Unveiling the Impact of Lactic Acid Bacteria on Blood Lipid Regulation for Cardiovascular Health
Abstract
1. Introduction
2. Lactic Acid Bacteria’s Metabolic Activities and Lipid Metabolism Modulation
3. Human Studies
4. Lactic Acid Bacteria’s Metabolic Activities and Lipid Modulation: Animal Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 14 January 2024).
- WHO. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 14 January 2024).
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Frąk, W.; Wojtasińska, A.; Lisińska, W.; Młynarska, E.; Franczyk, B.; Rysz, J. Pathophysiology of Cardiovascular Diseases: New Insights into Molecular Mechanisms of Atherosclerosis, Arterial Hypertension, and Coronary Artery Disease. Biomedicines 2022, 10, 1938. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e563–e595. [Google Scholar] [CrossRef] [PubMed]
- Dyńka, D.; Kowalcze, K.; Charuta, A.; Paziewska, A. The Ketogenic Diet and Cardiovascular Diseases. Nutrients 2023, 15, 3368. [Google Scholar] [CrossRef] [PubMed]
- Onuh, J.O.; Dawkins, N.L.; Aluko, R.E. Cardiovascular disease protective properties of blueberry polyphenols (Vaccinium corymbosum): A concise review. Food Prod. Process. Nutr. 2023, 5, 27. [Google Scholar] [CrossRef]
- Yuan, F.; Dong, H.; Gong, J.; Wang, D.; Hu, M.; Huang, W.; Fang, K.; Qin, X.; Qiu, X.; Yang, X.; et al. A Systematic Review and Meta-analysis of Randomized Controlled Trials on the Effects of Turmeric and Curcuminoids on Blood Lipids in Adults with Metabolic Diseases. Adv. Nutr. 2019, 10, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Zhang, Z.; Zheng, T.Z.; Bassig, B.A.; Mao, C.; Liu, X.; Zhu, Y.; Shi, K.; Ge, J.; Yang, Y.J.; et al. Green tea consumption and risk of cardiovascular and ischemic related diseases: A meta-analysis. Int. J. Cardiol. 2016, 202, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Gille, D.; Schmid, A.; Walther, B.; Vergères, G. Fermented Food and Non-Communicable Chronic Diseases: A Review. Nutrients 2018, 10, 448. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Mathur, H.; Beresford, T.P.; Cotter, P.D. Health Benefits of Lactic Acid Bacteria (LAB) Fermentates. Nutrients 2020, 12, 1679. [Google Scholar] [CrossRef]
- Salehi, S.O.; Karimpour, F.; Imani, H.; Ghatee, M.A.; Pirouze, M.; Bahramfard, T. Effects of an Iranian traditional fermented food consumption on blood glucose, blood pressure, and lipid profile in type 2 diabetes: A randomized controlled clinical trial. Eur. J. Nutr. 2022, 61, 3367–3375. [Google Scholar] [CrossRef] [PubMed]
- Ostadrahimi, A.; Taghizadeh, A.; Mobasseri, M.; Farrin, N.; Payahoo, L.; Beyramalipoor Gheshlaghi, Z.; Vahedjabbari, M. Effect of probiotic fermented milk (kefir) on glycemic control and lipid profile in type 2 diabetic patients: A randomized double-blind placebo-controlled clinical trial. Iran. J. Public. Health 2015, 44, 228–237. [Google Scholar] [PubMed]
