Abstract
The South China Botanical Garden (SCBG), one of the largest and oldest botanical gardens in China, conserves important plant germplasms of endangered species. Therefore, ensuring tree health and studying the associated mycobiome of the phyllosphere is essential to maintaining its visual aesthetics. During a survey of plant-associated microfungal species in SCBG, we collected several coelomycetous taxa. Phylogenetic relationships were evaluated based on the analyses of ITS, LSU, RPB2, and β-tubulin loci. The morphological features of the new collections were compared with those of existing species, emphasizing close phylogenetic affinities. Based on the morphological comparisons and multi-locus phylogeny, we introduce three new species. These are Ectophoma phoenicis sp. nov., Remotididymella fici-microcarpae sp. nov., and Stagonosporopsis pedicularis-striatae sp. nov. In addition, we describe a new host record for Allophoma tropica in the Didymellaceae. Detailed descriptions and illustrations are provided along with notes comparing allied species.
1. Introduction
The South China Botanical Garden (SCBG), Chinese Academy of Sciences, Guangzhou was established in 1929 [1]. This is the largest modern botanical garden in Guangzhou, Guangdong Province, and it extends over 2854 acres comprising around 2400 plant species including alpine, arctic, aquatic, Mediterranean, tropical, and desert examples [1]. SCBG is one of the top plant germplasm conservation institutions in China and it contains 710 of the verified Red List plant taxa [2]. There are more than 14,000 trees including Chinese endemic species in ex-situ conservation [3]. Tree health and disease management are rather important in SCBG.
Didymellaceae was established by de Gruyter et al. [4] to accommodate Phoma and other allied genera. Aveskamp et al. [5] delimited the boundaries of the Didymellaceae based on morphology and combined ITS, LSU, SSU, and βtubulin loci analyses. Subsequent revisions of the Didymellaceae resolved generic boundaries and revealed more natural evolutionary relationships [6,7,8,9,10,11]. Currently, the family encompasses more than 5400 species in 44 accepted genera [12,13,14] (Species Fungorum. http://www.speciesfungorum.org/Names/Names.asp, accessed on 14 August 2022). Species of the Didymellaceae are cosmopolitan and distributed in a wide range of hosts and habitats [5,15,16]. Some species are plant pathogens causing leaf and stem lesions and fruit rots [5,7,15,17], while some species such as Allophoma hayatii Babaahm. and Mehr-Koushk., Calophoma petasitis Tibpromma, Camporesi, and K.D. Hyde are saprobes on dead plant parts [18,19,20]. Few species such as Phoma herbarum Westend. and Juxtiphoma eupyrena (Sacc.) Valenz-Lopez, Crous, Stchigel, and Guarro and Cano have been reported as human and animal pathogens [21,22].
In a survey of fungal species associated with plants in botanical gardens, we collected several saprobic, hyaline-spored, and didymellaceae-like coelomycetous taxa from the SCBG. The main objectives of this study are to analyze the taxonomic placement of our didymellaceous collections and then to describe the taxonomic novelties using morphology and the multi-locus phylogeny of ITS, LSU, RPB2, and β-tubulin sequences. Our study revealed that three of our isolates are new to science. Herein, we introduce these isolates as novel species namely Ectophoma phoenicis sp. nov., Remotididymella fici-microcarpae sp. nov., and Stagonosporopsis pedicularis-striatae sp. nov. A new collection of Allophoma tropica (R. Schneid. and Boerema) Qian Chen and L. Cai is described herein as a new host record. Detailed descriptions, illustrations, and notes are provided.
2. Material and Methods
2.1. Sampling Sites, Specimens, and Isolates
Dead plant specimens were collected from the south China Botanical Garden, Guangzhou, Guangdong Province, China (Figure 1), between June to September 2021. Specimens were detached from the host using sterile blades and packed in sterilized paper bags. The collection site is characterized by a tropical climate with abundant sunshine and rainfall throughout the year. The average annual temperature is 22 °C with around 2125 mm of rainfall per year [23].

Figure 1.
Entrance of Southern China Botanical Garden.
The collected dead samples (petioles, sepals, and stems) were brought to the laboratory in sterilized paper bags and examined with a stereomicroscope (Carl Zeiss Discovery V8). Conidia were cultured following the method described by Senanayake et al. [24]. The germinated conidia were aseptically transferred into fresh potato dextrose agar (PDA) plates, incubated at 25 °C in the dark to obtain pure cultures, and later transferred to PDA slants and stored at 4 °C for further study. Colony characters were recorded from PDA cultures. Fungarium specimens are deposited at the Herbarium of Zhongkai University of Agriculture and Engineering (ZHKU), and all the ex-type and living cultures are deposited at the Culture Collection of Zhongkai University of Agriculture and Engineering (ZHKUCC). Index Fungorum numbers (https://www.indexfungorum.org, accessed on 1 June 2022) and Facesoffungi numbers [25] were registered for the new species.
2.2. Morphological Studies
Microscopic mounts of fruiting structures in sterilized tap water were examined and photographed using a stereomicroscope fitted with a camera (ZEISS Axiocam 208). The micromorphological characteristics such as the structure of the conidiomatal wall, conidiogenous cells, and conidia were studied and photographed using a compound microscope (Nikon Eclipse 80i) fitted with a digital camera (Canon 450D). All microscopic measurements were made with the Tarosoft image framework (v. 0.9.0.7).
2.3. DNA Extraction, PCR Amplification, and Sequencing
Fresh mycelia grown on PDA for two weeks at 25 °C in the dark were used for DNA extraction using a fungal genomic DNA extraction kit (Biospin DNA Extraction Kit, Bioer Technology, Co. Ltd., Hangzhou, China) following the manufacturer’s protocols. Polymerase chain reactions (PCR) and sequencing were carried out for the complete ITS region, part of the LSU ribosomal DNA, the RNA polymerase II subunit (RPB2) gene, and the β-tubulin gene. The PCR amplification reactions were carried out with the following protocols in Table 1. The total volume of the PCR reaction was 25 µL containing 1 µL of DNA template, 1 µL of each forward and reverse primer, 12.5 µL of 2 × PCR Master Mix, and 9.5 µL of double-distilled sterilized water (ddH2O). All the PCR thermal cycles include a final hold at 4 °C.

