On a Composite Obtained by Thermolysis of Cu-Doped Glycine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Characterization
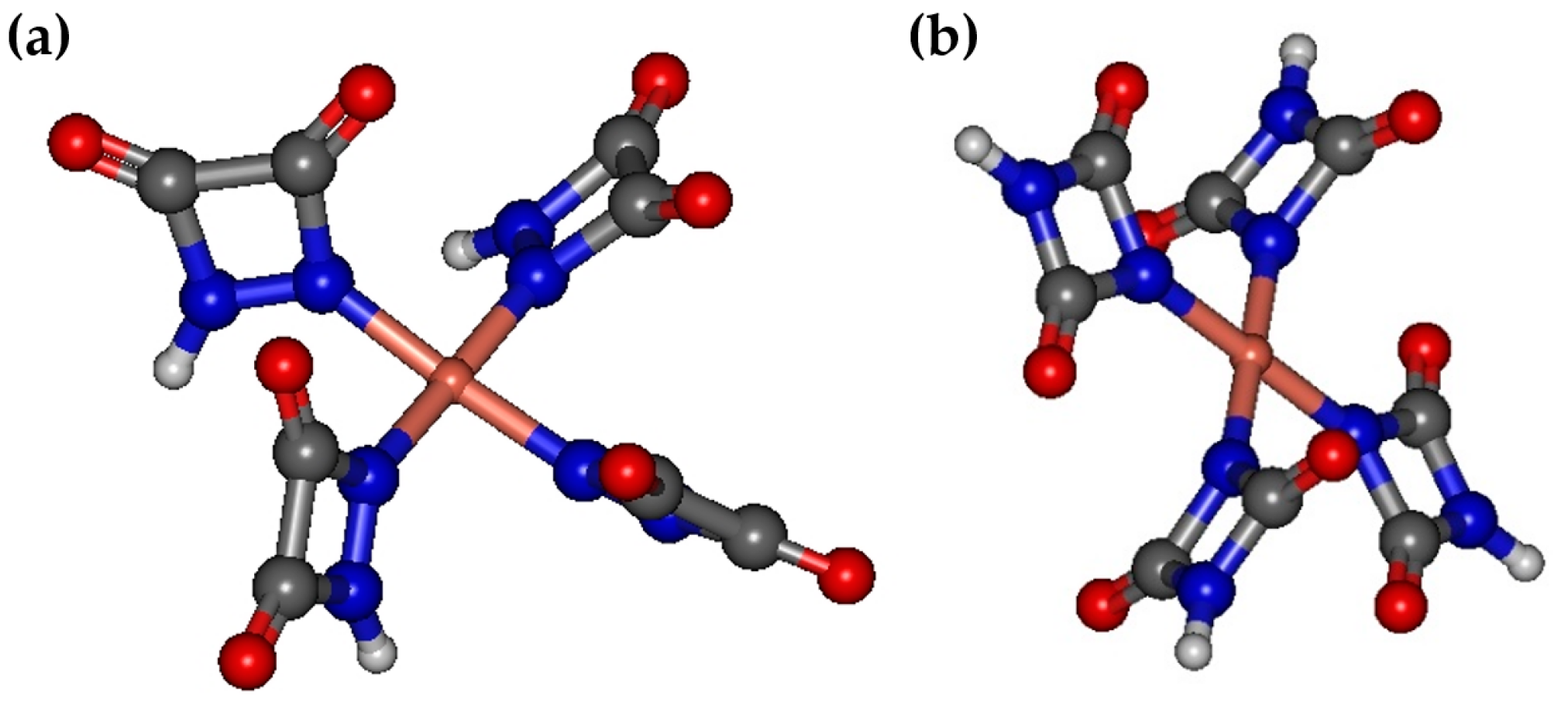
2.3. Computations
3. Results
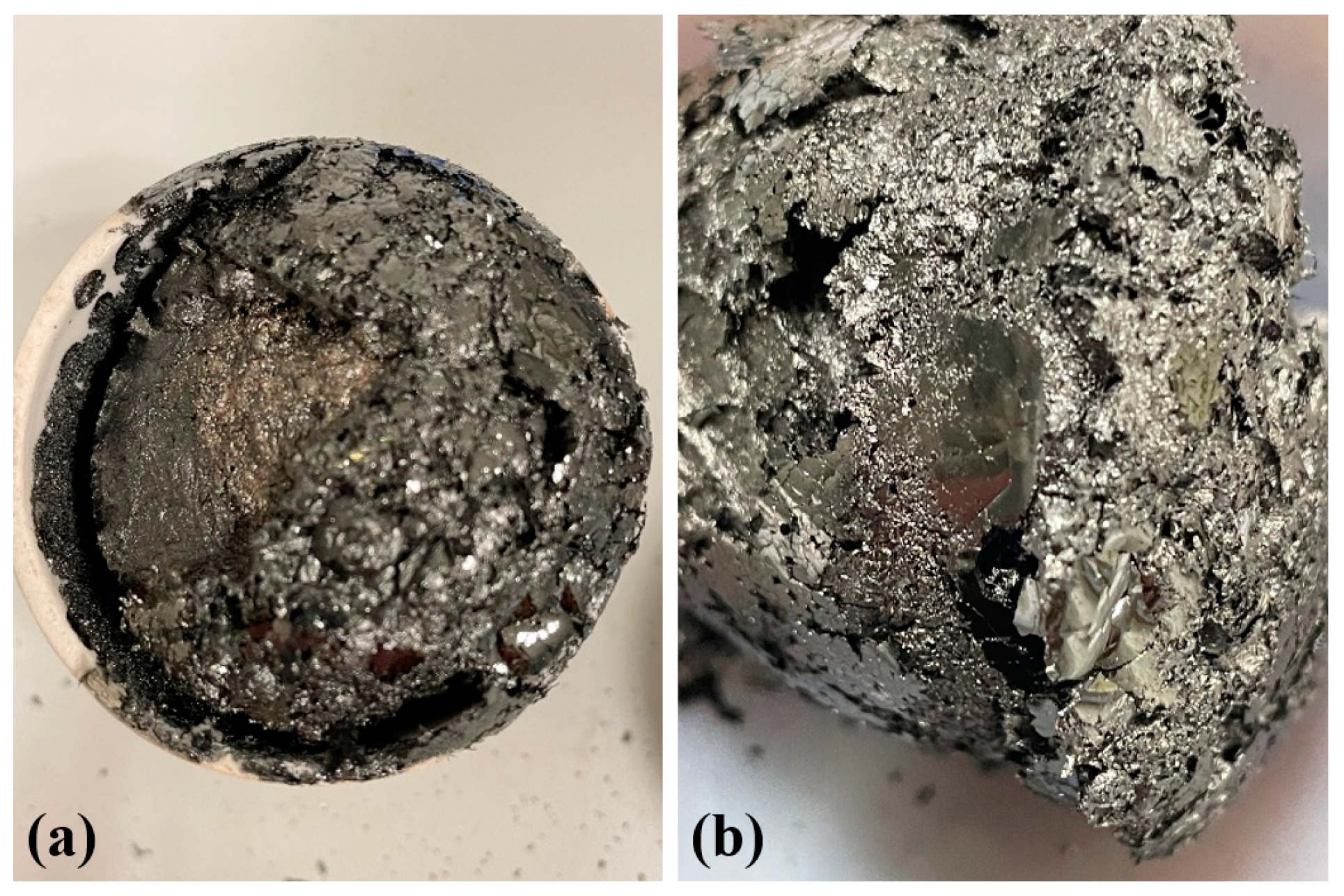
3.1. Pyrolysis Yields
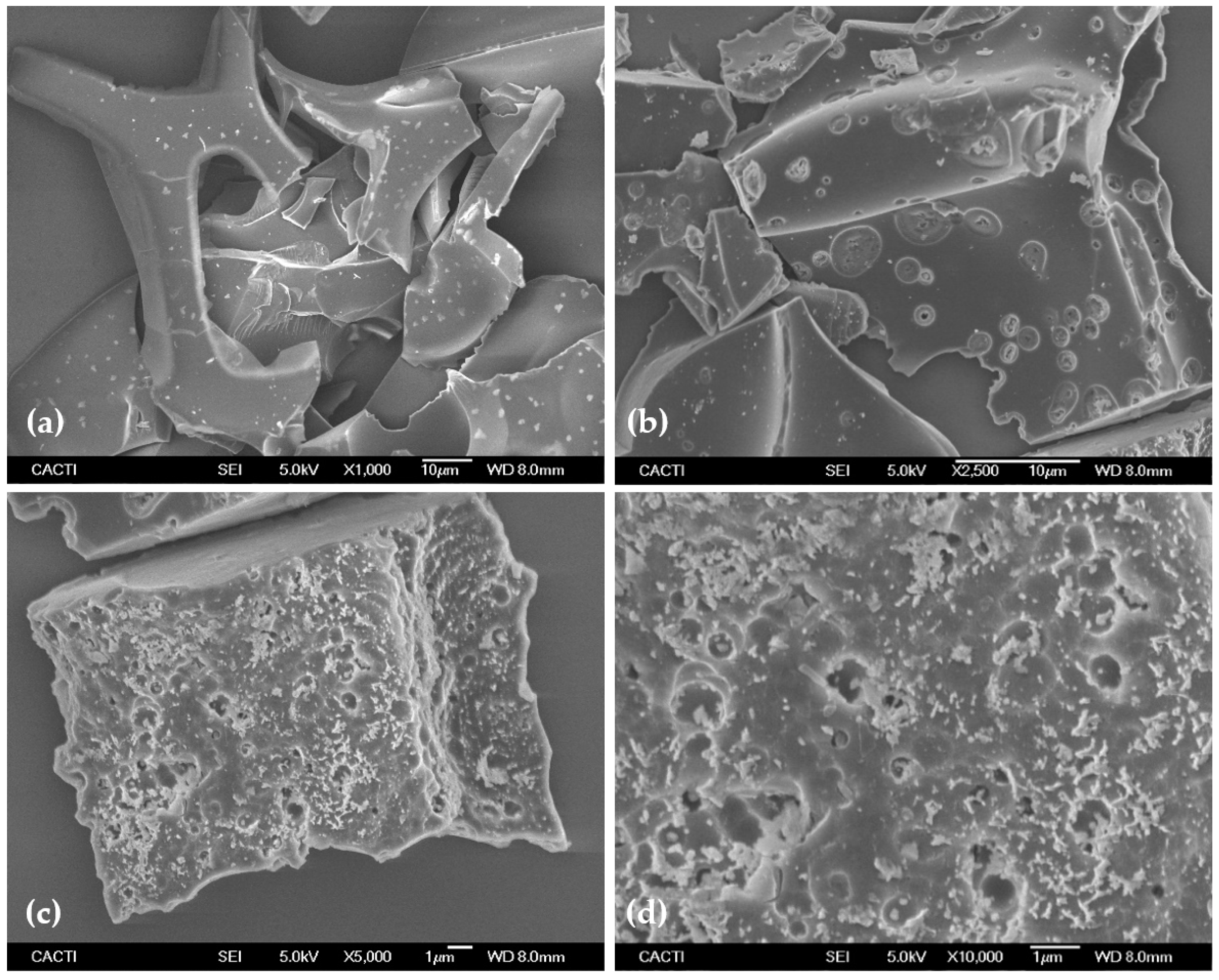
3.2. Morphological Characterization by TEM and STEM
3.3. Morphological Characterization by FESEM
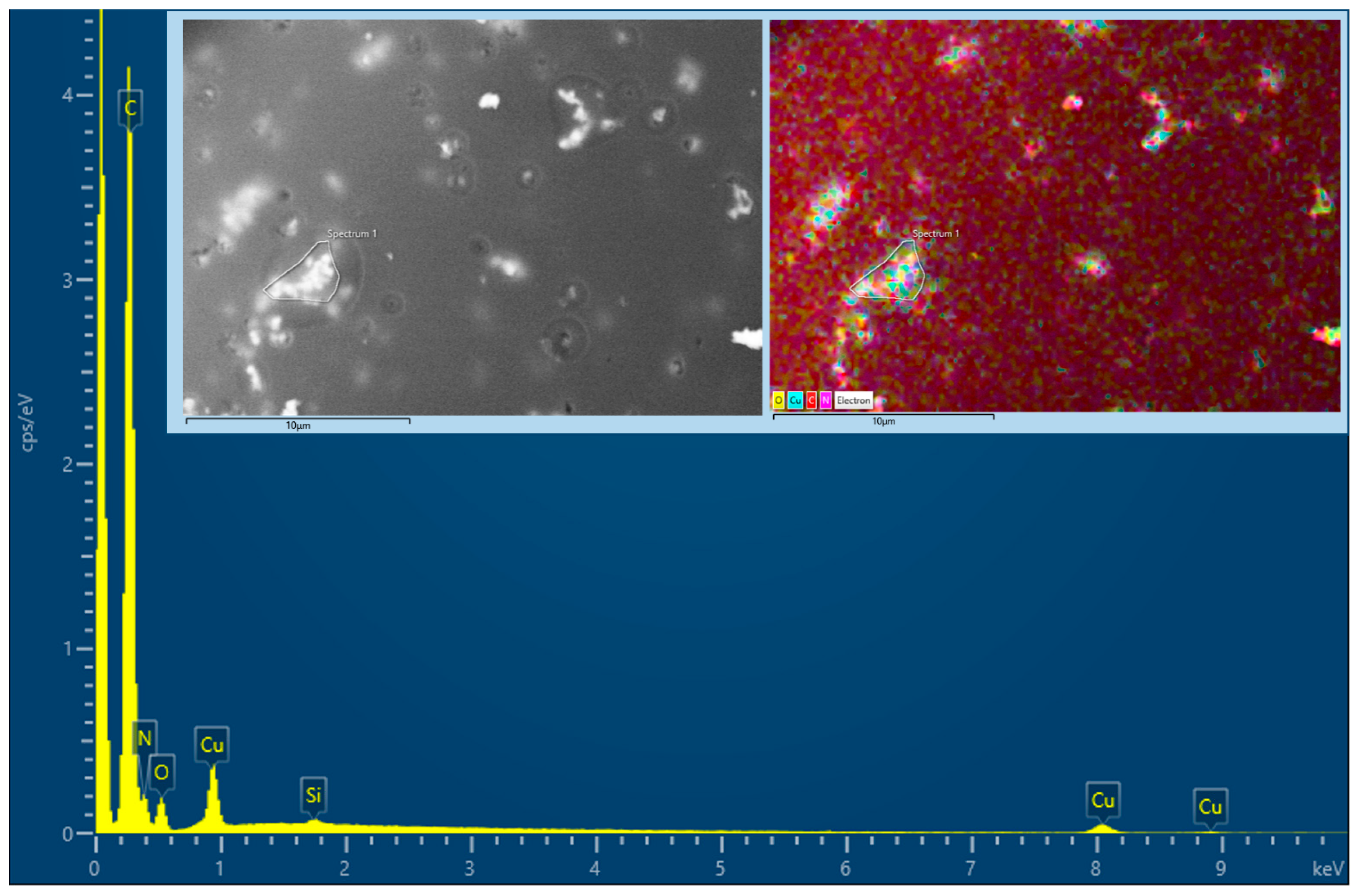
3.4. Multielemental Analysis by EDS
3.5. CHNS Characterization
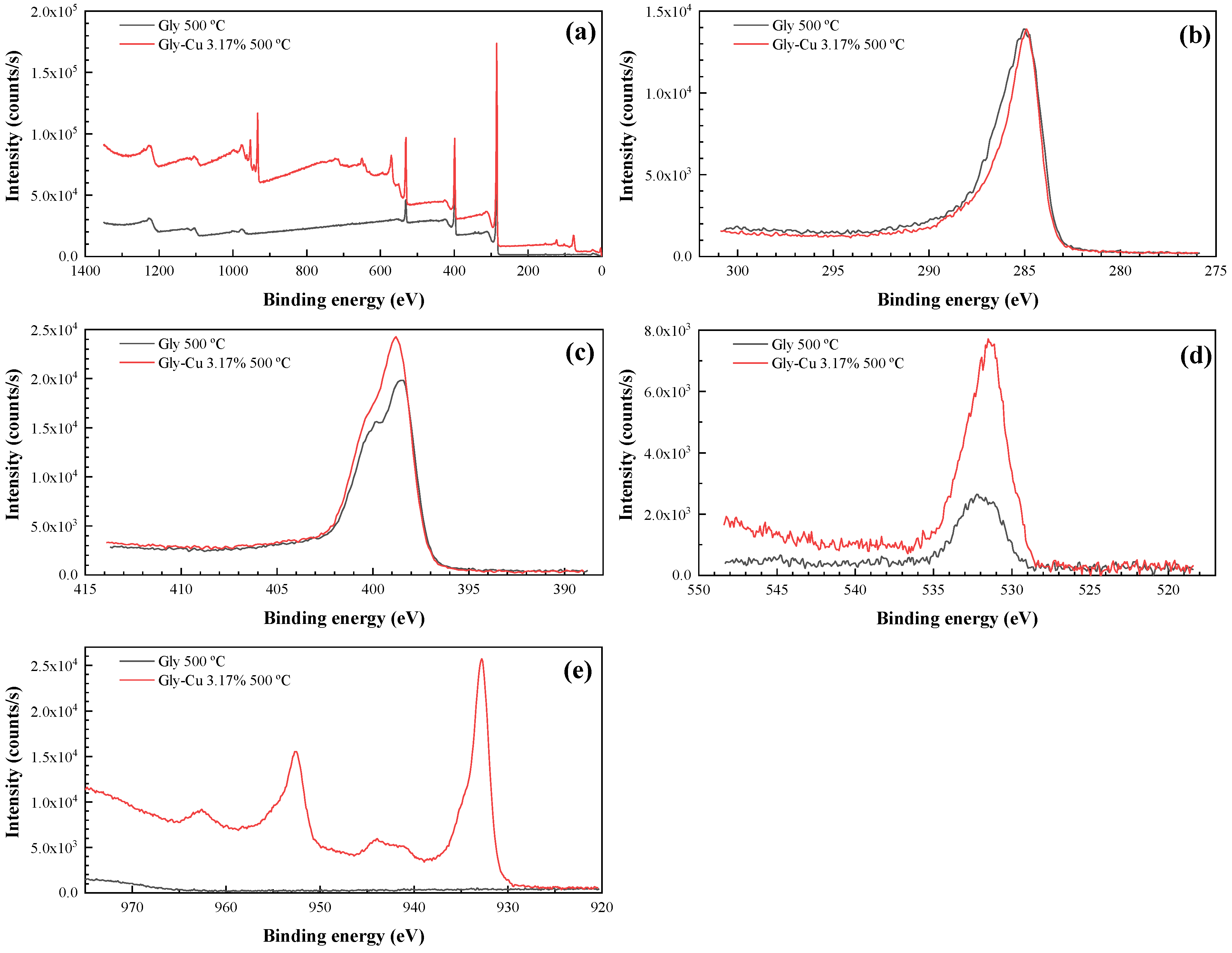
3.6. XPS Characterization
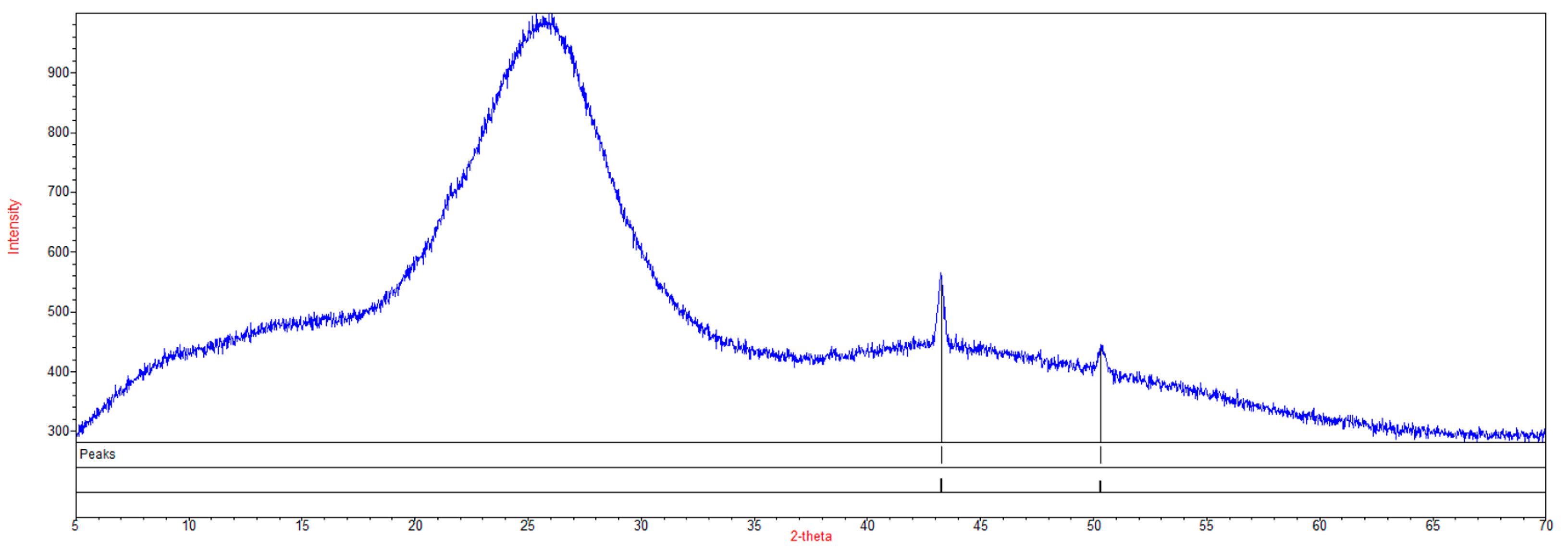
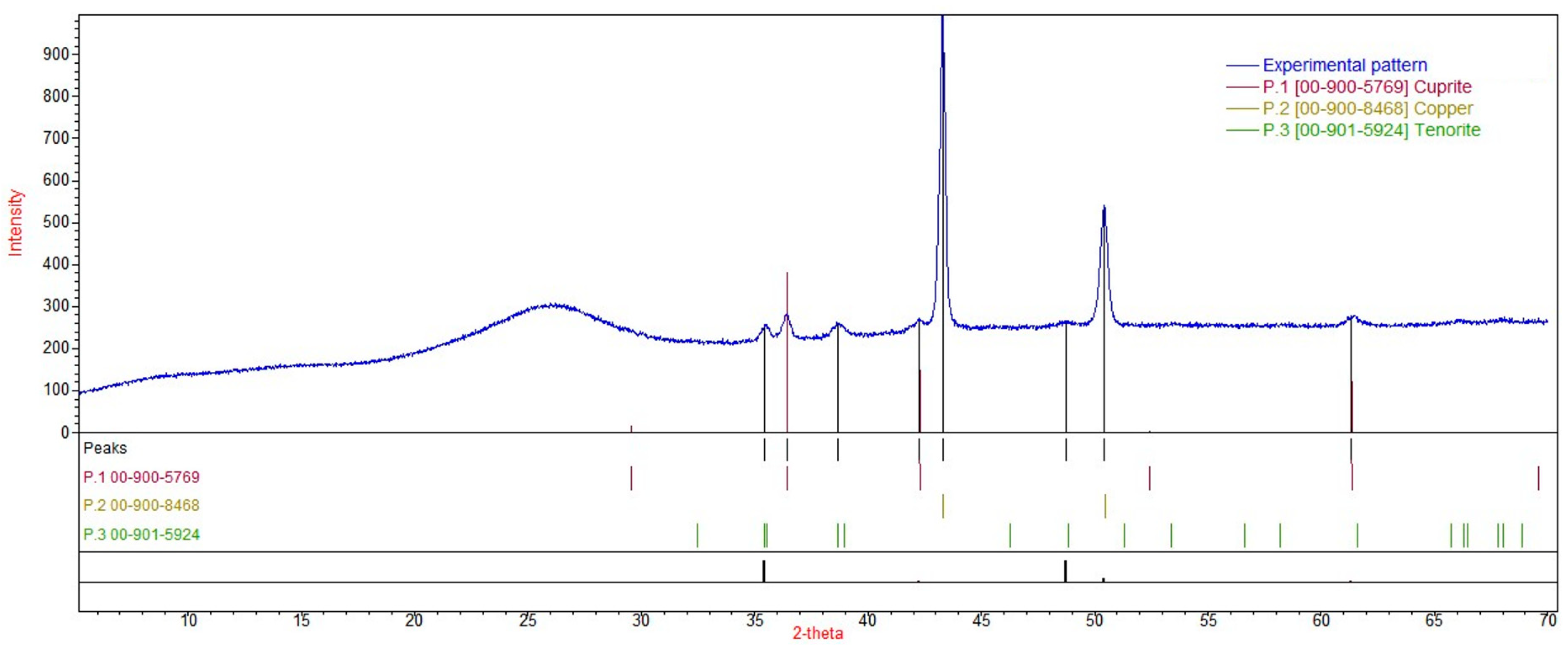
3.7. X-ray Powder Diffraction Characterization
3.8. Vibrational Characterization
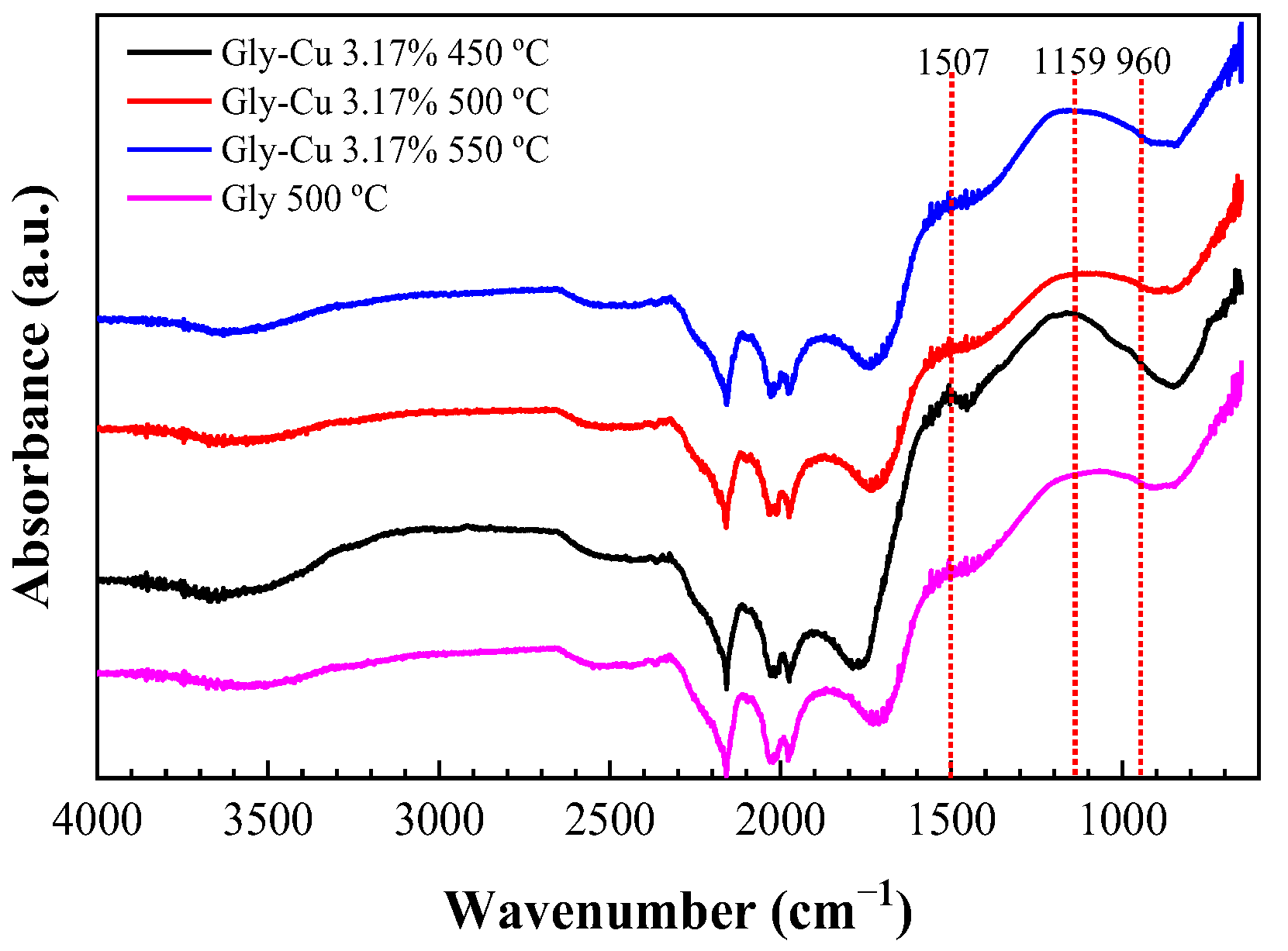
3.8.1. Infrared Spectra
3.8.2. Raman Spectra
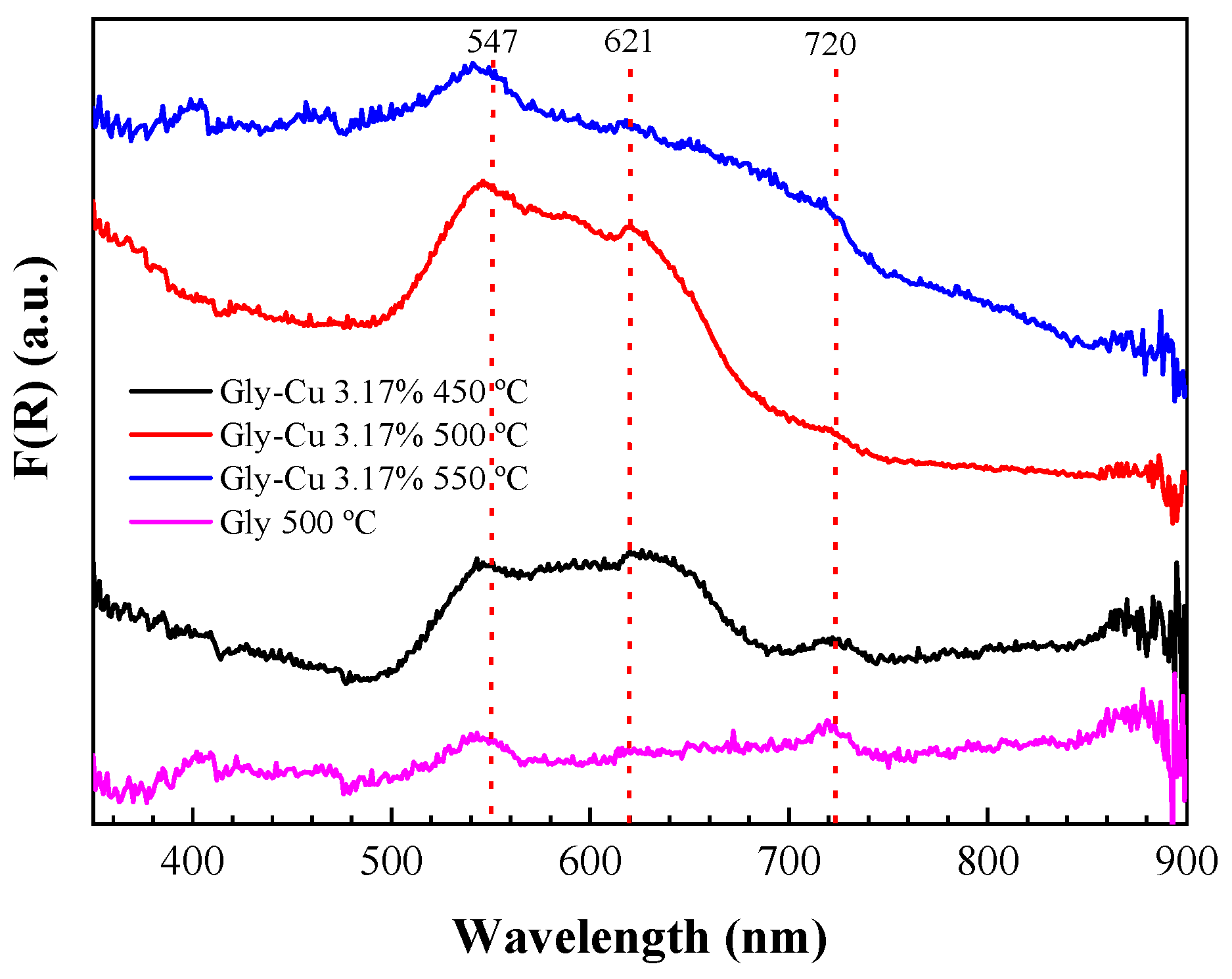
3.9. UV–Vis Spectra
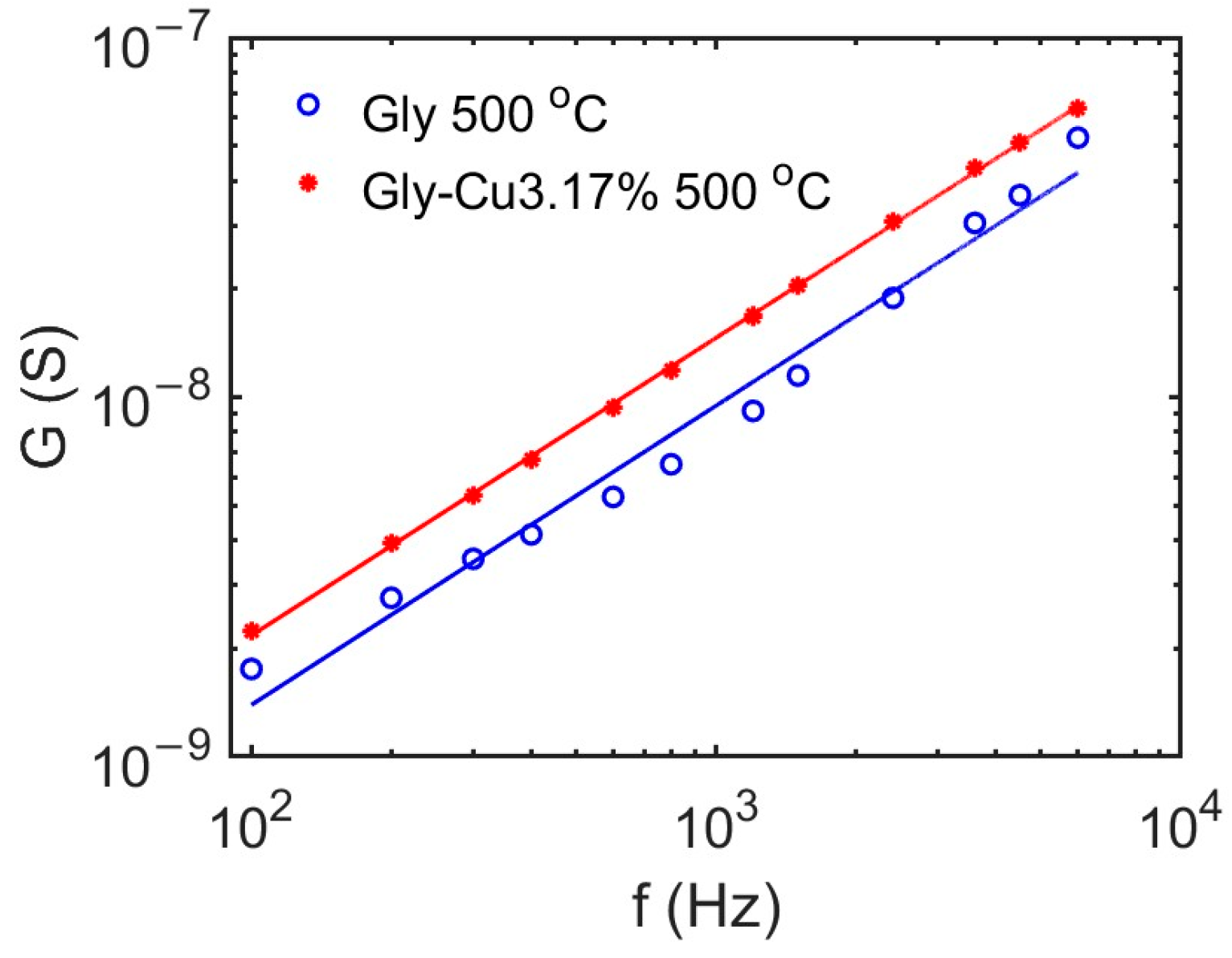
3.10. Conductivity Measurements
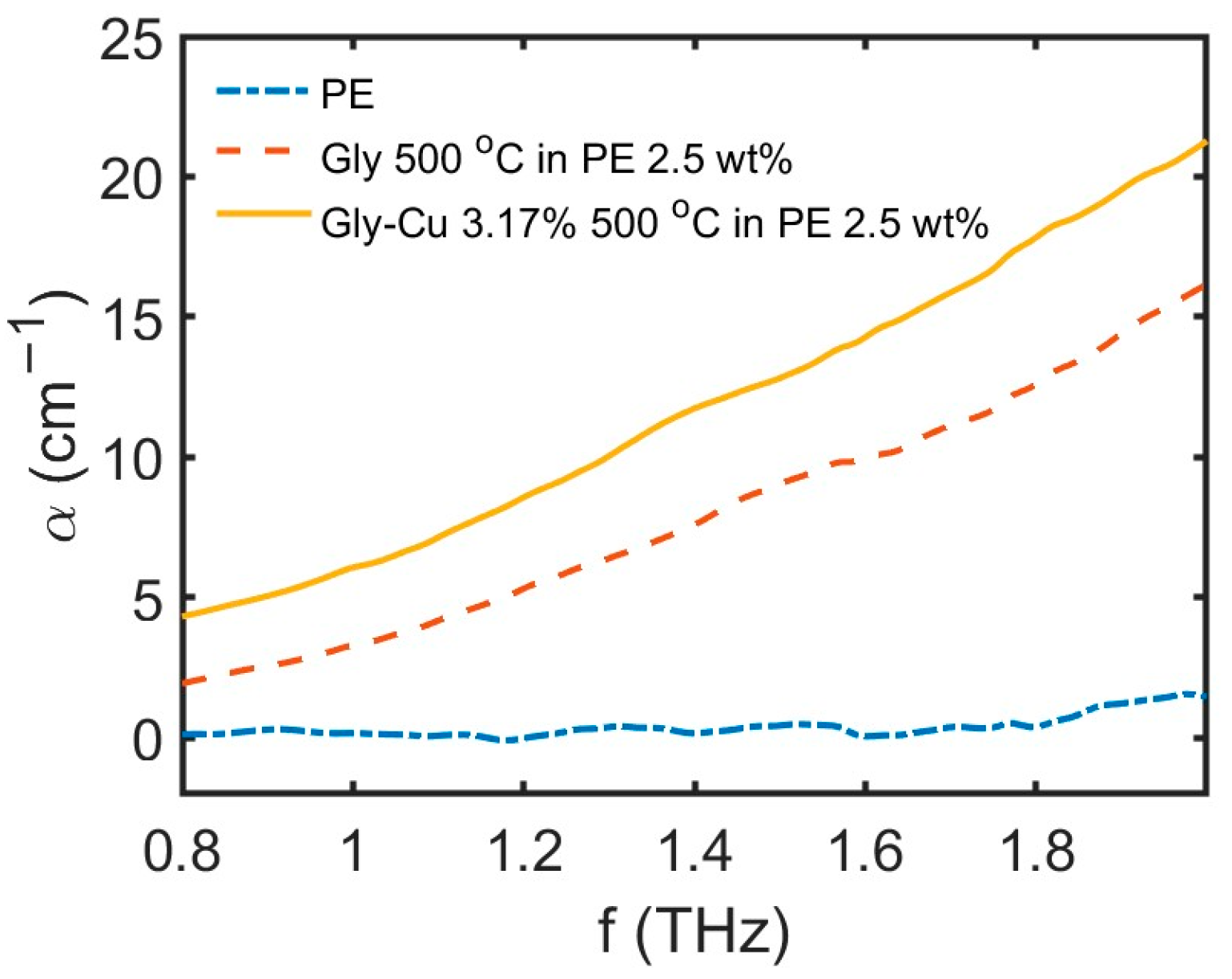
3.11. THz Absorption Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, S.; Bharti, H. A review on copper-graphite composite material fabrication and its mechanical properties. Int. J. Adv. Res. Innov. Ideas Educ. 2016, 2, 594–599. [Google Scholar]
- Hidalgo-Manrique, P.; Lei, X.; Xu, R.; Zhou, M.; Kinloch, I.A.; Young, R.J. Copper/graphene composites: A review. J. Mater. Sci. 2019, 54, 12236–12289. [Google Scholar] [CrossRef]
- Cantürk, S.B.; Kováčik, J. Review of recent development in copper/carbon composites prepared by infiltration technique. Energies 2022, 15, 5227. [Google Scholar] [CrossRef]
- Subramaniam, C.; Yamada, T.; Kobashi, K.; Sekiguchi, A.; Futaba, D.N.; Yumura, M.; Hata, K. One hundred fold increase in current carrying capacity in a carbon nanotube–copper composite. Nat. Commun. 2013, 4, 2202. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, C.; Yasuda, Y.; Takeya, S.; Ata, S.; Nishizawa, A.; Futaba, D.; Yamada, T.; Hata, K. Carbon nanotube-copper exhibiting metal-like thermal conductivity and silicon-like thermal expansion for efficient cooling of electronics. Nanoscale 2014, 6, 2669–2674. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, C.; Lecouturier, F.; Mesguich, D.; Ferreira, N.; Chevallier, G.; Estournès, C.; Weibel, A.; Laurent, C. High strength—High conductivity double-walled carbon nanotube—Copper composite wires. Carbon 2016, 96, 212–215. [Google Scholar] [CrossRef]
- Xu, G.; Zhao, J.; Li, S.; Zhang, X.; Yong, Z.; Li, Q. Continuous electrodeposition for lightweight, highly conducting and strong carbon nanotube-copper composite fibers. Nanoscale 2011, 3, 4215. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.; Markina, A.A.; Porotnikov, M.V.; Ahmad, F. A review of carbon fiber materials in automotive industry. IOP Conf. Ser. Mater. Sci. Eng. 2020, 971, 032011. [Google Scholar] [CrossRef]
- Wazeer, A.; Das, A.; Abeykoon, C.; Sinha, A.; Karmakar, A. Composites for electric vehicles and automotive sector: A review. Green Energy Intell. Transp. 2023, 2, 100043. [Google Scholar] [CrossRef]
- Li, X.; Zhou, C.; Overman, N.; Ma, X.; Canfield, N.; Kappagantula, K.; Schroth, J.; Grant, G. Copper carbon composite wire with a uniform carbon dispersion made by friction extrusion. J. Manuf. Process. 2021, 65, 397–406. [Google Scholar] [CrossRef]
- Graça, I.; Seixas, T.; Ferro, A.C.; Guedes, M. Nanostructured copper-carbon nanotubes composites for aircraft applications. Aircr. Eng. Aerosp. Technol. 2018, 90, 1042–1049. [Google Scholar] [CrossRef]
- Sundaram, R.M.; Sekiguchi, A.; Sekiya, M.; Yamada, T.; Hata, K. Copper/carbon nanotube composites: Research trends and outlook. R. Soc. Open Sci. 2018, 5, 180814. [Google Scholar] [CrossRef] [PubMed]
- Kute, A.D.; Gaikwad, R.P.; Warkad, I.R.; Gawande, M.B. A review on the synthesis and applications of sustainable copper-based nanomaterials. Green Chem. 2022, 24, 3502–3573. [Google Scholar] [CrossRef]