- Zhao, X.; Zhong, X.; Liu, X.; Wang, X.; Gao, X. Therapeutic and Improving Function of Lactobacilli in the Prevention and Treatment of Cardiovascular-Related Diseases: A Novel Perspective From Gut Microbiota. Front. Nutr. 2021, 8, 693412. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Nagpal, R.; Kumar, R.; Hemalatha, R.; Verma, V.; Kumar, A.; Chakraborty, C.; Singh, B.; Marotta, F.; Jain, S.; et al. Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseases. Exp. Diabetes Res. 2012, 2012, 902917. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Lin, P.P.; Hsieh, Y.M.; Zhang, Z.Y.; Wu, H.C.; Huang, C.C. Cholesterol-lowering potentials of lactic acid bacteria based on bile-salt hydrolase activity and effect of potent strains on cholesterol metabolism in vitro and in vivo. Sci. World J. 2014, 2014, 690752. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Zhang, H.; Guo, B.; Chen, W.; Wu, Z.; Wu, Y. Preparation, partial characterization and bioactivity of exopolysaccharides from Lactobacillus casei LC2W. Carbohydr. Polym. 2008, 74, 353–357. [Google Scholar] [CrossRef]
- Korcz, E.; Varga, L. Exopolysaccharides from lactic acid bacteria: Techno-functional application in the food industry. Trends Food Sci. Technol. 2021, 110, 375–384. [Google Scholar] [CrossRef]
- Sørensen, H.M.; Rochfort, K.D.; Maye, S.; MacLeod, G.; Brabazon, D.; Loscher, C.; Freeland, B. Exopolysaccharides of Lactic Acid Bacteria: Production, Purification and Health Benefits towards Functional Food. Nutrients 2022, 14, 2938. [Google Scholar] [CrossRef]
- Lee, D.K.; Jang, S.; Baek, E.H.; Kim, M.J.; Lee, K.S.; Shin, H.S.; Chung, M.J.; Kim, J.E.; Lee, K.O.; Ha, N.J. Lactic acid bacteria affect serum cholesterol levels, harmful fecal enzyme activity, and fecal water content. Lipids Health Dis. 2009, 8, 21. [Google Scholar] [CrossRef]
- Bourgin, M.; Kriaa, A.; Mkaouar, H.; Mariaule, V.; Jablaoui, A.; Maguin, E.; Rhimi, M. Bile Salt Hydrolases: At the Crossroads of Microbiota and Human Health. Microorganisms 2021, 9, 1122. [Google Scholar] [CrossRef]
- Ishimwe, N.; Daliri, E.B.; Lee, B.H.; Fang, F.; Du, G. The perspective on cholesterol-lowering mechanisms of probiotics. Mol. Nutr. Food Res. 2015, 59, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Singhania, R.R.; Pandey, A.; Chincholkar, S.B. Probiotic bile salt hydrolase: Current developments and perspectives. Appl. Biochem. Biotechnol. 2010, 162, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Korcz, E.; Kerényi, Z.; Varga, L. Dietary fibers, prebiotics, and exopolysaccharides produced by lactic acid bacteria: Potential health benefits with special regard to cholesterol-lowering effects. Food Funct. 2018, 9, 3057–3068. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Xu, M.; Huang, N.; Yuan, Z. Meta-analysis of the effect of probiotics or synbiotics on the risk factors in patients with coronary artery disease. Front. Cardiovasc. Med. 2023, 10, 1154888. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Guo, X.; Zhou, Y.; Cao, G. The Effects of Probiotics/Synbiotics on Glucose and Lipid Metabolism in Women with Gestational Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2023, 15, 1375. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Hashiguchi, M.; Shiga, T.; Tamura, H.O.; Mochizuki, M. Meta-Analysis: Effects of Probiotic Supplementation on Lipid Profiles in Normal to Mildly Hypercholesterolemic Individuals. PLoS ONE 2015, 10, e0139795. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guo, M.J.; Gao, Q.; Yang, J.F.; Yang, L.; Pang, X.L.; Jiang, X.J. The effects of probiotics on total cholesterol: A meta-analysis of randomized controlled trials. Medicine 2018, 97, e9679. [Google Scholar] [CrossRef] [PubMed]
