ViSCNOVAS: A Novel Classification System for Hyaluronic Acid-Based Gels in Orthobiologic Products and Regenerative Medicine
Abstract
1. Introduction
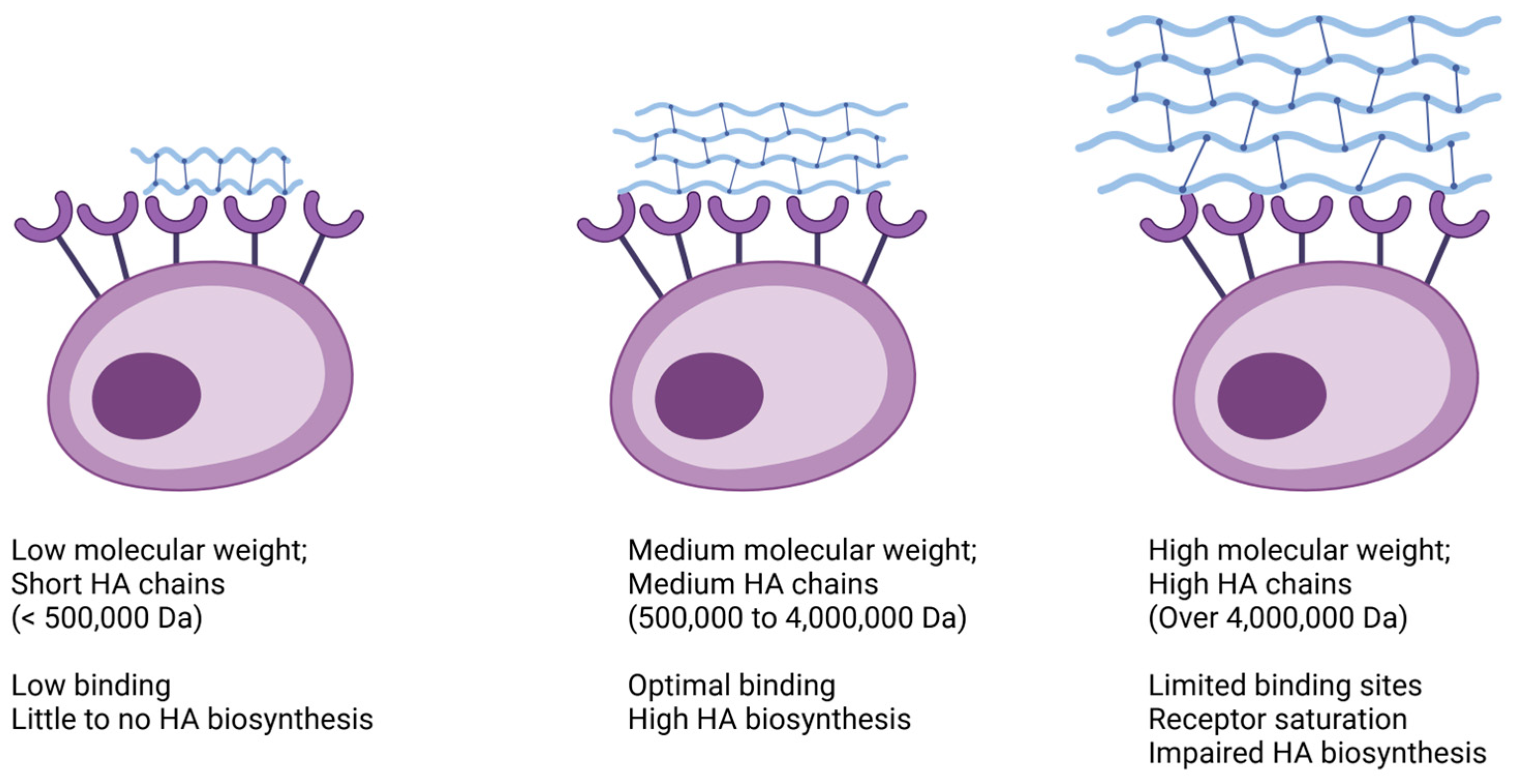
2. Evolution of Hyaluronic Acid
3. ViSCNOVAS Classification
- Vi(25): Viscosity of 25 pascal-seconds, suitable for applications requiring high resistance to flow.
