Synthesis of Flexible Polyamide Aerogels Cross-Linked with a Tri-Isocyanate
Abstract
:1. Introduction
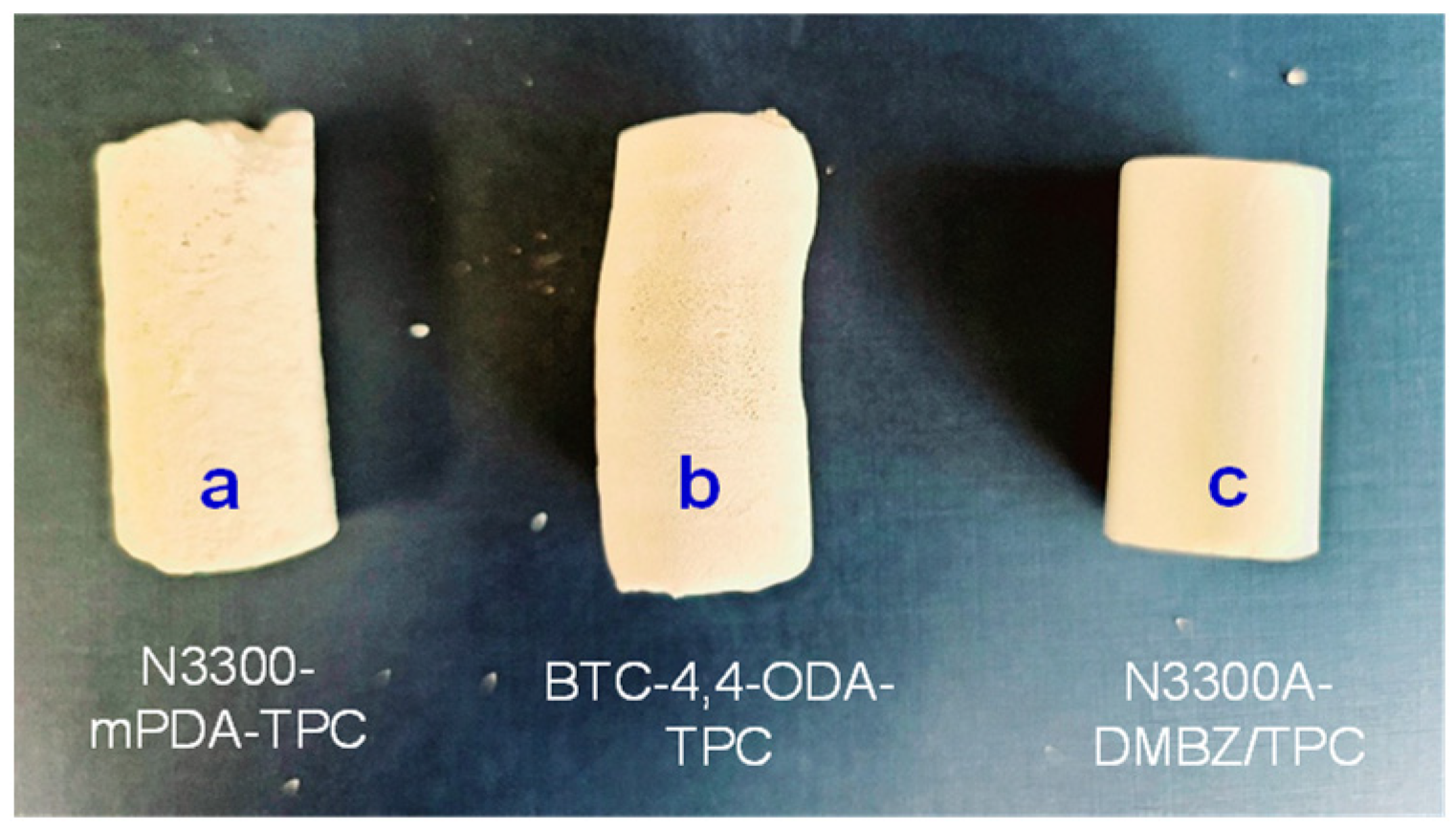
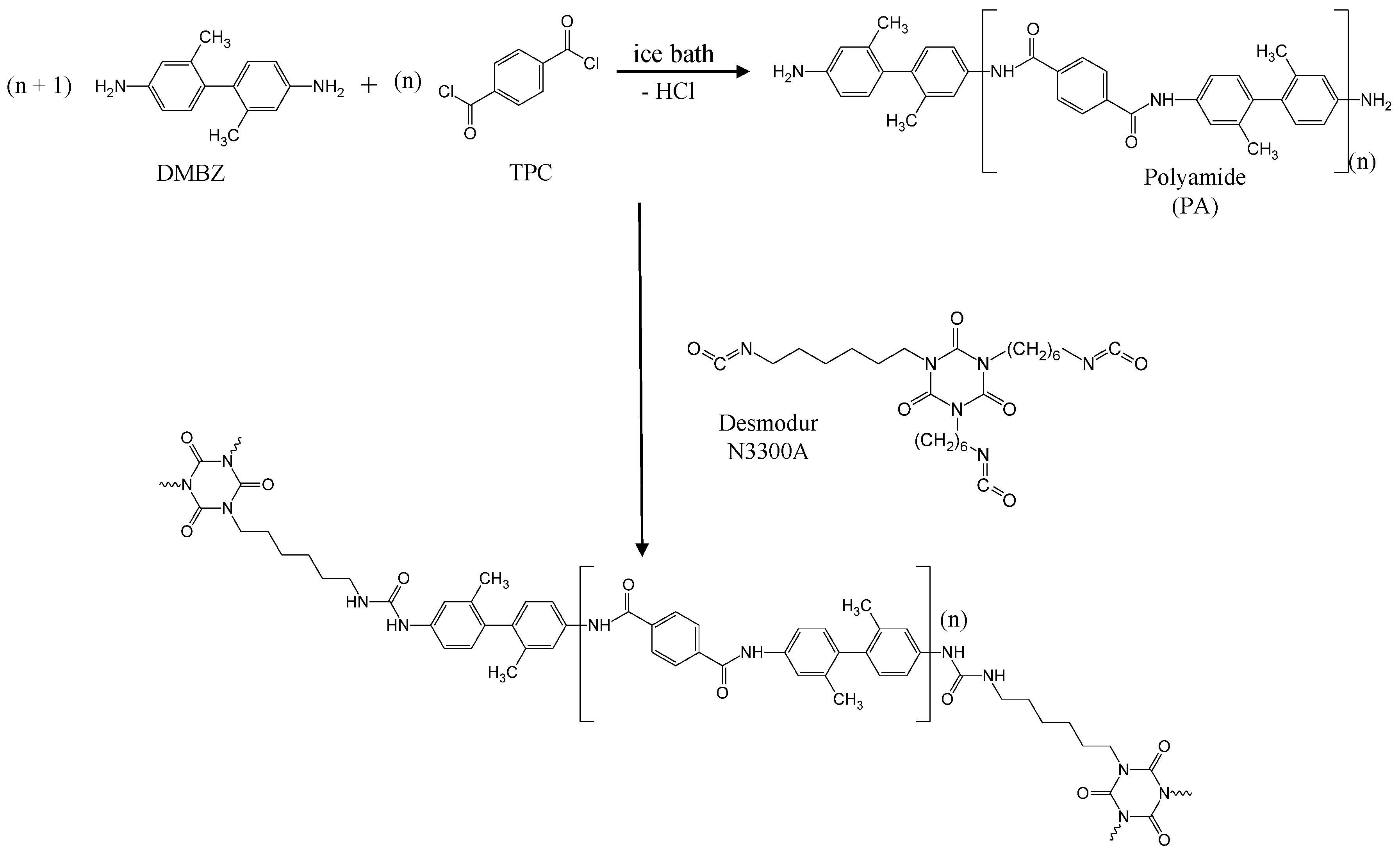
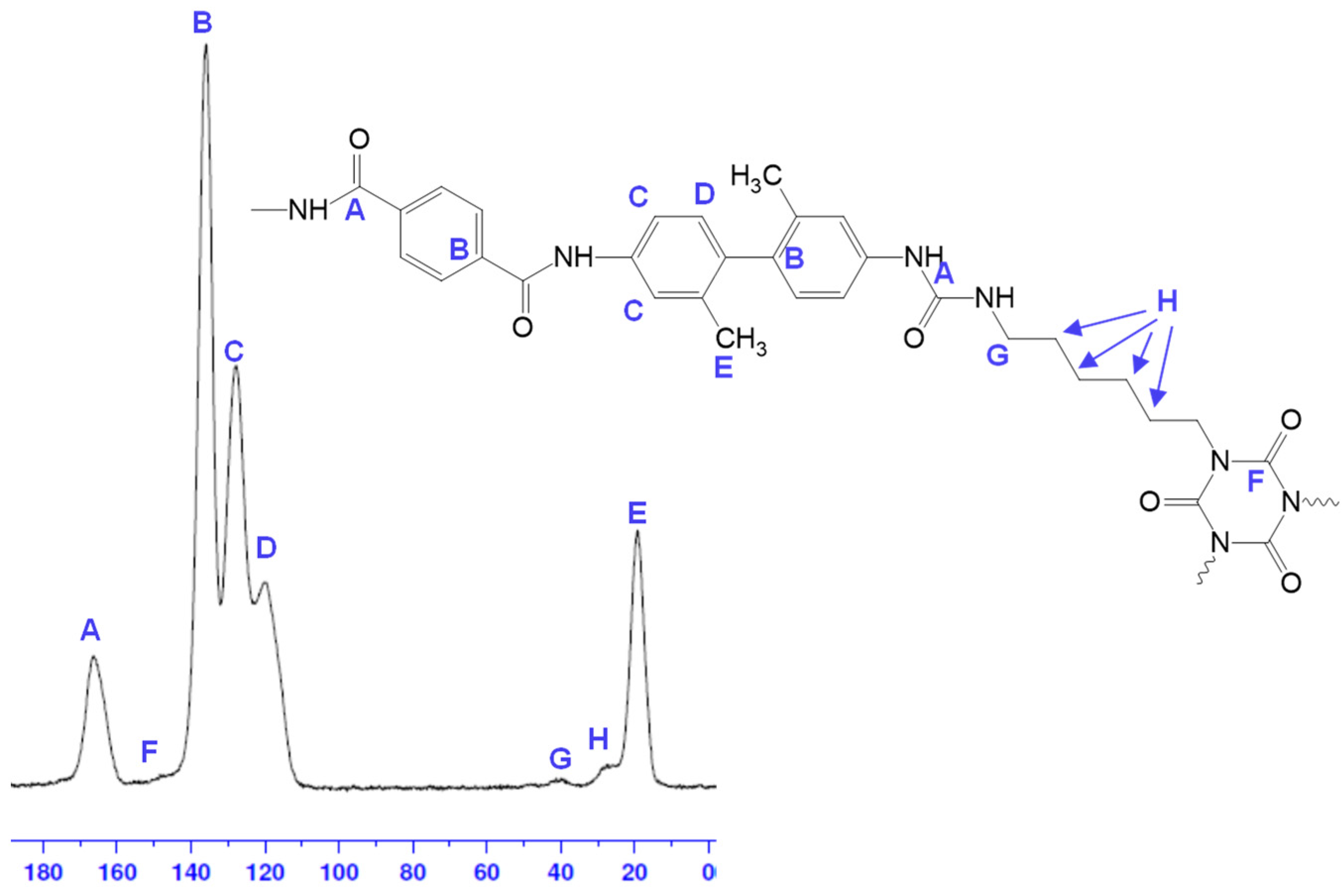
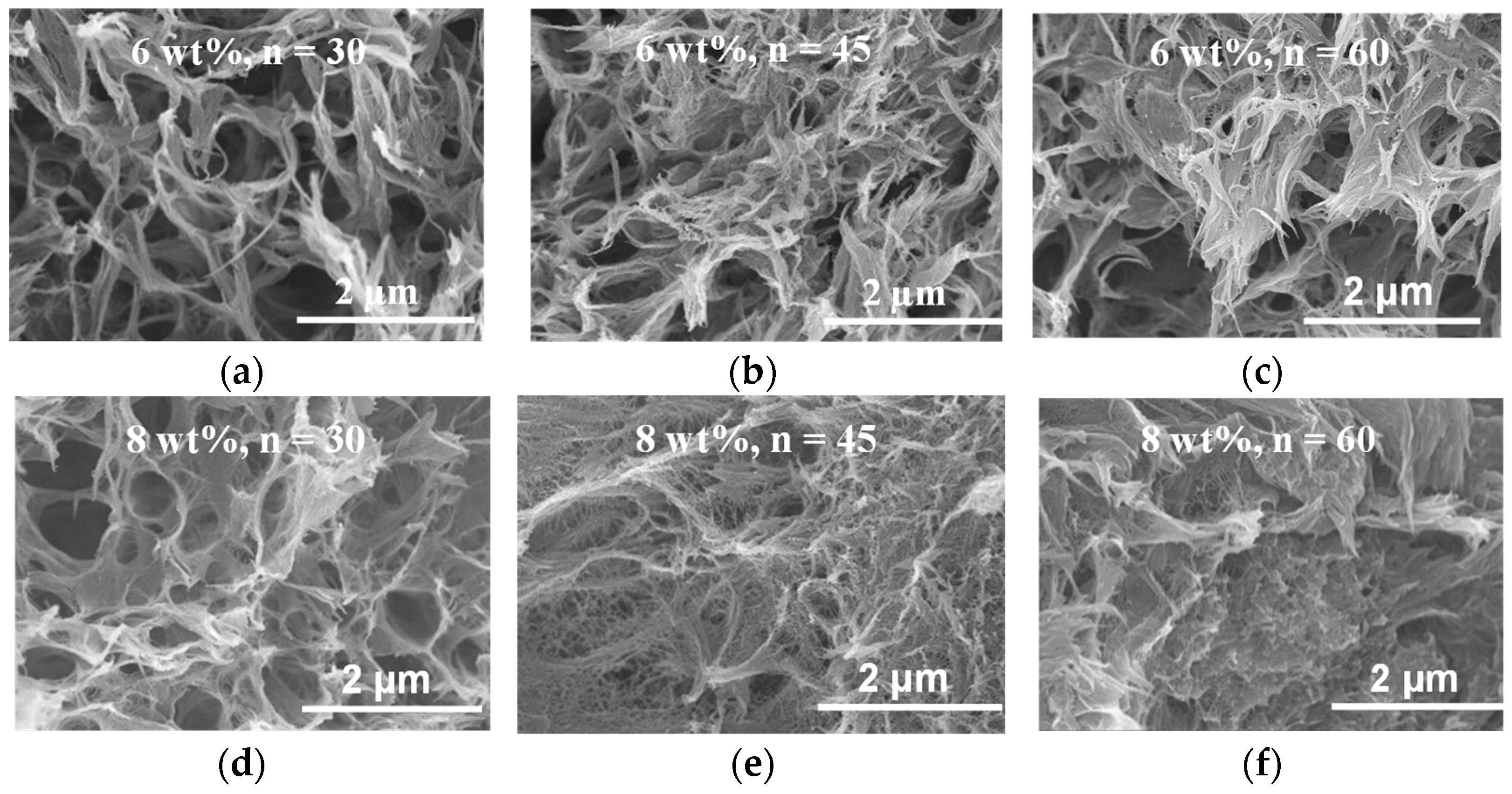
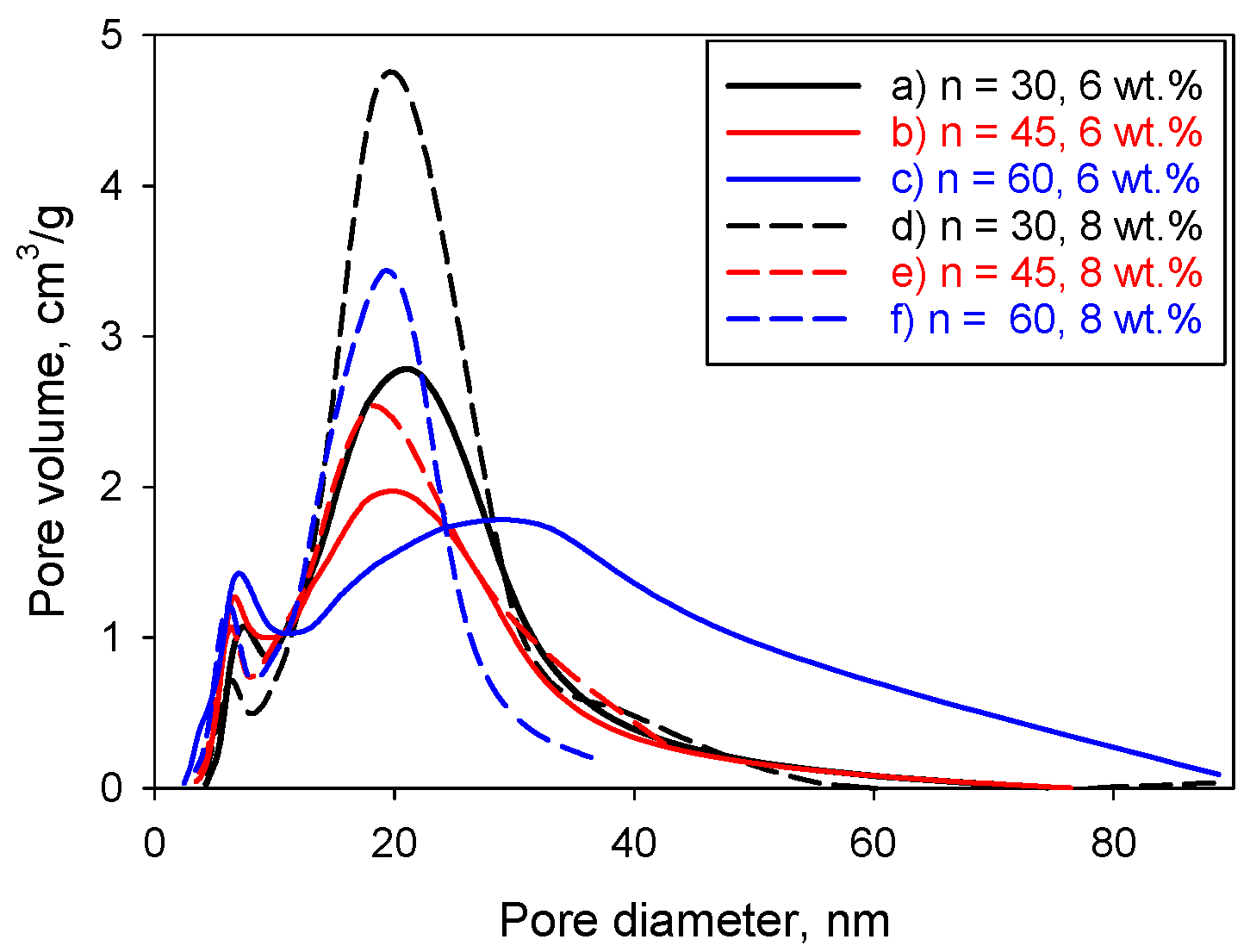
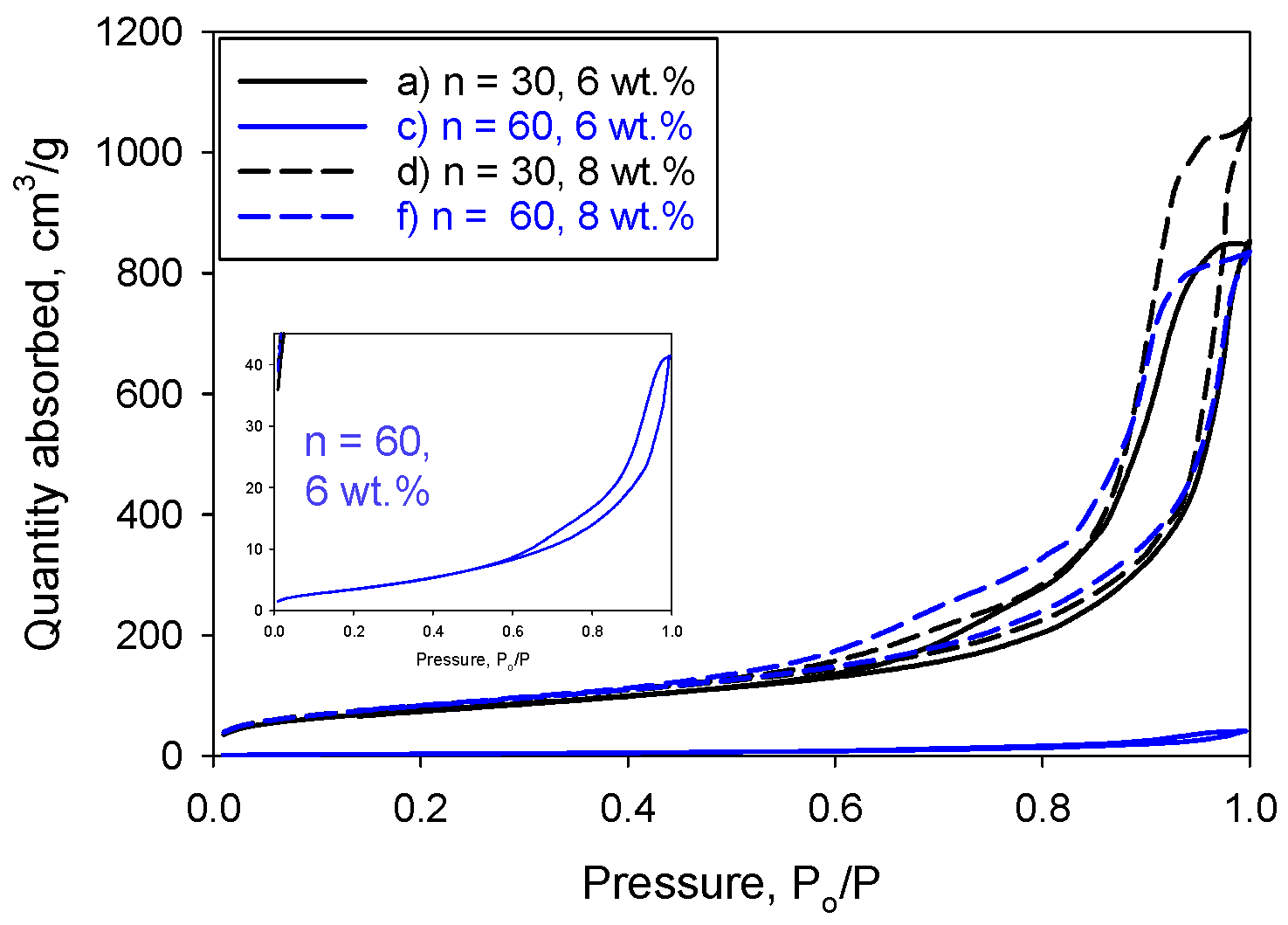
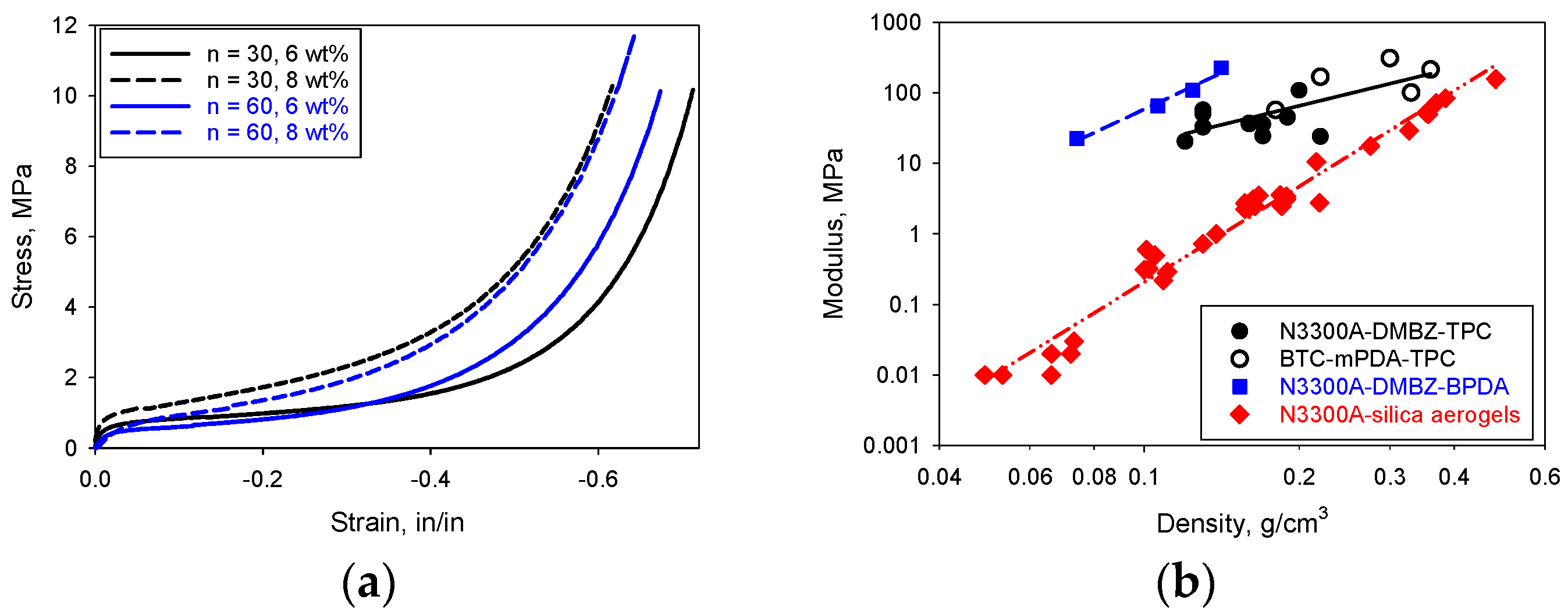
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
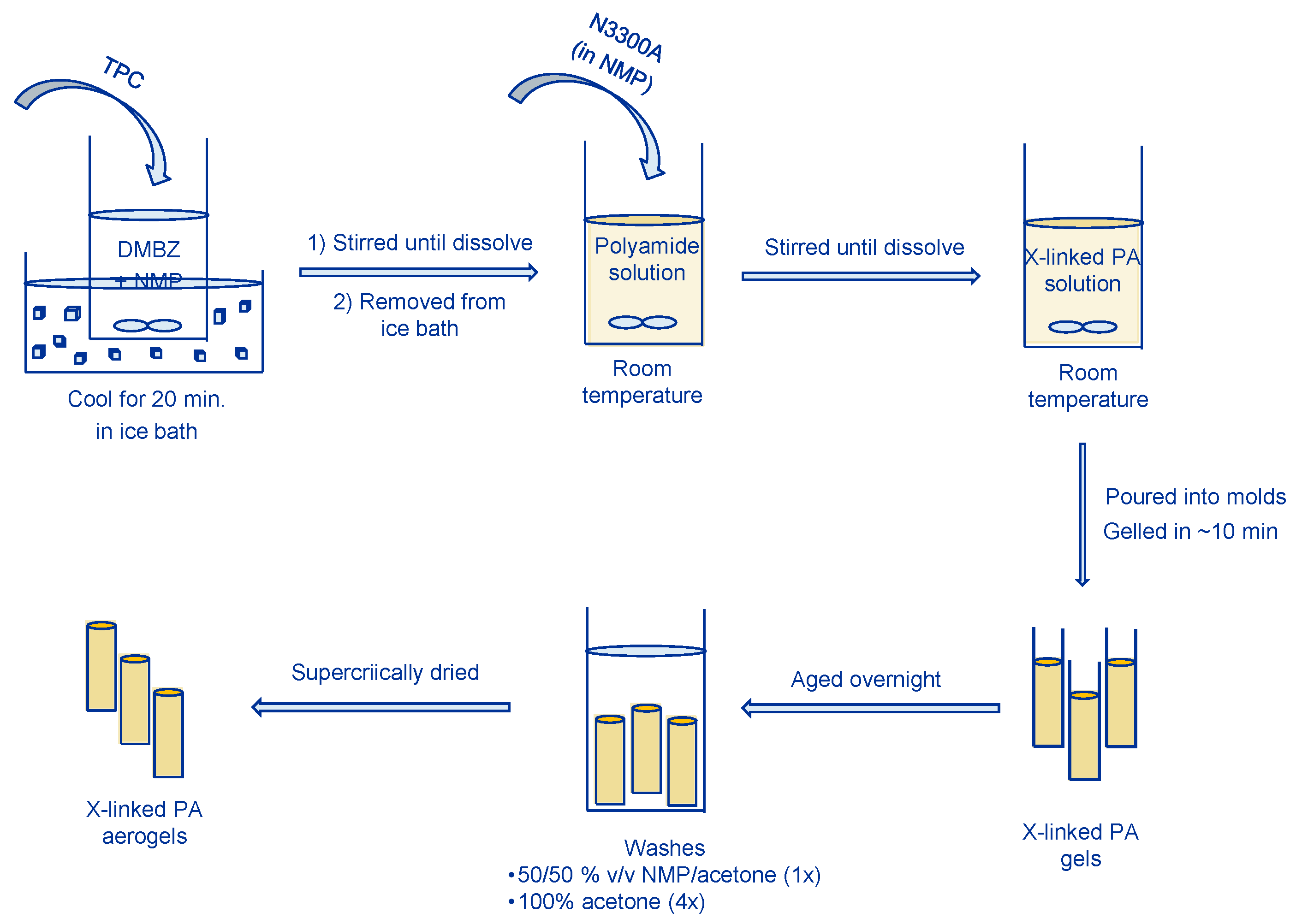
4.2. Synthesis of N3300A Cross-Linked Polyamide Aerogels
4.3. Instrumentation
4.4. Mechanical Characterization
4.5. Physical Characterizations
4.6. Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meador, M.B.; Vivod, S.L.; Nguyen, B.; Guo, H.; Viggiano, R.P. Aerogels from Engineering Polymers: Polyimide and Polyamide Aerogels. In Springer Handbook of Aerogel; Springer: Cham, Switzerland, 2023; Chapter 22. [Google Scholar]
- Pekala, R.W.; Alviso, C.T.; Kong, F.M.; Hulsey, S.S. Aerogels Derived from Multifunctional Organic Monomers. J. Non-Cryst. Solids 1992, 145, 90–98. [Google Scholar] [CrossRef]
- Vivod, S.L.; Meador, M.A.B.; Pugh, C.; Wilkosz, M.; Calomino, K.; McCorkle, L. Toward Improved Optical Transparency of Polyimide Aerogels. ACS Appl. Mater. Interfaces 2020, 12, 8622–8633. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Liu, Z.; Wu, X.; Li, G.; Fang, D.; Zhang, X. Nanofibrous Kevlar Aerogel Films and Their Phase-Change Composites for Highly Efficient Infrared Stealth. ACS Nano 2019, 13, 2236–2245. [Google Scholar] [CrossRef]
