The Anti-Methanogenic Activity of Lovastatin in Batch Cultures Using Rumen Inoculum from Sheep, Goats, and Cows
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Treatments
2.2. Experimental Procedures
2.3. In Vitro 24 h Incubation
2.3.1. Analysis of Gas Profile
2.3.2. Determination of Volatile Fatty Acids and Ammonia Nitrogen Concentrations
2.4. In Vitro 72 h Incubation
2.5. Statistical Analysis
3. Results and Discussion
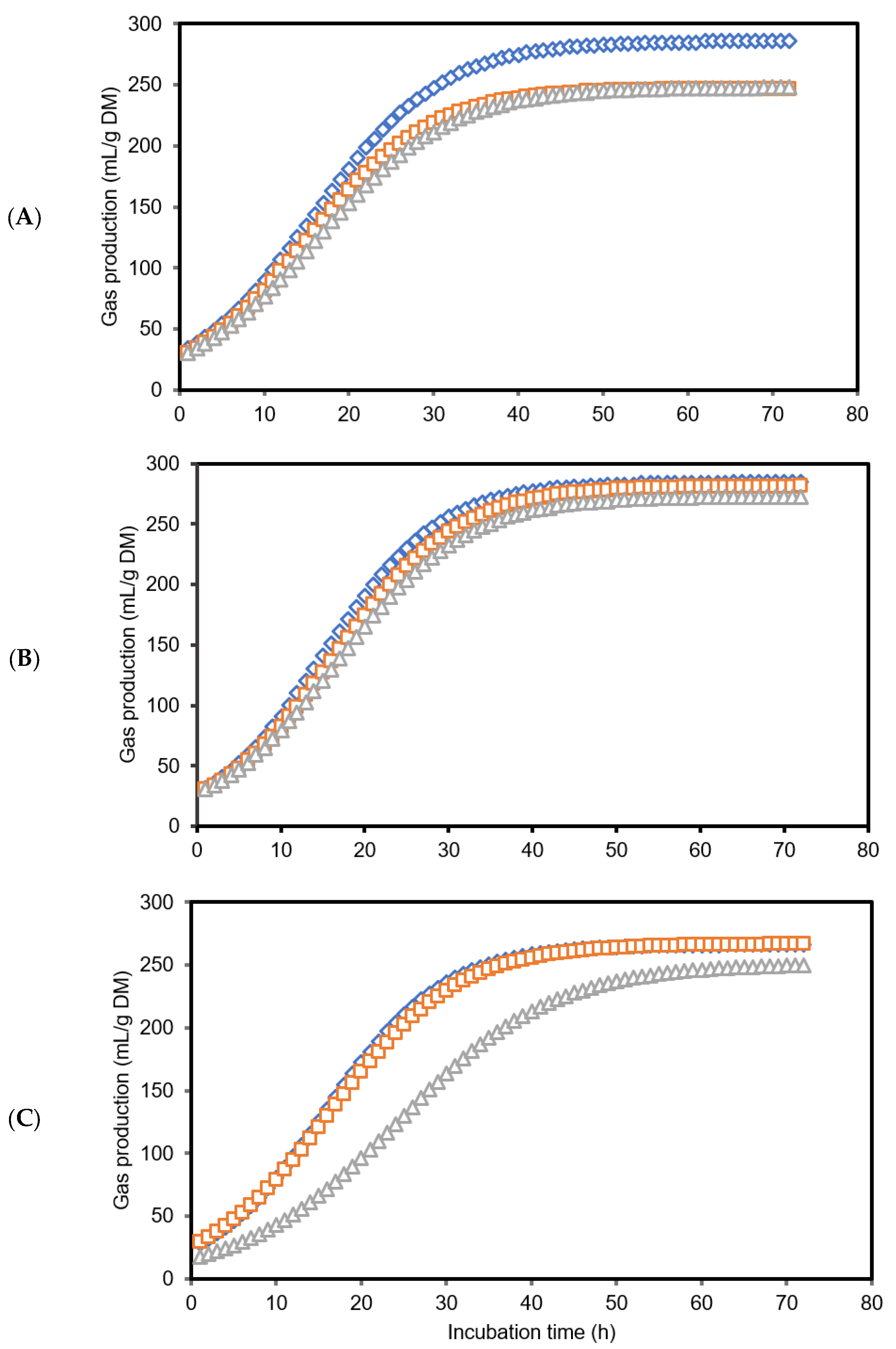
3.1. Gas Production Kinetics and Methane Production
3.2. In Vitro Degradability
3.3. Fermentation Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yusuf, R.O.; Noor, Z.Z.; Abba, A.H.; Hassan, M.A.A.; Din, M.F.M. Methane Emission by Sectors: A Comprehensive Review of Emission Sources and Mitigation Methods. Renew. Sust. Energy Rev. 2012, 16, 5059–5070. [Google Scholar] [CrossRef]
- Stavert, A.R.; Saunois, M.; Canadell, J.G.; Poulter, B.; Jackson, R.B.; Regnier, P.; Lauerwald, R.; Raymond, P.A.; Allen, G.H.; Patra, P.K.; et al. Regional Trends and Drivers of the Global Methane Budget. Glob. Change Biol. 2012, 28, 182–200. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Peng, S.; Ciais, P.; Saunois, M.; Dangal, S.R.C.; Herrero, M.; Havlík, P.; Tian, H.; Bousquet, P. Revisiting Enteric Methane Emissions from Domestic Ruminants and their δ13CCH4 Source Signature. Nat. Commun. 2019, 10, 3420. [Google Scholar] [CrossRef]
- Van, A.A. Methane. A review. J. Integr. Environ. Sci. 2012, 9, 5–30. [Google Scholar]
- Min, B.R.; Solaiman, S.; Waldrip, H.M.; Parker, D.; Todd, R.W.; Brauer, D. Dietary Mitigation of Enteric Methane Emissions from Ruminants: A Review of Plant Tannins Mitigation Options. Anim. Nutr. 2020, 6, 231–246. [Google Scholar] [CrossRef]
- Scoones, I. Livestock, Methane, and Climate Change: The Politics of Global Assessments. Interdiscip. Rev. Clim. Change 2022, 14, e790. [Google Scholar] [CrossRef] [PubMed]
- Zubir, M.A.; Bong, C.P.C.; Ishak, S.A.; Ishak, S.A.; Ho, W.S.; Hashim, H. The Trends and Projections of Greenhouse Gas Emission by the Livestock Sector in Malaysia. Clean Technol. Environ. Policy 2022, 24, 363–377. [Google Scholar] [CrossRef]
- Johnson, K.A.; Johnson, D.E. Methane Emissions from Cattle. J. Anim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.N. Dietary manipulation: A sustainable way to mitigate methane emissions from ruminants. J. Sci. Biotechnol. 2018, 60, 15. [Google Scholar] [CrossRef]
- Jose, S.; Dollinger, J. Silvopasture: A Sustainable Livestock Production System. Agrofor. Syst. 2019, 93, 1–9. [Google Scholar] [CrossRef]
