Development of Scalable Elastic Gelatin Hydrogel Films Crosslinked with Waterborne Polyurethane for Enhanced Mechanical Properties and Strain Recovery
Abstract
:1. Introduction
2. Results and Discussion
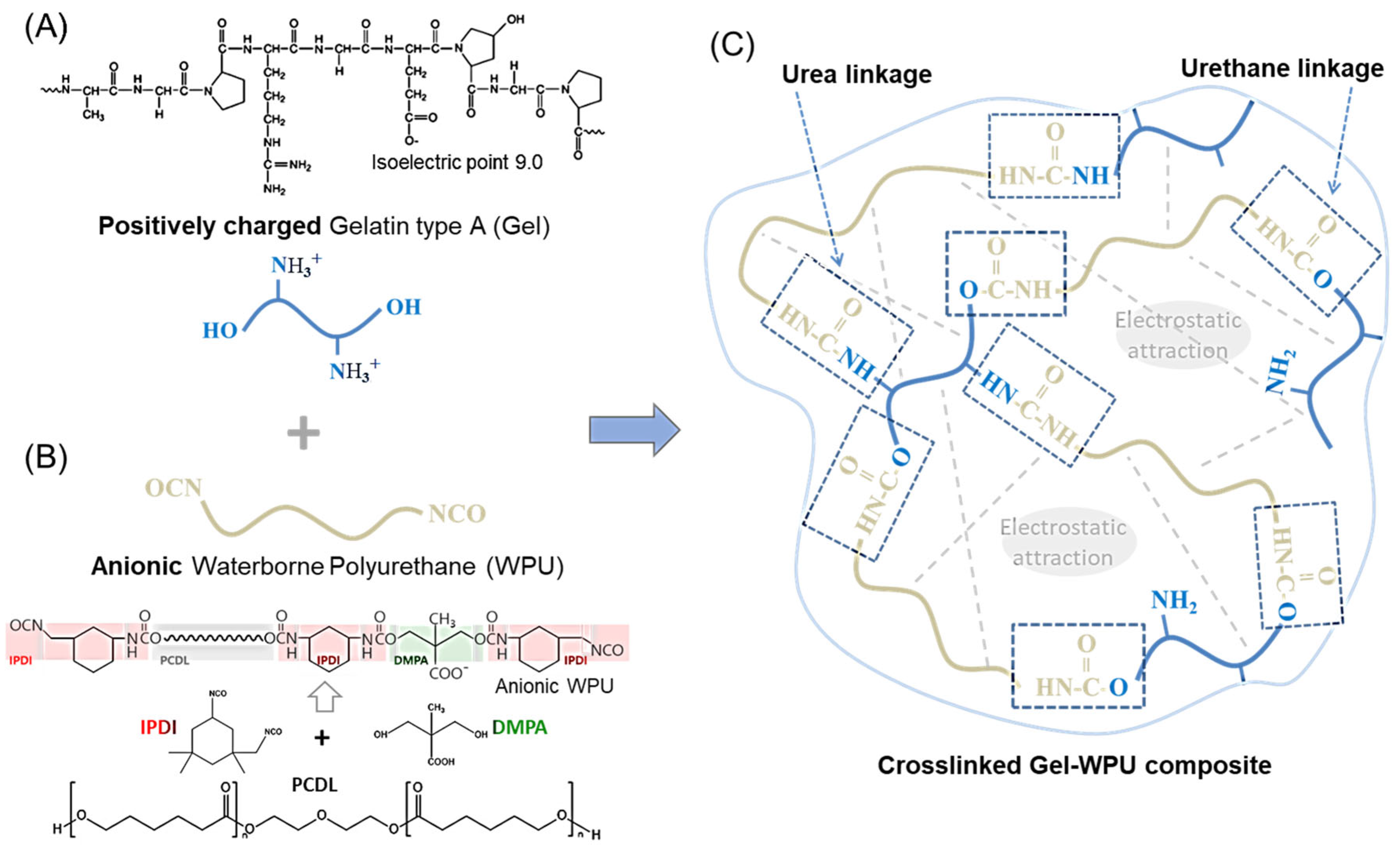
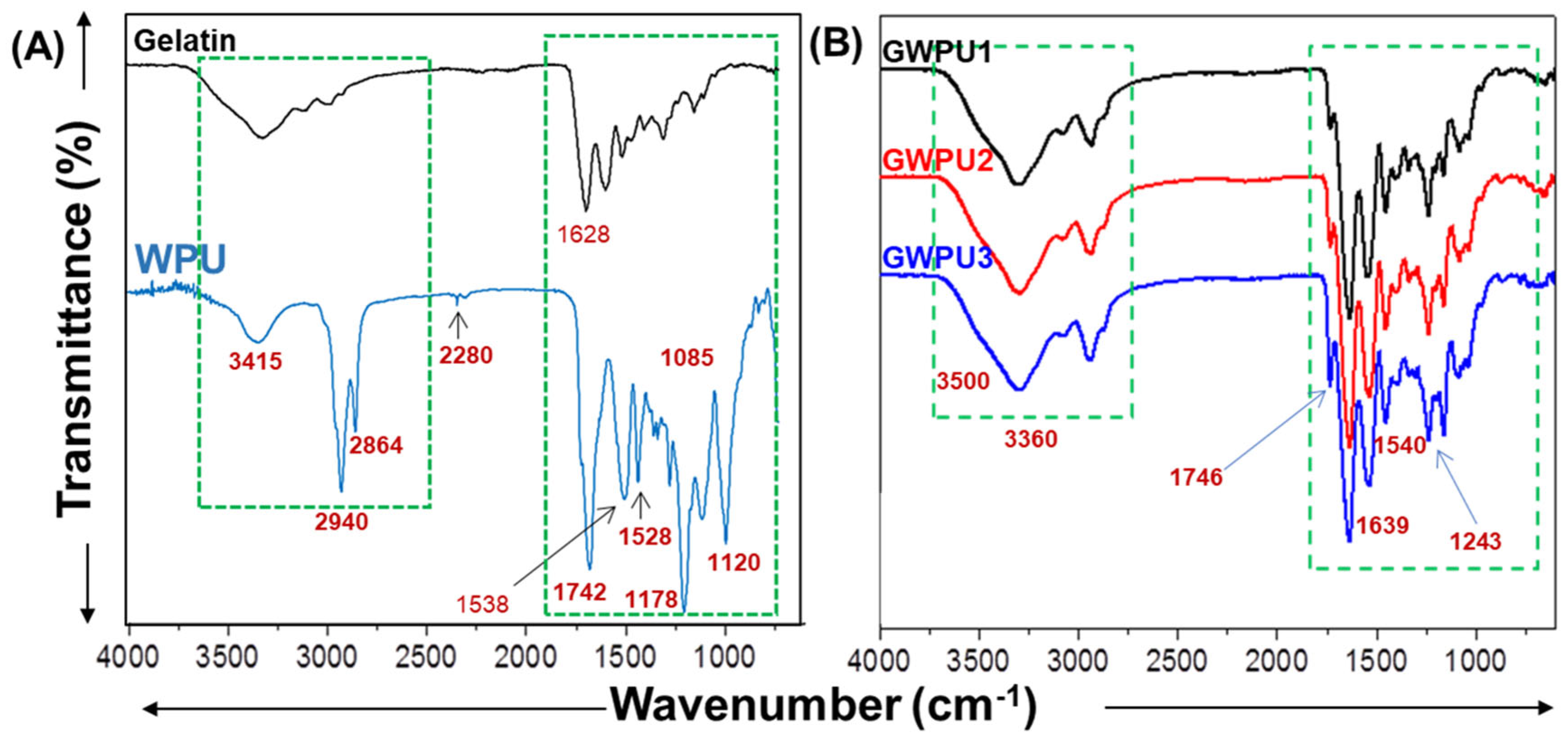
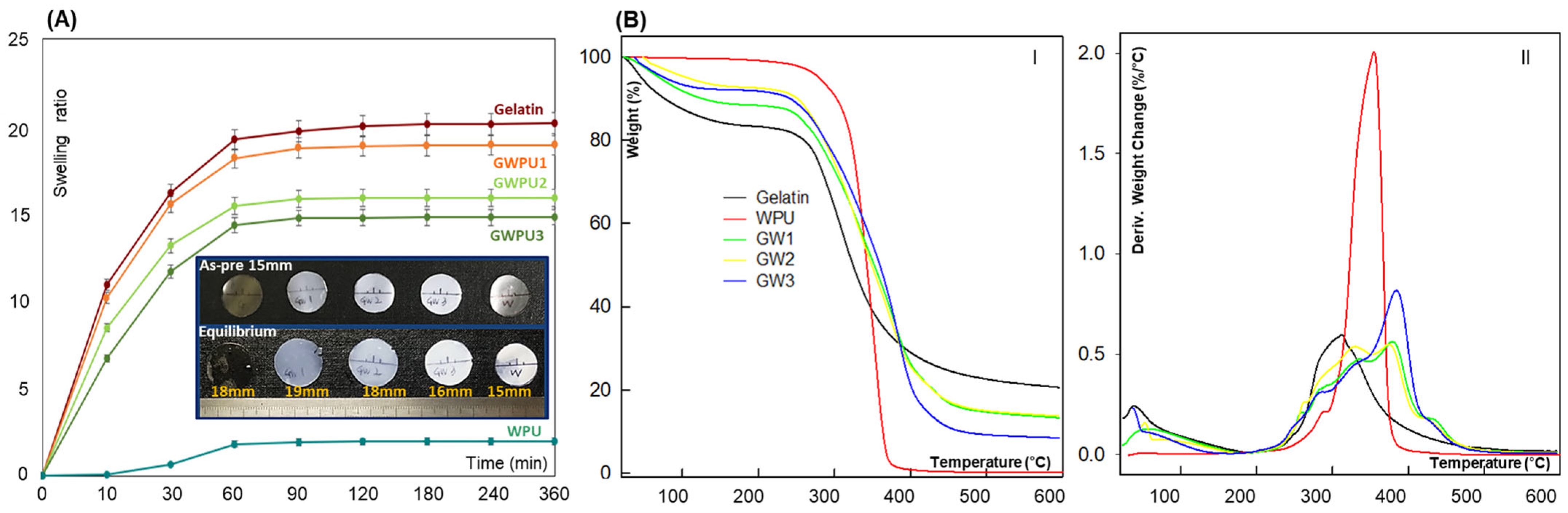
2.1. Characterization of Gelatin-Based Hydrogel
2.2. Mechanical Characterization of Gelatin-Based Hydrogel
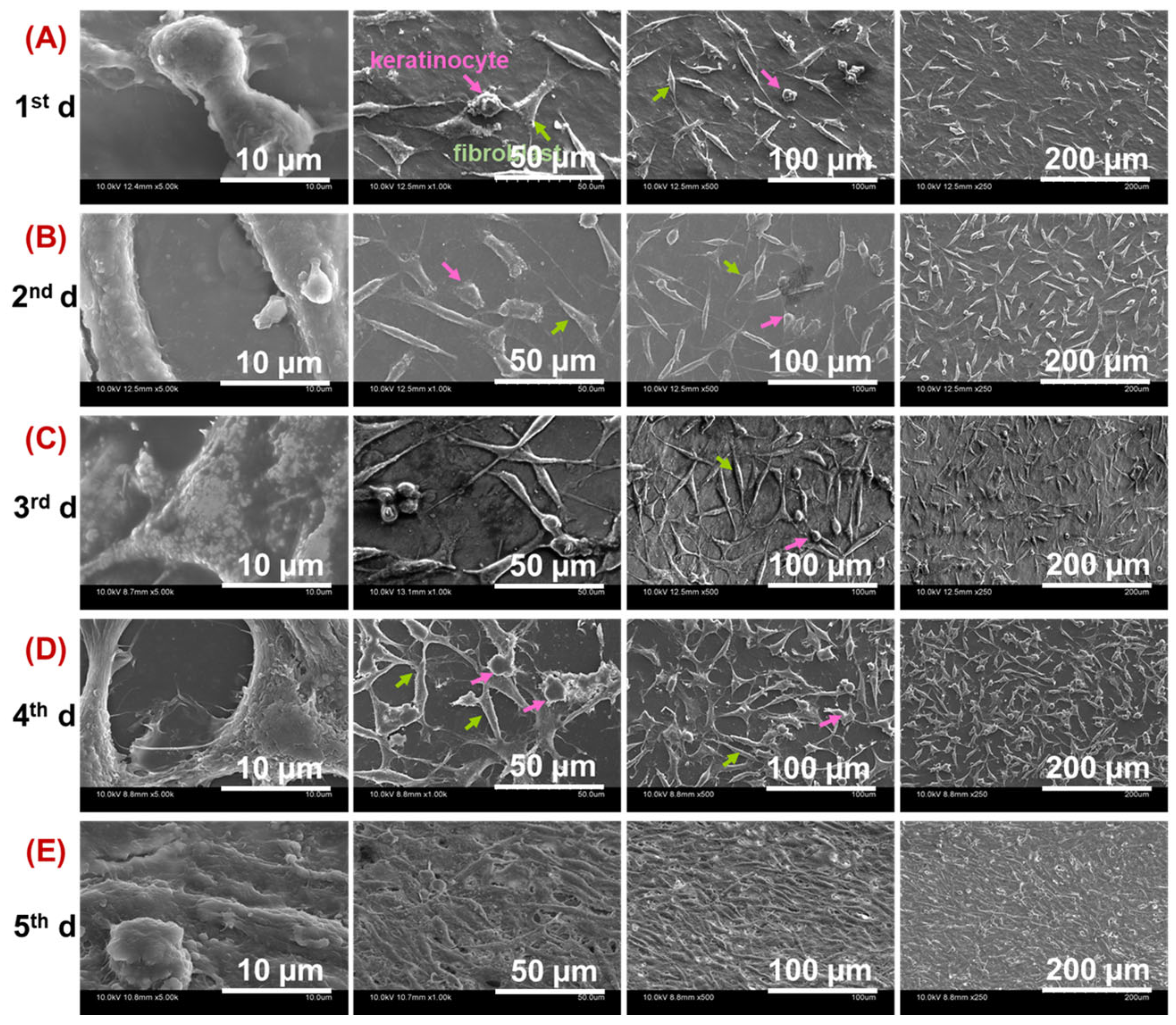
2.3. Cell Viability and Proliferation on Prepared Gelatin-Based Hydrogels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Water Polyurethane Dispersion (WPU)
4.3. Synthesis of Gelatin Hydrogels
4.4. Characterization
4.4.1. Rheological Analysis
4.4.2. SEM
4.4.3. FTIR
4.4.4. XPS
4.4.5. Swelling
4.4.6. TGA
4.4.7. Mechanical Behavior
4.4.8. Recovery
4.4.9. Cell Biocompatibility
4.4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, S.; Pang, H.; Li, Z.; Wang, Z.; Kang, H.; Zhang, W.; Zhang, S.; Li, J.; Li, L. Polyurethane as high-functionality crosslinker for constructing thermally driven dual-crosslinking plant protein adhesion system with integrated strength and ductility. Chem. Eng. J. 2021, 422, 130152. [Google Scholar] [CrossRef]
- Forrester, M.; Becker, A.; Hohmann, A.; Hernandez, N.; Lin, F.-Y.; Bloome, N.; Johnson, G.; Dietrich, H.; Marcinko, J.; Williams, R.C.; et al. RAFT thermoplastics from glycerol: A biopolymer for development of sustainable wood adhesives. Green Chem. 2020, 22, 6148–6156. [Google Scholar] [CrossRef]
- Wang, Y.; Zhai, W.; Cheng, S.; Li, J.; Zhang, H. Surface-functionalized design of blood-contacting biomaterials for preventing coagulation and promoting hemostasis. Friction 2023, 11, 1371–1394. [Google Scholar] [CrossRef]
