Gelatin-Based Scaffolds with Carrageenan and Chitosan for Soft Tissue Regeneration
Abstract
:1. Introduction
2. Results and Discussion
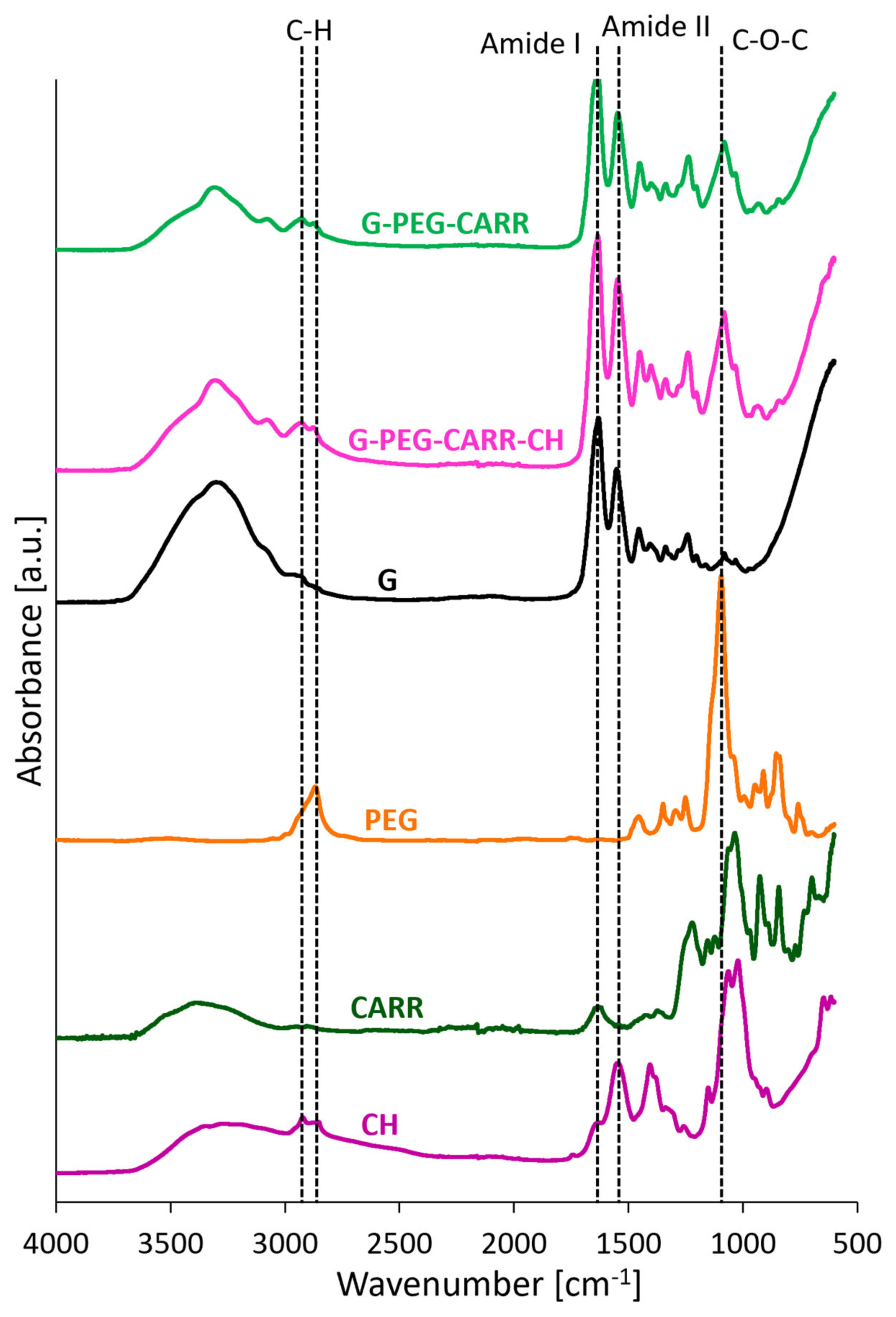
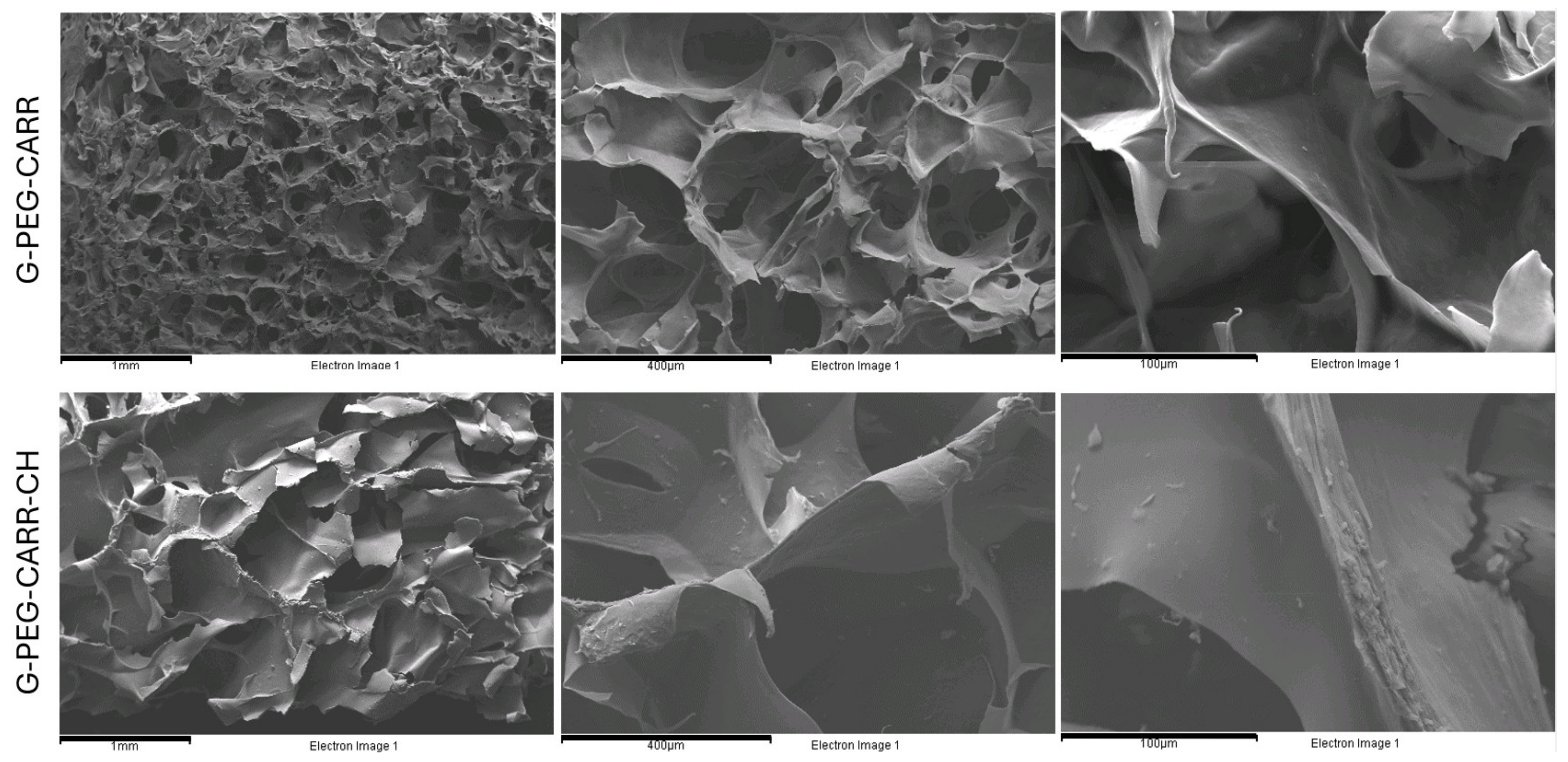
2.1. Physicochemical Properties of the Hydrogels
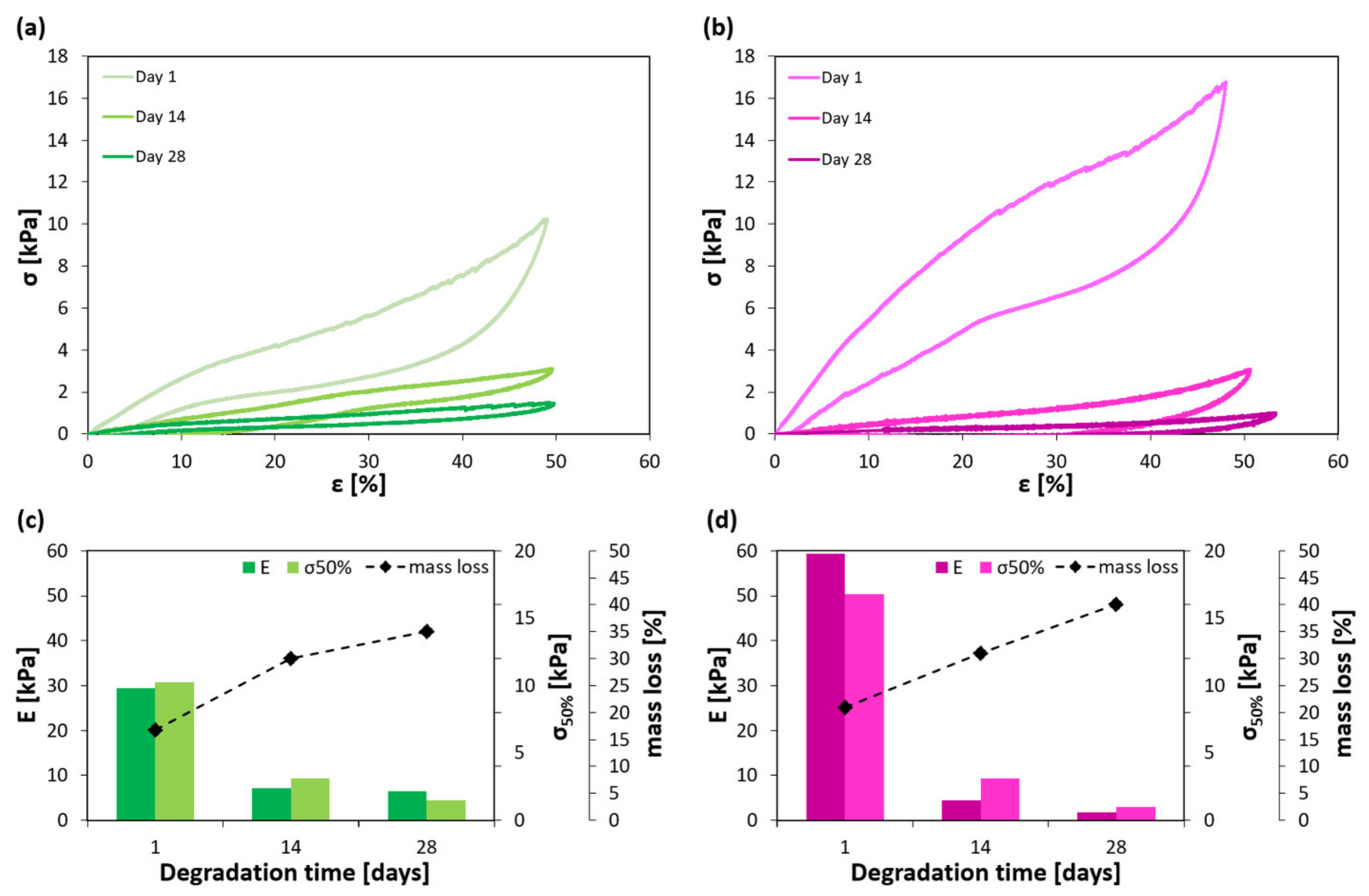
2.2. Mechanical Properties of the Hydrogels
2.3. Results of In Vitro Studies on Hydrogel Scaffolds
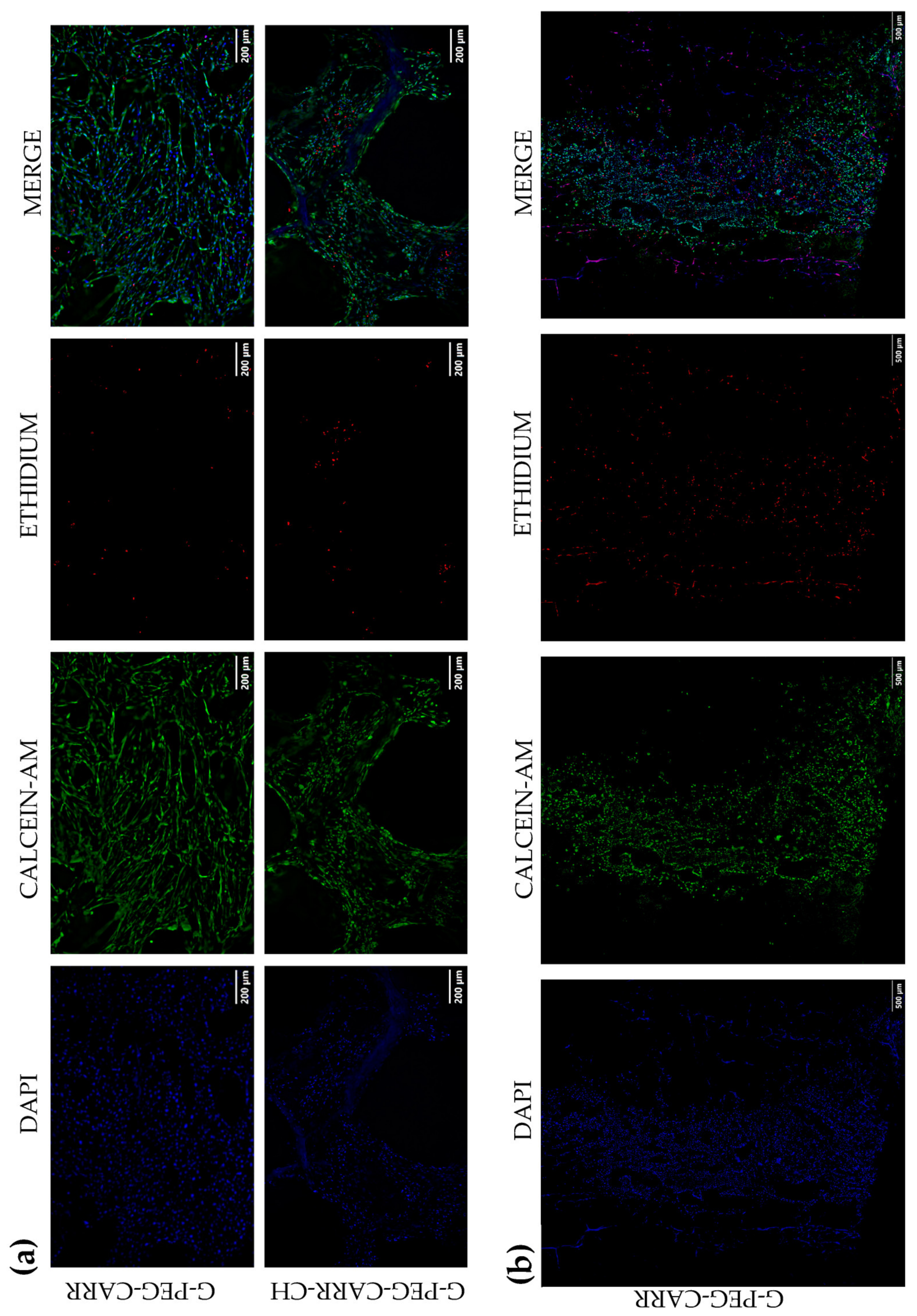
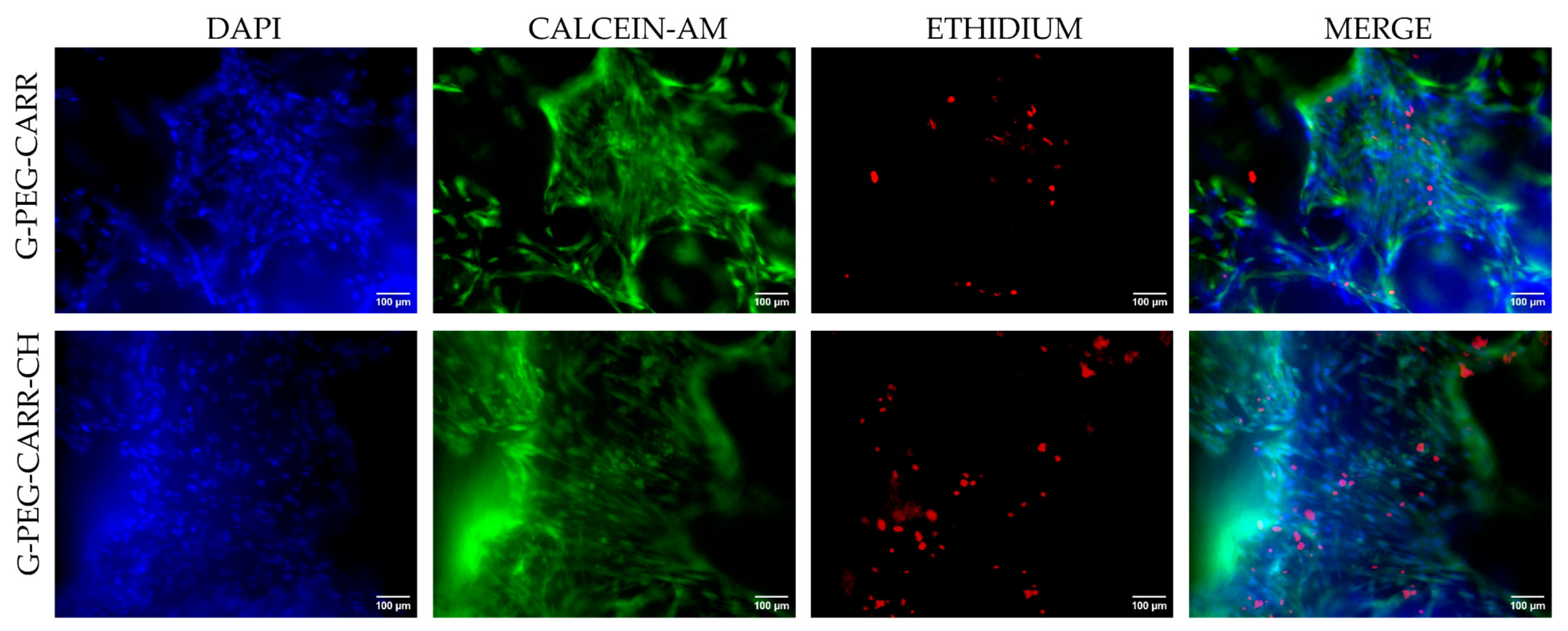
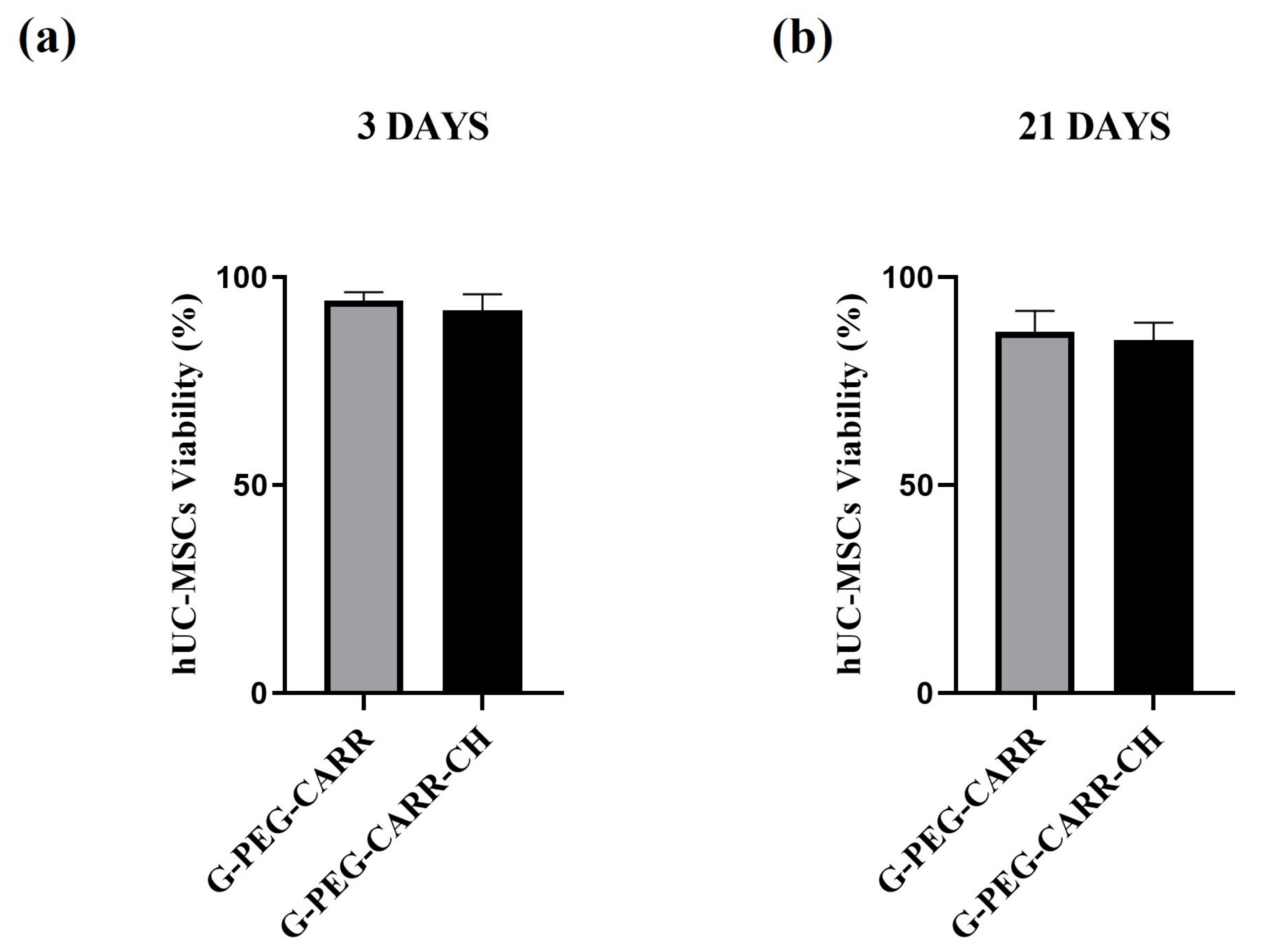
2.3.1. hUC-MSCs Maintained Viability into the G-PEG-CARR and G-PEG-CARR-CH Scaffolds
2.3.2. hUC-MSCs Proliferate with an Increasing Trend into the Scaffolds
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Hydrogels
4.2.1. Preparation of the G-PEG-CARR Hydrogel
4.2.2. Preparation of the G-PEG-CARR-CH Hydrogel
4.3. Chemical Structure and Morphology Analysis
4.4. Evaluation of Apparent Density, Porosity, Swelling and Mass Loss
4.5. Mechanical Characterization
4.6. In Vitro Biological Characterization
4.6.1. Human Umbilical Cord-Derived Mesenchymal Stem Cells Culture
4.6.2. Human Platelet Lysate Production
4.6.3. hUC-MSCs Cell Proliferation and Cell Viability Assay
4.6.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abalymov, A.; Parakhonskiy, B.; Skirtach, A.G. Polymer-and Hybrid-Based Biomaterials for Interstitial, Connective, Vascular, Nerve, Visceral and Musculoskeletal Tissue Engineering. Polymers 2020, 12, 620. [Google Scholar] [CrossRef] [PubMed]
