Impact of Long-Term Bottle Aging on Color Transition, Polymers, and Aromatic Compounds in Mulberry Wine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analysis of Pigments and Colors
2.3. Analysis of Tannins
2.4. Analysis of Pyranoanthocyanin and Polymeric Pigments
2.5. Analysis of Polysaccharides and Proteins
2.6. Analysis of Volatile Compounds
3. Results and Discussion
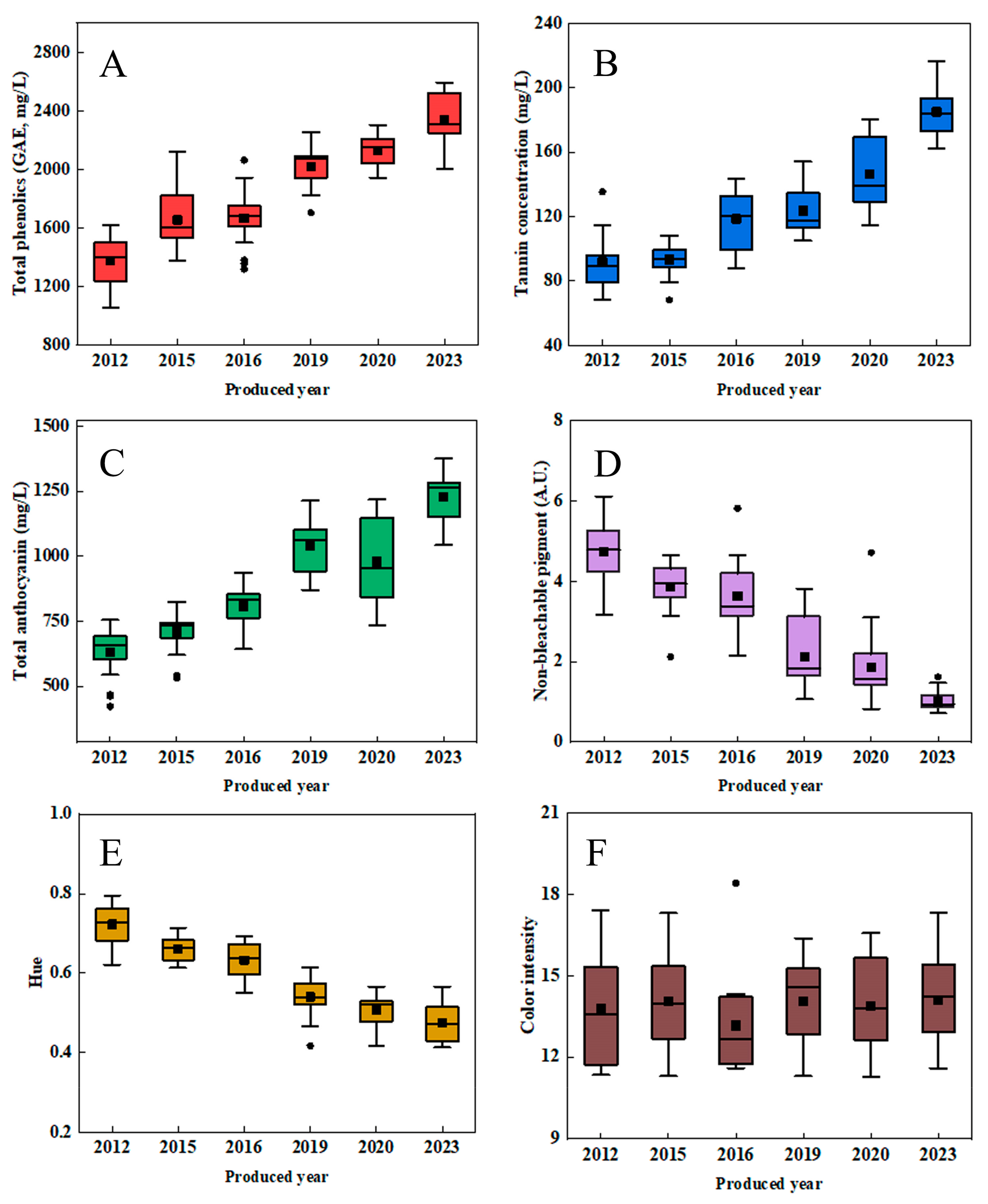
3.1. Impact of Aging on the Composition of Phenolic Compounds in Mulberry Wine
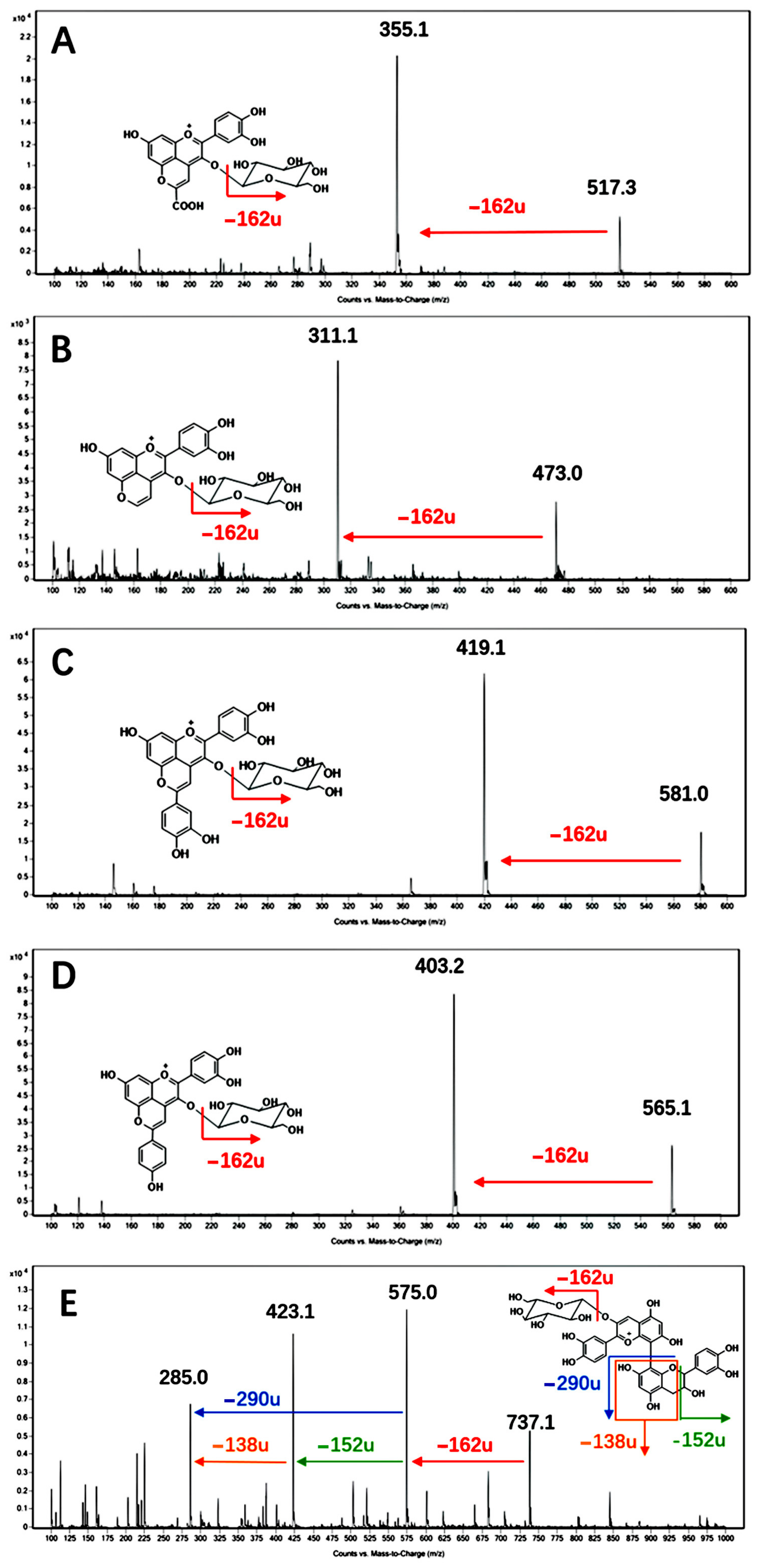
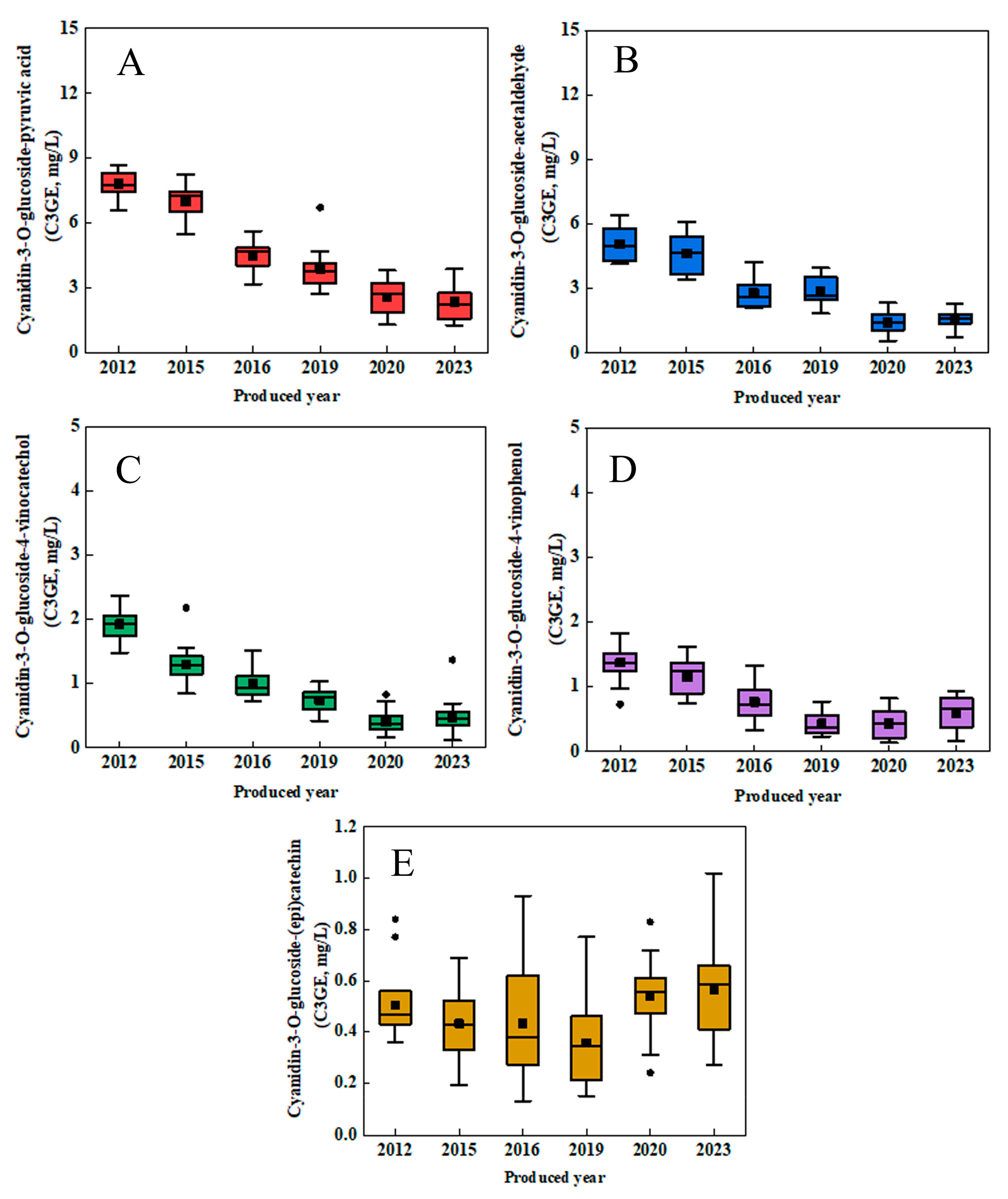
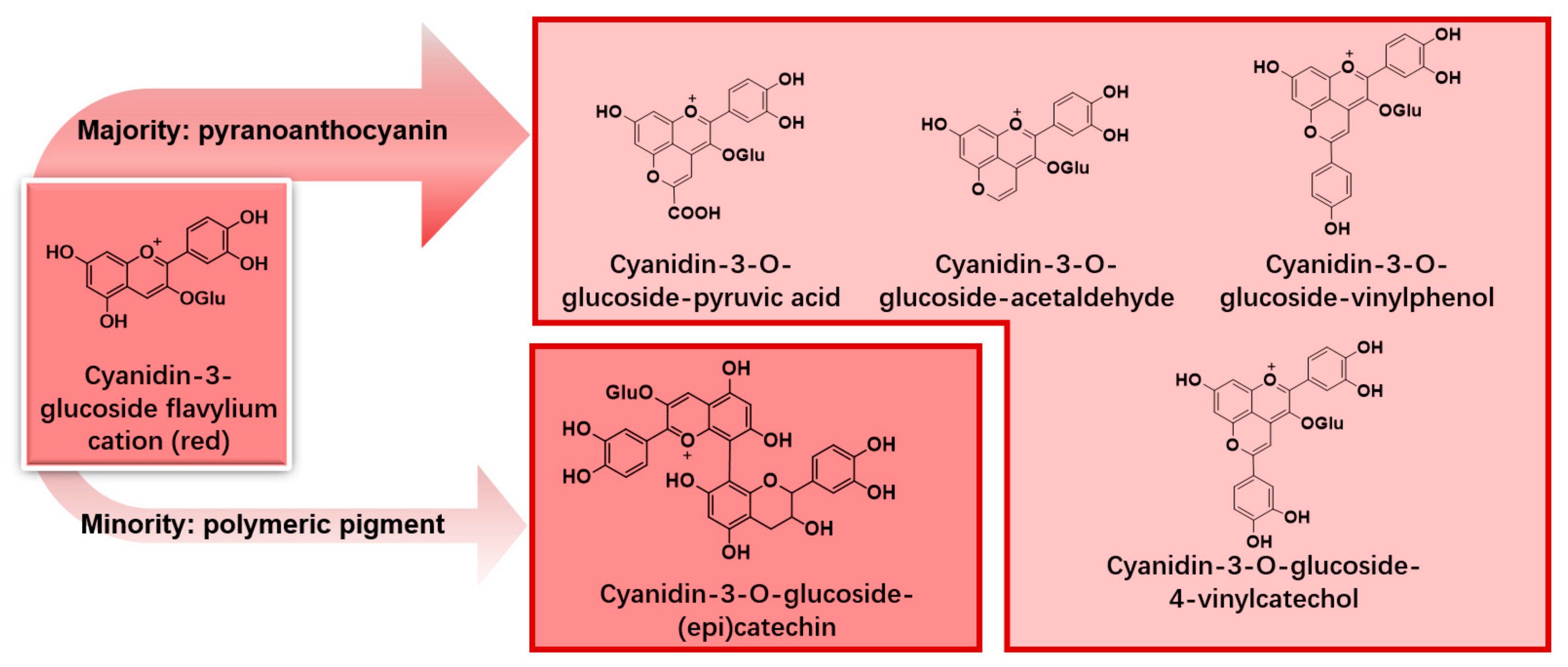
3.2. Pyranoanthocyanin and Polymeric Pigments
3.3. The Impact of Aging on Polysaccharides and Proteins
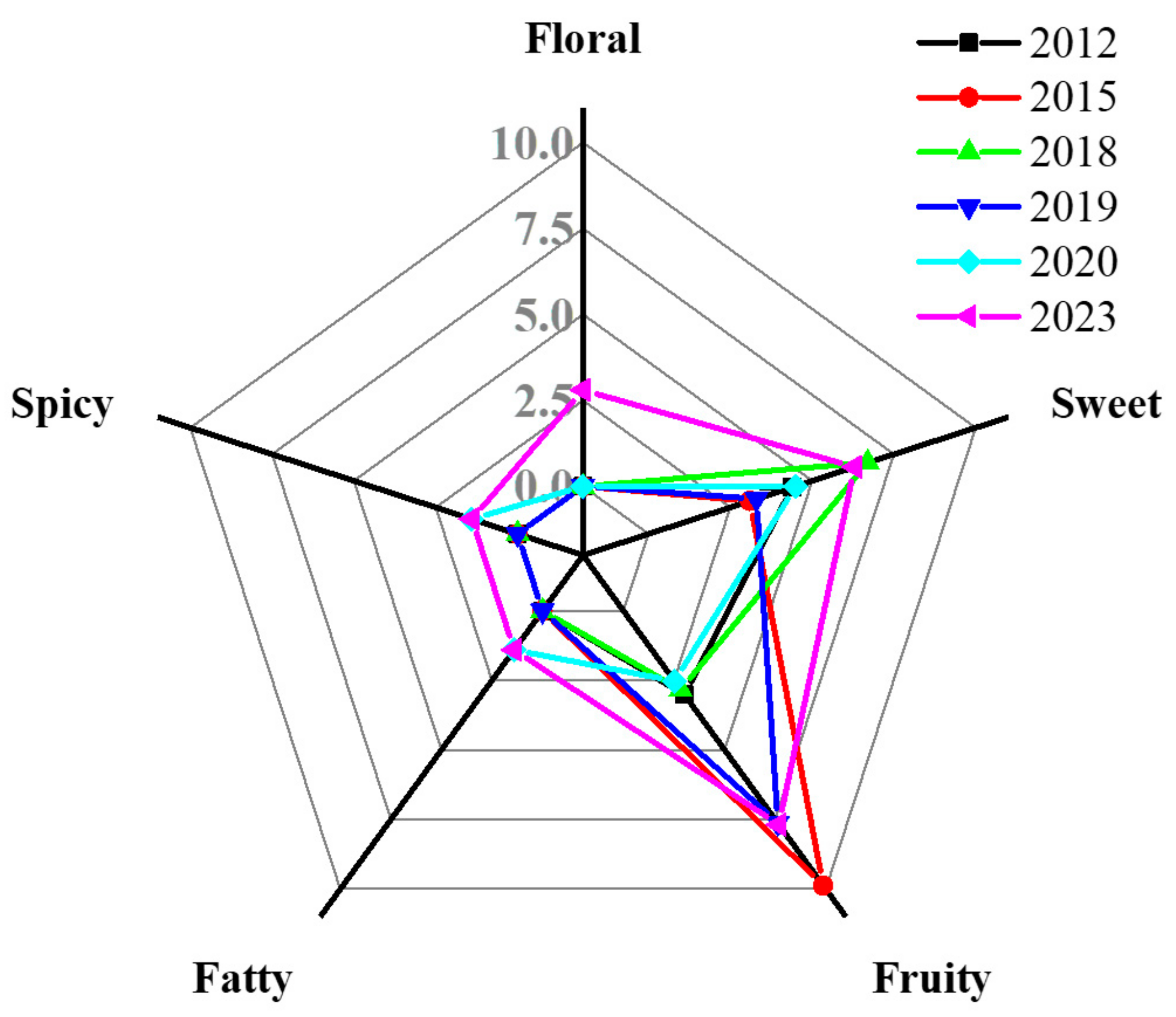
3.4. Impact of Aging on Volatile Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, P.; Hu, T.-G.; Linhardt, R.J.; Liao, S.-T.; Wu, H.; Zou, Y.-X. Mulberry: A review of bioactive compounds and advanced processing technology. Trends Food Sci. Technol. 2019, 83, 138–158. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhao, L. The Mulberry (Morus alba L.) Fruit—A Review of Characteristic Components and Health Benefits. J. Agric. Food Chem. 2017, 65, 10383–10394. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Nie, W.-J. Chemical properties in fruits of mulberry species from the Xinjiang province of China. Food Chem. 2015, 174, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Calín-Sánchez, A.; Martínez-Nicolás, J.; Munera-Picazo, S.; Carbonell-Barrachina, A.; Legua, P.; Hernández, F. Bioactive Compounds and Sensory Quality of Black and White Mulberries Grown in Spain. Plant Foods Hum. Nutr. 2013, 68, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.M.; Xiao, G.S.; Chen, W.D.; Xu, Y.J.; Wu, J.J. Quantification and purification of mulberry anthocyanins with macroporous resins. J. Biomed. Biotechnol. 2004, 5, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.H.; Suh, H.J. Antioxidant activities of five different mulberry cultivars in Korea. LWT—Food Sci. Technol. 2007, 40, 955–962. [Google Scholar] [CrossRef]
- Echave, J.; Barral, M.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Bottle Aging and Storage of Wines: A Review. Molecules 2021, 26, 713. [Google Scholar] [CrossRef] [PubMed]
