Abstract
The ecological success of lichens is related to both myco- and photobionts which condition the physiological limits of the lichen symbioses and thus affect their ecological niches and geographic ranges. A particular type of lichen, called cephalolichen, is characterized by housing both green algal and cyanobacterial symbionts—the latter is restricted to special structures called cephalodia. In this type of lichen, questions related to specialization within species or within individuals are still unsolved as different patterns have previously been observed. In order to study the variability at the intrathalline, intraspecific, and interspecific level, cyanobionts from different cephalodia within the same thalli and from different thalli were genetically analysed in three cephalolichen species at two different forests (18 thalli, 90 cephalodia). The results showed variability in the cephalodial Nostoc OTUs in all the studied species, both at the intrathalline and intraspecific levels. The variability of Nostoc OTUs found in different cephalodia of the same thallus suggests low specialization in this relationship. Additionally, differences in OTU diversity in the three studied species and in the two forests were found. The variability observed may confer an increased ecological plasticity and an advantage to colonize or persist under additional or novel habitats or conditions.
1. Introduction
Lichenization is a successful nutritional strategy, with almost 20% of all fungal species being lichenized [] and dominating about 8% of the land surface of the world []. According to photobiont association, lichens are divided into two groups. Bipartite lichens are characterized by a mycobiont establishing with green algae (chlorolichen) or cyanobacteria (cyanolichen), in which the photosynthetic partner (phycobiont) provides carbon products to the mycobiont. In tripartite lichens (cephalolichens), the fungus is simultaneously associated with both green algae (photobiont) and cyanobacteria (cyanobiont). In these lichens, the photobiont is distributed through the thallus, providing fixed carbon, and the cyanobiont is often confined to special structures, called cephalodia, supplying the fixed nitrogen. In this case, cyanobionts are specialized for nitrogen fixation; they are predominantly heterotrophic and show an increase in heterocyst proportions over 30% of non-symbiotic cyanobacterial cells and lichenized cyanobionts of bipartite cyanolichens [,].
Although lichens are compound organisms, and the physiological limits of the lichen symbiosis are driven by the association as an integrated whole, some aspects are specific to the photosynthetic symbiont []. Thus, photobionts are involved in photosynthesis, secondary compound production, nitrogen fixation, etc., and their preferences regarding abiotic conditions may limit the ecological niches and geographic ranges of lichens [,,,]. In this respect, the specialization of the fungal–photobiont association is related to the potential acclimation to local environmental conditions and colonization of new niches and geographic regions. Thus, low specialization between myco- and photobionts may preclude limitations if mycobionts have the ability to lichenize with different photobionts [] as they can expand their ecological niches and geographic distributions outside the physiological limits imposed by a single photobiont species. On the other hand, specialized species may have narrower geographical distributions and ecological niches [,]. While there are different attributes to characterize specialization in biotic interactions, the classification of organisms as specialists or generalists has been mostly based on the number of interacting partners [,]; however, see []. The variability in the photobiont partner is, thus, related to the specialization of the mycobiont for the photobiont and may be observed at different levels (biological, geographical, or ecological) [,,,,,]. At the biological level (individual, species, or genus levels), numerous studies have shown contrasting patterns: from a high specialization of mycobiont towards the photobiont, i.e., [,,,,], to a common pattern of generalization (i.e., a high number of interacting photobionts) [,,]. When comparing specialization between bi-membered (cyanolichens and chlorolichens) and tri-membered (cephalolichens) lichen species, several patterns have been found. A high specialization hypothesized for cephalolichens [] differed from the results of [], showing that cephalolichens are more generalized than cyanolichens, and from other studies, which found no differences between both [,]. At the intrathalline (individual) level, and concerning cephalolichens specifically, it has been shown that most tripartite lichens contain the same Nostoc strain in all cephalodia of individual thalli [], with the exception of Peltigera venosa, Lobaria pulmonaria, and three species of Pannaria (P. farinosa, P. sphinctrina, and P. lobulifera), which housed different cyanobionts in different cephalodia [,,]. However, this conclusion is based on very few studies analysing specifically the intrathalline variation in cephalodia. Additionally, concerning species with green algae, it has been shown that the co-occurrence of several photobionts in individual lichen thalli is relatively common [,,,,]. At the community scale, it has been revealed that lichens do not show a one-to-one relationship as different species of cyanobacteria are shared between different lichen species [,,,], like the Lichen Guilds theory proposes [,].