Table 1.
Gene regions, respective primer pairs, and thermal cycler conditions used in this study.
The PCR products were observed on a 1% agarose electrophoresis gel stained with ethidium bromide. Purification and sequencing of PCR products were carried out at Sunbiotech Company, Beijing, China. Sequence quality was checked, and sequences were condensed with DNASTAR Lasergene v. 7.1 [26]. Sequences derived in this study were deposited in GenBank and accession numbers were obtained (Table 2).

Table 2.
Taxa used in the present phylogenetic analyses and GenBank numbers of their sequences.
2.4. Phylogenetic Analyses
BLASTn searches were made using NCBI BLASTn (https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 18 March 2022). The newly generated sequences assist in taxon sampling for phylogenetic analyses. All the ex-type strains of species were included if available, and other authentic strains were selected when sequences from ex-type strains were unavailable. [27,28,29,30,31,32,33] were followed to obtain sequences from GenBank (Table 2). The concatenated ITS, LSU, RPB2, and β-tubulin sequence dataset for the family Didymellaceae comprised 123 strains with the outgroup taxon as Leptosphaeria conoidea (De Not.) Sacc. (CBS 616.75) and Leptosphaeria doliolum (Pers.) Ces. & De Not. (CBS 505.75). DNA sequences of all the single gene regions were aligned using the online version of MAFFT v. 7.0362 [34] with default settings and manually adjusted using BioEdit 7.1.3 [35] when necessary.
Maximum likelihood analysis was performed by RAxML [36] implemented in raxmlGUIv. 1.5 [37] using the ML + rapid bootstrap setting and the GTR + I + G model of nucleotide substitution with 1000 replicates. For the Bayesian inference (BI) analyses, the optimal substitution model for the combined datasets was determined to be GTR + I + G using the MrModeltest software v. 2.2 [38]. The BI analyses were computed in MrBayes v. 3.2.6 [39] with four simultaneous Markov chain Monte Carlo chains from random trees over 5 M generations (trees were sampled every 1000th generation). The distribution of log-likelihood scores was observed to check whether sampling was in the stationary phase and Tracer v1.5 was used to check if further runs were required to reach convergence [40]. The consensus tree and posterior probabilities were calculated after discarding the first 20% of the sampled trees as burn-in. The phylogram was visualized in FigTree v. 1.4 [41].
2.5. PHI Analyses
The PHI test was performed using SplitsTree4 v. 4.17.1 to determine the recombination level within phylogenetically closely related species. The concatenated four-locus dataset (ITS + LSU + RPB2 + β-tubulin) was used for the analyses. PHI test results (Φw) ≥0.05 indicated no significant recombination within the dataset. The relationships between closely related taxa were visualized in split graphs with both the Log-Det transformation and splits decomposition options.
3. Results
3.1. Phylogenetic Analyses
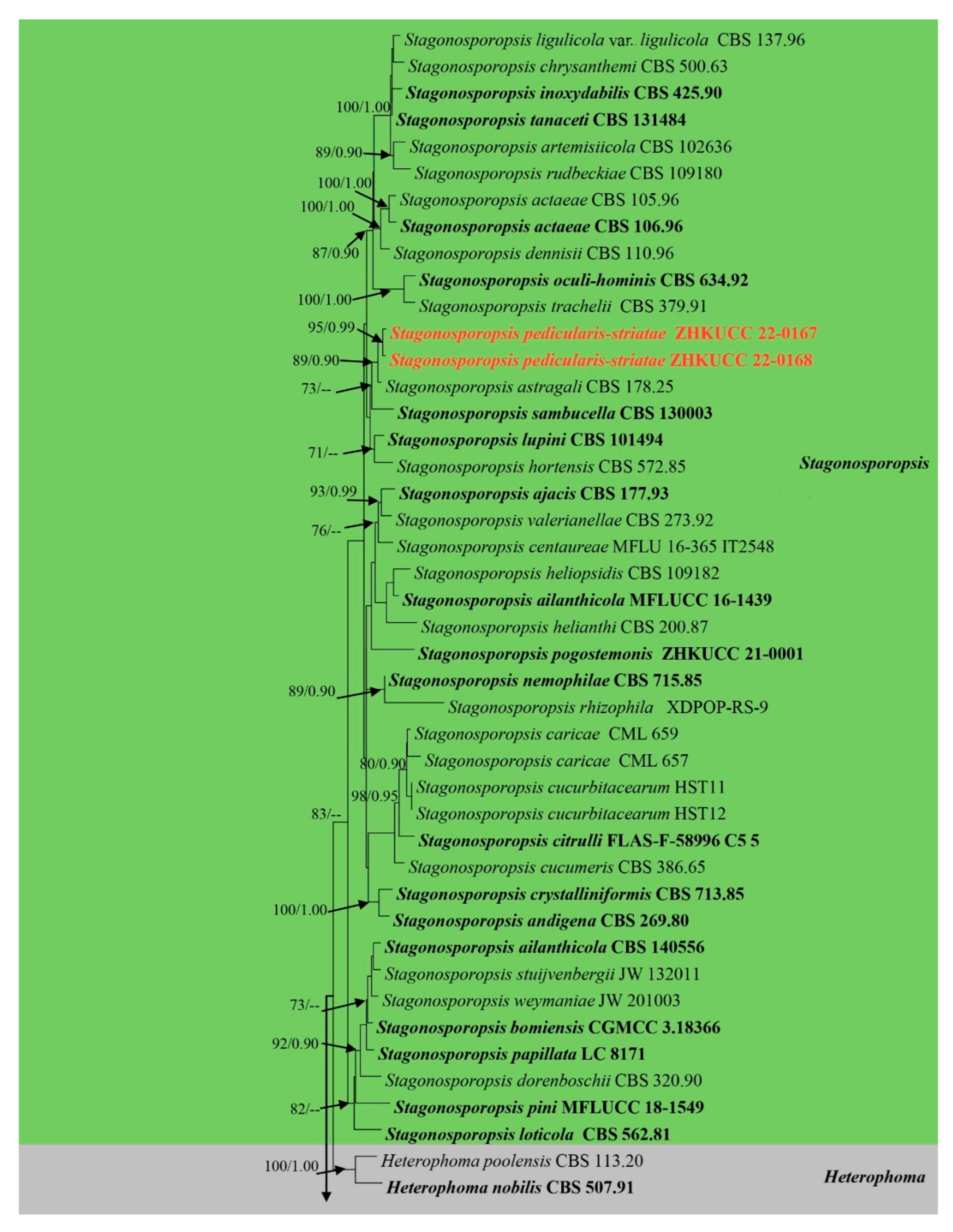
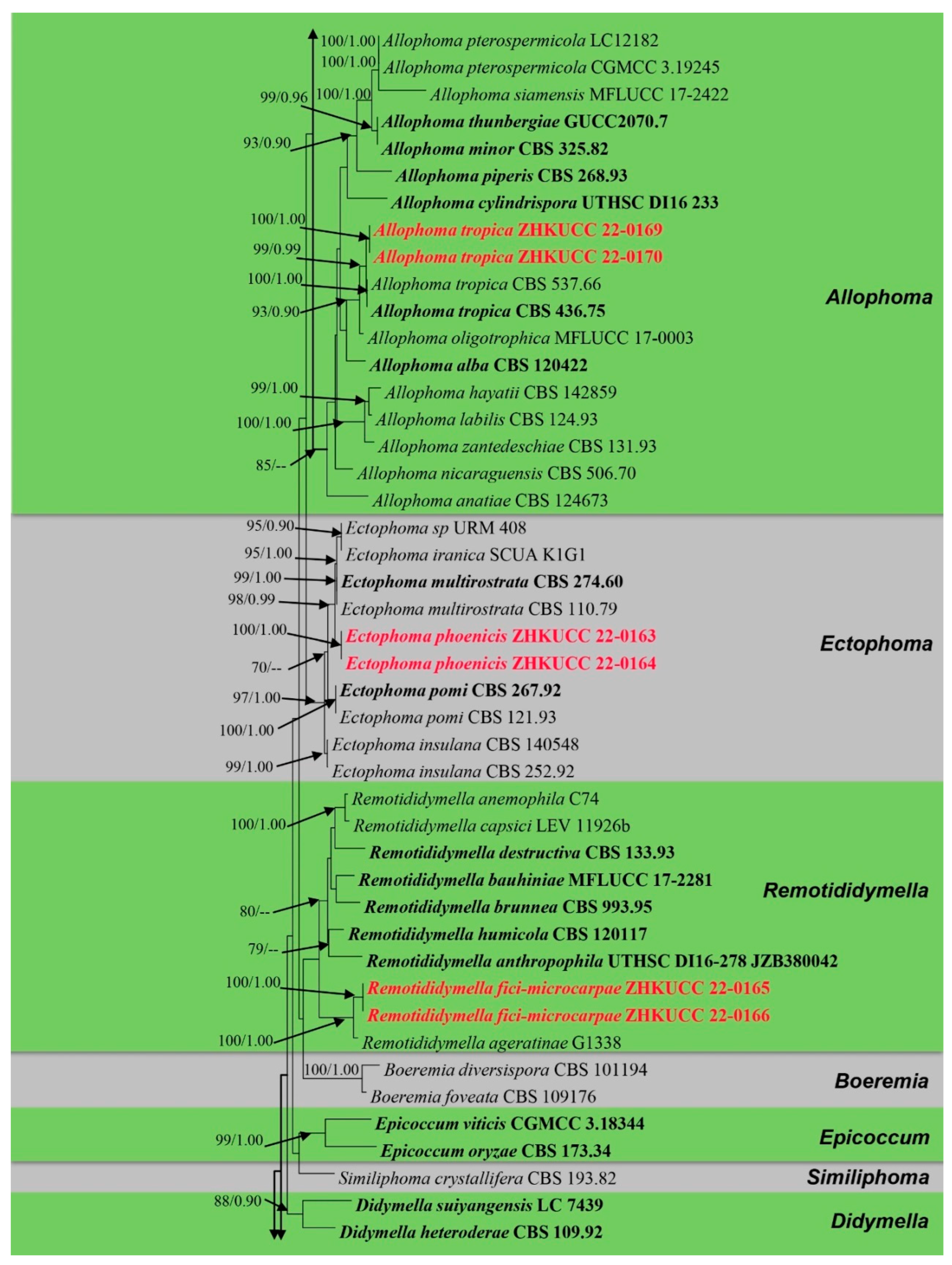
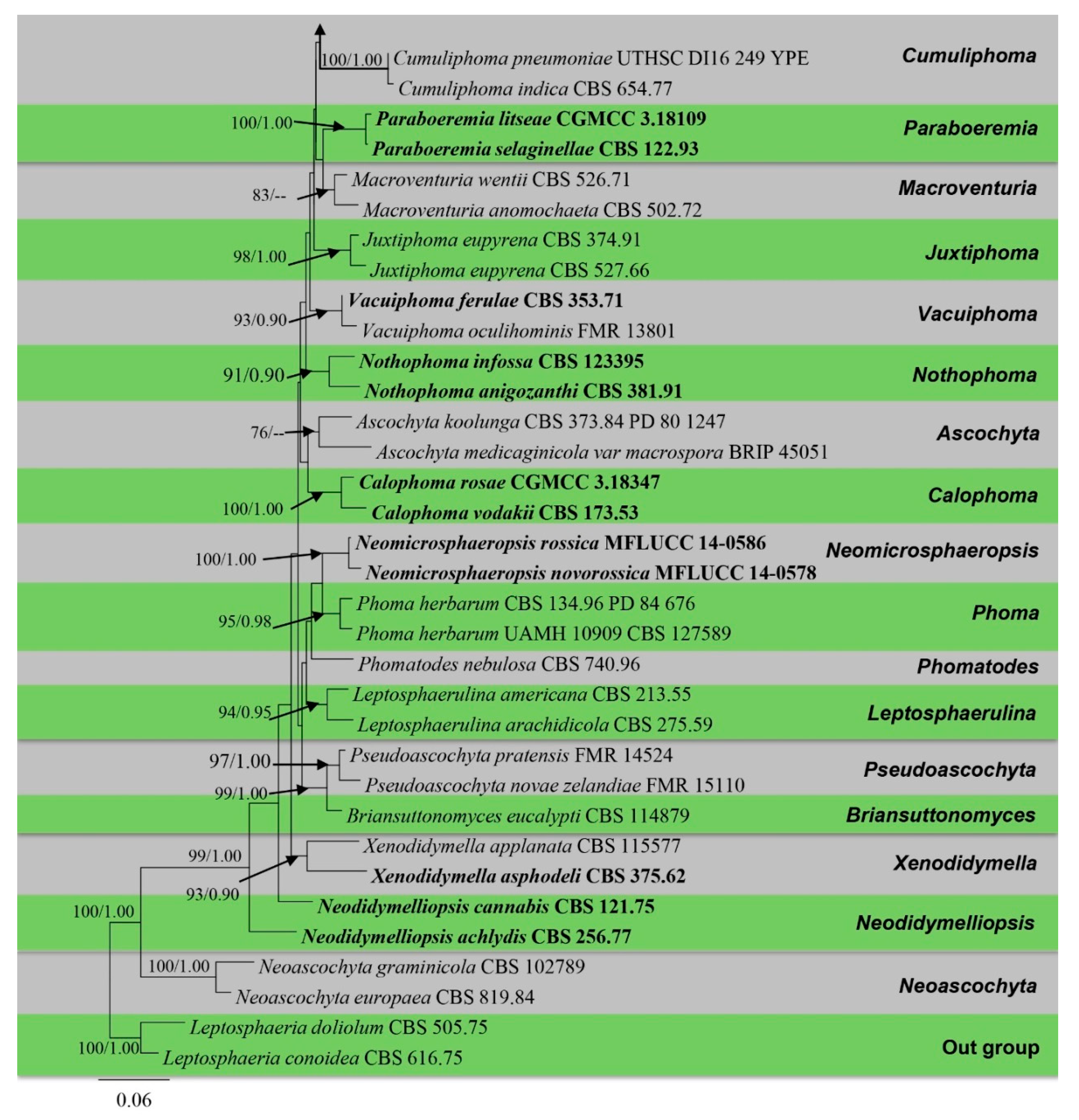
The alignment comprised 2308 nucleotide characters (488 of ITS, 893 of LSU, 595 of RPB2, 332 of β-tubulin). Maximum likelihood analysis yielded the best ML tree (Figure 2) with a likelihood value of −25,904.846543 and the following model parameters: estimated base frequencies A = 0.246664, C = 0.258093, G = 0.262080, and T = 0.233164; substitution rates AC = 1.535206, AG = 4.889606, AT = 1.722258, CG = 0.744297, CT = 10.883910, and GT = 1.0; proportion of invariable sites I = 0.587394; gamma distribution shape parameter: α = 0.536998. The alignment contained a total of 857 distinct alignment patterns and 9.44% of undetermined characters.
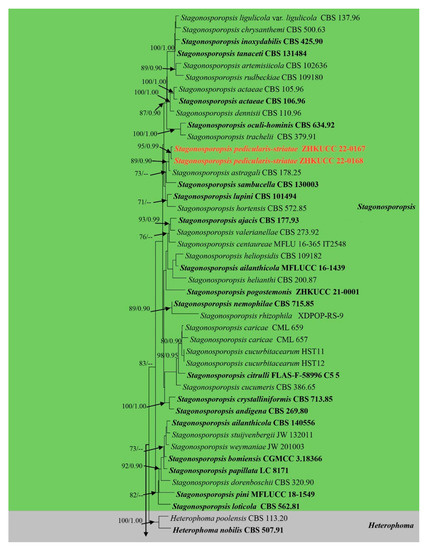
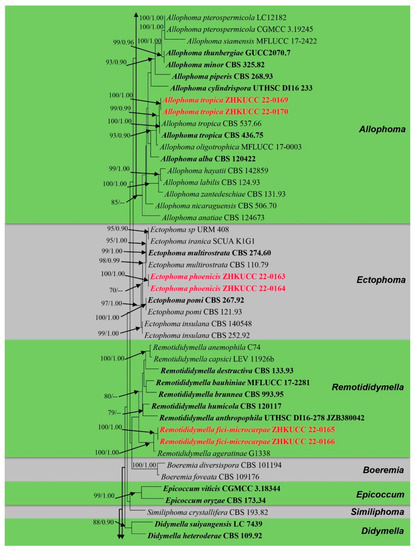
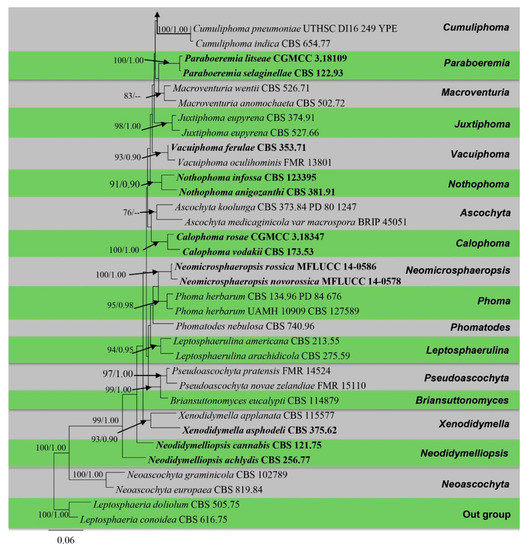
Figure 2.
Phylogram generated from a maximum likelihood analysis based on a combined ITS, LSU, RPB2, and β-tubulin sequence alignment. Maximum likelihood bootstrap support values greater than 70% and Bayesian posterior probabilities greater than 0.90 are given at the nodes. The tree is rooted with Leptosphaeria conoidea (CBS 616.75) and Leptosphaeria doliolum (CBS 505.75). Ex-type strains are given in bold and the newly generated sequences are indicated in red bold.
After discarding the first 20% of generations in the Bayesian analyses, 4000 trees remained from which the 50% consensus tree and Bayesian Interference (BI) posterior probabilities were calculated (Figure 2). All individual trees generated under different criteria from single gene datasets were similar in topology and not significantly different from the final trees generated from the concatenated datasets of the Didymellaceae. Topologies of the ML and Bayesian trees were similar to each other and there were no significant differences.
The phylogenetic analyses of this study (Figure 2) showed that the Didymellaceae comprises a total of 27 well-supported genera. We included 32 sequences from four new collections representing eight isolates in this analysis. Newly generated sequences from two isolates (ZHKUCC 22-0167, ZHKUCC 22-0168) grouped with Stagonosporopsis astragali (Cooke and Harkn.) Aveskamp, and Gruyter and Verkley (CBS 178.25) with strong statistical support (89% in ML, 0.90 in BI). Furthermore, another two isolates (ZHKUCC 22-0169, ZHKUCC 22-0170) grouped with the type strain (CBS 537.66) and another strain (CBS 436.75) of Allophoma tropica with a strong support value (99% in ML, 0.99 in BI). Other two isolates (ZHKUCC 22-0163, ZHKUCC 22-0164) form a distinct basal clade to Ectophoma multirostrata (P.N. Mathur, S.K. Menon and Thirum.) Valenz.-Lopez, Cano, Crous, Guarro and Stchigel-E. Iranica M. Mehrabi, Larki and Farokhinejad subclade with strong statistical support (98% in ML, 0.99 in BI). Moreover, two new isolates (ZHKUCC 22-0165, ZHKUCC 22-0166) form a sister clade to Remotididymella ageratinae H.B. Zhang, A.L. Yang and L. Chen (G1338) with strong support value (100% in ML, 1.00 in BI).
