Hydrogels for Antitumor and Antibacterial Therapy
Abstract
:1. Introduction
2. Hydrogel for Anti-Tumor Therapy
2.1. Antitumor Therapeutic Hydrogel for Drug Delivery in Chemotherapy
2.2. Antitumor Hydrogel for Immunotherapy and Radiotherapy
2.3. Antitumor Hydrogel for Phototherapy
2.3.1. Antitumor Therapy Hydrogel for PTT
2.3.2. Antitumor Therapy Hydrogel for PDT
2.3.3. Antitumor Hydrogel for PTT/PDT Combination Therapy
2.4. Antitumor Hydrogel for Sonodynamic Therapy (SDT)
3. Hydrogel for Antibacterial Treatment
3.1. Hydrogel for Wound Healing of Bacterial Infection Type
3.1.1. Inorganic Antibacterial Agent Hydrogel for Wound Healing
3.1.2. Antibiotic-Loaded Hydrogel for Wound Healing
3.1.3. Inherently Antibacterial Active Hydrogel for Wound Healing
3.1.4. Other Hydrogels for Wound Healing
3.2. Antibacterial Hydrogel for Other Applications
4. Co-Purpose Hydrogel for Anti-Tumor and Bacterial Infections
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, L.; Feng, X.; Pei, Y.; Wang, J.; Ding, J.; Chen, L. Alpha-cyclodextrin concentration-controlled thermo-sensitive supramolecular hydrogels. Mater. Sci. Eng. C 2018, 82, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Chen, J.; Zhao, S.; Ding, J.; Chen, X. Thermo-sensitive polypeptide hydrogel for locally sequential delivery of two-pronged antitumor drugs. Acta Biomater. 2017, 58, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Wang, Z.; Chen, B.; Dai, W.; Zhang, H.; He, B.; Wang, X.; Wang, Y.; Zhang, Q. Localized co-delivery of collagenase and trastuzumab by thermosensitive hydrogels for enhanced antitumor efficacy in human breast xenograft. Drug Deliv. 2018, 25, 1495–1503. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, H.; Lei, X.; Lau, J.N.Y.; Yuan, M.; Wang, X.; Zhang, F.; Zhou, F.; Qi, S.; Shu, B.; et al. A systematic review and meta-analysis of clinical effectiveness and safety of hydrogel dressings in the management of skin wounds. Front. Bioeng. Biotechnol. 2019, 7, 342. [Google Scholar] [CrossRef] [Green Version]
- Bai, X.; Lu, S.; Liu, H.; Cao, Z.; Ning, P.; Wang, Z.; Gao, C.; Ni, B.; Ma, D.; Liu, M. Polysaccharides based injectable hydrogel compositing bio-glass for cranial bone repair. Carbohydr. Polym. 2017, 175, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Miri, A.K.; Hosseinabadi, H.G.; Cecen, B.; Hassan, S.; Zhang, Y.S. Permeability mapping of gelatin methacryloyl hydrogels. Acta Biomater. 2018, 77, 38–47. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.Y.; Zhao, Y.; Yang, Y.; Wang, W.; Wu, C.; Yang, B.; Zhang, Z.; Zhang, L.; Liu, Y.; et al. pH-switchable antimicrobial nanofiber networks of hydrogel eradicate biofilm and rescue stalled healing in chronic wounds. ACS Nano 2019, 13, 11686–11697. [Google Scholar] [CrossRef]
- Peng, H.; Huang, Q.; Yue, H.; Li, Y.; Wu, M.; Liu, W.; Zhang, G.; Fu, S.; Zhang, J. The antitumor effect of cisplatin-loaded thermosensitive chitosan hydrogel combined with radiotherapy on nasopharyngeal carcinoma. Int. J. Pharm. 2019, 556, 97–105. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional hydrogels as wound dressing to enhance wound healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Mao, J.J.; Pillai, G.G.; Andrade, C.J.; Ligibel, J.A.; Basu, P.; Cohen, L.; Khan, I.A.; Mustian, K.M.; Puthiyedath, R.; Dhiman, K.S.; et al. Integrative oncology: Addressing the global challenges of cancer prevention and treatment. CA Cancer J. Clin. 2022, 72, 144–164. [Google Scholar] [CrossRef]
- Tan, B.; Huang, L.; Wu, Y.; Liao, J. Advances and trends of hydrogel therapy platform in localized tumor treatment: A review. J. Biomed. Mater. Res. A 2021, 109, 404–425. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Wang, X.; Song, G.; Liu, T.; He, B.; Zhang, H.; Wang, X.; Zhang, Q. Combination antitumor therapy with targeted dual-nanomedicines. Adv. Drug Deliv. Rev. 2017, 115, 23–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.H.; Huang, B.S.; Horng, H.C.; Yeh, C.C.; Chen, Y.J. Wound healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Xiao, L.; He, B.; Zhang, Q.; Nie, T.; Yang, X.; Wu, D.; Cheng, H.; Li, P.; Wang, Q. Oxygen-tuned nanozyme polymerization for the preparation of hydrogels with printable and antibacterial properties. J. Mater. Chem. B 2017, 5, 1518–1524. [Google Scholar] [CrossRef] [PubMed]
- Browne, K.; Chakraborty, S.; Chen, R.; Willcox, M.D.; Black, D.S.; Walsh, W.R.; Kumar, N. A new era of antibiotics: The clinical potential of antimicrobial peptides. Int. J. Mol. Sci. 2020, 21, 7047. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, Z.; Wang, Y.; Feng, L. Near-infrared light-triggered synergistic phototherapy for antimicrobial therapy. ACS Appl. Bio Mater. 2020, 3, 1730–1737. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, Y.; Wang, Y.; Jiang, H.; Wang, X. Advances and challenges in metallic nanomaterial synthesis and antibacterial applications. J. Mater. Chem. B 2020, 8, 4764–4777. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Hu, T.; Han, Y.; Guo, B. Mussel-inspired, antibacterial, conductive, antioxidant, injectable composite hydrogel wound dressing to promote the regeneration of infected skin. J. Colloid Interface Sci. 2019, 556, 514–528. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef]