- S(4): Storage at 4 degrees Celsius, indicating the optimal temperature for maintaining product stability.
- C(M): Chain structure is mixed, combining both linear and cross-linked HA.
- N(1): Number 1 indicates minimal cross-linking, ideal for superficial applications such as fine-line correction.
- O(B): Origin from bacterial source (biofermentation), which might be relevant for certain clinical decisions or patient preferences.
- V(3): Volume of 3 mL to be injected per joint, appropriate for moderate joint conditions.
- A(30): Total amount of 30 mg of HA in the formulation.
- S(M): Size with medium molecular weight (1–3 MDa), suitable for a balanced efficacy in terms of tissue penetration and duration.
3.1. Coding on the Product
3.2. Digits and Decimal Places
- Viscosity (Vi): Usually expressed as a whole number but can include decimals if needed for precision.
- Storage (S): Whole number representing temperature. Decimals may also be included for fine tuning.
- Chain (C): Single letter (L, R, M) denoting chain structure.
- Numbers (N): Single digit (1–5) indicating the degree of cross-linking or complexity.
- Origin (O): Single letter (A for animal, B for bacterial).
- Volume (V): Whole number or decimals, depending on the precision needed for the dosage.
- Amount (A): Total amount of HA per package, typically expressed as a whole number.
- Size (S): Single letter or abbreviation denoting molecular weight category.
3.3. Expanded Examples of ViSCNOVAS Classification
- Example: Ostenil®
- Viscosity (Vi): Vi(20)
- Storage (S): S(4)
- Chain (C): C(L)
- Numbers (N): N(2)
- Origin (O): O(B)
- Volume (V): V(2)
- Amount (A): A(20)
- Size (S): S(H)
- Printed on the Product: Vi(20) S(4) C(L) N(2) O(B) V(2) A(20) S(H)
- Interpretation: This product has a viscosity of 20 Pa·s, should be stored at 4 °C, has a linear chain structure, a cross-linking degree of 2, is derived from bacterial sources, has a typical injection volume of 2 mL, contains 20 mg in the package, and has a high molecular weight.
- Products with multiple components: For products with multiple components, each product’s primary component will be classified using the ViSCNOVAS system, and any additional components will be listed separately in the product description. For instance, Ostenil® Plus would be labeled as follows:
- Primary component (HA):
- Viscosity (Vi): Vi(40)
- Storage (S): S(4)
- Chain (C): C(M)
- Numbers (N): N(3)
- Origin (O): O(B)
- Volume (V): V(2)
- Amount (A): A(40)
- Size (S): S(H)
- Additional component:
- Mannitol: 10 mg (1.0%)
- Printed on the product:
- Vi(40) S(4) C(M) N(3) O(B) V(2) A(40) S(H) | Additional component: Mannitol 10 mg (1.0%)
- Products with combined molecular weights: For products with multiple molecular weights, the “Size (S)” variable will list each molecular weight category present in the product, separated by a slash (/).
- Example: Reneha Vis
- Primary component (HA):
- Viscosity (Vi): Vi(15.4)
- Storage (S): S(4)
- Chain (C): C(M)
- Numbers (N): N(3)
- Origin (O): O(B)
- Volume (V): V(2)
- Amount (A): A(22.4)
- Size (S): S(L/H)
- Printed on the product: Vi(15.4) S(4) C(M) N(3) O(B) V(2) A(22.4) S(L/H)
4. Analysis of ViSCNOVAS Classification Variables
4.1. Viscosity
4.2. Storage
4.3. Chain
4.4. Numbers
- N(1): No or minimal cross-linking, suitable for superficial applications like fine-line correction.
- N(2): Slightly higher cross-linking, appropriate for moderate-depth tissue integration.
- N(3): Intermediate cross-linking, used for joint lubrication in moderate arthritis.