- Edgar, L.; McNamara, K.; Wong, T.; Tamburrini, R.; Katari, R.; Orlando, G. Heterogeneity of Scaffold Biomaterials in Tissue Engineering. Materials 2016, 9, 332. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef] [PubMed]
- Neves, S.C.; Moroni, L.; Barrias, C.C.; Granja, P.L. Leveling Up Hydrogels: Hybrid Systems in Tissue Engineering. Trends Biotechnol. 2020, 38, 292–315. [Google Scholar] [CrossRef] [PubMed]
- González-Díaz, E.C.; Varghese, S. Hydrogels as Extracellular Matrix Analogs. Gels 2016, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Smagina, V.; Yudaev, P.; Kuskov, A.; Chistyakov, E. Polymeric Gel Systems Cytotoxicity and Drug Release as Key Features for their Effective Application in Various Fields of Addressed Pharmaceuticals Delivery. Pharmaceutics 2023, 15, 830. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, B.; Qian, Y.; Wang, Q.; Han, R.; Hao, T.; Shu, Y.; Zhang, Y.; Yao, F.; Wang, C. Iota-carrageenan/chitosan/gelatin scaffold for the osteogenic differentiation of adipose-derived MSCs in vitro. J. Biomed. Mater. Res. Part B 2015, 103B, 1498–1510. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging Role of Hydrogels in Drug Delivery Systems, Tissue Engineering and Wound Management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Peng, J.; Xiao, H.; Xu, X.; Qian, Z. Polysaccharide Hydrogels: Functionalization, Construction and Served as Scaffold for Tissue Engineering. Carbohydr. Polym. 2022, 278, 118952. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Shahruzzaman, M.; Biswas, S.; Nurus Sakib, M.; Rashid, T.U. Chitosan Based Bioactive Materials in Tissue Engineering Applications—A Review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef] [PubMed]