- Bindon, K.A.; McCarthy, M.G.; Smith, P.A. Development of wine colour and non-bleachable pigments during the fermentation and ageing of (Vitis vinifera L. cv.) Cabernet Sauvignon wines differing in anthocyanin and tannin concentration. LWT—Food Sci. Technol. 2014, 59, 923–932. [Google Scholar] [CrossRef]
- He, F.; Liang, N.-N.; Mu, L.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Anthocyanins and Their Variation in Red Wines II. Anthocyanin Derived Pigments and Their Color Evolution. Molecules 2012, 17, 1483–1519. [Google Scholar] [CrossRef]
- Vidal, S.; Cartalade, D.; Souquet, J.-M.; Fulcrand, H.; Cheynier, V. Changes in Proanthocyanidin Chain Length in Winelike Model Solutions. J. Agric. Food Chem. 2002, 50, 2261–2266. [Google Scholar] [CrossRef]
- Mouls, L.; Fulcrand, H. UPLC-ESI-MS study of the oxidation markers released from tannin depolymerization: Toward a better characterization of the tannin evolution over food and beverage processing. J. Mass Spectrom. 2012, 47, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Guadalupe, Z.; Ayestarán, B. Polysaccharide Profile and Content during the Vinification and Aging of Tempranillo Red Wines. J. Agric. Food Chem. 2007, 55, 10720–10728. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.B.; Piçarra-Pereira, M.A.; Monteiro, S.; Loureiro, V.l.B.; Teixeira, A.R. The wine proteins. Trends Food Sci. Technol. 2001, 12, 230–239. [Google Scholar] [CrossRef]
- Coelho, C.; Julien, P.; Nikolantonaki, M.; Noret, L.; Magne, M.; Ballester, J.; Gougeon, R.D. Molecular and Macromolecular Changes in Bottle-Aged White Wines Reflect Oxidative Evolution–Impact of Must Clarification and Bottle Closure. Front. Chem. 2018, 6, 95. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Wang, J.; Wang, H.; Zhao, Q.; Zhang, F.; Ge, Q.; Li, C.; Gamboa, G.G.; Fang, Y.; Sun, X. Wine aging and artificial simulated wine aging: Technologies, applications, challenges, and perspectives. Food Res. Int. 2022, 153, 110953. [Google Scholar] [CrossRef] [PubMed]
- Carpena, M.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Wine Aging Technology: Fundamental Role of Wood Barrels. Foods 2020, 9, 1160. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, M.D.; Dambergs, R.G.; Herderich, M.J.; Smith, P.A. High Throughput Analysis of Red Wine and Grape PhenolicsAdaptation and Validation of Methyl Cellulose Precipitable Tannin Assay and Modified Somers Color Assay to a Rapid 96 Well Plate Format. J. Agric. Food Chem. 2007, 55, 4651–4657. [Google Scholar] [CrossRef] [PubMed]