- N(4): High cross-linking, suitable for deep tissue integration and sustained release in severe conditions.
- N(5): Maximum cross-linking, ideal for long-term applications such as chronic arthritis management.
4.5. Origin
4.6. Volume
4.7. Amount
4.8. Size
5. Author’s Note
- Personalized treatment strategies:
- Enhanced decision-making:
- Improved standardization:
- Broad applicability:
- Educational tool:
- Facilitating research:
- Regulatory guidance:
6. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef] [PubMed]
- Rossatto, A.; Trocado dos Santos, J.; Zimmer Ferreira Arlindo, M.; Saraiva de Morais, M.; Denardi de Souza, T.; Saraiva Ogrodowski, C. Hyaluronic Acid Production and Purification Techniques: A Review. Prep. Biochem. Biotechnol. 2023, 53, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, Y.; Li, J.; Du, G.; Chen, J. Microbial Production of Hyaluronic Acid: Current State, Challenges, and Perspectives. Microb. Cell Fact. 2011, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef]
- Hermans, J.; Bierma-Zeinstra, S.M.A.; Bos, P.K.; Niesten, D.D.; Verhaar, J.A.N.; Reijman, M. The Effectiveness of High Molecular Weight Hyaluronic Acid for Knee Osteoarthritis in Patients in the Working Age: A Randomised Controlled Trial. BMC Musculoskelet. Disord. 2019, 20, 196. [Google Scholar] [CrossRef] [PubMed]
- Muneta, T.; Koga, H.; Ju, Y.-J.; Mochizuki, T.; Sekiya, I. Hyaluronan Injection Therapy for Athletic Patients with Patellar Tendinopathy. J. Orthop. Sci. 2012, 17, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Jiang, D.; Noble, P.W. Hyaluronan as a Therapeutic Target in Human Diseases. Adv. Drug Deliv. Rev. 2016, 97, 186–203. [Google Scholar] [CrossRef] [PubMed]
- Hintze, V.; Schnabelrauch, M.; Rother, S. Chemical Modification of Hyaluronan and Their Biomedical Applications. Front. Chem. 2022, 10, 830671. [Google Scholar] [CrossRef] [PubMed]
- Ranga, A.; Lutolf, M.P.; Hilborn, J.; Ossipov, D.A. Hyaluronic Acid Hydrogels Formed in Situ by Transglutaminase-Catalyzed Reaction. Biomacromolecules 2016, 17, 1553–1560. [Google Scholar] [CrossRef]
- Schanté, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Chemical Modifications of Hyaluronic Acid for the Synthesis of Derivatives for a Broad Range of Biomedical Applications. Carbohydr. Polym. 2011, 85, 469–489. [Google Scholar] [CrossRef]
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic Acid: Redefining Its Role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef]
- McCain, J.P.; Balazs, E.A.; de la Rua, H. Preliminary Studies on the Use of a Viscoelastic Solution in Arthroscopic Surgery of the Temporomandibular Joint. J. Oral. Maxillofac. Surg. 1989, 47, 1161–1168. [Google Scholar] [CrossRef]
- Altman, R.D.; Moskowitz, R. Intraarticular Sodium Hyaluronate (Hyalgan) in the Treatment of Patients with Osteoarthritis of the Knee: A Randomized Clinical Trial. Hyalgan Study Group. J. Rheumatol. 1998, 25, 2203–2212. [Google Scholar]