- Zhang, X.; Li, N.; Hu, Z.; Yu, J.; Wang, Y.; Zhu, J. Direct Fabrication of poly(p-phenylene terephthalamide) Aerogel and Its Composites with Great Thermal Insulation and Infrared Stealth. Chem. Eng. J. 2020, 388, 124310. [Google Scholar] [CrossRef]
- Hua, Y.; Cui, W.; Ji, Z.; Wang, X.; Wu, Z.; Liu, Y.; Li, Y. Binary Polyamide-Imide Fibrous Superelastic Aerogels for Fire-Retardant and High Temperature Air Filtration. Polymers 2022, 14, 4933. [Google Scholar] [CrossRef] [PubMed]
- Meador, M.A.B.; McMillon, E.; Sandberg, A.; Barrios, E.; Wilmoth, N.G.; Mueller, C.H.; Miranda, F.A. Dielectric and Other Properties of Polyimide Aerogels Containing Fluorinated Blocks. ACS Appl. Mater. Interfaces 2014, 6, 6062–6068. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Yue, X.; Zhang, X.; Chen, Y.; Liu, J.; Shi, Z.; Wang, Z.; Yan, X.; Liang, Z. A Polyimide Aerogel Separator with Electron Cloud Design to Boost Oi-Ion Transport for Sable Li Metal Batteries. Proc. Natl. Acad. Sci. USA 2023, 51, e2314264120. [Google Scholar] [CrossRef]
- Wei, H.; Hu, X.; Deng, Y.; Wei, X.; Yang, Z.; Han, G. Distributed Activation Energy Treatment of Polyimide Aerogel and Its Blocking Effect on Thermal Runaway Propagation of Ternary Battery. J. Energy Storage 2024, 90, 111744. [Google Scholar] [CrossRef]
- Shi, L.; Lin, Y.; Zhao, G.; Jiang, M.; Liu, Y.; Zhuang, X. Lightweight Aramid Nanofiber Aerogel with a Hierarchical Cellular Structure for Thermal Insulation. ACS Appl. Polym. Mater. 2022, 4, 9035–9312. [Google Scholar] [CrossRef]
