Plant-Based Nanovesicular Gel Formulations Applied to Skin for Ameliorating the Anti-Inflammatory Efficiency
Abstract
1. Introduction
2. Nanovesicular Drug Delivery Systems
2.1. Transfersomes
2.2. Ethosomes
2.3. Transethosomes
2.4. Glycerosomes
2.5. Bilosomes
2.6. Phytosomes
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Kiss, A.L. Inflammation in Focus: The Beginning and the End. Pathol. Oncol. Res. 2022, 27, 1610136. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hong, Y.; Huang, H. Triptolide attenuates inflammatory response in membranous glomerulo-nephritis rat via downregulation of NF-κB signaling pathway. Kidney Blood Press. Res. 2016, 41, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Bagad, A.S.; Joseph, J.A.; Bhaskaran, N.; Agarwal, A. Comparative evaluation of anti-inflammatory activity of curcuminoids, turmerones, and aqueous extract of Curcuma longa. Adv. Pharmacol. Pharm. Sci. 2013, 2013, 805756. [Google Scholar]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Ferrero-Miliani, L.; Nielsen, O.; Andersen, P.; Girardin, S. Chronic inflammation: Importance of NOD2 and NALP3 in interleukin-1β generation. Clin. Exp. Immunol. 2007, 147, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-H.; Baek, S.J. Molecular targets of dietary polyphenols with anti-inflammatory properties. Yonsei Med. J. 2005, 46, 585. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J.; Sasieni, P.; Myles, J.; Tyrer, J. Estimating the effect of treatment in a proportional hazards model in the presence of non-compliance and contamination. J. R. Stat. Soc. Ser. B Stat. Methodol. 2007, 69, 565–588. [Google Scholar] [CrossRef]
- Mukhopadhyay, N.; Shukla, A.; Makhal, P.N.; Kaki, V.R. Natural product-driven dual COX-LOX inhibitors: Overview of recent studies on the development of novel anti-inflammatory agents. Heliyon 2023, 9, e14569. [Google Scholar] [CrossRef]
- Yunus, M.H.M.; Nordin, A.; Kamal, H. Pathophysiological perspective of osteoarthritis. Medicina 2020, 56, 614. [Google Scholar] [CrossRef]
- Bertolini, A.; Ottani, A.; Sandrini, M. Selective COX-2 inhibitors and dual acting anti-inflammatory drugs: Critical remarks. Curr. Med. Chem. 2002, 9, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.; Noakes, T.; Schwellnus, M.; Windt, A.; Bowerbank, P. Non-steroidal antiinflammatory drugs fail to enhance healing of acute hamstring injuries treated with physiotherapy. S. Afr. Med. J. 1995, 85, 517–522. [Google Scholar] [PubMed]
- Gupta, M.; Chauhan, D.N.; Sharma, V.; Chauhan, N.S. Novel Drug Delivery Systems for Phytoconstituents; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Pandey, R.; Bhairam, M.; Shukla, S.S.; Gidwani, B. Colloidal and vesicular delivery system for herbal bioactive constituents. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2021, 29, 415–438. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Li, W.; Liao, W.; Jiang, H.; Liu, Y.; Cao, J.; Lu, W.; Feng, Y. Nano-Drug Delivery Systems Based on Natural Products. Int. J. Nanomed. 2024, 19, 541–569. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi-Kia, F.; Lorigooini, Z.; Amini-Khoei, H. Medicinal plants: Past history and future perspective. J. Herbmed Pharmacol. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Lukhele, B.S.; Bassey, K.; Witika, B.A. The Utilization of Plant-Material-Loaded Vesicular Drug Delivery Systems in the Management of Pulmonary Diseases. Curr. Issues Mol. Biol. 2023, 45, 9985–10017. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Harwansh, R.K.; Bhattacharyya, S. Bioavailability of herbal products: Approach toward improved pharmacokinetics. In Evidence-Based Validation of Herbal Medicine; Elsevier: Amsterdam, The Netherlands, 2015; pp. 217–245. [Google Scholar]
- Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Zafar, A.; Alsaidan, O.A.; Alruwaili, N.K.; Gilani, S.J.; Rizwanullah, M. Recent advancement in chitosan-based nanoparticles for improved oral bioavailability and bioactivity of phytochemicals: Challenges and perspectives. Polymers 2021, 13, 4036. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhao, W.; Ma, Q.; Gao, Y.; Wang, W.; Zhang, X.; Dong, Y.; Zhang, T.; Liang, Y.; Han, S.; et al. Functional nano-systems for transdermal drug delivery and skin therapy. Nanoscale Adv. 2023, 5, 1527–1558. [Google Scholar] [CrossRef]
- Ghasemian, M. A different look at pulsed glucocorticoid protocols Is high dose oral prednisolone really necessary just after initiation of pulse therapy? J. Case Rep. Practice 2015, 3, 1–3. [Google Scholar]
- Ghasemian, M.; Owlia, S.; Owlia, M.B. Review of Anti-Inflammatory Herbal Medicines. Adv. Pharmacol. Sci. 2016, 2016, 9130979. [Google Scholar] [CrossRef]
- Waheed, A.; Ahad, A.; Gupta, D.K.; Aqil, M.; Al-Jenoobi, F.I.; Al-Mohizea, A.M. Nanovesicles for the treatment of skin disorders. In Applications of Nanovesicular Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2022; pp. 285–302. [Google Scholar]
- Myneni, G.S.; Radha, G.; Soujanya, G. Novel vesicular drug delivery systems: A review. J. Pharm. Res. 2021, 11, 1650–1664. [Google Scholar]
- Mosallam, S.; Albash, R.; Abdelbari, M.A. Advanced vesicular systems for antifungal drug delivery. AAPS PharmSciTech 2022, 23, 206. [Google Scholar] [CrossRef] [PubMed]
- Rao, B.N.; Reddy, K.R.; Mounika, B.; Fathima, S.R.; Tejaswini, A. Vesicular drug delivery system: A review. Int. J. ChemTech Res. 2019, 12, 39e53. [Google Scholar] [CrossRef]
- Nasr, M.; Mansour, S.; Mortada, N.D.; Elshamy, A. Vesicular aceclofenac systems: A comparative study between liposomes and niosomes. J. Microencapsul. 2008, 25, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Bseiso, E.A.; Nasr, M.; Sammour, O.; Abd El Gawad, N.A. Recent advances in topical formulation carriers of antifungal agents. Indian J. Dermatol. Venereol. Leprol. 2015, 81, 457. [Google Scholar] [CrossRef]
- Carter, P.; Narasimhan, B.; Wang, Q. Biocompatible nanoparticles and vesicular systems in transdermal drug delivery for various skin diseases. Int. J. Pharm. 2019, 555, 49–62. [Google Scholar] [CrossRef]
- Chauhan, B.P. Hybrid Nanomaterials: Synthesis, Characterization, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391. [Google Scholar] [CrossRef]
- Abdallah, M.H.; Elsewedy, H.S.; AbuLila, A.S.; Almansour, K.; Unissa, R.; Elghamry, H.A.; Soliman, M.S. Quality by design for optimizing a novel liposomal jojoba oil-based emulgel to ameliorate the anti-inflammatory effect of brucine. Gels 2021, 7, 219. [Google Scholar] [CrossRef]
- Eroğlu, İ.; İbrahim, M. Liposome–ligand conjugates: A review on the current state of art. J. Drug Target. 2020, 28, 225–244. [Google Scholar] [CrossRef]
- Li, T.; Cipolla, D.; Rades, T.; Boyd, B.J. Drug nanocrystallisation within liposomes. J. Control. Release 2018, 288, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Muzzalupo, R.; Mazzotta, E. Do niosomes have a place in the field of drug delivery? Expert Opin. Drug Deliv. 2019, 16, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Barani, M.; Mirzaei, M.; Torkzadeh-Mahani, M.; Lohrasbi-Nejad, A.; Nematollahi, M.H. A new formulation of hydrophobin-coated niosome as a drug carrier to cancer cells. Mater. Sci. Eng. C 2020, 113, 110975. [Google Scholar] [CrossRef] [PubMed]
- Nematollahi, M.H.; Pardakhty, A.; Torkzadeh-Mahanai, M.; Mehrabani, M.; Asadikaram, G. Changes in physical and chemical properties of niosome membrane induced by cholesterol: A promising approach for niosome bilayer intervention. RSC Adv. 2017, 7, 49463–49472. [Google Scholar] [CrossRef]