- Yamamuro, S.; Takahashi, M.; Satomi, K.; Sasaki, N.; Kobayashi, T.; Uchida, E.; Kawauchi, D.; Nakano, T.; Fujii, T.; Narita, Y.; et al. Lomustine and nimustine exert efficient antitumor effects against glioblastoma models with acquired temozolomide resistance. Cancer Sci. 2021, 112, 4736–4747. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, Q.; Wang, S.; Dai, F.; Cheng, X.; Cheng, X.; Chen, W.; Zhang, M.; Chen, D. In vitro additive antitumor effects of dimethoxycurcumin and 5-fluorouracil in colon cancer cells. Cancer Med. 2017, 6, 1698–1706. [Google Scholar] [CrossRef] [PubMed]
- Macedo, L.B.; Nogueira-Librelotto, D.R.; Vargas, J.D.; Scheeren, L.E.; Vinardell, M.P.; Rolim, C.M.B. Poly (varepsilon-caprolactone) nanoparticles with pH-responsive behavior improved the in vitro antitumor activity of methotrexate. AAPS PharmSciTech 2019, 20, 165. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, S.; Xiao, L.; Dong, X.; Lu, Q.; Kaplan, D.L. Injectable and pH-responsive silk nanofiber hydrogels for sustained anticancer drug delivery. ACS Appl. Mater. Interfaces 2016, 8, 17118–17126. [Google Scholar] [CrossRef] [PubMed]
- Burgy, O.; Wettstein, G.; Bellaye, P.S.; Decologne, N.; Racoeur, C.; Goirand, F.; Beltramo, G.; Hernandez, J.F.; Kenani, A.; Camus, P.; et al. Deglycosylated bleomycin has the antitumor activity of bleomycin without pulmonary toxicity. Sci. Transl. Med. 2016, 8, 326ra20. [Google Scholar] [CrossRef]
- Yoo, Y.; Yoon, S.J.; Kim, S.Y.; Lee, D.W.; Um, S.; Hyun, H.; Hong, S.O.; Yang, D.H. A local drug delivery system based on visible light-cured glycol chitosan and doxorubicin-hydrochloride for thyroid cancer treatment in vitro and in vivo. Drug Deliv. 2018, 25, 1664–1671. [Google Scholar] [CrossRef] [Green Version]
- Florczak, A.; Deptuch, T.; Kucharczyk, K.; Dams-Kozlowska, H. Systemic and local silk-based drug delivery systems for cancer therapy. Cancers 2021, 13, 5389. [Google Scholar] [CrossRef]
- Zhu, C.; Ding, Z.; Lu, Q.; Lu, G.; Xiao, L.; Zhang, X.; Dong, X.; Ru, C.; Kaplan, D.L. Injectable silk-vaterite composite hydrogels with tunable sustained drug release capacity. ACS Biomater. Sci. Eng. 2019, 5, 6602–6609. [Google Scholar] [CrossRef]
- Dehshahri, A.; Kumar, A.; Madamsetty, V.S.; Uzieliene, I.; Tavakol, S.; Azedi, F.; Fekri, H.S.; Zarrabi, A.; Mohammadinejad, R.; Thakur, V.K. New horizons in hydrogels for methotrexate delivery. Gels 2021, 7, 2. [Google Scholar] [CrossRef]
- Saeednia, L.; Yao, L.; Cluff, K.; Asmatulu, R. Sustained releasing of methotrexate from injectable and thermosensitive chitosan-carbon nanotube hybrid hydrogels effectively controls tumor cell growth. ACS Omega 2019, 4, 4040–4048. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, J.; Lu, Y. Doxorubicin and CD-CUR inclusion complex coloaded in thermosensitive hydrogel PLGA-PEG-PLGA localized administration for osteosarcoma. Int. J. Oncol. 2020, 57, 433–444. [Google Scholar] [CrossRef]
- Mekonnen, T.W.; Andrgie, A.T.; Darge, H.F.; Birhan, Y.S.; Hanurry, E.Y.; Chou, H.Y.; Lai, J.Y.; Tsai, H.C.; Yang, J.M.; Chang, Y.H. Bioinspired composite, pH-responsive sodium deoxycholate hydrogel and generation 4.5 poly(amidoamine) dendrimer improves cancer treatment efficacy via doxorubicin and resveratrol co-delivery. Pharmaceutics 2020, 12, 1069. [Google Scholar] [CrossRef] [PubMed]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; He, C.; Chen, X. Injectable hydrogels as local depots at tumor sites for antitumor immunotherapy and immune-based combination therapy. Macromol. Biosci. 2021, 21, 2100039. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Zang, J.; Gao, S.; Gao, Q.; Duan, W.; Li, X.; Xu, W.; Zhang, Y. Nitric oxide donor hybrid compounds as promising anticancer agents. Drug Discov. Ther. 2016, 10, 276–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Francis, D.M.; Sestito, L.F.; Archer, P.A.; Manspeaker, M.P.; O’Melia, M.J.; Thomas, S.N. Thermosensitive hydrogel releasing nitric oxide donor and anti-CTLA-4 micelles for anti-tumor immunotherapy. Nat. Commun. 2022, 13, 1479. [Google Scholar] [CrossRef]
- Liu, M.; Cao, Z.; Zhang, R.; Chen, Y.; Yang, X. Injectable supramolecular hydrogel for locoregional immune checkpoint blockade and enhanced cancer chemo-immunotherapy. ACS Appl. Mater. Interfaces 2021, 13, 33874–33884. [Google Scholar] [CrossRef]
- Head, H.W.; Dodd, G.D. Thermal ablation for hepatocellular carcinoma. Gastroenterology 2004, 127, S167–S178. [Google Scholar] [CrossRef]
- Cao, Y.; Zhou, Y.; Pan, J.; Zhong, X.; Ding, J.; Jing, X.; Sun, S. A general strategy towards an injectable microwave-sensitive immune hydrogel for combined percutaneous microwave ablation and immunotherapy. Chem. Eng. J. 2021, 422, 13011. [Google Scholar] [CrossRef]
- Marill, J.; Mohamed Anesary, N.; Paris, S. DNA damage enhancement by radiotherapy-activated hafnium oxide nanoparticles improves cGAS-STING pathway activation in human colorectal cancer cells. Radiother. Oncol. 2019, 141, 262–266. [Google Scholar] [CrossRef]