- Teng, B.; Petrie, P.R.; Smith, P.A.; Bindon, K.A. Comparison of water addition and early-harvest strategies to decrease alcohol concentration in Vitis vinifera cv. Shiraz wine: Impact on wine phenolics, tannin composition and colour properties. Aust. J. Grape Wine Res. 2020, 26, 158–171. [Google Scholar] [CrossRef]
- Teng, B.; Hayasaka, Y.; Smith, P.A.; Bindon, K.A. Precipitation of Tannin-Anthocyanin Derivatives in Wine is Influenced by Acetaldehyde Concentration and Tannin Molecular Mass with Implications for the Development of Nonbleachable Pigments. J. Agric. Food Chem. 2021, 69, 4804–4815. [Google Scholar] [CrossRef] [PubMed]
- Amico, V.; Napoli, E.M.; Renda, A.; Ruberto, G.; Spatafora, C.; Tringali, C. Constituents of grape pomace from the Sicilian cultivar ‘Nerello Mascalese’. Food Chem. 2004, 88, 599–607. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; Ai, J.; Huang, W.; Zhan, J.; You, Y. Formation of vinylphenolic pyranoanthocyanins by selected indigenous yeasts displaying high hydroxycinnamate decarboxylase activity during mulberry wine fermentation and aging. Food Microbiol. 2023, 113, 104272. [Google Scholar] [CrossRef]
- Teng, B.; Petrie, P.R.; Espinase Nandorfy, D.; Smith, P.; Bindon, K. Pre-Fermentation Water Addition to High-Sugar Shiraz Must: Effects on Wine Composition and Sensory Properties. Foods 2020, 9, 1193. [Google Scholar] [CrossRef]
- Butkhup, L.; Jeenphakdee, M.; Jorjong, S.; Samappito, S.; Samappito, W.; Chowtivannakul, S. HS-SPME-GC-MS analysis of volatile aromatic compounds in alcohol related beverages made with mulberry fruits. Food Sci. Biotechnol. 2011, 20, 1021. [Google Scholar] [CrossRef]
- Muñoz, D.; Peinado, R.A.; Medina, M.; Moreno, J. Biological aging of sherry wines under periodic and controlled microaerations with Saccharomyces cerevisiae var. capensis: Effect on odorant series. Food Chem. 2007, 100, 1188–1195. [Google Scholar] [CrossRef]
- Cliff, M.A.; King, M.C.; Schlosser, J. Anthocyanin, phenolic composition, colour measurement and sensory analysis of BC commercial red wines. Food Res. Int. 2007, 40, 92–100. [Google Scholar] [CrossRef]
- Oliveira, J.; Fernandes, V.; Miranda, C.; Santos-Buelga, C.; Silva, A.; de Freitas, V.; Mateus, N. Color Properties of Four Cyanidin–Pyruvic Acid Adducts. J. Agric. Food Chem. 2006, 54, 6894–6903. [Google Scholar] [CrossRef] [PubMed]