- Arslan, B.; Yilmaz, I. The effect of kefir consumption on the lipid profile for individuals with normal and dyslipidemic properties: A randomized controlled trial. Rev. Nutr. 2022, 35, e210098. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Farnworth, E.R.; Savard, T.; Chabot, D.; Mafu, A.; Jones, P.J. Kefir consumption does not alter plasma lipid levels or cholesterol fractional synthesis rates relative to milk in hyperlipidemic men: A randomized controlled trial [ISRCTN10820810]. BMC Complement. Altern. Med. 2002, 2, 1. [Google Scholar] [CrossRef]
- Fathi, Y.; Ghodrati, N.; Zibaeenezhad, M.J.; Faghih, S. Kefir drink causes a significant yet similar improvement in serum lipid profile, compared with low-fat milk, in a dairy-rich diet in overweight or obese premenopausal women: A randomized controlled trial. J. Clin. Lipidol. 2017, 11, 136–146. [Google Scholar] [CrossRef]
- Jones, M.L.; Martoni, C.J.; Prakash, S. Cholesterol lowering and inhibition of sterol absorption by Lactobacillus reuteri NCIMB 30242: A randomized controlled trial. Eur. J. Clin. Nutr. 2012, 66, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Ghizi, A.C.d.S.; de Almeida Silva, M.; Moraes, F.S.D.A.; da Silva, C.L.; Endringer, D.C.; Scherer, R.; Lenz, D.; de Lima, E.M.; Brasil, G.A.; Maia, J.F.; et al. Kefir improves blood parameters and reduces cardiovascular risks in patients with metabolic syndrome. Pharma Nutr. 2021, 16, 100266. [Google Scholar] [CrossRef]
- Ivey, K.L.; Hodgson, J.M.; Kerr, D.A.; Thompson, P.L.; Stojceski, B.; Prince, R.L. The effect of yoghurt and its probiotics on blood pressure and serum lipid profile; a randomised controlled trial. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, F.; Ishida, Y.; Aihara, K.; Sawada, D.; Ashida, N.; Sugawara, T.; Aoki, Y.; Takehara, I.; Takano, K.; Fujiwara, S. Effect of fragmented Lactobacillus amylovorus CP1563 on lipid metabolism in overweight and mildly obese individuals: A randomized controlled trial. Microb. Ecol. Health Dis. 2016, 27, 30312. [Google Scholar] [CrossRef]
- Agerholm-Larsen, L.; Raben, A.; Haulrik, N.; Hansen, A.S.; Manders, M.; Astrup, A. Effect of 8 week intake of probiotic milk products on risk factors for cardiovascular diseases. Eur. J. Clin. Nutr. 2000, 54, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, M.C.; Lajo, T.; Carrión, J.M.; Cuñé, J. A randomized clinical trial evaluating a proprietary mixture of Lactobacillus plantarum strains for lowering cholesterol. Mediterr. J. Nutr. Metab. 2016, 9, 125–135. [Google Scholar] [CrossRef]
- Jones, M.L.; Martoni, C.J.; Parent, M.; Prakash, S. Cholesterol-lowering efficacy of a microencapsulated bile salt hydrolase-active Lactobacillus reuteri NCIMB 30242 yoghurt formulation in hypercholesterolaemic adults. Br. J. Nutr. 2012, 107, 1505–1513. [Google Scholar] [CrossRef]
- Rezazadeh, L.; Alipour, B.; Jafarabadi, M.A.; Gargari, B.P. Evaluation of the effects of probiotic yoghurt on inflammation and cardiometabolic risk factors in subjects with metabolic syndrome: A randomised controlled trial. Int. Dairy. J. 2020, 101, 104577. [Google Scholar] [CrossRef]
