Fed-Batch Bioreactor Cultivation of Bacillus subtilis Using Vegetable Juice as an Alternative Carbon Source for Lipopeptides Production: A Shift towards a Circular Bioeconomy
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Media
2.2. Shake Flask Cultivation
2.3. Fed-Batch Cultivation
2.4. Lipopeptides Extraction
2.5. LC-MS/MS Analyses of Lipopeptides
2.6. Other Analytical Analysis
2.7. Data Analysis
3. Results and Discussion
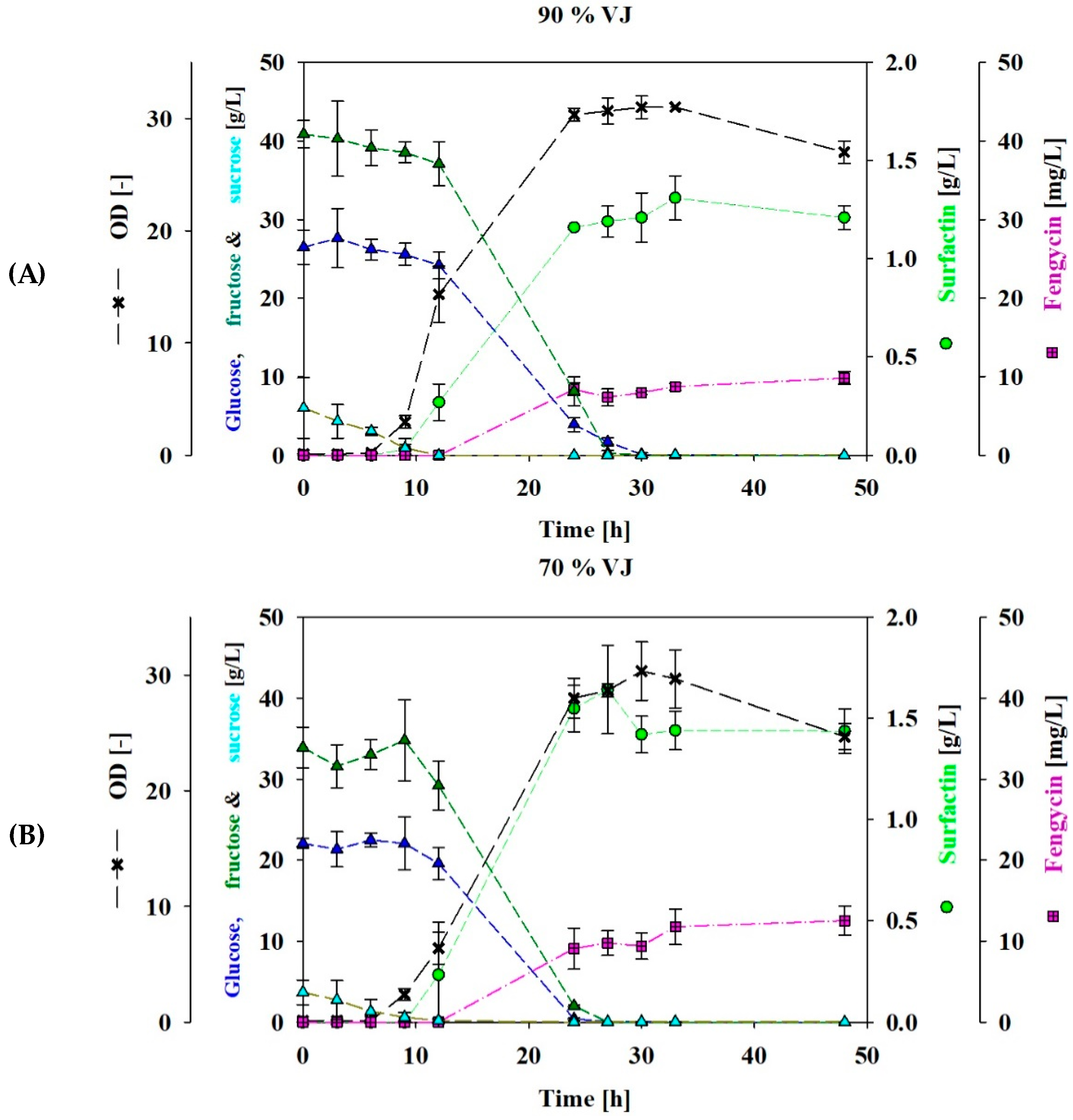
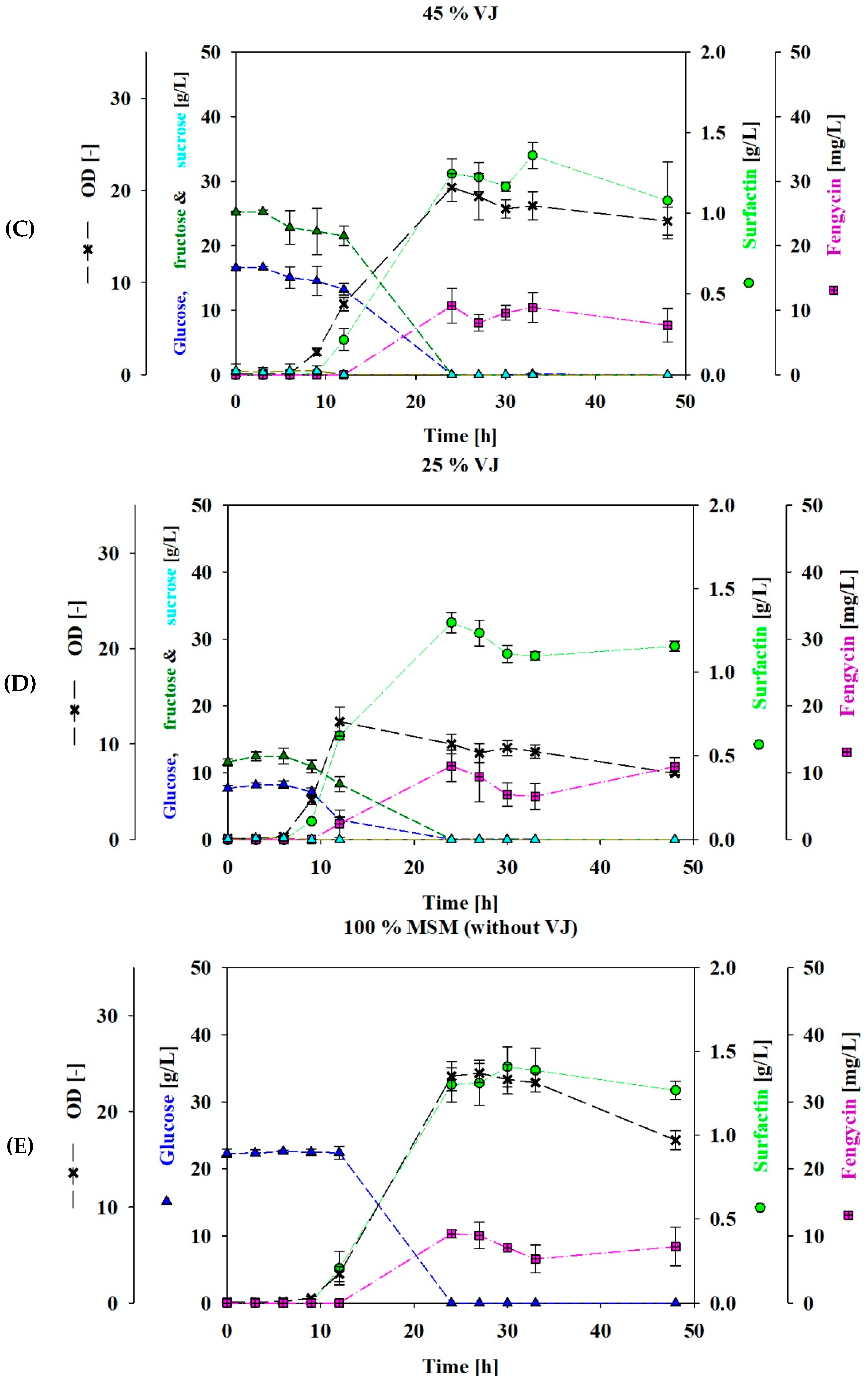
3.1. Shake Flasks Cultivation Using VJ as a Carbon Source
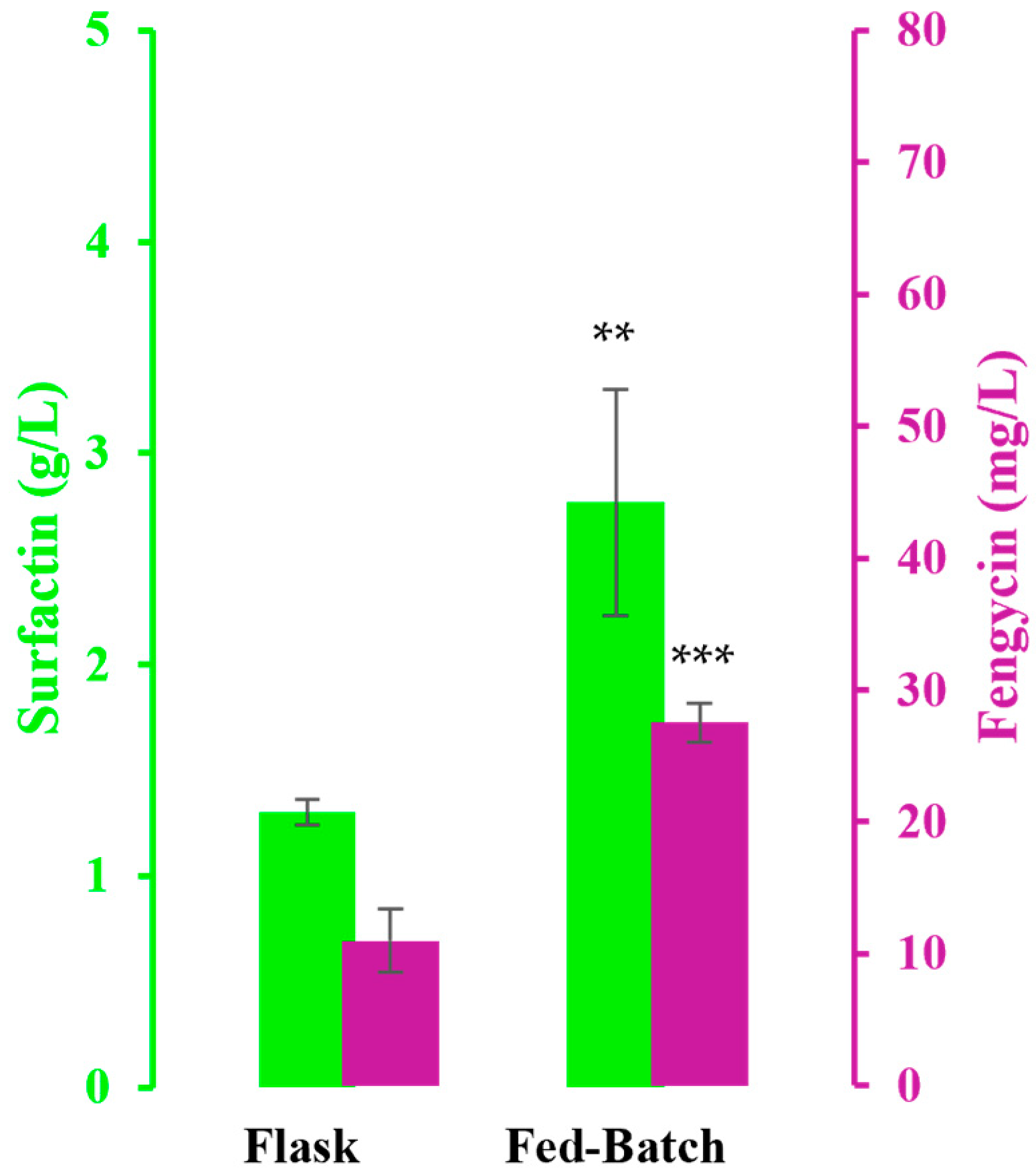
3.2. Fed-Batch Bioreactor Cultivation
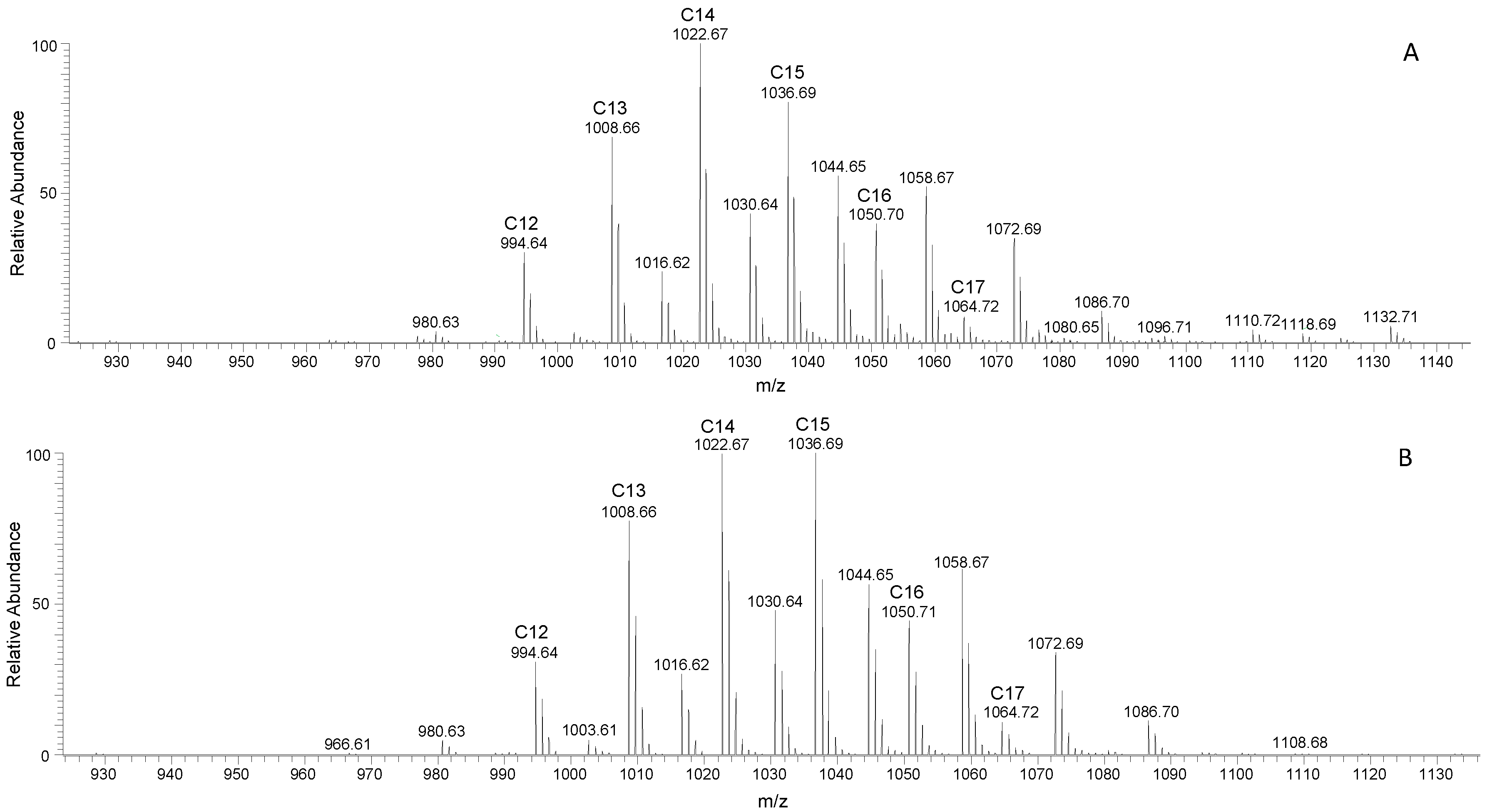