- Honan, M.; Feng, X.; Tricarico, J.M.; Kebreab, E. Feed Additives as a Strategic Approach to Reduce Enteric Methane Production in Cattle: Modes of Action, Effectiveness and Safety. Anim. Prod. Sci. 2021, 62, 1303–1317. [Google Scholar] [CrossRef]
- Ábrego-García, A.; Poggi-Varaldo, H.M.; Robles-González, V.; Ponce-Noyola, T.; Calva-Calva, G.; Ríos-Leal, E.; Estrada-Bárcenas, D.; Mendoza-Vargas, A. Lovastatin as a Supplement to Mitigate Rumen Methanogenesis: An Overview. J. Sci. Biotechnol. 2021, 12, 131. [Google Scholar]
- Gottlieb, K.; Wacher, V.; Sliman, J.; Pimentel, M. Review Article: Inhibition of Methanogenic Archaea by Statins as a Targeted Management Strategy for Constipation and Related Disorders. Aliment. Pharmacol. Ther. 2016, 4, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Tobert, J. Lovastatin and Beyond: The History of the HMG-Coa Reductase Inhibitors. Nat. Rev. Drug. Discov. 2003, 2, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Khonkhaeng, B.; Cherdthong, A. Improving Nutritive Value of Purple Field Corn Residue and Rice Straw by Culturing with White-Rot Fungi. J. Fungi 2020, 6, 69. [Google Scholar] [CrossRef]
- Ábrego-García, A.; Poggi-Varaldo, H.M.; Mendoza-Vargas, A.; Mercado-Valle, F.G.; Rios-Leal, E.; Ponce-Noyola, T.; Calva-Calva, G. Effects of Fermented Oat Straw as a Lovastatin-Carrier on In Vitro Methane Production and Rumen Microbiota. Front. Energy 2021, 9, 630701. [Google Scholar]
- Joch, M.; Vadroňová, M.; Výborná, A.; Jochová, K. Inhibition of In Vitro Rumen Methane Production by Three Statins. Ann. Anim. Sci. 2022, 22, 271–282. [Google Scholar] [CrossRef]
- Bueno, I.C.S.; Brandi, R.A.; Franzolin, R.; Benetel, G.; Fagundes, G.M.; Abdalla, A.L.; Louvandini, H.; Muir, J.P. In Vitro Methane Production and Tolerance to Condensed Tannins in Five Ruminant Species. Anim. Feed. Sci. Technol. 2015, 205, 1–9. [Google Scholar] [CrossRef]
- Martinez-Fernandez, G.; Denman, S.E.; Yang, C.; Cheung, J.; Mitsumori, M.; McSweeney, C.S. Methane Inhibition Alters the Microbial Community, Hydrogen Flow, and Fermentation Response in the Rumen of Cattle. Front. Microbiol. 2016, 7, 1122. [Google Scholar] [CrossRef]
- Leahy, S.C.; Janssen, P.H.; Attwood, G.T.; Mackie, R.I.; McAllister, T.A.; Kelly, W.J. Electron Flow: Key to Mitigating Ruminant Methanogenesis. Trends Microbiol. 2022, 30, 209–212. [Google Scholar] [CrossRef]
- Ma, J.; Zhong, P.; Li, Y.; Sun, Z.; Sun, X.; Aung, M.; Hao, L.; Cheng, Y.; Zhu, W. Hydrogenosome, Pairing Anaerobic Fungi and H2-Utilizing Microorganisms Based on Metabolic Ties to Facilitate Biomass Utilization. J. Fungi 2022, 8, 338. [Google Scholar] [CrossRef] [PubMed]
