The Correlation between Amino Acids and Biogenic Amines in Wines without Added Sulfur Dioxide
Abstract
:1. Introduction
2. Materials and Methods
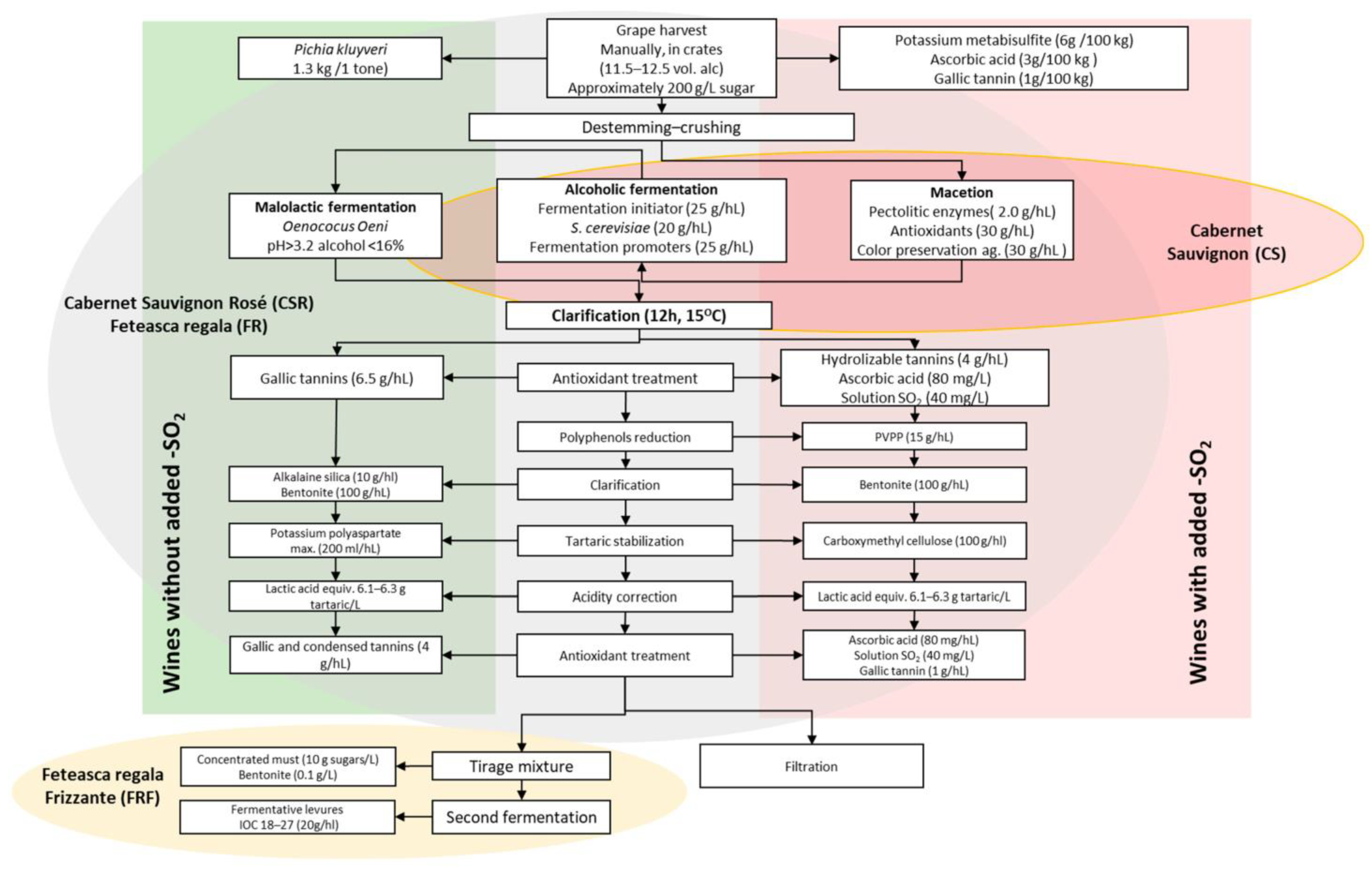
2.1. Wine Preparation Procedures
2.2. Reagents and Reference Materials
2.3. Chromatographic Conditions
2.4. Statistical Analysis
2.5. Sensory Analysis
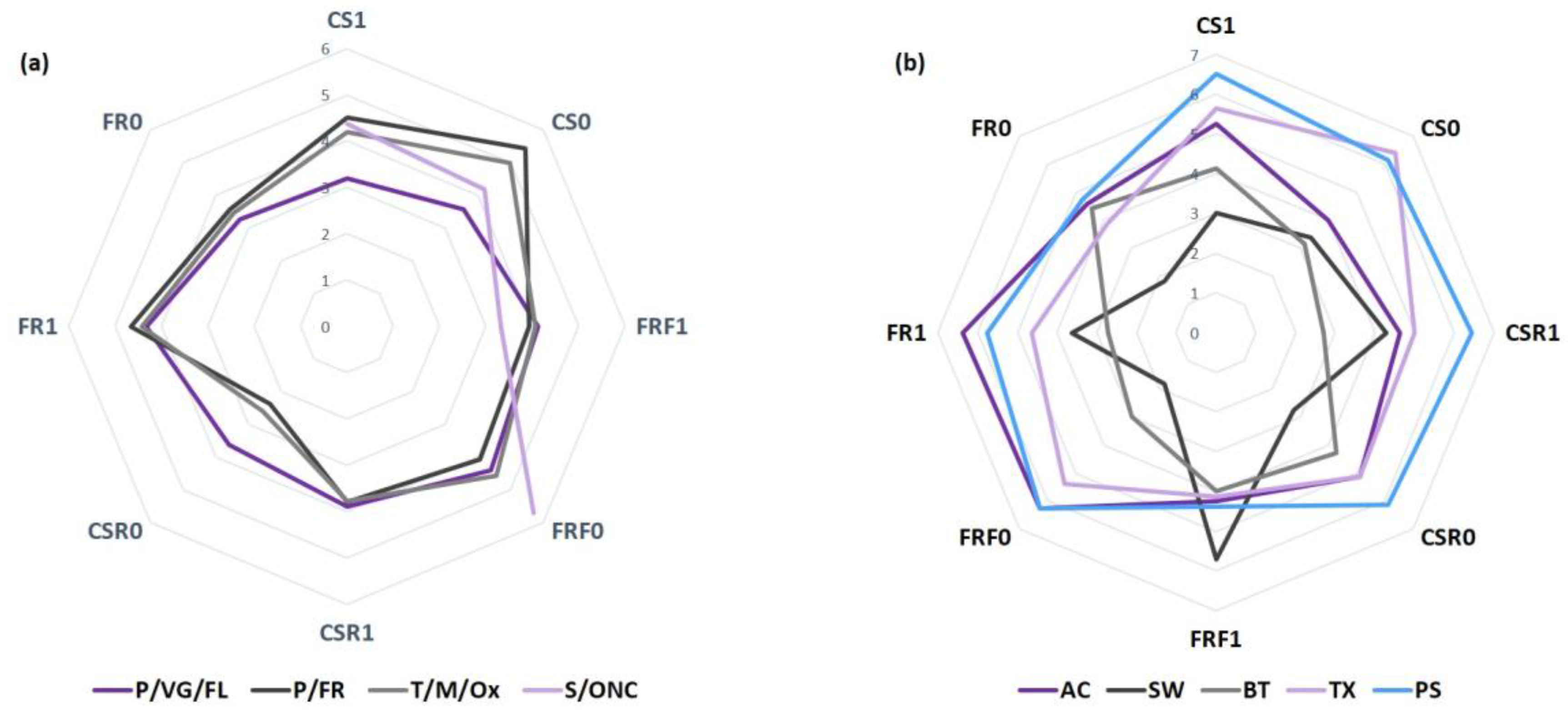
3. Results
3.1. Distribution of Amino Acids and Biogenic Amines in the Chromatographic Method
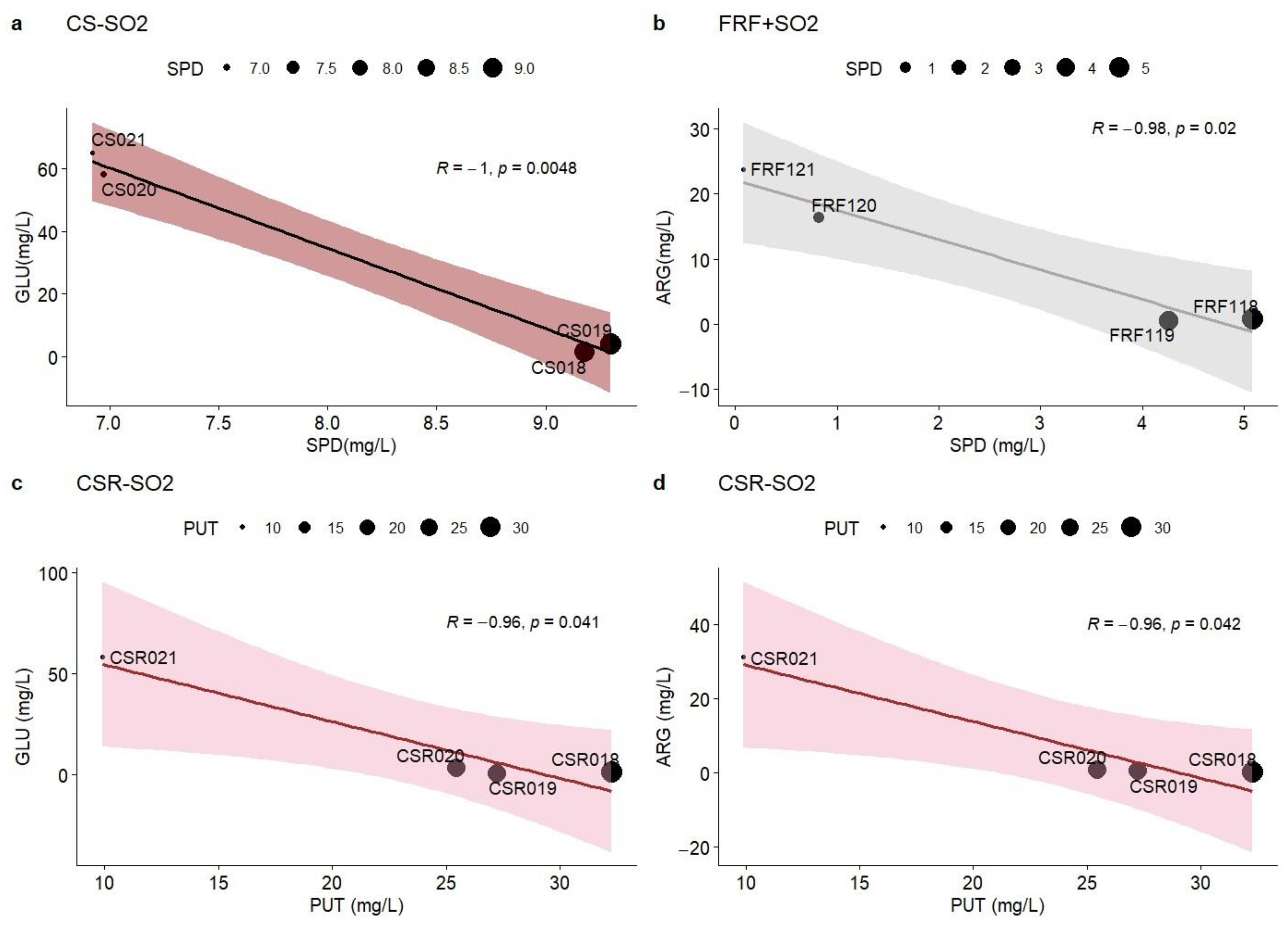
3.2. Amino Acids’ Concentration in Relation to Biogenic Amines
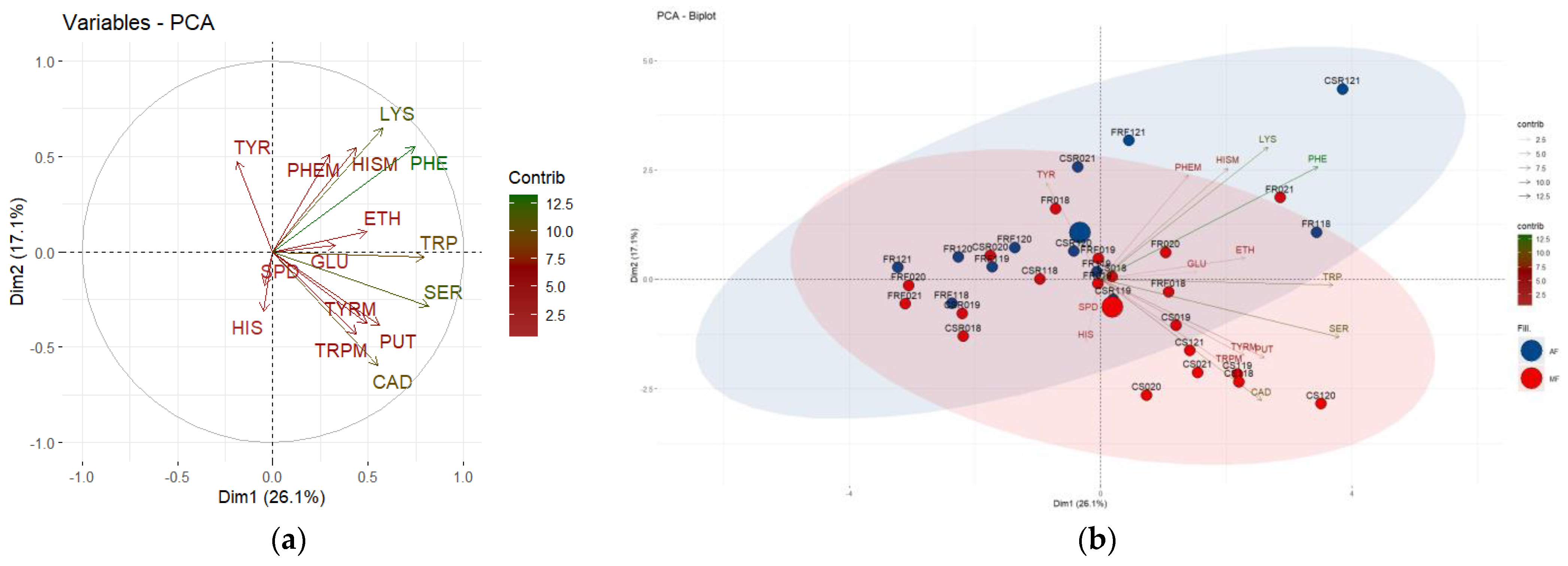
3.3. Principal Component Analysis
4. Discussion
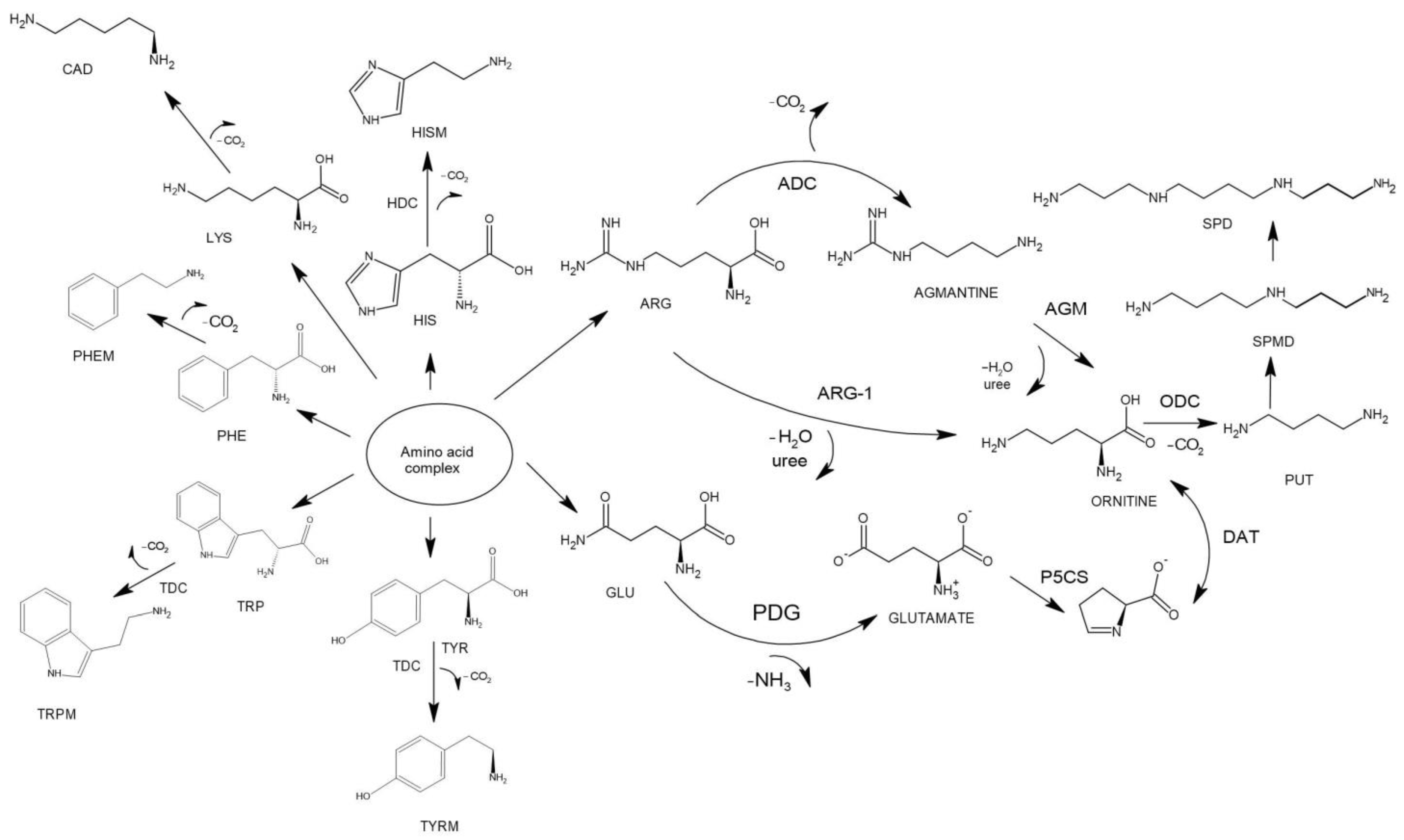
4.1. Proposed Mechanisms of Biogenic Amines’ Production in Wine Samples
4.2. Influence of Wine Stability on Production of Biogenic Amines
4.3. Sensory Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zamora, F. Biochemistry of Alcoholic Fermentation. In Wine Chemistry and Biochemistry; Victoria Moreno-Arribas, M., Carmen Polo, M., Eds.; Springer: New York, NY, USA, 2009; pp. 3–26. [Google Scholar]
- Cosme, F.; Vilela, A. Chitin and chitosan in the alcoholic and non-alcoholic beverage industry: An overview. Appl. Sci. 2021, 11, 11427. [Google Scholar] [CrossRef]
- López-Rituerto, E.; Avenoza, A.; Busto, J.H.; Peregrina, J.M. Evidence of metabolic transformations of amino acids into higher alcohols through 13c nmr studies of wine alcoholic fermentation. J. Agric. Food Chem. 2010, 58, 4923–4927. [Google Scholar] [CrossRef] [PubMed]
- Lilly, M.; Bauer, F.F.; Styger, G.; Lambrechts, M.G.; Pretorius, I.S. The effect of increased branched-chain amino acid transaminase activity in yeast on the production of higher alcohols and on the flavour profiles of wine and distillates. FEMS Yeast Res. 2006, 6, 726–743. [Google Scholar] [CrossRef] [PubMed]
- García-Ruiz, A.; González-Rompinelli, E.M.; Bartolomé, B.; Moreno-Arribas, M.V. Potential of wine-associated lactic acid bacteria to degrade biogenic amines. Int. J. Food Microbiol. 2011, 148, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, H. Alternative methods of sulfur dioxide used in wine production. J. Microbiol. Biotechnol. Food Sci. 2020, 9, 675–687. [Google Scholar] [CrossRef]