- Rezvani, M.; Hesari, J.; Peighambardoust, S.H.; Manconi, M.; Hamishehkar, H.; Escribano-Ferrer, E. Potential application of nanovesicles (niosomes and liposomes) for fortification of functional beverages with Isoleucine-Proline-Proline: A comparative study with central composite design approach. Food Chem. 2019, 293, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Barde, L.; Dighe, N. A review on niosomes: As a vesicular drug delivery system. Stud. Indian Place Names 2020, 40, 281–288. [Google Scholar]
- Abdallah, M.H.; Elghamry, H.A.; Khalifa, N.E.; Khojali, W.M.; Khafagy, E.-S.; Lila, A.S.A.; El-Horany, H.E.-S.; El-Housiny, S. Ginger Extract-Loaded Sesame Oil-Based Niosomal Emulgel: Quality by Design to Ameliorate Anti-Inflammatory Activity. Gels 2022, 8, 737. [Google Scholar] [CrossRef]
- Abdelbari, M.A.; Elshafeey, A.H.; Abdelbary, A.A.; Mosallam, S. Implementing Nanovesicles for Boosting the Skin Permeation of Non-steroidal Anti-inflammatory Drugs. AAPS PharmSciTech 2023, 24, 195. [Google Scholar] [CrossRef]
- Yasam, V.R.; Jakki, S.L.; Natarajan, J.; Kuppusamy, G. A review on novel vesicular drug delivery: Proniosomes. Drug Deliv. 2014, 21, 243–249. [Google Scholar] [CrossRef]
- Wu, P.-S.; Li, Y.-S.; Kuo, Y.-C.; Tsai, S.-J.J.; Lin, C.-C. Preparation and Evaluation of Novel Transfersomes Combined with the Natural Antioxidant Resveratrol. Molecules 2019, 24, 600. [Google Scholar] [CrossRef]
- Patel, R.; Singh, S.; Singh, S.; Sheth, N.; Gendle, R. Development and characterization of curcumin loaded transfersome for transdermal delivery. J. Pharm. Sci. Res. 2009, 1, 71. [Google Scholar]
- Umam, N.; Ahmad, M.; Kushwaha, P. Design and fabrication of Sesamol-loaded transfersomal gel for wound healing: Physicochemical characterization and in-vivo evaluation. Drug Dev. Ind. Pharm. 2023, 49, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Pandit, A.P.; Omase, S.B.; Mute, V.M. A chitosan film containing quercetin-loaded transfersomes for treatment of secondary osteoporosis. Drug Deliv. Transl. Res. 2020, 10, 1495–1506. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, D.A.; Fatima, Z.; Kaur, C.D. Antipsoriatic and anti-inflammatory studies of Berberis aristata extract loaded nanovesicular gels. Pharmacogn. Mag. 2017, 13, S587. [Google Scholar]
- Ansari, S.A.; Qadir, A.; Warsi, M.H.; Mujeeb, M.; Aqil, M.; Mir, S.R.; Sharma, S. Ethosomes-based gel formulation of karanjin for treatment of acne vulgaris: In vitro investigations and preclinical assessment. 3 Biotech 2021, 11, 456. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Bondi, A.; Pula, W.; Contado, C.; Baldisserotto, A.; Manfredini, S.; Boldrini, P.; Sguizzato, M.; Montesi, L.; Benedusi, M.; et al. Ethosomes for Curcumin and Piperine Cutaneous Delivery to Prevent Environmental-Stressor-Induced Skin Damage. Antioxidants 2024, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Paolino, D.; Lucania, G.; Mardente, D.; Alhaique, F.; Fresta, M. Ethosomes for skin delivery of ammonium glycyrrhizinate: In vitro percutaneous permeation through human skin and in vivo anti-inflammatory activity on human volunteers. J. Control. Release 2005, 106, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.H.; Lila, A.S.A.; Unissa, R.; Elsewedy, H.S.; Elghamry, H.A.; Soliman, M.S. Brucine-Loaded Ethosomal Gel: Design, Optimization, and Anti-inflammatory Activity. AAPS PharmSciTech 2021, 22, 269. [Google Scholar] [CrossRef]
- Alam, P.; Shakeel, F.; Foudah, A.I.; Alshehri, S.; Salfi, R.; Alqarni, M.H.; Aljarba, T.M. Central Composite Design (CCD) for the Optimisation of Ethosomal Gel Formulation of Punica granatum Extract: In Vitro and In Vivo Evaluations. Gels 2022, 8, 511. [Google Scholar] [CrossRef]