On the other hand, factors driving photobiont selection are diverse and related to phylogenetic relationships, reproductive strategy, the availability of photobionts, or ecological factors [,,,,,,,,]. In a recent study, the authors showed that photobiont availability, the function of the cyanobiont (principal photobiont for cyanolichens or secondary photobiont for cephalolichens), and the mycobiont species were factors related with specialization in cyanobacterial lichens [].
Based on this previous study, of which shows a high variability of Nostoc phylogroups in different lichen species along a latitudinal gradient in Chile, a question arose on whether this variability was also present at the intrathalline (individual) level, and if this variation may be related with the geographic gradient studied. As stated before, this variability had been observed in several tripartite lichen species previously [,,]. Thus, the aims of this study were to investigate if the individual thalli of tripartite lichens host different Nostoc genotypes depending on the species and on the studied forest. For this purpose, three cephalolichens species were selected at two different forests at different latitudes in Chile, and different cephalodia per thallus were genetically characterized.
2. Materials and Methods
Two forests from a previous study carried out in Chile and sampled between 2017 and 2018 were used (Parque Nacional Torres del Paine and Isla Navarino) []. Forest stands were mostly formed by Notofagus pumilio with over 65% of cover. Within these forests, three species of Peltigerales with cephalodia were collected: Pannaria farinosa, Nephroma antarcticum, and Pseudocyphellaria granulata. From each species, 6 thalli were selected (3 at each forest), and from each thallus, 5 different cephalodia were analysed. Samples were air-dried and stored at −20 °C. DNA from different cephalodia in each thallus was extracted using Chelex® 100 Chelating Resin (Bio-Rad, Hercules, CA, USA). Region rbcLX was amplified using primers CW and CX [] and the following program: 95 °C 15 min; 35 cycles of 1 min at 95 °C, 30 s at 54 °C, 30 s at 72 °C; and 10 min at 72 °C. The PCR products were sequenced at Macrogen Spain service (www.macrogen.com (accessed on 21 September 2020)) using the same primers employed in the PCR.
The obtained sequences were edited and aligned using Geneious Prime v. 2021.0.1 software (https://www.geneious.com (accessed on 11 January 2021)). Some samples failed in the PCR or were too short and were discarded. Ambiguous regions and introns were delimited manually and excluded for the analysis using AliView v. 1.26, Uppsala, Sweeden []. Nostoc sequences were grouped into operational taxonomic units (OTUs). The delimitation of Nostoc OTUs was based on the ASAP method (Assemble Species by Automatic Partitioning) [], which proposes partitions of species hypotheses using genetic distances calculated between DNA sequences. Additionally, we performed a phylogenetic and network analysis. The ASAP analysis was carried out in the webserver (https://bioinfo.mnhn.fr/abi/public/asap/ (accessed on 9 March 2021)), applying the Jukes–Cantor (JC69) model of substitution (groups below 0.01 probability were split, the 10 best scores were kept, and −1 was the seed value). Partitions included in the 0.001–0.01 range of genetic distances were selected. A maximum likelihood phylogenetic analysis (ML) was conducted with RAxML v. 8.2.12, Karlsruhe, Germany [], assuming the GTRGAMMA model. The node support was estimated with the rapid bootstrap algorithm, using 1000 pseudoreplicates. A haplotype network was constructed using the TCS method [], as implemented in PopART v. 1.7, Dunedin, New Zeland (http://popart.otago.ac.nz (accessed on 17 July 2023)).
3. Results
A total of 61 cyanobacterial consensus sequences were obtained (Table 1). The best partition obtained in ASAP was selected based on the lower score. Thus, a total of nine OTUs were found consorting with the three species (Table 1). This result is congruent with the ML phylogenetic analyses and the haplotype network, as shown in Figure 1 and Figure 2. Nostoc OTUs were shared between the species. Thus, OTUs 2 (present in 25 cephalodia out of 58), 1 (11), 3 (7) and 6 (6) were the most abundant, while the rest of OTUs (except 4) were only found in 1 cephalodia (Table 1; Figure 3).

Table 1.
Voucher and GenBank numbers of the species studied with thalli, cephalodia, and forest information and OTU classification based on the ASAP analysis, ML phylogenetic analysis, and TCS haplotype network. TP: Torres del Paine, IN: Navarino Island.