- Barker, H.E.; Paget, J.T.; Khan, A.A.; Harrington, K.J. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Rev. Cancer 2015, 15, 409–425. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, M.; Wang, B.; Wang, P.; Liu, Y.; Zhao, Y.; Li, K.; Song, G.; Zhang, X.; Tan, W. NIR-II driven plasmon-enhanced catalysis for a timely supply of oxygen to overcome hypoxia-Induced radiotherapy tolerance. Angew. Chem. Int. Ed. 2019, 58, 15069–15075. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Huang, X.; Zhu, Z.; Wong, M.; Watkins, S.; Zhao, Q.; Herberman, R.; Gorelik, E. Immune response against 3LL lewis lung carcinoma potentiates the therapeutic efficacy of endostatin. J. Immunol. 2001, 24, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Gao, Q.; Tang, J.; Jiang, Y.Q.; Yang, L.S.; Shi, X.X.; Chen, Y.; Zhang, Y.; Fu, S.Z.; Lin, S. Anti-tumor effect of local injectable hydrogel-loaded endostatin alone and in combination with radiotherapy for lung cancer. Drug Deliv. 2021, 28, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Mirrahimi, M.; Khateri, M.; Beik, J.; Ghoreishi, F.S.; Dezfuli, A.S.; Ghaznavi, H.; Shakeri-Zadeh, A. Enhancement of chemoradiation by co-incorporation of gold nanoparticles and cisplatin into alginate hydrogel. J. Biomed. Mater. Res. Part B 2019, 107, 2658–2663. [Google Scholar] [CrossRef]
- Vanpouille-Box, C.; Formenti, S.C.; Demaria, S. Toward precision radiotherapy for use with immune checkpoint blockers. Clin. Cancer Res. 2018, 24, 259–265. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Shen, F.; Tian, L.; Tao, H.; Xiong, Z.; Xu, J.; Liu, Z. ATP-responsive smart hydrogel releasing immune adjuvant synchronized with repeated chemotherapy or radiotherapy to boost antitumor immunity. Adv. Mater. 2021, 33, 2007910. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, P.; Zhang, G.; Zhang, L.; Liu, X.; Hu, C.; Yang, X.; Sroka, R.; Zhou, Z.; Wang, X. Conventional versus daylight photodynamic therapy for actinic keratosis: A randomized and prospective study in China. Photodiagn. Photodyn. Ther. 2018, 24, 366–371. [Google Scholar] [CrossRef]
- Gao, G.; Sun, X.B.; Liang, G.L. Nanoagent-promoted mild-temperature photothermal therapy for cancer treatment. Adv. Funct. Mater. 2021, 31, 2100738. [Google Scholar] [CrossRef]
- Ma, K.; Xing, R.; Jiao, T.; Shen, G.; Chen, C.; Li, J.; Yan, X. Injectable self-assembled dipeptide-based nanocarriers for tumor delivery and effective in vivo photodynamic therapy. ACS Appl. Mater. Interfaces 2016, 8, 30759–30767. [Google Scholar] [CrossRef]
- Xue, F.; Wen, Y.; Wei, P.; Gao, Y.; Zhou, Z.; Xiao, S.; Yi, T. A smart drug: A pH-responsive photothermal ablation agent for golgi apparatus activated cancer therapy. Chem. Commun. 2017, 53, 6424–6427. [Google Scholar] [CrossRef]
- Liang, Y.; Hao, Y.; Wu, Y.; Zhou, Z.; Li, J.; Sun, X.; Liu, Y.N. Integrated hydrogel platform for programmed antitumor therapy based on near infrared-triggered hyperthermia and vascular disruption. ACS Appl. Mater. Interfaces 2019, 11, 21381–21390. [Google Scholar] [CrossRef] [PubMed]
- Dabbagh Moghaddam, F.; Akbarzadeh, I.; Marzbankia, E.; Farid, M.; Khaledi, L.; Hossein Reihani, A.; Javidfar, M.; Mortazavi, P. Delivery of melittin-loaded niosomes for breast cancer treatment: An in vitro and in vivo evaluation of anti-cancer effect. Cancer Nanotechnol. 2021, 12, 14. [Google Scholar] [CrossRef]
- Guha, S.; Ferrie, R.P.; Ghimire, J.; Ventura, C.R.; Wu, E.; Sun, L.; Kim, S.Y.; Wiedman, G.R.; Hristova, K.; Wimley, W.C. Applications and evolution of melittin, the quintessential membrane active peptide. Biochem. Pharmacol. 2021, 193, 114769. [Google Scholar] [CrossRef]
- Jin, H.; Zhao, G.; Hu, J.; Ren, Q.; Yang, K.; Wan, C.; Huang, A.; Li, P.; Feng, J.P.; Chen, J.; et al. Melittin-containing hybrid peptide hydrogels for enhanced photothermal therapy of glioblastoma. ACS Appl. Mater. Interfaces 2017, 9, 25755–25766. [Google Scholar] [CrossRef] [PubMed]
- Gangrade, A.; Gawali, B.; Jadi, P.K.; Naidu, V.G.M.; Mandal, B.B. Photo-electro active nanocomposite silk hydrogel for spatiotemporal controlled release of chemotherapeutics: An in vivo approach toward suppressing solid tumor growth. ACS Appl. Mater. Interfaces 2020, 12, 27905–27916. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Dai, X.; Yang, J.; Zhang, B.; Zhao, D.; Li, C.; Yin, Z.; Zhao, Y.; Liu, B. Injectable polypeptide-engineered hydrogel depot for amplifying the anti-tumor immune effect induced by chemo-photothermal therapy. J. Mater. Chem. B 2020, 8, 8623–8633. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Ager, B.; Lloyd, S.; Torgeson, A.; Denney, M.; Gaffney, D.; Kharofa, J.; Lin, S.H.; Koong, A.C.; Anzai, Y.; et al. Hypoxia imaging in upper gastrointestinal tumors and application to radiation therapy. J. Gastrointest. Oncol. 2018, 9, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Quintero, M.; Mackenzie, N.; Brennan, P.A. Hypoxia-inducible factor 1 (Hif-1) in cancer. Eur. J. Surg. Oncol. 2004, 30, 465–468. [Google Scholar] [CrossRef]
- Son, S.W.; Yun, B.D.; Song, M.G.; Lee, J.K.; Choi, S.Y.; Kuh, H.J.; Park, J.K. The hypoxia-long noncoding RNA interaction in solid cancers. Int. J. Mol. Sci. 2021, 22, 7261. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Wang, M.; Schmid, T.; Xin, Z.; Kozhuharova, L.; Yu, W.K.; Huang, Y.; Cai, F.; Biskup, E. Hypoxia in breast cancer-scientific translation to therapeutic and diagnostic clinical applications. Front. Oncol. 2021, 11, 652266. [Google Scholar] [CrossRef]
