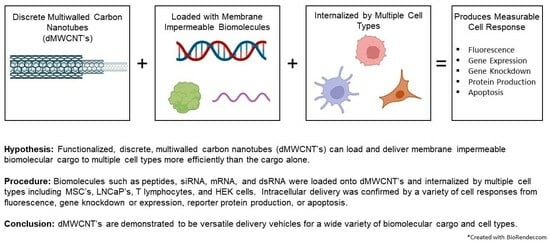
Discrete Multiwalled Carbon Nanotubes for Versatile Intracellular Transport of Functional Biomolecular Complexes
Abstract
1. Introduction
2. Materials and Methods
2.1. CNT Functionalization and Discretization
2.2. Cell Lines
2.3. MWCNT Internalization
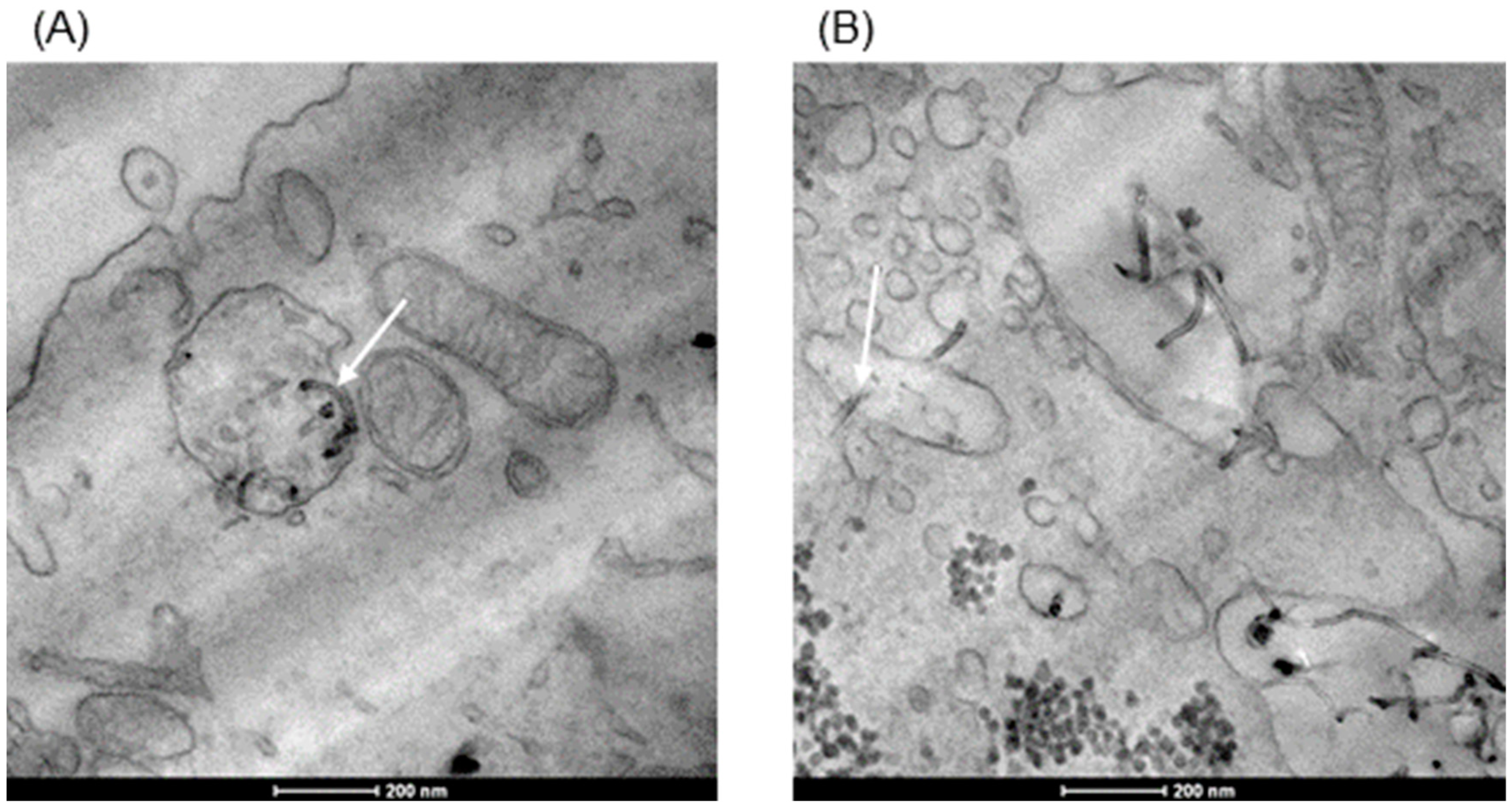
2.4. Sample Preparation and TEM Imaging
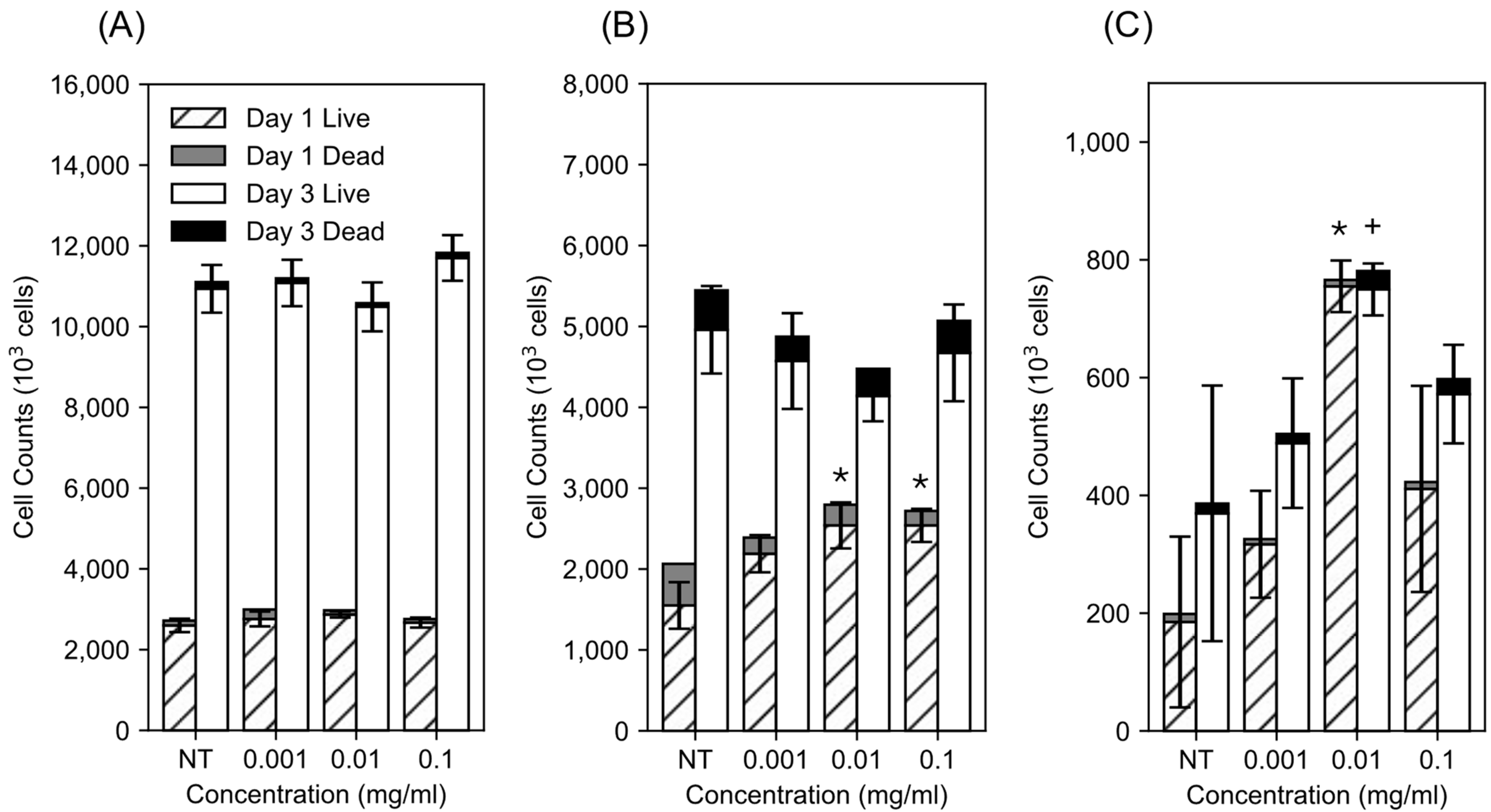
2.5. Proliferation and Live/Dead Assay
2.6. KLA Delivery Study
2.7. mRNA Delivery Study
2.8. siRNA Delivery Study
2.9. T-Cell Activation via PolyI:C
2.10. Statistical Analysis
3. Results
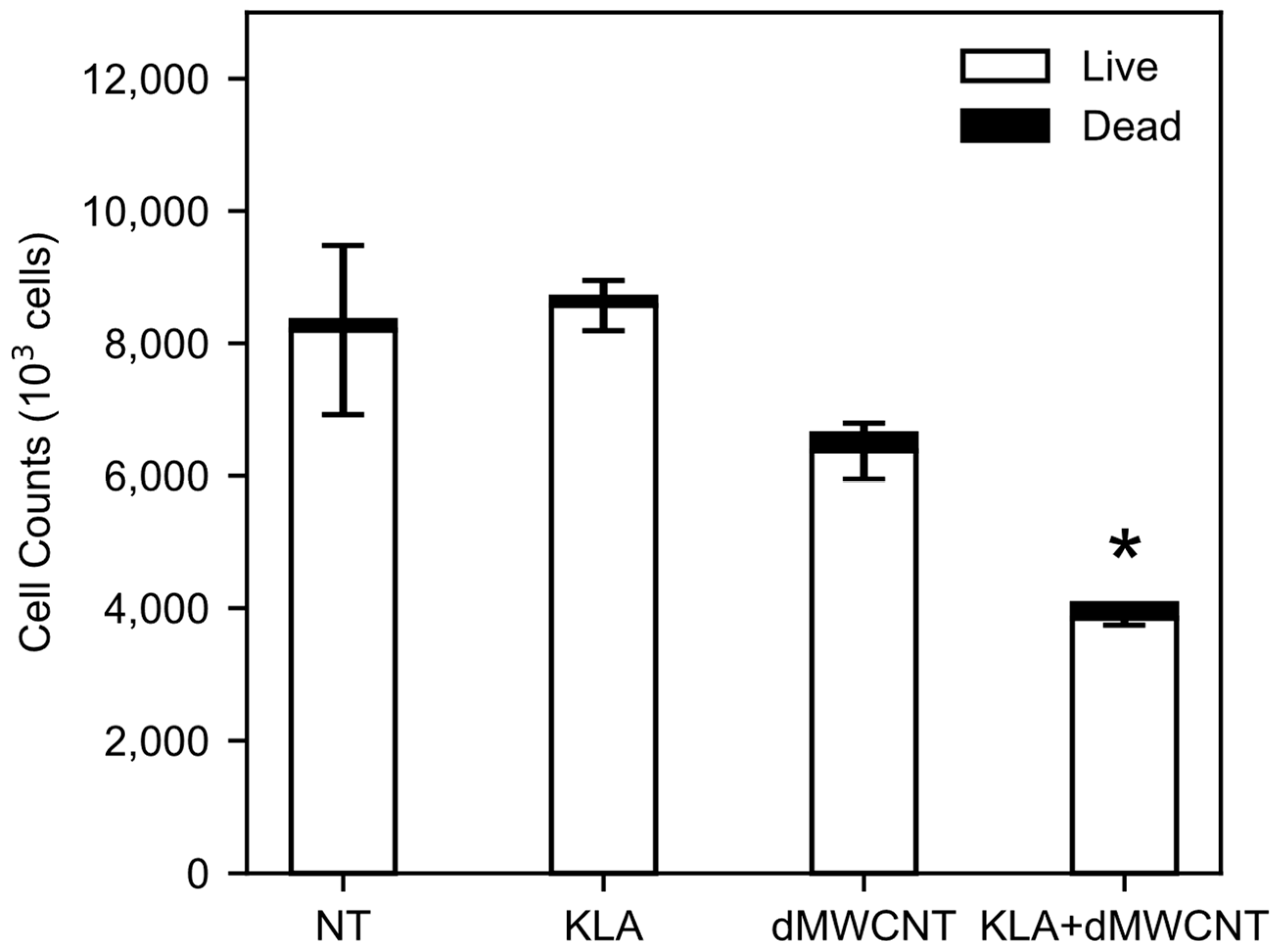
3.1. Impact of dMWCNTs on Cell Proliferation
3.2. Internalization of dMWCNTs into the Cell
3.3. Intracellular Delivery of KLA Peptide
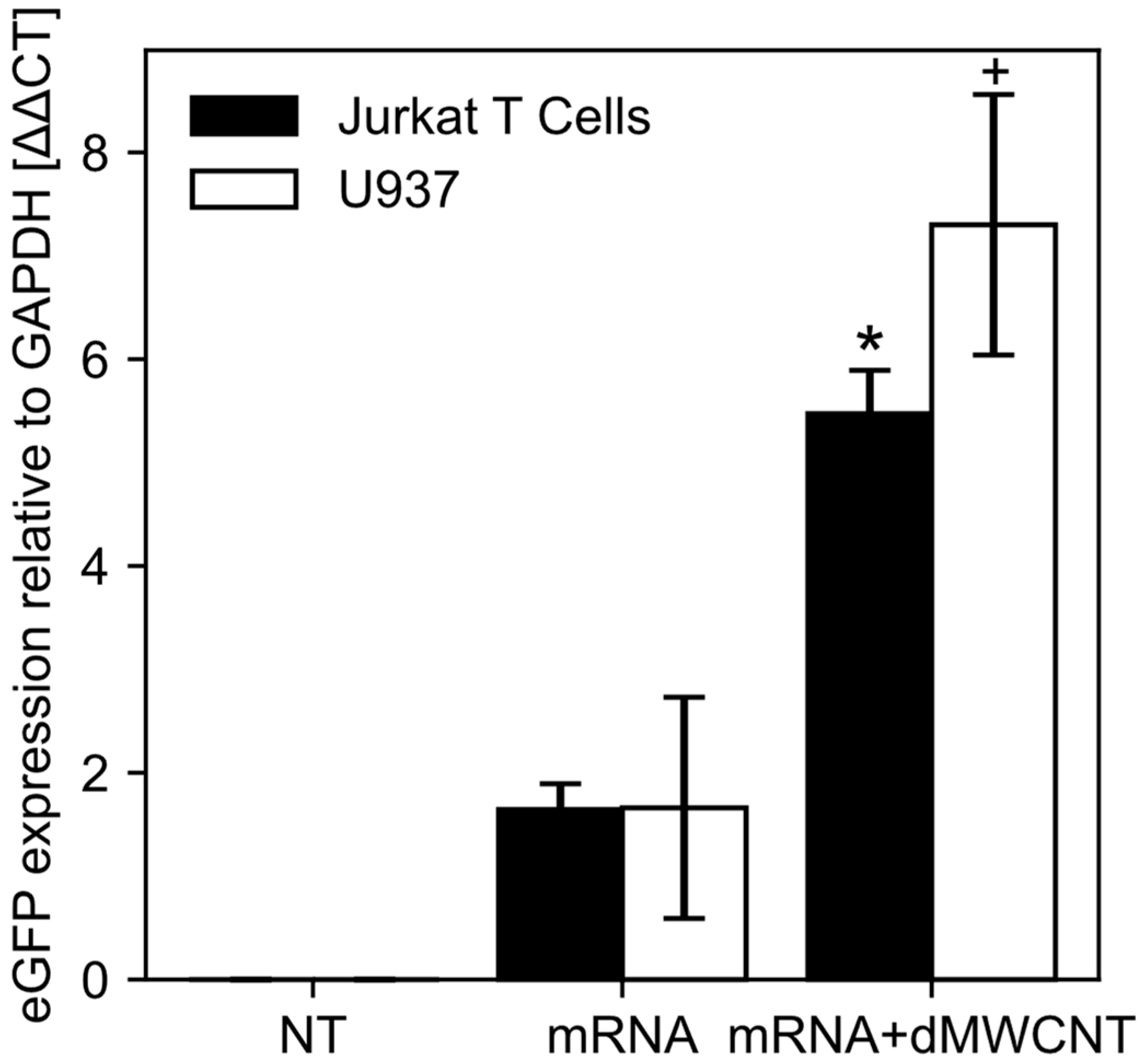
3.4. Intracellular Delivery of mRNA
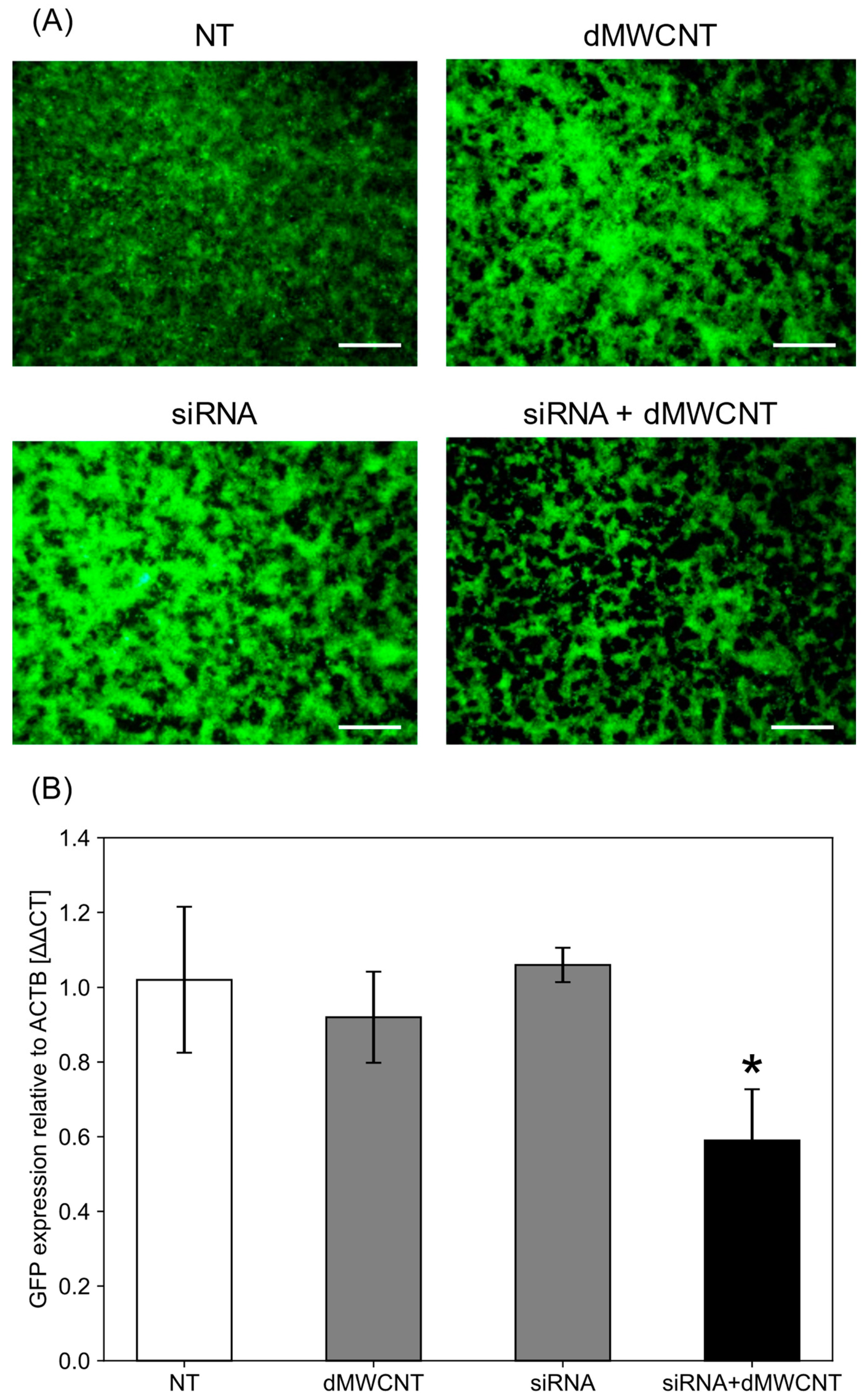
3.5. Intracellular Delivery of siRNA
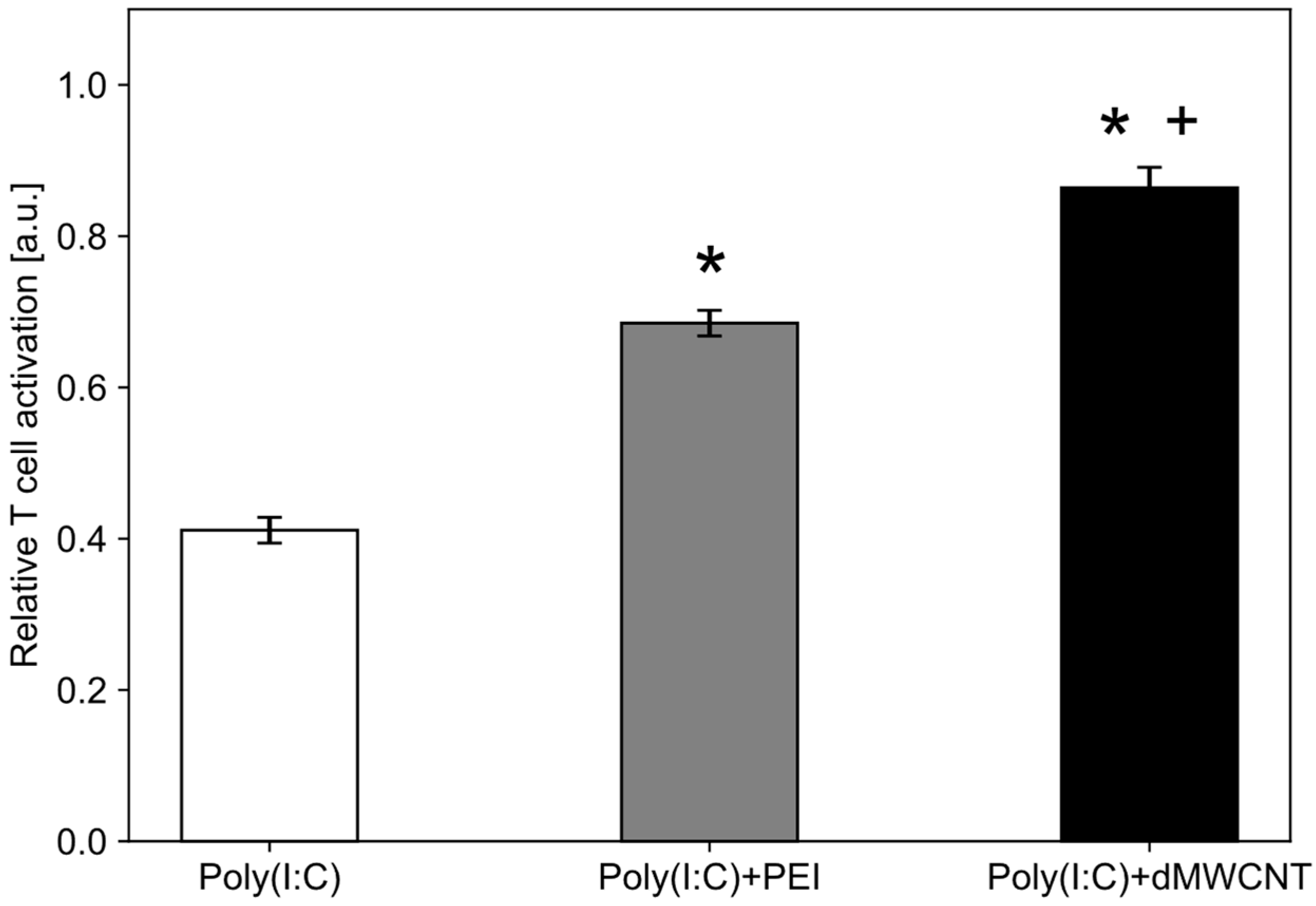
3.6. Intracellular Delivery of Synthetic dsRNA
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stewart, M.P.; Sharei, A.; Ding, X.; Sahay, G.; Langer, R.; Jensen, K.F. In vitro and ex vivo strategies for intracellular delivery. Nature 2016, 538, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Satapathy, S.R.; Dutta, T. Delivery Strategies for mRNA Vaccines. Pharm. Med. 2022, 36, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.; Singh, A.; Sharma, P.K.; Fuloria, N.K. Smart carbon nanotubes for drug delivery system: A comprehensive study. J. Drug Deliv. Sci. Technol. 2020, 58, 101811. [Google Scholar] [CrossRef]