- Santos, M.C.; Nunes, C.; Saraiva, A.J.; Coimbra, J.A. Chemical and physical methodologies for the replacement/reduction of sulfur dioxide use during winemaking: Review of their potentialities and limitations. Eur. Food Res. Technol. 2012, 234, 1–12. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Yang, Y.P.; Peng, Q.; Han, Y. Biogenic amines in wine: A review. Int. J. Food Sci. Technol. 2015, 50, 1523–1532. [Google Scholar] [CrossRef]
- Stojanović, Z.; Kos, J. Detection of Metabolites of Microbial Origin in Beverages with Harmful Effect on Human Health—Biogenic Amines and Mycotoxins. In Safety Issues in Beverage Production; Grumezescu, A., Holban, A.M., Eds.; Elsevier: San Diego, CA, USA, 2020; pp. 39–77. [Google Scholar]
- Lonvaud-Funel, A. Biogenic amines in wines: Role of lactic acid bacteria. FEMS Microbiol. Lett. 2001, 199, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Casas-Godoy, L.; Arellano-Plaza, M.; Kirchmayr, M.; Barrera-Martínez, I.; Gschaedler-Mathis, A. Preservation of non-Saccharomyces yeasts: Current technologies and challenges. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3464–3503. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Vejarano, R.; González CCallejo, M.J.; Suárez-Lepe, J.A. Emerging preservation technologies in grapes for winemaking. Trends Food Sci. Technol. 2017, 67, 36–43. [Google Scholar] [CrossRef]
- Mendes Ferreira, A.; Mendes-Faia, A. The role of yeasts and lactic acid bacteria on the metabolism of organic acids during winemaking. Foods 2020, 9, 1231. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.; Pereira, A.C.; Pérez Trujillo, J.P.; Cacho, J.; Marques, J.C. Amino acids and biogenic amines evolution during the estufagem of fortified wines. J. Chem. 2015, 2015, 494285. [Google Scholar] [CrossRef]
- Scutarașu, E.C.; Luchian, C.E.; Cioroiu, I.B.; Trincă, L.C.; Cotea, V.V. Increasing amino acids content of white wines with enzymes treatments. Agronomy 2022, 12, 1406. [Google Scholar] [CrossRef]
