Eco-Friendly Production of Polyvinyl Alcohol/Carboxymethyl Cellulose Wound Healing Dressing Containing Sericin
Abstract
1. Introduction
2. Results and Discussion
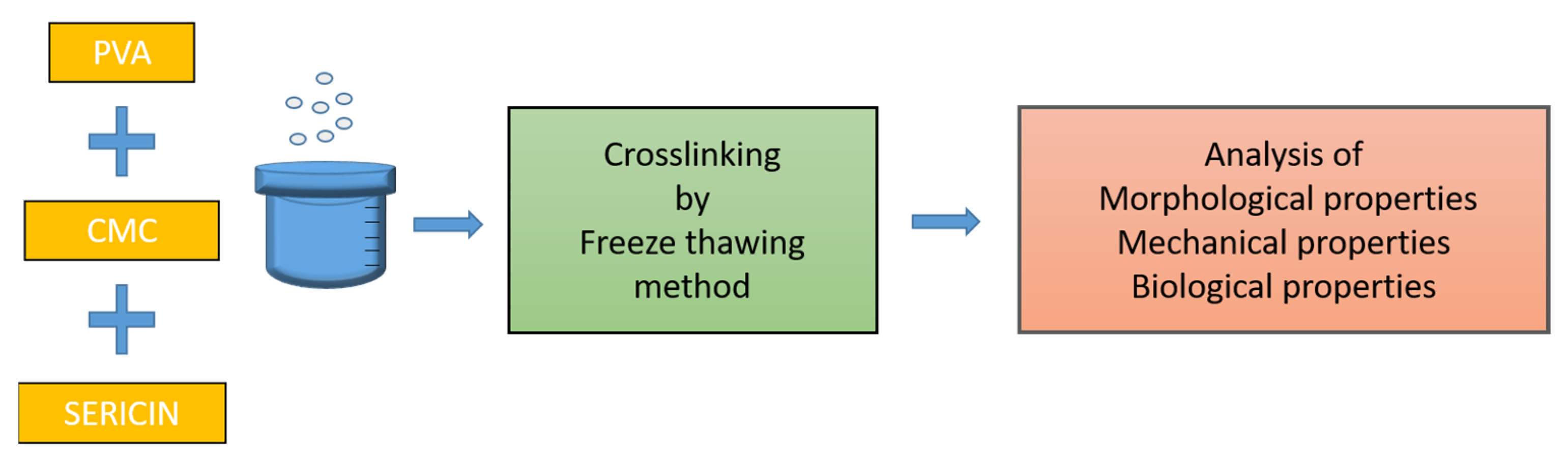
2.1. Eco-Friendly Preparation Method
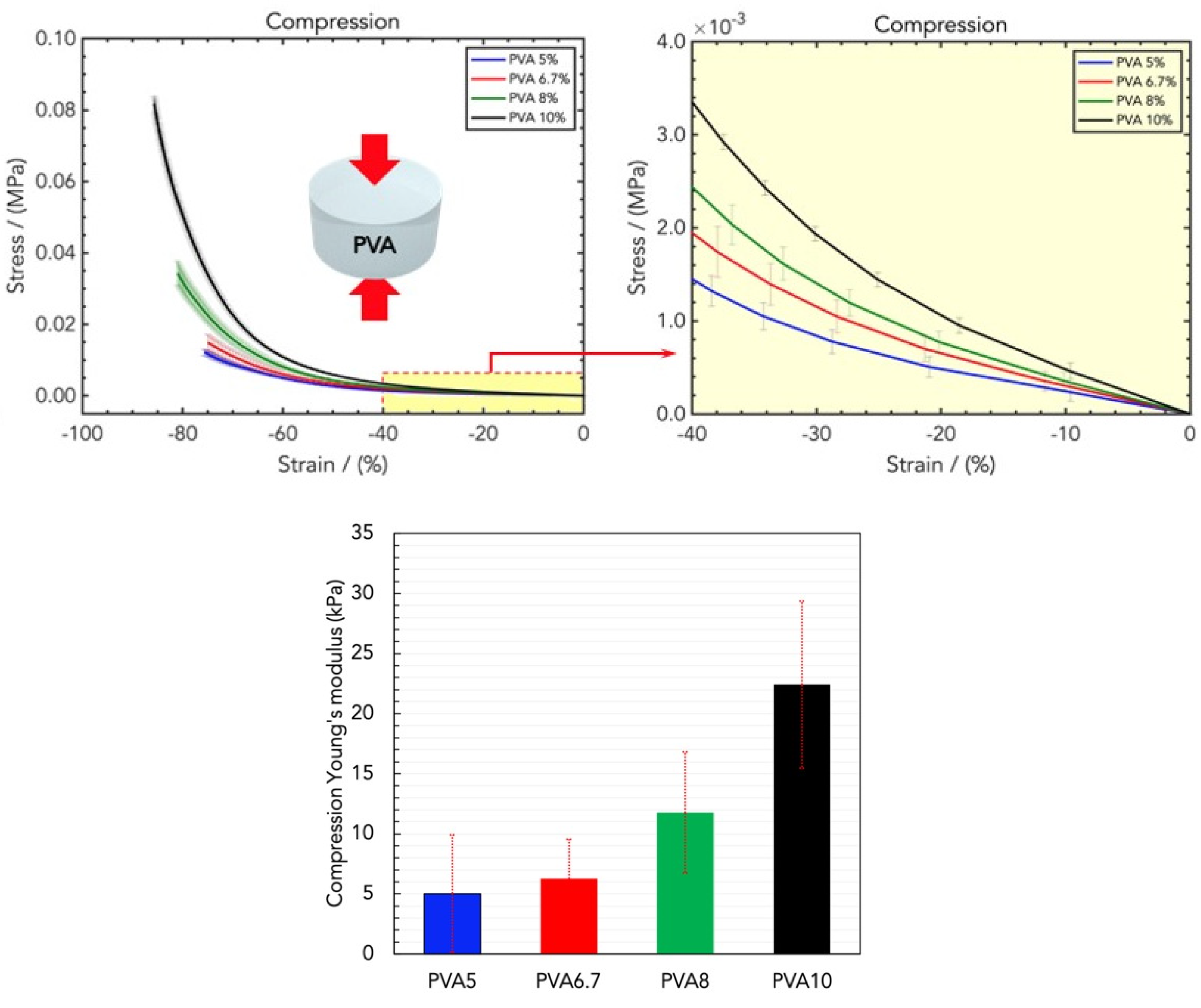
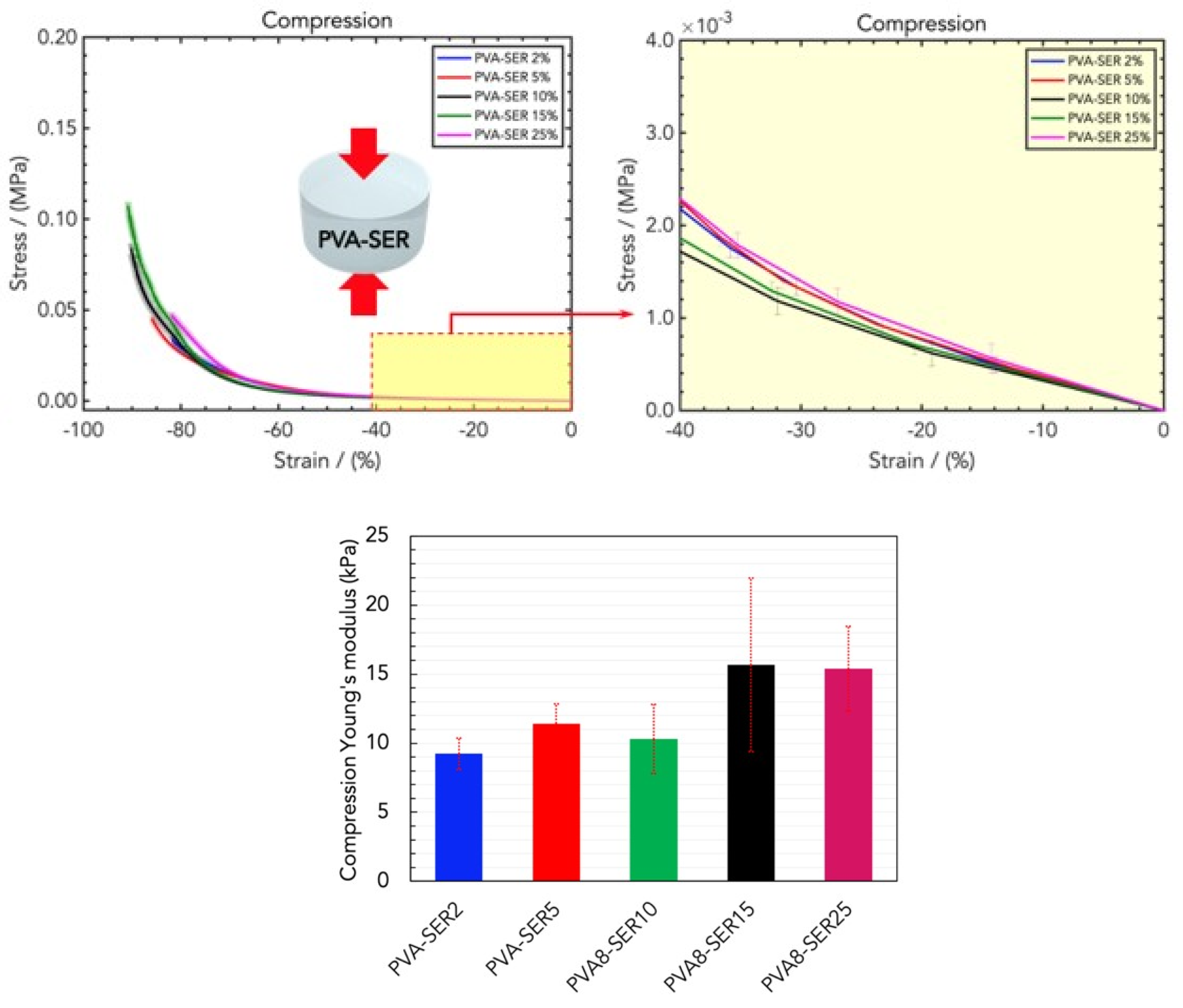
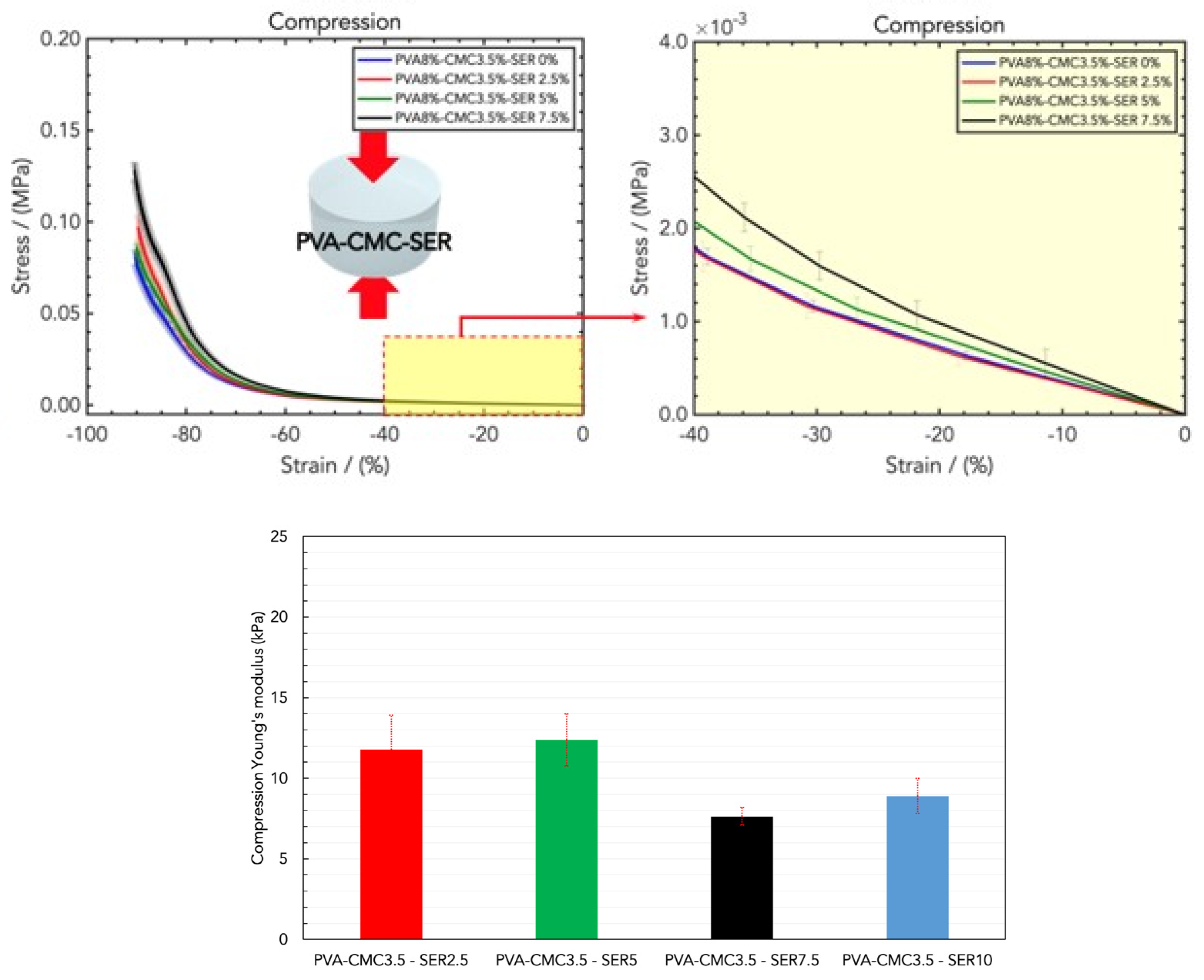
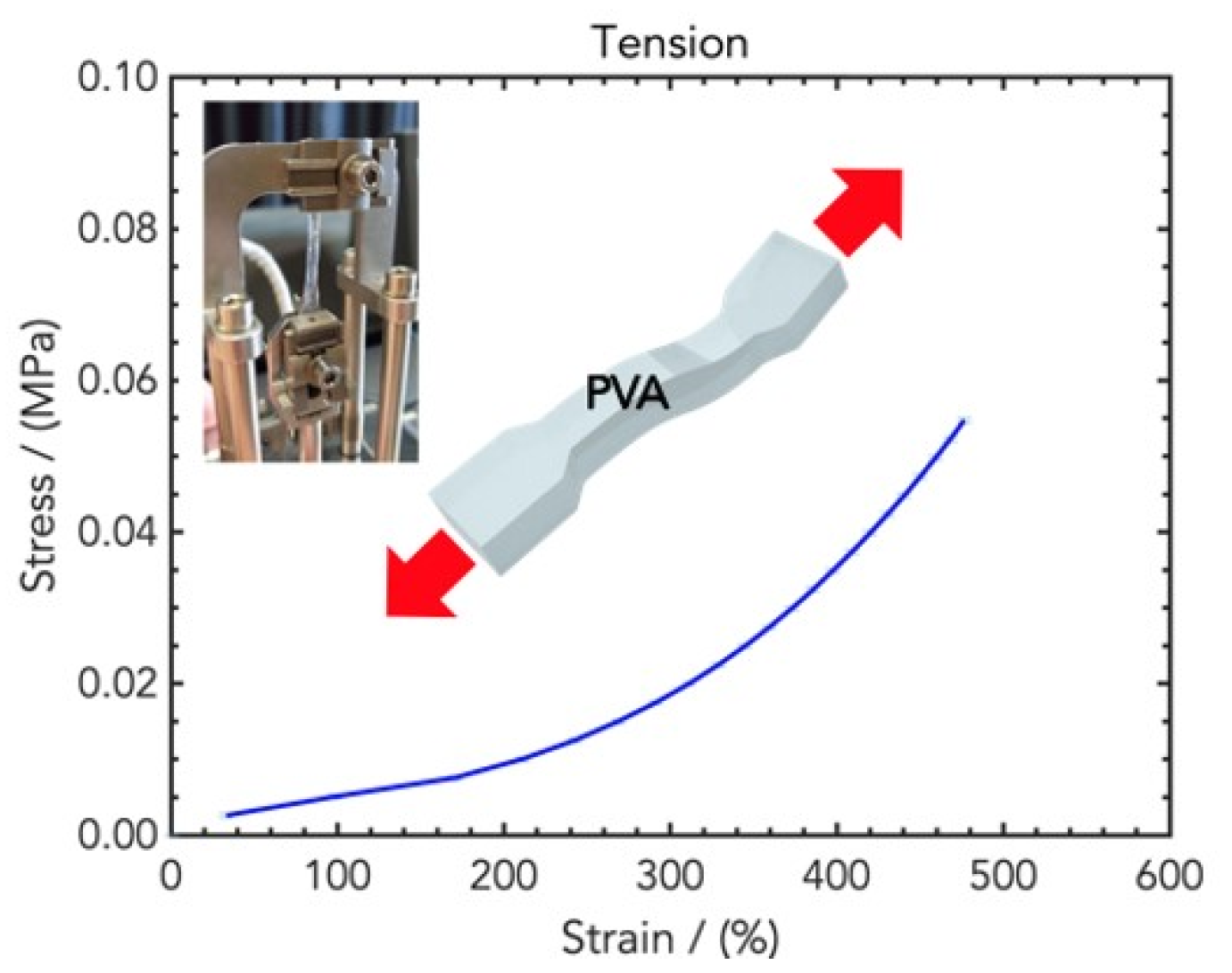
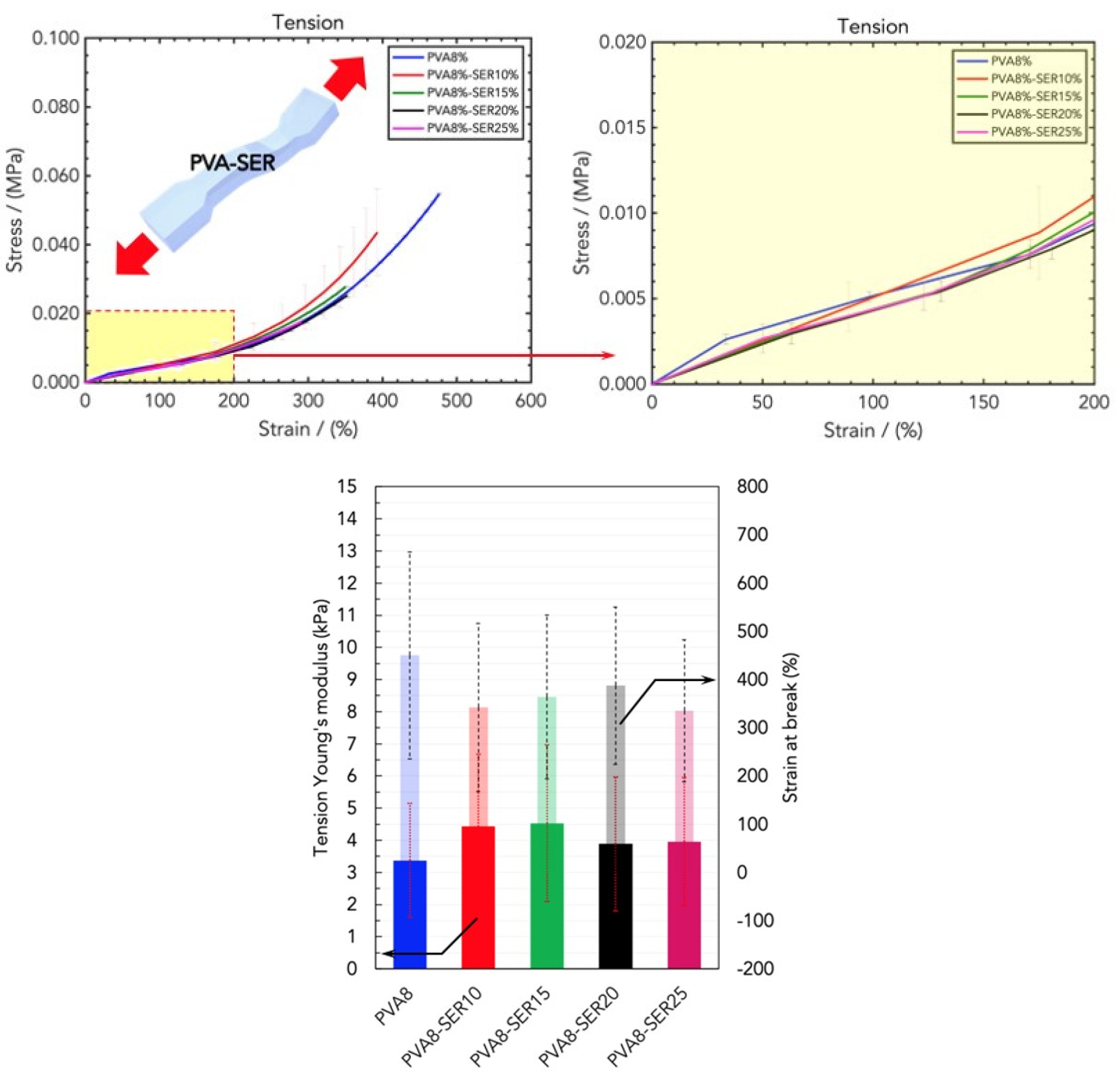
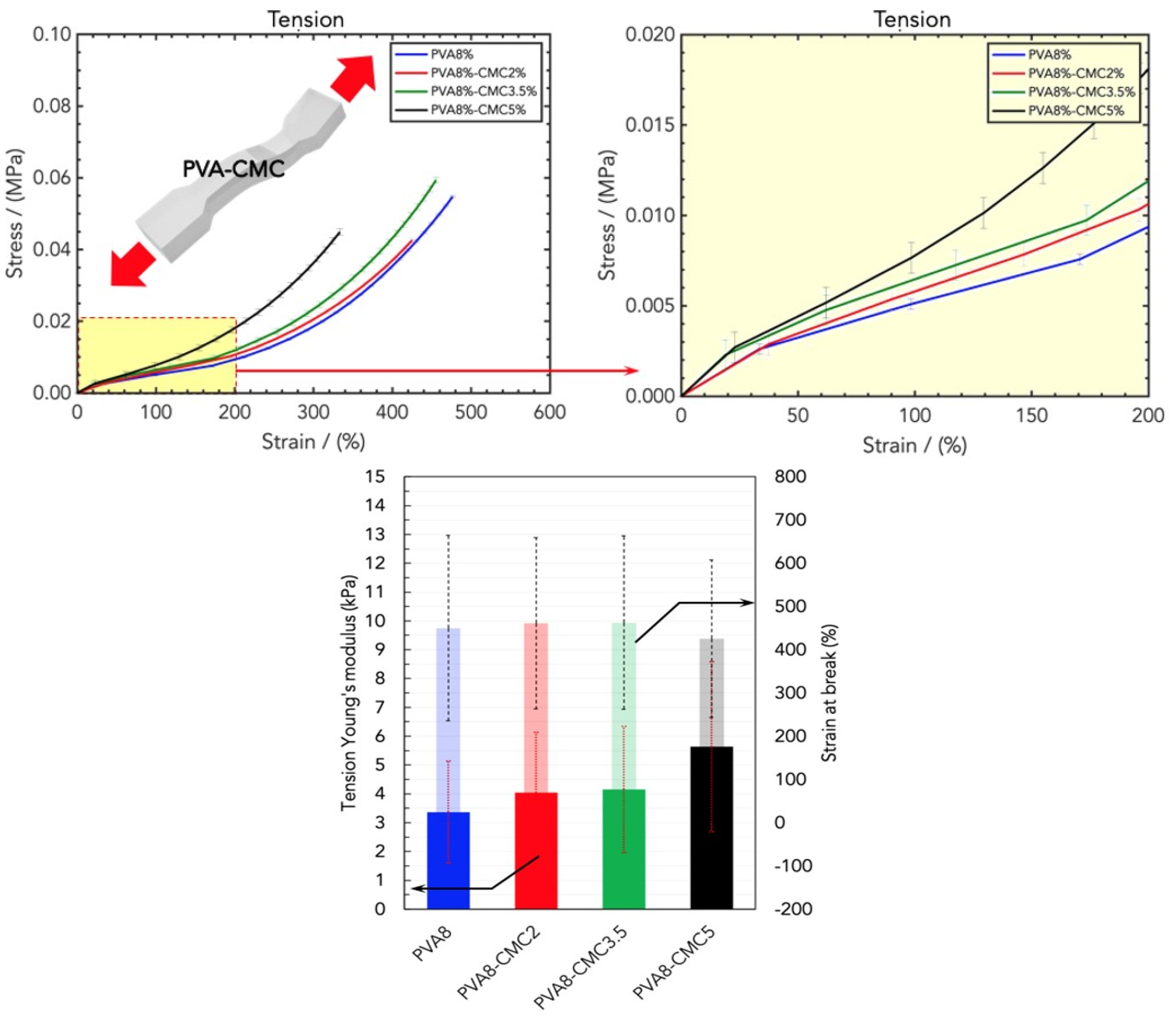
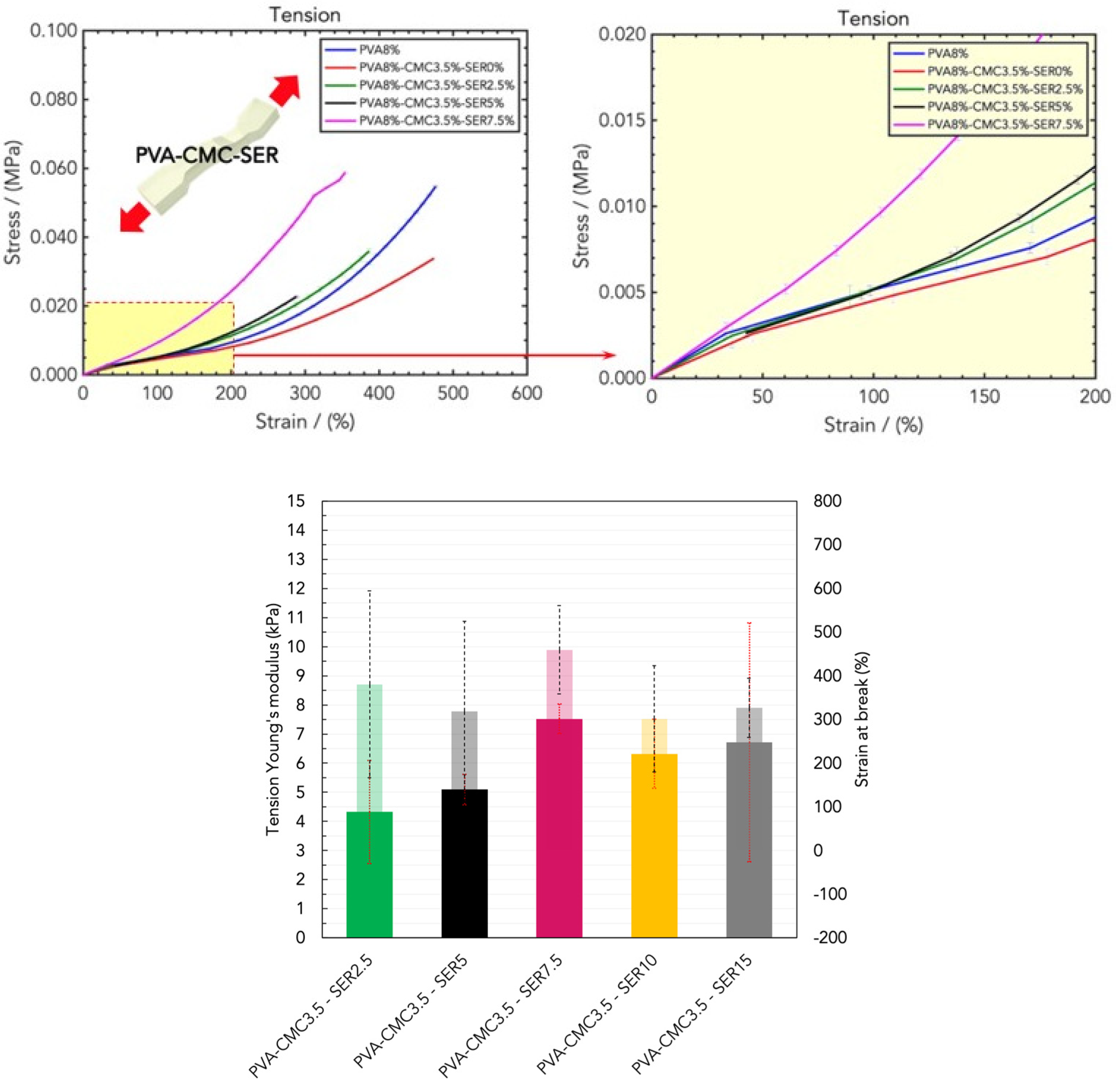
2.2. Mechanical Properties of Wound Healing Dressings
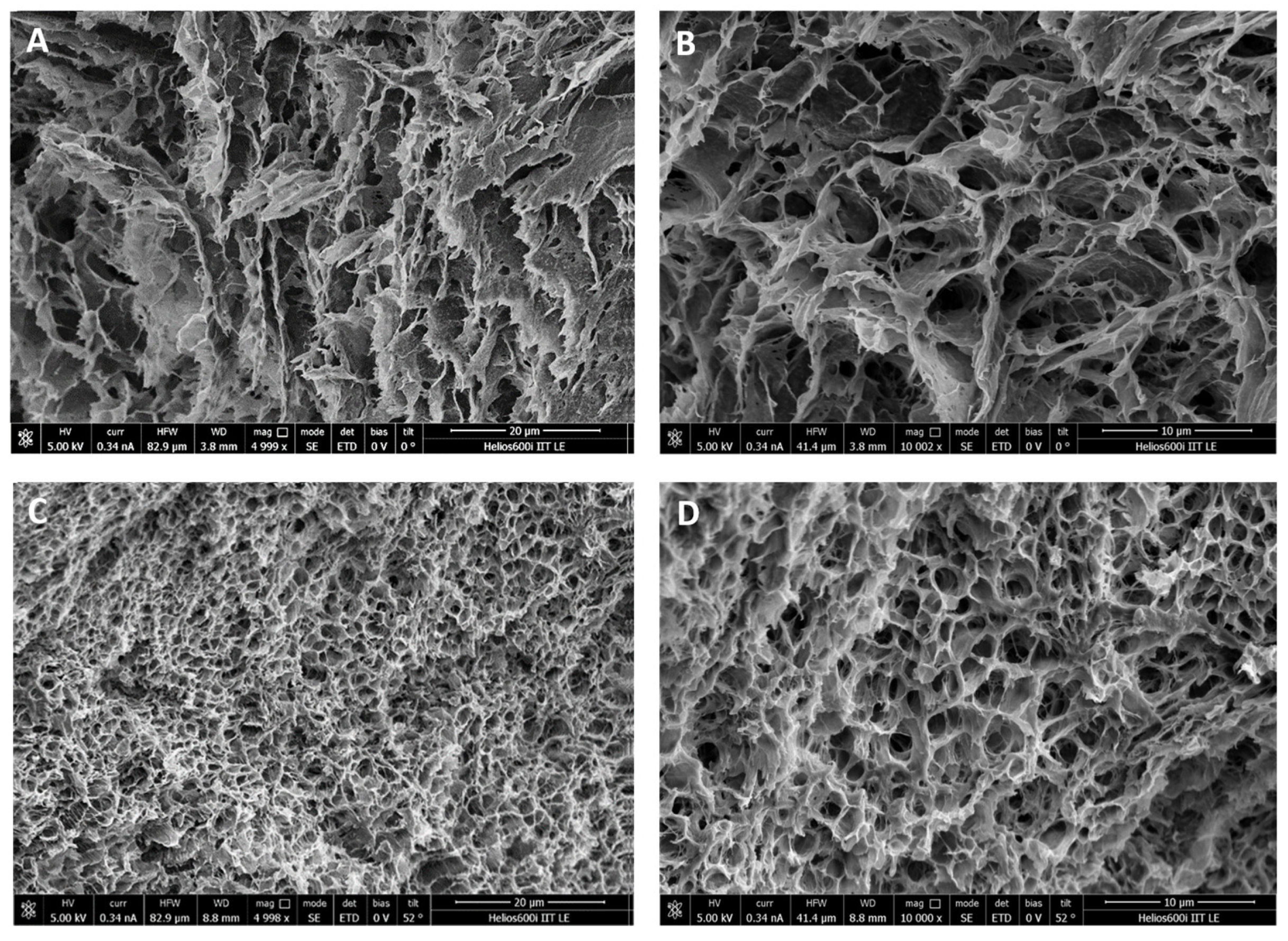
2.3. Morphological Characterization of the Wound Dressings
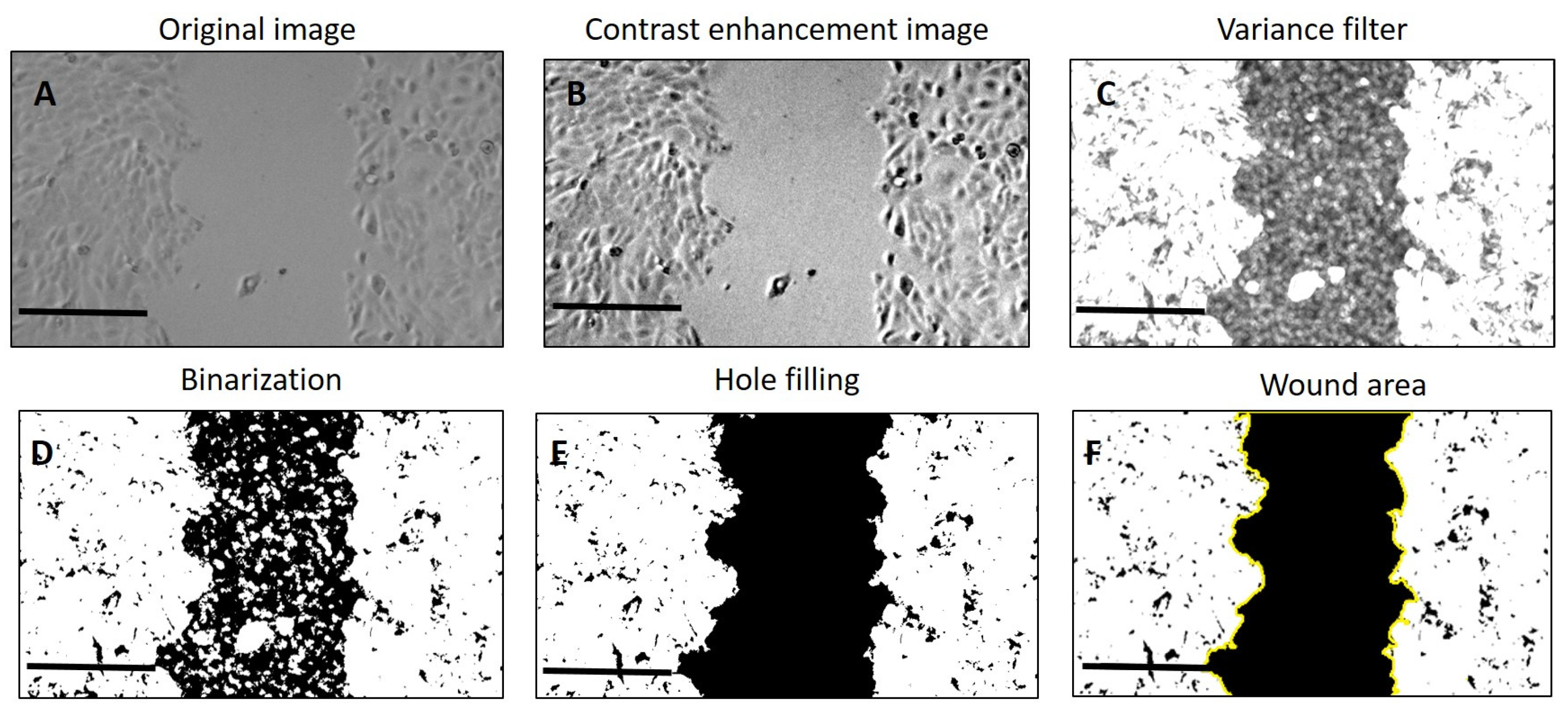
2.4. Biological Properties of Wound-Healing Dressings
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Hydrogel Wound Healing Dressing Preparation
4.3. Mechanical Tensile and Compression Measurements
4.4. Morphological Characterization by SEM Analysis
4.5. Wound Scratch Assay
4.6. Ex Vivo Experiments
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blacklow, S.O.; Li, J.; Freedman, B.R.; Zeidi, M.; Chen, C.; Mooney, D.J. Bioinspired mechanically active adhesive dressings to accelerate wound closure. Sci. Adv. 2019, 5, eaaw3963. [Google Scholar] [CrossRef] [PubMed]
- Alven, S.; Aderibigbe, B.A. Fabrication of Hybrid Nanofibers from Biopolymers and Poly (Vinyl Alcohol)/Poly (ε-Caprolactone) for Wound Dressing Applications. Polymers 2021, 13, 2104. [Google Scholar] [CrossRef] [PubMed]
- Junker, J.P.; Kamel, R.A.; Caterson, E.J.; Eriksson, E. Clinical Impact Upon Wound Healing and Inflammation in Moist, Wet, and Dry Environments. Adv. Wound Care 2013, 2, 348–356. [Google Scholar] [CrossRef]
- Stan, D.; Tanase, C.; Avram, M.; Apetrei, R.; Mincu, N.-B.; Mateescu, A.L.; Stan, D. Wound healing applications of creams and “smart” hydrogels. Exp. Dermatol. 2021, 30, 1218–1232. [Google Scholar] [CrossRef] [PubMed]