- Sairaman, S.; Nivedhitha, M.S.; Shrivastava, D.; Al Onazi, M.A.; Algarni, H.A.; Mustafa, M.; Alqahtani, A.R.; AlQahtani, N.; Teja, K.V.; Janani, K.; et al. Biocompatibility and Antioxidant Activity of a Novel Carrageenan Based Injectable Hydrogel Scaffold Incorporated with Cissus Quadrangularis: An in Vitro Study. BMC Oral Health 2022, 22, 377. [Google Scholar] [CrossRef] [PubMed]
- Graceffa, V.; Zeugolis, D.I. Carrageenan Enhances Chondrogenesis and Osteogenesis in Human Bone Marrow Stem Cell Culture. Eur. Cell Mater. 2019, 37, 310–332. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, B.; Ki, J.S. Biological Activity of Algal Derived Carrageenan: A Comprehensive Review in Light of Human Health and Disease. Int. J. Biol. Macromol. 2023, 238, 124085. [Google Scholar] [CrossRef] [PubMed]
- Rode, M.P.; Batti Angulski, A.B.; Gomes, F.A.; da Silva, M.M.; Jeremias, T.d.S.; de Carvalho, R.G.; Iucif Vieira, D.G.; Oliveira, L.F.C.; Fernandes Maia, L.; Trentin, A.G.; et al. Carrageenan Hydrogel as a Scaffold for Skin-Derived Multipotent Stromal Cells Delivery. J. Biomater. Appl. 2018, 33, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Neamtu, B.; Barbu, A.; Negrea, M.O.; Berghea-Neamțu, C.Ș.; Popescu, D.; Zăhan, M.; Mireșan, V. Carrageenan-Based Compounds as Wound Healing Materials. Int. J. Mol. Sci. 2022, 23, 9117. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Tamayol, A.; Uquillas, J.A.; Akbari, M.; Bertassoni, L.E.; Cha, C.; Camci-Unal, G.; Dokmeci, M.R.; Peppas, N.A.; Khademhosseini, A. 25th Anniversary Article: Rational Design and Applications of Hydrogels in Regenerative Medicine. Adv. Mater. 