- Zhou, T.J.; Xing, L.; Fan, Y.T.; Cui, P.F.; Jiang, H.L. Inhibition of breast cancer proliferation and metastasis by strengthening host immunity with a prolonged oxygen-generating phototherapy hydrogel. J. Control. Release 2019, 309, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Wang, Y.; Li, S.; Zhang, M.; Xu, M. One-pot synthesis chlorin e6 nano-precipitation for colorectal cancer treatment Ce6 NPs for colorectal cancer treatment. IET Nanobiotechnol. 2021, 15, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, D.; Shi, Y.; Zou, J.; Zhao, Q.; Zhang, Q.; Huang, W.; Shao, J.; Xie, X.; Dong, X. Black phosphorus nanosheets immobilizing Ce6 for imaging-guided photothermal/photodynamic cancer therapy. ACS Appl. Mater. Interfaces 2018, 10, 12431–12440. [Google Scholar] [CrossRef]
- Sun, X.; Sun, J.; Lv, J.; Dong, B.; Liu, M.; Liu, J.; Sun, L.; Zhang, G.; Zhang, L.; Huang, G.; et al. Ce6-C6-TPZ co-loaded albumin nanoparticles for synergistic combined PDT-chemotherapy of cancer. J. Mater. Chem. B 2019, 7, 5797–5807. [Google Scholar] [CrossRef] [PubMed]
- Nafiujjaman, M.; Revuri, V.; Park, H.K.; Keun Kwon, I.; Cho, K.J.; Lee, Y.K. Enhanced photodynamic properties of graphene quantum dot conjugated Ce6 nanoparticles for targeted cancer therapy and imaging. Chem. Lett. 2016, 45, 997–999. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Zhang, L.; Liu, Y.; Sun, S.; Yin, Z.; Zhang, L.; Li, A.; Lu, G.; Wu, A.; Zeng, L. Ce6/Mn2+-chelated polydopamine@black-TiO2 nanoprobes for enhanced synergistic phototherapy and magnetic resonance imaging in 4T1 breast cancer. Nanoscale 2020, 12, 1801–1810. [Google Scholar] [CrossRef]
- Karuppusamy, S.; Hyejin, K.; Kang, H.W. Nanoengineered chlorin e6 conjugated with hydrogel for photodynamic therapy on cancer. Colloids Surf. B 2019, 181, 778–788. [Google Scholar] [CrossRef]
- Xu, X.; Zeng, Z.; Huang, Z.; Sun, Y.; Huang, Y.; Chen, J.; Ye, J.; Yang, H.; Yang, C.; Zhao, C. Near-infrared light-triggered degradable hyaluronic acid hydrogel for on-demand drug release and combined chemo-photodynamic therapy. Carbohydr. Polym. 2020, 229, 115394. [Google Scholar] [CrossRef]
- Wen, K.; Wu, L.; Wu, X.; Lu, Y.; Duan, T.; Ma, H.; Peng, A.; Shi, Q.; Huang, H. Precisely tuning photothermal and photodynamic effects of polymeric nanoparticles by controlled copolymerization. Angew. Chem. Int. Ed. 2020, 59, 12756–12761. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, D.; Pan, J.; Xia, C.; Fan, L.; Pu, Y.; Zhang, Q.; Ni, Y.H.; Wang, J.; Hu, Q. A near infrared light-triggered human serum albumin drug delivery system with coordination bonding of indocyanine green and cisplatin for targeting photochemistry therapy against oral squamous cell cancer. Biomater. Sci. 2019, 7, 5270–5282. [Google Scholar] [CrossRef]
- Liu, C.; Ruan, C.; Shi, R.; Jiang, B.P.; Ji, S.; Shen, X.C. A near infrared-modulated thermosensitive hydrogel for stabilization of indocyanine green and combinatorial anticancer phototherapy. Biomater. Sci. 2019, 7, 1705–1715. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, G.; You, M.; Song, E.; Shukoor, M.I.; Zhang, K.; Altman, M.B.; Chen, Y.; Zhu, Z.; Huang, C.Z.; et al. Assembly of aptamer switch probes and photosensitizer on gold nanorods for targeted photothermal and photodynamic cancer therapy. ACS Nano 2012, 6, 5070–5077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, J.; Yi, Y.; Wu, G.; Liu, W. Gold nanotriangles: Green synthesis and PDT & PTT effect. Mater. Lett. 2017, 187, 148–150. [Google Scholar]
- Jin, X.; Yao, S.; Qiu, F.; Mao, Z.; Wang, B. A multifunctional hydrogel containing gold nanorods and methylene blue for synergistic cancer phototherapy. Colloids Surf. A Physicochem. Eng. Asp. 2021, 614, 126154. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, N.; Cao, Z.; Zhang, Z.; Zhu, Z.; Sun, G.; Jin, L.; Yang, X. Injectable hydrogel-mediated combination of hyperthermia ablation and photo-enhanced chemotherapy in the NIR-II window for tumor eradication. Biomater. Sci. 2021, 9, 3516–3525. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Pi, W.; Hao, M.; Li, Y.; An, H.; Li, Q.; Zhang, P.; Wen, Y. An injectable and biodegradable nano-photothermal DNA hydrogel enhances penetration and efficacy of tumor therapy. Biomater. Sci. 2021, 9, 4904–4921. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Zheng, Y.; Chen, Y. Micro/nanoparticle-augmented sonodynamic therapy (SDT): Breaking the depth shallow of photoactivation. Adv. Mater. 2016, 28, 8097–8129. [Google Scholar] [CrossRef]
- She, J.; Zhou, X.; Zhang, Y.; Zhang, R.; Li, Q.; Zhu, W.; Meng, Z.; Liu, Z. Thermo-triggered in situ chitosan-based gelation system for repeated and enhanced sonodynamic therapy post a single injection. Adv. Healthc. Mater. 2021, 10, e2001208. [Google Scholar] [CrossRef]
- Qin, X.; Wu, C.; Niu, D.; Qin, L.; Wang, X.; Wang, Q.; Li, Y. Peroxisome inspired hybrid enzyme nanogels for chemodynamic and photodynamic therapy. Nat. Commun. 2021, 12, 5243. [Google Scholar] [CrossRef]
- Zhang, E.; Zhao, X.; Hu, J.; Wang, R.; Fu, S.; Qin, G. Antibacterial metals and alloys for potential biomedical implants. Bioact. Mater. 2021, 6, 2569–2612. [Google Scholar] [CrossRef]
- Gedik, G.; Aksit, A.; Engin, B.; Paksu, U. Production of metal oxide containing antibacterial coated textile material and investigation of the mechanism of action. Fibers Polym. 2019, 19, 2548–2563. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Eckhard, U.; Delgado, L.M.; De Roo Puente, Y.J.D.; Hoyos-Nogues, M.; Gil, F.J.; Perez, R.A. Antibacterial approaches in tissue engineering using metal ions and nanoparticles: From mechanisms to applications. Bioact. Mater. 2021, 6, 4470–4490. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Park, S.J. Antibacterial behavior of transition-metals-decorated activated carbon fibers. J. Colloid Interface Sci. 2008, 325, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Forghani, F.; Kong, X.; Liu, D.; Ye, X.; Chen, S.; Ding, T. Antibacterial applications of metal-organic frameworks and their composites. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1397–1419. [Google Scholar] [CrossRef] [Green Version]