- Sood, A.; Ji, S.M.; Kumar, A.; Han, S.S. Enzyme-Triggered Crosslinked Hybrid Hydrogels for Bone Tissue Engineering. Materials 2022, 15, 6383. [Google Scholar] [CrossRef] [PubMed]
- Asim, S.; Hayhurst, E.; Callaghan, R.; Rizwan, M. Ultra-low content physio-chemically crosslinked gelatin hydrogel improves encapsulated 3D cell culture. Int. J. Biol. Macromol. 2024, 264, 130657. [Google Scholar] [CrossRef]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Y.; Zhang, M.; Yang, D.; Huang, W.; Zheng, A.; Cao, L. High-Performance MXene Hydrogel for Self-Propelled Marangoni Swimmers and Water-Enabled Electricity Generator. Adv. Sci. 2024, 2408161. [Google Scholar] [CrossRef]
- Choudhary, A.; Sharma, A.; Singh, A.; Han, S.S.; Sood, A. Strategy and Advancement in Hybrid Hydrogel and Their Applications: Recent Progress and Trends. Adv. Eng. Mater. 2024, 26, 2400944. [Google Scholar] [CrossRef]
- Singhmar, R.; Son, Y.; Jo, Y.J.; Zo, S.; Min, B.K.; Sood, A.; Han, S.S. Fabrication of alginate composite hydrogel encapsulated retinoic acid and nano Se doped biphasic CaP to augment in situ mineralization and osteoimmunomodulation for bone regeneration. Int. J. Biol. Macromol. 2024, 275, 133597. [Google Scholar] [CrossRef]
- Lin, X.; Zhao, X.; Xu, C.; Wang, L.; Xia, Y. Progress in the mechanical enhancement of hydrogels: Fabrication strategies and underlying mechanisms. J. Polym. Sci. 2022, 60, 2525–2542. [Google Scholar] [CrossRef]
- Yuk, H.; Wu, J.; Zhao, X. Hydrogel interfaces for merging humans and machines. Nat. Rev. Mater. 2022, 7, 935–952. [Google Scholar] [CrossRef]
