astorga, A.M.; Jiranek, V. Rapid method for proline determination in grape juice and wine. J. Agric. Food Chem. 2012, 60, 4259–4264. [Google Scholar] [CrossRef] [PubMed]
- Ough, C.S. Proline content of grapes and wines. VITIS 1968, 7, 321. [Google Scholar]
- Valero, E.; Millán, C.; Ortega, J.M.; Mauricio, J.C. Concentration of amino acids in wine after the end of fermentation by Saccharomyces cerevisiae strains. J. Sci. Food Agric. 2003, 83, 830–835. [Google Scholar] [CrossRef]
- Wang, J.; Li, W.; Li, Z.; Wu, W.; Tang, X. Analysis and evaluation of the characteristic taste components in portobello mushroom. J. Food Sci. 2018, 83, 1542–1551. [Google Scholar] [CrossRef]
- Zhao, C.J.; Schieber, A.; Gänzle, M.G. Formation of taste-active amino acids, amino acid derivatives and peptides in food fermentations—A Review. Food Res. Int. 2016, 89, 39–47. [Google Scholar] [CrossRef]
- Bamforth, C.W. Beer haze. J. Am. Soc. Brew. Chem. 1999, 57, 81–90. [Google Scholar] [CrossRef]
- Omura, F.; Fujita, A.; Miyajima, K.; Fukui, N. Engineering of yeast Put4 permease and its application to lager yeast for efficient proline assimilation. Biosci. Biotechnol. Biochem. 2005, 69, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Komoto, K.; Okamoto, S.; Hamada, M.; Obana, N.; Samori, M.; Imamura, T. Japanese Consumer perceptions of genetically modified food: Findings from an international comparative study. Interact. J. Med. Res. 2016, 5, e5850. [Google Scholar] [CrossRef] [PubMed]
- Boundy-Mills, K.L.; Glantschnig, E.; Roberts, I.N.; Yurkov, A.; Casaregola, S.; Daniel, H.M.; Groenewald, M.; Turchetti, B. Yeast culture collections in the twenty-first century: New opportunities and challenges. Yeast 2016, 33, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, A.; Tanikawa, T.; Takagi, H. Inhibitory effect of arginine on proline utilization in Saccharomyces cerevisiae. Yeast 2020, 37, 531–540. [Google Scholar] [CrossRef]