- Dallas, C.; Ricardo-da-Silva, J.M.; Laureano, O. Interactions of Oligomeric Procyanidins in Model Wine Solutions Containing Malvidin-3-Glucoside and Acetaldehyde. J. Sci. Food Agric. 1996, 70, 493–500. [Google Scholar] [CrossRef]
- Teng, B.; Hayasaka, Y.; Smith, P.A.; Bindon, K.A. Effect of Grape Seed and Skin Tannin Molecular Mass and Composition on the Rate of Reaction with Anthocyanin and Subsequent Formation of Polymeric Pigments in the Presence of Acetaldehyde. J. Agric. Food Chem. 2019, 67, 8938–8949. [Google Scholar] [CrossRef]
- Song, W.; Wang, H.-J.; Bucheli, P.; Zhang, P.-F.; Wei, D.-Z.; Lu, Y.-H. Phytochemical Profiles of Different Mulberry (Morus sp.) Species from China. J. Agric. Food Chem. 2009, 57, 9133–9140. [Google Scholar] [CrossRef]
- Hayasaka, Y.; Birse, M.; Eglinton, J.; Herderich, M. The effect of Saccharomyces cerevisiae and Saccharomyces bayanus yeast on colour properties and pigment profiles of a Cabernet Sauvignon red wine. Aust. J. Grape Wine Res. 2007, 13, 176–185. [Google Scholar] [CrossRef]
- Hayasaka, Y.; Kennedy, J.A. Mass spectrometric evidence for the formation of pigmented polymers in red wine. Aust. J. Grape Wine Res. 2003, 9, 210–220. [Google Scholar] [CrossRef]
- Carew, A.L.; Smith, P.; Close, D.C.; Curtin, C.; Dambergs, R.G. Yeast Effects on Pinot noir Wine Phenolics, Color, and Tannin Composition. J. Agric. Food Chem. 2013, 61, 9892–9898. [Google Scholar] [CrossRef] [PubMed]
- Bindon, K.; Varela, C.; Kennedy, J.; Holt, H.; Herderich, M. Relationships between harvest time and wine composition in Vitis vinifera L. cv. Cabernet Sauvignon 1. Grape and wine chemistry. Food Chem. 2013, 138, 1696–1705. [Google Scholar] [CrossRef]
- Burtch, C.E.; Mansfield, A.K.; Manns, D.C. Reaction Kinetics of Monomeric Anthocyanin Conversion to Polymeric Pigments and Their Significance to Color in Interspecific Hybrid Wines. J. Agric. Food Chem. 2017, 65, 6379–6386. [Google Scholar] [CrossRef] [PubMed]
- Bindon, K.A.; Carew, A.L.; Mierczynska-Vasilev, A.; Kassara, S.; Kerslake, F.; Smith, P.A. Characterization of macromolecular complexes in red wine: Composition, molecular mass distribution and particle size. Food Chem. 2016, 199, 838–846. [Google Scholar] [CrossRef]