- Kolarz, G.; Kotz, R.; Hochmayer, I. Long-Term Benefits and Repeated Treatment Cycles of Intra-Articular Sodium Hyaluronate (Hyalgan) in Patients with Osteoarthritis of the Knee. Semin. Arthritis Rheum. 2003, 32, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Migliore, A.; Bizzi, E.; De Lucia, O.; Delle Sedie, A.; Bentivegna, M.; Mahmoud, A.; Foti, C. Differences among Branded Hyaluronic Acids in Italy, Part 1: Data from In Vitro and Animal Studies and Instructions for Use. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2016, 9, 89–101. [Google Scholar] [CrossRef]
- Maheu, E.; Rannou, F.; Reginster, J.Y. Efficacy and Safety of Hyaluronic Acid in the Management of Osteoarthritis: Evidence from Real-Life Setting Trials and Surveys. Semin. Arthritis Rheum. 2016, 45, S28–S33. [Google Scholar] [CrossRef] [PubMed]
- Balazs, E.A.; Laurent, T.C.; Jeanloz, R.W. Nomenclature of Hyaluronic Acid. Biochem. J. 1986, 235, 903. [Google Scholar] [CrossRef]
- Iaconisi, G.N.; Lunetti, P.; Gallo, N.; Cappello, A.R.; Fiermonte, G.; Dolce, V.; Capobianco, L. Hyaluronic Acid: A Powerful Biomolecule with Wide-Ranging Applications—A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 10296. [Google Scholar] [CrossRef]
- Momen-Heravi, F.; Balaj, L.; Alian, S.; Trachtenberg, A.J.; Hochberg, F.H.; Skog, J.; Kuo, W.P. Impact of Biofluid Viscosity on Size and Sedimentation Efficiency of the Isolated Microvesicles. Front. Physiol. 2012, 3, 162. [Google Scholar] [CrossRef] [PubMed]
- Fundarò, S.P.; Salti, G.; Malgapo, D.M.H.; Innocenti, S. The Rheology and Physicochemical Characteristics of Hyaluronic Acid Fillers: Their Clinical Implications. Int. J. Mol. Sci. 2022, 23, 10518. [Google Scholar] [CrossRef]
- Mori, S.; Naito, M.; Moriyama, S. Highly Viscous Sodium Hyaluronate and Joint Lubrication. Int. Orthop. 2002, 26, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, M.; Casasco, A.; Ferrara, F.; Raichi, M.; Spinelli, G. Hyaluronic Acid Fillers, Needle Contamination by Fastidious Microorganisms, and Risk of Complications. Dermatol. Surg. 2023, 49, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Pontello, R.; Kondo, R.N.; Pavezzi, P.D.; Nicolacopulos, T.; Kippert, J.P.; Lena, C. Cross-Sectional Study of the Microbiological Safety Profile of Reusing Hyaluronic Acid Fillers. J. Cosmet. Dermatol. 2018, 17, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Snetkov, P.; Zakharova, K.; Morozkina, S.; Olekhnovich, R.; Uspenskaya, M. Hyaluronic Acid: The Influence of Molecular Weight on Structural, Physical, Physico-Chemical, and Degradable Properties of Biopolymer. Polymers 2020, 12, 1800. [Google Scholar] [CrossRef] [PubMed]
- Simulescu, V.; Mondek, J.; Kalina, M.; Pekař, M. Kinetics of Long-Term Degradation of Different Molar Mass Hyaluronan Solutions Studied by SEC-MALLS. Polym. Degrad. Stab. 2015, 111, 257–262. [Google Scholar] [CrossRef]
- Marinho, A.; Nunes, C.; Reis, S. Hyaluronic Acid: A Key Ingredient in the Therapy of Inflammation. Biomolecules 2021, 11, 1518. [Google Scholar] [CrossRef] [PubMed]
- Sze, J.H.; Brownlie, J.C.; Love, C.A. Biotechnological Production of Hyaluronic Acid: A Mini Review. 3 Biotech. 2016, 6, 67. [Google Scholar] [CrossRef] [PubMed]
- Dovedytis, M.; Liu, Z.J.; Bartlett, S. Hyaluronic Acid and Its Biomedical Applications: A Review. Eng. Regen. 2020, 1, 102–113. [Google Scholar] [CrossRef]
- Müller-Lierheim, W.G.K. Why Chain Length of Hyaluronan in Eye Drops Matters. Diagnostics 2020, 10, 511. [Google Scholar] [CrossRef]