- Sun, J.; Zhuo, S.; Zhang, R. Highly Transparent, Temperature-Resistant, and Flexible Polyimide Aerogels for Solar Energy Collection. ACS Appl. Mater. Interfaces 2023, 15, 37957–37965. [Google Scholar] [CrossRef]
- Noroozi, M.; Panahi-Sarmad, M.; Abrisham, M.; Amirkiai, A.; Asghari, N.; Golbaten-Mofrad, H.; Karimpour-Matlagh, N.; Goodarzi, V.; Bahramian, A.R.; Zahiri, B. Nanostructure of Aerogels and Their Applications in Thermal Energy Insulation. ACS Appl. Energy Mater. 2019, 2, 5319–5349. [Google Scholar] [CrossRef]
- Liu, Z.; Lyu, J.; Fang, D.; Zhang, X. Nanofibrous Kevlar Aerogel Threads for Thermal Insulation in Harsh Environments. ACS Nano 2019, 13, 5703–5711. [Google Scholar] [CrossRef]
- Lee, G.-H.; Lingappan, N.; Kang, H.W.; Jeon, I.; Lee, W. Three-Dimentional Nanostructures of Crosslinked Aramid Nanofibers with Exceptional Mechanical and Thermal Insulation Characteristics. Appl. Surf. Sci. 2024, 660, 159993. [Google Scholar] [CrossRef]
- Williams, J.C.; Meador, M.A.B.; McCorkle, L.; Mueller, C.; Wilmoth, N. Synthesis and Properties of Step-Growth Polyamide Aerogels Cross-linked with Triacid Chlorides. Chem. Mater. 2014, 26, 4163–4171. [Google Scholar] [CrossRef]
- Ren, H.; Zhu, J.; Bi, Y.; Xu, Y.; Zhang, L. Facile Fabrication of Mechanical Monolithic Polyamide Aerogels via a Modified Sol-Gel Method. J. Sol-Gel Sci. Technol. 2017, 82, 417–423. [Google Scholar] [CrossRef]