- Ungerfeld, E.M. Metabolic Hydrogen Flows in Rumen Fermentation: Principles and Possibilities of Interventions. Front. Microbiol. 2020, 11, 589. [Google Scholar] [CrossRef] [PubMed]
- Jayanegara, A.; Goel, G.; Makkar, H.P.S.; Becker, K. Divergence between Purified Hydrolysable and Condensed Tannin Effects on Methane Emission, Rumen Fermentation and Microbial Population In Vitro. Anim. Feed. Sci. Technol. 2015, 209, 60–68. [Google Scholar] [CrossRef]
- Schilde, M.; von Soosten, D.; Hüther, L.; Kersten, S.; Meyer, U.; Zeyner, A.; Dänicke, S. Dose–Response Effects of 3-Nitrooxypropanol Combined with Low- And High-Concentrate Feed Proportions in the Dairy Cow Ration on Fermentation Parameters in a Rumen Simulation Technique. Animals 2021, 11, 1784. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, M.; Lin, B.; Ma, Z.Y.; Ungerfeld, E.M.; Wu, T.T.; Wen, J.N.; Zhang, X.M.; Deng, J.P.; Tan, Z.L. Association of Fibre Degradation with Ruminal Dissolved Hydrogen in Growing Beef Bulls Fed with Two Types of Forages. Br. J. Nutr. 2021, 125, 601–610. [Google Scholar] [CrossRef]
- Ramos-Morales, E.; Arco-Pérez, A.; Martín-García, A.I.; Yáñez-Ruiz, D.R.; Frutos, P.; Hervás, G. Use of stomach tubing as an alternative to rumen cannulation to study ruminal fermentation and microbiota in sheep and goats. Anim. Feed. Sci. Technol. 2014, 198, 57–66. [Google Scholar] [CrossRef]
- SAGARPA. Norma Oficial Mexicana NOM-062-ZOO-1999. Especificaciones Técnicas para la Producción, Cuidado y Uso de los Animales de Laboratorio. 2001. Secretaria de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación; Diario Oficial de la Federación: Ciudad de México, Mexico, 2001. [Google Scholar]
- AOAC. Association of Official Analytical Chemists. Official Methods of Analysis of the AOAC, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, S.M.; Mendoza, G.D.; Miranda, R.L.A.; Gutiérrez, C.G. In Vitro Gas Production and Ruminal Fermentation of Glycerol, Propylene Glycol and Molasses. Anim. Feed. Sci. Technol. 2009, 154, 112–118. [Google Scholar] [CrossRef]
- Ábrego-García, A.; Héctor, M.P.V.; Robles, G.V.S.; Rios, L.E.; Ponce, N.T.; Calva, C.G.; Estrada, B.D.A.; Mendoza, V.A. In Vitro Inhibition of Rumen Methanogenesis of a High-Grain Diet Using Lovastatin. Rev. Int. Contam. Ambient. 2022, 38, 491–500. [Google Scholar]
- Martínez-Hernández, B.E.; Salvador-Flores, O.; Miranda-Romero, L.A. Indicador de Calentamiento Global a Partir de la Fermentación Ruminal de Alimentos con diferentes Niveles de Energía y Proteína. Pastos Forrajes 2020, 42, 285–289. [Google Scholar]