- Rocha, S.l.M.; Rodrigues, F.; Coutinho, P.; Delgadillo, I.; Coimbra, M.A. Volatile composition of Baga red wine: Assessment of the identification of the would-be impact odourants. Anal. Chim. Acta 2004, 513, 257–262. [Google Scholar] [CrossRef]
- García-Carpintero, E.G.; Sánchez-Palomo, E.; Gallego, M.A.G.; González-Viñas, M.A. Volatile and sensory characterization of red wines from cv. Moravia Agria minority grape variety cultivated in La Mancha region over five consecutive vintages. Food Res. Int. 2011, 44, 1549–1560. [Google Scholar] [CrossRef]
- Zea, L.; Moyano, L.; Moreno, J.; Cortes, B.; Medina, M. Discrimination of the aroma fraction of Sherry wines obtained by oxidative and biological ageing. Food Chem. 2001, 75, 79–84. [Google Scholar] [CrossRef]
- Peinado, R.A.; Moreno, J.; Bueno, J.E.; Moreno, J.A.; Mauricio, J.C. Comparative study of aromatic compounds in two young white wines subjected to pre-fermentative cryomaceration. Food Chem. 2004, 84, 585–590. [Google Scholar] [CrossRef]
- Perestrelo, R.; Fernandes, A.; Albuquerque, F.F.; Marques, J.C.; Câmara, J.S. Analytical characterization of the aroma of Tinta Negra Mole red wine: Identification of the main odorants compounds. Anal. Chim. Acta 2006, 563, 154–164. [Google Scholar] [CrossRef]
| Polysaccharide Component | 2012 | 2015 | 2018 | 2019 | 2020 | 2023 |
|---|---|---|---|---|---|---|
| Monosaccharides | ||||||
| Mannose | 166.7 ± 1.4 d | 168.4 ± 5.9 d | 332.2 ± 5.3 a | 340.9 ± 2.7 a | 285.2 ± 4.8 c | 306.7 ± 5.4 b |
| Rhamnose | 78.1 ± 1.3 b | 84.4 ± 1.0 a | 79.4 ± 1.3 b | 71.8 ± 1.2 c | 86.0 ± 1.8 a | 92.0 ± 1.7 a |
| Glucuronic acid | 29.7 ± 1.0 a | 17.6 ± 1.4 c | 15.5 ± 0.3 c | 24.4 ± 1.2 b | 14.2 ± 0.3 c | 15.9 ± 1.1 c |
| Galacturonic acid | 78.9 ± 1.3 a | 18.8 ± 1.0 b | 19.2 ± 1.2 c | 12.8 ± 0.2 d | 14.3 ± 0.8 d | 18.3 ± 0.9 c |
| Glucose | 80.9 ± 2.0 b | 82.0 ± 1.4 b | 61.3 ± 2.4 c | 56.1 ± 1.3 c | 89.6 ± 2.5 a | 62.5 ± 1.2 c |
| Galactose | 157.0 ± 2.0 b | 199.8 ± 2.0 a | 74.4 ± 2.4 d | 73.1 ± 2.1 d | 68.7 ± 1.4 c | 74.7 ± 2.1 d |
| Arabinose | 64.5 ± 1.2 c | 97.1 ± 1.1 a | 71.9 ± 2.2 b | 63.1 ± 1.1 c | 66.9 ± 1.6 c | 71.6 ± 0.8 b |
| Total polysaccharides | 656.0 ± 7.6 a | 668.2 ± 8.3 a | 654.1 ± 6.8 a | 642.4 ± 4.5 b | 625.1 ± 7.7 c | 641.7 ± 7.2 b |
| Amino acids | ||||||
| Asparagine | 3.6 ± 0.3 c | 4.5 ± 0.3 bc | 6.6 ± 0.2 a | 5.1 ± 0.3 b | 1.5 ± 0.0 d | 3.7 ± 0.0 c |