- Gould, L.; Abadir, P.; Brem, H.; Carter, M.; Conner-Kerr, T.; Davidson, J.; DiPietro, L.; Falanga, V.; Fife, C.; Gardner, S.; et al. Chronic wound repair and healing in older adults: Current status and future research. Wound Repair Regen. 2015, 23, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Insuasti-Cruz, E.; Suárez-Jaramillo, V.; Mena Urresta, K.A.; Pila-Varela, K.O.; Fiallos-Ayala, X.; Dahoumane, S.A.; Alexis, F. Natural Biomaterials from Biodiversity for Healthcare Applications. Adv. Healthc. Mater. 2022, 11, 2101389. [Google Scholar] [CrossRef]
- Bernal-Chávez, S.A.; Romero-Montero, A.; Hernández-Parra, H.; Peña-Corona, S.I.; Del Prado-Audelo, M.L.; Alcalá-Alcalá, S.; Cortés, H.; Kiyekbayeva, L.; Sharifi-Rad, J.; Leyva-Gómez, G. Enhancing chemical and physical stability of pharmaceuticals using freeze-thaw method: Challenges and opportunities for process optimization through quality by design approach. J. Biol. Eng. 2023, 17, 35. [Google Scholar] [CrossRef] [PubMed]
- Waresindo, W.X.; Luthfianti, H.R.; Priyanto, A.; Hapidin, D.A.; Edikresnha, D.; Aimon, A.H.; Suciati, T.; Khairurrijal, K. Freeze–thaw hydrogel fabrication method: Basic principles, synthesis parameters, properties, and biomedical applications. Mater. Res. Express 2023, 10, 024003. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Singh, V.K.; Chauhan, S. A review on mechanical and water absorption properties of polyvinyl alcohol based composites/films. J. Mech. Behav. Mater. 2017, 26, 213–222. [Google Scholar] [CrossRef]
- Djumaev, A.; Tashmukhamedova, S. Physical and chemical properties of PVA-CMC based hydrogel carrier loaded with herbal hemostatic agent for application as wound dressings. Natl. J. Physiol. Pharm. Pharmacol. 2020, 10, 905–909. [Google Scholar]