- McCullough, H. The Determination of Ammonia in Whole Blood by a Direct Colorimetric Method. Clin. Chim. Acta 1967, 17, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Romero, L.A.; Tirado-González, D.N.; Tirado-Estrada, G.; Améndola-Massiotti, R.; Sandoval-González, L.; Ramírez-Valverde, R.; Salem, A.Z.M. Quantifying Non-fibrous Carbohydrates, Acid Detergent Fiber and Cellulose of Forage Through an In Vitro Gas Production Technique. J. Sci. Food. Agric. 2020, 100, 3099–3110. [Google Scholar] [CrossRef] [PubMed]
- Pitt, R.E.; Cross, T.L.; Pell, A.N.; Schofield, P.; Doane, P.H. Use of In Vitro Gas Production Models in Ruminal Kinetics. Math. Biosci. 1999, 159, 145–163. [Google Scholar] [CrossRef] [PubMed]
- Roman-Garcia, Y.; Denton, B.L.; Mitchell, K.E.; Lee, C.; Socha, M.T.; Firkins, J.L. Conditions Stimulating Neutral Detergent Fiber Degradation by Dosing Branched-Chain Volatile Fatty Acids. I: Comparison with Branched-Chain Amino Acids and Forage Source in Ruminal Batch Cultures. J. Dairy Sci. 2021, 104, 6739–6755. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Espinoza, M.; Bernal-Barragán, H.; Vásquez-Aguilar, N.C.; Cantu-Silva, I.; Cotera-Correa, M.; Estrada-Castillón, A.E.; González-Rodríguez, H.; Sarquis-Ramírez, J.I. Cell-Wall Composition and Digestibility of Five Native Shrubs of the Tamaulipan Thornscrub in Northeastern Mexico. Trop. Subtrop. 2021, 24, 15. [Google Scholar] [CrossRef]
- Zhao, C.; Hu, Y.; Chen, H.; Li, B.; Cao, L.; Xia, J.; Yin, Y. An in vitro evaluation of the effects of different statins on the structure and function of human gut bacterial community. PLoS ONE 2020, 15, e0230200. [Google Scholar] [CrossRef]
- Osorio-Teran, A.I.; Mendoza-Martínez, G.D.; Miranda-Romero, L.A.; Martínez-Gomez, D.; Hernández-García, P.A.; Martínez-García, J.A. Effect of Calcium Propionate and Monensin on In Vitro Digestibility and Gas Production. Rev. Bras. De Zootec. 2017, 46, 348–353. [Google Scholar] [CrossRef]
- Yáñez-Ruiz, D.R.; Bannink, A.; Dijkstra, J.; Kebreab, E.; Morgavi, D.P.; O’Kiely, P.; Reynolds, C.K.; Schwarm, A.; Shingfield, K.J.; Yu, Z.; et al. Design, Implementation and Interpretation of In Vitro Batch Culture Experiments to Assess Enteric Methane Mitigation in Ruminants—A Review. Anim. Feed. Sci. Technol. 2016, 216, 1–18. [Google Scholar] [CrossRef]
- Langda, S.; Zhang, C.; Zhang, K.; Gui, B.; Ji, D.; Deji, C.; Cuoji, A.; Wang, X.; Wu, Y. Diversity and Composition of Rumen Bacteria, Fungi, and Protozoa in Goats and Sheep Living in the Same High-Altitude Pasture. Animals 2020, 10, 186. [Google Scholar] [CrossRef]