- Stankic, S.; Suman, S.; Haque, F.; Vidic, J. Pure and multi metal oxide nanoparticles: Synthesis, antibacterial and cytotoxic properties. J. Nanobiotechnol. 2016, 14, 73. [Google Scholar] [CrossRef] [Green Version]
- Udegova, E.S.; Gildeeva, K.A.; Rukosueva, T.V.; Baker, S. Metal nanoparticle antibacterial effect on antibiotic-resistant strains of bacteria. Russ. J. Infect. Immun. 2021, 11, 771–776. [Google Scholar] [CrossRef]
- El-Mekkawi, D.M.; Selim, M.M.; Hamdi, N.; Hassan, S.A.; Ezzat, A. Studies on the influence of the physicochemical characteristics of nanostructured copper, zinc and magnesium oxides on their antibacterial activities. J. Environ. 2018, 6, 5608–5615. [Google Scholar] [CrossRef]
- Malachová, K.; Praus, P.; Rybková, Z.; Kozák, O. Antibacterial and antifungal activities of silver, copper and zinc montmorillonites. Appl. Clay Sci. 2011, 53, 642–645. [Google Scholar] [CrossRef]
- Gokcekaya, O.; Ueda, K.; Ogasawara, K.; Kanetaka, H.; Narushima, T. In vitro evaluation of Ag-containing calcium phosphates: Effectiveness of Ag-incorporated beta-tricalcium phosphate. Mater. Sci. Eng. C 2017, 75, 926–933. [Google Scholar] [CrossRef]
- Xie, Y.; Yue, L.; Zheng, Y.; Zhao, L.; Liang, C.; He, W.; Liu, Z.; Sun, Y.; Yang, Y. The antibacterial stability of poly(dopamine) in-situ reduction and chelation nano-Ag based on bacterial cellulose network template. Appl. Surf. Sci. 2019, 491, 383–394. [Google Scholar] [CrossRef]
- Prusty, K.; Swain, S. Polypropylene oxide/polyethylene oxide-cellulose hybrid nanocomposite hydrogels as drug delivery vehicle. J. Appl. Polym. Sci. 2021, 138, e49921. [Google Scholar] [CrossRef]
- Haidari, H.; Kopecki, Z.; Bright, R.; Cowin, A.J.; Garg, S.; Goswami, N.; Vasilev, K. Ultrasmall agnp-impregnated biocompatible hydrogel with highly effective biofilm elimination properties. ACS Appl. Mater. Interfaces 2020, 12, 41011–41025. [Google Scholar] [CrossRef] [PubMed]
- He, S.T.; Yao, J.N.; Wang, Y.P.; Jiang, P.; Shi, D.X.; Xie, S.S.; Pang, S.J.; Gao, H.J. Self-assembled two-dimensional ordered array of silver nanoparticles. Acta Phys. Sin. 2001, 50, 765–768. [Google Scholar]
- Cheng, L.; Wang, X.; Gong, F.; Liu, T.; Liu, Z. 2D nanomaterials for cancer theranostic applications. Adv. Mater. 2020, 32, e1902333. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, J.; Yu, Y.; Su, Z.; Zhang, L.; Chen, X. Facile synthesis of rGO–MoS2–Ag nanocomposites with long-term antimicrobial activities. Nanotechnology 2020, 31, 125101. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.F.; Li, M.Y.; Liu, J.; Hu, Y.D.; Tang, K.Y. MoS2@PDA@Ag/PVA hybrid hydrogel with excellent light-responsive antibacterial activity and enhanced mechanical properties for wound dressing. Macromol. Mater. Eng. 2022, 307, 2100654. [Google Scholar] [CrossRef]
- Kong, Y.; Hou, Z.; Zhou, L.; Zhang, P.; Ouyang, Y.; Wang, P.; Chen, Y.; Luo, X. Injectable self-healing hydrogels containing CuS nanoparticles with abilities of hemostasis, antibacterial activity, and promoting wound healing. ACS Biomater. Sci. Eng. 2021, 7, 335–349. [Google Scholar] [CrossRef]
- Wu, C.; Xu, Q.H. Stable and functionable mesoporous silica-coated gold nanorods as sensitive localized surface plasmon resonance (LSPR) nanosensors. Langmuir 2009, 25, 9441–9446. [Google Scholar] [CrossRef]
- Wickramasinghe, S.; Ju, M.; Milbrandt, N.B.; Tsai, Y.H.; Navarreto-Lugo, M.; Visperas, A.; Klika, A.; Barsoum, W.; Higuera-Rueda, C.A.; Samia, A.C.S. Photoactivated gold nanorod hydrogel composite containing d-amino acids for the complete eradication of bacterial biofilms on metal alloy implant materials. ACS Appl. Nano Mater. 2020, 3, 5862–5873. [Google Scholar] [CrossRef]
- Jiang, S.; Lin, K.; Cai, M. ZnO nanomaterials: Current advancements in antibacterial mechanisms and applications. Front. Chem. 2020, 8, 580. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Wang, R.; Yuan, P.; Fan, Z.; Yang, S. Preparation and properties of ZnO/sodium alginate bi-layered hydrogel films as novel wound dressings. New J. Chem. 2019, 43, 8684–8693. [Google Scholar] [CrossRef]
- Li, X.X.; Dong, J.Y.; Li, Y.H.; Zhong, J.; Yu, H.; Yu, Q.Q.; Lei, M. Fabrication of Ag–ZnO@carboxymethyl cellulose/K-carrageenan/graphene oxide/konjac glucomannan hydrogel for effective wound dressing in nursing care for diabetic foot ulcers. Appl. Nanosci. 2019, 10, 729–738. [Google Scholar] [CrossRef]
- Xiang, Y.; Mao, C.; Liu, X.; Cui, Z.; Jing, D.; Yang, X.; Liang, Y.; Li, Z.; Zhu, S.; Zheng, Y.; et al. Rapid and superior bacteria killing of carbon quantum dots/ZnO decorated injectable folic acid-conjugated PDA hydrogel through dual-light triggered ROS and membrane permeability. Small 2019, 15, e1900322. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Xiang, Y.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Pan, H.; Wang, X.; Chu, P.K.; Wu, S. Photo-inspired antibacterial activity and wound healing acceleration by hydrogel embedded with Ag/Ag@AgCl/ZnO nanostructures. ACS Nano 2017, 11, 9010–9021. [Google Scholar] [CrossRef] [PubMed]