2014, 26, 85–124. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yang, J.; Fang, J.; Zhou, Y.; Candi, E.; Wang, J.; Hua, D.; Shao, C.; Shi, Y. The Secretion Profile of Mesenchymal Stem Cells and Potential Applications in Treating Human Diseases. Signal Transduct. Target. Ther. 2022, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Maxson, S.; Lopez, E.A.; Yoo, D.; Danilkovitch-Miagkova, A.; LeRoux, M.A.; Danilkovitch-miagkova, A.; Leroux, M.A. Concise Review: Role of Mesenchymal Stem Cells in Wound Repair. Stem Cells Transl. Med. 2012, 1, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.N.; Naeem, B.K.; Ali, A.; Salim, A.; Khan, I. Expansion of Human Umbilical Cord Derived Mesenchymal Stem Cells in Regenerative Medicine. World J. Stem Cells 2024, 16, 410–433. [Google Scholar] [CrossRef] [PubMed]
- Oeller, M.; Laner-plamberger, S.; Krisch, L.; Rohde, E.; Strunk, D.; Schallmoser, K. Human Platelet Lysate for Good Manufacturing Practice-Compliant Cell Production. Int. J. Mol. Sci. 2021, 22, 5178. [Google Scholar] [CrossRef] [PubMed]
- Re, F.; Sartore, L.; Moulisova, V.; Cantini, M.; Almici, C.; Bianchetti, A.; Chinello, C.; Dey, K.; Agnelli, S.; Manferdini, C.; et al. 3D Gelatin-Chitosan Hybrid Hydrogels Combined with Human Platelet Lysate Highly Support Human Mesenchymal Stem Cell Proliferation and Osteogenic Differentiation. J. Tissue Eng. 2019, 10, 2041731419845852. [Google Scholar] [CrossRef] [PubMed]