- Ceylan, S.; Göktürk, D.; Bölgen, N. Effect of crosslinking methods on the structure and biocompatibility of polyvinyl alcohol/gelatin cryogels. Bio-Med. Mater. Eng. 2016, 27, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Bai, J.; Shao, K.; Tang, W.; Zhao, X.; Lin, D.; Huang, S.; Chen, C.; Ding, Z.; Ye, J. Poly(vinyl alcohol) Hydrogels: The Old and New Functional Materials. Int. J. Polym. Sci. 2021, 2021, 16. [Google Scholar] [CrossRef]
- Bates, N.M.; Puy, C.; Jurney, P.L.; McCarty, O.J.T.; Hinds, M.T. Evaluation of the Effect of Crosslinking Method of Poly(Vinyl Alcohol) Hydrogels on Thrombogenicity. Cardiovasc. Eng. Technol. 2020, 11, 448–455. [Google Scholar] [CrossRef]
- Adelnia, H.; Ensandoost, R.; Shebbrin Moonshi, S.; Gavgani, J.N.; Vasafi, E.I.; Ta, H.T. Freeze/thawed polyvinyl alcohol hydrogels: Present, past and future. Eur. Polym. J. 2022, 164, 110974. [Google Scholar] [CrossRef]
- Koosha, M.; Aalipour, H.; Sarraf Shirazi, M.J.; Jebali, A.; Chi, H.; Hamedi, S.; Wang, N.; Li, T.; Moravvej, H. Physically Crosslinked Chitosan/PVA Hydrogels Containing Honey and Allantoin with Long-Term Biocompatibility for Skin Wound Repair: An In Vitro and In Vivo Study. J. Funct. Biomater. 2021, 12, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Hasan, M.S. Recent Developments of Carboxymethyl Cellulose. Polymers 2021, 13, 1345. [Google Scholar] [CrossRef]
- Verma, N.; Pramanik, K.; Singh, A.K.; Biswas, A. Design of magnesium oxide nanoparticle incorporated carboxy methyl cellulose/poly vinyl alcohol composite film with novel composition for skin tissue engineering. Mater. Technol. 2022, 37, 706–716. [Google Scholar] [CrossRef]
- Kanikireddy, V.; Varaprasad, K.; Jayaramudu, T.; Karthikeyan, C.; Sadiku, R. Carboxymethyl cellulose-based materials for infection control and wound healing: A review. Int. J. Biol. Macromol. 2020, 164, 963–975. [Google Scholar] [CrossRef]