- Tedesco, F.; Siesto, G.; Pietrafesa, R.; Romano, P.; Salvia, R.; Scieuzo, C.; Falabella, P.; Capece, A. Chemical methods for microbiological control of winemaking: An overview of current and future applications. Beverages 2022, 8, 58. [Google Scholar] [CrossRef]
- Costantini, A.; Vaudano, E.; Pulcini, L.; Carafa, T.; Garcia-Moruno, E. An overview on biogenic amines in wine. Beverages 2019, 5, 19. [Google Scholar] [CrossRef]
- Henríquez-Aedoa, K.; Galarce-Bustos, O.; Aqueveque, P.; García, A.; Aranda, M. Dynamic of biogenic amines and precursor amino acids during Cabernet Sauvignon vinification. LWT 2018, 97, 238–244. [Google Scholar] [CrossRef]
- Smit, A.Y.; du Toit, W.J.; du Toit, M. Biogenic amines in wine: Understanding the headache. South Afr. J. Enol. Vitic. 2008, 29, 109–127. [Google Scholar] [CrossRef]
- Cruzat, V.; Rogero, M.M.; Keane, K.N.; Curi, R.; Newsholme, P. Glutamine: Metabolism and immune function, supplementation and clinical translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Clemente, G.S.; van Waarde, A.; Antunes, I.F.; Dömling, A.; Elsinga, P.H. Arginase as a potential biomarker of disease progression: A molecular imaging perspective. Int. J. Mol. Sci. 2020, 21, 5291. [Google Scholar] [CrossRef]
- Ayestaran, B.; Acin, C.; Corroza, M.; Garrido, J. Changes in free amino acid concentration during stabilization and aging of wines derived from garnacha and viura musts clarified by static sedimentation. Food Control 1996, 7, 157–163. [Google Scholar] [CrossRef]
- González-Royo, E.; Pascual, O.; Kontoudakis, N.; Esteruelas, M.; Esteve-Zarzoso, B.; Mas, A.; Canals, J.M.; Zamora, F. Oenological consequences of sequential inoculation with non-Saccharomyces yeasts (Torulaspora delbrueckii or Metschnikowia pulcherrima) and Saccharomyces cerevisiae in base wine for sparkling wine production. Eur. Food Res. Technol. 2015, 240, 999–1012. [Google Scholar] [CrossRef]
- Doeun, D.; Davaatseren, M.; Chung, M.S. Biogenic amines in foods. Food Sci. Biotechnol. 2017, 26, 1463–1474. [Google Scholar] [CrossRef] [PubMed]