- Reljic, S.; Cuadrado-Collados, C.; Oliveira Jardim, E.; Farrando-Perez, J.; Martinez-Escandell, M.; Silvestre-Albero, J. Activated carbon materials with a rich surface chemistry prepared from L-cysteine amino acid. Fluid Phase Equilib. 2022, 558, 113446. [Google Scholar] [CrossRef]
- Wei, L.; Guo, L.; Jiang, J.; Liu, M.; Yang, T. Influence of Fe2O3 on glycine pyrolysis characteristics and nitrogen conversion. CIESC J. 2019, 70, 1942–1950. [Google Scholar] [CrossRef]
- Wang, W.; Tesio, A.Y.; Olivares-Marín, M.; Gómez Romero, P.; Tonti, D. Facile preparation of glycine-based mesoporous graphitic carbons with embedded cobalt nanoparticles. J. Mater. Sci. 2022, 57, 13403–13413. [Google Scholar] [CrossRef]
- Leng, L.; Yang, L.; Zu, H.; Yang, J.; Ai, Z.; Zhang, W.; Peng, H.; Zhan, H.; Li, H.; Zhong, Q. Insights into glycine pyrolysis mechanisms: Integrated experimental and molecular dynamics/DFT simulation studies. Fuel 2023, 351, 128949. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Yang, X.; Hu, L.; Liu, Y.; Wang, C. Evaluate the pyrolysis pathway of glycine and glycylglycine by TG–FTIR. J. Anal. Appl. Pyrolysis 2007, 80, 247–253. [Google Scholar] [CrossRef]
- Hao, J.; Guo, J.; Ding, L.; Xie, F.; Xia, Q.; Xie, J. TG-FTIR, Py-two-dimensional GC–MS with heart-cutting and LC–MS/MS to reveal hydrocyanic acid formation mechanisms during glycine pyrolysis. J. Therm. Anal. Calorim. 2013, 115, 667–673. [Google Scholar] [CrossRef]
- Weiss, I.M.; Muth, C.; Drumm, R.; Kirchner, H.O.K. Thermal decomposition of the amino acids glycine, cysteine, aspartic acid, asparagine, glutamic acid, glutamine, arginine and histidine. BMC Biophys. 2018, 11, 2. [Google Scholar] [CrossRef]
- Shao, Y.; Hu, Z.-y.; Yao, Y.; Wei, X.-r.; Gao, X.-m.; Wu, Z.-x. Glycine-derived nitrogen-doped ordered mesoporous carbons with a bimodal mesopore size distribution for supercapacitors and oxygen reduction. New Carbon Mater. 2022, 37, 259–276. [Google Scholar] [CrossRef]