- Kiessling, G.; Schneider, J.; Jahreis, G. Long-term consumption of fermented dairy products over 6 months increases HDL cholesterol. Eur. J. Clin. Nutr. 2002, 56, 843–849. [Google Scholar] [CrossRef]
- Higashikawa, F.; Noda, M.; Awaya, T.; Nomura, K.; Oku, H.; Sugiyama, M. Improvement of constipation and liver function by plant-derived lactic acid bacteria: A double-blind, randomized trial. Nutrition 2010, 26, 367–374. [Google Scholar] [CrossRef]
- Schaafsma, G.; Meuling, W.J.; van Dokkum, W.; Bouley, C. Effects of a milk product, fermented by Lactobacillus acidophilus and with fructo-oligosaccharides added, on blood lipids in male volunteers. Eur. J. Clin. Nutr. 1998, 52, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-L.; Zhang, L.-Q.; Yang, Y.; Yin, B.-C.; Ye, B.-C.; Zhou, Y. Advances in the role and mechanism of lactic acid bacteria in treating obesity. Food Bioeng. 2022, 1, 101–115. [Google Scholar] [CrossRef]
- Karamali, M.; Dadkhah, F.; Sadrkhanlou, M.; Jamilian, M.; Ahmadi, S.; Tajabadi-Ebrahimi, M.; Jafari, P.; Asemi, Z. Effects of probiotic supplementation on glycaemic control and lipid profiles in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Diabetes Metab. 2016, 42, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, J.; Tian, Z.; Si, Y.; Chen, H.; Gan, J. The Mechanisms of the Potential Probiotic Lactiplantibacillus plantarum against Cardiovascular Disease and the Recent Developments in its Fermented Foods. Foods 2022, 11, 2549. [Google Scholar] [CrossRef] [PubMed]
- De Assis Gadelha, D.D.; de Brito Alves, J.L.; da Costa, P.C.T.; da Luz, M.S.; de Oliveira Cavalcanti, C.; Bezerril, F.F.; Almeida, J.F.; de Campos Cruz, J.; Magnani, M.; Balarini, C.M.; et al. Lactobacillus group and arterial hypertension: A broad review on effects and proposed mechanisms. Crit. Rev. Food Sci. Nutr. 2024, 64, 3839–3860. [Google Scholar] [CrossRef] [PubMed]
- Sert, M.; Özer, Z. The Effect of Microbiota on Cardiovascular Health and Disease. Turk. J. Card. Nur 2019, 10, 25–32. [Google Scholar] [CrossRef]
- Albano, C.; Morandi, S.; Silvetti, T.; Casiraghi, M.C.; Manini, F.; Brasca, M. Lactic acid bacteria with cholesterol-lowering properties for dairy applications: In vitro and in situ activity. J. Dairy. Sci. 2018, 101, 10807–10818. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Sharma, H.; Melekoglu, E.; Ozogul, F. Recent developments in dairy kefir-derived lactic acid bacteria and their health benefits. Food Biosci. 2022, 46, 101592. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, X.; Wang, W.; Gu, L.; Hu, C.; Sun, H.; Xu, C.; Hou, J.; Jiang, Z. Lactobacillus paracasei 24 Attenuates Lipid Accumulation in High-Fat Diet-Induced Obese Mice by Regulating the Gut Microbiota. J. Agric. Food Chem. 2022, 70, 4631–4643. [Google Scholar] [CrossRef]
- Witkowski, M.; Weeks, T.L.; Hazen, S.L. Gut Microbiota and Cardiovascular Disease. Circ. Res. 2020, 127, 553–570. [Google Scholar] [CrossRef]
- Qian, B.; Zhang, K.; Li, Y.; Sun, K. Update on gut microbiota in cardiovascular diseases. Front. Cell Infect. Microbiol. 2022, 12, 1059349. [Google Scholar] [CrossRef]