- Héberger, K.; Csomós, E.; Simon-Sarkadi, L. Principal component and linear discriminant analyses of free amino acids and biogenic amines in hungarian wines. J. Agric. Food Chem. 2003, 51, 8055–8060. [Google Scholar] [CrossRef] [PubMed]
- Płotka-Wasylka, J.; Simeonov, V.; Morrison, C.; Namieśnik, J. Impact of selected parameters of the fermentation process of wine and wine itself on the biogenic amines content: Evaluation by application of chemometric tools. Microchem. J. 2018, 142, 187–194. [Google Scholar] [CrossRef]
- Arena, M.E.; Landete, J.M.; Manca de Nadra, M.C.; Pardo, I.; Ferrer, S. Factors affecting the production of putrescine from agmatine by Lactobacillus hilgardii X1B isolated from wine. J. Appl. Microbiol. 2008, 105, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Anlı, R.E.; Bayram, M. Biogenic amines in wines. Food Rev. Int. 2009, 25, 86–102. [Google Scholar] [CrossRef]
- Paramithiotis, S.; Stasinou, V.; Tzamourani, A.; Kotseridis, Y.; Dimopoulou, M. Malolactic fermentation—Theoretical advances and practical considerations. Fermentation 2022, 8, 521. [Google Scholar] [CrossRef]
- Esposito, F.; Montuori, P.; Schettino, M.; Velotto, S.T.; Stasi SRomano, R.; Cirillo, T. Level of biogenic amines in red and white wines, dietary exposure and histamine-mediated symptoms upon wine ingestion. Molecules 2019, 24, 3629. [Google Scholar] [CrossRef] [PubMed]
- Beneduce, L.; Romano, A.; Capozzi, V.; Lucas, P.; Barnavon, L.; Bach, B.; Vuchot, P.; Grieco, F.; Spano, G. Biogenic amine in wines. Ann. Microbiol. 2010, 60, 573–578. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, C.; Gonçalves, C.; Castillo, M.; Câmara, J.S. An approach of the Madeira wine chemistry. Beverages 2020, 6, 12. [Google Scholar] [CrossRef]
- Jacob, F.F.; Striegel, L.; Rychlik, M.; Hutzler, M.; Methner, F.J. Spent yeast from brewing processes: A biodiverse starting material for yeast extract production. Fermentation 2019, 5, 51. [Google Scholar] [CrossRef]
- Karamanidou, A.; Kallithraka, S.; Hatzidimitriou, E. Fining of red wines: Effects on their analytical and sensory parameters. J. Int. Sci. Vigne. Vin. 2010, 45, 47–60. [Google Scholar] [CrossRef]