3.2. Taxonomy
Allophoma Q. Chen & L. Cai, Stud. Mycol. 82: 162 (2015).
Type species: Allophoma tropica (R. Schneid. and Boerema) Q. Chen and L. Cai. Chen et al.
Notes: Allophoma was introduced to accommodate phoma-like taxa with various-shaped conidia [7]. Currently, 14 species are accepted in the genus [14] (Species Fungorum. http://www.speciesfungorum.org/Names/Names.asp, accessed on 14 August 2022). Allophoma species are phytopathogens or saprobes on leaves, pods, and stems and some are human and animal pathogens [33,42,43]. Allophoma labilis (Sacc.) Qian Chen and L. Cai often causes leaf necrosis, canker, and stem lesions, or stem rot in various plants [18,44,45,46,47,48], and A. tropica can cause necrosis in a wide variety of ornamental plants [42]. In addition, A. cylindrispora Valenz-Lopez, Stchigel, Guarro and Cano was isolated from a lesion of the human eye [43] and A. oligotrophica Qian Chen, Crous and L. Cai is an air borne fungus [49].
Allophoma tropica (R. Schneid. and Boerema) Q. Chen and L. Cai, Stud. Mycol. 82: 164 (2015).
Basionym: Phoma tropica R. Schneid. and Boerema, Phytopath. Z. 83 (4): 361 (1975)
Index Fungorum number: IF814071; Facesoffungi number: FoF 13240, Figure 3.
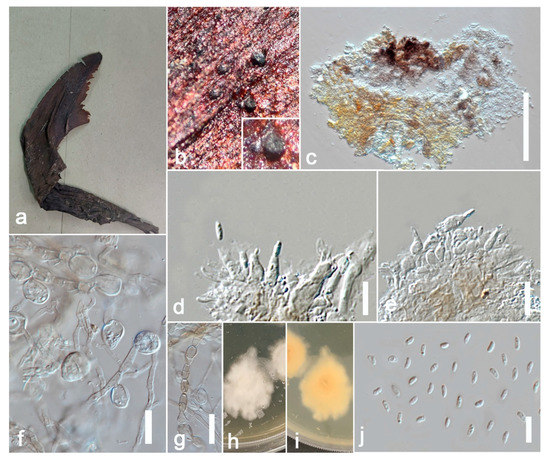
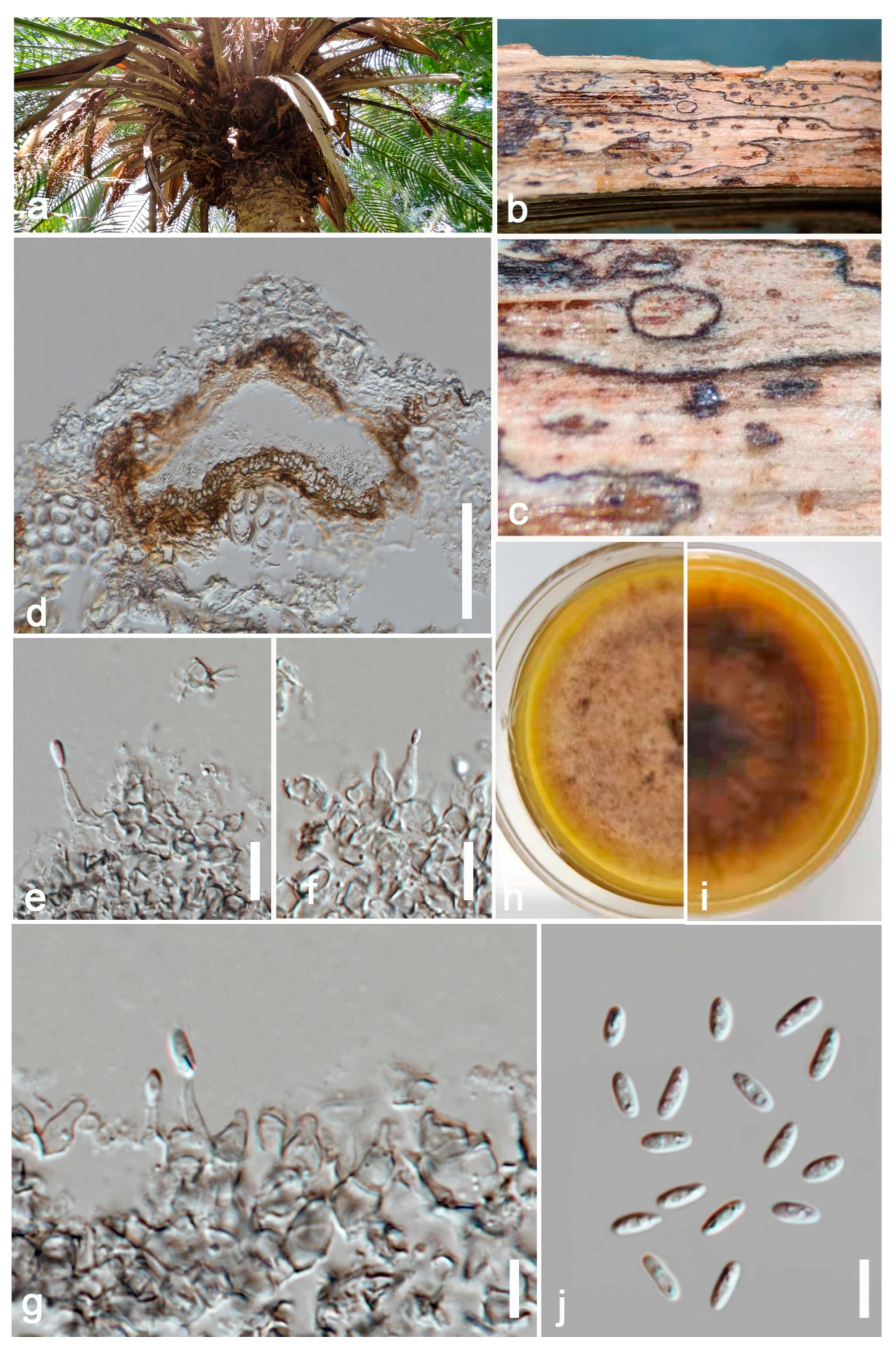
Figure 3.
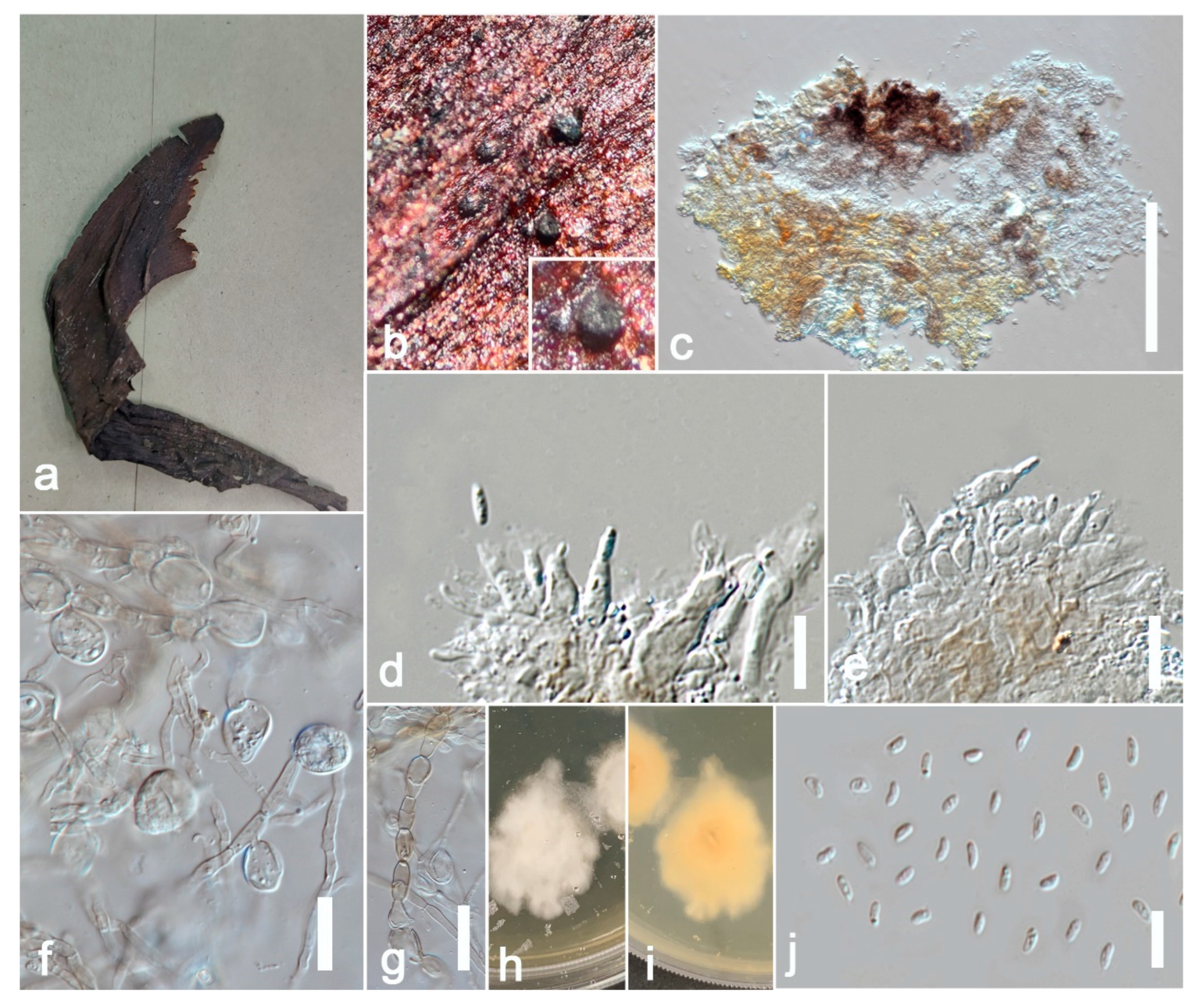
Allophoma tropica (ZHKU 22-0099) (a) Examined material. (b) Conidiomata on the substrate. (c) Vertical section of a conidioma. (d,e) Conidiogenous cells attached to conidia. (j) Conidia. (f,g) Chlamydospores in cultures. (h,i) Colonies on PDA (i from the bottom). Scale bars: e = 200 μm, f–j = 10 μm.
Saprobic on the sepals of Canna sp. Sexual morph: Undetermined. Asexual morph: Coelomycetous. Appearing as black spots. Hyphae 4–6 μm wide, initially hyaline, become olivaceous to brown, septate. Conidiomata 80–250 μm high, 100–300 μm diam. (x = 210 × 280 μm, n = 20), pycnidial, mostly solitary, rarely aggregated, superficial to erumpent, uniloculate, subglobose to irregular, dark brown, glabrous, conspicuous dark ostiole. Ostiole single, dark, distinct, non-papillate or sometimes slightly papillate. Pycnidial wall 5–10 μm thick (x = 8 μm, n = 10), 1–3-layered, composed of pale brown cells of textura angularis. Conidiophores are reduced to conidiogenous cells. Conidiogenous cells 5–8 × 3–4 μm (x = 7 × 3.6 μm, n = 10), phialidic, ampulliform to doliiform, hyaline, smooth. Conidia 3–5 × 1–2.5 μm (x = 4 × 2 μm, n = 20), ellipsoidal, smooth and thin-walled, hyaline, aseptate, with small guttules. Conidial exudates not observed. Chlamydospores globose 10–25 μm diam. (x = 20 μm, n = 20) to irregular 10–15 × 6–15 μm (x = 13 × 10 μm, n =20), unicellular or multicellular, intercalary or terminal, solitary or in chains, smooth, verruculose or incidentally tuberculate, subhyaline to pale brown.
Culture characters: Colonies on PDA reaching 5 cm after 7 days at 25 °C in the dark, white to off-white, aerial mycelium less, circular, flat, smooth to slightly waved margin; reverse off-white. Cultures were not sporulating and no pigments were produced.
Material examined: China, Guangdong Province, Guangzhou City, South China Botanical Garden (23°11′12″ N 113°21′51″ E), on sepals of Canna sp. (Cannaceae), 17 June 2021, N.D. Kularathnage, NDK 71-3 (ZHKU 22-0099), living cultures ZHKUCC 22-0169, ZHKUCC 22-0170.