- Dey, K.; Agnelli, S.; Re, F.; Russo, D.; Lisignoli, G.; Manferdini, C.; Bernardi, S.; Gabusi, E.; Sartore, L. Rational Design and Development of Anisotropic and Mechanically Strong Gelatin-Based Stress Relaxing Hydrogels for Osteogenic/Chondrogenic Differentiation. Macromol. Biosci. 2019, 19, 1900099. [Google Scholar] [CrossRef]
- Re, F.; Sartore, L.; Borsani, E.; Ferroni, M.; Baratto, C.; Mahajneh, A.; Smith, A.; Dey, K.; Almici, C.; Guizzi, P.; et al. Mineralization of 3D Osteogenic Model Based on Gelatin-Dextran Hybrid Hydrogel Scaffold Bioengineered with Mesenchymal Stromal Cells: A Multiparametric Evaluation. Materials 2021, 14, 3852. [Google Scholar] [CrossRef] [PubMed]
- Fung, Y.-C. Biomechanics: Mechanical Properties of Living Tissues; Springer: New York, NY, USA, 1981. [Google Scholar]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014; ISBN 9781139878326. [Google Scholar]
- Ní Annaidh, A.; Bruyère, K.; Destrade, M.; Gilchrist, M.D.; Otténio, M. Characterization of the Anisotropic Mechanical Properties of Excised Human Skin. J. Mech. Behav. Biomed. Mater. 2012, 5, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Bader, D.L.; Bowker, P. Mechanical Characteristics of Skin and Underlying Tissues In Vivo. Biomaterials 1983, 4, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Little, C.J.; Bawolin, N.K.; Chen, X. Mechanical Properties of Natural Cartilage and Tissue-Engineered Constructs. Tissue Eng. Part B Rev. 2011, 17, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O. Viscoelastic Hydrogels for 3D Cell Culture. Biomater. Sci. 2017, 5, 1480–1490. [Google Scholar] [CrossRef] [PubMed]