- Buxaderas, S.; López-Tamames, E. Sparkling wines: Features and trends from tradition. Adv. Food Nutr. 2012, 66, 1–45. [Google Scholar]
- Espinase Nandorfy, D.; Watson, F.; Likos, D.; Siebert, T.; Bindon, K.; Kassara, S.; Shellie, R.; Keast, R.; Francis, I.L. Influence of amino acids, and their interaction with volatiles and polyphenols, on the sensory properties of red wine. Aust. J. Grape Wine Res. 2022, 28, 621–637. [Google Scholar] [CrossRef]
- Dimopoulou, M.; Troianou, V.; Paramithiotis, S.; Proksenia, N.; Kotseridis, Y. Evaluation of malolactic starters in white and rosé winemaking of Moschofilero wines. Appl. Sci. 2022, 12, 5722. [Google Scholar] [CrossRef]
- Prida, A.; Chatonnet, P. Impact of oak-derived compounds on the olfactory perception of barrel-aged wines. Am. J. Enol. Vitic. 2010, 61, 408–413. [Google Scholar] [CrossRef]
- Lloret, A.; Boido, E.; Lorenzo, D.; Medina, K.; Carrau, F.; Dellacassa, E. Aroma variation in Tannat wines: Effect of malolactic fermentation on ethyl lactate level and its enantiomeric distribution. IJFS 2002, 14, 175–180. [Google Scholar]
- Ferreira, A.C.S.; De Pinho, P.G.; Rodrigues, P.; Hogg, T. Kinetics of oxidative degradation of white wines and how they are affected by selected technological parameters. J. Agric. Food Chem. 2002, 50, 5919–5924. [Google Scholar] [CrossRef]


| [M + H]+ (m/z) | [M − N]+ (m/z) | CE (V) | Retention Time (min) | |
|---|---|---|---|---|
| L-Serine (SER) | 106.1 | 60.18 | 10 | 1.67 |
| L-glutamine (GLU) | 147.21 | 84.11 | 7 | 1.67 |
| L-Phenylalanine (PHE) | 166.1 | 120.16 | 10 | 6.71 |
| L-Tryptophan (TRP) | 205.1 | 146.02 | 10 | 8.30 |
| L-Tyrosine (TYR) | 182.19 | 91.05 | 27 | 4.01 |
| L-Lysine (LYS) | 147.19 | 84.11 | 15 | 2.89 |
| L-Arginine (ARG) | 175.2 | 70.2 | 11 | 2.52 |
| L-Histidine (HIS) | 156.1 | 110.5 | 10 | 3.22 |
| Phenylamine (PHEM) | 120.2 | 102.06 | 14 | 8.91 |
| Tryptamine (TRPM) | 161.3 | 144.07 | 10 | 7.72 |
| Ethanolamine (ETH) | 177.25 | 160.05 | 8 | 4.27 |
| Tyramine (TYRM) | 138.1 | 121.12 | 8 | 3.85 |
| Histamine (HISM) | 112.1 | 95.1 | 12 | 3.71 |
| Putrescine (PUT) | 89.15 | 72.21 | 8 | 3.48 |
| Cadaverine (CAD) | 103.1 | 86.14 | 7 | 3.62 |
| Spermidine (SPD) | 203.2 | 112.12 | 10 | 9.92 |