Hosts and distribution: on Aphelandra sp. from the Netherlands, on Saintpaulia ionantha from Germany, on Gossypium sp. from Bolivia [42], on Lactuca sativa from Italy [50], on Syzygium cumini from India [46], and on sepals of Canna sp. from China (the present study).
Notes: The multi-locus phylogeny of ITS, LSU, RPB2, and β-tubulin showed that our isolates (ZHKUCC 22-0169 and ZHKUCC 22-0170) are affiliated with Allophoma tropica (CBS 537.66, CBS 436.75) with high statistical support (ML/BI 99/0.99) (Figure 2). A single gene comparison of ITS, LSU, RPB2, and β-tubulin of our isolates (ZHKUCC 22-0169, ZHKUCC 22-0170) with the type strain of Allophoma tropica (CBS 436.75) revealed base pair differences of 2/488, 1/893, 0/595, and 2/332, respectively. Our specimen fits well with the type collection of Allophoma tropica (DSM 63365) [51] in all morphological aspects [52].
No species of Allophoma had been reported from Canna sp., and our collection is the first Allophoma species reported from this host genus. Two species, A. pterospermicola Qian Chen and L. Cai and A. thunbergiae Jun Yuan and Yong Wang, have been described from Guangxi and Guizhou Provinces in China [49,53], and A. tropica is the third species reported in the country.
Ectophoma Valenz-Lopez, Cano, Crous, Guarro and Stchigel, Stud. Mycol. 90: 34 (2017)
Type species: Ectophoma multirostrata (P.N. Mathur, S.K. Menon and Thirum.) Valenz-Lopez, J.F. Cano, Crous, Guarro, and Stchigel.
Notes: Ectophoma was established to accommodate two phoma-like taxa, Phoma multirostrata (P.N. Mathur, S.K. Menon and Thirum.) Dorenb. and Boerema and P. pereupyrena Gruyter, Noordel. and Boerema which formed a distinct lineage in the Didymellaceae [28]. Currently, there are four accepted species (Species Fungorum. http://www.speciesfungorum.org/Names/Names.asp, accessed on 14 August 2022). This genus is characterized by pycnidial conidiomata with one or more short necks, phialidic conidiogenous cells, and aseptate conidia [28]. Ectophoma species have been isolated from different woody and herbaceous plants as opportunistic plant pathogens or from soil inhabitants [51,54]. Ectophoma species have been reported from Greece, India, Iran, South Africa, and The Netherlands.
Ectophoma phoenicis Kular. sp. nov.
Index Fungorum number: IF900158; Facesoffungi number: FoF 13241; Figure 4.
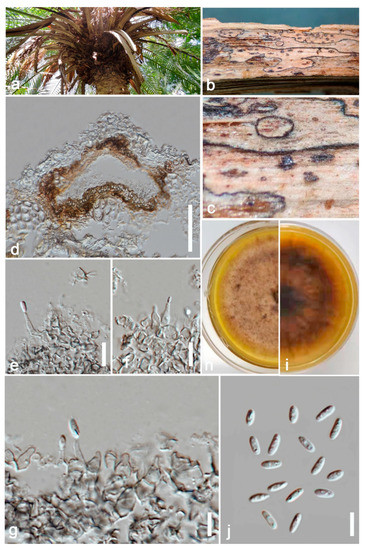
Figure 4.
Ectophoma phoenicis (ZHKU 22-0093, holotype). (a) Host. (b,c) Conidiomata on the substrate. (d) Vertical section of a conidioma. (e–g) Conidiogenous cells attached to conidia. (j) Conidia. (h,i) Colonies on PDA (i) from the bottom. Scale bars: d = 100 μm, e–h = 15 μm.
Etymology: in reference to the host genus, Phoenix.
Saprobic on dead petioles of Phoenix roebelenii O’Brien. Sexual morph: Undetermined. Asexual morph: Coelomycetous, Conidiomata 80–110 μm high, 100–130 μm wide (x = 100 × 120 μm, n = 10), pycnidial, solitary or rarely aggregated, immersed in substrate, conical to subglobose, brown to blackish brown, coriaceous, ostiolate, apapillate. Pycnidial wall comprises 4–7 layered, brown cells of textura angularis. Conidiophores are reduced to conidiogenous cells. Conidiogenous cells 15–20 μm high, 3–8 μm wide (x = 18 × 5 μm, n = 10), phialidic, cylindrical to doliiform, discrete, hyaline to grey, smooth. Conidia 6–8 × 2–4 μm (x = 7 × 3 μm, n = 20), oblong to ellipsoidal, aseptate, with polar guttules, hyaline, smooth, thin-walled, straight.
Culture characters: Colonies on PDA reaching 4 cm diam. after 7 days of incubation at 25 °C, circular, flat, floccose, filiform, pale brown to greyish brown, darker in the central area, then paler ring and darker ring around the center; reverse brown. Hyphae pale brown, branched, septate. No pigments or chlamydospores observed.
Material examined: China, Guangdong Province, Guangzhou City, South China Botanical Garden (23°11′12″ N 113°21′51″ E) on dead petioles of Phoenix roebelenii (Arecaceae), 17 June 2021, N.D. Kularathnage, NDK 78-1 (ZHKU 22-0093, holotype), ex-type culture ZHKUCC 22-0163; ibid. NDK 78-2 (ZHKU 22-0094, isotype), ex-type culture ZHKUCC 22-0164.
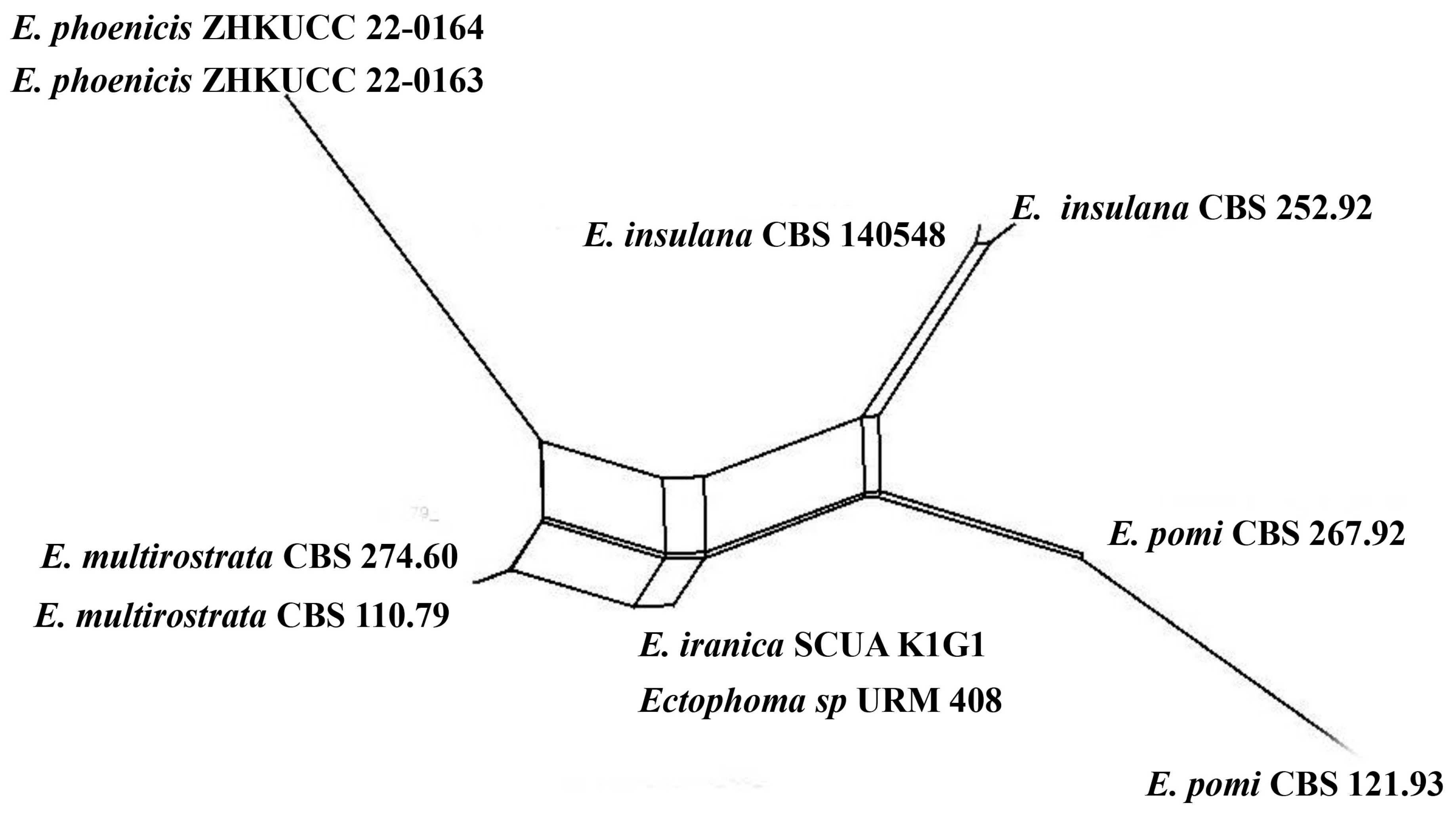
Notes: The multi-locus analysis of ITS, LSU, RPB2, and β-tubulin showed that our isolates (ZHKUCC 22-0163, ZHKUCC 22-0164) clustered within Ectophoma and formed a sister clade to E. iranica and E. multirostrata with 98% in ML and 0.99 in BI support values (Figure 2). A single gene comparison of ITS, RPB2, and β-tubulin locus of our isolates (ZHKUCC 22-0163, ZHKUCC 22-0164) with the type strain of E.iranica (CBS 144681) and E. multirostrata (CBS 274.60) revealed the base pair differences of 7/488, N/A, 3/332 and 6/488, 6/595, 2/332, respectively. The LSU sequences of all Ectophoma species are identical. The genetic distinctness and phylogenetic stability of our isolates were further confirmed by PHI analysis of the Ectophoma clade. The result showed that Φw = 0.99 and this means there was no significant genetic recombination (Φw ≥ 0.05) between these novel isolates with existing Ectophoma species (Figure 5).
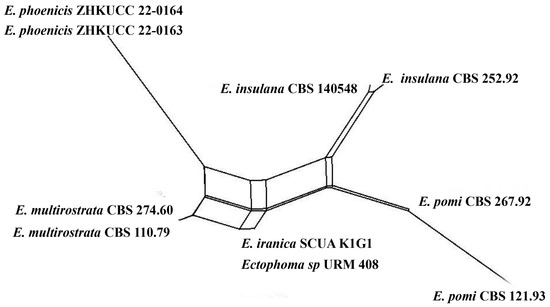
Figure 5.
The results of a PHI test of Ectophoma clade using both LogDet transformation and splits decomposition. PHI test results Φw = 0.99.
Morphologically, our collection differs from E. iranica by its floccose, grey colonies, apapillate pycnidia, cylindrical to doliiform, hyaline to grey conidiogenous cells, and ellipsoidal, straight conidia. In contrast, E. iranica has pale brown to greyish brown colonies, conidiomata with 1–2(3)-narrowed necks, and hyaline to pale brown, oblong to ellipsoidal, straight or sometimes very slightly curved conidia [29]. Ectophoma iranica is a phytopathogen that forms leaf spots on Dracaena compacta and Catharanthus roseus [29] while our collections are saprobes. Furthermore, our collections differ from E. multirostrata by its bi-guttulated conidia, and conidiomata with single ostiole while E. multirostrata is characterized by eguttulate conidia or sometimes with 2–3, polar guttules and necks with several ostioles. Ectophoma multirostrata is a plurivorous opportunistic plant pathogen isolated from soil and also from some plant samples [51] and our species is a saprobe.
Remotididymella Valenz-Lopez, Crous, Cano, Guarro and Stchigel, Stud. Mycol. 90: 35 (2017).
Type species: Remotididymella destructiva (Plowr.) ValenzuelaLopez, Cano, Crous, Guarro and Stchigel.
Notes:Remotididymella is characterized by aseptate, hyaline, smooth- and thin-walled, allantoid or cylindrical, guttulate conidia. Currently, there are eight species listed under this genus (https://www.indexfungorum.org, accessed on 1 June 2022) and the sexual morph has been reported only for R. bauhiniae Jayasiri, E.B.G. Jones and K.D. Hyde [48]. Species of Remotididymella have been reported from Ageratina adenophora, Bauhinia sp., Capsicum annuum, Lycopersicon sp., and Solanum sp. as saprobes or pathogens. However, some species have been isolated from air, soil in tropical forests, and human respiratory tract [12,28,32,48]. Remotididymella species have been reported from China, Guadeloupe, Papua New Guinea, Thailand, The Republic of Fiji, and the United States.
Remotididymella fici-microcarpae Kular. sp. nov.