- Kojic, N.; Panzer, M.J.; Leisk, G.G.; Raja, W.K.; Kojic, M.; Kaplan, D.L. Ion electrodiffusion governs silk electrogelation. Soft Matter 2012, 8, 6897–6905. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.; Maji, B.; Yadav, H. Progress on green crosslinking of polysaccharide hydrogels for drug delivery and tissue engineering applications. Carbohydr. Polym. 2024, 326, 121584. [Google Scholar] [CrossRef]
- Shin, E.J.; Choi, S.M. Advances in Waterborne Polyurethane-Based Biomaterials for Biomedical Applications. Adv. Exp. Med. Biol. 2018, 1077, 251–283. [Google Scholar]
- Hsieh, C.-T.; Hsu, S.-h. Double-Network Polyurethane-Gelatin Hydrogel with Tunable Modulus for High-Resolution 3D Bioprinting. ACS Appl. Mater. Interfaces 2019, 11, 32746–32757. [Google Scholar] [CrossRef]
- Rodrigues, I.C.P.; Woigt, L.F.; Pereira, K.D.; Luchessi, A.D.; Lopes, É.S.N.; Webster, T.J.; Gabriel, L.P. Low-cost hybrid scaffolds based on polyurethane and gelatin. J. Mater. Res. Technol. 2020, 9, 7777–7785. [Google Scholar] [CrossRef]
- Díez-García, I.; Lemma, M.R.d.C.; Barud, H.S.; Eceiza, A.; Tercjak, A. Hydrogels based on waterborne poly(urethane-urea)s by physically cross-linking with sodium alginate and calcium chloride. Carbohydr. Polym. 2020, 250, 116940. [Google Scholar] [CrossRef]
- Chen, R.-D.; Huang, C.-F.; Hsu, S.-h. Composites of waterborne polyurethane and cellulose nanofibers for 3D printing and bioapplications. Carbohydr. Polym. 2019, 212, 75–88. [Google Scholar] [CrossRef]
- Lee, T.J.; Kwon, S.H.; Kim, B.K. Biodegradable sol–gel coatings of waterborne polyurethane/gelatin chemical hybrids. Prog. Org. Coat. 2014, 77, 1111–1116. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, M.; Wang, Y.; Li, Y.; Han, W.; Dang, X. Silver Nanoparticle-Loaded Gelatin-Based Nanocomposite Films toward Enhanced Mechanical Properties and Antibacterial Activity. ACS Appl. Bio Mater. 2022, 5, 2193–2201. [Google Scholar] [CrossRef]