- Henrotin, Y.; Raman, R.; Richette, P.; Bard, H.; Jerosch, J.; Conrozier, T.; Chevalier, X.; Migliore, A. Consensus Statement on Viscosupplementation with Hyaluronic Acid for the Management of Osteoarthritis. Semin. Arthritis Rheum. 2015, 45, 140–149. [Google Scholar] [CrossRef]
- Khunmanee, S.; Jeong, Y.; Park, H. Crosslinking Method of Hyaluronic-Based Hydrogel for Biomedical Applications. J. Tissue Eng. 2017, 8, 2041731417726464. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A.; Kjøniksen, A.-L.; Nyström, B. Characterization of the Chemical Degradation of Hyaluronic Acid during Chemical Gelation in the Presence of Different Cross-Linker Agents. Carbohydr. Res. 2007, 342, 2776–2792. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.H.; Kim, D.H.; Yune, J.H.; Kwon, H.C.; Shin, D.-M.; Sohn, H.; Lee, K.H.; Choi, B.; Kim, E.S.; Kang, J.H.; et al. In Vitro Toxicity Assessment of Crosslinking Agents Used in Hyaluronic Acid Dermal Filler. Toxicol. Vitr. 2021, 70, 105034. [Google Scholar] [CrossRef] [PubMed]
- Sciabica, S.; Barbari, R.; Fontana, R.; Tafuro, G.; Semenzato, A.; Traini, D.; Silva, D.M.; Reis, L.G.D.; Canilli, L.; Terno, M.; et al. A Safe-by-Design Approach for the Synthesis of a Novel Cross-Linked Hyaluronic Acid with Improved Biological and Physical Properties. Pharmaceuticals 2023, 16, 431. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, R.M.; Sandkvist, U.; Lundgren, B. Degradation of Hylauronic Acid Fillers Using Hyaluronidase in an In Vivo Model. J. Drugs Dermatol. 2018, 17, 548–553. [Google Scholar] [PubMed]
- Rodriguez-Marquez, C.D.; Arteaga-Marin, S.; Rivas-Sánchez, A.; Autrique-Hernández, R.; Castro-Muñoz, R. A Review on Current Strategies for Extraction and Purification of Hyaluronic Acid. Int. J. Mol. Sci. 2022, 23, 6038. [Google Scholar] [CrossRef] [PubMed]
- Ucm, R.; Aem, M.; Lhb, Z.; Kumar, V.; Taherzadeh, M.J.; Garlapati, V.K.; Chandel, A.K. Comprehensive Review on Biotechnological Production of Hyaluronic Acid: Status, Innovation, Market and Applications. Bioengineered 2022, 13, 9645–9661. [Google Scholar] [CrossRef] [PubMed]
- Graciela, C.-Q.; José Juan, E.-C.; Gieraldin, C.-L.; Xóchitl Alejandra, P.-M.; Gabriel, A.-Á. Hyaluronic Acid—Extraction Methods, Sources and Applications. Polymers 2023, 15, 3473. [Google Scholar] [CrossRef] [PubMed]
- Shikina, E.V.; Kovalevsky, R.A.; Shirkovskaya, A.I.; Toukach, P.V. Prospective Bacterial and Fungal Sources of Hyaluronic Acid: A Review. Comput. Struct. Biotechnol. J. 2022, 20, 6214–6236. [Google Scholar] [CrossRef]
- Ferreira, R.G.; Azzoni, A.R.; Santana, M.H.A.; Petrides, D. Techno-Economic Analysis of a Hyaluronic Acid Production Process Utilizing Streptococcal Fermentation. Processes 2021, 9, 241. [Google Scholar] [CrossRef]
- Baron, D.; Flin, C.; Porterie, J.; Despaux, J.; Vincent, P. Hyaluronic Acid Single Intra-Articular Injection in Knee Osteoarthritis: A Multicenter Open Prospective Study (ART-ONE 75) with Placebo Post Hoc Comparison. Curr. Ther. Res. Clin. Exp. 2018, 88, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Yu, T.; Li, Z.; Wang, J.; Jiang, Y.; Liu, Y. Short- and Long-Term Outcomes of Postoperative Intrauterine Application of Hyaluronic Acid Gel: A Meta-Analysis of Randomized Controlled Trials. J. Minim. Invasive Gynecol. 2022, 29, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-H.; Tsai, T.-F. Safety and Effectiveness of Hyaluronic Acid Fillers With Lidocaine for Full-Face Treatment in Asian Patients. J. Drugs Dermatol. 2020, 19, 836–842. [Google Scholar] [CrossRef] [PubMed]