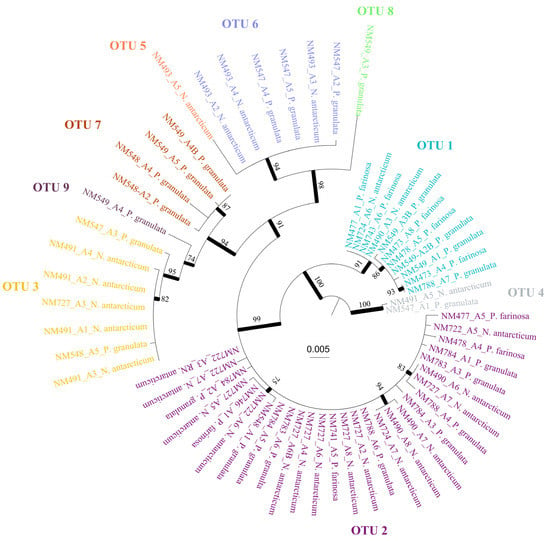
Figure 1.
Best tree from the ML analysis of the rbcLX region. Bootstrap values ≥70% are indicated on or below the branches and with thicker lines. OTUs delimited based on the ASAP results are depicted in the tree and represented with different colours.
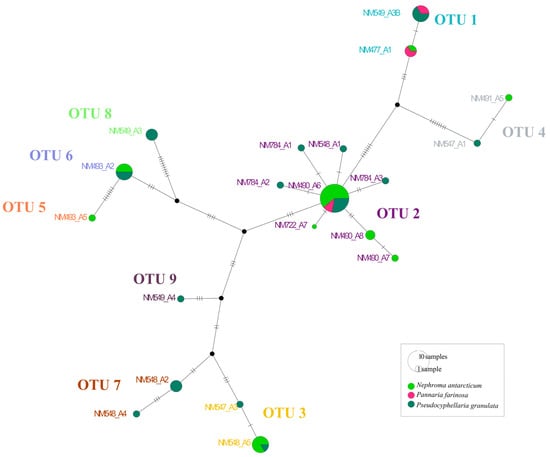
Figure 2.
The TCS haplotype network for Nostoc rbcLX sequences. The size of the pie chart is proportional to the number of thalli belonging to the haplotype, and the colour is according to the 3 studied species. The dash on the line represents one mutational step of the haplotype sequence. Black-filled circles indicate missing haplotypes. Haplotypes are grouped in OTUs obtained by the ASAP analysis.
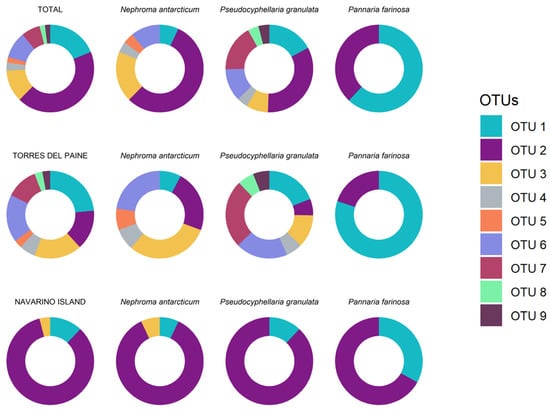
Figure 3.
The abundance (richness) of Nostoc OTUs found in the studied species at the different levels.
The obtained results were different in the different species and in both forests (Table 1 and Figure 3). Thus, Pseudocyphellaria granulata had the highest variability in OTUs followed by Nephroma antarcticum, which also showed high variability. The results in Pannaria farinosa showed lower variability in its cyanobionts. In addition, the two OTUs found in Pannaria farinosa (OTU 1 and 2) were shared with the other two lichen species. Also, OTUs 3, 4 and 6 were found in both Pseudocyphellaria granulata and Nephroma antarcticum. On the other hand, OTU 5 was exclusive from Nephroma antarcticum, while OTUs 7, 8, and 9 were only found in Pseudocyphellaria granulata.
When comparing forests, a significant difference was observed between Torres del Paine and Navarino Island, as the latter showed lower diversity in the cyanobionts found, and there were also differences in their abundances. Nine OTUs were found in Torres del Paine with a dominance of OTU 1; meanwhile, three OTUs were found in Navarino, with OTU 2 being dominant. Nonetheless, the OTUs from Navarino Island were a subset of those from Torres del Paine, showing a nested pattern.
The three species also showed differences in their cyanobionts depending on the forest. Nephroma antarcticum had a similar proportion of OTUs 2 and 3 in Torres del Paine, while Pseudocyphellaria granulata had a higher proportion of OTU 7. All three species showed a dominance of OTU 2 in Navarino.