- Meador, M.A.B.; Malow, E.J.; Silva, R.; Wright, S.; Quade, D.; Vivod, S.L.; Guo, H.; Guo, J.; Cakmak, M. Mechanically Strong, Flexible Polyimide Aerogels Cross-Linked with Aromatic Triamine. ACS Appl. Mater. Interfaces 2012, 4, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Meador, M.A.B.; Alemán, C.R.; Hanson, K.; Ramirez, N.; Vivod, S.L.; Wilmoth, N.; McCorkle, L. Polyimide Aerogels with Amide Cross-Links: A Low Cost Alternative for Mechanically Strong Polymer Aerogels. ACS Appl. Mater. Interfaces 2015, 7, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.N.; Meador, A.B.; Scheiman, S.; McCorkle, L. Polyimide Aerogels Using Triisocyanate as Cross-linker. ACS Appl. Mater. Inerfaces 2017, 9, 27313–27321. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.C.; Nguyen, B.N.; McCorkle, L.; Scheiman, D.; Griffin, J.S.; Steiner, S.A., III; Meador, M.A.B. Highly Porous, Rigid-Rod Polyamide Aerogels with Superior Mechanical Properties and Unsual High Thermal Conductivity. ACS Appl. Mater. Interfaces 2017, 9, 1801–1809. [Google Scholar] [CrossRef] [PubMed]
- Moldován, K.; Forgács, A.; Paul, G.; Marchese, L.; Len, A.; Dudás, Z.; Kéki, S.; Fábián, I.; Kalmár, J. Mechanism of Hydration Induced Stiffening and Subsequent Plasticization of Polyamide Aerogels. Adv. Mater. Interfaces 2023, 10, 2300109. [Google Scholar] [CrossRef]
- He, S.; Zhang, Y.; Shi, X.; Bi, Y.; Luo, X.; Zhang, L. Rapid and Facile Synthesis of a Low-Cost Monolithic Polyamide Aerogel via Sol-Gel Technology. Mater. Lett. 2015, 144, 82–84. [Google Scholar] [CrossRef]
- Ren, H.; Zhu, J.; Bi, Y.; Xu, Y.; Zhang, L. Facile Fabrication of Multifunctional Monolithic Polyamide Aerogels. J. Porous Mater. 2017, 24, 1165–1173. [Google Scholar] [CrossRef]