- Shin, J.-Y.; Lee, D.Y.; Yoon, J.I.; Song, Y.-S. Effect of CMC Concentration on Cell Growth Behavior of PVA/CMC Hydrogel. Macromol. Res. 2020, 28, 813–819. [Google Scholar] [CrossRef]
- Capanema, N.S.; Mansur, A.A.; Carvalho, I.C.; Carvalho, S.M.; Mansur, H.S. Bioengineered Water-Responsive Carboxymethyl Cellulose/Poly(vinyl alcohol) Hydrogel Hybrids for Wound Dressing and Skin Tissue Engineering Applications. Gels 2023, 9, 166. [Google Scholar] [CrossRef] [PubMed]
- Thönes, S.; Rother, S.; Wippold, T.; Blaszkiewicz, J.; Balamurugan, K.; Moeller, S.; Ruiz-Gómez, G.; Schnabelrauch, M.; Scharnweber, D.; Saalbach, A. Hyaluronan/collagen hydrogels containing sulfated hyaluronan improve wound healing by sustained release of heparin-binding EGF-like growth factor. Acta Biomater. 2019, 86, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo-Quiceno, N.; Rueda-Mira, S.; Marín, J.F.S.; Álvarez-López, C. Development of a novel silk sericin-based hydrogel film by mixture design. J. Polym. Res. 2023, 30, 120. [Google Scholar] [CrossRef]
- Ekasurya, W.; Sebastian, J.; Puspitasari, D.; Asri, P.P.P.; Asri, L.A.T.W. Synthesis and Degradation Properties of Sericin/PVA Hydrogels. Gels 2023, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Puspitasari, D.; Anwar, A.M.; Ananda, D.S.G.; Reza, G.; Jusuf, A.; Asri, L.A.T.W. Porous Sericin/PVA/Moringa oleifera Hydrogels: Physical Properties and Hyperelastic Model. Procedia Struct. Integr. 2024, 52, 410–417. [Google Scholar] [CrossRef]
- Su, D.; Ding, S.; Shi, W.; Huang, X.; Jiang, L. Bombyx mori silk-based materials with implication in skin repair: Sericin versus regenerated silk fibroin. J. Biomater. Appl. 2019, 34, 36–46. [Google Scholar] [CrossRef]
- Das, G.; Shin, H.-S.; Campos, E.V.R.; Fraceto, L.F.; del Pilar Rodriguez-Torres, M.; Mariano, K.C.F.; de Araujo, D.R.; Fernández-Luqueño, F.; Grillo, R.; Patra, J.K. Sericin based nanoformulations: A comprehensive review on molecular mechanisms of interaction with organisms to biological applications. J. Nanobiotechnology 2021, 19, 30. [Google Scholar] [CrossRef] [PubMed]