| Glutamic acid | 9.0 ± 0.3 b | 9.9 ± 0.4 b | 11.4 ± 0.2 a | 5.7 ± 0.4 c | 3.9 ± 0.3 d | 11.6 ± 0.8 a |
| Serine | 38.4 ± 0.4 a | 32.7 ± 2.2 ab | 44.7 ± 3.1 a | 39.9 ± 2.1 a | 19.2 ± 0.4 c | 46.2 ± 1.1 a |
| Histidine | 18.6 ± 0.2 b | 24.3 ± 1.0 a | 13.2 ± 1.4 c | 7.8 ± 0.4 d | 11.4 ± 1.2 c | 13.3 ± 0.8 c |
| Glycine | 13.2 ± 0.1 a | 10.5 ± 1.0 bc | 11.7 ± 0.2 b | 9.9 ± 0.1 c | 8.1 ± 0.2 d | 13.0 ± 0.5 a |
| Threonine | 25.8 ± 1.0 a | 19.5 ± 1.2 b | 19.5 ± 1.4 b | 20.4 ± 0.5 b | 14.1 ± 0.0 c | 20.7 ± 1.2 b |
| Arginine | 8.7 ± 0.2 b | 9.3 ± 0.2 a | 9.9 ± 0.1 a | 9.3 ± 0.3 ab | 8.4 ± 0.3 b | 9.7 ± 0.3 a |
| Alanine | 10.8 ± 0.1 a | 6.9 ± 0.3 c | 9.0 ± 0.3 b | 8.7 ± 0.2 b | 7.2 ± 0.3 c | 6.2 ± 0.7 c |
| Tyrosine | 5.1 ± 0.0 b | 4.2 ± 0.0 d | 5.7 ± 0.1 a | 4.5 ± 0.0 c | 3.3 ± 0.1 e | 4.1 ± 0.1 d |
| Cysteine | 1.2 ± 0.1 bc | 1.2 ± 0.0 cd | 2.1 ± 0.1 a | 1.5 ± 0.0 b | 1.2 ± 0.0 c | 2.0 ± 0.3 a |
| Valine | 3.1 ± 0.1 a | 0.1 ± 0.0 c | 1.5 ± 0.1 b | 1.2 ± 0.2 b | 0.1 ± 0.2 c | 0.1 ± 0.0 c |
| Methionine | 6.9 ± 0.4 b | 4.8 ± 0.1 c | 8.4 ± 0.3 a | 9.3 ± 0.5 a | 6.3 ± 0.0 b | 6.5 ± 0.2 b |
| Isoleucine | 5.4 ± 0.3 bc | 4.8 ± 0.2 c | 6.9 ± 0.2 a | 5.7 ± 0.1 b | 3.9 ± 0.1 d | 6.7 ± 0.3 a |
| Phenylaniline | 12.6 ± 0.3 ab | 10.5 ± 0.3 b | 13.8 ± 0.1 a | 11.1 ± 0.2 b | 12.3 ± 0.1 ab | 10.3 ± 0.5 b |
| Leucine | 4.5 ± 0.1 b | 7.5 ± 0.1 a | 7.8 ± 0.2 a | 4.8 ± 0.2 b | 3.6 ± 0.1 c | 4.7 ± 0.2 b |
| Lysine | 21.1 ± 0.3 a | 12.6 ± 0.4 d | 18.9 ± 0.1 b | 21.0 ± 0.2 a | 17.1 ± 0.1 c | 12.5 ± 0.1 d |
| Proline | 12.3 ± 0.2 a | 11.7 ± 0.2 a | 12.6 ± 0.2 a | 12.3 ± 0.1 a | 12.0 ± 0.2 a | 11.9 ± 0.2 a |
| Total amino acids | 200.8 ± 4.3 a | 175.4 ± 4.1 b | 204.2 ± 7.3 a | 178.7 ± 7.1 b | 134.2 ± 4.3 c | 183.2 ± 6.4 b |
| Volatile Compound | Odor Description | Odor Perception Threshold (µg/L) | Produced Year | |||||
|---|---|---|---|---|---|---|---|---|
| 2012 | 2015 | 2018 | 2019 | 2020 | 2023 | |||
| Acetate esters | ||||||||
| Isoamyl acetate | Banana, fruity, sweet | 160 | 177.8 ± 6.4 d | 164.0 ± 6.59 d | 194.7 ± 7.3 c | 243.5 ± 4.1 b | 266.7 ± 7.9 a | 269.1 ± 10.2 a |
| phenethyl acetate | Roses, floral, honey | 250 | 188.9 ± 2.5 c | 197.3 ± 2.1 c | 212.6 ± 15.0 c | 234.6 ± 2.0 b | 265.7 ± 1.0 a | 282.3 ± 2.5 a |
| Ethyl acetate | Pineapple, fruity, balsamic | 12,000 | 2370.0 ± 23.5 e | 2793.0 ± 17.3 d | 3090.0 ± 20.0 c | 3460.0 ± 24.8 b | 4570.0 ± 42.3 a | 5894.6 ± 57.3 a |