- Jeon, Y.B.; Lee, J.-J.; Chang, H.C. Characterization of juice fermented with Lactobacillus plantarum EM and its cholesterol-lowering effects on rats fed a high-fat and high-cholesterol diet. Food Sci. Nutr. 2019, 7, 3622–3634. [Google Scholar] [CrossRef]
- Zafar, H.; Ain, N.U.; Alshammari, A.; Alghamdi, S.; Raja, H.; Ali, A.; Siddique, A.; Tahir, S.D.; Akbar, S.; Arif, M.; et al. Lacticaseibacillus rhamnosus FM9 and Limosilactobacillus fermentum Y57 Are as Effective as Statins at Improving Blood Lipid Profile in High Cholesterol, High-Fat Diet Model in Male Wistar Rats. Nutrients 2022, 14, 1654. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, P.N.; Patel, A.; Modi, H.A.; Prajapati, J.B. Hypocholesterolemic Effect of Potential Probiotic Lactobacillus fermentum Strains Isolated from Traditional Fermented Foods in Wistar Rats. Probiotics Antimicrob. Proteins 2020, 12, 1002–1011. [Google Scholar] [CrossRef]
- Tian, L.; Liu, R.; Zhou, Z.; Xu, X.; Feng, S.; Kushmaro, A.; Marks, R.S.; Wang, D.; Sun, Q. Probiotic Characteristics of Lactiplantibacillus Plantarum N-1 and Its Cholesterol-Lowering Effect in Hypercholesterolemic Rats. Probiotics Antimicrob. Proteins 2022, 14, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Vij, R.; Kapila, S.; Khan, S.H.; Kumar, N.; Meena, S.; Kapila, R. Milk fermented with probiotic strains Lactobacillus rhamnosus MTCC: 5957 and Lactobacillus rhamnosus MTCC: 5897 ameliorates the diet-induced hypercholesterolemia in rats. Ann. Microbiol. 2019, 69, 483–494. [Google Scholar] [CrossRef]
- Banjoko, I.O.; Adeyanju, M.M.; Ademuyiwa, O.; Adebawo, O.O.; Olalere, R.A.; Kolawole, M.O.; Adegbola, I.A.; Adesanmi, T.A.; Oladunjoye, T.O.; Ogunnowo, A.A.; et al. Hypolipidemic effects of lactic acid bacteria fermented cereal in rats. Lipids Health Dis. 2012, 11, 170. [Google Scholar] [CrossRef]
- Lee, M.; Yun, Y.-R.; Choi, E.J.; Song, J.H.; Kang, J.Y.; Kim, D.; Lee, K.W.; Chang, J.Y. Anti-obesity effect of vegetable juice fermented with lactic acid bacteria isolated from kimchi in C57BL/6J mice and human mesenchymal stem cells. Food Funct. 2023, 14, 1349–1356. [Google Scholar] [CrossRef]
- Yi, R.; Tan, F.; Zhou, X.; Mu, J.; Li, L.; Du, X.; Yang, Z.; Zhao, X. Effects of Lactobacillus fermentum CQPC04 on Lipid Reduction in C57BL/6J Mice. Front. Microbiol. 2020, 11, 573586. [Google Scholar] [CrossRef]
- Yun, Y.-R.; Kwon, M.-S.; Lee, H.-J.; Lee, W.; Lee, J.-E.; Hong, S.W. Anti-obesity activity of lactic acid bacteria-starter-based kimchi in high-fat diet-induced obese mice. J. Funct. Foods 2024, 112, 105966. [Google Scholar] [CrossRef]
- Wu, N.; Sarna, L.K.; Hwang, S.Y.; Zhu, Q.; Wang, P.; Siow, Y.L.; Karmin, O. Activation of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase during high fat diet feeding. Biochim. Biophys. Acta 2013, 1832, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- Tricarico, P.M.; Crovella, S.; Celsi, F. Mevalonate Pathway Blockade, Mitochondrial Dysfunction and Autophagy: A Possible Link. Int. J. Mol. Sci. 2015, 16, 16067–16084. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.M.; DeBose-Boyd, R.A. Posttranslational Regulation of HMG CoA Reductase, the Rate-Limiting Enzyme in Synthesis of Cholesterol. Annu. Rev. Biochem. 2021, 90, 659–679. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Gong, K.; Xu, S.; Zhang, F.; Meng, X.; Han, J. Regulation of cholesterol homeostasis in health and diseases: From mechanisms to targeted therapeutics. Signal Transduct. Target. Ther. 2022, 7, 265. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, B.; Zhou, X.; Wang, Y.; Wang, H.; Jia, S.; Mu, J. Combined Lowering Effects of Rosuvastatin and L. acidophilus on Cholesterol Levels in Rat. J. Microbiol. Biotechnol. 2019, 29, 473–481. [Google Scholar] [CrossRef]
- Gan, Y.; Tang, M.-W.; Tan, F.; Zhou, X.-R.; Fan, L.; Xie, Y.-X.; Zhao, X. Anti-obesity effect of Lactobacillus plantarum CQPC01 by modulating lipid metabolism in high-fat diet-induced C57BL/6 mice. J. Food Biochem. 2020, 44, e13491. [Google Scholar] [CrossRef]
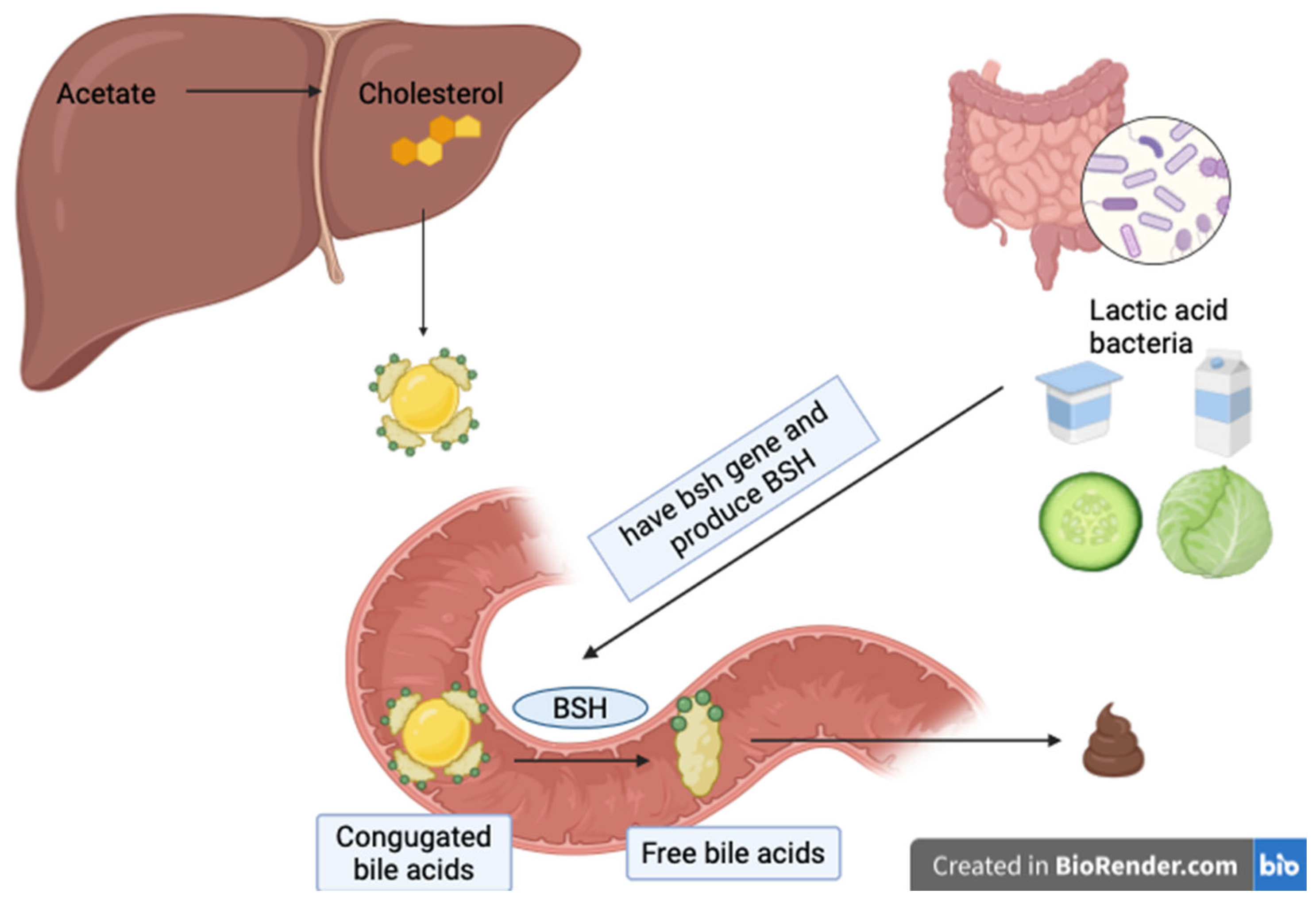
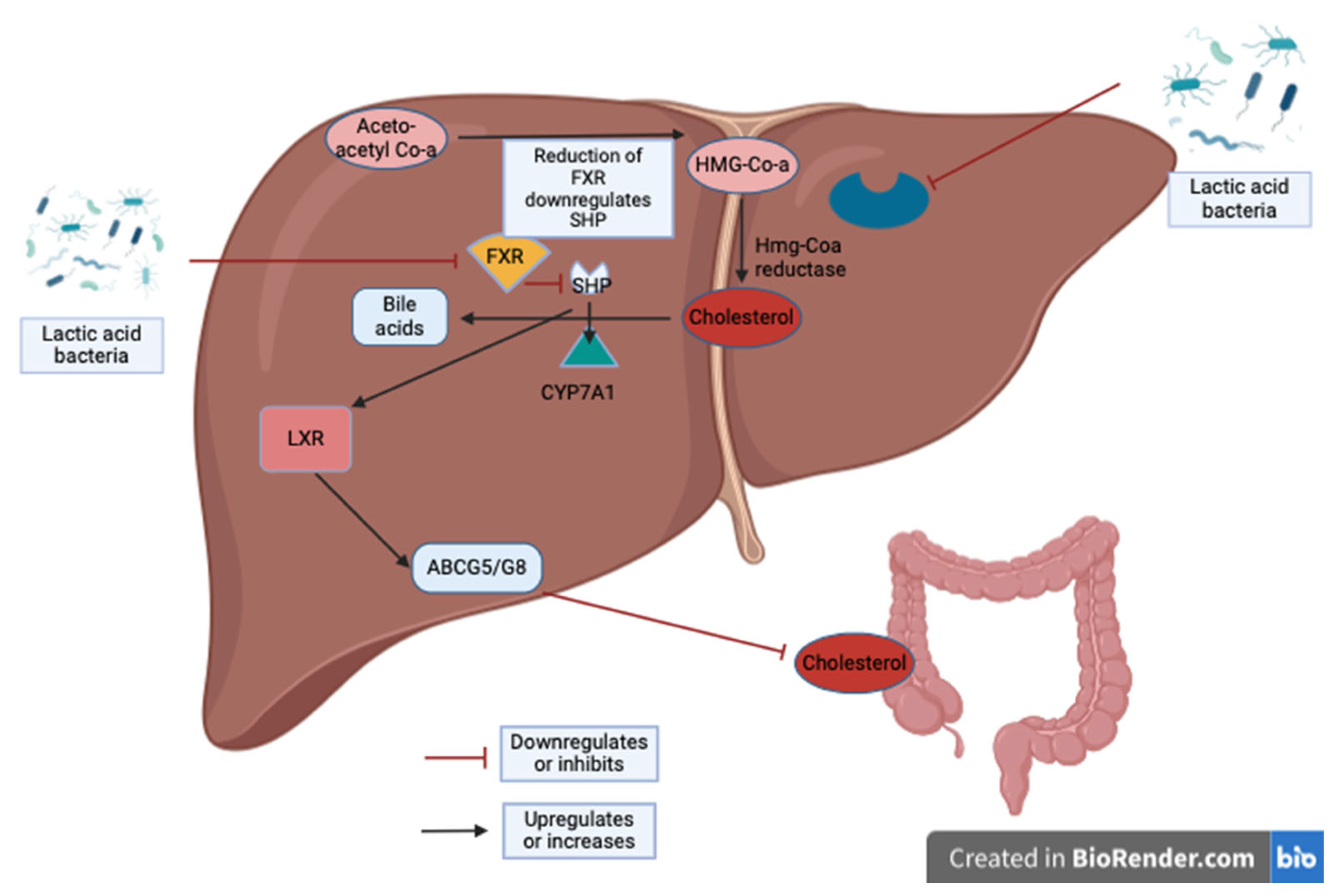
| Study Design | LAB Species | Intervention | Subjects | Duration | Main Results | Reference |
|---|---|---|---|---|---|---|
| Randomized controlled clinical trial | Not specified | Iranian fermented food Ash-Kardeh (250 g/day) | 23 type 2 diabetes (intervention) 23 type 2 diabetes (control group) | 6 weeks | While TC decreased, HDL-C increased significantly. | [13] |
| Randomized controlled clinical trial | L. acidophilus La5 and Bifidobacterium animalis subsp. lactis Bb12 | Yoghurt or capsule form | 156 overweight men or women | 6 weeks | Neither capsule nor yogurt supplementation changed levels of blood lipids and blood pressure. | [35] |
| Randomized clinical trial | L. amylovorus | Beverages with powder CP1563 powder | 100 overweight or obese females or males | 12 weeks | Triglyceride, TC, LDL-C and diastolic blood pressure showed significant reductions. | [36] |
| Randomized, double-blind, placebo- and compliance-controlled parallel study. |
| Fermented milk (yogurt) (450 mL/day) | 80 healthy overweight or obese | 8 weeks | When body weight variations were adjusted, LDL-cholesterol decreased by 8.4% in the commercial product group (p < 0.05). | [37] |
| Randomized clinical trial | L. plantarum | Probiotic form | 60 patients, mean BMI 26.2 kg/m2 | 16 weeks | When compared with a placebo, the lactic acid bacteria receiving group had more reductions in LDL-C and triglycerides and increases in HDL-C | [38] |
| Randomized clinical trial | L. casei, L. acidophilus and Bifidobacteria | Probiotic fermented milk (kefir) (600 mL/day) or conventional fermented milk (600 mL/day) (for the control group) | 60 diabetic patients | 8 weeks | Total cholesterol significantly decreased in the probiotic fermented group. | [14] |
| Randomized clinical trial | L. reuteri | Capsules with L. reuteri and capsules with placebo | 127 patients with hypercholesterolemia | 9 weeks | Comparatively, LDL-C was reduced by 11.64%, and total cholesterol was reduced by 9.14%. | [33] |
| Randomized crossover trial | Not specified | Kefir (500 mL/day) or milk (500 mL/day) | 13 patients (mildly hyperlipidemic) | 4 weeks | There were no changes in TC, LDL-C, HDL-C and triglyceride levels. | [31] |
| Randomized clinical trial | L. reuteri | Yogurts containing microencapsulated L. reuteri and placebo yogurt | 114 patients with hypercholesterolemia | 6 weeks | Significant reductions in LDL-C and TC in L. reuteri, including yogurt. | [39] |