- Ersel, M.; Uyanikgil, Y.; Karbek Akarca, F.; Ozcete, E.; Altunci, Y.A.; Karabey, F.; Cavusoglu, T.; Meral, A.; Yigitturk, G.; Oyku Cetin, E. Effects of Silk Sericin on Incision Wound Healing in a Dorsal Skin Flap Wound Healing Rat Model. Med. Sci. Monit. 2016, 22, 1064–1078. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.S.; Costa, E.C.; Reis, S.; Spencer, C.; Calhelha, R.C.; Miguel, S.P.; Ribeiro, M.P.; Barros, L.; Vaz, J.A.; Coutinho, P. Silk Sericin: A Promising Sustainable Biomaterial for Biomedical and Pharmaceutical Applications. Polymers 2022, 14, 4931. [Google Scholar] [CrossRef]
- Ye, X.; Zhao, S.; Wu, M.; Ruan, J.; Tang, X.; Wang, X.; Zhong, B. Role of sericin 1 in the immune system of silkworms revealed by transcriptomic and proteomic analyses after gene knockout. FEBS Open Bio 2021, 11, 2304–2318. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Seidi, O.; Ribeiro, N.; Colaço, R.; Serro, A.P. Tribomechanical Comparison between PVA Hydrogels Obtained Using Different Processing Conditions and Human Cartilage. Materials 2019, 12, 3413. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Kundu, S.C. Sericin–carboxymethyl cellulose porous matrices as cellular wound dressing material. J. Biomed. Mater. Res. Part A 2014, 102, 1928–1940. [Google Scholar] [CrossRef] [PubMed]
- Reizabal, A.; Costa, C.M.; Pérez-Álvarez, L.; Vilas-Vilela, J.L.; Lanceros-Méndez, S. Silk Fibroin as Sustainable Advanced Material: Material Properties and Characteristics, Processing, and Applications. Adv. Funct. Mater. 2023, 33, 2210764. [Google Scholar] [CrossRef]
- Reis, T.C.; Castleberry, S.; Rego, A.M.B.; Aguiar-Ricardo, A.; Hammond, P.T. Three-dimensional multilayered fibrous constructs for wound healing applications. Biomater. Sci. 2016, 4, 319–330. [Google Scholar] [CrossRef]