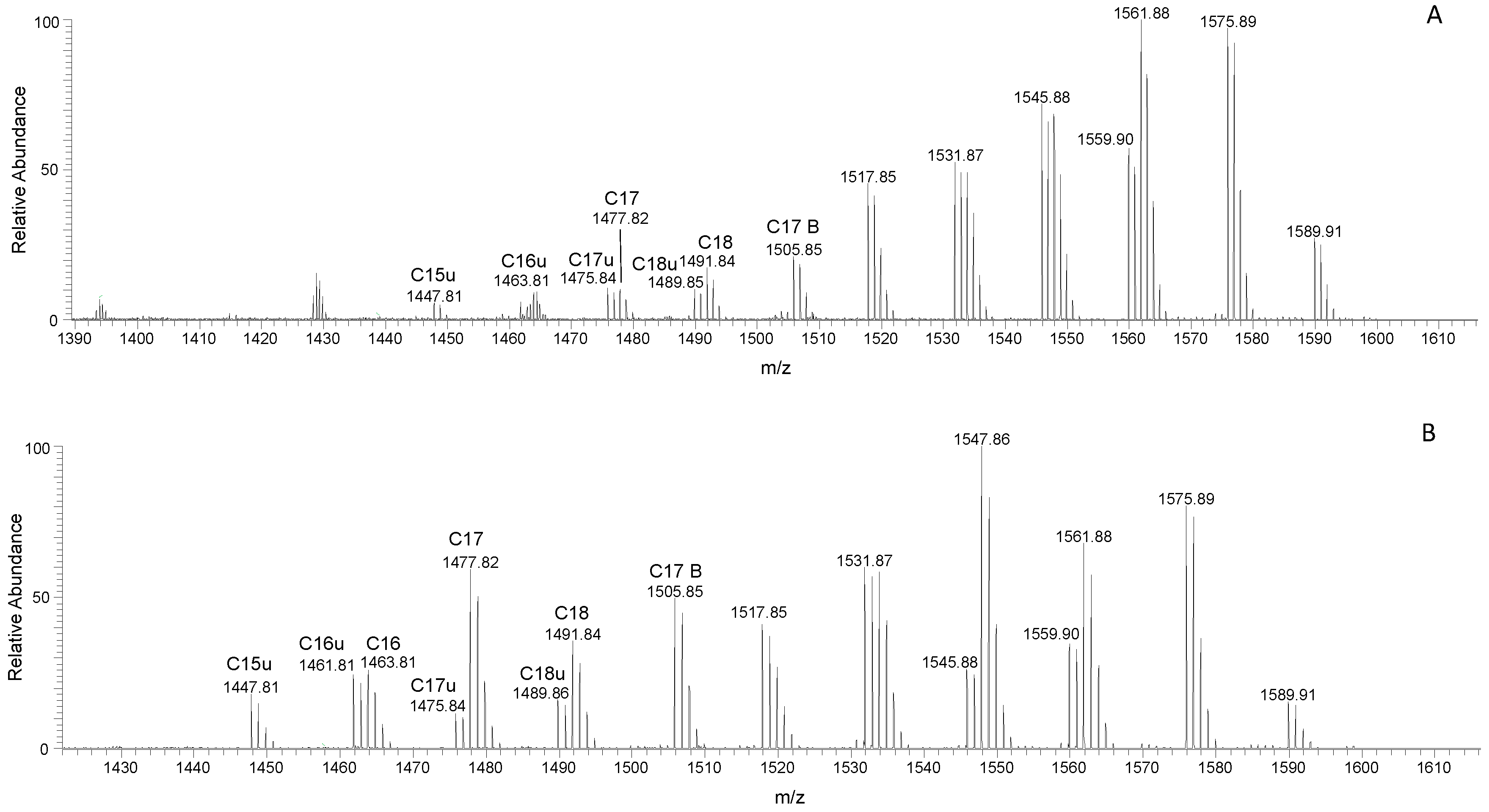
3.3. Surfactin and Fengycin Characterization through LC-MS/MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaur, J.; Rani, G.; Yogalakshmi, K. Problems and Issues of Food Waste-Based Biorefineries. In Food Waste to Valuable Resources; Elsevier: Amsterdam, The Netherlands, 2020; pp. 343–357. [Google Scholar]
- D’Adamo, I.; Desideri, S.; Gastaldi, M.; Tsagarakis, K.P. Sustainable Food Waste Management in Supermarkets. Sustain. Prod. Consum. 2023, 43, 204–216. [Google Scholar] [CrossRef]
- Melikoglu, M.; Lin, C.S.K.; Webb, C. Analysing Global Food Waste Problem: Pinpointing the Facts and Estimating the Energy Content. Cent. Eur. J. Eng. 2013, 3, 157–164. [Google Scholar] [CrossRef]