All the species showed variability in their cephalodial Nostoc at the intrathaline (individual) and intraspecific levels. All thalli except one of N. antarcticum (five in total) had two different OTUs per thallus. In Pseudocyphellaria granulata, three thalli presented between three and four Nostoc OTUs. In this species, two thalli from Navarino only had one OTU and other sample had two OTUs. Despite the poor results obtained in Pannaria farinosa, as only nine cephalodia were successfully sequenced, the studied thalli had two OTUs.
4. Discussion
In relation to the variability of the photobiont partners in lichenized fungi, many basic questions remain unknown. An important step is to learn about this variability of photobionts in lichens at different levels, from the individual to the ecological communities. The results from this study, based on the richness of cyanobionts in tripartite lichens (cephalolichens), showed differences in the Nostoc OTUs from cephalodia at different scales: thallus, species, and forests.
At the lowest level, the thallus (individual), a high variability of cyanobionts was found in different cephalodia of the same thallus. Although there was a difference in the number of OTUs per thalli between the three studied species, all three showed the ability to harbour more than one and different Nostoc OTUs within a thallus. The coexistence inside a single lichen thallus of different Trebouxia species has been previously demonstrated [,,,], but it has rarely been studied in tripartite lichens (e.g., in Lobaria pulmonaria, Peltigera venosa and Pannaria) [,,]. In the case of Lobaria pulmonaria [], only one of the studied thalli showed different Nostoc genotypes in different cephalodia. The results obtained in Pannaria showed non identical Nostoc 16S sequences from different cephalodia in the same thallus in three Pannaria individuals belonging to P. farinosa, P. sphinctrina and P. lobulifera []. However, the genetic distance between these sequences is not enough to consider them as different haplotypes, at least in P. sphinctrina and P. lobulifera. In addition, previous morphological studies not using molecular data observed different cyanobacterial morphotypes within single thalli and occasionally even in the same cephalodium [,].
The ecological significance of photobiont coexistence in the same thallus is not clear. Additionally, it is not clear if this occurrence is a widespread phenomenon. On the one hand, the same mycobiont with different photobionts has shown differences in various aspects of its physiology []. On the other hand, [] demonstrated that the contribution of the secondary photobionts was marginal in most thalli. Additionally, [] suggested the possibility of the existence of different degrees of lichenization with different Nostoc strains, ranging from loosely associated colonies to well-corticated cephalodia in Peltigera venosa. It is unlikely that a loose association between the myco- and cyanobiont occurs in cephalodia—it has been shown that there is a high specific biorecognition process involved in the acquisition of the cephalodial Nostoc [], performed by specific lectins produced and secreted by the mycobiont. Thus, lectins produced by a vegetative fungal component in Peltigera aphthosa were shown to have a similar function in selecting the compatible cephalodial cyanobacterium as lectins produced by germinating spores. The observed photobiont diversity was already predicted to operate on the level of single cyanolichen thalli—especially in the case of cephalodiate species [].
At the species level (comparing different thalli from the same species), numerous studies have shown contrasting patterns: from a high specialization of mycobiont towards the photobiont [,,,,], to a common pattern of generalization (i.e., a high number of interacting photobionts) [,,,]. Previous results [] have shown that the identity of cyanobionts was related with the species identity of the lichen-forming fungus rather than the geographical area where the lichen was growing. In this previous example, the same lichen species collected in Sweden and Finland (i.e., Peltigera aphthosa, P. canina and Nephroma arcticum) had the same identical intron sequences in different samples of the same lichen species. Conversely, in the present study, the location (forests) determined the cyanobiont variability. Thus, in Navarino Island, there was a lower diversity of cyanobionts for the three studied species, suggesting that environmental variables may determine the cyanobiont pool found in ecological communities [].
In many cases [,], one fungal species can associate with more than one symbiont, and these are often also shared by several taxonomically unrelated cyanolichen species. This is also in line with the Lichen Guild theory [], where different species shared the same Nostoc genotype. As in our results, the most common Nostoc OTUs were shared between the three species, pointing out the existence of facilitation in a community context where some species may act as core species (source of cyanobionts), whereas others may act as fringe species, capturing their photobionts from the former ones []. Species with the ability to associate with many OTUs (generalized) may host compatible OTUs for other more specialized species, with the former acting as a core species, as could be Pseudocyphellaria granulata in the current study.