- Koch, M.; Włodarczyk-Biegun, M.K. Faithful scanning electron microscopic (SEM) visualization of 3D printed alginate-based scaffolds. Bioprinting 2020, 20, e00098. [Google Scholar] [CrossRef]
- Rahman, M.S.; Islam, M.M.; Islam, M.S.; Zaman, A.; Ahmed, T.; Biswas, S.; Sharmeen, S.; Rashid, T.U.; Rahman, M.M. Morphological Characterization of Hydrogels. In Cellulose-Based Superabsorbent Hydrogels; Mondal, M.I.H., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 819–863. [Google Scholar]
- Dong, X.; Zhao, S.-X.; Yin, X.-L.; Wang, H.-Y.; Wei, Z.-G.; Zhang, Y.-Q. Silk sericin has significantly hypoglycaemic effect in type 2 diabetic mice via anti-oxidation and anti-inflammation. Int. J. Biol. Macromol. 2020, 150, 1061–1071. [Google Scholar] [CrossRef]
- Basu, P.; Narendrakumar, U.; Arunachalam, R.; Devi, S.; Manjubala, I. Characterization and Evaluation of Carboxymethyl Cellulose-Based Films for Healing of Full-Thickness Wounds in Normal and Diabetic Rats. ACS Omega 2018, 3, 12622–12632. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Dey, S.; Kundu, S.C. Skin equivalent tissue-engineered construct: Co-cultured fibroblasts/keratinocytes on 3D matrices of sericin hope cocoons. PLoS ONE 2013, 8, e74779. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, R.; Tao, G.; Wang, P.; Zuo, H. A Novel AgNPs/Sericin/Agar Film with Enhanced Mechanical Property and Antibacterial Capability. Molecules 2018, 23, 1821. [Google Scholar] [CrossRef]
- Gonzalez, A.C.d.O.; Costa, T.F.; Andrade, Z.d.A.; Medrado, A.R.A.P. Wound healing-A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef]
- Hofmann, E.; Schwarz, A.; Fink, J.; Kamolz, L.-P.; Kotzbeck, P. Modelling the Complexity of Human Skin In Vitro. Biomedicines 2023, 11, 794. [Google Scholar] [CrossRef] [PubMed]