- Bin Jardan, Y.A.; Ahad, A.; Raish, M.; Al-Jenoobi, F.I. Preparation and Characterization of Transethosome Formulation for the Enhanced Delivery of Sinapic Acid. Pharmaceutics 2023, 15, 2391. [Google Scholar] [CrossRef]
- Moolakkadath, T.; Aqil, M.; Ahad, A.; Imam, S.S.; Iqbal, B.; Sultana, Y.; Mujeeb, M.; Iqbal, Z. Development of transethosomes formulation for dermal fisetin delivery: Box–Behnken design, optimization, in vitro skin penetration, vesicles–skin interaction and dermatokinetic studies. Artif. Cells Nanomed. Biotechnol. 2018, 46, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.S.; Hofni, A.; Abourehab, M.A.S.; Abdel-Rahman, I.A.M. Ginger Extract-Loaded Transethosomes for Effective Transdermal Permeation and Anti-Inflammation in Rat Model. Int. J. Nanomed. 2023, 18, 1259–1280. [Google Scholar] [CrossRef] [PubMed]
- Adin, S.N.; Gupta, I.; Aqil, M.; Mujeeb, M. Baicalin loaded transethosomes for rheumatoid arthritis: Development, characterization, pharmacokinetic and pharmacodynamic evaluation. J. Drug Deliv. Sci. Technol. 2023, 81, 104209. [Google Scholar] [CrossRef]
- Abdulbaqi, I.M.; Darwis, Y.; Assi, R.A.; Khan, N.A.K. Transethosomal gels as carriers for the transdermal delivery of colchicine: Statistical optimization, characterization, and ex vivo evaluation. Drug Des. Dev. Ther. 2018, 12, 795–813. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Li, Z.; Li, N.; Feng, N. Essential oil-mediated glycerosomes increase transdermal paeoniflorin delivery: Optimization, characterization, and evaluation in vitro and in vivo. Int. J. Nanomed. 2017, 12, 3521–3532. [Google Scholar] [CrossRef]
- Alam, M.S.; Sultana, N.; Rashid, M.A.; Alhamhoom, Y.; Ali, A.; Waheed, A.; Ansari, M.S.; Aqil, M.; Mujeeb, M. Quality by Design-Optimized Glycerosome-Enabled Nanosunscreen Gel of Rutin Hydrate. Gels 2023, 9, 752. [Google Scholar] [CrossRef]
- Elkomy, M.H.; Alruwaili, N.K.; Elmowafy, M.; Shalaby, K.; Zafar, A.; Ahmad, N.; Alsalahat, I.; Ghoneim, M.M.; Eissa, E.M.; Eid, H.M. Surface-modified bilosomes nanogel bearing a natural plant alkaloid for safe management of rheumatoid arthritis inflammation. Pharmaceutics 2022, 14, 563. [Google Scholar] [CrossRef]
- Das, M.K.; Kalita, B. Design and evaluation of phyto-phospholipid complexes (phytosomes) of rutin for transdermal application. J. Appl. Pharm. Sci. 2014, 4, 051–057. [Google Scholar] [CrossRef]
- Ju Ho, P.; Jun Sung, J.; Ki Cheon, K.; Jin Tae, H. Anti-inflammatory effect of Centella asiatica phytosome in a mouse model of phthalic anhydride-induced atopic dermatitis. Phytomedicine 2018, 43, 110–119. [Google Scholar] [CrossRef]
- Rajput, R.P.S.; Chakravarty, C.; Bhardwaj, S.K. Preparation and Evaluation of Phytosome of Herbal Plant of Lawsonia inermis L. for Topical Application. J. Innov. Pharm. Sci. 2019, 3, 9–11. [Google Scholar]
- Arun Ramachandran, A.B.; Archana, A.; Mohammad Ismail, M. Formulation and Evaluation of Herbal Anti-Inflammatory Phytosome Gel Containing Methanolic Extract of Crotalaria Biflora. Int. J. Pharm. Res. Appl. 2023, 8, 35–46. [Google Scholar]
- Bhasin, B.; Londhe, V.Y. An overview of transfersomal drug delivery. Int. J. Pharm. Sci. Res. 2018, 9, 2175–2184. [Google Scholar]
- Opatha, S.A.T.; Titapiwatanakun, V.; Chutoprapat, R. Transfersomes: A promising nanoencapsulation technique for transdermal drug delivery. Pharmaceutics 2020, 12, 855. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.H.; Abu Lila, A.S.; Shawky, S.M.; Almansour, K.; Alshammari, F.; Khafagy, E.-S.; Makram, T.S. Experimental Design and Optimization of Nano-Transfersomal Gel to Enhance the Hypoglycemic Activity of Silymarin. Polymers 2022, 14, 508. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Yadav, T.; Tickoo, O.; Sudhakar, K.; Pandey, N.; Wafa, A.; Rana, P.; Monika, M. Transfersomes as a Surfactant-based Ultradeformable Liposome. In BIO Web of Conferences; EDP Sciences: Les Ulis, France, 2024; Volume 86, p. 01021. [Google Scholar]