- Cossu, F.L.; Poddighe, M.; Stagi, L.; Anedda, R.; Innocenzi, P. The birth of fluorescence from thermally polymerized glycine. Macromol. Chem. Phys. 2022, 223, 2200052. [Google Scholar] [CrossRef]
- Siegel, P.H. Terahertz technology. IEEE Trans. Microw. Theory Tech. 2002, 50, 910–928. [Google Scholar] [CrossRef]
- Venkatachalam, S.; Zeranska-Chudek, K.; Zdrojek, M.; Hourlier, D. Carbon-based terahertz absorbers: Materials, applications, and perspectives. Nano Sel. 2020, 1, 471–490. [Google Scholar] [CrossRef]
- Castle, J.E. Practical Surface Analysis by Auger and X-ray Photoelectron Spectroscopy; John Wiley & Sons, Ltd.: Chichester, UK, 1984; Volume 6, p. 302. [Google Scholar]
- Rodríguez-Carvajal, J. Recent developments of the program FULLPROF. Comm. Powder Diffr. (IUCr) Newsl. 2001, 26, 12–19. [Google Scholar]
- Zanatta, A.R. Revisiting the optical bandgap of semiconductors and the proposal of a unified methodology to its determination. Sci. Rep. 2019, 9, 11225. [Google Scholar] [CrossRef]
- Duvillaret, L.; Garet, F.; Coutaz, J.L. A reliable method for extraction of material parameters in terahertz time-domain spectroscopy. IEEE J. Sel. Top. Quantum Electron. 1996, 2, 739–746. [Google Scholar] [CrossRef]
- Chamorro-Posada, P.; Vazquez-Cabo, J.; Rubinos-Lopez, O.; Martin-Gil, J.; Hernandez-Navarro, S.; Martin-Ramos, P.; Sanchez-Arevalo, F.M.; Tamashausky, A.V.; Merino-Sanchez, C.; Dante, R.C. THz TDS study of several sp(2) carbon materials: Graphite, needle coke and graphene oxides. Carbon 2016, 98, 484–490. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods V: Modification of NDDO approximations and application to 70 elements. J. Mol. Model. 2007, 13, 1173–1213. [Google Scholar] [CrossRef] [PubMed]
- Ridley, J.; Zerner, M. An intermediate neglect of differential overlap technique for spectroscopy: Pyrrole and the azines. Theor. Chim. Acta 1973, 32, 111–134. [Google Scholar] [CrossRef]