| T | V. | ETH | SER | PUT | GLU | TYRM | TYR | CAD | LYS | HYSM | HYS | PHEM | PHE | TRPM | TRP | SPD | ARG |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MF | CS1 | 12.31 ± 1.8 | 20.18 ± 2.9 | 35.91 ± 2.3 | 14.29 ± 12.9 | 14.62 ± 1.5 | 1.19 ± 0.8 | 4.44 ± 1.0 | 22.61 ± 2.9 | 0.63 ± 0.0 | 67.66 ± 0.6 | 0.12 ± 0.02 | 13.82 ± 1.0 | 0.06 ± 0.02 | 4.42 ± 0.4 | 7.27 ± 0.1 | 9.42 ± 7.4 |
| AF | CSR1 | 9.51 ± 1.9 | 10.02 ± 1.5 | 17.01 ± 2.4 | 13.67 ± 10.9 | 1.97 ± 1.5 | 2.87 ± 1.3 | 1.44 ± 0.6 | 41.54 ± 9.0 | 2.00 ± 1.6 | 67.60 ± 0.5 | 0.15 ± 0.1 | 18.65 ± 3.9 | 0.06 ± 0.02 | 2.73 ± 0.7 | 5.49 ± 1.4 | 12.40 ± 9.2 |
| AF | FR1 | 10.03 ± 2.9 | 10.13 ± 4.2 | 23.77 ± 5.5 | 4.38 ± 2.3 | 0.12 ± 0.04 | 3.86 ± 1.6 | 0.55 ± 0.2 | 26.85 ± 7.5 | 2.67 ± 2.2 | 68.64 ± 2.3 | 0.17 ± 0.0 | 12.04 ± 3.4 | 0.04 ± 0.0 | 2.88 ± 1.3 | 9.79 ± 7.2 | 5.37 ± 1.8 |
| AF | FRF1 | 12.78 ± 2.3 | 7.13 ± 1.8 | 20.78 ± 3.2 | 2.22 ± 1.0 | 0.43 ± 0.3 | 4.48 ± 1.3 | 0.28 ± 0.0 | 24.13 ± 6.5 | 0.44 ± 0.1 | 66.79 ± 1.1 | 0.15 ± 0.1 | 12.43 ± 2.8 | 0.03 ± 0.01 | 1.98 ± 0.8 | 2.56 ± 1.2 | 10.40 ± 5.8 |
| MF | CS0 | 13.18 ± 2.6 | 11.73 ± 1.9 | 31.25 ± 2.9 | 32.19 ± 17.1 | 4.85 ± 3.2 | 2.52 ± 0.6 | 4.36 ± 1.2 | 23.15 ± 8.0 | 0.85 ± 0.1 | 68.03 ± 1.0 | 0.09 ± 0.01 | 13.28 ± 2.9 | 0.06 ± 0.01 | 2.83 ± 0.8 | 8.09 ± 0.7 | 15.16 ± 8.0 |
| MF | CSR0 | 6.06 ± 2.3 | 5.25 ± 0.6 | 23.72 ± 4.8 | 15.63 ± 14.2 | 0.59 ± 0.2 | 4.53 ± 1.2 | 0.92 ± 0.3 | 24.06 ± 4.0 | 1.08 ± 0.6 | 64.83 ± 4.1 | 0.09 ± 0.02 | 11.03 ± 2.1 | 0.04 ± 0.01 | 1.37 ± 0.8 | 4.05 ± 1.7 | 8.14 ± 7.7 |
| MF | FR0 | 11.93 ± 1.3 | 13.67 ± 2.3 | 26.26 ± 7.0 | 8.94 ± 6.5 | 0.06 ± 0.06 | 5.79 ± 2.1 | 1.28 ± 0.5 | 41.87 ± 5.3 | 2.33 ± 2.0 | 66.96 ± 1.4 | 0.14 ± 0.01 | 18.20 ± 2.7 | 0.05 ± 0.0 | 2.24 ± 0.7 | 2.94 ± 0.9 | 8.03 ± 4.2 |
| MF | FRF0 | 14.58 ± 1.3 | 6.85 ± 3.6 | 15.86 ± 4.7 | 1.32 ± 0.3 | 0.25 ± 0.1 | 3.77 ± 1.0 | 1.48 ± 0.8 | 13.21 ± 2.6 | 0.17 ± 0.0 | 70.97 ± 0.6 | 0.21 ± 0.1 | 9.79 ± 3.2 | 0.05 ± 0.02 | 0.85 ± 0.1 | 0.99 ± 0.2 | 13.13 ± 5.4 |