- Nishimura, A.; Tanahashi, R.; Takagi, H. The yeast α-arrestin Art3 is a key regulator for arginine-induced endocytosis of the high-affinity proline transporter Put4. Biochem. Biophys. Res. Commun. 2020, 531, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Tanahashi, R.; Nishimura, A.; Nguyen, M.; Sitepu, I.; Fox, G.; Boundy-Mills, K.; Takagi, H. Large-scale screening of yeast strains that can utilize proline. Biosci. Biotechnol. Biochem. 2023, 87, 358–362. [Google Scholar] [CrossRef]
- Bamforth, C.W. Scientific Principles of Malting and Brewing; American Society of Brewing Chemists: St. Paul, MN, USA, 2006. [Google Scholar]
- Boctor, F.N. An improved method for colorimetric determination of proline with isatin. Anal. Biochem. 1971, 43, 66–70. [Google Scholar] [CrossRef]
- Postigo, V.; García, M.; Cabellos, J.M.; Arroyo, T. Wine Saccharomyces yeasts for beer fermentation. Fermentation 2021, 7, 290. [Google Scholar] [CrossRef]
- Nishimura, A.; Ichikawa, K.; Nakazawa, H.; Tanahashi, R.; Morita, F.; Sitepu, I.; Boundy-Mills, K.; Fox, G.; Takagi, H. The Cdc25/Ras/cAMP-dependent protein kinase A signaling pathway regulates proline utilization in wine yeast Saccharomyces cerevisiae under a wine fermentation model. Biosci. Biotechnol. Biochem. 2022, 86, 1318–1326. [Google Scholar] [CrossRef]
- Tanahashi, R.; Nishimura, A.; Morita, F.; Nakazawa, H.; Taniguchi, A.; Ichikawa, K.; Nakagami, K.; Boundy-Mills, K.; Takagi, H. The arginine transporter Can1 acts as a transceptor for regulation of proline utilization in the yeast Saccharomyces cerevisiae. Yeast 2023, 40, 333–348. [Google Scholar] [PubMed]
- Johansson, L.; Nikulin, J.; Juvonen, R.; Krogerus, K.; Magalhães, F.; Mikkelson, A.; Nuppunen-Puputti, M.; Sohlberg, E.; de Francesco, G.; Perretti, G.; et al. Sourdough cultures as reservoirs of maltose-negative yeasts for low-alcohol beer brewing. Food Microbiol. 2021, 94, 103629. [Google Scholar]
- Vicente, J.; Ruiz, J.; Belda, I.; Benito-Vázquez, I.; Marquina, D.; Calderón, F.; Santos, A.; Benito, S. The genus Metschnikowia in enology. Microorganisms 2020, 8, 1038. [Google Scholar] [PubMed]
- Osburn, K.; Amaral, J.; Metcalf, S.R.; Nickens, D.M.; Rogers, C.M.; Sausen, C.; Caputo, R.; Miller, J.; Li, H.; Tennessen, J.M.; et al. Primary souring: A novel bacteria-free method for sour beer production. Food Microbiol. 2018, 70, 76–84. [Google Scholar]
