Three New Species of Fusicolla (Hypocreales) from China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Morphological Studies
2.2. DNA Extraction, PCR Amplification, Sequencing and Phylogenetic Analyses
3. Results
3.1. Phylogeny
3.2. Taxonomy
- Fusicolla aeria Z.Q. Zeng & W.Y. Zhuang, sp. nov., Figure 2.

- Fusicolla coralloidea Z.Q. Zeng & W.Y. Zhuang, sp. nov., Figure 3.
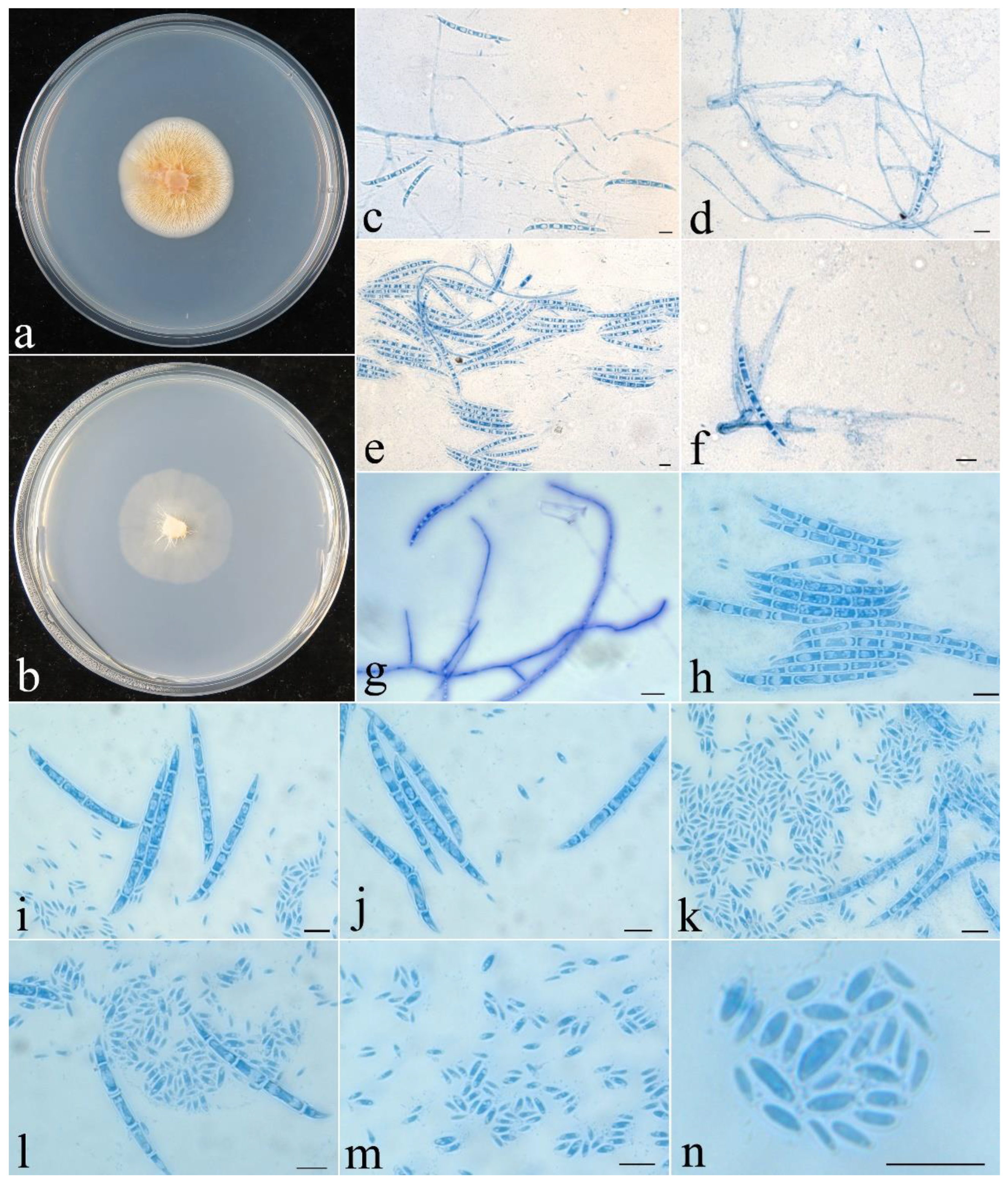
- Fusicolla filiformis Z.Q. Zeng & W.Y. Zhuang, sp. nov., Figure 4.