- Boroushaki, M.T.; Fanoudi, S.; Mollazadeh, H.; Boroumand-Noughabi, S.; Hosseini, A. Reno-protective effect of rheum turkestanicum against gentamicin-induced nephrotoxicity. Iran. J. Basic Med. Sci. 2019, 22, 328–333. [Google Scholar]
- Niyompanich, J.; Chuysinuan, P.; Pavasant, P.; Supaphol, P. Development of thermoresponsive poloxamer in situ gel loaded with gentamicin sulfate for cavity wounds. J. Polym. Res. 2021, 28, 128. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, W.; Li, Q.; Liu, X.; Guo, Z. Preparation of cross-linked chitosan quaternary ammonium salt hydrogel films loading drug of gentamicin sulfate for antibacterial wound dressing. Mar. Drugs 2021, 19, 479. [Google Scholar] [CrossRef]
- Makarov, G.I.; Makarova, T.M. A noncanonical binding site of chloramphenicol revealed via molecular dynamics simulations. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2940–2947. [Google Scholar] [CrossRef]
- Ingebrigtsen, S.G.; Didriksen, A.; Johannessen, M.; Skalko-Basnet, N.; Holsaeter, A.M. Old drug, new wrapping—A possible comeback for chloramphenicol? Int. J. Pharm. 2017, 526, 538–546. [Google Scholar] [CrossRef] [Green Version]
- Sayyafan, M.S.; Ramzi, M.; Salmanpour, R. Clinical assessment of topical erythromycin gel with and without zinc acetate for treating mild-to-moderate acne vulgaris. J. Dermatol. Treat. 2020, 31, 730–733. [Google Scholar] [CrossRef]
- Alavi, T.; Rezvanian, M.; Ahmad, N.; Mohamad, N.; Ng, S.F. Pluronic-F127 composite film loaded with erythromycin for wound application: Formulation, physicomechanical and in vitro evaluations. Drug Deliv. Transl. Res. 2019, 9, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Ren, J.; Wang, H. Injectable micromotor@hydrogel system for antibacterial therapy. Chem. Eur. J. 2022, 28, e202103867. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.N.; Rybak, M.J. Beta-lactam combinations with vancomycin show synergistic activity against vancomycin-susceptible Staphylococcus aureus, vancomycin-intermediate S. aureus (visa), and heterogeneous visa. Antimicrob. Agents Chemother. 2018, 62, e00157-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, C.H.; Chen, C.S.; Chen, Y.C.; Jiang, N.E.; Farn, C.J.; Shen, Y.S.; Hsu, M.L.; Chang, C.H. Vancomycin-loaded oxidized hyaluronic acid and adipic acid dihydrazide hydrogel: Bio-compatibility, drug release, antimicrobial activity, and biofilm model. J. Microbiol. Immunol. Infect. 2020, 53, 525–531. [Google Scholar] [CrossRef]
- Cai, D.; Chen, S.; Wu, B.; Chen, J.; Tao, D.; Li, Z.; Dong, Q.; Zou, Y.; Chen, Y.; Bi, C.; et al. Construction of multifunctional porcine acellular dermal matrix hydrogel blended with vancomycin for hemorrhage control, antibacterial action, and tissue repair in infected trauma wounds. Mater. Today Bio 2021, 12, 100127. [Google Scholar] [CrossRef]
- Boot, W.; Schmid, T.; D’Este, M.; Guillaume, O.; Foster, A.; Decosterd, L.; Richards, R.G.; Eglin, D.; Zeiter, S.; Moriarty, T.F. A hyaluronic acid hydrogel loaded with gentamicin and vancomycin successfully eradicates chronic methicillin-resistant Staphylococcus aureus orthopedic infection in a sheep model. Antimicrob. Agents Chemother. 2021, 65, e01840-20. [Google Scholar] [CrossRef]
- Yan, J.; Ji, Y.; Huang, M.; Li, T.; Liu, Y.; Lü, S.; Liu, M. Nucleobase-inspired self-adhesive and inherently antibacterial hydrogel for wound dressing. ACS Mater. Lett. 2020, 2, 1375–1380. [Google Scholar] [CrossRef]
- Zhou, L.; Lei, D.; Wang, Q.; Luo, X.; Chen, Y. Biocompatible polyphosphorylcholine hydrogels with inherent antibacterial and nonfouling behavior effectively promote skin wound healing. ACS Appl. Bio Mater. 2020, 3, 5357–5366. [Google Scholar] [CrossRef]
- Zheng, B.; Ye, J.; Yang, Y.; Huang, Y.; Xiao, M. Self-healing polysaccharide-based injectable hydrogels with antibacterial activity for wound healing. Carbohydr. Polym. 2022, 275, 118770. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, Y.; Liu, G.; Wei, Y.; Wu, Y.; Tao, L. Antibacterial self-healing hydrogel via the Ugi reaction. ACS Appl. Polym. Mater. 2019, 2, 404–410. [Google Scholar] [CrossRef]
- Liu, W.; Ou-Yang, W.; Zhang, C.; Wang, Q.; Pan, X.; Huang, P.; Zhang, C.; Li, Y.; Kong, D.; Wang, W. Synthetic polymeric antibacterial hydrogel for methicillin-resistant Staphylococcus aureus-Infected wound healing: Nanoantimicrobial self-assembly, drug- and cytokine-free strategy. ACS Nano 2020, 14, 12905–12917. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Han, H.; Li, M.; Li, N.; Wang, Q.; Qin, X.; Wang, X.; Yu, J.; Zhou, Y.; Li, Y.; et al. Nanofibers reinforced injectable hydrogel with self-healing, antibacterial, and hemostatic properties for chronic wound healing. J. Colloid Interface Sci. 2021, 596, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qiu, L.; Wang, C.; Gao, Z.; Zhou, S.; Cui, P.; Jiang, P.; Hu, H.; Ni, X.; Du, X.; et al. Nanodot-doped peptide hydrogels for antibacterial phototherapy and wound healing. Biomater. Sci. 2022, 10, 654–664. [Google Scholar] [CrossRef]