| YEAR | ETH | SER | PUT | GLU | TYRM | TYR | CAD | LYS | HISM | HIS | PHEM | PHE | TRPM | TRP | SPD | ARG |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2018 | 9.05 ± 1.70 | 11.38 ± 2.4 | 27.78 ± 4.5 | 1.21 ± 0.1 | 3.39 ± 2.1 | 3.35 ± 0.8 | 1.92 ± 0.6 | 28.53 ± 3.9 | 2.51 ± 1.3 | 69.00 ± 0.8 | 0.14 ± 0.03 | 14.49 ± 1.8 | 0.06 ± 0.01 | 2.07 ± 0.8 | 4.84 ± 1.1 | 4.13 ± 3.1 |
| 2019 | 12.42 ± 1.4 | 11.34 ± 1.4 | 26.77 ± 2.2 | 2.09 ± 0.5 | 2.18 ± 1.5 | 2.77 ± 0.5 | 2.47 ± 0.7 | 26.55 ± 3.0 | 0.47 ± 0.1 | 68.37 ± 0.5 | 0.13 ± 0.03 | 13.11 ± 1.1 | 0.05 ± 0.002 | 2.04 ± 0.4 | 4.73 ± 1.1 | 3.51 ± 2.2 |
| 2020 | 10.43 ± 1.4 | 10.68 ± 2.9 | 22.35 ± 2.9 | 9.54 ± 6.9 | 2.42 ± 1.9 | 3.27 ± 0.7 | 1.57 ± 0.7 | 23.62 ± 4.1 | 0.51 ± 0.1 | 67.97 ± 1.1 | 0.13 ± 0.02 | 11.77 ± 1.6 | 0.04 ± 0.01 | 2.38 ± 0.5 | 7.58 ± 3.5 | 10.35 ± 3.0 |
| 2021 | 13.30 ± 1.5 | 9.07 ± 2.3 | 20.37 ± 4.0 | 33.47 ± 9.0 | 3.46 ± 2.2 | 5.11 ± 1.4 | 1.41 ± 0.7 | 30.00 ± 8.2 | 1.59 ± 0.7 | 65.39 ± 2.0 | 0.15 ± 0.03 | 15.25 ± 3.3 | 0.05 ± 0.01 | 3.16 ± 0.4 | 3.42 ± 1.1 | 23.04 ± 4.5 |
| CS | CSR | FR | FRF | |||||
|---|---|---|---|---|---|---|---|---|
| (r/p) | −SO2 | +SO2 | −SO2 | +SO2 | −SO2 | +SO2 | −SO2 | +SO2 |
| SPD:ARG | −0.991 * | 0.300 | 0.561 | −0.915 | −0.991 * | 0.363 | 0.201 | −0.980 * |
| 0.01 | 0.70 | 0.44 | 0.09 | 0.01 | 0.64 | 0.80 | 0.02 | |
| TRPM:TRP | 0.956 * | −0.814 | −0.763 | −0.995 * | 0.999 * | 0.909 | 0.903 | −0.736 |
| 0.04 | 0.19 | 0.24 | 0.01 | 0.00 | 0.09 | 0.10 | 0.26 | |
| PHEM:PHE | 0.862 | −0.408 | 0.694 | 0.682 | −0.189 | 0.601 | 0.998 * | 0.912 |
| 0.14 | 0.59 | 0.31 | 0.32 | 0.81 | 0.40 | 0.00 | 0.09 | |
| HYSM:HYS | −0.376 | −0.632 | −0.987 * | −0.247 | 0.390 | 0.563 | −0.857 | 0.453 |
| 0.62 | 0.37 | 0.01 | 0.75 | 0.61 | 0.44 | 0.14 | 0.55 | |
| CAD:LYS | −0.786 | 0.919 | −0.508 | 0.499 | −0.906 | 0.964 * | 0.998 * | −0.593 |
| 0.21 | 0.08 | 0.49 | 0.50 | 0.09 | 0.04 | 0.00 | 0.41 | |
| TYRM:TYR | 0.742 | 0.768 | 0.915 | −0.652 | −0.859 | 0.861 | 0.176 | 0.493 |
| 0.26 | 0.23 | 0.08 | 0.35 | 0.14 | 0.14 | 0.82 | 0.51 | |
| ETH:SER | 0.703 | −0.047 | 0.625 | −0.403 | 0.385 | 0.939 | 0.823 | 0.919 |
| 0.30 | 0.95 | 0.38 | 0.60 | 0.62 | 0.06 | 0.18 | 0.08 | |
| PUT:GLU | −0.052 | −0.747 | −0.883 | −0.256 | 0.555 | −0.757 | 0.767 | −0.909 |