- De Meyere, B.; Mir-Mir, S.; Peñas, J.; Camenisch, C.C.; Hedén, P. Stabilized Hyaluronic Acid Gel for Volume Restoration and Contouring of the Buttocks: 24-Month Efficacy and Safety. Aesthetic Plast. Surg. 2014, 38, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.V.; Fabi, S.G.; Greene, R. Correction of Age-Related Midface Volume Loss With Low-Volume Hyaluronic Acid Filler. JAMA Facial Plast. Surg. 2017, 19, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.C.; Lowe, P.M. Fractional Filling with the Microdepot Technique as an Alternative to Bolus Hyaluronic Acid Injections in Facial Volume Restoration. Australas. J. Dermatol. 2011, 52, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Concoff, A.; Sancheti, P.; Niazi, F.; Shaw, P.; Rosen, J. The Efficacy of Multiple versus Single Hyaluronic Acid Injections: A Systematic Review and Meta-Analysis. BMC Musculoskelet. Disord. 2017, 18, 542. [Google Scholar] [CrossRef] [PubMed]
- Goodman, G.J.; Magnusson, M.R.; Callan, P.; Roberts, S.; Hart, S.; McDonald, C.B.; Clague, M.; Rudd, A.; Bekhor, P.S.; Liew, S.; et al. A Consensus on Minimizing the Risk of Hyaluronic Acid Embolic Visual Loss and Suggestions for Immediate Bedside Management. Aesthet. Surg. J. 2020, 40, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Corduff, N.; Juniarti, L.; Lim, T.S.; Lin, F.; Mariwalla, K.; Pavicic, T.; Quiambao, A.; Siew, T.W.; Suwanchinda, A.; Tseng, F.W.; et al. Current Practices in Hyaluronic Acid Dermal Filler Treatment in Asia Pacific and Practical Approaches to Achieving Safe and Natural-Looking Results. Clin. Cosmet. Investig. Dermatol. 2022, 15, 1213–1223. [Google Scholar] [CrossRef]
- Moon, H.-J.; Gao, Z.-W.; Hu, Z.-Q.; Wang, H.; Wang, X.-J. Expert Consensus on Hyaluronic Acid Filler Facial Injection for Chinese Patients. Plast. Reconstr. Surg. Glob. Open 2020, 8, e3219. [Google Scholar] [CrossRef]
- Dıracoglu, D.; Sezikli, S.; Dernek, B.; Yildirim, M.A.; Sen, E.I. Different Doses of Hyaluronic Acid Injections in Patients with Knee Osteoarthritis: A Multicenter, Randomized, Prospective, Single-Blind, Clinical Study. J. Back. Musculoskelet. Rehabil. 2023, 37, 629–639. [Google Scholar] [CrossRef]
- Abate, M.; Vanni, D.; Pantalone, A.; Salini, V. Hyaluronic Acid in Knee Osteoarthritis: Preliminary Results Using a Four Months Administration Schedule. Int. J. Rheum. Dis. 2017, 20, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Babu, A.; Channaveera, C.; Gupta, A.; Mittal, M.K.; Johnson, D.S. Clinical and Radiological Efficacy of Single-Dose Intra-Articular High-Molecular-Weight Hyaluronic Acid in Knee Osteoarthritis. J. Fam. Med. Prim. Care 2023, 12, 1692–1696. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lee, J.; Cho, Y.-D.; Kim, S.; Seol, Y.-J.; Lee, Y.-M.; Koo, K.