- Huang, J.-P. Two-Parts Adhesive System. Patent EP 3 371 232 B1, 1 June 2021. [Google Scholar]
- Wilczek, L.; Nagata, I. Preparation and Use of Reactive Organosilicon Compounds. Patent US2006/0128920 A1, 15 June 2006. [Google Scholar]
- Hafeman, A.E.; Li, B.; Yoshii, T.; Zienkiewicz, K.; Davidson, J.M.; Guelcher, S.C. Injectable Biodegradable Polyurethane Scaffolds with Release of Platelet-derived Growht Factor for Tissue Repair and Regeneration. Pharma. Res. 2008, 25, 2387–2399. [Google Scholar] [CrossRef] [PubMed]
- Bang, A.; Buback, C.; Sotiriou-Leventis, C.; Leventis, N. Flexible Aerogels from Hyperbranched Polyurethanes: Probing the Role of Molecular Rigidity with Poly(Urethane Acrylates) Versus Poly(Urethane Norbornenes). Chem. Mater. 2014, 26, 6979–6993. [Google Scholar] [CrossRef]
- Leventis, N.; Sotiriou-Leventis, C.; Mulik, S. Three-Dimensional Pourous Polyurea Networks and Methods of Manufacture. Patent US 2012/01528846 A1, 21 June 2012. [Google Scholar]
- Leventis, N. Polyurea Aerogels: Synthesis, Material Properties, and Applications. Polymers 2022, 14, 969. [Google Scholar] [CrossRef]
- Nguyen, B.N.; Meador, M.A.B.; Medoro, A.; Arendt, V.; Randall, J.; McCorkle, L.; Shonkwiler, B. Elastic Behavior of Methyltrimethoxysilane Based Aerogels Reinforced with Tri-Isocyanate. ACS Appl. Mater. Interfaces 2010, 2, 1430–1443. [Google Scholar] [CrossRef] [PubMed]
- Donthula, S.; Mandal, C.; Leventis, T.; Schisler, J.; Saeed, A.M.; Sotiriou-Leventis, C.; Leventis, N. Shape Memory Superelastic Poly(isocyanuated-urethane) Aerogels (PIR-PUR) for Deployable Panels and Biomimetic Applications. Chem. Mater. 2017, 29, 4461–4477. [Google Scholar] [CrossRef]