- Saleemi, M.A.; Kong, Y.L.; Yong, P.V.C.; Wong, E.H. An overview of recent development in therapeutic drug carrier system using carbon nanotubes. J. Drug Deliv. Sci. Technol. 2020, 59, 101855. [Google Scholar] [CrossRef]
- Dilliard, S.A.; Siegwart, D.J. Passive, active and endogenous organ-targeted lipid and polymer nanoparticles for delivery of genetic drugs. Nat. Rev. Mater. 2023, 8, 282–300. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.P.; Sawant, K.K. Polyethylenimine: A versatile, multifunctional non-viral vector for nucleic acid delivery. Mater. Sci. Eng. C 2016, 68, 904–918. [Google Scholar] [CrossRef] [PubMed]
- Olden, B.R.; Cheng, Y.; Yu, J.L.; Pun, S.H. Cationic polymers for non-viral gene delivery to human T cells. J. Control. Release 2018, 282, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed]
- Canatella, P.J.; Karr, J.F.; Petros, J.A.; Prausnitz, M.R. Quantitative Study of Electroporation-Mediated Molecular Uptake and Cell Viability. Biophys. J. 2001, 80, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, M.; Shinozaki, F.; Motoyoshi, Y.; Sugiyama, T.; Yamamoto, S.; Sueishi, M. Sonoporation: Gene transfer using ultrasound. World J. Methodol. 2013, 3, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Sainz, R.; Li, S.; Dumortier, H.; Lacerda, L.; Kostarelos, K.; Giordani, S.; Prato, M. Medicinal Chemistry and Pharmacological Potential of Fullerenes and Carbon Nanotubes. In Carbon Materials: Chemistry and Physics; Springer: Dordrecht, The Netherlands, 2008; pp. 23–50. [Google Scholar] [CrossRef]
- Amenta, V.; Aschberger, K. Carbon nanotubes: Potential medical applications and safety concerns. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2015, 7, 371–386. [Google Scholar] [CrossRef] [PubMed]