-T. The Optimal Dosage of Hyaluronic Acid for Bone Regeneration in Rat Calvarial Defects. J. Periodontal Implant. Sci. 2022, 53, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Safali, S.; Ertaş, E.S.; Özdemir, A.; Cataklı, D. Evaluation of Single and Multiple Hyaluronic Acid Injections at Different Concentrations with High Molecular Weight in the Treatment of Knee Osteoarthritis. BMC Musculoskelet. Disord. 2024, 25, 164. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.-H.; Lu, P.W.-A.; Lin, C.-W.; Lu, E.W.-H.; Yang, S.-F. Different Molecular Weights of Hyaluronan Research in Knee Osteoarthritis: A State-of-the-Art Review. Matrix Biol. 2023, 117, 46–71. [Google Scholar] [CrossRef] [PubMed]
- Onuma, H.; Sugihara, T. Comparison of the Efficacy of Two High-Molecular-Weight Hyaluronic Acid Products for Patients With Knee Osteoarthritis. Orthopedics 2022, 45, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Juncan, A.M.; Moisă, D.G.; Santini, A.; Morgovan, C.; Rus, L.-L.; Vonica-Țincu, A.L.; Loghin, F. Advantages of Hyaluronic Acid and Its Combination with Other Bioactive Ingredients in Cosmeceuticals. Molecules 2021, 26, 4429. [Google Scholar] [CrossRef]
- Hu, L.; Nomura, S.; Sato, Y.; Takagi, K.; Ishii, T.; Honma, Y.; Watanabe, K.; Mizukami, Y.; Muto, J. Anti-Inflammatory Effects of Differential Molecular Weight Hyaluronic Acids on UVB-Induced Calprotectin-Mediated Keratinocyte Inflammation. J. Dermatol. Sci. 2022, 107, 24–31. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, Y.; Yang, H.; Qiu, P.; Cong, Z.; Zou, Y.; Song, L.; Guo, J.; Anastassiades, T.P. A Low Molecular Weight Hyaluronic Acid Derivative Accelerates Excisional Wound Healing by Modulating Pro-Inflammation, Promoting Epithelialization and Neovascularization, and Remodeling Collagen. Int. J. Mol. Sci. 2019, 20, 3722. [Google Scholar] [CrossRef]
- Schlesinger, T.; Rowland Powell, C. Efficacy and Safety of a Low-Molecular Weight Hyaluronic Acid Topical Gel in the Treatment of Facial Seborrheic Dermatitis. J. Clin. Aesthet. Dermatol. 2012, 5, 20–23. [Google Scholar] [PubMed]

| Source | Product | Concentration (Per mL) | Total Amount in Package | Molecular Weight | Indications |
|---|---|---|---|---|---|
| Biofermentation | Ostenil® | 20 mg (2%) | 20 mg | High molecular weight (1–2 MDa) | Used for treating mild to moderate osteoarthritis in the knee. |
| Biofermentation | OrthoVisc® | 15 mg (1.5%) | 30 mg | High molecular weight (1.1–2.9 MDa) | Provides joint lubrication and pain relief in knee osteoarthritis. |
| Biofermentation | OrthoVisc® mini | 15 mg (1.5%) | 15 mg | High molecular weight (1.4 MDa) | Suitable for smaller joints affected by osteoarthritis. |
| Biofermentation | Synolis® VA | HA: 20 mg (2%) Sorbitol: 40 mg (4%) | 60 mg | High molecular weight (2.1 MDa) | Used for joint lubrication and cushioning in osteoarthritis. |
| Biofermentation | RenehaVis® | Low MW HA: 15.4 mg (1.5%) High MW HA: 7 mg (0.7%) | 22.4 mg | Low (<1 MDa) and high (2 MDa) MW | Provides dual-action treatment for osteoarthritis, addressing both pain and inflammation. |
| Biofermentation | MonoVisc® | 80 mg (8%) | 80 mg | High molecular weight (1–2.9 MDa) | Used for single-injection knee osteoarthritis treatment, providing long-lasting relief. |
| Biofermentation | Ostenil® Plus | HA: 40 mg (4%) Mannitol: 10 mg (1.0%) | 50 mg | High molecular weight (1–2 MDa) | Provides extended joint lubrication and pain relief in osteoarthritis. |