- Bigdeloo, M.; Teymourian, T.; Kowsari, E.; Ramakrishna, S.; Ehsani, A. Sustainability and Circular Economy of Food Wastes: Waste Reduction Strategies, Higher Recycling Methods, and Improved Valorization. Mater. Circ. Econ. 2021, 3, 3. [Google Scholar] [CrossRef]
- Rohini, C.; Geetha, P.; Vijayalakshmi, R.; Mini, M.; Pasupathi, E. Global Effects of Food Waste. J. Pharmacogn. Phytochem. 2020, 9, 690–699. [Google Scholar]
- Jones, R.; Speight, R.; Blinco, J.; O’Hara, I. Biorefining within Food Loss and Waste Frameworks: A Review. Renew. Sustain. Energy Rev. 2022, 154, 111781. [Google Scholar] [CrossRef]
- UNEP DTU Partnership and United Nations Environment Programme. Reducing Consumer Food Waste Using Green and Digital Technologies; UNEP DTU Partnership: Copenhagen, Denmark; Nairobi, Kenya, 2021. [Google Scholar]
- De Laurentiis, V.; Mancini, L.; Casonato, C.; Boysen-Urban, K.; De Jong, B.; M’Barek, R.; Sanyé Mengual, E.; Sala, S. Setting the Scene for an EU Initiative on Food Waste Reduction Targets. Publ. Off. Eur. Union Luxemb. Doi 2023, 10, 13859. [Google Scholar]
- Scialabba, N.E. Food Wastage Footprint Full-Cost Accounting: Final Report; FAO: Rome, Italy, 2014; ISBN 978-92-5-108512-7. Available online: http://www.fao.org/3/a-i3991e.pdf (accessed on 6 October 2020).