| Prospective self-controlled clinical trial | Kefir was analyzed to confirm LAB content | Kefir (250 mL/day) | 13 patients with hyperlipidemic symptoms, 10 healthy patients | 8 weeks | For both groups, TC, LDL-C and triglyceride levels decreased while HDL-C levels increased. | [30] |
| Randomized clinical trial | Kefir | Kefir (1.6 mL/kg for females, 1.9 mL/kg for males) or placebo (curd) | 48 patients with metabolic syndrome (24 kefir, 24 curd-receiving group) | 12 weeks | In female group, TC-triglyceride and ox-LDL concentrations decreased while HDL-C levels increased. | [34] |
| Randomized clinical trial | Not specified | Kefir or milk or control group | 75 overweight or obese premenopausal women | 8 weeks | In kefir group, significantly lower levels of TC and LDL-C were observed when compared with control group. | [32] |
| Randomized controlled trial | L. acidophilus, Bifidobacterium lactis | 300 g daily regular yogurt or 300 g probiotic yogurt | 44 patients with metabolic syndrome | 8 weeks | When compared with the baseline, triglycerides decreased and HDL-C increased significantly in probiotic yogurt group. | [40] |
| Randomized crossover trial | L. acidophilus 145, B. longum 913 | 300 g/day probiotic yogurt and normal yogurt | 14 hypercholesterolemic 15 normocholesterolemic | 6 months | In probiotic yogurt group, HDL-C significantly increased whereas there was no cholesterol-lowering effect. | [41] |
| Randomized controlled trial | L. plantarum SN35N, L. plantarum SN13T, (type C) or Lactococcus lactis A6 and Streptococcus thermophilus 510 (normal yogurt) | 100 g/day yogurt made from plant-derived LAB or normal yogurt | 68 healthy adults with symptoms of constipation or diarrhea | 6 weeks | TC and LDL-C significantly decreased in L. plantarum SN13T-derived and normal yogurt groups. | [42] |
| Randomized two-way crossover trial | L. acidophilus (two strains) and traditional yogurt starters | 3 × 125 mL of yogurt (with Lactobacillus acidophilus) or normal yogurt | 30 healthy male adults | 3 weeks | Consumption of the test product resulted in significantly lower values for TC and LDL-C. | [43] |
| Animals | Intervention | LAB Species | Main Results | Proposed Mechanism/s | Reference |
|---|---|---|---|---|---|
| 5-week-old Sprague Dawley male rats (n:32) | Four groups: 1. Normal diet 2. High-fat/high-cholesterol diet 3. High-fat/high-cholesterol diet + nonfermented control juice; and 4. High-fat/high-cholesterol diet + fermented EM juice Duration: 6-weeks | L. plantarum EM |
|
| [54] |
| 7–8-week-old C57BL/6 J male mice (n:48) | Four groups: 1. Normal diet 2. High-fat diet 3. High-fat diet + positive control 4. High-fat diet + LP24 Duration: 8-weeks | L. paracasei 24 |
|
| [51] |
| 7–8-week-old male Wistar rats (n:45) | Nine groups: 1. Normal diet 2. The remaining eight groups: high-fat diet with one probiotic strain from each: FM9, Y57, FM6, Y55, FM16, Y59, FM12 and Y63 Duration: 30 days | L. rhamnosus FM9, L. fermentum Y57 L. fermentum FM12, L. fermentum FM16, L. fermentum Y55, L. rhamnosus Y59 and L. fermentum Y63 |
|
| [55] |