- Cialdai, F.; Risaliti, C.; Monici, M. Role of fibroblasts in wound healing and tissue remodeling on Earth and in space. Front. Bioeng. Biotechnol. 2022, 10, 958381. [Google Scholar] [CrossRef]
- Tariq, M.; Tahir, H.M.; Butt, S.A.; Ali, S.; Ahmad, A.B.; Raza, C. Silk derived formulations for accelerated wound healing in diabetic mice. PeerJ 2021, 9, e10232. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.-Y.; Kweon, H.; Oh, J.-H. Sericin for Tissue Engineering. Appl. Sci. 2020, 10, 8457. [Google Scholar]
- Kunz, R.I.; Brancalhão, R.M.C.; Ribeiro, L.d.F.C.; Natali, M.R.M. Silkworm Sericin: Properties and Biomedical Applications. BioMed Res. Int. 2016, 2016, 8175701. [Google Scholar] [CrossRef]
- Martínez-Mora, C.; Mrowiec, A.; García-Vizcaíno, E.M.; Alcaraz, A.; Cenis, J.L.; Nicolás, F.J. Fibroin and sericin from Bombyx mori silk stimulate cell migration through upregulation and phosphorylation of c-Jun. PLoS ONE 2012, 7, e42271. [Google Scholar] [CrossRef] [PubMed]
- Siritientong, T.; Angspatt, A.; Ratanavaraporn, J.; Aramwit, P. Clinical potential of a silk sericin-releasing bioactive wound dressing for the treatment of split-thickness skin graft donor sites. Pharm. Res. 2014, 31, 104–116. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Shi, X.; Qin, S.; Liu, J.; Lv, Q.; Liu, J.; Li, Q.s.; Wang, Z.; Wang, L. Development and Application of an Advanced Biomedical Material-Silk Sericin. Adv. Mater. 2024, 36, e2311593. [Google Scholar] [CrossRef]
- ASTM E8/E8M-22; Standard Test Methods for Tension Testing of Metallic Materials. 03.01. ASTM International: West Conshohocken, PA, USA, 2024.
- Grada, A.; Otero-Vinas, M.; Prieto-Castrillo, F.; Obagi, Z.; Falanga, V. Research Techniques Made Simple: Analysis of Collective Cell Migration Using the Wound Healing Assay. J. Investig. Dermatol. 2017, 137, e11–e16. [Google Scholar] [CrossRef]
- Suarez-Arnedo, A.; Torres Figueroa, F. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef]