- Abdallah, M.H.; Lila, A.S.A.; Anwer, M.K.; Khafagy, E.-S.; Mohammad, M.; Soliman, M.S. Formulation, development and evaluation of ibuprofen loaded nano-transferosomal gel for the treatment of psoriasis. J. Pharm. Res. Int. 2019, 31, 1–8. [Google Scholar] [CrossRef]
- Van Hoogevest, P.; Wendel, A. The use of natural and synthetic phospholipids as pharmaceutical excipients. Eur. J. Lipid Sci. Technol. 2014, 116, 1088–1107. [Google Scholar] [CrossRef] [PubMed]
- Rother, M.; Lavins, B.J.; Kneer, W.; Lehnhardt, K.; Seidel, E.J.; Mazgareanu, S. Efficacy and safety of epicutaneous ketoprofen in Transfersome (IDEA-033) versus oral celecoxib and placebo in osteoarthritis of the knee: Multicentre randomised controlled trial. Ann. Rheum. Dis. 2007, 66, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Ethosomes: A promising tool for transdermal delivery of drug. Pharma Info. Net 2007, 5, 345–350. [Google Scholar]
- Meesa, R.; Srinivas, A. Ethosomes as novel drug delivery carriers-a review. Indo Am. J. Pharm. Res. 2016, 3, 1639–1643. [Google Scholar]
- Abdallah, M.H.; Elghamry, H.A.; Khalifa, N.E.; Khojali, W.M.; Khafagy, E.-S.; Shawky, S.; El-Horany, H.E.-S.; El-Housiny, S. Development and optimization of erythromycin loaded transethosomes cinnamon oil based emulgel for antimicrobial efficiency. Gels 2023, 9, 137. [Google Scholar] [CrossRef]
- Cevc, G. Lipid vesicles and other colloids as drug carriers on the skin. Adv. Drug Deliv. Rev. 2004, 56, 675–711. [Google Scholar] [CrossRef] [PubMed]
- Abdulbaqi, I.M.; Darwis, Y.; Khan, N.A.K.; Assi, R.A.; Khan, A.A. Ethosomal nanocarriers: The impact of constituents and formulation techniques on ethosomal properties, in vivo studies, and clinical trials. Int. J. Nanomed. 2016, 11, 2279–2304. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R. The new era of vesicular drug delivery system: A review. Int. J. Innov. Res. Sci. Eng. Technol. 2021, 8, 661–668. [Google Scholar]
- Bhalaria, M.; Naik, S.; Misra, A. Ethosomes: A novel delivery system for antifungal drugs in the treatment of topical fungal diseases. Indian J. Exp. Biol. 2009, 47, 368–375. [Google Scholar] [PubMed]
- Guzel, I.; Gungor, S.; Erdal, M. Improved skin penetration and deposition of naftifine from transethosomes and transethosomal gel formulations. Farmacia 2022, 70, 514–521. [Google Scholar] [CrossRef]
- Shaji, J.; Bajaj, R. Transethosomes: A new prospect for enhanced transdermal delivery. Int. J. Pharm. Sci. Res. 2018, 9, 2681–2685. [Google Scholar]
- Mahmoud, D.B.; ElMeshad, A.N.; Fadel, M.; Tawfik, A.; Ramez, S.A. Photodynamic therapy fortified with topical oleyl alcohol-based transethosomal 8-methoxypsoralen for ameliorating vitiligo: Optimization and clinical study. Int. J. Pharm. 2022, 614, 121459. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A.; Qureshi, O.S.; Kim, H.-S.; Cha, J.-H.; Kim, H.-S.; Kim, J.-K. Improved skin permeation of methotrexate via nanosized ultradeformable liposomes. Int. J. Nanomed. 2016, 11, 3813–3824. [Google Scholar]
- Chowdary, P.; Padmakumar, A.; Rengan, A.K. Exploring the potential of transethosomes in therapeutic delivery: A comprehensive review. MedComm–Biomater. Appl. 2023, 2, e59. [Google Scholar] [CrossRef]
- Bajaj, K.J.; Parab, B.S.; Shidhaye, S.S. Nano-transethosomes: A novel tool for drug delivery through skin. Indian J. Pharm. Educ. Res. 2021, 55, 1–10. [Google Scholar] [CrossRef]