- Brancoli, P.; Rousta, K.; Bolton, K. Life Cycle Assessment of Supermarket Food Waste. Resour. Conserv. Recycl. 2017, 118, 39–46. [Google Scholar] [CrossRef]
- Mattsson, L.; Williams, H.; Berghel, J. Waste of Fresh Fruit and Vegetables at Retailers in Sweden–Measuring and Calculation of Mass, Economic Cost and Climate Impact. Resour. Conserv. Recycl. 2018, 130, 118–126. [Google Scholar] [CrossRef]
- Eriksson, M.; Strid, I.; Hansson, P.-A. Food Losses in Six Swedish Retail Stores: Wastage of Fruit and Vegetables in Relation to Quantities Delivered. Resour. Conserv. Recycl. 2012, 68, 14–20. [Google Scholar] [CrossRef]
- Scholz, K.; Eriksson, M.; Strid, I. Carbon Footprint of Supermarket Food Waste. Resour. Conserv. Recycl. 2015, 94, 56–65. [Google Scholar] [CrossRef]
- Papargyropoulou, E.; Lozano, R.; Steinberger, J.K.; Wright, N.; bin Ujang, Z. The Food Waste Hierarchy as a Framework for the Management of Food Surplus and Food Waste. J. Clean. Prod. 2014, 76, 106–115. [Google Scholar] [CrossRef]
- Liakou, V.; Pateraki, C.; Palaiogeorgou, A.-M.; Kopsahelis, N.; de Castro, A.M.; Freire, D.M.G.; Nychas, G.-J.E.; Papanikolaou, S.; Koutinas, A. Valorisation of Fruit and Vegetable Waste from Open Markets for the Production of 2, 3-Butanediol. Food Bioprod. Process. 2018, 108, 27–36. [Google Scholar] [CrossRef]
- Mitrea, L.; Calinoiu, L.F.; Teleky, B.E.; Szabo, K.; Martău, A.G.; Nemes, S.A.; Plamada, D.; Pascuta, M.S.; Barta, G.; Varvara, R.A. Fruit and Vegetable Wastes for Biobased Chemicals. In Fruit and Vegetable Waste Utilization and Sustainability; Elsevier: Amsterdam, The Netherlands, 2023; pp. 43–76. [Google Scholar]
- Dahiya, S.; Kumar, A.N.; Sravan, J.S.; Chatterjee, S.; Sarkar, O.; Mohan, S.V. Food Waste Biorefinery: Sustainable Strategy for Circular Bioeconomy. Bioresour. Technol. 2018, 248, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Magama, P.; Chiyanzu, I.; Mulopo, J. A Systematic Review of Sustainable Fruit and Vegetable Waste Recycling Alternatives and Possibilities for Anaerobic Biorefinery. Bioresour. Technol. Rep. 2022, 18, 101031. [Google Scholar] [CrossRef]
- Nagarajan, D.; Lee, D.-J.; Chang, J.-S. Circular Bioeconomy: An Introduction. In Biomass, Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2021; pp. 3–23. [Google Scholar]
- Tan, E.C.; Lamers, P. Circular Bioeconomy Concepts—A Perspective. Front. Sustain. 2021, 2, 701509. [Google Scholar] [CrossRef]
- Gaur, V.K.; Sharma, P.; Sirohi, R.; Varjani, S.; Taherzadeh, M.J.; Chang, J.-S.; Ng, H.Y.; Wong, J.W.; Kim, S.-H. Production of Biosurfactants from Agro-Industrial Waste and Waste Cooking Oil in a Circular Bioeconomy: An Overview. Bioresour. Technol. 2022, 343, 126059. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.S.; Koul, Y.; Varjani, S.; Pandey, A.; Ngo, H.H.; Chang, J.-S.; Wong, J.W.; Bui, X.-T. A Critical Review on Various Feedstocks as Sustainable Substrates for Biosurfactants Production: A Way towards Cleaner Production. Microb. Cell Factories 2021, 20, 120. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, B.; Cai, Q.; Ling, J.; Lee, K.; Chen, B. Fish Waste Based Lipopeptide Production and the Potential Application as a Bio-Dispersant for Oil Spill Control. Front. Bioeng. Biotechnol. 2020, 8, 734. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, C.; Shi, Y.; Huang, M.; Song, Z.; Simal-Gandara, J.; Li, N.; Shi, J. Classification, Application, Multifarious Activities and Production Improvement of Lipopeptides Produced by Bacillus. Crit. Rev. Food Sci. Nutr. 2023, 1–14. [Google Scholar] [CrossRef]
- Hamdache, A.; Azarken, R.; Lamarti, A.; Aleu, J.; Collado, I.G. Comparative Genome Analysis of Bacillus spp. and Its Relationship with Bioactive Nonribosomal Peptide Production. Phytochem. Rev. 2013, 12, 685–716. [Google Scholar] [CrossRef]
- Zhao, H.; Shao, D.; Jiang, C.; Shi, J.; Li, Q.; Huang, Q.; Rajoka, M.S.R.; Yang, H.; Jin, M. Biological Activity of Lipopeptides from Bacillus. Appl. Microbiol. Biotechnol. 2017, 101, 5951–5960. [Google Scholar] [CrossRef] [PubMed]
- Kanlayavattanakul, M.; Lourith, N. Lipopeptides in Cosmetics. Int. J. Cosmet. Sci. 2010, 32, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Al-Bahry, S.; Al-Wahaibi, Y.; Elshafie, A.; Al-Bemani, A.; Joshi, S.; Al-Makhmari, H.; Al-Sulaimani, H. Biosurfactant Production by Bacillus subtilis B20 Using Date Molasses and Its Possible Application in Enhanced Oil Recovery. Int. Biodeterior. Biodegrad. 2013, 81, 141–146. [Google Scholar] [CrossRef]
- Umar, A.; Zafar, A.; Wali, H.; Siddique, M.P.; Qazi, M.A.; Naeem, A.H.; Malik, Z.A.; Ahmed, S. Low-Cost Production and Application of Lipopeptide for Bioremediation and Plant Growth by Bacillus subtilis SNW3. AMB Express 2021, 11, 165. [Google Scholar] [CrossRef] [PubMed]