- Ji, Y.; Han, Z.; Ding, H.; Xu, X.; Wang, D.; Zhu, Y.; An, F.; Tang, S.; Zhang, H.; Deng, J.; et al. Enhanced eradication of bacterial/fungi biofilms by glucose oxidase-modified magnetic nanoparticles as a potential treatment for persistent endodontic infections. ACS Appl. Mater. Interfaces 2021, 13, 17289–17299. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Niu, D.; Shi, J.; Wang, L. A three-in-one ZIFs-derived CuCo(O)/GOx@PCNs hybrid cascade nanozyme for immunotherapy/enhanced starvation/photothermal therapy. ACS Appl. Mater. Interfaces 2021, 13, 11683–11695. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Du, X.; Jiang, L.; Luo, H.; Wang, F.; Wang, J.; Qiu, L.; Liu, L.; Liu, X.; Wang, X.; et al. Glucose oxidase-loaded antimicrobial peptide hydrogels: Potential dressings for diabetic wound. J. Nanosci. Nanotechnol. 2020, 20, 2087–2094. [Google Scholar] [CrossRef]
- Huang, T.; Yuan, B.; Jiang, W.; Ding, Y.; Jiang, L.; Ren, H.; Tang, J. Glucose oxidase and Fe3O4/TiO2/Ag3PO4 co-embedded biomimetic mineralization hydrogels as controllable ROS generators for accelerating diabetic wound healing. J. Mater. Chem. B 2021, 9, 6190–6200. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Zhang, D.; Chen, C. Intrinsic oxidase-like nanoenzyme Co4S3/Co(OH)2 hybrid nanotubes with broad-spectrum antibacterial activity. ACS Appl. Mater. Interfaces 2020, 12, 29614–29624. [Google Scholar] [CrossRef]
- Li, Y.; Fu, R.; Duan, Z.; Zhu, C.; Fan, D. Construction of multifunctional hydrogel based on the tannic acid-metal coating decorated MoS2 dual nanozyme for bacteria-infected wound healing. Bioact. Mater. 2022, 9, 461–474. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, S.; Chen, X.; Wang, L.; Yang, W. A self-assembled fmoc-diphenylalanine hydrogel-encapsulated Pt nanozyme as oxidase- and peroxidase-pike breaking pH limitation for potential antimicrobial application. Chem. Eur. J. 2022, 28, e202104247. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Y.; Liu, H.; Wang, L. Tailoring DNA self-assembly to build hydrogels. Top. Curr. Chem. 2020, 378, 32. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Yao, C.; Kou, X.; Tang, J.; Luo, D.; Yang, D. A fluorescent biofunctional DNA hydrogel prepared by enzymatic polymerization. Adv. Healthc. Mater. 2018, 7, 1700998. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Pi, W.; Cheng, S.; Gu, Z.; Zhang, K.; Min, T.; Zhang, W.; Du, H.; Zhang, P.; Wen, Y. Multifunctional DNA hydrogels with hydrocolloid-cotton structure for regeneration of diabetic infectious wounds. Adv. Funct. Mater. 2021, 31, 2106167. [Google Scholar] [CrossRef]
- Jiang, X.; Li, M.; Guo, X.; Yang, M.; Rasooly, A. Self-assembled DNA-THPS hydrogel as a topical antibacterial agent for wound healing. ACS Appl. Bio Mater. 2019, 2, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Burlibasa, M.; Muntianu, L.A.S.; Tanase, G.; Bucur, M.B.; Comes, C.A.; Ionescu, C.A. Study on microbial contamination of biomaterials in medical practice. Metal. Int. 2010, 15, 163–166. [Google Scholar]
- Ding, H.; Xu, S.; Wang, J.; Fan, Z.; Huang, Z.; Wu, H.; Pi, P.; Cheng, J.; Wen, X. A conductive, antibacterial, and antifouling hydrogel based on zwitterion. J. Appl. Polym. Sci. 2022, 139, e51648. [Google Scholar] [CrossRef]
- Thieme, L.; Hartung, A.; Tramm, K.; Klinger-Strobel, M.; Jandt, K.D.; Makarewicz, O.; Pletz, M.W. MBEC versus MBIC: The Lack of differentiation between biofilm reducing and inhibitory effects as a current problem in biofilm methodology. Biol. Proced. Online 2019, 21, 18. [Google Scholar] [CrossRef]
- Porter, G.C.; Schwass, D.R.; Tompkins, G.R.; Bobbala, S.K.R.; Medlicott, N.J.; Meledandri, C.J. AgNP/Alginate nanocomposite hydrogel for antimicrobial and antibiofilm applications. Carbohydr. Polym. 2021, 251, 117017. [Google Scholar] [CrossRef]
- Du, C.; Gao, D.; Gao, M.; Yuan, H.; Liu, X.; Wang, B.; Xing, C. Property regulation of conjugated oligoelectrolytes with polyisocyanide to achieve efficient photodynamic antibacterial biomimetic hydrogels. ACS Appl. Mater. Interfaces 2021, 13, 27955–27962. [Google Scholar] [CrossRef]
- Elkihel, A.; Christie, C.; Vernisse, C.; Ouk, T.; Lucas, R.; Chaleix, V.; Sol, V. Xylan-based cross-linked hydrogel for photodynamic antimicrobial chemotherapy. ACS Appl. Bio Mater. 2021, 4, 7204–7212. [Google Scholar] [CrossRef]
- Xie, Y.; Gan, C.; Li, Z.; Liu, W.; Yang, D.; Qiu, X. Fabrication of a lignin-copper sulfide-incorporated PVA hydrogel with near-infrared-activated photothermal/photodynamic/peroxidase-like performance for combating bacteria and biofilms. ACS Biomater. Sci. Eng. 2022, 8, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Chu, C.R.; Payne, K.A.; Marra, K.G. Injectable in situ forming biodegradable chitosan–hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials 2009, 30, 2499–2506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horner, P.J.; Gage, F.H. Regenerating the damaged central nervous system. Nature 2000, 407, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Ma, Z.; Gao, C.; Gong, Y.; Shen, J. Cartilage tissue engineering PLLA scaffold with surface immobilized collagen and basic fibroblast growth factor. Biomaterials 2005, 26, 1253–1259. [Google Scholar] [CrossRef]
- Kim, S.; Fan, J.; Lee, C.S.; Lee, M. Dual functional lysozyme-chitosan conjugate for tunable degradation and antibacterial activity. ACS Appl. Bio Mater. 2020, 3, 2334–2343. [Google Scholar] [CrossRef]