- Dey, K.; Agnelli, S.; Sartore, L. Dynamic Freedom: Substrate Stress Relaxation Stimulates Cell Responses. Biomater. Sci. 2019, 7, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Webber, R.E.; Creton, C.; Brown, H.R.; Gong, J.P. Large Strain Hysteresis and Mullins Effect of Tough Double-Network Hydrogels. Macromolecules 2007, 40, 2919–2927. [Google Scholar] [CrossRef]
- Zhan, L.; Qu, S.; Xiao, R. A Review on the Mullins Effect in Tough Elastomers and Gels. Acta Mech. Solida Sin. 2024, 37, 181–214. [Google Scholar] [CrossRef]
- Dey, K.; Agnelli, S.; Borsani, E.; Sartore, L. Degradation-Dependent Stress Relaxing Semi-Interpenetrating Networks of Hydroxyethyl Cellulose in Gelatin-PEG Hydrogel with Good Mechanical Stability and Reversibility. Gels 2021, 7, 277. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Gu, L.; Klumpers, D.; Darnell, M.; Bencherif, S.A.; Weaver, J.C.; Huebsch, N.; Lee, H.P.; Lippens, E.; Duda, G.N.; et al. Hydrogels with Tunable Stress Relaxation Regulate Stem Cell Fate and Activity. Nat. Mater. 2015, 15, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Viidik, A. Functional Properties of Collagenous Tissues. Int. Rev. Connect. Tissue Res. 1973, 6, 127–215. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.G.; Fung, Y.-C. Mechanical Properties of the Heart Muscle in the Passive State. J. Biomech. 1973, 6, 597–616. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Han, T.; Yang, Q.; Wang, J.; Feng, B.; Jia, Y.; Wei, Z.; Xu, F. Viscoelastic Cell Microenvironment: Hydrogel-Based Strategy for Recapitulating Dynamic ECM Mechanics. Adv. Funct. Mater. 2021, 31, 2100848. [Google Scholar] [CrossRef]