- Henderson, G.; Cox, F.; Ganesh, S.; Jonker, A.; Young, W.; Janssen, P.H. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci. Rep. 2015, 5, 14567. [Google Scholar] [CrossRef]
- Wang, K.; Xiong, B.; Zhao, X. Could Propionate Formation Be Used to Reduce Enteric Methane Emission in Ruminants? Sci. Total Environ. 2022, 855, 158867. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; Fukuma, N.; Hanada, M.; Nishida, T. The Efficacy of Plant-Based Bioactives Supplementation to Different Proportion of Concentrate Diets on Methane Production and Rumen Fermentation Characteristics In Vitro. Animals 2021, 11, 1029. [Google Scholar] [CrossRef] [PubMed]
- Lashof, D.A.; Ahuja, D.R. Relative Contributions of Greenhouse Gas Emissions to Global Warming. Nature 1990, 344, 529–531. [Google Scholar] [CrossRef]
- Albores-Moreno, S.; Alayon-Gamboa, J.A.; Miranda-Romero, L.A.; Jiménez-Ferrer, G.; Ku-Vera, J.C.; Vargas-Villamil, L. Nutritional Composition, In Vitro Degradation and Potential Fermentation of Tree Species Grazed by Ruminants in Secondary Vegetation (Acahual) of Deciduous Forest. J. Anim. Plant Sci. 2018, 28, 1263–1275. [Google Scholar]
- Zhang, L.; Chung, J.; Jiang, Q.; Sun, R.; Zhang, J.; Zhong, Y.; Ren, N. Characteristics of Rumen Microorganisms Involved in Anaerobic Degradation of Cellulose at Various pH Values. RSC Adv. 2017, 7, 40303–40310. [Google Scholar] [CrossRef]
- Prusty, S.; Kundu, S.; Kumar, V. Nutrient Utilization and Methane Emissions in Murrah Buffalo Calves Fed on Diets with Different Methanogenic Potential. Livest. Sci. 2017, 202, 89–95. [Google Scholar] [CrossRef]
- Soliva, C.R.; Amelchanka, S.L.; Duval, S.M.; Kreuzer, M. Ruminal Methane Inhibition Potential of Various Pure Compounds in Comparison with Garlic Oil as Determined with a Rumen Simulation Technique (Rusitec). Br. J. Nutr. 2011, 106, 114–122. [Google Scholar] [CrossRef]
- O’Brien, M.; Navarro-Villa, A.; Purcell, P.J.; Boland, T.M.; O’Kiely, p. Reducing In Vitro Rumen Methanogenesis for Two Contrasting Diets Using a Series of Inclusion Rates of Different Additives. Anim. Prod. Sci. 2013, 54, 141–157. [Google Scholar] [CrossRef][Green Version]
- Miller, T.L.; Wolin, M.J. Inhibition of Growth of Methane-Producing Bacteria of the Ruminant Forestomach by Hydroxymethylglutaryl-Scoa Reductase Inhibitors. J. Dairy Sci. 2001, 84, 1445–1448. [Google Scholar] [CrossRef]
- Mohd, A.P.; Jahromi, M.F.; Ariff, M.O.; Ebrahimi, M.; Candyrine, S.C.L.; Liang, J.B. Aspergillus Terreus Treated Rice Straw Suppresses Methane Production and Enhances Feed Digestibility in Goats. Trop. Anim. Health Prod. 2018, 50, 565–571. [Google Scholar] [CrossRef]