- Other Fusicolla Species Recorded in China
- Fusicolla aquaeductuum (Radlk. & Rabenh.) Gräfenhan, Seifert & Schroers, in Gräfenhan, Schroers, Nirenberg & Seifert, Stud. Mycol. 68: 100, 2011.
- Fusicolla gigas Chang Liu, Z.Q. Zeng & W.Y. Zhuang, in Crous et al., Fungal Systematics and Evolution 9: 192, 2022.
- Fusicolla guangxiensis Z.Q. Zeng, Chang Liu & W.Y. Zhuang, in Crous et al., Fungal Systematics and Evolution 9: 192, 2022.
- Fusicolla matuoi (Hosoya & Tubaki) Gräfenhan & Seifert, in Gräfenhan, Schroers, Nirenberg & Seifert, Stud. Mycol. 68: 101, 2011.
- Fusicolla violacea Gräfenhan & Seifert, in Gräfenhan, Schroers, Nirenberg & Seifert, Stud. Mycol. 68: 101, 2011.
- Key to the Known Species of Fusicolla in China
| 1. Forming macroconidia and microconidia on PDA | 2 |
| 1. Only forming macroconidia on PDA | 6 |
| 2. Microconidia ellipiosoid, rod-shaped to falcate | 3 |
| 2. Microconidia subcylindrical, curved to C-shaped | 4 |
| 3. Producing pale orange-yellow pigment on PDA | F. coralloidea |
| 3. Producing purple pigment on PDA | F. violacea |
| 4. Aerial mycelium abundant on PDA | F. aeria |
| 4. Aerial mycelium absent to spare on PDA | 5 |
| 5. Colony on PDA light yellow to deep orange | F. matuoi |
| 5. Colony on PDA pinkish orange | F. gigas |
| 6. Macroconidia filiform | F. filiformis |
| 6. Macroconidia falcate | 7 |
| 7. Producing orange-yellow pigment on PDA | F. guangxiensis |
| 7. Producing pink pigment on PDA | F. aquaeductuum |
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonorden, H.F. Handbuch der Allgemeinen Mykologie; Schweizerbart: Stuttgart, Germany, 1851; pp. 1–336. [Google Scholar]
- Gräfenhan, T.; Schroers, H.J.; Nirenberg, H.I.; Seifert, K.A. An overview of the taxonomy, phylogeny, and typification of nectriaceous fungi in Cosmospora, Acremonium, Fusarium, Stilbella, and Volutella. Stud. Mycol. 2011, 68, 79–113. [Google Scholar] [CrossRef] [PubMed]
- Lombard, L.; Van der Merwe, N.A.; Groenewald, J.Z.; Crous, P.W. Generic concepts in Nectriaceae. Stud. Mycol. 2015, 80, 189–245. [Google Scholar] [CrossRef] [PubMed]
- Lechat, C.; Rossman, A.R. A new species of Fusicolla (Hypocreales), F. ossicola, from Belgium. Ascomycete.org 2017, 9, 225–228. [Google Scholar]
- Jones, E.B.G.; Devadatha, B.; Abdel-Wahab, M.A.; Dayarathne, M.C.; Zhang, S.N.; Hyde, K.D.; Liu, J.K.; Bahkali, A.H.; Sarma, V.V.; Tibell, S.; et al. Phylogeny of new marine Dothideomycetes and Sordariomycetes from mangroves and deep-sea sediments. Bot. Mar. 2019, 63, 155–181. [Google Scholar] [CrossRef]
- Dayarathne, M.C.; Jones, E.B.G.; Maharachchikumbura, S.S.N.; Devadatha, B.; Sarma, V.V.; Khongphinitbunjong, K.; Chomnunti, P.; Hyde, K.D. Morphomolecular characterization of microfungi associated with marine based habitats. Mycosphere 2020, 11, 1–188. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Jaeduk, G.; Hye, Y.M. Diversity of fungi in brackish water in Korea. Kor. J. Mycol. 2020, 48, 457–473. [Google Scholar]
- Singh, S.K.; Rana, S.; Bhat, J.D.; Singh, P.N. Morphology and phylogeny of a novel species of Fusicolla (Hypocreales, Nectriaceae), isolated from the air in the Western Ghats, India. J. Fungal Res. 2020, 4, 258–265. [Google Scholar]
- Liu, C.; Zhuang, W.Y.; Yu, Z.H.; Zeng, Z.Q. Two new species of Fusicolla (Hypocreales) from China. Phytotaxa 2022, 536, 165–174. [Google Scholar] [CrossRef]