| Hexyl acetate | Green, floral | 1500 | 139.0 ± 8.7 a | 115.9 ± 8.3 b | 150.3 ± 7.5 a | 71.0 ± 5.2 c | 0.0 | 67.5 ± 5.4 c |
| Ethyl ester | ||||||||
| Ethyl lactate | Acid, medicine | 150,000 | 9337.0 ± 85.2 b | 7168.0 ± 24.1 d | 9005.0 ± 52.2 c | 8920.0 ± 25.5 c | 9644.0 ± 40.8 a | 12,400.3 a |
| 2-methylbutyrate | Fruity, apple aroma | — | 22.9 ± 2.3 b | 41.1 ± 1.0 b | 31.3 ± 12.0 b | 31.8 ± 3.1 b | 52.4 ± 2.7 a | 346.5 ± 11.3 a |
| Ethyl hexanoate | Fruity, green apple, banana | 80 | 297.4 ± 41.4 b | 299.1 ± 37.3 b | 285.0 ± 11.6 b | 288.0 ± 5.6 b | 343.5 ± 7.3 a | 1952.7 ± 18.4 a |
| Diethyl succinate | Cheese, earthy, spicy | 1400 | 786.0 ± 13.3 b | 769.0 ± 8.2 b | 615.8 ± 33.8 b | 690.5 ± 19.7 b | 1932.0 ± 26.3 a | 1921.9 ± 22.1 a |
| Ethyl octanoate | Sweet, floral, banana, pear | 240 | 95.2 ± 6.1 b | 75.2 ± 4.1 b | 53.0 ± 6.1 b | 64.4 ± 5.2 b | 107.0 ± 3.4 a | 65.1 ± 4.7 c |
| Ethyl dodecanoate | Fruity, apple aroma | 1500 | 11,200 ± 118 b | 88,300 ± 170 a | 11,100 ± 80 c | 64,400 ± 120 c | 13,400 ± 200 c | 66,523 ± 314 a |
| Ethyl palmitate | Fruity, sweet, cream | 1000 | 54.8 ± 12.1 b | 14.0 ± 2.2 c | 33.1 ± 4.1 b | 36.4 ± 5.2 b | 62.8 ± 3.1 a | 18.3 ± 2.6 ab |
| Ethyl 3-hydroxypropionate | — | — | 13.9 ± 4.2 ab | 14.5 ± 6.8 a | 9.3 ± 4.7 ab | 21.3 ± 0.7 a | 16.7 ± 1.7 ab | 8.2 ± 5.0 a |
| Ethyl 2-methylbutanoate | Fruity | 18,000 | 6.7 ± 2.4 a | 9.8 ± 1.1 a | 7.9 ± 5.0 a | 10.4 ± 1.7 a | ND | 15.6 ± 3.1 a |
| Ethyl pentanoate | Fruity, apple | 5 | 28.1 ± 8.1 a | 17.3 ± 5.7 a | 10.4 ± 3.5 a | 17.8 ± 7.4 a | ND | ND |
| Ethyl sorbate | — | — | 25.8 ± 8.2 a | 13.1 ± 3.2 a | 21.3 ± 8.0 a | 20.1 ± 2.6 a | ND | ND |
| Ethyl octadecanoate | — | — | 11.8 ± 2.2 b | 7.0 ± 2.0 ab | 18.3 ± 2.0 ab | 20.1 ± 4.9 a | ND | ND |
| Ethyl propionate | Sweet, fruity, pear-like | 2100 | 13.0 ± 2.1 a | 6.1 ± 2.6 a | 14.2 ± 8.9 a | 7.3 ± 4.8 a | 0.0 | ND |
| Diethyl dodecanoate | —- | — | 14.9 ± 8.1 a | 7.3 ± 0.7 b | 19.0 ± 1.7 a | 18.4 ± 0.8 a | ND | |
| Higher alcohols | ||||||||
| 2-Phenylethanol | Flowery, rose, honey | 10,000 | 32,800 ± 199 b | 21,200 ± 123 b | 55,100 ± 122 a | 18,100 ± 180 b | 28,000 ± 100 b | 35,700 ± 100 b |
| Nonanol | Fatty, mild, green, melon | 6000 | 332 ± 15.6 bc | 1176.7 ± 5.7 a | 747.9 ± 1.1 ab | 128.5 ± 1.2 c | 444.2 ± 1.6 bc | 126.7 ± 3.3 c |