- Zerner, M.C.; Loew, G.H.; Kirchner, R.F.; Mueller-Westerhoff, U.T. An intermediate neglect of differential overlap technique for spectroscopy of transition-metal complexes. Ferrocene. J. Am. Chem. Soc. 1980, 102, 589–599. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Bansal, V.; Collins, D.; Luxton, T.; Kumar, N.; Shah, S.; Walker, V.K.; Shah, V. Assessing the impact of copper and zinc oxide nanoparticles on soil: A field study. PLoS ONE 2012, 7, e42663. [Google Scholar] [CrossRef] [PubMed]
- Altomare, A.; Corriero, N.; Cuocci, C.; Falcicchio, A.; Moliterni, A.; Rizzi, R. QUALX2.0: A qualitative phase analysis software using the freely available database POW_COD. J. Appl. Crystallogr. 2015, 48, 598–603. [Google Scholar] [CrossRef]
- Demadis, K.D.; Famelis, N.; Cabeza, A.; Aranda, M.A.G.; Colodrero, R.M.P.; Infantes-Molina, A. 2D Corrugated magnesium carboxyphosphonate materials: Topotactic transformations and interlayer “decoration” with ammonia. Inorg. Chem. 2012, 51, 7889–7896. [Google Scholar] [CrossRef]
- Patil, B.; Prabhakar, B.; Kulkarni, V. Magnetic and spectral studies of copper (II) complexes with schiff bases derived from o-amino-benzoyl hydrazide. Curr. Sci. 1979, 48, 974–976. [Google Scholar]
- Dante, R.C.; Chamorro-Posada, P.; Vázquez-Cabo, J.; Rubiños-López, Ó.; Sánchez-Árevalo, F.M.; Huerta, L.; Martín-Ramos, P.; Lartundo-Rojas, L.; Ávila-Vega, C.F.; Rivera-Tapia, E.D.; et al. Nitrogen-carbon graphite-like semiconductor synthesized from uric acid. Carbon 2017, 121, 368–379. [Google Scholar] [CrossRef]
- Cioci, A.; Dante, D.G.; Martín-Ramos, P.; Chamorro-Posada, P.; Marabello, D.; Benzi, P.; Valsania, M.C.; Dante, R.C. CuTPP films grown on ceramic and ITO-coated glasses. Fuller. Nanotub. Carbon Nanostruct. 2023, 31, 856–867. [Google Scholar] [CrossRef]