- Jinturkar, K.A.; Rathi, M.N.; Misra, A. Challenges in Delivery of Therapeutic Genomics and Proteomics; Elsevier: Amsterdam, The Netherlands, 2011; pp. 83–126. [Google Scholar] [CrossRef]
- Thurnherr, T.; Brandenberger, C.; Fischer, K.; Diener, L.; Manser, P.; Maeder-Althaus, X.; Kaiser, J.-P.; Krug, H.F.; Rothen-Rutishauser, B.; Wick, P. A comparison of acute and long-term effects of industrial multiwalled carbon nanotubes on human lung and immune cells in vitro. Toxicol. Lett. 2011, 200, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Firme, C.P.; Bandaru, P.R. Toxicity issues in the application of carbon nanotubes to biological systems. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Herlem, G.; Picaud, F.; Girardet, C.; Micheau, O. Nanocarriers for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 469–529. [Google Scholar] [CrossRef]
- Shannahan, J. The biocorona: A challenge for the biomedical application of nanoparticles. Nanotechnol. Rev. 2017, 6, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.K.; Srivastava, R. Drug Delivery With Carbon-Based Nanomaterials as Versatile Nanocarriers: Progress and Prospects. Front. Nanotechnol. 2021, 3, 644564. [Google Scholar] [CrossRef]
- Kam, N.W.S.; Dai, H. Carbon Nanotubes as Intracellular Protein Transporters: Generality and Biological Functionality. J. Am. Chem. Soc. 2005, 127, 6021–6026. [Google Scholar] [CrossRef] [PubMed]
- Kam, N.W.S.; Liu, Z.; Dai, H. Carbon Nanotubes as Intracellular Transporters for Proteins and DNA: An Investigation of the Uptake Mechanism and Pathway. Angew. Chem. Int. Ed. 2006, 45, 577–581. [Google Scholar] [CrossRef] [PubMed]
- d’Amora, M.; Giordani, S. Smart Nanoparticles for Biomedicine; Elsevier: Amsterdam, The Netherlands, 2018; pp. 103–113. [Google Scholar] [CrossRef]