Index Fungorum number: IF900161; Facesoffungi number: FoF 13242; Figure 6.
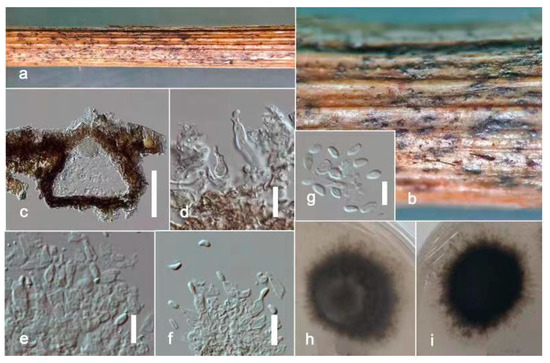
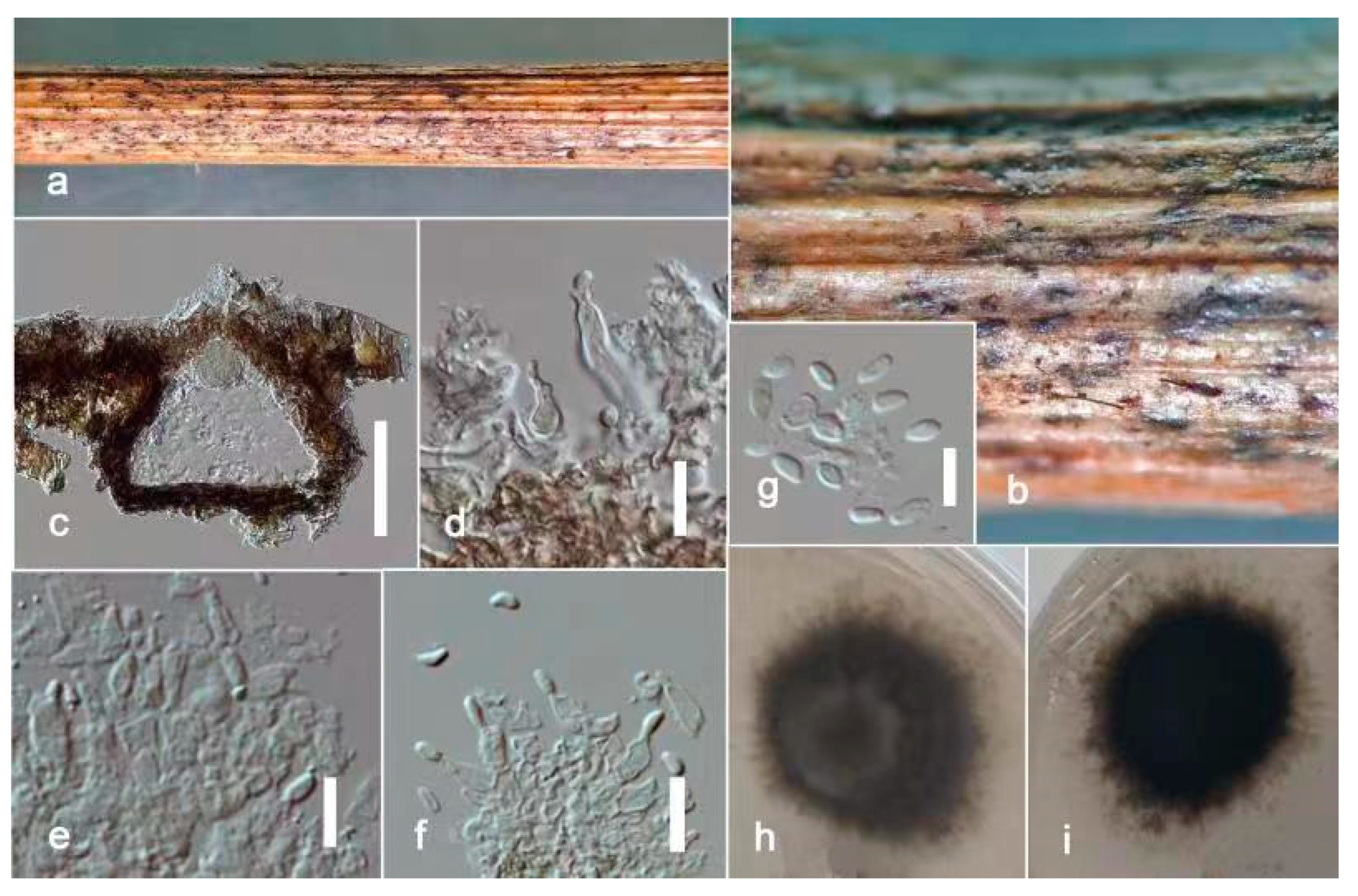
Figure 6.
Remotididymella fici-microcarpae (ZHKU 22-0095, holotype). (a,b) Conidiomata on the substrate. (c) Vertical section of a conidioma. (d–f) Conidiogenous cells attached to conidia. (g) Conidia. (h) Surface view of colony on PDA. (i) Reverse view of colony on PDA. Scale bars: c = 100 μm, d–g = 15 μm.
Etymology: in reference to the host name Ficus microcarpa.
Saprobic on dead stems of Ficus microcarpa L.f Sexual morph: Undetermined. Asexual morph: Conidiomata 180–230 × 130–180 μm, (x = 200 × 150 μm, n = 20), pycnidial, dark brown, mostly solitary, rarely aggregated, immersed, glabrous, conical to irregularly-shaped, with a single papillate ostiolar neck. Pycnidial wall 15–25 μm thick (x = 19 μm, n = 10), 6–8-layered, composed of brown, flattened cells of textura angularis. Conidiogenous cells 12–15 × 8–11 μm (x = 14 × 9 μm, n = 20), phialidic, ampulliform, hyaline, smooth-walled. Conidia 5–8 × 3.5–4.5 μm (x = 7 × 4 μm, n = 20), mostly fusiform to allantoid, aseptate, hyaline, smooth, thin-walled, indistinct guttules.
Culture characters: Colonies on PDA reached 6 cm diam. after 7 days, circular, flat, filiform margin, center pale, margin darker, margin comprised of filiform hyphal tips, aerial mycelia less, brown, reverse pale brown. Cultures not sporulating and no pigments were produced.
Material examined: China, Guangdong Province, Guangzhou City, South China Botanical Garden (23°11′12″ N 113°21′51″ E), on stems of Ficus microcarpa (Moraceae), 17 June 2021, Senanayake I.C, S2 3-1 (ZHKU 22-0095, holotype), ex-type culture ZHKUCC 22-0165, ibid. S2 3-1 A (ZHKU 22-0096, isotype), ex-type living culture ZHKUCC 22-0166.
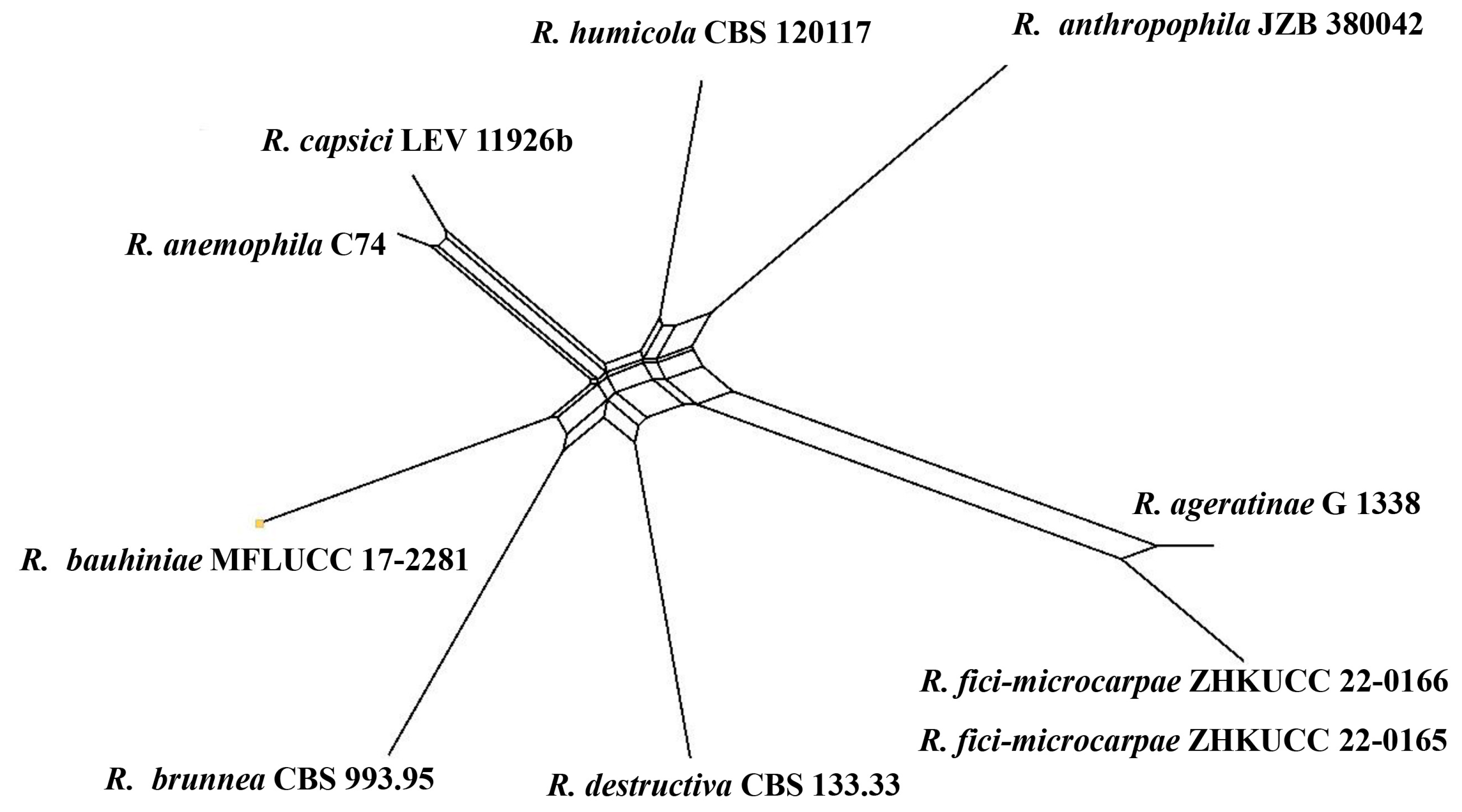
Notes: The combined gene analysis of ITS, LSU, RPB2, and β-tubulin (Figure 2) showed that our isolates (ZHKUCC 22-0165, ZHKUCC 22-0166) grouped with the type strain of Remotididymella ageratinae (CGMCC 3.19991) with a strong support value (100% in ML, 1.00 in BI). Result of PHI analysis of Remotididymella species showed a value of Φw = 0.26 and there was no significant genetic recombination (Φw ≥ 0.05) between these novel species of Remotididymella and other species in this genus (Figure 7). A comparison of the DNA sequences of ITS, LSU, RPB2, and β-tubulin locus of our isolates (ZHKUCC 22-0165, ZHKUCC 22-0166) with the type strain of R. ageratinae revealed base pair differences of 6/488, 8/893, 9/595, 3/332, respectively.
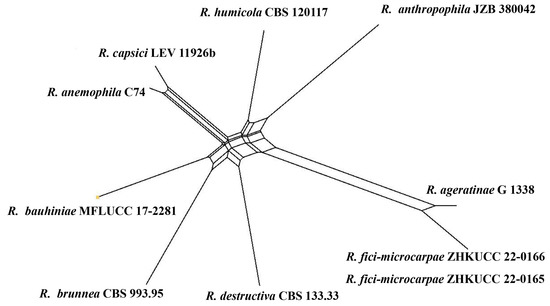
Figure 7.
The results of a PHI test of closely related taxa (Remotididymella clade) using both LogDet transformation and splits decomposition. PHI test results. Φw = 0.26.
Morphologically, our collections differ from R. ageratinae by having small conidiomata (180–230 × 130–180 μm), large conidiogenous cells (12–15 × 8–11 μm), and fusiform to allantoid conidia with indistinct guttules, whereas R. ageratinae has large conidiomata (107–409 × 121–503 µm), small conidiogenous cells (8–9 × 10 µm), and oblong to cylindrical, obovoid, sometimes slightly curved to reniform conidia with distinct guttules. Therefore, we introduce our collection as Remotididymella fici-microcarpae sp. nov. based on morphology and phylogeny.
Stagonosporopsis Died., Annls mycol. 10 (2): 142 (1912).
Type species: Stagonosporopsis hortensis (Sacc. and Malbr.) Petr.