- Crous, P.W.; Sandoval-Denis, M.; Costa, M.M.; Groenewald, J.Z.; van Iperen, A.L.; Starink-Willemse, M.; Hernández-Restrepo, M.; Kandemir, H.; Ulaszewski, B.; de Boer, W.; et al. Fusarium and allied fusarioid taxa (FUSA). 1. Fungal Syst. Evol. 2022, 9, 161–200. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, T.; Qu, Z.; Li, H.; Yang, Z. Contribution of filamentous fungi to the musty odorant 2,4,6-trichloroanisole in water supply reservoirs and associated drinking water treatment plants. Chemosphere 2017, 182, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.K.; Jeewon, R.; Hyde, K.D.; Bhat, D.; Wen, T.C. Novel taxa within Nectriaceae: Cosmosporella gen. nov. and Aquanectria sp. nov. from freshwater habitats in China. Cryptogam. Mycol. 2018, 39, 169–192. [Google Scholar] [CrossRef]
- Li, W.; Long, Y.; Mo, F.; Shu, R.; Yin, X.; Wu, X.; Zhang, R.; Zhang, Z.; He, L.; Chen, T.; et al. Antifungal activity and biocontrol mechanism of Fusicolla violacea J-1 against soft rot in Kiwifruit caused by Alternaria alternata. J. Fungi 2021, 7, 937. [Google Scholar] [CrossRef] [PubMed]
- Nirenberg, H.I. Studies on the morphologic and biologic differentiation in Fusarium section Liseola. Mitt. Biol. Bundesanst. Land-Forstw. 1976, 169, 1–117. [Google Scholar]
- Wang, L.; Zhuang, W.Y. Designing primer sets for amplification of partial calmodulin genes from penicillia. Mycosystema 2004, 23, 466–473. [Google Scholar]
- Nowrousian, M.; Kück, U.; Loser, K.; Weltring, K.M. The fungal acl1 and acl2 genes encode two polypeptides with homology to the N- and C-terminal parts of the animal ATP citrate lyase polypeptide. Curr. Genet. 2000, 37, 189–193. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfland, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- Rehner, S.A.; Samuels, G.J. Taxonomy and phylogeny of Gliocladium analyzed from nuclear large subunit ribosomal DNA sequences. Mycol. Res. 1994, 98, 625–634. [Google Scholar] [CrossRef]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA Polymerase II Subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- O’Donnell, K.; Cigelnik, E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgin, D.G. The ClustalX windows interface: Flexible strategies for multiple sequences alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Nylander, J.A.A. MrModeltest v2, Program distributed by the author; Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004.
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Chernomor, O.; von Haeseler, A.; Minh, B.Q. Terrace aware data structure for phylogenomic inference from supermatrices. Mol. Syst. Biol. 2016, 65, 997–1008. [Google Scholar] [CrossRef]
- Page, R.D. TreeView: An application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 1996, 12, 357–358. [Google Scholar] [PubMed]
- Hosoya, T.; Tubaki, K. Fusarium matuoi sp. nov. and its teleomorph Cosmospora matuoi sp. nov. Mycoscience 2004, 45, 261–270. [Google Scholar] [CrossRef]
- O’Donnell, K. Fusarium and its near relatives. In The Fungal Holomorph: Mitotic, Meiotic and Pleomorphic Speciation in Fungal Systematic; Reynolds, D.R., Taylor, J.W., Eds.; CAB International: Wallingford, UK, 1993; pp. 224–233. [Google Scholar]
- Rossman, A.Y.; Samuels, G.J.; Rogerson, C.T.; Lowen, R. Genera of Bionectriaceae, Hypocreaceae and Nectriaceae (Hypocreales, Ascomycetes). Stud. Mycol. 1999, 42, 1–248. [Google Scholar]
- Summerbell, R.C.; Schroers, H.J. Analysis of phylogenetic relationship of Cylindrocarpon lichenicola and Acremonium falciforme to the Fusarium solani species complex and a review of similarities in the spectrum of opportunistic infections caused by these fungi. J. Clin. Microbiol. 2002, 40, 2866–2875. [Google Scholar] [CrossRef] [PubMed]