- Bergman, D.J.; Imry, Y. Critical behavior of the complex dielectric constant near the percolation threshold of a heterogeneous material. Phys. Rev. Lett. 1977, 39, 1222–1225. [Google Scholar] [CrossRef]
- Song, Y.; Noh, T.W.; Lee, S.-I.; Gaines, J.R. Experimental study of the three-dimensional ac conductivity and dielectric constant of a conductor-insulator composite near the percolation threshold. Phys. Rev. B 1986, 33, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Jagannadham, K. Volume fraction of graphene platelets in copper-graphene composites. Metall. Mater. Trans. A 2013, 44, 552–559. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 97th ed.; CRC Press: Boca Raton, FL, USA, 2016; 2670p. [Google Scholar] [CrossRef]
- Perry, D.L. Handbook of Inorganic Compounds; CRC Press: Boca Raton, FL, USA, 2016; 581p. [Google Scholar] [CrossRef]
- Pan, C.; Gaur, A.P.S.; Lynn, M.; Olson, M.P.; Ouyang, G.; Cui, J. Enhanced electrical conductivity in graphene–copper multilayer composite. AIP Adv. 2022, 12, 015310. [Google Scholar] [CrossRef]
- Khattak, M.I.; Anab, M.; Muqarrab, N. A duo of graphene-copper based wideband planar plasmonic antenna analysis for lower region of terahertz (THz) communications. Prog. Electromagn. Res. C 2021, 111, 83–96. [Google Scholar] [CrossRef]
- Song, R.; Mao, B.; Wang, Z.; Hui, Y.; Zhang, N.; Fang, R.; Zhang, J.; Wu, Y.; Ge, Q.; Novoselov, K.S.; et al. Comparison of copper and graphene-assembled films in 5G wireless communication and THz electromagnetic-interference shielding. Proc. Natl. Acad. Sci. USA 2023, 120, e2209807120. [Google Scholar] [CrossRef]


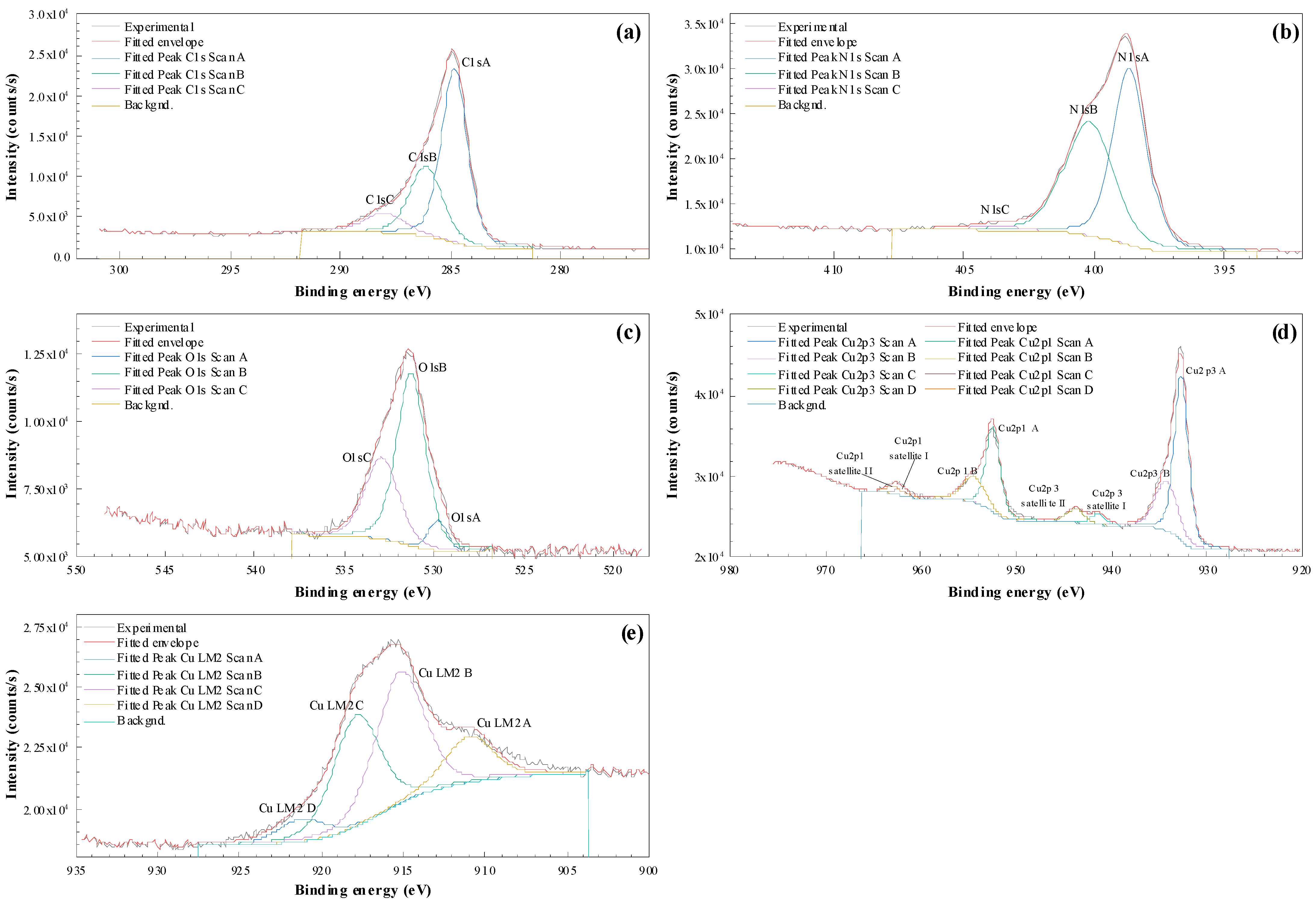


| Element/Transition Core Lines | SCOFIELD SF |
|---|---|
| C1s | 1 |
| O1s | 2.930 |
| N1s | 1.8 |
| Cu2p3 | 16.730 |
| Temperature (°C) | Glycine | Glycine + 0.5% Copper (II) Acetate | Glycine + 10% Copper (II) Acetate |
|---|---|---|---|
| 450 | 34.2% | 38.1% | 38.8% |
| 500 | 29.2% | 30.7% | 33.8% |
| 550 | 4.8% | 5.3% | 7.1% |