- Moshtagh, B.; Hawboldt, K.; Zhang, B. Optimization of Biosurfactant Production by Bacillus subtilis N3-1P Using the Brewery Waste as the Carbon Source. Environ. Technol. 2018, 40, 3371–3380. [Google Scholar] [CrossRef] [PubMed]
- Gudiña, E.J.; Fernandes, E.C.; Rodrigues, A.I.; Teixeira, J.A.; Rodrigues, L.R. Biosurfactant Production by Bacillus subtilis Using Corn Steep Liquor as Culture Medium. Front. Microbiol. 2015, 6, 126037. [Google Scholar]
- Herrmann, L.W.; Letti, L.A.J.; de Oliveira Penha, R.; Soccol, V.T.; Rodrigues, C.; Soccol, C.R. Bacillus Genus Industrial Applications and Innovation: First Steps towards a Circular Bioeconomy. Biotechnol. Adv. 2023, 70, 108300. [Google Scholar] [CrossRef] [PubMed]
- Vahidinasab, M.; Lilge, L.; Reinfurt, A.; Pfannstiel, J.; Henkel, M.; Morabbi Heravi, K.; Hausmann, R. Construction and Description of a Constitutive Plipastatin Mono-Producing Bacillus subtilis. Microb. Cell Factories 2020, 19, 205. [Google Scholar] [CrossRef] [PubMed]
- Reuß, D.R.; Thürmer, A.; Daniel, R.; Quax, W.J.; Stülke, J. Complete Genome Sequence of Bacillus subtilis Subsp. subtilis Strain∆ 6. Genome Announc. 2016, 4, 10–1128. [Google Scholar]
- Wenzel, M.; Müller, A.; Siemann-Herzberg, M.; Altenbuchner, J. Self-Inducible Bacillus subtilis Expression System for Reliable and Inexpensive Protein Production by High-Cell-Density Fermentation. Appl. Environ. Microbiol. 2011, 77, 6419–6425. [Google Scholar] [CrossRef]
- Klausmann, P.; Hennemann, K.; Hoffmann, M.; Treinen, C.; Aschern, M.; Lilge, L.; Morabbi Heravi, K.; Henkel, M.; Hausmann, R. Bacillus subtilis High Cell Density Fermentation Using a Sporulation-Deficient Strain for the Production of Surfactin. Appl. Microbiol. Biotechnol. 2021, 105, 4141–4151. [Google Scholar] [CrossRef] [PubMed]
- Vahidinasab, M.; Adiek, I.; Hosseini, B.; Akintayo, S.O.; Abrishamchi, B.; Pfannstiel, J.; Henkel, M.; Lilge, L.; Voegele, R.T.; Hausmann, R. Characterization of Bacillus velezensis UTB96, Demonstrating Improved Lipopeptide Production Compared to the Strain B. velezensis FZB42. Microorganisms 2022, 10, 2225. [Google Scholar] [CrossRef] [PubMed]
- Geissler, M.; Oellig, C.; Moss, K.; Schwack, W.; Henkel, M.; Hausmann, R. High-Performance Thin-Layer Chromatography (HPTLC) for the Simultaneous Quantification of the Cyclic Lipopeptides Surfactin, Iturin A and Fengycin in Culture Samples of Bacillus Species. J. Chromatogr. B 2017, 1044, 214–224. [Google Scholar] [CrossRef]
- Hoffmann, M.; Fernandez Cano Luna, D.S.; Xiao, S.; Stegemüller, L.; Rief, K.; Heravi, K.M.; Lilge, L.; Henkel, M.; Hausmann, R. Towards the Anaerobic Production of Surfactin Using Bacillus subtilis. Front. Bioeng. Biotechnol. 2020, 8, 554903. [Google Scholar] [CrossRef] [PubMed]
- Sandrin, C.; Peypoux, F.; Michel, G. Coproduction of surfactin and iturin A, lipopeptides with surfactant and antifungal properties, by Bacillus subtilis. Biotechnol. Appl. Biochem. 1990, 12, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, M.; Pastore, G.M. Production and Properties of a Surfactant Obtained from Bacillus subtilis Grown on Cassava Wastewater. Bioresour. Technol. 2006, 97, 336–341. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Schmidt, V.K.; Moraes, P.A.D.; Cesca, K.; Pereira, L.P.S.; de Andrade, L.M.; Mendes, M.A.; de Oliveira, D.; de Andrade, C.J. Enhanced Production of Surfactin Using Cassava Wastewater and Hydrophobic Inducers: A Prospection on New Homologues. World J. Microbiol. Biotechnol. 2023, 39, 82. [Google Scholar] [CrossRef] [PubMed]
- Willenbacher, J.; Yeremchuk, W.; Mohr, T.; Syldatk, C.; Hausmann, R. Enhancement of Surfactin Yield by Improving the Medium Composition and Fermentation Process. Amb Express 2015, 5, 57. [Google Scholar] [CrossRef] [PubMed]
- Desai, J.D.; Banat, I.M. Microbial Production of Surfactants and Their Commercial Potential. Microbiol. Mol. Biol. Rev. 1997, 61, 47–64. [Google Scholar]
- Wei, Y.; Wang, L.; Chang, J. Optimizing Iron Supplement Strategies for Enhanced Surfactin Production with Bacillus subtilis. Biotechnol. Prog. 2004, 20, 979–983. [Google Scholar] [CrossRef]
- Kim, H.-S.; Yoon, B.-D.; Lee, C.-H.; Suh, H.-H.; Oh, H.-M.; Katsuragi, T.; Tani, Y. Production and Properties of a Lipopeptide Biosurfactant from Bacillus subtilis C9. J. Ferment. Bioeng. 1997, 84, 41–46. [Google Scholar] [CrossRef]
- Abushady, H.; Bashandy, A.; Aziz, N.; Ibrahim, H. Molecular Characterization of Bacillus subtilis Surfactin Producing Strain and the Factors Affecting Its Production. Int. J. Agric. Biol. 2005, 7, 337–344. [Google Scholar]