| 2-Methylpropanol | Medicinal, wine-like | 150,000 | 92.1 ± 29.6 a | 89.0 ± 31.4 a | 458.1 ± 103.2 a | 463.1 ± 205.6 a | 69.6 ± 10.4 a | 372.6 ± 10.3 a |
| Hexanol | Herbaceous, grass, woody | 8000 | 39.5 ± 8.0 a | 32.8 ± 1.4 a | 67.5 ± 17.8 a | 58.8 ± 10.5 a | 69.6 ± 4.1 a | 71.3 ± 5.1 a |
| 1-Pentanol | Fruity | 64,000 | 63.4 ± 6.1 a | 76.3 ± 5.7 a | 69.1 ± 9.6 a | 74.5 ± 3.4 a | 76.7 ± 5.9 a | 78.4 ± 2.1 a |
| 2,5-Dimethyl phenylethanol | — | — | 4.3 ± 0.1 a | 3.6 ± 0.1 a | 6.1 ± 1.4 a | 11.0 ± 4.7 a | ND | ND |
| 1,10-Decadiol | — | — | 10.6 ± 1.9 a | 9.0 ± 1.0 a | 5.7 ± 0.5 a | 7.7 ± 0.1 a | ND | ND |
| Pentaethylene glycol | — | — | 50.1 ± 7.6 a | 70.9 ± 46.8 a | 6.1 ± 1.4 a | 2.9 ± 0.2 a | ND | ND |
| Pentadiol | — | — | 48.3 ± 14.0 a | 11.4 ± 0.8 b | 32.3 ± 7.5 ab | 2.1 ± 0.0 b | ND | ND |
| 2,3-butanediol | Fruity | 150,000 | 34.8 ± 5.5 a | 15.8 ± 3.1 b | 8.2 ± 4.0 b | 16.2 ± 1.6 b | ND | ND |
| 2-propanol | Alcohol-like, ripe fruit | 306,000 | 24.4 ± 11.6 b | 19.5 ± 2.1 b | 67.4 ± 7.1 a | 69.7 ± 15.3 a | ND | ND |
| Volatile acids | ||||||||
| Formic acid | Vinegar, pungent | 200,000 | 17.9 ± 1.1 a | 27.1 ± 7.1 a | 18.2 ± 0.1 a | 24.1 ± 1.6 a | 23.0 ± 7.4 a | 18.3 ± 4.1 a |
| 2,6-dihydroxy benzoic acid | — | — | 11.5 ± 0.1 c | 12.6 ± 5.5 c | 9.1 ± 1.3 c | 19.1 ± 1.2 b | 77.8 ± 11.9 a | 23.6 ± 4.2 b |
| Palmitic acid | — | — | 23.8 ± 7.6 b | 15.6 ± 2.6 b | 58.1 ± 4.7 ab | 45.5 ± 12.4 b | 98.2 ± 10.9 a | 43.2 ± 8.4 b |
| Octadecanoic acid | — | — | 8.2 ± 1.9 a | 7.6 ± 3.3 a | 22.9 ± 4.4 a | 21.5 ± 8.5 a | 53.0 ± 7.1 a | 26.7 ± 4.1 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, J.; Peng, H.; Zhang, W.; Yuan, L.; Liu, Y.; Kang, W.; Teng, B. Impact of Long-Term Bottle Aging on Color Transition, Polymers, and Aromatic Compounds in Mulberry Wine. Fermentation 2024, 10, 271. https://doi.org/10.3390/fermentation10060271
Cai J, Peng H, Zhang W, Yuan L, Liu Y, Kang W, Teng B. Impact of Long-Term Bottle Aging on Color Transition, Polymers, and Aromatic Compounds in Mulberry Wine. Fermentation. 2024; 10(6):271. https://doi.org/10.3390/fermentation10060271
Chicago/Turabian StyleCai, Jieling, Huihui Peng, Wanqin Zhang, Ling Yuan, Yang Liu, Wenyu Kang, and Bo Teng. 2024. "Impact of Long-Term Bottle Aging on Color Transition, Polymers, and Aromatic Compounds in Mulberry Wine" Fermentation 10, no. 6: 271. https://doi.org/10.3390/fermentation10060271