- Bele, M.; Gaberscek, M.; Dominko, R.; Drofenik, J.; Zupan, K.; Komac, P.; Kocevar, K.; Musevic, I.; Pejovnik, S. Gelatin-pretreated carbon particles for potential use in lithium ion batteries. Carbon 2002, 40, 1117–1122. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, T.; Kuang, H.; Sun, X.; Zhu, J.; Shi, Y.; Wang, C.; Mo, X.; Lu, S.; Hong, T. Preparation and evaluation of poly(ester-urethane) urea/gelatin nanofibers based on different crosslinking strategies for potential applications in vascular tissue engineering. RSC Adv. 2018, 8, 35917–35927. [Google Scholar] [CrossRef]
- Kocen, R.; Gasik, M.; Gantar, A.; Novak, S. Viscoelastic behaviour of hydrogel-based composites for tissue engineering under mechanical load. Biomed. Mater. 2017, 12, 025004. [Google Scholar] [CrossRef] [PubMed]
- Pruksawan, S.; Lim, J.W.R.; Lee, Y.L.; Lin, Z.; Chee, H.L.; Chong, Y.T.; Chi, H.; Wang, F. Enhancing hydrogel toughness by uniform cross-linking using modified polyhedral oligomeric silsesquioxane. Commun. Mater. 2023, 4, 75. [Google Scholar] [CrossRef]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 86, 325–332. [Google Scholar] [CrossRef]
- Lian, M.; Fan, J.; Shi, Z.; Zhang, S.; Li, H.; Yin, J. Gelatin-assisted fabrication of graphene-based nacre with high strength, toughness, and electrical conductivity. Carbon 2015, 89, 279–289. [Google Scholar] [CrossRef]