- Davis, D.; Lynch, H.; Varley, J. The Production of Surfactin in Batch Culture by Bacillus subtilis ATCC 21332 Is Strongly Influenced by the Conditions of Nitrogen Metabolism. Enzyme Microb. Technol. 1999, 25, 322–329. [Google Scholar] [CrossRef]
- Heryani, H.; Putra, M.D. Kinetic Study and Modeling of Biosurfactant Production Using Bacillus Sp. Electron. J. Biotechnol. 2017, 27, 49–54. [Google Scholar] [CrossRef]
- Alonso, S.; Martin, P.J. Impact of Foaming on Surfactin Production by Bacillus subtilis: Implications on the Development of Integrated in Situ Foam Fractionation Removal Systems. Biochem. Eng. J. 2016, 110, 125–133. [Google Scholar] [CrossRef]
- Yeh, M.; Wei, Y.; Chang, J. Enhanced Production of Surfactin from Bacillus subtilis by Addition of Solid Carriers. Biotechnol. Prog. 2005, 21, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Hmidet, N.; Ben Ayed, H.; Jacques, P.; Nasri, M. Enhancement of Surfactin and Fengycin Production by Bacillus mojavensis A21: Application for Diesel Biodegradation. BioMed Res. Int. 2017, 2017, 5893123. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Qian, S.; Muhammad, U.; Jiang, X.; Han, J.; Lu, Z. Effect of Fructose on Promoting Fengycin Biosynthesis in Bacillus amyloliquefaciens Fmb-60. J. Appl. Microbiol. 2016, 121, 1653–1664. [Google Scholar] [CrossRef]
- van Niekerk, K.; Pott, R.W. The Effect of Carbon Source on the Growth and Lipopeptide Production of Bacillus circulans. Biocatal. Agric. Biotechnol. 2023, 53, 102841. [Google Scholar] [CrossRef]
- Dauner, M.; Storni, T.; Sauer, U. Bacillus subtilis Metabolism and Energetics in Carbon-Limited and Excess-Carbon Chemostat Culture. J. Bacteriol. 2001, 183, 24. [Google Scholar] [CrossRef]
- Sheppard, J.D.; Mulligan, C.N. The Production of Surfactin by Bacillus subtilis Grown on Peat Hydrolysate. Appl. Microbiol. Biotechnol. 1987, 27, 110–116. [Google Scholar] [CrossRef]
- Minihane, B.; Brown, D. Fed-Batch Culture Technology. Biotechnol. Adv. 1986, 4, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Stülke, J.; Hillen, W. Regulation of Carbon Catabolism in Bacillus Species. Annu. Rev. Microbiol. 2000, 54, 849–880. [Google Scholar] [CrossRef] [PubMed]
- Buffing, M.F.; Link, H.; Christodoulou, D.; Sauer, U. Capacity for Instantaneous Catabolism of Preferred and Non-Preferred Carbon Sources in Escherichia coli and Bacillus subtilis. Sci. Rep. 2018, 8, 11760. [Google Scholar] [CrossRef] [PubMed]
- Giro, M.E.A.; Martins, J.J.L.; Rocha, M.V.P.; Melo, V.M.M.; Gonçalves, L.R.B. Clarified Cashew Apple Juice as Alternative Raw Material for Biosurfactant Production by Bacillus subtilis in a Batch Bioreactor. Biotechnol. J. 2009, 4, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Ponte Rocha, M.V.; Gomes Barreto, R.V.; Melo, V.M.M.; Barros Gonçalves, L.R. Evaluation of Cashew Apple Juice for Surfactin Production by Bacillus subtilis LAMI008. Appl. Biochem. Biotechnol. 2009, 155, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Coutte, F.; Lecouturier, D.; Ait Yahia, S.; Leclère, V.; Béchet, M.; Jacques, P.; Dhulster, P. Production of Surfactin and Fengycin by Bacillus subtilis in a Bubbleless Membrane Bioreactor. Appl. Microbiol. Biotechnol. 2010, 87, 499–507. [Google Scholar] [CrossRef]
- Amin, G. Exponential Fed-Batch Strategy for Enhancing Biosurfactant Production by Bacillus subtilis. Water Sci. Technol. 2014, 70, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Bouassida, M.; Mnif, I.; Ghribi, D. Enhanced Biosurfactant Production by Bacillus subtilis SPB1 Using Developed Fed-Batch Fermentation: Effects of Glucose Levels and Feeding Systems. Bioprocess Biosyst. Eng. 2023, 46, 555–563. [Google Scholar] [CrossRef]
- Ganesan, N.G.; Rangarajan, V. A Comparative Study on Surfactin Production from Various Fruit Juices for Diverse Applications. Mater. Today Proc. 2023, 72, 2660–2667. [Google Scholar] [CrossRef]
- Zhi, Y.; Wu, Q.; Xu, Y. Production of Surfactin from Waste Distillers’ Grains by Co-Culture Fermentation of Two Bacillus amyloliquefaciens Strains. Bioresour. Technol. 2017, 235, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.-R.; Wei, S.-Y.; Ding, M.-Z.; Hou, Z.-J.; Wang, D.-J.; Xu, Q.-M.; Cheng, J.-S.; Yuan, Y.-J. Enhancing Fengycin Production in the Co-Culture of Bacillus subtilis and Corynebacterium glutamicum by Engineering Proline Transporter. Bioresour. Technol. 2023, 383, 129229. [Google Scholar] [CrossRef]
- Hewitt, C.J.; Nienow, A.W. The Scale-up of Microbial Batch and Fed-batch Fermentation Processes. Adv. Appl. Microbiol. 2007, 62, 105–135. [Google Scholar] [PubMed]
- Bhatia, L.; Jha, H.; Sarkar, T.; Sarangi, P.K. Food Waste Utilization for Reducing Carbon Footprints towards Sustainable and Cleaner Environment: A Review. Int. J. Environ. Res. Public Health 2023, 20, 2318. [Google Scholar] [CrossRef]