| Element (wt%) | Glycine Thermolysate (Gly 500 °C) | Cu-doped Glycine Thermolysate (Gly-Cu 3.17% 500 °C) | ||||
|---|---|---|---|---|---|---|
| Site 1 | Site 2 | Site 3 | Site 1 | Site 2 | Site 3 | |
| C | 61.1 | 62.1 | 69.7 | 63.0 | 58.4 | 58.3 |
| N | 29.6 | 27.7 | 25.5 | 22.0 | 23.2 | 26.5 |
| O | 7.5 | 8.3 | 4.6 | 7.5 | 9.1 | 8.7 |
| Cu | - | - | - | 7.3 | 8.8 | 3.5 |
| Suggested formula | C12HxN5O (64.3%C, 27.6%N, 6.8%O) | C50CuHxN17O5 (59.9%C, 23.9%N, 8.4%O, 6.5%Cu) | ||||
| Sample | C | N | O | Cu | Si | Cl | C/N | C/O |
|---|---|---|---|---|---|---|---|---|
| Gly 500 °C | 68.97 | 24.02 | 6.32 | - | 0.54 | 0.15 | 2.9 | 10.9 |
| Gly-Cu 3.17% 500 °C | 68.70 | 17.46 | 10.39 | 2.44 | 1.02 | - | 3.9 | 6.6 |
| Sample | C1s A C–C, CHx (284.8 eV) | C1s B C-NH3+, C–O–C, C–O–H (285.6–286.5 eV) | C1s COO– (288–289 eV) |
|---|---|---|---|
| Gly 500 °C | 284.8/45.54% | 286.13/37.47% | 288/9.37% |
| Gly-Cu 3.17% 500 °C | 284.8/59.09% | 286.1/30.21% | 288/10.7% |
| Sample | N1s A –NH2 | N1s B amine –NH– (400.0 eV), –N–(C=O) (400.50 eV) | N1s C doped imine –NH+– (402.00 eV) nitrite (NO2) (403.9 eV) |
|---|---|---|---|
| Gly 500 °C | 398.53/49.09% | 400.17/46.26% | 402/4.65% |
| Gly-Cu 3.17% 500 °C | 398.64/51.3% | 400.2/46.94% | 403.8/1.76% |
| Cu2p3/2 A | Cu2p3/2 B | Satellite I | Satellite II | Cu LMM2 A | Cu LMM2 B | Cu LMM2 C | Cu LMM2 D |
|---|---|---|---|---|---|---|---|
| 932.71/62.37% | 934.28/27.5% | 941.45/4.19% | 943.8/5.94% | 921.12 | 917.75 | 915.09 | 910.88 |
| Cu2p3/2 (I) | Cu2p3/2 (II) | Cu L2–3M45M45 Average Peak Position Peaks 2–3 | Auger Parameter (I) | Auger Parameter (II) | |
|---|---|---|---|---|---|
| From high-resolution spectrum | 932.71 | 934.28 | 916.42 | 1849.13 Cu2O− Cu1+ | 1850.7 Cu(OH)2− Cu2+ |
| From survey | 932.9 | 916.42 | 1849.34 Cu2O Cu1+ |
| Gly 450 °C | Gly 500 °C | Gly 550 °C | Gly–Cu 0.16% 500 °C | Gly–Cu 3.17% 500 °C | Assignment |
|---|---|---|---|---|---|