- Nguyen, B.N.; Scheiman, D.; Meador, M.A.B.; Guo, J.; Hamilton, B.; McCorkle, L. Effect of Urea Links in the Backbone of Polyimide Aerogels. ACS Appl. Polym. Mater. 2021, 3, 2027–2037. [Google Scholar] [CrossRef]
- Cashman, J.L.; Nguyen, B.N.; Dosa, B.; Meador, M.A.B. Flexible Polyimide Aerogels Derived from the Use of a Neopentyl Spacer in the Backbone. ACS Appl. Polym. Mater. 2020, 2, 2179–2189. [Google Scholar] [CrossRef]
- Zang, G.; Dass, A.; Rawashdeh, B.-M.M.; Thomas, J.; Counsil, J.A.; Sotiriou-Leventis, C.; Fabrizio, E.F.; Ilhan, F.; Vassilaras, P.; Scheiman, D.A.; et al. Isocyanate-crosslinked silica aerogel monoliths: Preparation and characterization. J. Non-Cryst. Solids 2004, 350, 152–164. [Google Scholar] [CrossRef]
- Weigold, L.; Mohite, D.P.; Mahadik-Khanolkar, S.; Leventis, N.; Reichenauer, G. Correlation of Microstructure and Thermal Conductivity in Nanoporous Solids: The case of Polyurea Aerogels Synthesized from an Aliphatic Tri-isocyante and Water. J. Non-Cryst. Solids 2013, 368, 105–111. [Google Scholar] [CrossRef]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.M.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendation for the Characterization of Porous Solids. Pure Appl Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Techincal Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- ASTM D695-10; Standard Test Method for Compressive Properties of Rigid Plastics. ASTM International: West Conshohocken, PA, USA, 2015.