- Adamu, H.; Bello, U.; Yuguda, A.U.; Tafida, U.I.; Jalam, A.M.; Sabo, A.; Qamar, M. Production Processes, Techno-Economic and Policy Challenges of Bioenergy Production from Fruit and Vegetable Wastes. Renew. Sustain. Energy Rev. 2023, 186, 113686. [Google Scholar] [CrossRef]
- Hoffmann, M.; Mück, D.; Grossmann, L.; Greiner, L.; Klausmann, P.; Henkel, M.; Lilge, L.; Weiss, J.; Hausmann, R. Surfactin from Bacillus subtilis Displays Promising Characteristics as O/W-Emulsifier for Food Formulations. Colloids Surf. B Biointerfaces 2021, 203, 111749. [Google Scholar] [CrossRef] [PubMed]
- Théatre, A.; Cano-Prieto, C.; Bartolini, M.; Laurin, Y.; Deleu, M.; Niehren, J.; Fida, T.; Gerbinet, S.; Alanjary, M.; Medema, M.H. The Surfactin-like Lipopeptides from Bacillus spp.: Natural Biodiversity and Synthetic Biology for a Broader Application Range. Front. Bioeng. Biotechnol. 2021, 9, 623701. [Google Scholar] [CrossRef]
- Kecskeméti, A.; Bartal, A.; Bóka, B.; Kredics, L.; Manczinger, L.; Shine, K.; Alharby, N.S.; Khaled, J.M.; Varga, M.; Vágvölgyi, C. High-Frequency Occurrence of Surfactin Monomethyl Isoforms in the Ferment Broth of a Bacillus subtilis Strain Revealed by Ion Trap Mass Spectrometry. Molecules 2018, 23, 2224. [Google Scholar] [CrossRef]

| Parameter | Concentration |
|---|---|
| Total nitrogen (g/100 g) | 0.90 ± 0.10 |
| Fructose (g/100 g) | 2.89 ± 0.28 |
| Glucose (g/100 g) | 1.96 ± 0.23 |
| Sucrose (g/100 g) | 0.12 ± 0.02 |
| Mineral composition (mg/kg): | |
| Copper | 0.274 ± 0.107 |
| Zinc | 0.596 ± 0.206 |
| Calcium | 112 ± 15 |
| Iron | 0.972 ± 0.312 |
| Magnesium | 43.10 ± 5.60 |
| Potassium | 1060 ± 140 |
| Manganese | 1.13 ± 0.35 |
| Organic acids (g/L): | |
| Maleic acid | 0.914 ± 0.01 |
| Lactic acid | 0.089 ± 0.03 |
| Formic acid | 0.089 ± 0.01 |
| Medium Composition | ||
|---|---|---|
| 100% MSM | - | 100 mL MSM (+20 g/L Glucose) |
| 90% VJ | 90 mL of VJ | 10 mL dd-water + salts |
| 70% VJ | 70 mL of VJ | 30 mL dd-water + salts |
| 45% VJ | 45 mL of VJ | 55 mL dd-water + salts |
| 25% VJ | 25 mL of VJ | 75 mL dd-water + salts |
| Surfactin | Fengycin | |||||||
|---|---|---|---|---|---|---|---|---|
| µ (h−1) | Yx/s (g/g) | Yp/s (g/g) | Yp/x (g/g) | qp/x (g/gꞏh) | Yp/s (mg/g) | Yp/x (mg/g) | qp/x (mg/gꞏh) | |
| MSM | 0.193 | 0.188 | 0.046 | 0.263 | 0.009 | 0.369 | 1.886 | 0.079 |
| 90% VJ | 0.172 | 0.075 | 0.011 | 0.184 | 0.006 | 0.080 | 1.580 | 0.033 |
| 70%VJ | 0.170 | 0.085 | 0.021 | 0.249 | 0.01 | 0.126 | 2.209 | 0.046 |
| 45% VJ | 0.206 | 0.088 | 0.021 | 0.323 | 0.01 | 0.253 | 2.288 | 0.095 |
| 25% VJ | 0.368 | 0.240 | 0.054 | 0.568 | 0.02 | 0.457 | 4.809 | 0.200 |
| Surfactin | Fengycin | |||||||
|---|---|---|---|---|---|---|---|---|
| µ (h−1) | Yx/s (g/g) | Yp/s (g/g) | Yp/x (g/g) | qp/x (g/gꞏh) | Yp/s (mg/g) | Yp/x (mg/g) | qp/x (mg/gꞏh) | |
| Fed-batch fermentation | 0.398 | 0.289 | 0.086 | 0.297 | 0.019 | 0.852 | 0.018 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gugel, I.; Vahidinasab, M.; Benatto Perino, E.H.; Hiller, E.; Marchetti, F.; Costa, S.; Pfannstiel, J.; Konnerth, P.; Vertuani, S.; Manfredini, S.; et al. Fed-Batch Bioreactor Cultivation of Bacillus subtilis Using Vegetable Juice as an Alternative Carbon Source for Lipopeptides Production: A Shift towards a Circular Bioeconomy. Fermentation 2024, 10, 323. https://doi.org/10.3390/fermentation10060323
Gugel I, Vahidinasab M, Benatto Perino EH, Hiller E, Marchetti F, Costa S, Pfannstiel J, Konnerth P, Vertuani S, Manfredini S, et al. Fed-Batch Bioreactor Cultivation of Bacillus subtilis Using Vegetable Juice as an Alternative Carbon Source for Lipopeptides Production: A Shift towards a Circular Bioeconomy. Fermentation. 2024; 10(6):323. https://doi.org/10.3390/fermentation10060323
Chicago/Turabian StyleGugel, Irene, Maliheh Vahidinasab, Elvio Henrique Benatto Perino, Eric Hiller, Filippo Marchetti, Stefania Costa, Jens Pfannstiel, Philipp Konnerth, Silvia Vertuani, Stefano Manfredini, and et al. 2024. "Fed-Batch Bioreactor Cultivation of Bacillus subtilis Using Vegetable Juice as an Alternative Carbon Source for Lipopeptides Production: A Shift towards a Circular Bioeconomy" Fermentation 10, no. 6: 323. https://doi.org/10.3390/fermentation10060323
APA StyleGugel, I., Vahidinasab, M., Benatto Perino, E. H., Hiller, E., Marchetti, F., Costa, S., Pfannstiel, J., Konnerth, P., Vertuani, S., Manfredini, S., & Hausmann, R. (2024). Fed-Batch Bioreactor Cultivation of Bacillus subtilis Using Vegetable Juice as an Alternative Carbon Source for Lipopeptides Production: A Shift towards a Circular Bioeconomy. Fermentation, 10(6), 323. https://doi.org/10.3390/fermentation10060323