Notes: There are 52 accepted species in this genus (Species Fungorum. http://www.speciesfungorum.org/Names/Names.asp, accessed on 14 August 2022). Members of the Stagonosporopsis are saprobes on dead plant materials, and some species have been reported from house dust and garden soil [12]. Some species of Stagonosporopsis can cause devastating diseases on a wide range of economically important plants, including those found in farmlands, forests, grasslands, and other natural ecosystems [5]. Stagonosporopsis species have been reported as severe pathogens of some crops and ornamentals in several countries, including Australia China, France, India, Italy, Turkey, and the United States [55,56,57,58,59].
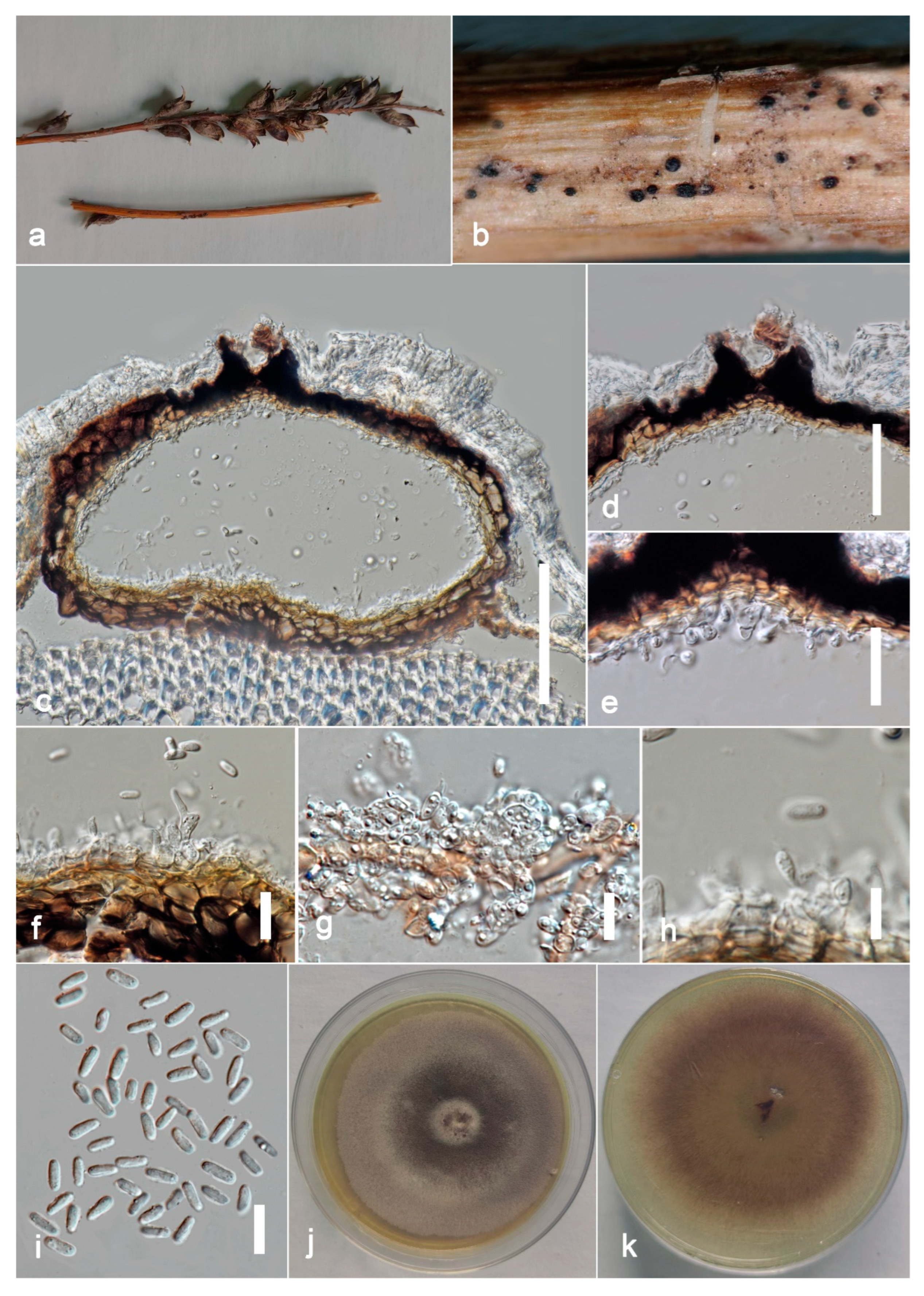
Stagonosporopsis pedicularis-striatae Kular. sp. nov.
Index Fungorum number: IF900160; Facesoffungi number: FoF 13243, Figure 8.
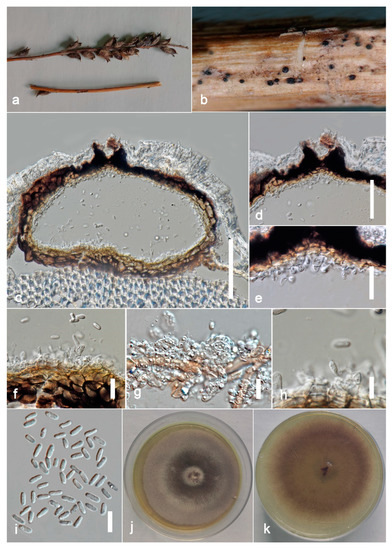
Figure 8.
Stagonosporopsis pedicularis-striatae (ZHKU 22-0097, holotype). (a) Host. (b) Material examined. (c) Vertical section of a conidioma. (d) Papilla. (e,f) Conidiogenous cells attached to conidia. (g,h) Conidiogenous cells. (i) Conidia. (j) Colony on PDA (k) from the bottom. Scale bars: c = 100 μm, d = 40 μm, e–i = 10 μm.
Etymology: in reference to the host name Pedicularis striata.
Saprobic on dead stems of Pedicularis striata Pallas. Sexual morph: Undetermined. Asexual morph: Coelomycetous. Conidiomata 150–200 × 300–400 μm (x = 180 × 350 μm, n = 10), pycnidial, solitary or aggregated, scattered, subglobose, coriaceous, brown to dark brown, thin-walled, glabrous, ostiolate. Ostiole single, slightly papillate. Pycnidial wall 10–15 μm thick, pseudoparenchymatous, 4–5-layered, composed with brown, angularis to globosa cells. Conidiophores arereduced to conidiogenous cells. Conidiogenous cells 4–6 × 4–5 μm (x = 5.5 × 4.5 μm, n = 20), phialidic, subglobose, hyaline, smooth. Conidia 7–9 × 3–5 μm (x = 8 × 4 μm, n = 20), oblong to ellipsoid, with ends rounded, smooth and thin-walled, aseptate, small guttulate.
Culture characters: Colonies on PDA reached 7 cm diam. after 7 days, circular, flat, smooth, entire margin, aerial mycelia concentrated at the margin, brown, center dark, margin pale; reverse pale brown, center pale, margin darker. Cultures not sporulating and no pigments are produced.
Material examined: China, Guangdong Province, Guangzhou City, South China Botanical Garden (23°11′12″ N 113°21′51″ E) on dead petioles of Pedicularis striata (Orobanchaceae), 17 June 2021, Kularathnage N.D., S1 1010 (ZHKU 22-0097, holotype), ex-type culture ZHKUCC 22-0167; ibid. S1-1002 (ZHKU 22-0098, isotype), ex-type culture ZHKUCC 22-0168.
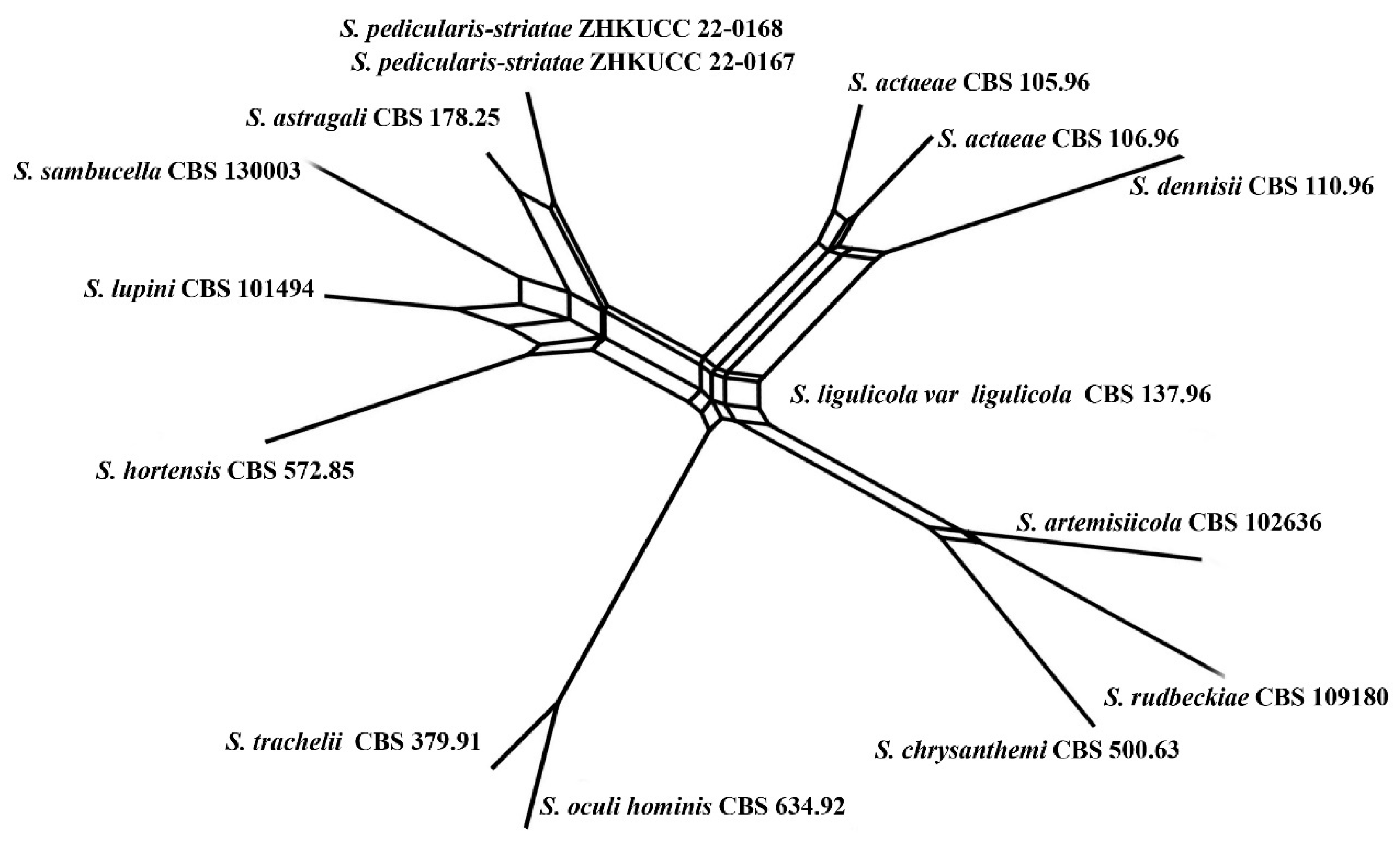
Notes: Our isolates (ZHKUCC 22-0167, ZHKUCC 22-0168) grouped with Stagonosporopsis astragali (CBS 178.25), forming a well-supported (89% in ML, 0.90 in BI) distinct clade in the combined gene analysis of ITS, LSU, RPB2 and β-tubulin (Figure 2). The result of PHI analysis of Stagonosporopsis species in this study shows Φw = 0.18 and there was no significant genetic recombination between these novel isolates and other Stagonosporopsis species (Figure 9). Comparison of the DNA sequences of ITS, LSU, RPB2, and β-tubulin locus of our isolates (ZHKUCC 22-0167, ZHKUCC 22-0168) with the type strain of S. astragali reveals the base pair differences of 5/488, 9/893, 7/595, 15/332 respectively.
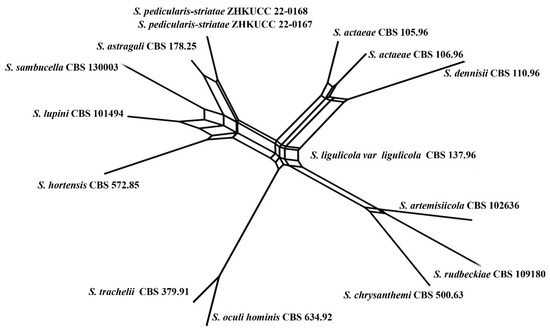
Figure 9.
The results of PHI test of closely related taxa (Stagonosporopsis sub clade) using both LogDet transformation and splits decomposition. PHI test results. Φw = 0.18.