| 3074 | 3086 | 3072 | 3044 | - | C–H stretching; amide B mode; O–H stretching |
| - | - | - | 2880 | 2659 | CH2 stretching |
| 2223 | - | 2161 | - | - | CH3 asymmetrical stretch; C=C stretch; C=O stretching |
| 1583 | - | - | - | - | graphene/graphite; amide II stretching |
| - | (1564) | - | 1563 | 1564 | asymmetrical stretching of C=O in Cu carboxylates |
| - | - | - | 1507 | 1493 | ν (C=C); asymmetrical flexion of the C–H bond in CH3 |
| 1206 | 1212 | 1216 | 1204 | 1216 | N–H stretching; asymmetrical vibration COO– |
| - | - | - | 1159 | 1155 | CN stretching |
| - | - | - | - | 1081 | C–O bond stretching |
| - | - | - | - | 1008 | Cu–N stretching mode |
| 711 | 712 | 715 | - | - | C–H bending vibration |
| - | - | - | 479 | Cu–N stretching vibration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamorro-Posada, P.; Dante, R.C.; Martín-Gil, J.; Dante, D.G.; Cioci, A.; Vázquez-Cabo, J.; Rubiños-López, Ó.; Mediavilla-Martínez, I.; Martín-Ramos, P. On a Composite Obtained by Thermolysis of Cu-Doped Glycine. C 2024, 10, 49. https://doi.org/10.3390/c10020049
Chamorro-Posada P, Dante RC, Martín-Gil J, Dante DG, Cioci A, Vázquez-Cabo J, Rubiños-López Ó, Mediavilla-Martínez I, Martín-Ramos P. On a Composite Obtained by Thermolysis of Cu-Doped Glycine. C. 2024; 10(2):49. https://doi.org/10.3390/c10020049
Chicago/Turabian StyleChamorro-Posada, Pedro, Roberto C. Dante, Jesús Martín-Gil, Denisse G. Dante, Alma Cioci, José Vázquez-Cabo, Óscar Rubiños-López, Irene Mediavilla-Martínez, and Pablo Martín-Ramos. 2024. "On a Composite Obtained by Thermolysis of Cu-Doped Glycine" C 10, no. 2: 49. https://doi.org/10.3390/c10020049
APA StyleChamorro-Posada, P., Dante, R. C., Martín-Gil, J., Dante, D. G., Cioci, A., Vázquez-Cabo, J., Rubiños-López, Ó., Mediavilla-Martínez, I., & Martín-Ramos, P. (2024). On a Composite Obtained by Thermolysis of Cu-Doped Glycine. C, 10(2), 49. https://doi.org/10.3390/c10020049