Several mechanisms have been proposed to explain the differences in specialization in lichens. Previous studies suggest that geographic and ecological factors including macroclimatic variables can drive the differences in the specialization of the association in both cyano- and chlorolichens [,]. In general, a low specialization towards phycobionts allows for the host to associate with ecologically diversified or locally adapted algae, thereby broadening the lichen ecological amplitude [,]. For instance, a previous study analysed Nostoc cyanobionts from five lichen species in maritime Antarctica and determined that lichens from those regions were more generalized than lichens from temperate and boreal regions; this was regarded as lichens adapting to extreme environmental stress []. However, this finding opposed the results obtained here. In addition, environmental factors have been found to affect the Nostoc pool at the community scale []. In Navarino Island, the diversity of Nostoc genotypes found was lower than in Torres del Paine, harbouring a subset of the genotypes found at northern locations (i.e., Torres del Paine). The ability of mycobiont species to interact with different cyanobionts in different localities, even though they are present, may allow for some fungal hosts to associate with the cyanobacterial genotypes that are optimally adapted to the conditions [].
Our study also notices the high variability of Nostoc OTUs (low specialization) at different biological and spatial scales in cephalolichens. This high variability of partners, even at the intrathalline level, could be due to a low dependence on the cyanobiont in those tri-membered lichens, as the main photosynthetic partner is green algae, which conducts the photosynthetic activity, whereas the cyanobiont’s main function is nitrogen fixation [,]. In addition, this study emphasizes the importance of the contextualization of the scale, as results based on one site could change when widening the spatial scale, and thus limit the understanding of specialization. Also, it highlights the importance of determining the local availability of cyanobionts, as differences in specialization between locations may be due to a different pool of the cyanobionts available to interact.
Author Contributions
Conceptualization, C.R.-A. and M.P.; methodology, C.R.-A. and M.P.; formal analysis, C.R.-A., N.M. and M.P.; investigation, C.R.-A., N.M. and M.P.; resources, C.R.-A., I.M., G.A. and M.P.; data curation, M.P.; writing—original draft preparation, C.R.-A. and M.P.; writing—review and editing, C.R.-A., N.M., G.A., I.M. and M.P.; visualization, C.R.-A. and M.P.; supervision, M.P.; project administration, M.P and I.M.; funding acquisition, M.P. and I.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by THE SPANISH “MINISTERIO DE ECONOMÍA Y COMPETITIVIDAD”, project NOTHODIVERSITY CGL2016-80562-P.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Sequences produced in this study are deposited in GenBank. Lichen samples used are deposited in Herbarium ARAN-Fungi.
Acknowledgments
The authors thank the logistical support offered by the Corporación Nacional Forestal (CONAF), and the attention and useful recommendations of administrative managers and forest guards of each of the National Parks or Reserves sampled. Work assistance during fieldwork was provided by Pilar Hurtado, Noelia Fernández and Luca Di Nuzzo. Diego Alarcón, Maritza A. K. Mihoc and Graciela Valencia helped with the correct treatment of the collected samples. We especially thank Manuel Rojo for fieldwork and trait measurements assistance and Raquel Pino for comments on the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kirk, P.M.; Cannon, P.F.; Minter, D.W.; Stalpers, J.A. Dictionary of the Fungi, 10th ed.; Cabi: Wallingford, UK, 2008; p. 640. [Google Scholar]
- Ahmadjian, V. Lichens are more important than you think. BioScience 1995, 45, 123–124. [Google Scholar] [CrossRef]
- Kershaw, K.A. Physiological Ecology of Lichens; Cambridge University Press: Cambridge, UK, 1985; p. 293. [Google Scholar]