- Lacerda, L.; Raffa, S.; Prato, M.; Bianco, A.; Kostarelos, K. Cell-penetrating CNTs for delivery of therapeutics. Nano Today 2007, 2, 38–43. [Google Scholar] [CrossRef]
- Hong, H.; Gao, T.; Cai, W. Molecular imaging with single-walled carbon nanotubes. Nano Today 2009, 4, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Pantarotto, D.; Singh, R.; McCarthy, D.; Erhardt, M.; Briand, J.; Prato, M.; Kostarelos, K.; Bianco, A. Functionalized Carbon Nanotubes for Plasmid DNA Gene Delivery. Angew. Chem. Int. Ed. 2004, 43, 5242–5246. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.E.; Gass, M.; Muller, K.; Skepper, J.N.; Midgley, P.A.; Welland, M. Direct imaging of single-walled carbon nanotubes in cells. Nat. Nanotechnol. 2007, 2, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Kostarelos, K.; Lacerda, L.; Pastorin, G.; Wu, W.; Wieckowski, S.; Luangsivilay, J.; Godefroy, S.; Pantarotto, D.; Briand, J.-P.; Muller, S.; et al. Cellular uptake of functionalized carbon nanotubes is independent of functional group and cell type. Nat. Nanotechnol. 2007, 2, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Broughton, D.L.; Yan, B. Endosomal Leakage and Nuclear Translocation of Multiwalled Carbon Nanotubes: Developing a Model for Cell Uptake. Nano Lett. 2009, 9, 4370–4375. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yu, S.; Wang, C.; Kong, J. Tracking the Endocytic Pathway of Recombinant Protein Toxin Delivered by Multiwalled Carbon Nanotubes. Acs Nano 2010, 4, 6483–6490. [Google Scholar] [CrossRef] [PubMed]
- Kraszewski, S.; Bianco, A.; Tarek, M.; Ramseyer, C. Insertion of Short Amino-Functionalized Single-Walled Carbon Nanotubes into Phospholipid Bilayer Occurs by Passive Diffusion. PLoS ONE 2012, 7, e40703. [Google Scholar] [CrossRef] [PubMed]