- Parale, V.G.; Kim, T.; Choi, H.; Phdtare, V.D.; Dhavale, R.P.; Kanamori, D.; Park, H.-H. Mechanicanlly Strengthened Aerogels through Multiscale, Multicompositional, and Multidimensional Approaches: A Review. Adv. Mater. 2024, 36, 202307772. [Google Scholar]
- Niculescu, A.-G.; Tudorache, D.-I.; Bocioagă, M.; Mihaiescu, D.E.; Hadibarata, T.; Grumezescu, A.M. An Updated Overview of Silica Aerogel-Based Nanomaterials. Nanomaterials 2024, 14, 469. [Google Scholar] [CrossRef]
- Czlonka, S.; Bertino, M.; Kósny, J.; Shukla, N. Density and Shrinkage as Guiding Criteria for the Optimization of the Thermal Conductiviy of Poly(urethane)-Class Aerogel. J. Sol-Gel Sci. Technol. 2020, 93, 149–167. [Google Scholar] [CrossRef]

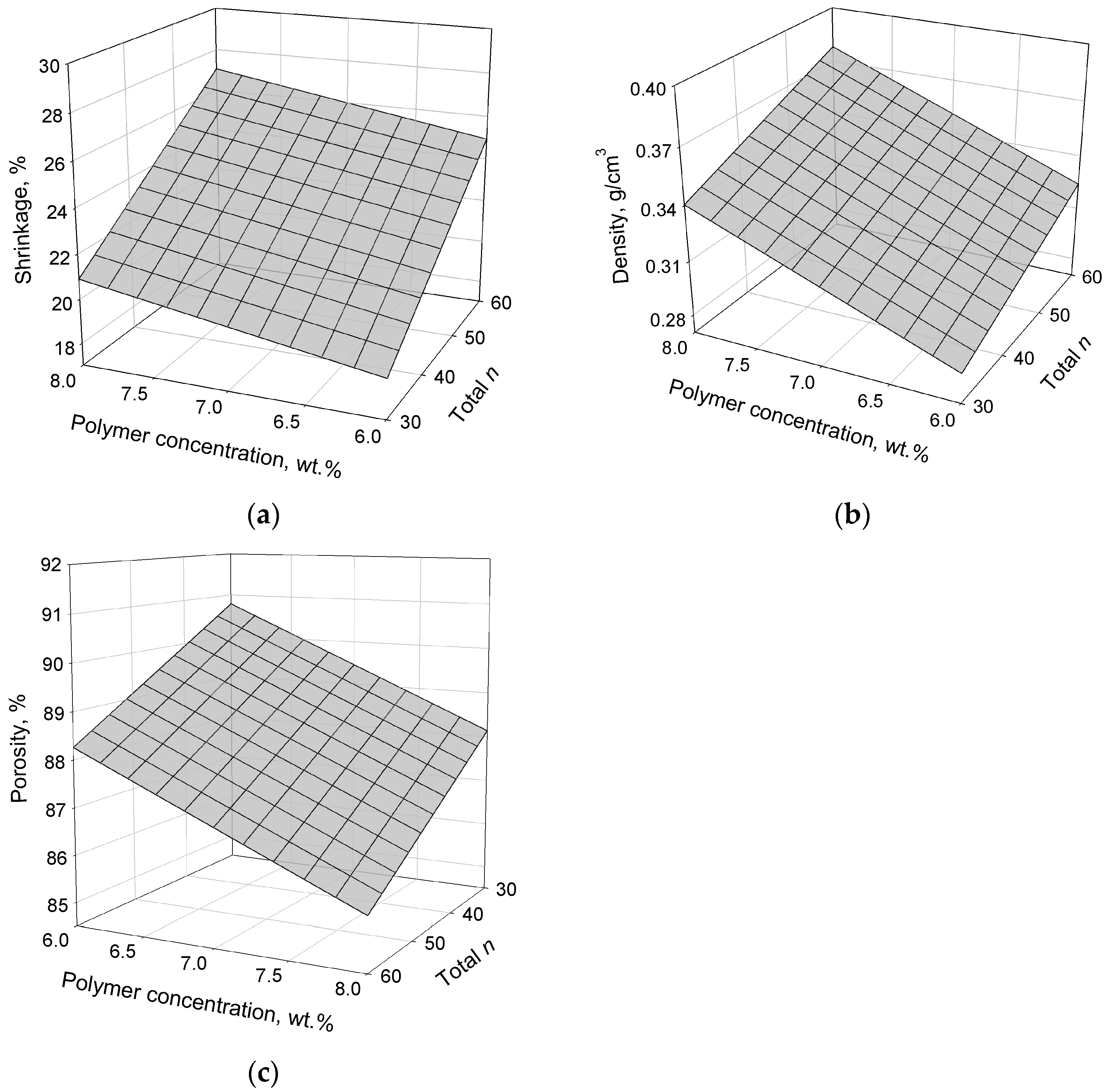

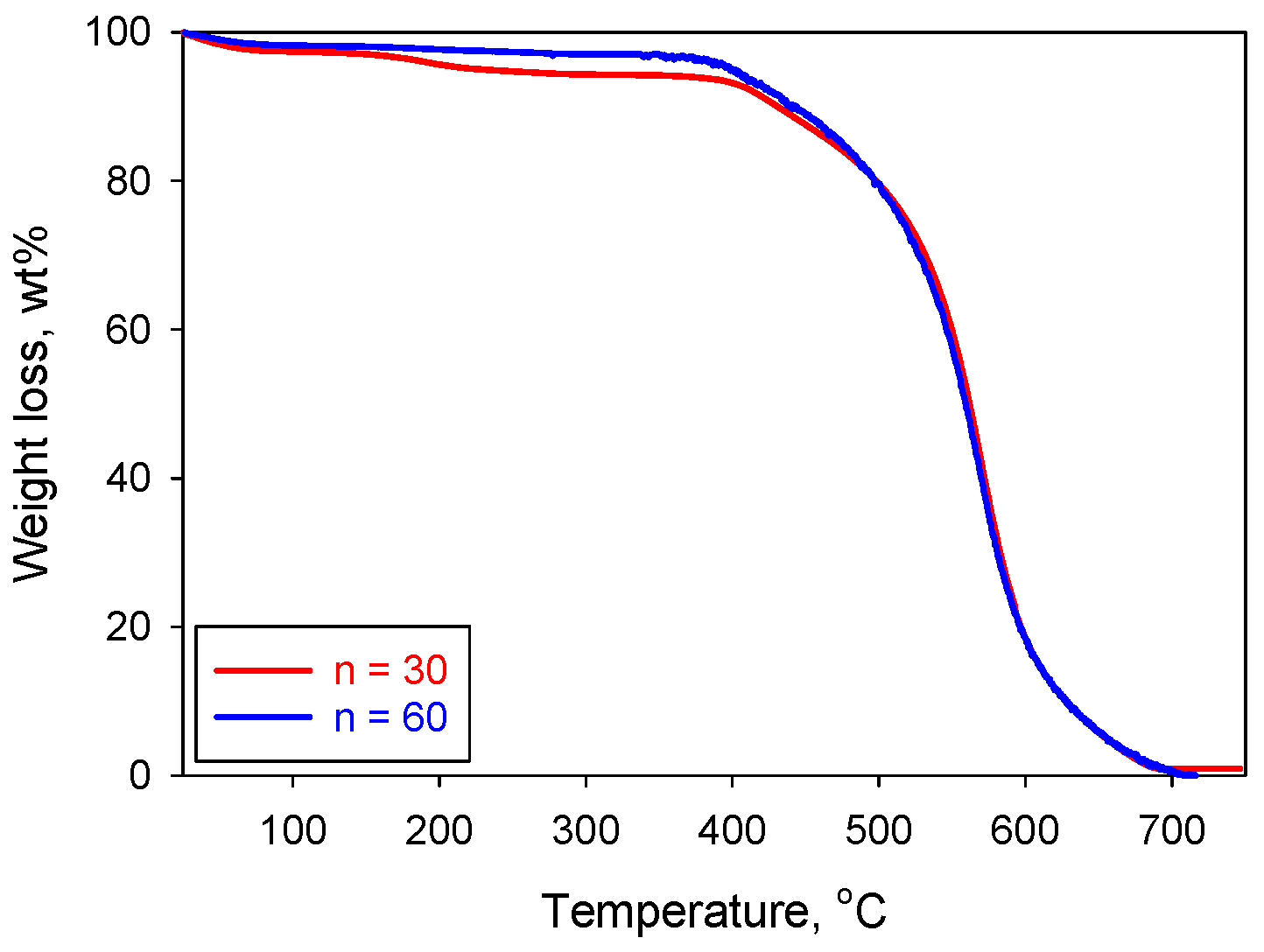

| Run # | Polymer Conc. (wt.%) | Repeat Unit (n) | Density (mg/cm3) | Shrinkage (%) | Porosity (%) | BET Surface Area (m2/g) | Young’s Modulus (M(Pa) | Decomposition Temperature of Polyamide (°C) |
|---|---|---|---|---|---|---|---|---|
| 1 | 8 | 60 | 22.1 | 26.46 | 82 | 308 | 24.18 | 570 |
| 2 | 6 | 40 | 13.3 | 21.47 | 90 | 284 | 50.89 | 540 |
| 3 | 8 | 40 | 19.8 | 24.48 | 84 | 295 | 109.02 | 554 |