- Rai, A.N. Nitrogen metabolism. In CRC Handbook of Lichenology, 1st ed.; Galun, M., Ed.; CRC Press Inc.: Boca Raton, FL, USA, 1998; Volume 1, pp. 201–237. [Google Scholar]
- Stanton, D.E.; Ormond, A.; Koch, N.M.; Colesie, C. Lichen ecophysiology in a changing climate. Am. J. Bot. 2023, 110, e16131. [Google Scholar] [CrossRef] [PubMed]
- Peksa, O.; Škaloud, P. Do photobionts influence the ecology of lichens? A case study of environmental preferences in symbiotic green alga Asterochloris (Trebouxiophyceae). Mol. Ecol. 2011, 20, 3936–3948. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Mendoza, F.; Domaschke, S.; García, M.A.; Jordan, P.; Martín, M.P.; Printzen, C. Population structure of mycobionts and photobionts of the widespread lichen Cetraria aculeata. Mol. Ecol. 2011, 20, 1208–1232. [Google Scholar] [CrossRef] [PubMed]
- Rolshausen, G.; Grande, F.D.; Sadowska-Deś, A.D.; Otte, J.; Schmitt, I. Quantifying the climatic niche of symbiont partners in a lichen symbiosis indicates mutualist mediated niche expansions. Ecography 2018, 41, 1380–1392. [Google Scholar] [CrossRef]
- Rolshausen, G.; Hallman, U.; Grande, F.D.; Otte, J.; Knudsen, K.; Schmitt, I. Expanding the mutualistic niche: Parallel symbiont turnover along climatic gradients. Proc. R. Soc. B 2020, 287, 20192311. [Google Scholar] [CrossRef]
- Muggia, L.; Pérez-Ortega, S.; Kopun, T.; Zellnig, G.; Grube, M. Photobiont selectivity leads to ecological tolerance and evolutionary divergence in a polymorphic complex of lichenized fungi. Ann. Bot. 2014, 114, 463–475. [Google Scholar] [CrossRef]
- Leavitt, S.D.; Kraichak, E.; Nelsen, M.P.; Altermann, S.; Divakar, P.K.; Alors, D.; Esslinger, T.L.; Crespo, A.; Lumbsch, T. Fungal specificity and selectivity for algae play a major role in determining lichen partnerships across diverse ecogeographic regions in the lichen-forming family Parmeliaceae (Ascomycota). Mol. Ecol. 2015, 24, 3779–3797. [Google Scholar] [CrossRef]
- Magain, N.; Miadlikowska, J.; Goffinet, B.; Sérusiaux, E.; Lutzoni, F. Macroevolution of specificity in cyanolichens of the genus Peltigera Section Polydactylon (Lecanoromycetes, Ascomycota). Syst. Biol. 2017, 66, 74–99. [Google Scholar]
- Blüthgen, N.; Menzel, F.; Blüthgen, N. Measuring specialization in species interaction networks. BMC Ecol. 2006, 6, 9. [Google Scholar] [CrossRef][Green Version]
- Sahli, H.F.; Conner, J.K. Characterizing ecological generalization in plant-pollination systems. Oecologia 2006, 148, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Arribas, C.; Prieto, M.; Aragón, G.; López-Angulo, J.; Escudero, A.; Martínez, I. Specialization: A new multidimensional and integrative perspective. Ecology 2023, 13, e10296. [Google Scholar]
- Fox, L.R.; Morrow, P.A. Specialization: Species property or local phenomenon? Science 1981, 211, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Fedrowitz, K.; Kaasalainen, U.; Rikkinen, J. Geographic mosaic of symbiont selectivity in a genus of epiphytic cyanolichens. Ecol. Evol. 2012, 2, 2291–2303. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Magain, N.; Miadlikowska, J.; Coyle, J.R.; Truong, C.; Lutzoni, F. Bioclimatic factors at an intrabiome scale are more limiting than cyanobiont availability for the lichen-forming genus Peltigera. Am. J. Bot. 2018, 105, 1198–1211. [Google Scholar] [CrossRef]
- Herrera, P.; Suárez, J.P.; Sánchez-Rodríguez, A.; Molina, M.C.; Prieto, M.; Méndez, M. Many broadly-shared mycobionts characterize mycorrhizal interactions of two coexisting epiphytic orchids in a high elevation tropical forest. Fungal Ecol. 2019, 39, 26–36. [Google Scholar] [CrossRef]
- Rodríguez-Arribas, C.; Martínez, I.; Aragón, G.; Zamorano-Elgueta, C.; Cavieres, L.; Prieto, M. Specialization patterns in symbiotic associations: A community perspective over spatial scales. Ecol. Evol. 2023, 13, e10296. [Google Scholar] [CrossRef]
- Paulsrud, P.; Rikkinen, J.; Lindblad, P. Cyanobiont specificity in some Nostoc-containing lichens and in a Peltigera aphthosa photosymbiodeme. New Phytol. 1998, 139, 517–524. [Google Scholar] [CrossRef]