| 4–6-week-old Wistar albino rats (either sex) | Seven groups: 1. Normal diet 2. Atherogenic diet 3. Atherogenic diet + standard antihyperlipidemic drug 4. Atherogenic diet + curd having 107 probiotic strain PD2 5. Atherogenic diet + curd having 109 probiotic strain PD2 6. Atherogenic diet + curd having 107 probiotic strain PH5 7. Atherogenic diet + curd having 109 probiotic strain PH5 Duration: 4 weeks | L. fermentum PD2 and PH5 |
|
| [56] |
| 8-week-old male Sprague Dawley rats | Three groups: 1. High-fat diet group 2. High-fat diet + simvastatin 3. High-fat diet + L. plantarum N-1 Duration: 30 days | L. plantarum N-1 |
|
| [57] |
| 6-week-old male Wistar rats | Five groups: 1. Standard diet 2. High-cholesterol diet 3. High-cholesterol diet + milk 4. High-cholesterol diet + L. rhamnosus MTCC:5957 fermented milk 5. High-cholesterol diet+ L. rhamnosus MTCC:5897 fermented milk Duration: 3 months | L. rhamnosus MTCC:5957 and L. rhamnosus MTCC:5897 |
|
| [58] |
| Wistar strain albino male rats | Three groups: 1. Normal diet 2. High-fat fermented diet 3. High-fat unfermented diet Duration: 4 weeks | L. acidophilus (DSM 20242), Bifidobacterium bifidum (DSM 20082) and L. helveticus (CK60) |
|
| [59] |
| Six-week-old male C57BL/6J mice | Seven groups 1. Normal diet 2. High-fat diet 3. High-fat diet+ Companilactobacillus allii WiKim39 4. High-fat diet + L. lactis WiKim0124 5. High-fat diet + non-fermented vegetable juice 6. High-fat diet + C. allii WiKim39-fermented vegetable juice 7. High-fat diet + L. lactis WiKim0124-fermented vegetable juice | Companilactobacillus allii WiKim39 and L. lactis WiKim0124 |
|
| [60] |
| 6-week-old C57BL/6J mice (half male and half female) | Six groups: 1. Low-fat diet 2. High-fat diet 3. High-fat diet + L-carnitine 4. High-fat diet+ L. fermentum CQPC04 (108 CFU/mL) 5. High-fat diet+ L. fermentum CQPC04 (109 CFU/mL) 6. High-fat diet+ L. delbrueckii subsp. Bulgaricus (109 CFU/mL) | L. fermentum CQPC04 |
|
| [61] |
| 6-week-old male C57BL/6N mice | Four groups: 1. Normal diet 2. High-fat diet 3. High-fat diet+ LAB-uninoculated kimchi 4. High-fat diet+ LAB-inoculated kimchi Duration: 8 weeks | Leuconostoc mesenteroides KCKM0828 |
|
| [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yilmaz, B.; Arslan, N.; Şahin, T.Ö.; Ağadündüz, D.; Ozogul, F.; Rocha, J.M.F. Unveiling the Impact of Lactic Acid Bacteria on Blood Lipid Regulation for Cardiovascular Health. Fermentation 2024, 10, 350. https://doi.org/10.3390/fermentation10070350
Yilmaz B, Arslan N, Şahin TÖ, Ağadündüz D, Ozogul F, Rocha JMF. Unveiling the Impact of Lactic Acid Bacteria on Blood Lipid Regulation for Cardiovascular Health. Fermentation. 2024; 10(7):350. https://doi.org/10.3390/fermentation10070350
Chicago/Turabian StyleYilmaz, Birsen, Neslihan Arslan, Teslime Özge Şahin, Duygu Ağadündüz, Fatih Ozogul, and João Miguel F. Rocha. 2024. "Unveiling the Impact of Lactic Acid Bacteria on Blood Lipid Regulation for Cardiovascular Health" Fermentation 10, no. 7: 350. https://doi.org/10.3390/fermentation10070350
APA StyleYilmaz, B., Arslan, N., Şahin, T. Ö., Ağadündüz, D., Ozogul, F., & Rocha, J. M. F. (2024). Unveiling the Impact of Lactic Acid Bacteria on Blood Lipid Regulation for Cardiovascular Health. Fermentation, 10(7), 350. https://doi.org/10.3390/fermentation10070350