| Rooster Comb | Synvisc® | 16 mg (80% HMW HA cross-linked; 20% gel) | 16 mg | High molecular weight (6 MDa) | Treats severe osteoarthritis with enhanced viscosity for better joint lubrication. |
| Rooster Comb | Synvisc® One | 48 mg (80% HMW HA cross-linked; 20% gel) | 48 mg | High molecular weight (6 MDa) | Single-dose treatment for severe osteoarthritis with long-lasting effects. |
| Biofermentation | Suprahyal Duo | 10 mg (1%) | 10 mg | Medium molecular weight (1 MDa) | Used for mild osteoarthritis, providing moderate joint lubrication. |
| Biofermentation | Euflexxa | 10 mg (1%) | 10 mg | High molecular weight (2.4–3.6 MDa) | Provides effective joint lubrication for knee osteoarthritis, reducing pain and improving function. |
| Biofermentation | Polireumin | 10 mg (1%) | 10 mg | Low to medium molecular weight (0.5–1 MDa) | Suitable for mild osteoarthritis and smaller joints, providing joint cushioning and lubrication. |
| Biofermentation | Reviscon PLUS | 16 mg (1.6%) | 16 mg | High molecular weight (3 MDa) | Used for treating moderate to severe osteoarthritis, providing sustained joint lubrication and pain relief. |
| Biofermentation | Biovisc PLUS | 20 mg (2%) | 20 mg | High molecular weight (3.2 MDa) | Provides effective lubrication and cushioning for knee osteoarthritis, reducing pain and improving mobility. |
| Letter | Relates to | Description | Possible Values |
|---|---|---|---|
| Vi | Viscosity | Pascal-second (Pa·s) or kg·m−1·s−1 | Numerical values (e.g., 10, 25) |
| S | Storage | Temperature (degrees Celsius) | Numerical values (e.g., 4, −20) |
| C | Chain | Linear (L), reticulated (R), or mixed (M) | L, R, or M |
| N | Numbers | Degree of cross-linking | 1, 2, 3, 4, 5 |
| O | Origin | Source of HA | A (animal), B (bacterial) |
| V | Volume | Typical volume to be injected (mL) per joint or tendon | Numerical values (e.g., 1, 3) |
| A | Amount | Total amount in package (mg) | Numerical values (e.g., 20, 45) |
| S | Size | Molecular weight (Da) | Single (e.g., L, M) or combined (e.g., L/H, ML/M) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, F.R.; Pires, L.; Martins, R.A.; Costa, B.R.; Santos, G.S.; Lana, J.F. ViSCNOVAS: A Novel Classification System for Hyaluronic Acid-Based Gels in Orthobiologic Products and Regenerative Medicine. Gels 2024, 10, 510. https://doi.org/10.3390/gels10080510
Costa FR, Pires L, Martins RA, Costa BR, Santos GS, Lana JF. ViSCNOVAS: A Novel Classification System for Hyaluronic Acid-Based Gels in Orthobiologic Products and Regenerative Medicine. Gels. 2024; 10(8):510. https://doi.org/10.3390/gels10080510
Chicago/Turabian StyleCosta, Fábio Ramos, Luyddy Pires, Rubens Andrade Martins, Bruno Ramos Costa, Gabriel Silva Santos, and José Fábio Lana. 2024. "ViSCNOVAS: A Novel Classification System for Hyaluronic Acid-Based Gels in Orthobiologic Products and Regenerative Medicine" Gels 10, no. 8: 510. https://doi.org/10.3390/gels10080510
APA StyleCosta, F. R., Pires, L., Martins, R. A., Costa, B. R., Santos, G. S., & Lana, J. F. (2024). ViSCNOVAS: A Novel Classification System for Hyaluronic Acid-Based Gels in Orthobiologic Products and Regenerative Medicine. Gels, 10(8), 510. https://doi.org/10.3390/gels10080510