| 0.95 | 0.25 | 0.12 | 0.74 | 0.45 | 0.24 | 0.23 | 0.09 | |
| Variety | AC | TA | VA | SO2 Free | SO2 Total | RS | pH | |
|---|---|---|---|---|---|---|---|---|
| CS | +SO2 | 13.4 ± 0.07 | 6.7 ± 1.01 | 0.43 ± 0.06 | 20.3 ± 0.28 | 49.2 ± 1.06 | 4.5 ± 0.73 | 3.6 ± 0.01 |
| −SO2 | 13.4 ± 0.20 | 6.6 ± 0.32 | 0.66 ± 0.03 | 4.9 ± 0.11 | 10.1 ± 0.29 | 2.9 ± 0.90 | 3.1 ± 0.02 | |
| CSr | +SO2 | 12.3 ± 0.95 | 6.9 ± 0.90 | 0.47 ± 0.07 | 41.0 ± 1.7 | 50.6 ± 11.54 | 9.0 ± 0.60 | 3.1 ± 0.06 |
| −SO2 | 13.6 ± 0.03 | 6.1 ± 0.07 | 0.63 ± 0.07 | 4.7 ± 0.3 | 10.2 ± 0.35 | 0.7 ± 0.01 | 3.1 ± 0.03 | |
| FR | +SO2 | 12.1 ± 0.56 | 6.1 ± 0.03 | 0.48 ± 0.04 | 38.5 ± 0.7 | 50.5 ± 4.95 | 8.0 ± 1.31 | 3.1 ± 0.04 |
| −SO2 | 12.2 ± 0.07 | 6.1 ± 0.10 | 0.34 ± 0.01 | 7.5 ± 3.5 | 12.0 ± 2.82 | 1.6 ± 0.03 | 3.2 ± 0.07 | |
| FRF | +SO2 | 12.9 ± 0.42 | 6.0 ± 0.74 | 0.37 ± 0.18 | 36.0 ± 1.8 | 56.5 ± 9.19 | 1.8 ± 0.04 | 3.2 ± 0.05 |
| −SO2 | 11.3 ± 0.70 | 6.1 ± 0.03 | 0.34 ± 0.04 | 4.9 ± 0.21 | 10.5 ± 0.70 | 0.7 ± 0.35 | 3.2 ± 0.07 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macoviciuc, S.; Niculaua, M.; Nechita, C.-B.; Cioroiu, B.-I.; Cotea, V.V. The Correlation between Amino Acids and Biogenic Amines in Wines without Added Sulfur Dioxide. Fermentation 2024, 10, 302. https://doi.org/10.3390/fermentation10060302
Macoviciuc S, Niculaua M, Nechita C-B, Cioroiu B-I, Cotea VV. The Correlation between Amino Acids and Biogenic Amines in Wines without Added Sulfur Dioxide. Fermentation. 2024; 10(6):302. https://doi.org/10.3390/fermentation10060302
Chicago/Turabian StyleMacoviciuc, Sorin, Marius Niculaua, Constantin-Bogdan Nechita, Bogdan-Ionel Cioroiu, and Valeriu V. Cotea. 2024. "The Correlation between Amino Acids and Biogenic Amines in Wines without Added Sulfur Dioxide" Fermentation 10, no. 6: 302. https://doi.org/10.3390/fermentation10060302
APA StyleMacoviciuc, S., Niculaua, M., Nechita, C. -B., Cioroiu, B. -I., & Cotea, V. V. (2024). The Correlation between Amino Acids and Biogenic Amines in Wines without Added Sulfur Dioxide. Fermentation, 10(6), 302. https://doi.org/10.3390/fermentation10060302