Morphologically, our collections are different from Stagonosporopsis astragali by short papillate, larger conidiomata (150–200 × 300–400 μm), and oblong to ellipsoid conidia without polar guttules. In contrast, S. astragali is an opportunistic pathogen producing smaller conidiomata (80–180 μm diam.) with 0–3 papillate ostioles, and cylindrical to allantoid conidia with numerous polar guttules. Therefore, we introduce this collection as Stagonosporopsis pedicularis-striatae sp. nov. This fungus has often recorded in North America (United States and Canada) on stems of various species of Astragalus [51].
4. Discussion and Conclusions
In a survey of the diversity and species richness of plant-associated fungi in the South China Botanical Garden, we collected several saprobic, hyaline-spored asexual species in the Didymellaceae. The Didymellaceae has recently undergone extensive revision based on its phylogenetic relationships and morphological characteristics [5,7,60]. In this study, we identified and introduced three new species in Didymellaceae (Ectophoma phoenicis, Remotididymella fici-microcarpae, and Stagonosporopsis pedicularis-striatae) along with a new host and locality record of Allophoma tropica based on polyphasic approaches according to the procedure of [61,62,63]. The guidelines for introducing novel species have been discussed in [64,65] were followed.
We described and illustrated a new locality report of Allophoma tropica. Allophoma tropica was recorded for the first time as a saprobe on leaves of Streptocarpus ionanthus and this study is the first record of Allophoma species on Canna in China. Furthermore, this study reported morphological characters of chlamydospores from cultures of Allophoma for the first time. The type collection of Allophoma tropica obtained from Streptocarpus ionanthus (H. Wendl.) Christenh. as a pathogen and morphological characters were obtained from pycnidia in culture while morphological characters of our collection were obtained from conidiomata on the substrate. Therefore, there are negligible differences in the morphological characteristics of fungi on a substrate and in media. There are four Ectophoma species listed in (Species Fungorum. http://www.speciesfungorum.org/Names/Names.asp, accessed on 14 August 2022). The type species of Ectophoma, E. multirostrata has been isolated from poultry farm soil and the live stem of Cucumis sativus in a greenhouse [28]. Ectophoma iranica and E. pomi are plant pathogens isolated from leaf spots of Dracaena compacta, Catharanthus roseus [29], and Coffea arabica [28]. Ectophoma insulana was isolated from the fruit of Olea europaea and from house dust [12]. Morphologically, our collections differ from E. iranica, and E. multirostrata and also our collection of Ectophoma phoenicis isolated from the dead petioles of Phoenix roebelenii are saprobic fungi. As such, this is the first record of saprobic behavior of Ectophoma species. Our collection of Ectophoma phoenicis was identified and introduced as a new species belonging to the genus Ectophoma.
Remotididymella fici-microcarpae is the first species in this genus reported from China and also from Ficus microcarpa. This was collected from dead stems as saprobes. However, Remotididymella species show a wide variation of life modes including plant and human pathogens and saprobes [12,32,48]. Morphologically, our collections differ from R. ageratinae by having immersed, brown, small conidiomata, large conidiogenous cells, and fusiform to allantoid conidia with indistinct guttules. Our collection of Remotididymella fici-microcarpae was identified and introduced as a new species belonging to the genus Remotididymella.
There are several Stagonosporopsis species that have been reported from China, and most of them are plant pathogens [31]. Stagonosporopsis vannaccii Baroncelli, Cafà, Castro, Boufleur and Massola was reported from leaf spots on Crassocephalum crepidioides in Guangxi [66] while Stagonosporopsis cucurbitacearum (Fr.) Aveskamp, Gruyter and Verkley are the cause of pumpkin gummy stem blight, which is one of the most devastating pumpkin crop diseases in North-East China [67]. Stagonosporopsis pogostemonis M. Luo, Y.H. Huang and Manawas. was reported from leaf spots and as a stem blight on Pogostemon cablin in South China [31]. However, Stagonosporopsis pedicularis-striatae was collected from dead stems as a saprobe. Morphologically, our collections differ from Stagonosporopsis astragali by their subglobose, slightly papillate, comparatively larger conidiomata (150–200 × 300–400 μm), and oblong to ellipsoid conidia. In terms of phylogenetic and morphological differences, we identified and introduced Stagonosporopsis pedicularis-striatae as a new species belonging to the genus Stagonosporopsis.
Author Contributions
Conceptualization, N.D.K.; Methodology, N.D.K.; formal analysis, N.D.K.; resources, N.D.K. and I.C.S.; data curation, N.D.K.; writing—original draft preparation, N.D.K.; writing—review and editing, N.D.K., I.C.S., D.N.W., M.D., S.L.S., J.S., B.X. and W.D.; supervision, W.D.; project administration, W.D.; funding acquisition, M.D., J.S. and W.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research study is supported by the National Natural Science Foundation of China (Grant no. 32200015) and the Talent Program of Zhongkai University of Agricultural and Engineering (KA22016B787). National Natural Science Foundation of China (31860008; U1803232), and the foundations of Guangdong Provincial Department of Education (2022KCXTD015; 2022ZDJS020). National Natural Science Foundation of China (Nos. 32100012) and Talent Program of Zhongkai University of Agricultural and Engineering (KA200540852). Talent Program of Zhongkai University of Agricultural and Engineering (KA22016B746) and the Key Laboratory of Green Prevention and Control on Fruits and Vegetables in South China, Guangdong (KA21031C502).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All sequence data are available in NCBI GenBank following the accession numbers in the manuscript.
Acknowledgments
Nuwan Kularathnage thanks the Innovative Institute for Plant Health, Zhongkai University of Agriculture and Engineering, Guangzhou, China for providing research facilities and Mae Fah Luang University, Chiang Rai, Thailand for providing a Ph.D. scholarship. Nuwan Kularathnage thanks to Shaun Pennycook for nomenclatural advices.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huang, H.W. The Chinese Botanical Gardens; Chinees Forestry Press: Beijing, China, 2018. [Google Scholar]
- Huang, H.W. The Chinese Botanical Gardens; EDP Sciences: Les Ulis, France, 2022; p. 437. [Google Scholar]
- Ren, H.; Jian, S.G.; Liu, H.X.; Zhang, Q.M.; Lu, H.F. Advances in the reintroduction of rare and endangered wild plant species. Sci. China Life Sci. 2014, 57, 603–609. [Google Scholar] [CrossRef]
- de Gruyter, J.; Aveskamp, M.M.; Woudenberg, J.H.C.; Verkley, G.J.M.; Groenewald, J.Z.; Crous, P.W. Molecular phylogeny of Phoma and allied anamorph genera towards a reclassification of the Phoma complex. Mycol. Res. 2009, 113, 508–519. [Google Scholar] [CrossRef]
- Aveskamp, M.M.; de Gruyter, J.; Woudenberg, J.H.C.; Verkley, G.J.M.; Crous, P.W. Highlights of the Didymellaceae: A polyphasic approach to characterise Phoma and related pleosporalean genera. Stud. Mycol. 2010, 65, 1–60. [Google Scholar] [CrossRef]
- Ariyawansa, H.A.; Hyde, K.D.; Jayasiri, S.C.; Buyck, B.; Chethana, K.T.; Dai, D.Q.; Dai, Y.C.; Daranagama, D.A.; Jayawardena, R.S.; Lücking, R.; et al. Fungal diversity notes 111–252—Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2015, 75, 27–274. [Google Scholar] [CrossRef]
- Chen, Q.; Jiang, J.R.; Zhang, G.Z.; Crous, P.W. Resolving the Phoma enigma. Stud. Mycol. 2015, 82, 137–217. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Hyde, K.D.; Wanasinghe, D.N.; Papizadeh, M.; Goonasekara, I.D.; Camporesi, E.; Bhat, D.J.; McKenzie, E.H.; Phillips, A.J.; Diederich, P.; et al. Taxonomy and phylogeny of dematiaceous coelomycetes. Fungal Divers. 2016, 77, 1–316. [Google Scholar]
- Thambugala, K.M.; Daranagama, D.A.; Phillips, A.; Bulgakov, T.; Bhat, D.J.; Camporesi, E.; Bahkali, A.H.; Eungwanichayapant, P.D.; Liu, Z.-Y.; Hyde, K.D. Microfungi on Tamarix. Fungal Divers. 2017, 82, 239–306. [Google Scholar] [CrossRef]
- Wanasinghe, D.N.; Jeewon, R.; Peršoh, D.; Jones, E.B.G.; Camporesi, E.; Bulgakov, T.S.; Gafforov, Y.S.; Hyde, K.D. Taxonomic circumscription and phylogenetics of novel didymellaceous taxa with brown muriform spores. Stud. Fungi 2018, 3, 152–175. [Google Scholar] [CrossRef]
- Hongsanan, S.; Hyde, K.D.; Phookamsak, R.; Wanasinghe, D.N.; McKenzie, E.H.; Sarma, V.V.; Lücking, R.; Boonmee, S.; Bhat, J.D.; Liu, N.G.; et al. Refined families of Dothideomycetes: Orders and families incertaesedis in Dothideomycetes. Fungal Divers. 2020, 105, 17–318. [Google Scholar] [CrossRef]
- Hou, L.W.; Hernández-Restrepo, M.; Groenewald, J.Z.; Cai, L.; Crous, P.W. Citizen science project reveals high diversity in Didymellaceae (Pleosporales, Dothideomycetes). MycoKeys 2020, 65, 49–99. [Google Scholar] [CrossRef]
- Keirnan, E.C.; Tan, Y.P.; Laurence, M.H.; Mertin, A.A.; Liew, E.C.; Summerell, B.A.; Shivas, R.G. Cryptic diversity found in Didymellaceae from Australian native legumes. MycoKeys 2021, 78, 1–20. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Hyde, K.D.; Dai, D.Q.; Sánchez-García, M.; Goto, B.T.; Saxena, R.K.; Erdoğdu, M.; Selçuk, F.; Rajeshkumar, K.C.; Aptroot, A.; et al. Outline of fungi and fungus-like taxa 2021. Mycosphere 2022, 13, 53–453. [Google Scholar] [CrossRef]
- Aveskamp, M.M.; de Gruyter, J.; Crous, P.W. Biology and recent developments in the systematics of Phoma, a complex genus of major quarantine significance. Fungal Divers. 2008, 31, 1–18. [Google Scholar]
- Marin-Felix, Y.; Groenewald, J.Z.; Cai, L.; Chen, Q.; Marincowitz, S.; Barnes, I.; Bensch, K.; Braun, U.; Camporesi, E.; Damm, U.; et al. Genera of phytopathogenic fungi: GOPHY 1. Stud. Mycol. 2017, 86, 99–216. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Hyde, K.D.; Divakar, P.K.; Rajeshkumar, K.C.; Weerahewa, D.; Delgado, G.; Wang, Y.; Fu, L. Notes for genera update—Ascomycota: 6616–6821. Mycosphere 2018, 9, 115–140. [Google Scholar] [CrossRef]
- Babaahmadi, G.; Mehrabi-Koushki, M.; Hayati, J. Allophoma hayatii sp. nov.; an undescribed pathogenic fungus causing dieback of Lantana camara in Iran. Mycol. Prog. 2018, 17, 365–379. [Google Scholar] [CrossRef]
- Tibpromma, S.; Hyde, K.D.; Jeewon, R.; Maharachchikumbura, S.S.N.; Liu, J.K.; Bhat, D.J.; Jones, E.B.G.; McKenzie, E.H.C.; Camporesi, E.; Bulgakov, T.S.; et al. Fungal diversity notes 491–602: Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2017, 83, 1–261. [Google Scholar] [CrossRef]