- Vieira, T.; Silva, J.C.; Borges, J.P.; Henriques, C. Synthesis, electrospinning and in vitro test of a new biodegradable gelatin-based poly(ester urethane urea) for soft tissue engineering. Eur. Polym. J. 2018, 103, 271–281. [Google Scholar] [CrossRef]
- Bankoti, K.; Rameshbabu, A.P.; Datta, S.; Maity, P.P.; Goswami, P.; Datta, P.; Ghosh, S.K.; Mitra, A.; Dhara, S. Accelerated healing of full thickness dermal wounds by macroporous waterborne polyurethane-chitosan hydrogel scaffolds. Mater. Sci. Eng. C 2017, 81, 133–143. [Google Scholar] [CrossRef]
- Vashist, A.; Shahabuddin, S.; Gupta, Y.K.; Ahmad, S. Polyol induced interpenetrating networks: Chitosan–methylmethacrylate based biocompatible and pH responsive hydrogels for drug delivery system. J. Mater. Chem. B 2013, 1, 168–178. [Google Scholar] [CrossRef]
- Garrett, J.T.; Xu, R.; Cho, J.; Runt, J. Phase separation of diamine chain-extended poly(urethane) copolymers: FTIR spectroscopy and phase transitions. Polymer 2003, 44, 2711–2719. [Google Scholar] [CrossRef]
- Shi, Y.; Zhan, X.; Luo, Z.; Zhang, Q.; Chen, F. Quantitative IR characterization of urea groups in waterborne polyurethanes. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 2433–2444. [Google Scholar] [CrossRef]
- Li, S.; Kong, X.; Feng, S. Preparation of uniform poly(urea–siloxane) microspheres through precipitation polymerization. RSC Adv. 2015, 5, 90313–90320. [Google Scholar] [CrossRef]
- Sarkar, S.; Chourasia, A.; Maji, S.; Sadhukhan, S.; Kumar, S.; Adhikari, B. Synthesis and characterization of gelatin based polyester urethane scaffold. Bull. Mater. Sci. 2006, 29, 475–484. [Google Scholar] [CrossRef]
- Zhang, M.; Dai, Y.; Wen, L.; Wang, H.; Chu, J. Maskless Surface Modification of Polyurethane Films by an Atmospheric Pressure He/O2 Plasma Microjet for Gelatin Immobilization. Micromachines 2018, 9, 195. [Google Scholar] [CrossRef]