- Al-Jamal, K.T.; Nerl, H.; Müller, K.H.; Ali-Boucetta, H.; Li, S.; Haynes, P.D.; Jinschek, J.R.; Prato, M.; Bianco, A.; Kostarelos, K.; et al. Cellular uptake mechanisms of functionalized multi-walled carbon nanotubes by 3D electron tomography imaging. Nanoscale 2011, 3, 2627–2635. [Google Scholar] [CrossRef] [PubMed]
- Raffa, V.; Ciofani, G.; Vittorio, O.; Riggio, C.; Cuschieri, A. Physicochemical properties affecting cellular uptake of carbon nanotubes. Nanomedicine 2010, 5, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Schluesener, H.J. Multi-walled carbon nanotubes affect drug transport across cell membrane in rat astrocytes. Nanotechnology 2010, 21, 105104. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, L.; Russier, J.; Pastorin, G.; Herrero, M.A.; Venturelli, E.; Dumortier, H.; Al-Jamal, K.T.; Prato, M.; Kostarelos, K.; Bianco, A. Translocation mechanisms of chemically functionalized carbon nanotubes across plasma membranes. Biomaterials 2012, 33, 3334–3343. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, L.; Ali-Boucetta, H.; Kraszewski, S.; Tarek, M.; Prato, M.; Ramseyer, C.; Kostarelos, K.; Bianco, A. How do functionalized carbon nanotubes land on, bind to and pierce through model and plasma membranes. Nanoscale 2013, 5, 10242–10250. [Google Scholar] [CrossRef] [PubMed]
- Wick, P.; Manser, P.; Limbach, L.K.; Dettlaff-Weglikowska, U.; Krumeich, F.; Roth, S.; Stark, W.J.; Bruinink, A. The degree and kind of agglomeration affect carbon nanotube cytotoxicity. Toxicol. Lett. 2007, 168, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xue, X.; Hu, X.; Wei, M.; Zhang, C.; Ge, G.; Liang, X. Structure-Dependent Mitochondrial Dysfunction and Hypoxia Induced with Single-Walled Carbon Nanotubes. Small 2014, 10, 2859–2869. [Google Scholar] [CrossRef] [PubMed]