- Tennakoon, D.S.; Bhat, D.J.; Chang-Hsin, K.; Hyde, K.D. Leaf litter saprobic Dictyosporiaceae (Pleosporales, Dothideomycetes): Pseudocoleophoma zingiberacearum sp. nov. from Hedychium coronarium. Kavaka 2019, 53, 1–67. [Google Scholar] [CrossRef]
- Bakerspigel, A.; Lowe, D.; Rostas, A. The isolation of Phoma eupyrena from a human lesion. Arch. Dermatol. 1981, 117, 362–363. [Google Scholar] [CrossRef]
- Gkoutselis, G.; Rohrbach, S.; Harjes, J.; Obst, M.; Brachmann, A.; Horn, M.A.; Rambold, G. Microplastics accumulate fungal pathogens in terrestrial ecosystems. Sci. Rep. 2021, 11, 13214. [Google Scholar] [CrossRef]
- Kularathnage, N.D.; Wanasinghe, D.N.; Senanayake, I.C.; Yang, Y.; Manawasinghe, I.S.; Phillips, A.J.L.; Hyde, K.D.; Dong, W.; Song, J. Microfungi associated with ornamental palms: Byssosphaeria phoenicis sp.nov. (Melanommataceae) and Pseudocoleophoma rhapidis sp. nov. (Dictyosporiaceae) from south China. Phytotaxa 2022, 568, 149–169. [Google Scholar] [CrossRef]
- Senanayake, I.C.; Rathnayaka, A.R.; Marasinghe, D.S.; Calabon, M.S.; Gentekaki, E.; Lee, H.B.; Hurdeal, V.G.; Pem, D.; Dissanayake, L.S.; Wijesinghe, S.N.; et al. Morphological approaches in studying fungi: Collection, examination, isolation, sporulation and preservation. Mycosphere 2020, 11, 2678–2754. [Google Scholar] [CrossRef]
- Jayasiri, S.C.; Hyde, K.D.; Abd-Elsalam, K.A.; Abdel-Wahab, M.A.; Ariyawansa, H.A.; Bhat, J.; Buyck, B.; Dai, Y.C.; Ertz, D.; Hidayat, I.; et al. The faces of fungi database: Fungal names linked with morphology, molecular and human attributes. Fungal Divers. 2015, 74, 18–357. [Google Scholar] [CrossRef]
- de Oliveira, D.A.S.; Debing, Y.; Dieryck, I.; Lyimu, W.M.; Paeshuyse, J. Genome sequences and phylogeny of two duck Hepatitis B viruses. Microbiol. Resour. Announc. 2021, 10, e01327-20. [Google Scholar]
- Jayasiri, S.C.; Hyde, K.D.; Jones, E.G.; Ariyawansae, H.A.; Bahkali, A.H.; Elgorban, A.M.; Kang, J.C. A new hysteriform Dothideomycetes (Gloniaceae, Pleosporomycetidae Incertae sedis), Purpurepithecium murisporum gen. et sp.nov.on pine cone scales. Cryptogam. Mycol. 2017, 38, 241–251. [Google Scholar] [CrossRef]
- Valenzuela-Lopez, N.; Cano-Lira, J.F.; Guarro, J.; Sutton, D.A.; Wiederhold, N.; Crous, P.W.; Stchigel, A.M. Coelomycetous Dothideomycetes with emphasis on the families Cucurbitariaceae and Didymellaceae. Stud. Mycol. 2018, 90, 1–69. [Google Scholar] [CrossRef]
- Larki, R.; Mehrabi-Koushki, M.; Farokhinejad, R. Ectophoma iranica sp.nov. and new hosts and records of Allophoma spp. in Iran. J. Phytopathol. 2019, 167, 538–545. [Google Scholar] [CrossRef]
- Li, W.J.; McKenzie, E.H.C.; Liu, J.K.; Bhat, D.J.; Dai, D.Q.; Camporesi, E.; Tian, Q.; Maharachchikumbura, S.S.N.; Luo, Z.L.; Shang, Q.J.; et al. Taxonomy and phylogeny of hyaline-spored coelomycetes. Fungal Divers. 2020, 100, 279–801. [Google Scholar]
- Dong, Z.Y.; Huang, Y.H.; Manawasinghe, I.S.; Wanasinghe, D.N.; Liu, J.W.; Shu, Y.X.; Zhao, M.P.; Xiang, M.M.; Luo, M. Stagonosporopsis pogostemonis: A novel ascomycete fungus causing leaf spot and stem blight on Pogostemon cablin (Lamiaceae) in South China. Pathogens 2021, 10, 1093. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.-L.; Chen, L.; Fang, K.; Dong, X.-F.; Li, Y.-X.; Zhang, H.-B.; Yu, Z.-F. Remotididymella ageratinae sp. nov. and Remotididymella anemophila sp.nov., two novel species isolated from the invasive weed Ageratina adenophora in PR China. Int. J. Syst. Evol. Microbiol. 2021, 71, 004572. [Google Scholar] [CrossRef]
- Yuan, M.; Jiang, Z.; Bi, G.; Nomura, K.; Liu, M.; Wang, Y.; Cai, B.; Zhou, J.-M.; He, S.Y.; Xin, X.-F. Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature 2021, 592, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Stamatakis, A.; Alachiotis, N. Time and memory efficient likelihood-based tree searches on phylogenomic alignments with missing data. Bioinformatics 2010, 26, i132–i139. [Google Scholar] [CrossRef]
- Silvestro, D.; Michalak, I. raxmlGUI: A graphical front-end for RAxML. Org. Divers. Evol. 2012, 12, 335–337. [Google Scholar] [CrossRef]
- Nylander, J.A.A. MrModeltest 2.0. Program distributed by the author. In Evolutionary Biology Centre; Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2.efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Drummond, A.J. Tracer v1, 4. 2017. Available online: http://beast.bio.ed.ac.uk/Tracer (accessed on 26 August 2022).
- Rambaut, A. FigTree v1. 4.0. A Graphical Viewer of Phylogenetic Trees. 2012. Available online: http://tree.bio.ed.ac.uk/software/figtree (accessed on 25 August 2022).
- Schneider, R.; Boerema, G.H. Phoma tropica n. sp., ein a Gewächshauspflanzen häufig vorkommender, nicht pathogener Pilz. Phytopathol. Z. 1975, 83, 239–243. [Google Scholar] [CrossRef]
- Valenzuela-Lopez, N.; Sutton, D.A.; Cano-Lira, J.F.; Paredes, K.; Wiederhold, N.; Guarro, J.; Stchigel, A.M. Coelomycetous fungi in the clinical setting: Morphological convergence and cryptic diversity. J. Clin. Microbiol. 2017, 55, 552–567. [Google Scholar] [CrossRef]
- Zimowska, B. Characteristics and occurrence of Phoma spp. on herbs from the family Lamiaceae. Acta Sci. Pol. Hortorum Cultus 2011, 10, 213–224. [Google Scholar]
- Garibaldi, A.; Gilardi, G.; Ortu, G.; Gullino, M.L. First report of leaf spot of lettuce (Lactuca sativa L.) caused by Phoma tropica in Italy. Plant Dis. 2012, 96, 9. [Google Scholar] [CrossRef]
- Nagarjun, N.; Suryanarayana, V. Documentation, characterization and management of leaf spot of Syzygium cumini (L.) Skeels. J. Farm Sci. 2016, 29, 3. [Google Scholar]
- O’Neill, T.; Mayne, S. An Unusual Phoma stem rot of tomato. AHDB Hortic. 2016, 6–16. [Google Scholar]
- Jayasiri, S.C.; Hyde, K.D.; Jones, E.B.G.; McKenzie, E.H.C.; Jeewon, R.; Phillips, A.J.L.; Bhat, D.J.; Wanasinghe, D.N.; Liu, J.K.; Lu, Y.Z.; et al. Diversity, morphology and molecular phylogeny of Dothideomycetes on decaying wild seed pods and fruits. Mycosphere 2019, 10, 1–186. [Google Scholar] [CrossRef]
- Chen, Q.; Hou, L.; Duan, W.; Cai, L. Didymellaceae revisited. Stud. Mycol. 2017, 87, 105–159. [Google Scholar] [CrossRef] [PubMed]
- Gullino, M.L.; Gilardi, G.; Garibaldi, A. Evaluating severity of leaf spot of lettuce, caused by Allophoma tropica, under a climate change scenario. Phytopathol. Mediterr. 2017, 56, 235–241. [Google Scholar]
- Boerema, G.H.; de Gruyter, J.; Noordeloos, M.E.; Hamers, M.E.C. Phoma Identification Manual. Differentiation of Specific and Infra-Specific Taxa in Culture; CABI Publishing: Wallingford, UK, 2004; p. 470. [Google Scholar]
- de Gruyter, J.; Noordeloos, M.E. Contributions towards a monograph of Phoma (Coelomycetes)—I. 1. Section Phoma: Taxa with very small conidia in vitro. Persoonia 1992, 15, 71–92. [Google Scholar]
- Marin-Felix, Y.; Hernández-Restrepo, M.; Iturrieta-González, I.; García, D.; Gené, J.; Groenewald, J.Z.; Cai, L.; Chen, Q.; Quaedvlieg, W.; Schumacher, R.K. Genera of phytopathogenic fungi: GOPHY 3. Stud. Mycol. 2019, 94, 1–24. [Google Scholar] [CrossRef]
- Mathur, P.N.; Thirumalachar, M.J. Studies on some Indian soil fungi 1. Some new or noteworthy Sphaeropsidales. Sydowia 1959, 13, 143–146. [Google Scholar]
- Vaghefi, N.; Pethybridge, S.J.; Ford, R.; Nicolas, M.E.; Crous, P.W.; Taylor, P.W.J. Stagonosporopsis spp. associated with ray blight disease of Asteraceae. Australas. Plant Pathol. 2012, 41, 675–686. [Google Scholar] [CrossRef]
- Vaghefi, N.; Hay, F.S.; Ades, P.K.; Pethybridge, S.J.; Ford, R.; Taylor, P.W.J. Rapid changes in the genetic composition of Stagonosporopsis tanaceti population in Australian Pyrethrum fields. Phytopathology 2015, 105, 358–369. [Google Scholar] [CrossRef]
- Basım, E.; Basım, H.; Abdulai, M.; Baki, D.; Nurhan, Z. Identification and characterization of Didymella bryoniae causing gummy stem blight disease of watermelon (Citrullus lanatus) in Turkey. Crop Prot. 2016, 90, 150–156. [Google Scholar] [CrossRef]
- Zhang, C.; Qian, Y.; Zheng, X.; Zhou, Y.; Xiong, Q. Stagonosporopsis trachelii causes leaf spot on Ningpo Figwort (Scrophularia ningpoensis) in China. Australas. Plant Dis. Notes 2019, 14, 1–6. [Google Scholar] [CrossRef]
- Mahapatra, S.; Rao, E.S.; Sandeepkumar, G.M.; Sriram, S. Stagonosporopsis cucurbitacearum the causal agent of gummy stem blight of watermelon in India. Australas. Plant Dis. Notes 2020, 15, 1–3. [Google Scholar] [CrossRef]
- Aveskamp, M.M.; Verkley, G.J.; de Gruyter, J.; Murace, M.A.; Perello, A.; Woudenberg, J.H.; Groenewald, J.Z.; Crous, P.W. DNA phylogenyreveals polyphyly of Phoma section Peyronellaea and multiple taxonomic novelties. Mycologia 2009, 101, 363–382. [Google Scholar] [CrossRef]
- Chethana, K.W.T.; Manawasinghe, I.S.; Hurdeal, V.G.; Bhunjun, C.S.; Appadoo, M.A.; Gentekaki, E.; Raspé, O.; Promputtha, I.; Hyde, K.D. What are fungal species and how to delineate them. Fungal Divers. 2021, 109, 1–25. [Google Scholar] [CrossRef]
- Jayawardena, R.S.; Hyde, K.D.; de Farias, A.R.G.; Bhunjun, C.S.; Ferdinandez, H.S.; Manamgoda, D.S.; Udayanga, D.; Herath, I.S.; Thambugala, K.M.; Manawasinghe, I.S.; et al. What is a species in fungal plant pathogens? Fungal Divers. 2021, 109, 239–266. [Google Scholar] [CrossRef]
- Bhunjun, C.S.; Niskanen, T.; Suwannarach, N.; Wannathes, N.; Chen, Y.J.; McKenzie, E.H.; Maharachchikumbura, S.S.; Buyck, B.; Zhao, C.L.; Fan, Y.G.; et al. The numbers of fungi: Are the most speciose genera truly diverse? Fungal Divers. 2022, 114, 387–462. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Phillips, A.J.L.; Pereira, D.S.; Dai, D.-Q.; Aptroot, A.; Monteiro, J.S.; Druzhinina, I.S.; Cai, F.; Fan, X.; Selbmann, L.; et al. Forecasting the number of species of asexually reproducing fungi (Ascomycota and Basidiomycota). Fungal Divers. 2022, 114, 1–28. [Google Scholar] [CrossRef]
- Maharachchikumbura, S.S.N.; Chen, Y.; Ariyawansa, H.A.; Hyde, K.D.; Haelewaters, D.; Perera, R.H.; Samarakoon, M.C.; Wanasinghe, D.N.; Bustamante, D.E.; Liu, J.; et al. Integrative approaches for species delimitation in Ascomycota. Fungal Divers. 2021, 109, 155–179. [Google Scholar] [CrossRef]
- He, Y.L.; He, G.; Li, Q.Q.; Lin, W.; Yuan, G.Q. First report of Stagonosporopsis vannaccii causing leaf spot on Crassocephalum crepidioides in China. Plant Dis. 2020, 105, 1–499. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, J.; Zhang, L.; Xu, L.; Yan, C.; Gong, Z. Identification and characteristics of Stagonosporopsis cucurbitacearum pathogenic factors influencing pumpkin seeding survival in northeast China. J. Phytopathol. 2018, 167, 41–55. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).