- Iimaa, T.; Hirayama, T.; Shirakigawa, N.; Imai, D.; Yamao, T.; Yamashita, Y.; Baba, H.; Ijima, H. Antibacterial-agent-immobilized gelatin hydrogel as a 3D scaffold for natural and bioengineered tissues. Gels 2019, 5, 32. [Google Scholar] [CrossRef] [Green Version]
- Ducret, M.; Montembault, A.; Josse, J.; Pasdeloup, M.; Celle, A.; Benchrih, R.; Mallein-Gerin, F.; Alliot-Licht, B.; David, L.; Farges, J.C. Design and characterization of a chitosan-enriched fibrin hydrogel for human dental pulp regeneration. Dent. Mater. 2019, 35, 523–533. [Google Scholar] [CrossRef]
- Moreira, M.S.; Sarra, G.; Carvalho, G.L.; Gonçalves, F.; Caballero-Flores, H.V.; Pedroni, A.C.F.; Lascala, C.A.; Catalani, L.H.; Marques, M.M. Physical and biological properties of a chitosan hydrogel scaffold associated to photobiomodulation therapy for dental pulp regeneration: An in vitro and in vivo study. BioMed Res. Int. 2021, 2021, 6684667. [Google Scholar] [CrossRef]
- Jin, C.; Lagoudas, G.K.; Zhao, C.; Bullman, S.; Bhutkar, A.; Hu, B.; Ameh, S.; Sandel, D.; Liang, X.S.; Mazzilli, S.; et al. Commensal microbiota promote lung cancer development via gammadelta T cells. Cell 2019, 176, 998–1013. [Google Scholar] [CrossRef] [Green Version]
- Dejea, C.M.; Fathi, P.; Craig, J.M.; Boleij, A.; Taddese, R.; Geis, A.L.; Wu, X.Q.; Shields, C.E.D.; Hechenbleikner, E.M.; Huso, D.L.; et al. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science 2018, 359, 592–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Wang, X.; Wang, W.; Qu, X.; Song, X.; Zhang, Y.; Zhong, L.; Yang, D.P.; Dong, X.; Zhao, Y. Injectable hydrogel for postoperative synergistic photothermal-chemodynamic tumor and anti-infection therapy. Biomaterials 2022, 280, 121289. [Google Scholar] [CrossRef] [PubMed]
- Salahuddin, N.; Rehab, A.; Emad, S. Synthesis and efficacy of norfloxacin loaded onto magnetic hydrogel nanocomposites. RSC Adv. 2021, 11, 30183–30194. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Wang, S.; Zhou, L.; He, X.; Cheng, Z.; Cheng, F.; Liu, Z.; Wang, X.; Chen, Y.; Zhang, Q. Injectable multi-responsive micelle/nanocomposite hybrid hydrogel for bioenzyme and photothermal augmented chemodynamic therapy of skin cancer and bacterial infection. Chem. Eng. J. 2021, 404, 126439. [Google Scholar] [CrossRef]
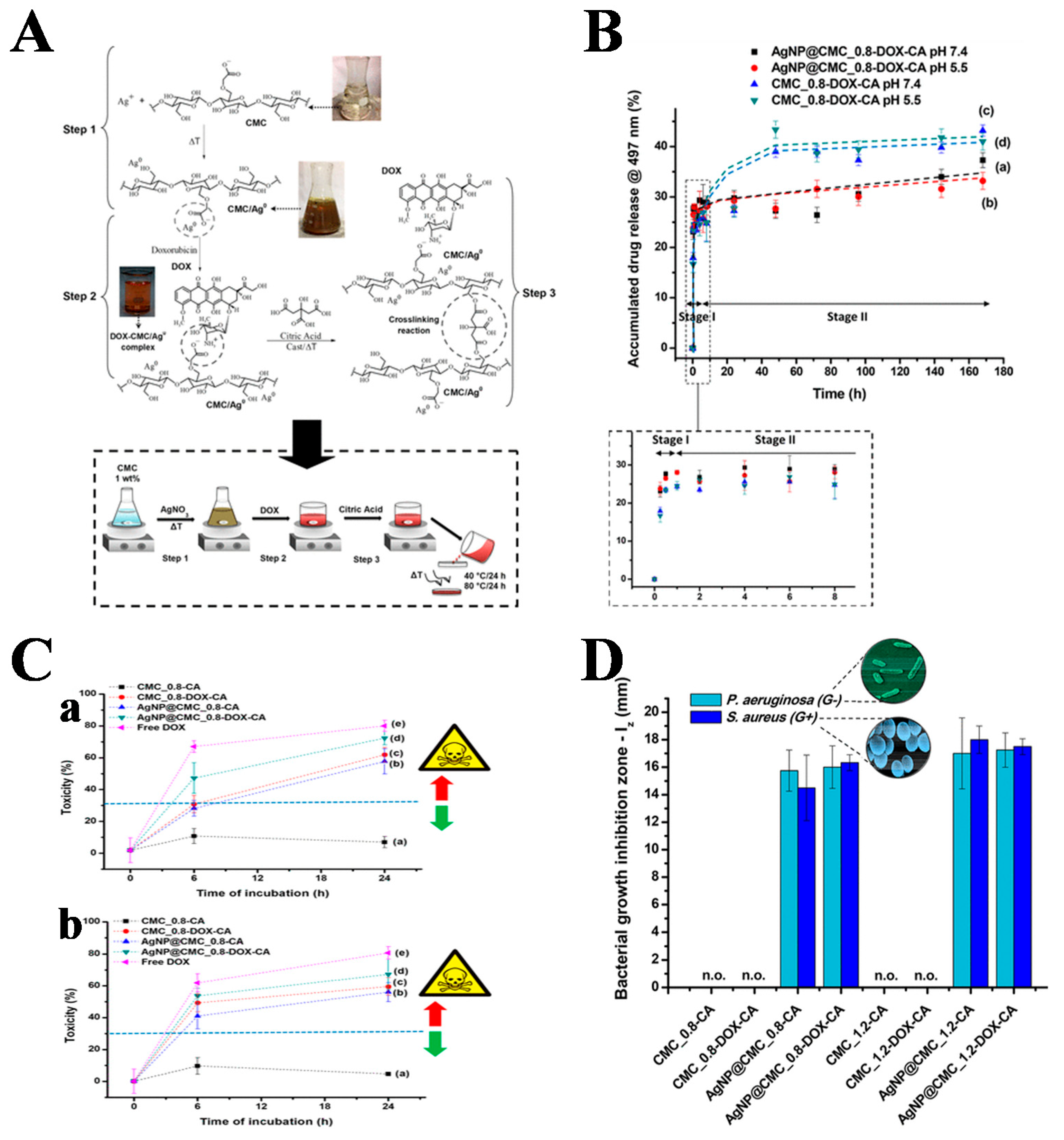
- Capanema, N.S.V.; Carvalho, I.C.; Mansur, A.A.P.; Carvalho, S.M.; Lage, A.P.; Mansur, H.S. Hybrid hydrogel composed of carboxymethylcellulose–silver nanoparticles–doxorubicin for anticancer and antibacterial therapies against melanoma skin cancer cells. ACS Appl. Nano Mater. 2019, 2, 7393–7408. [Google Scholar] [CrossRef]
- Tang, X.; Chen, X.; Zhang, S.; Gu, X.; Wu, R.; Huang, T.; Zhou, Z.; Sun, C.; Ling, J.; Liu, M.; et al. Silk-inspired in situ hydrogel with anti-tumor immunity enhanced photodynamic therapy for melanoma and infected wound healing. Adv. Funct. Mater. 2021, 31, 2101320. [Google Scholar] [CrossRef]



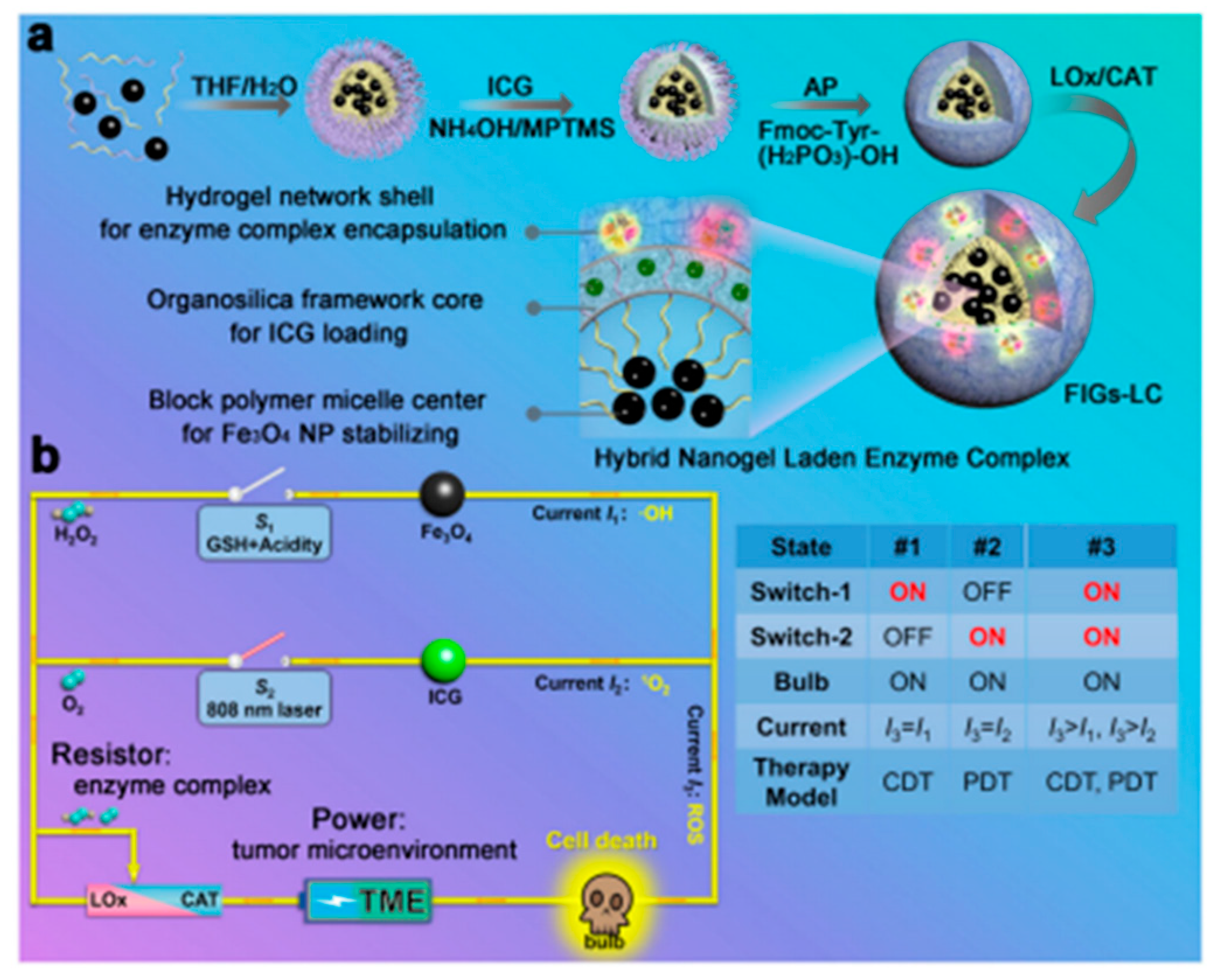

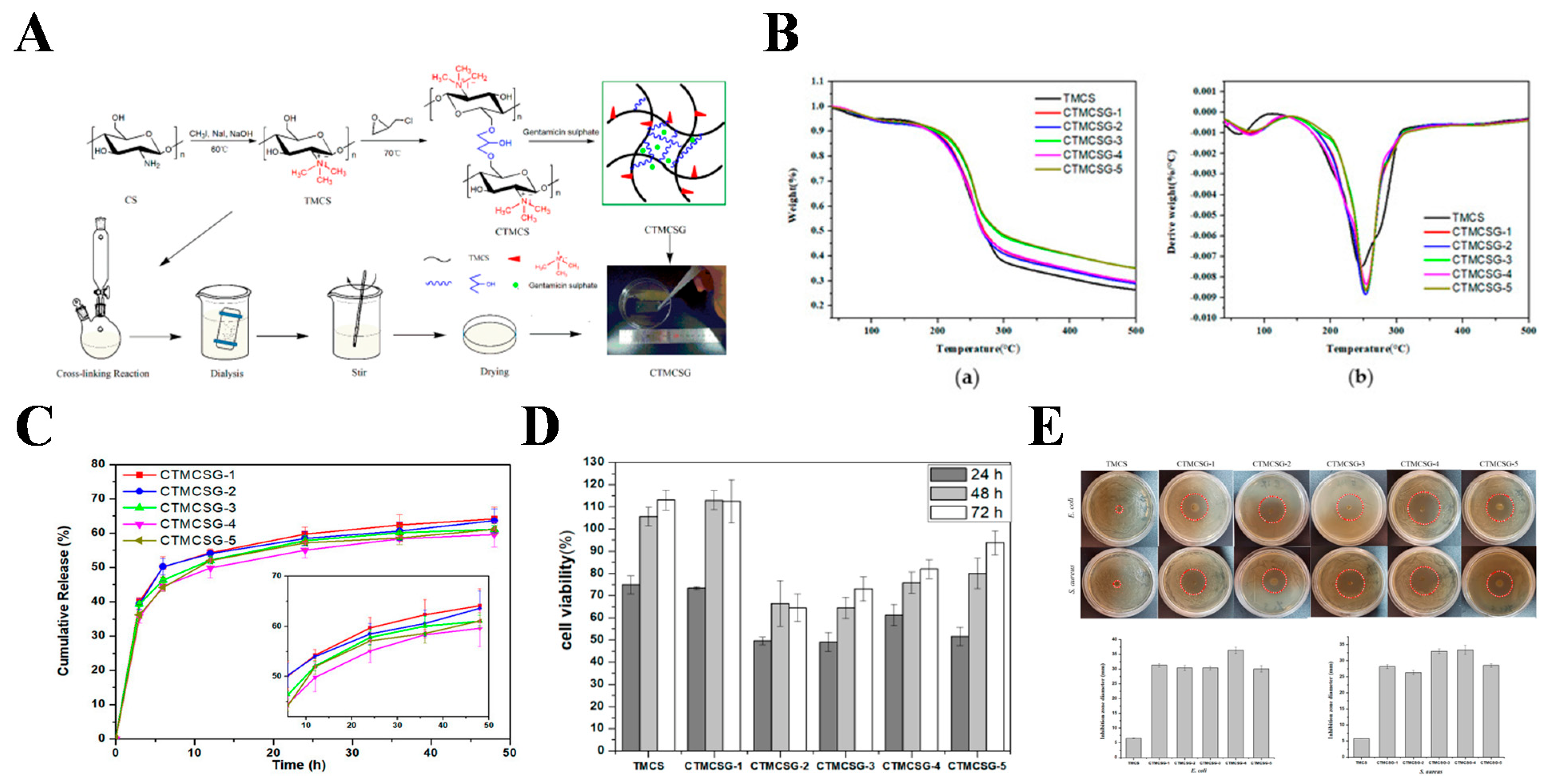
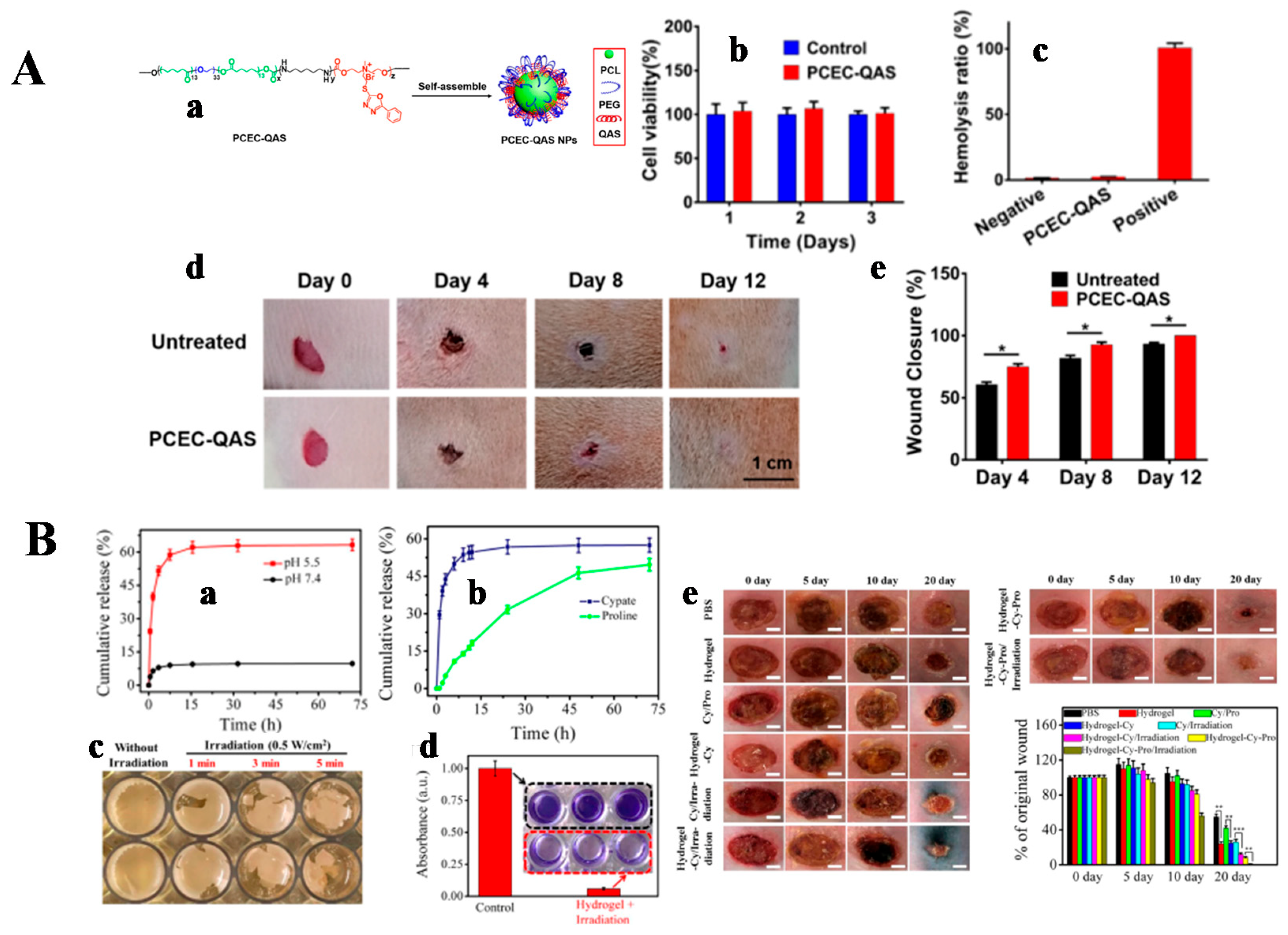
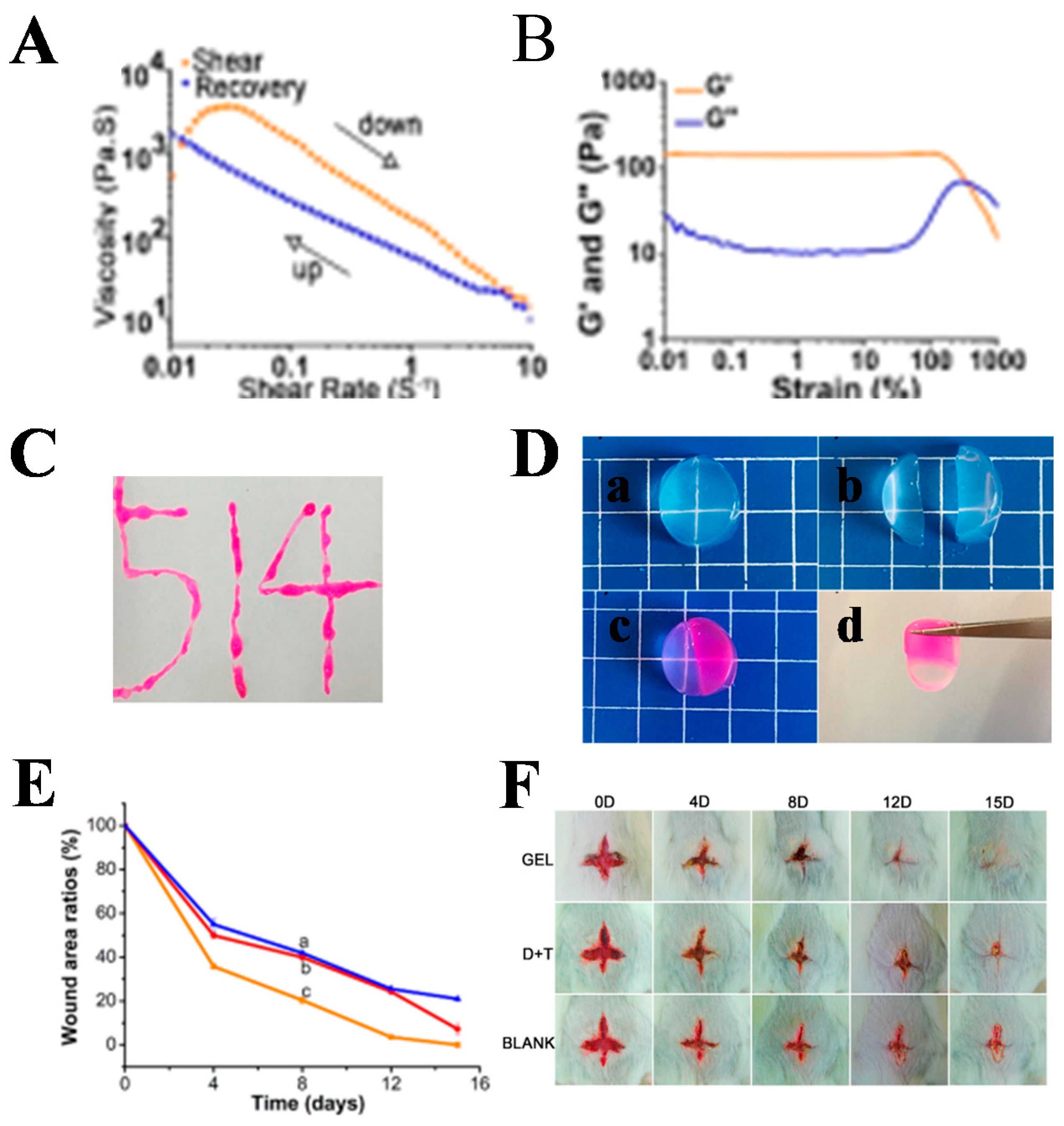
| Function | The Classification of Function Component | Function Component |
|---|---|---|
| antitumor | chemotherapy drug | DOX [25,27,30,31,36,55,56,68,75,76,155], MTX [29], CD-CUR [30], RESV [31], ES [43], cisplatin [44,70], CA4 [51], Melittin [54] |
| immune adjuvant | CTLA-4 [35] DPPA-1 [36], R837 [38], CpG ODN [46], Bestatin [56] | |
| phototherapy agent | PB [51], ICG [54,70,71,79] single-walled carbon nanotubes [55], Ag2S QD [56], Ce6 [67,156], PpIX [68], MB [74], AuNR [74,75], AgNR [75], black phosphorus quantum dots [76], BGN-Fe-Ag2S [152], Fe3O4 [153], MoS2@MnFe2O4 [154] | |
| acoustic sensitizer | TCPP [78], Fe3O4 [79] | |
| antibacterial | organic or inorganic antibacterial agent | Ag NP [92,132,138,155], MoS2@PDA@Ag [96], CuS NP [97,123,141], AuNR [99], ZnO [101], Ag-ZnO [102], C/ZnO [103], Ag/Ag@AgCl/ZnO [104], Fe3O4/TiO2/Ag3PO4 [127], PEI@BPQD [133], THPS [134], BGN-Fe-Ag2S [152], Fe3O4 [153], MoS2@MnFe2O4 [154] |
| antibiotic | gentamicin [106,107,116], chloramphenicol [109], erythromycin [111,112], vancomycin [114–116], NOR [153] | |
| inherently antibacterial hydrogel material | PDMAPS-co-PMA-Ade [117], chitosan [117,146,148,149,154], P(GMA-co-MPC) [118], MF-PEG [120], PCEC-QAS [121], IKFQFHFD peptide [7], RRRFRADA peptide [122], GIIKKIIKKIIKKI-GRADARADARADARADA-NH2 peptide [123], IKYLSVN peptide [126], PVA/PAM/PCBMAE [136] | |
| enzyme or nanoenzyme antimicrobial agent | GOx [126,127,154], MoS2@TA/Fe [129], Pt NP [130], lysozyme [146] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, X.; Wang, C.; Zhou, S.; Cui, P.; Hu, H.; Ni, X.; Jiang, P.; Wang, J. Hydrogels for Antitumor and Antibacterial Therapy. Gels 2022, 8, 315. https://doi.org/10.3390/gels8050315
Fang X, Wang C, Zhou S, Cui P, Hu H, Ni X, Jiang P, Wang J. Hydrogels for Antitumor and Antibacterial Therapy. Gels. 2022; 8(5):315. https://doi.org/10.3390/gels8050315
Chicago/Turabian StyleFang, Xiuling, Cheng Wang, Shuwen Zhou, Pengfei Cui, Huaanzi Hu, Xinye Ni, Pengju Jiang, and Jianhao Wang. 2022. "Hydrogels for Antitumor and Antibacterial Therapy" Gels 8, no. 5: 315. https://doi.org/10.3390/gels8050315
APA StyleFang, X., Wang, C., Zhou, S., Cui, P., Hu, H., Ni, X., Jiang, P., & Wang, J. (2022). Hydrogels for Antitumor and Antibacterial Therapy. Gels, 8(5), 315. https://doi.org/10.3390/gels8050315