- Falank, C.; Tasset, A.W.; Farrell, M.; Harris, S.; Everill, P.; Marinkovic, M.; Reagan, M.R. Development of medical-grade, discrete, multi-walled carbon nanotubes as drug delivery molecules to enhance the treatment of hematological malignancies. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 102025. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Zhang, F.; Hong, C.Q.; Giuliano, A.E.; Cui, X.J.; Zhou, G.J.; Zhang, G.J.; Cui, Y.K. Critical protein GAPDH and its regulatory mechanisms in cancer cells. Cancer Biol. Med. 2015, 12, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Barber, R.D.; Harmer, D.W.; Coleman, R.A.; Clark, B.J. GAPDH as a housekeeping gene: Analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiol. Genom. 2005, 21, 389–395. [Google Scholar] [CrossRef]
- de Jonge, H.J.; Fehrmann, R.S.; de Bont, E.S.; Hofstra, R.M.; Gerbens, F.; Kamps, W.A.; de Vries, E.G.; van der Zee, A.G.; te Meerman, G.J.; ter Elst, A. Evidence based selection of housekeeping genes. PLoS ONE 2007, 2, e898. [Google Scholar] [CrossRef]
- Boussif, O.; Lezoualc’h, F.; Zanta, M.A.; Mergny, M.D.; Scherman, D.; Demeneix, B.; Behr, J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297–7301. [Google Scholar] [CrossRef]
- Maruyama, K.; Haniu, H.; Saito, N.; Matsuda, Y.; Tsukahara, T.; Kobayashi, S.; Tanaka, M.; Aoki, K.; Takanashi, S.; Okamoto, M.; et al. Endocytosis of multiwalled carbon nanotubes in bronchial epithelial and mesothelial cells. BioMed Res. Int. 2015, 2015, 793186. [Google Scholar] [CrossRef] [PubMed]