| Sample Name | Composition (mG per G of Hydrogel) | ||
|---|---|---|---|
| PVA | CMC | Sericin | |
| PVA10 | 100 | 0 | 0 |
| PVA8 | 80 | 0 | 0 |
| PVA6.7 | 67 | 0 | 0 |
| PVA5 | 50 | 0 | 0 |
| PVA8/CMC2 | 80 | 1.6 | 0 |
| PVA8/CMC3.5 | 80 | 2.8 | 0 |
| PVA8/CMC5 | 80 | 4 | 0 |
| PVA8/CMC10 | 80 | 8 | 0 |
| PVA8/SER2 | 80 | 0 | 1.6 |
| PVA8/SER5 | 80 | 0 | 4 |
| PVA8/SER10 | 80 | 0 | 8 |
| PVA8/SER15 | 80 | 0 | 12 |
| PVA8/SER25 | 80 | 0 | 20 |
| PVA8/CMC3.5/SER2.5 | 80 | 2.8 | 2 |
| PVA8/CMC3.5/SER5 | 80 | 2.8 | 4 |
| PVA8/CMC3.5/SER7.5 | 80 | 2.8 | 6 |
| PVA8/CMC3.5/SER10 (SER1) | 80 | 2.8 | 8 |
| PVA8/CMC3.5/SER15 (SER2) | 80 | 2.8 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariello, M.; Binetti, E.; Todaro, M.T.; Qualtieri, A.; Brunetti, V.; Siciliano, P.; De Vittorio, M.; Blasi, L. Eco-Friendly Production of Polyvinyl Alcohol/Carboxymethyl Cellulose Wound Healing Dressing Containing Sericin. Gels 2024, 10, 412. https://doi.org/10.3390/gels10060412
Mariello M, Binetti E, Todaro MT, Qualtieri A, Brunetti V, Siciliano P, De Vittorio M, Blasi L. Eco-Friendly Production of Polyvinyl Alcohol/Carboxymethyl Cellulose Wound Healing Dressing Containing Sericin. Gels. 2024; 10(6):412. https://doi.org/10.3390/gels10060412
Chicago/Turabian StyleMariello, Massimo, Enrico Binetti, Maria Teresa Todaro, Antonio Qualtieri, Virgilio Brunetti, Pietro Siciliano, Massimo De Vittorio, and Laura Blasi. 2024. "Eco-Friendly Production of Polyvinyl Alcohol/Carboxymethyl Cellulose Wound Healing Dressing Containing Sericin" Gels 10, no. 6: 412. https://doi.org/10.3390/gels10060412
APA StyleMariello, M., Binetti, E., Todaro, M. T., Qualtieri, A., Brunetti, V., Siciliano, P., De Vittorio, M., & Blasi, L. (2024). Eco-Friendly Production of Polyvinyl Alcohol/Carboxymethyl Cellulose Wound Healing Dressing Containing Sericin. Gels, 10(6), 412. https://doi.org/10.3390/gels10060412