- Li, H.; Lian, X.; Guan, D. Crossover Behavior in Stress Relaxations of Poroelastic and Viscoelastic Dominant Hydrogels. Soft Matter. 2023, 19, 5443–5451. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Munar, M.L.; Ishikawa, K. Effects of macropore size in carbonate apatite honeycomb scaffolds on bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110848. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Baroud, G.; Bernstein, A.; Döbelin, N.; Galea, L.; Hesse, B.; Heuberger, R.; Meille, S.; Michel, P.; von Rechenberg, B.; et al. Characterization and distribution of mechanically competent mineralized tissue in micropores of β tricalcium phosphate bone substitutes. Mater. Today 2017, 20, 106–115. [Google Scholar] [CrossRef]
- Jeyachandran, D.; Cerruti, M. Glass, Ceramic, Polymeric, and Composite Scaffolds with Multiscale Porosity for Bone Tissue Engineering. Adv. Eng. Mater. 2023, 25, 2201743. [Google Scholar] [CrossRef]
- Webber, V.; de Carvalho, S.M.; Ogliari, P.J.; Hayashi, L.; Barreto, P.L.M. Optimization of the Extraction of Carrageenan from Kappaphycus Alvarezii Using Response Surface Methodology. Food Sci. Technol. 2012, 32, 812–818. [Google Scholar] [CrossRef]
- ISO 11137-1:2006; Sterilization of Health Care Products—Radiation: Part 1: Requirements for Development, Validation and Routine Control of a Sterilization Process for Medical Devices. ISO: Geneva, Switzerland, 2006.
- Kothapalli, C.R.; Shaw, M.T.; Wei, M. Biodegradable HA-PLA 3-D Porous Scaffolds: Effect of Nano-Sized Filler Content on Scaffold Properties. Acta Biomater. 2005, 1, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Doucet, C.; Ernou, I.; Zhang, Y.; Llense, J.R.; Begot, L.; Holy, X.; Lataillade, J.J. Platelet Lysates Promote Mesenchymal Stem Cell Expansion: A Safety Substitute for Animal Serum in Cell-Based Therapy Applications. J. Cell. Physiol. 2005, 205, 228–236. [Google Scholar] [CrossRef] [PubMed]