- Schroers, H.J.; Gräfenhan, T.; Nirenberg, H.I.; Seifert, K.A. A revision of Cyanonectria and Geejayessia gen. nov., and related species with Fusarium-like anamorphs. Stud. Mycol. 2011, 68, 115–138. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Lombard, L.; Sandoval-Denis, M.; Seifert, K.A.; Schroers, H.J.; Chaverri, P.; Gené, J.; Guarro, J.; Hirooka, Y.; Bensch, K.; et al. Fusarium: More than a node or a foot-shaped basal cell. Stud. Mycol. 2021, 98, 100116. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, S.; Yanagisawa, M.; Katoh, A.; Fujii, T.; Sano, T.; Matsukuma, S.; Furumai, T.; Fujiu, M.; Watanabe, K.; Yokose, K.; et al. Fusarium merismoides CORDA NR 6356, the source of the protein kinase C inhibitor, azepinostatin. taxonomy, yield improvement, fermentation and biological activity. J. Antibiot. 1994, 47, 639–647. [Google Scholar] [CrossRef] [PubMed]
- De Marchi, R.; Koss, M.; Ziegler, D.; De Respinis, S.; Petrini, O. Fungi in water samples of a full-scale water work. Mycol. Prog. 2018, 17, 467–478. [Google Scholar] [CrossRef]
- You, N.; Xu, J.; Wang, L.; Zhuo, L.; Zhou, J.; Song, Y.; Ali, A.; Luo, Y.; Yang, J.; Yang, W.; et al. Fecal fungi dysbiosis in nonalcoholic fatty liver disease. Obesity 2021, 29, 350–358. [Google Scholar] [CrossRef]
- Zhong, M.Y.; Xiong, Y.B.; Zhao, J.B.; Gao, Z.; Ma, J.S.; Wu, Z.X.; Song, Y.X.; Hong, X.H. Candida albicans disorder is associated with gastric carcinogenesis. Theranostics 2021, 11, 4945–4956. [Google Scholar] [CrossRef]
- Zang, J.; Xu, Y.; Xia, W.; Yu, D.; Gao, P.; Jiang, Q.; Yang, F. Dynamics and diversity of microbial community succession during fermentation of Suan yu, a Chinese traditional fermented fish, determined by high throughput sequencing. Food Res. Int. 2018, 111, 565–573. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Huang, Y.G. Structure and diversity analysis of mold community in main Maotai-flavor baijiu brewing areas of Maotai town using high-throughput sequencing. Food Sci. 2021, 42, 150–156. [Google Scholar]
- Clocchiatti, A.; Hannula, S.E.; van den Berg, M.; Hundscheid, M.P.J.; de Boer, W. Evaluation of phenolic root exudates as stimulants of saptrophic fungi in the rhizosphere. Front. Microbiol. 2021, 12, 644046. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, N.; Duan, B.; Wang, Q.; Wang, Y. Rhizosphere bacterial and fungal communities succession patterns related to growth of poplar fine roots. Sci. Total Environ. 2021, 756, 143839. [Google Scholar] [CrossRef]
- Hoch, H.C.; Abawi, G.S. Mycoparasitism of oospores of Pythium ultimum by Fusarium merismoides. Mycologia 1979, 71, 621–625. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Y.; Zhou, T.; Akkaya, M.S.; Wang, L.; Li, S.; Li, X. Biocontrol of Fusarium wilt disease in strawberries using bioorganic fertilizer fortified with Bacillus licheniformis X-1 and Bacillus methylotrophicus Z-1. 3 Biotech 2020, 10, 80. [Google Scholar] [CrossRef]
- Wang, M.; Xue, J.; Ma, J.; Feng, X.; Ying, H.; Xu, H. Streptomyces lydicus M01 regulates soil microbial community and alleviates foliar disease caused by Alternaria alternata on cucumbers. Front. Microbiol. 2020, 11, 942. [Google Scholar] [CrossRef] [PubMed]
- Forin, N.; Vizzini, A.; Nigris, S.; Ercole, E.; Voyron, S.; Girlanda, M.; Baldan, B. Illuminating type collections of nectriaceous fungi in Saccardo’s fungarium. Persoonia 2020, 45, 221–249. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.H.; Hyde, K.D.; Maharachchikumbura, S.S.N.; Jones, E.B.G.; Mckenzie, E.H.C.; Stadler, M.; Lee, H.B.; Samarakoon, M.C.; Ekanayaka, A.H.; Camporesi, E.; et al. Fungi on wild seeds and fruits. Mycosphere 2020, 11, 2108–2480. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Burgess, T.I.; Hardy, G.E.; Crane, C.; Barrett, S.; Cano-Lira, J.F.; Le Roux, J.J.; Thangavel, R.; Guarro, J.; et al. Fungal Planet description sheets: 469–557. Persoonia 2016, 37, 218–403. [Google Scholar] [CrossRef]