- Li, Q.; Guo, L.; Qiu, T.; Xiao, W.; Du, D.; Li, X. Synthesis of waterborne polyurethane containing alkoxysilane side groups and the properties of the hybrid coating films. Appl. Surf. Sci. 2016, 377, 66–74. [Google Scholar] [CrossRef]
- Jalaja, K.; Naskar, D.; Kundu, S.C.; James, N.R. Fabrication of cationized gelatin nanofibers by electrospinning for tissue regeneration. RSC Adv. 2015, 5, 89521–89530. [Google Scholar]
- Wang, S.; Li, K.; Zhou, Q. High strength and low swelling composite hydrogels from gelatin and delignified wood. Sci. Rep. 2020, 10, 17842. [Google Scholar] [CrossRef]
- Jutrzenka Trzebiatowska, P.; Santamaria Echart, A.; Calvo Correas, T.; Eceiza, A.; Datta, J. The changes of crosslink density of polyurethanes synthesised with using recycled component. Chemical structure and mechanical properties investigations. Prog. Org. Coat. 2018, 115, 41–48. [Google Scholar] [CrossRef]
- Peng, L.; Wang, H.; Dai, H.; Fu, Y.; Ma, L.; Zhu, H.; Yu, Y.; Li, L.; Wang, Q.; Zhang, Y. Preparation and characterization of gelatin films by transglutaminase cross-linking combined with ethanol precipitation or Hofmeister effect. Food Hydrocoll. 2021, 113, 106421. [Google Scholar] [CrossRef]
- Chiellini, E.; Cinelli, P.; Grillo Fernandes, E.; Kenawy el, R.S.; Lazzeri, A. Gelatin-based blends and composites. Morphological and thermal mechanical characterization. Biomacromolecules 2001, 2, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, X.; Zhang, D.; Wang, X.; Zhao, F.; Shi, P.; Pang, X. Coculture with human fetal epidermal keratinocytes promotes proliferation and migration of human fetal and adult dermal fibroblasts. Mol. Med. Rep. 2015, 11, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-M.; Lee, M.