- Piercey-Normore, M.D.; DePriest, P.T. Algal switching among lichen symbioses. Am. J. Bot. 2001, 88, 1490–1498. [Google Scholar] [CrossRef]
- Myllys, L.; Stenroos, S.; Thell, A.; Kuusinen, M. High cyanobiont selectivity of epiphytic lichens in old growth boreal forest of Finland. New Phytol. 2007, 173, 621–629. [Google Scholar] [CrossRef]
- O’Brien, H.E.; Miadlikowska, J.; Lutzoni, F. Assessing population structure and host specialization in lichenized cyanobacteria. New Phytol. 2013, 198, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, N.; Lumbsch, T.; Green, T.G.A.; Turk, R.; Pintado, A.; Sancho, L.; Schroeter, B. Lichen fungi have low cyanobiont selectivity in maritime Antarctica. New Phytol. 2003, 160, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Muggia, L.; Vancurova, L.; Škaloud, P.; Peksa, O.; Wedin, M.; Grube, M. The symbiotic playground of lichen thalli—A highly flexible photobiont association in rock-inhabiting lichens. FEMS Microbiol. Ecol. 2013, 85, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Deś, A.D.; Dal Grande, F.; Lumbsch, H.T.; Beck, A.; Otte, J.; Hur, J.S.; Kim, J.A.; Schmitt, I. Integrating coalescent and phylogenetic approaches to delimit species in the lichen photobiont Trebouxia. Mol. Phylogenet. Evol. 2014, 76, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Paulsrud, P.; Rikkinen, J.; Lindblad, P. Field investigations on cyanobacterial specificity in Peltigera aphthosa. New Phytol. 2001, 152, 117–123. [Google Scholar] [CrossRef]
- Pardo De la Hoz, C.J.; Magain, N.; Lutzoni, F.; Goward, T.; Restrepo, S.; Miadlikowska, J. Contrasting symbiotic patterns in two closely related lineages of trimembered lichens of the genus Peltigera. Front. Microbiol. 2019, 9, 2770. [Google Scholar] [CrossRef]
- Elvebakk, A.; Papaefthimiou, D.; Robertsen, E.; Liaimer, A. Phylogenetic patterns among Nostoc cyanobionts within bi- and tripartite lichens of the genus Pannaria. J. Phycol. 2008, 44, 1049–1059. [Google Scholar] [CrossRef]
- Paulsrud, P.; Rikkinen, J.; Lindblad, P. Spatial patterns of photobiont diversity in some Nostoc-containing lichens. New Phytol. 2000, 146, 291–299. [Google Scholar] [CrossRef]
- Bačkor, M.; Peksa, O.; Škaloud, P.; Bačkorová, M. Photobiont diversity in lichens from metal-rich substrata based on ITS rDNA sequences. Ecotoxicol. Environ. Saf. 2010, 73, 603–612. [Google Scholar] [CrossRef]
- Moya, P.; Molins, A.; Martinez-Alberola, F.; Muggia, L.; Barreno, E. Unexpected associated microalgal diversity in the lichen Ramalina farinacea is uncovered by pyrosequencing analyses. PLoS ONE 2017, 12, 1–21. [Google Scholar] [CrossRef]
- Vančurová, L.; Muggia, L.; Peksa, O.; Řídká, T.; Škaloud, P. The complexity of symbiotic interactions influences the ecological amplitude of the host: A case study in Stereocaulon (lichenized Ascomycota). Mol. Ecol. 2018, 27, 3016–3033. [Google Scholar] [CrossRef] [PubMed]
- Onuț-Brännström, I.; Benjamin, M.; Scofield, D.G.; Heiðmarsson, S.; Andersson, M.G.I.; Lindström, E.S.; Johannesson, H. Sharing of photobionts in sympatric populations of Thamnolia and Cetraria lichens: Evidence from high-throughput sequencing. Sci. Rep. 2018, 8, 4406. [Google Scholar] [CrossRef] [PubMed]
- Peksa, O.; Gebouská, T.; Škvorová, Z.; Vančurová, L.; Škaloud, P. The guilds in green algal lichens-an insight into the life of terrestrial symbiotic communities. FEMS Microbiol. Ecol. 2022, 98, fiac008. [Google Scholar] [CrossRef] [PubMed]
- Rikkinen, J.; Oksanen, I.; Lohtander, K. Lichen guilds share related cyanobacterial symbionts. Science 2002, 297, 357. [Google Scholar] [CrossRef]
- Rikkinen, J. Ecological and evolutionary role of photobiont-mediated guilds in lichens. Symbiosis 2003, 34, 99–110. [Google Scholar]
- Yahr, R.; Vilgalys, R.; DePriest, P.T. Geographic variation in algal partners of Cladonia subtenuis (Cladoniaceae) highlights the dynamic nature of a lichen symbiosis. New Phytol. 2006, 171, 847–860. [Google Scholar] [CrossRef]