- Mussagy, C.U.; Ribeiro, H.F.; Santos-Ebinuma, V.C.; Schuur, B.; Pereira, J.F.B. Rhodotorula sp.–based biorefinery: A source of valuable biomolecules. Appl. Microbiol. Biotechnol. 2022, 106, 7431–7447. [Google Scholar]
- Du, Y.-K.; Xin, W.; Xia, Y.; Zhu, M.; Qin, J.-L.; Pan, Z.-F.; Wu, R.-F.; Luo, G.-R.; Wu, P.-S.; Wu, Z.-Y.; et al. Analysis of fermentation control factors on volatile compounds of primary microorganisms in jiang-flavor daqu. J. Food Biochem. 2022, 46, e14277. [Google Scholar]
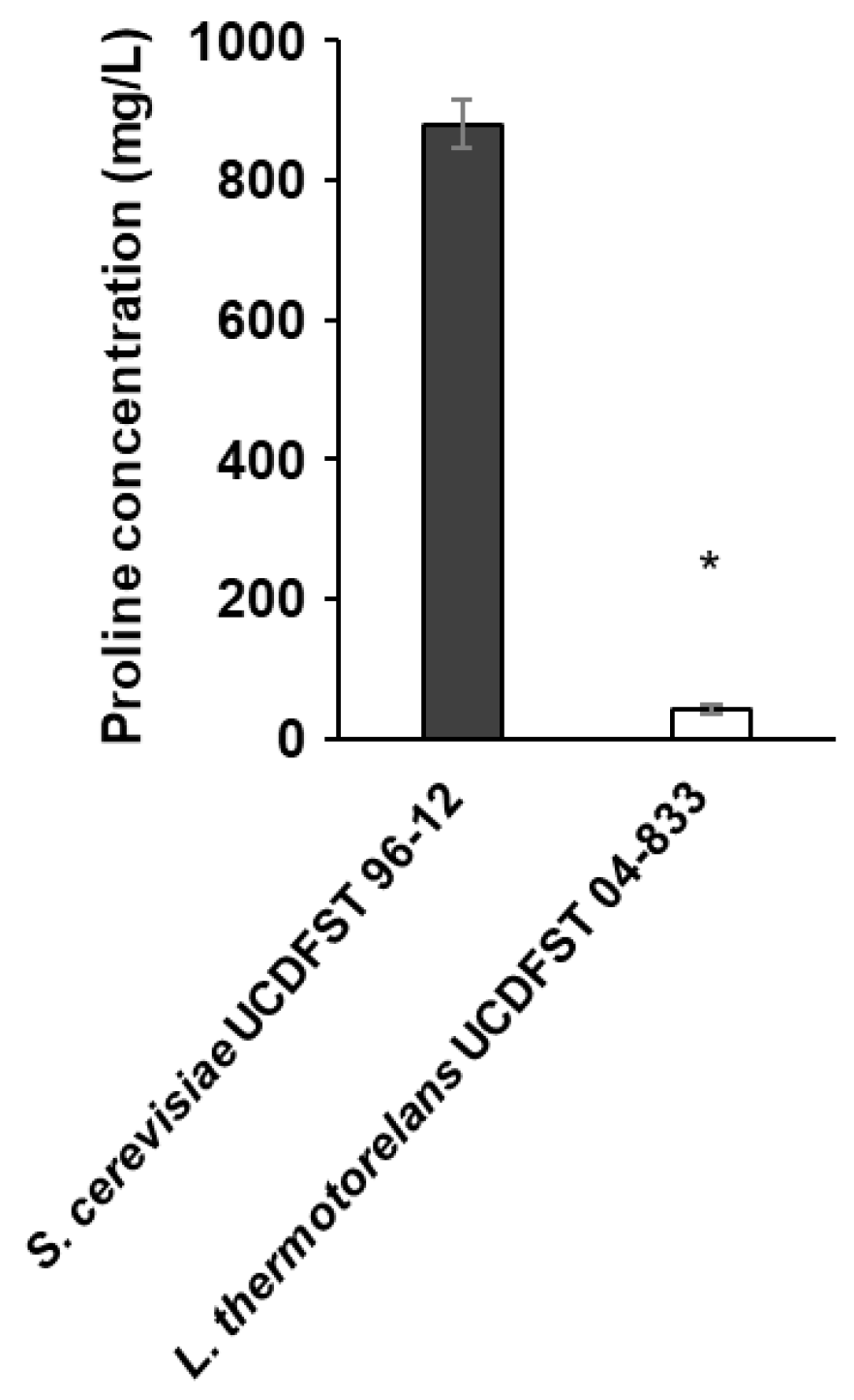

| Strain | Time (h) | Proline Concentration (mg/L) | |
|---|---|---|---|
| by Isatin | by A. A. Analyzer | ||
| S. cerevisiae UCDFST 96-12 | 24 | 990 ± 10.9 | 1066 ± 39.3 |
| 48 | 916 ± 22.9 | 1100 ± 9.2 | |
| 72 | 1003 ± 2.5 | 1162 ± 5.2 | |
| L. thermotorelans UCDFST 04-833 | 24 | 800 ± 18.4 | 807 ± 18.0 |
| 48 | 181 ± 0.0 | 208 ± 14.6 | |
| 72 | 103 ± 54.2 | 110 ± 54.9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanahashi, R.; Nishimura, A.; Nguyen, M.; Sitepu, I.; Fox, G.; Boundy-Mills, K.; Takagi, H. Isolation of Yeast Strains with Higher Proline Uptake and Their Applications to Beer Fermentation. J. Fungi 2023, 9, 1137. https://doi.org/10.3390/jof9121137
Tanahashi R, Nishimura A, Nguyen M, Sitepu I, Fox G, Boundy-Mills K, Takagi H. Isolation of Yeast Strains with Higher Proline Uptake and Their Applications to Beer Fermentation. Journal of Fungi. 2023; 9(12):1137. https://doi.org/10.3390/jof9121137
Chicago/Turabian StyleTanahashi, Ryoya, Akira Nishimura, Minh Nguyen, Irnayuli Sitepu, Glen Fox, Kyria Boundy-Mills, and Hiroshi Takagi. 2023. "Isolation of Yeast Strains with Higher Proline Uptake and Their Applications to Beer Fermentation" Journal of Fungi 9, no. 12: 1137. https://doi.org/10.3390/jof9121137
APA StyleTanahashi, R., Nishimura, A., Nguyen, M., Sitepu, I., Fox, G., Boundy-Mills, K., & Takagi, H. (2023). Isolation of Yeast Strains with Higher Proline Uptake and Their Applications to Beer Fermentation. Journal of Fungi, 9(12), 1137. https://doi.org/10.3390/jof9121137