- Crous, P.W.; Luangsa-ard, J.J.; Wingfield, M.J.; Carnegie, A.J.; Hernández-Restrepo, M.; Lombard, L.; Roux, J.; Barreto, R.W.; Baseia, I.G.; Cano-Lira, J.F.; et al. Fungal Planet description sheets: 785–867. Persoonia 2018, 41, 238–417. [Google Scholar] [CrossRef]

| Species | Herbarium/Strain Numbers | GenBank Accession Numbers | ||||
|---|---|---|---|---|---|---|
| acl1 | ITS | LSU | rpb2 | tub2 | ||
| F. acetilerea | BBA 63789 T | HQ897839 | HQ897790 | U88108 | HQ897701 | − |
| F. aeria | CGMCC 3.24908 T | OQ134105 a | OQ128334 a | OQ128338 a | OQ134111 a | OQ134100 a |
| CGMCC 3.24909 | OQ134106 a | OQ128335 a | OQ128339 a | OQ134112 a | OQ134101 a | |
| F. aquaeductuum | CBS 837.85 T | HQ897880 | KM231823 | KM231699 | HQ897744 | − |
| F. betae | BBA 64317 T | HQ897917 | − | − | HQ897781 | − |
| F. bharatavarshae | NFCCI 4423 T | − | MK152510 | MK152511 | MK157022 | MK376462 |
| F. cassiae-fistulae | MFLUCC 19-0318 T | − | NR171299 | NG073862 | − | − |
| F. coralloidea | CGMCC 3.24907 T | OQ134104 a | OQ128333 a | OQ128337 a | OQ134110 a | OQ134099 a |
| F. elongata | CBS 148934 T | ON759286 | ON763203 | ON763200 | ON759297 | ON745628 |
| F. epistroma | BBA 62201 T | HQ897901 | − | AF228352 | HQ897765 | − |
| F. filiformis | CGMCC 3.24910 T | OQ134103 a | OQ128332 a | OQ128336 a | OQ134109 a | OQ134098 a |
| F. gigantispora | MFLU 16-1206 T | − | MN047104 | MN017869 | − | − |
| F. gigas | CGMCC 3.20680 T | OQ134107 a | OK465362 | OK465449 | OQ134113 a | OQ134102 a |
| F. guangxiensis | CGMCC 3.20679 T | OQ134108 a | OK465363 | OK465450 | OQ134114 a | − |
| F. hughesii | NFCCI 4234 T | − | MG779450 | MG779452 | − | − |
| F. matuoi | CBS 581.78 T | HQ897858 | KM231822 | KM231698 | HQ897720 | KM232093 |
| F. melogrammae | CBS 141092 T | − | KX897140 | NG058275 | − | MW834305 |
| F. meniscoidea | CBS 110189 T | MW834043 | MW827613 | MW827654 | MW834010 | MW834306 |
| F. merismoides | CBS 186.34 T | − | MH855482 | MH866963 | − | − |
| F. ossicola | CBS 140161 T | − | NR161034 | MF628021 | MW834011 | MW834307 |
| F. quarantenae | URM 8367 T | − | MW553789 | MW553788 | MW556626 | MW556624 |
| F. septimanifiniscientiae | CBS 144935 T | − | MK069422 | MK069418 | − | MK069408 |
| F. siamensis | MFLUCC 172577 T | − | NR171300 | NG073863 | − | − |
| F. sporellula | CBS 110191 T | MW834044 | MW827614 | MW827655 | MW834012 | MW834308 |
| F. violacea | CBS 634.76 T | KM231059 | KM231824 | KM231700 | HQ897696 | KM232095 |
| Macroconia leptosphaeriae | CBS 100001 | HQ897891 | HQ897810 | KC291787 | HQ728164 | KM232097 |
| Microcera larvarum | CBS 738.79/AR 4580 | KM231060 | KM231825 | KM231701 | KM232387 | KC291935 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Z.-Q.; Zhuang, W.-Y. Three New Species of Fusicolla (Hypocreales) from China. J. Fungi 2023, 9, 572. https://doi.org/10.3390/jof9050572
Zeng Z-Q, Zhuang W-Y. Three New Species of Fusicolla (Hypocreales) from China. Journal of Fungi. 2023; 9(5):572. https://doi.org/10.3390/jof9050572
Chicago/Turabian StyleZeng, Zhao-Qing, and Wen-Ying Zhuang. 2023. "Three New Species of Fusicolla (Hypocreales) from China" Journal of Fungi 9, no. 5: 572. https://doi.org/10.3390/jof9050572
APA StyleZeng, Z.-Q., & Zhuang, W.-Y. (2023). Three New Species of Fusicolla (Hypocreales) from China. Journal of Fungi, 9(5), 572. https://doi.org/10.3390/jof9050572