- Otálora, M.A.G.; Martínez, I.; O’Brien, H.; Molina, M.C.; Aragón, G.; Lutzoni, F. Multiple origins of high reciprocal specificity at an intercontinental spatial scale among gelatinous lichens (Collemataceae, Lecanoromycetes). Mol. Phylogenet. Evol. 2010, 56, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Dal Grande, F.; Divakar, P.K.; Otte, J.; Crespo, A.; Schmitt, I. Fungal–algal association patterns in lichen symbiosis linked to macroclimate. New Phytol. 2017, 214, 317–329. [Google Scholar] [CrossRef]
- Mark, K.; Laanisto, L.; Bueno, C.G.; Niinemets, Ü.; Keller, C.; Scheidegger, C. Contrasting co-occurrence patterns of photobiont and cystobasidiomycete yeast associated with common epiphytic lichen species. New Phytol. 2020, 227, 1362–1375. [Google Scholar] [CrossRef] [PubMed]
- Rudi, K.; Skulberg, O.M.; Jakobsen, K.S. Evolution of cyanobacteria by exchange of genetic material among phyletically related strains. J. Bacteriol. 1998, 180, 3453–3461. [Google Scholar] [CrossRef]
- Larsson, A. AliView: A fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 2014, 30, 3276–3278. [Google Scholar] [CrossRef]
- Puillandre, N.; Brouillet, S.; Achaz, G. ASAP: Assemble species by automatic partitioning. Mol. Ecol. Resour. 2021, 21, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Clement, M.; Snell, Q.; Walker, P.; Posada, D.; Crandall, K. TCS: Estimating gene genealogies. In Proceedings of the Parallel and Distributed Processing Symposium, International Proceedings, Ft. Lauderdale, FL, USA, 15–19 April 2002; Volume 2, p. 184. [Google Scholar]
- Piercey-Normore, M.D.; Coxson, D.; Goward, T.; Goffinet, B. Phylogenetic position of a Pacific North American endemic cyanolichen, Nephroma occultum (Ascomycota, Peltigerales). Lichenologist 2006, 38, 441–456. [Google Scholar] [CrossRef]
- del Campo, E.M.; Gimeno, J.; de Nova, J.P.G.; Casano, L.M.; Gasulla, F.; García-Breijo, F.; Reig, F.J.; Barreno, E. South European populations of Ramalina farinacea (L.) Ach. Share different Trebouxia algae. Bibl. Lichenol. 2010, 105, 247–256. [Google Scholar]
- Blázquez, M.; Hernández-Moreno, L.S.; Gasulla, F.; Pérez-Vargas, I.; Pérez-Ortega, S. The role of photobionts as drivers of diversification in an island radiation of lichen-forming Fungi. Front. Microbiol. 2022, 12, 784182. [Google Scholar] [CrossRef] [PubMed]
- Jordan, W.P.; Rickson, F.R. Cyanophyte cephalodia in the lichen genus Nephroma. Am. J. Bot. 1971, 58, 562–568. [Google Scholar] [CrossRef]
- Tschermak-Woess, E. The algal partner. In CRC Handbook of Lichenology, 1st ed.; Galun, M., Ed.; CRC Press Inc.: Boca Raton, FL, USA, 1998; Volume 1, pp. 39–92. [Google Scholar]
- Casano, L.M.; del Campo, E.M.; García-Breijo, F.J.; Reig-Armiñana, J.; Gasulla, F.; Del Hoyo, A.; Guéra, A.; Barreno, E. Two Trebouxia algae with different physiological performances are ever-present in lichen thalli of Ramalina farinacea. Coexistence versus competition? Environ. Microbiol. 2011, 13, 806–818. [Google Scholar] [CrossRef] [PubMed]
- Lehr, H.; Galun, M.; Ott, S.; Jahns, H.M.; Fleminger, G. Cephalodia of the lichen Peltigera aphthosa (L.) Willd. Specific recognition of the compatible photobiont. Symbiosis 2000, 29, 357–365. [Google Scholar]
- Rikkinen, J. Molecular studies on cyanobacterial diversity in lichen symbioses. MycoKeys 2013, 6, 3–32. [Google Scholar] [CrossRef]
- O’Brien, H.E.; Miadlikowska, J.; Lutzoni, F. Assessing host specialization in symbiotic cyanobacteria associated with four closely related species of the lichen fungus Peltigera. Eur. J. Phycol. 2005, 40, 363–378. [Google Scholar] [CrossRef]
- Cardós, J.L.H.; Prieto, M.; Jylhä, M.; Aragón, G.; Molina, M.C.; Martínez, I.; Rikkinen, J. A case study on the re-establishment of the cyanolichen symbiosis: Where do the compatible photobionts come from? An. Bot. 2019, 124, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.N.; Soderback, E.; Bergman, B. Cyanobacterium–plant symbioses. New Phytol. 2000, 147, 449–481. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).