- Rani, D.; Sharma, V.; Singh, P.; Singh, R. Glycerosomes: A novel vesicular drug delivery system. Res. J. Pharm. Technol. 2022, 15, 921–926. [Google Scholar] [CrossRef]
- Manca, M.L.; Zaru, M.; Manconi, M.; Lai, F.; Valenti, D.; Sinico, C.; Fadda, A.M. Glycerosomes: A new tool for effective dermal and transdermal drug delivery. Int. J. Pharm. 2013, 455, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.H.; El-Horany, H.E.; El-Nahas, H.M.; Ibrahim, T.M. Tailoring Risperidone-Loaded Glycethosomal In Situ Gels Using Box-Behnken Design for Treatment of Schizophrenia-Induced Rats via Intranasal Route. Pharmaceutics 2023, 15, 2521. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Zeeshan, F.; Srivastava, D.; Awasthi, H. A Discursive Review of Recent Development and Patents on Glycerosomes. Recent Pat. Nanotechnol. 2023, 17, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Rani, A.; Singh, V.D.; Shah, P.; Sharma, S.; Kumar, S. Glycerosomes: Novel Nano-Vesicles for Efficient Delivery of Therapeutics. Recent Adv. Drug Deliv. Formul. 2023, 17, 173–182. [Google Scholar] [CrossRef]
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Arab, F.L.; Hoseinzadeh, A.; Mohammadi, F.S.; Rajabian, A.; Faridzadeh, A.; Mahmoudi, M. Immunoregulatory effects of nanocurcumin in inflammatory milieu: Focus on COVID-19. Biomed. Pharmacother. 2024, 171, 116131. [Google Scholar] [CrossRef]
- Marino, P.; Pepe, G.; Basilicata, M.G.; Vestuto, V.; Marzocco, S.; Autore, G.; Procino, A.; Gomez-Monterrey, I.M.; Manfra, M.; Campiglia, P. Potential Role of Natural Antioxidant Products in Oncological Diseases. Antioxidants 2023, 12, 704. [Google Scholar] [CrossRef]
- El Menshawe, S.F.; Aboud, H.M.; Elkomy, M.H.; Kharshoum, R.M.; Abdeltwab, A.M. A novel nanogel loaded with chitosan decorated bilosomes for transdermal delivery of terbutaline sulfate: Artificial neural network optimization, in vitro characterization and in vivo evaluation. Drug Deliv. Transl. Res. 2020, 10, 471–485. [Google Scholar] [CrossRef]
- Abdallah, M.H.; Shahien, M.M.; Alshammari, A.; Ibrahim, S.; Ahmed, E.H.; Atia, H.A.; Elariny, H.A. The Exploitation of Sodium Deoxycholate-Stabilized Nano-Vesicular Gel for Ameliorating the Antipsychotic Efficiency of Sulpiride. Gels 2024, 10, 239. [Google Scholar] [CrossRef]
- Stojančević, M.; Pavlović, N.; Goločorbin-Kon, S.; Mikov, M. Application of bile acids in drug formulation and delivery. Front. Life Sci. 2013, 7, 112–122. [Google Scholar] [CrossRef]
- Ahmed, S.; Kassem, M.A.; Sayed, S. Bilosomes as promising nanovesicular carriers for improved transdermal delivery: Construction, in vitro optimization, ex vivo permeation and in vivo evaluation. Int. J. Nanomed. 2020, 15, 9783–9798. [Google Scholar] [CrossRef] [PubMed]
- Bonifacio, B.V.; da Silva, P.B.; Ramos, M.A.d.S.; Negri, K.M.S.; Bauab, T.M.; Chorilli, M. Nanotechnology-based drug delivery systems and herbal medicines: A review. Int. J. Nanomed. 2014, 9, 1–15. [Google Scholar]
- Nagar, G. Phytosomes: A novel drug delivery for herbal extracts. Int. J. Pharm. Sci. Res. 2019, 4, 949–959. [Google Scholar]
- Bombardelli, E.; Curri, S.; Della Loggia, R.; Del Negro, P.; Gariboldi, P.; Tubaro, A. Complexes between phospholipids and vegetal derivates of biological interest. Fitoterapia 1989, 60, 29–37. [Google Scholar]
- Bhattacharya, S. Phytosomes: The new technology for enhancement of bioavailability of botanicals and nutraceuticals. Int. J. Health Res. 2009, 2, 225–232. [Google Scholar] [CrossRef]
- Kidd, P.; Head, K. A review of the bioavailability and clinical efficacy of milk thistle phytosome: A silybin-phosphatidylcholine complex (Siliphos). Altern. Med. Rev. 2005, 10, 193. [Google Scholar] [PubMed]