-W.; Shin, E.-J. One-Pot Processing of Regenerated Cellulose Nanoparticles/Waterborne Polyurethane Nanocomposite for Eco-friendly Polyurethane Matrix. Polymers 2019, 11, 356. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.M.; Shin, E.J.; Zo, S.M.; Kummara, M.R.; Kim, C.M.; Kumar, A.; Bae, H.J.; Sood, A.; Han, S.S. Development of Scalable Elastic Gelatin Hydrogel Films Crosslinked with Waterborne Polyurethane for Enhanced Mechanical Properties and Strain Recovery. Gels 2025, 11, 49. https://doi.org/10.3390/gels11010049
Choi SM, Shin EJ, Zo SM, Kummara MR, Kim CM, Kumar A, Bae HJ, Sood A, Han SS. Development of Scalable Elastic Gelatin Hydrogel Films Crosslinked with Waterborne Polyurethane for Enhanced Mechanical Properties and Strain Recovery. Gels. 2025; 11(1):49. https://doi.org/10.3390/gels11010049
Chicago/Turabian StyleChoi, Soon Mo, Eun Joo Shin, Sun Mi Zo, Madhusudana Rao Kummara, Chul Min Kim, Anuj Kumar, Han Jo Bae, Ankur Sood, and Sung Soo Han. 2025. "Development of Scalable Elastic Gelatin Hydrogel Films Crosslinked with Waterborne Polyurethane for Enhanced Mechanical Properties and Strain Recovery" Gels 11, no. 1: 49. https://doi.org/10.3390/gels11010049
APA StyleChoi, S. M., Shin, E. J., Zo, S. M., Kummara, M. R., Kim, C. M., Kumar, A., Bae, H. J., Sood, A., & Han, S. S. (2025). Development of Scalable Elastic Gelatin Hydrogel Films Crosslinked with Waterborne Polyurethane for Enhanced Mechanical Properties and Strain Recovery. Gels, 11(1), 49. https://doi.org/10.3390/gels11010049