| Hydrogel | Composition | Physical Properties | |||||
|---|---|---|---|---|---|---|---|
| G [wt%] | PEG [wt%] | CARR [wt%] | CH [wt%] | Apparent Density [g/cm3] | Porosity [%] | Swelling Ratio (24 h) [%] | |
| G-PEG-CARR | 75 | 17.5 | 7.5 | - | 0.074 ± 0.01 | 80 ± 5 | 550 ± 15 |
| G-PEG-CARR-CH | 69 | 17 | 6 | 8 | 0.080 ± 0.01 | 78 ± 7 | 830 ± 25 |
| Hydrogel | Tensile Properties | Compressive Properties | |||
|---|---|---|---|---|---|
| Modulus [kPa] | Stress at Break [kPa] | Strain at Break [%] | Modulus [kPa] | Stress at 50% [kPa] | |
| G-PEG-CARR | 1400 ± 200 | 130 ± 20 | 11 ± 2 | 31 ± 6 | 11 ± 2 |
| G-PEG-CARR-CH | 140 ± 20 | 36 ± 5 | 26 ± 9 | 63 ± 10 | 16 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasini, C.; Re, F.; Trenta, F.; Russo, D.; Sartore, L. Gelatin-Based Scaffolds with Carrageenan and Chitosan for Soft Tissue Regeneration. Gels 2024, 10, 426. https://doi.org/10.3390/gels10070426
Pasini C, Re F, Trenta F, Russo D, Sartore L. Gelatin-Based Scaffolds with Carrageenan and Chitosan for Soft Tissue Regeneration. Gels. 2024; 10(7):426. https://doi.org/10.3390/gels10070426
Chicago/Turabian StylePasini, Chiara, Federica Re, Federica Trenta, Domenico Russo, and Luciana Sartore. 2024. "Gelatin-Based Scaffolds with Carrageenan and Chitosan for Soft Tissue Regeneration" Gels 10, no. 7: 426. https://doi.org/10.3390/gels10070426