- Dewan, N.; Dasgupta, D.; Pandit, S.; Ahmed, P. Review on-Herbosomes, A new arena for drug delivery. J. Pharmacogn. Phytochem. 2016, 5, 104–108. [Google Scholar]
- Jain, N.; Gupta, B.P.; Thakur, N.; Jain, R.; Banweer, J.; Jain, D.K.; Jain, S. Phytosome: A novel drug delivery system for herbal medicine. Int. J. Pharm. Sci. Drug Res. 2010, 2, 224–228. [Google Scholar]
- Barani, M.; Sangiovanni, E.; Angarano, M.; Rajizadeh, M.A.; Mehrabani, M.; Piazza, S.; Gangadharappa, H.V.; Pardakhty, A.; Mehrbani, M.; Dell’Agli, M.; et al. Phytosomes as Innovative Delivery Systems for Phytochemicals: A Comprehensive Review of Literature. Int. J. Nanomed. 2021, 16, 6983–7022. [Google Scholar] [CrossRef] [PubMed]
- Dodle, T.; Mohanty, D.; Tripathy, B.; Panigrahy, A.B.; Sirikonda, S.; Kumar, L.; Kumar, C.P.; Gobinath, M.; Patro, C.S.; Bakshi, V. A critical review on phytosomes: Advancement and research on emerging nanotechnological tools. Curr. Bioact. Compd. 2023, 19, 89–99. [Google Scholar] [CrossRef]
- Kalita, B.; Das, M.K. Rutin–phospholipid complex in polymer matrix for long-term delivery of rutin via skin for the treatment of inflammatory diseases. Artif. Cells Nanomed. Biotechnol. 2018, 46, 41–56. [Google Scholar] [CrossRef] [PubMed]

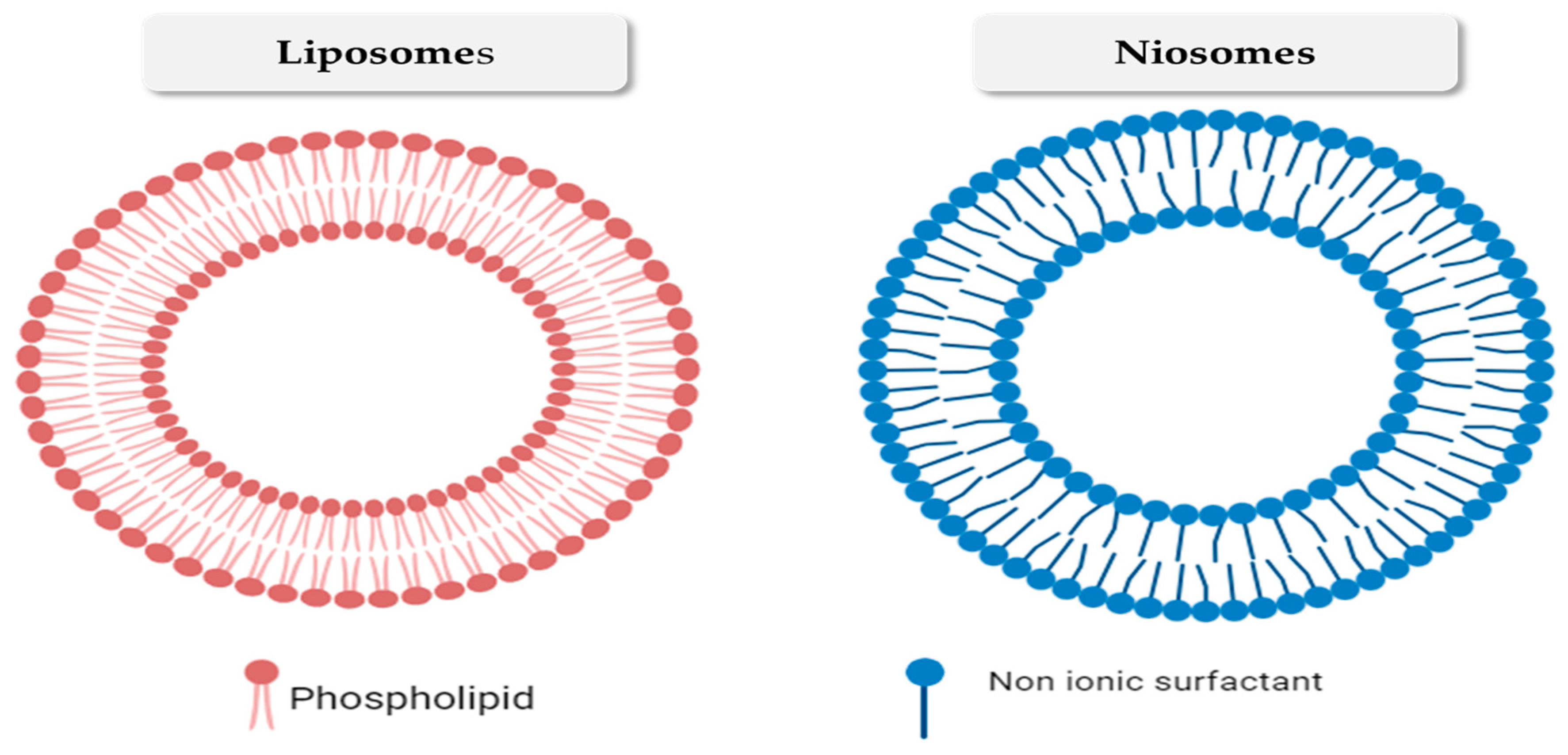
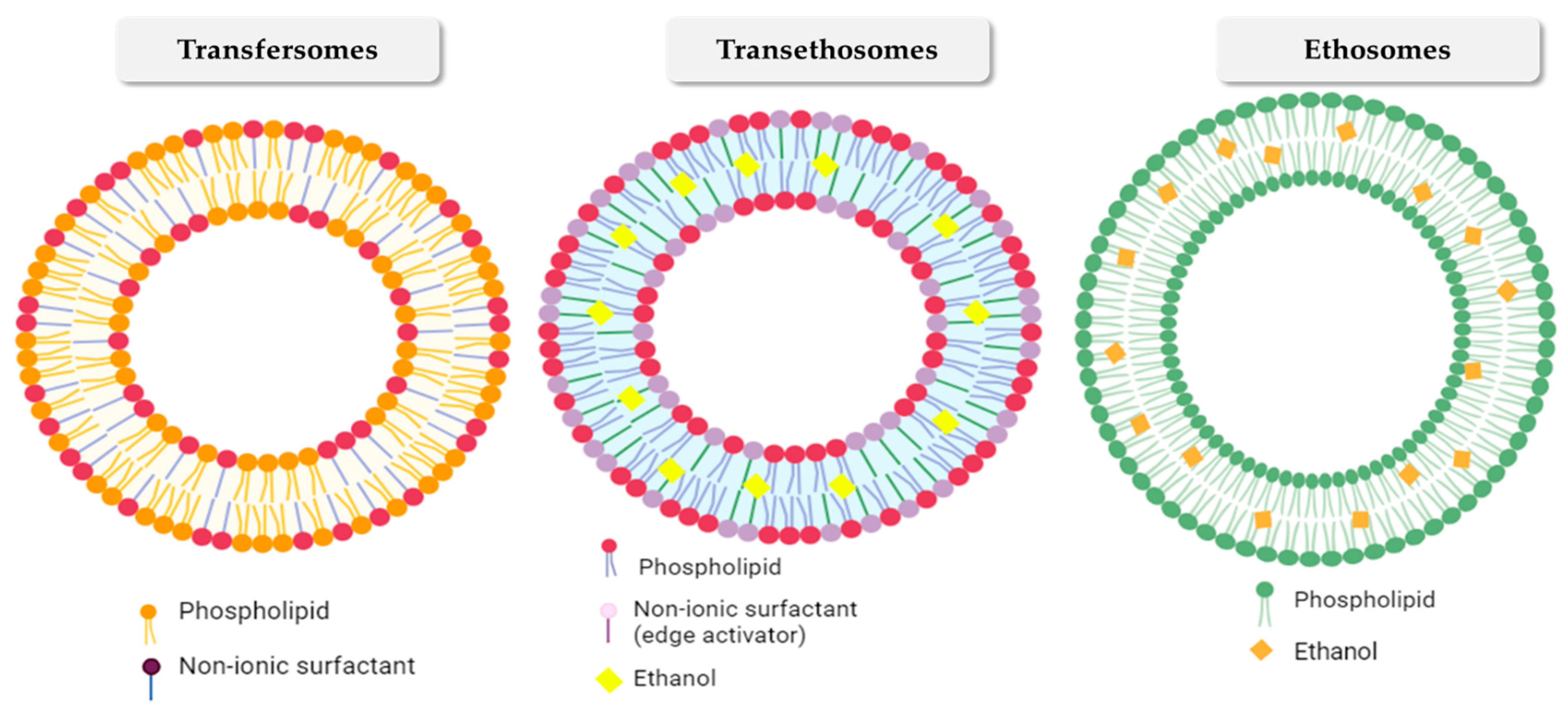
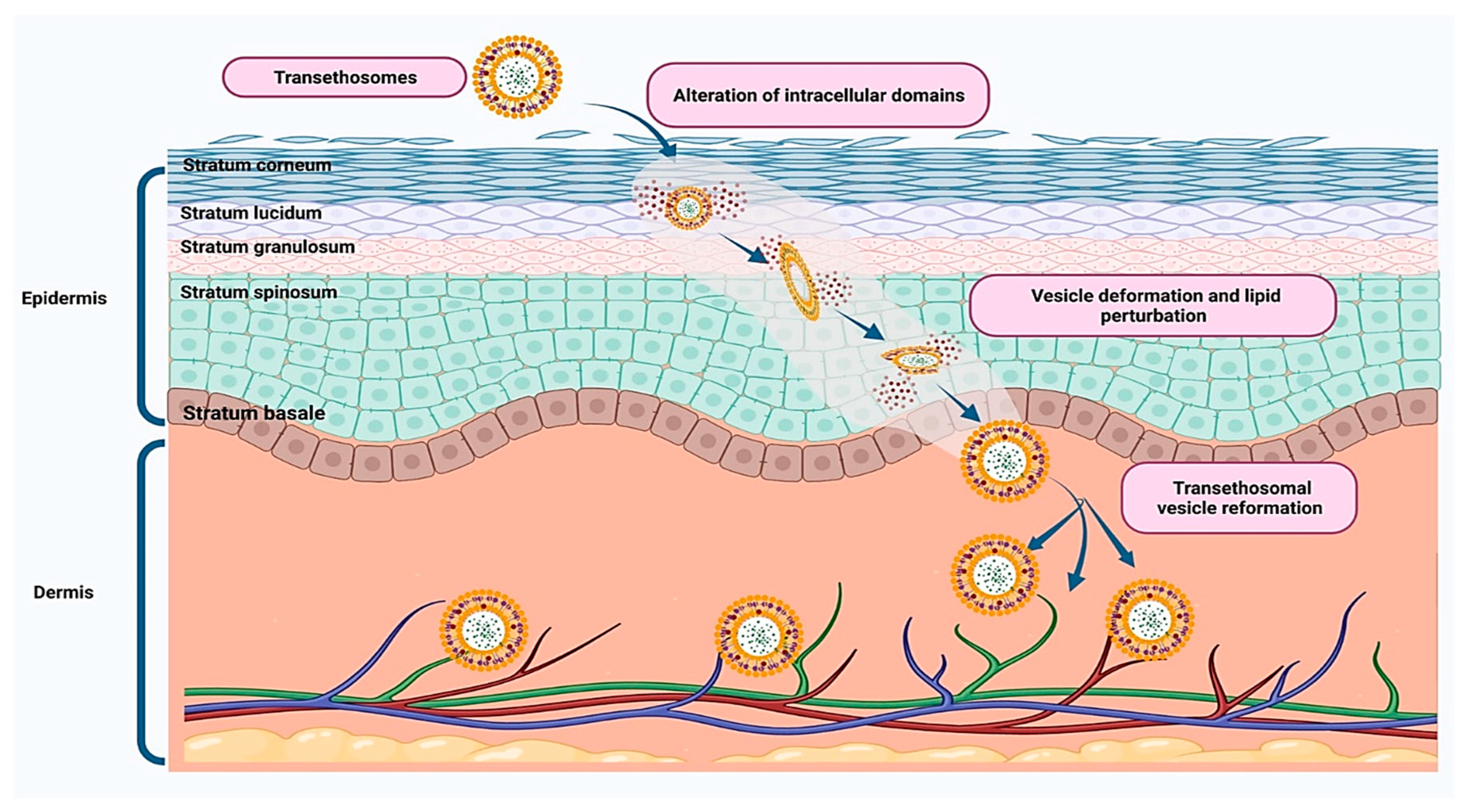
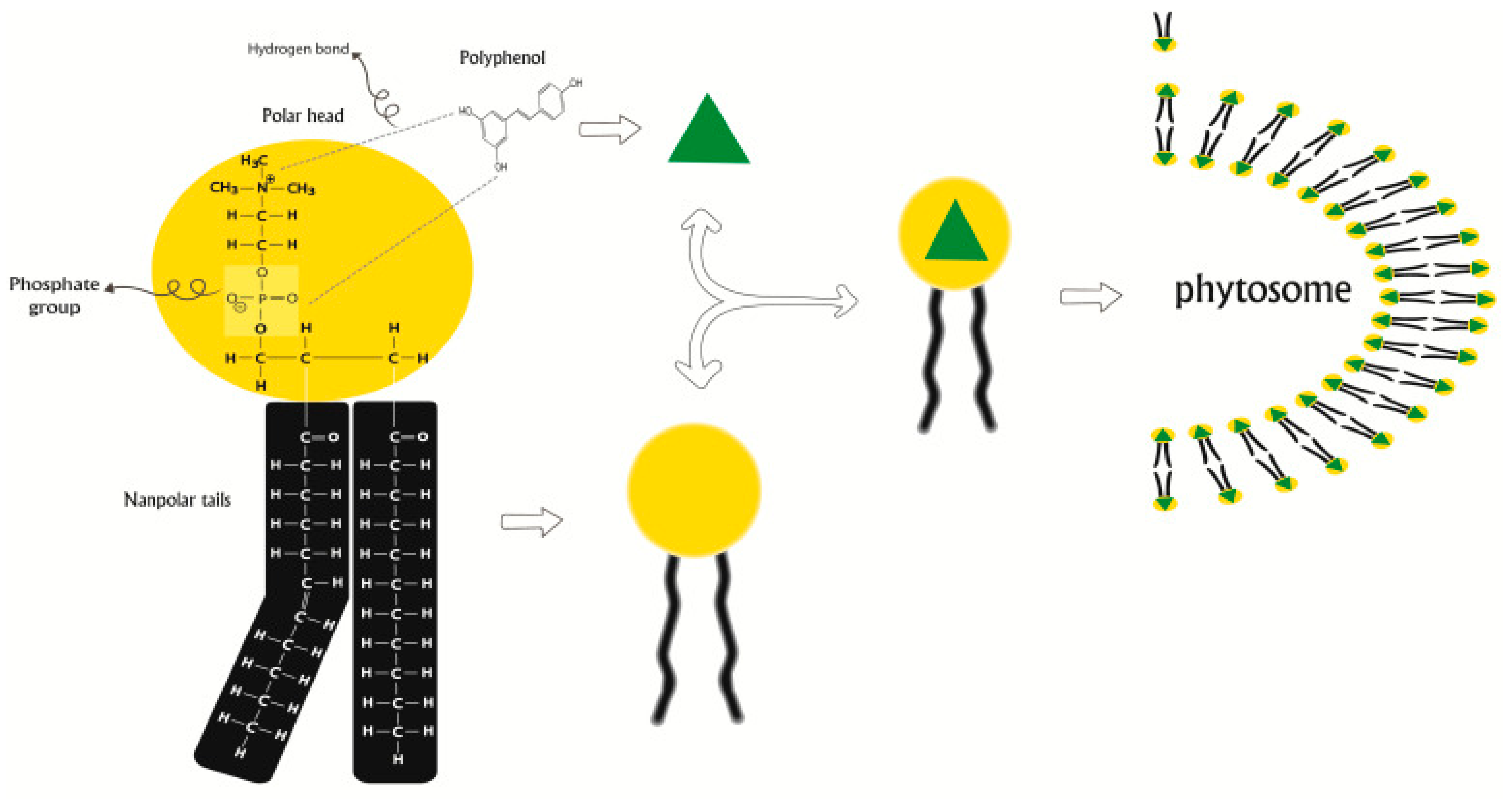
| Nanovesicular System | Plant-Based Compounds | Composition | Method of Preparation | Special Characteristics | References |
|---|---|---|---|---|---|
| Transfersomes | Resveratrol | Lecithin and edge activators (Tween 20, Tween 80 | High-pressure homogenization technique | High flexibility and stability, enhanced skin permeability and improved solubility, bioavailability, and safety of drug | [44] |
| Curcumin | Lecithin and edge activators (Tween 80, Span 80 | Modified hand shaking, lipid film hydration technique | Improved skin permeability of drug | [45] | |
| Sesamol | Phosphatidylcholine, Tween 80 and Span 80 edge activators | Thin-film hydration | Better skin penetration and deposition were observed in sesamol-loaded transfersomal gel | [46] | |
| Quercetin | Phosphatidylcholine and tween 80 | Thin lipid film hydration technique | Quercetin-loaded transfersomes were found to be a good alternative to oral administration of quercetin to treat osteoporosis | [47] | |
| Berberis aristata extract | Soyaphosphatidylcholine (SPC), edge activator (EA Tween 80/Span 80/sodium deoxycholate) | Modified lipid film hydration technique using rotary evaporator | Better therapeutic efficiency | [48] | |
| Ethosomes | Karanjin | Phospholipids 90 G, ethanol and phosphate buffer | Film hydration method | Greater flexibility, enhanced skin permeation and effective anti-inflammatory activity | [49] |
| Curcumin and piperine | Soybean lecithin, ethanol, water | Bulk cold method or by a microfluidic approach | Prolonged transdermal release of the drugs within the skin | [50] | |
| Ammonium glycyrrhizinate | Phospholipids 90 G, ethanol and water | Film hydration method | Higher percutaneous permeation and enhanced anti-inflammatory activity | [51] | |
| Brucine | Lecithin, cholesterol, ethanol and phosphate buffer pH 7.4 | Thin film hydration method | Greater skin permeability and higher anti-inflammatory activity | [52] | |
| Punica granatum extract | Lecithin, cholesterol, propylene glycol, ethanol, and water | Hot method followed by sonication or extrusion techniques | Greater potential of P. granatum ethosomal gel for enhancing its anti-inflammatory activity | [53] | |