- Marinkovic, M.; Tran, O.N.; Block, T.J.; Rakian, R.; Gonzalez, A.O.; Dean, D.D.; Yeh, C.-K.; Chen, X.-D. Native extracellular matrix, synthesized ex vivo by bone marrow or adipose stromal cells, faithfully directs mesenchymal stem cell differentiation. Matrix Biol. Plus 2020, 8, 100044. [Google Scholar] [CrossRef] [PubMed]
- Marinkovic, M.; Block, T.J.; Rakian, R.; Li, Q.; Wang, E.; Reilly, M.A.; Dean, D.D.; Chen, X.-D. One size does not fit all: Developing a cell-specific niche for in vitro study of cell behavior. Matrix Biol. 2016, 52, 426–441. [Google Scholar] [CrossRef]
- Foillard, S.; Jin, Z.; Garanger, E.; Boturyn, D.; Favrot, M.; Coll, J.; Dumy, P. Synthesis and Biological Characterization of Targeted Pro-Apoptotic Peptide. ChemBioChem 2008, 9, 2326–2332. [Google Scholar] [CrossRef] [PubMed]
- Capello, A.; Krenning, E.P.; Bernard, B.F.; Breeman, W.A.P.; Erion, J.L.; de Jong, M. Anticancer activity of targeted proapoptotic peptides. J. Nucl. Med. 2006, 47, 122–129. [Google Scholar] [PubMed]
- Zurita, A.J.; Troncoso, P.; Cardo, M.; Logothetis, C.J.; Pasqualini, R.; Arap, W. Combinatorial Screenings in Patients: The Interleukin-11 Receptor α as a Candidate Target in the Progression of Human Prostate Cancer. Cancer Res. 2004, 64, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Javadpour, M.M.; Juban, M.M.; Lo, W.-C.J.; Bishop, S.M.; Alberty, J.B.; Cowell, S.M.; Becker, C.L.; McLaughlin, M.L. De Novo Antimicrobial Peptides with Low Mammalian Cell Toxicity. J. Med. Chem. 1996, 39, 3107–3113. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.; Lee, S.; Kim, S.; Jang, S.; Yu, J.; Lee, Y. Apoptosis Inducing, Conformationally Constrained, Dimeric Peptide Analogs of KLA with Submicromolar Cell Penetrating Abilities. Biomacromolecules 2014, 15, 3746–3752. [Google Scholar] [CrossRef] [PubMed]