| 4 | 6 | 60 | 13.1 | 20.79 | 89 | 300 | 32.89 | 522 |
| 5 | 7 | 50 | 16.2 | 22.63 | 87 | 272 | 36.39 | 531 |
| 6 | 6 | 45 | 12.9 | 21.16 | 90 | 265 | 27.39 | 537 |
| 7 | 8 | 45 | 16.5 | 19.14 | 88 | 269 | 36.05 | 538 |
| 8 | 6 | 30 | 12.1 | 19.47 | 91 | 282 | 20.6 | 522 |
| 9 | 8 | 30 | 16.4 | 20.03 | 88 | 309 | 37.29 | 541 |
| 10 | 8 | 30 | 17.1 | 19.85 | 88 | 291 | (-) | 524 |
| 11 | 8 | 50 | 18.9 | 26.63 | 86 | 312 | 45.39 | 555 |
| 12 | 6 | 50 | 16.5 | 26.47 | 88 | 297 | 24.71 | 540 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheiman, D.A.; Guo, H.; Oosterbaan, K.J.; McCorkle, L.; Nguyen, B.N. Synthesis of Flexible Polyamide Aerogels Cross-Linked with a Tri-Isocyanate. Gels 2024, 10, 519. https://doi.org/10.3390/gels10080519
Scheiman DA, Guo H, Oosterbaan KJ, McCorkle L, Nguyen BN. Synthesis of Flexible Polyamide Aerogels Cross-Linked with a Tri-Isocyanate. Gels. 2024; 10(8):519. https://doi.org/10.3390/gels10080519
Chicago/Turabian StyleScheiman, Daniel A., Haiquan Guo, Katherine J. Oosterbaan, Linda McCorkle, and Baochau N. Nguyen. 2024. "Synthesis of Flexible Polyamide Aerogels Cross-Linked with a Tri-Isocyanate" Gels 10, no. 8: 519. https://doi.org/10.3390/gels10080519