| Transethosomes | Sinapic acid | Phospholipon 90 G, SDC, ethanol, phosphate-buffered saline | Thin film hydration method | Enhanced penetrability across the membrane, and improved vesicles flexibility | [54] |
| Fisetin | Lipoid S 100, sodium cholate and ethanol | Thin lipid film hydration technique | Deeper skin penetration and deposition | [55] | |
| Ginger extract | Phospholipon 90 G, cholesterol, edge activator (Span 80, Tween 80, and sodium deoxycholate) and ethanol | Cold injection technique | [56] | ||
| Baicalin | Phospholipon 90 G, Sodium cholate, ethanol | Thin film hydration method | High elasticity and skin deposition | [57] | |
| Colchicine | Phospholipon 90 G and surfactant (Tween 20®, sodium taurocholate, or Labrafil®) in ethanol | Cold method | Transfersomes is proven to be an alternative route to the oral route to overcome bioavailability problems and other side effects | [58] | |
| Glycerosomes | Paeoniflorin | Lipoid S 80, cholesterol, water and glycerol | Reverse-phase evaporation method | Superior transdermal flux, safe and applicable vehicle for the treatment of rheumatoid arthritis | [59] |
| Rutin | Phospholipid 90 G, cholesterol, water and glycerol | Thin film hydration method | Suitable alternation for administration of drug topically to maximize the therapeutic efficacy of the drugs | [60] | |
| Bilosomes | Berberine chloride | Cholesterol, soybean lecithin, sodium deoxycholate | Thin-film hydration technique | Chitosan-coated bilosomes, which contain berberine, have shown promise as a therapeutic method for managing inflammation in rheumatoid arthritis (RA). | [61] |
| Phytosomes | Rutin | Phosphatidylcholine | Refluxing followed by solvent evaporation | Rutin phytosomes improve skin absorption to treat inflammatory disorders and supply the medicine longer without oral administration complications | [62] |
| Centella asiatica | Phospholipid such as phosphatidylcholine, phophatidylethanolamine or phosphatidylserine, (dioxane, acetone, methylene chloride, or ethyl acetate) | Solvent evaporation, precipitation and anhydrous co-solvent lyophilization | Phytosome effectively reduces both skin inflammation and PA treatment-induced allergic responses | [63] | |
| Lawsonia inermis L. (Lawsone) | Lawsone and soya lecithin | Anti-solvent precipitation technique | The anti-inflammatory activity of lawsone phytosomal gel showed significant anti-inflammatory activity as compared to lawsone gel | [64] | |
| Methanolic extract of Crotalaria biflora | Plant extract with phosphatidylcholine | Rotary evaporation method | Development of herbal topical phytosomal gel with enhanced anti-inflammatory efficiency | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atia, H.A.; Shahien, M.M.; Ibrahim, S.; Ahmed, E.H.; Elariny, H.A.; Abdallah, M.H. Plant-Based Nanovesicular Gel Formulations Applied to Skin for Ameliorating the Anti-Inflammatory Efficiency. Gels 2024, 10, 525. https://doi.org/10.3390/gels10080525
Atia HA, Shahien MM, Ibrahim S, Ahmed EH, Elariny HA, Abdallah MH. Plant-Based Nanovesicular Gel Formulations Applied to Skin for Ameliorating the Anti-Inflammatory Efficiency. Gels. 2024; 10(8):525. https://doi.org/10.3390/gels10080525
Chicago/Turabian StyleAtia, Hanan Abdelmawgoud, Mona M. Shahien, Somaia Ibrahim, Enas Haridy Ahmed, Hemat A. Elariny, and Marwa H. Abdallah. 2024. "Plant-Based Nanovesicular Gel Formulations Applied to Skin for Ameliorating the Anti-Inflammatory Efficiency" Gels 10, no. 8: 525. https://doi.org/10.3390/gels10080525
APA StyleAtia, H. A., Shahien, M. M., Ibrahim, S., Ahmed, E. H., Elariny, H. A., & Abdallah, M. H. (2024). Plant-Based Nanovesicular Gel Formulations Applied to Skin for Ameliorating the Anti-Inflammatory Efficiency. Gels, 10(8), 525. https://doi.org/10.3390/gels10080525