- Martins, K.A.; Bavari, S.; Salazar, A.M. Vaccine adjuvant uses of poly-IC and derivatives. Expert Rev. Vaccines 2014, 14, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Puri, N.; Saxena, R.K. Uptake of poly-dispersed single-walled carbon nanotubes and decline of functions in mouse NK cells undergoing activation. J. Immunotoxicol. 2016, 13, 758–765. [Google Scholar] [CrossRef] [PubMed]
| Characterization Parameter | MWCNTs | dMWCNTs |
|---|---|---|
| % oxidized wt/wt | 0.3334% | 2.22% |
| % residuals wt/wt | 2.796% | 0.2036% |
| Density of -COOH groups | N/A | 0.09 mmol/g |
| Density of -OH groups | N/A | 0.17 mmol/g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo, K.; Tasset, A.; Marinkovic, M.; Foote, A. Discrete Multiwalled Carbon Nanotubes for Versatile Intracellular Transport of Functional Biomolecular Complexes. C 2024, 10, 37. https://doi.org/10.3390/c10020037
Castillo K, Tasset A, Marinkovic M, Foote A. Discrete Multiwalled Carbon Nanotubes for Versatile Intracellular Transport of Functional Biomolecular Complexes. C. 2024; 10(2):37. https://doi.org/10.3390/c10020037
Chicago/Turabian StyleCastillo, Kevin, Aaron Tasset, Milos Marinkovic, and Aaron Foote. 2024. "Discrete Multiwalled Carbon Nanotubes for Versatile Intracellular Transport of Functional Biomolecular Complexes" C 10, no. 2: 37. https://doi.org/10.3390/c10020037
APA StyleCastillo, K., Tasset, A., Marinkovic, M., & Foote, A. (2024). Discrete Multiwalled Carbon Nanotubes for Versatile Intracellular Transport of Functional Biomolecular Complexes. C, 10(2), 37. https://doi.org/10.3390/c10020037