- Getachew, G.; Robinson, P.H.; DePeters, E.J.; Taylor, S.J. Relationships between Chemical Composition, Dry Matter Degradation and In Vitro Gas Production of Several Ruminant Feeds. Anim. Feed. Sci. Technol. 2004, 111, 57–71. [Google Scholar] [CrossRef]
- Morgavi, D.P.; Martin, C.; Boudra, H. Fungal Secondary Metabolites from Monascus Spp. Reduce Rumen Methane Production In Vitro and In Vivo. J. Anim. Sci. 2013, 91, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Restrepo, C.A.; O’neill, C.J.; López-Villalobos, N.; Padmanabha, J.; McSweeney, C. Tropical Cattle Methane Emissions: The Role of Natural Statins Supplementation. Anim. Prod. Sci. 2014, 54, 1294–1299. [Google Scholar] [CrossRef]
- Roasa, J.; De Villa, R.; Mine, Y.; Tsao, R. Phenolics of Cereal, Pulse and Oilseed Processing By-Products and Potential Effects of Solid-State Fermentation on Their Bioaccessibility, Bioavailability and Health Benefits: A Review. Trends Food Sci. Technol. 2021, 116, 954–974. [Google Scholar] [CrossRef]
- Lan, W.; Yang, C. Ruminal Methane Production: Associated Microorganisms and The Potential of Applying Hydrogen-Utilizing Bacteria for Mitigation. Sci. Total Environ. 2019, 654, 1270–1283. [Google Scholar] [CrossRef] [PubMed]
- Janssen, P.H. Influence of Hydrogen on Rumen Methane Formation and Fermentation Balances through Microbial Growth Kinetics and Fermentation Thermodynamics. Anim. Feed. Sci. Technol. 2010, 160, 1–22. [Google Scholar] [CrossRef]
- Newbold, C.J.; de la Fuente, G.; Belanche, A.; Ramos-Morales, E.; McEwan, N.R. The Role of Ciliate Protozoa in the Rumen. Front. Microbiol. 2015, 6, 1313. [Google Scholar] [CrossRef]
- Ayemele, A.G.; Ma, L.; Park, T.; Xu, J.; Yu, Z.; Bu, D. Giant milkweed (Calotropis gigantea): A New Plant Resource to Inhibit Protozoa and Decrease Ammoniagenesis of Rumen Microbiota In Vitro Without Impairing Fermentation. Sci. Total Environ. 2020, 743, 140665. [Google Scholar] [CrossRef]
- Melo, L.; Caldas, I.S.; Azevedo, M.A.; Gonçalves, K.R.; da Silva do Nascimento, A.F.; Figueiredo, V.P.; de Figueiredo Diniz, L.; de Lima, W.G.; Torres, R.M.; Bahia, M.T.; et al. Low Doses of Simvastatin Therapy Ameliorate Cardiac Inflammatory Remodeling in Trypanosoma Cruzi-Infected Dogs. Am. J. Trop. Med. Hyg. 2011, 84, 325–331. [Google Scholar] [CrossRef]
- Martín-Navarro, C.M.; Lorenzo-Morales, J.; Machin, R.P.; López-Arencibia, A.; García-Castellano, J.M.; de Fuentes, I.; Loftus, B.; Maciver, S.K.; Valladares, B.; Piñero, J.E. Inhibition of 3-Hydroxy-3-Methylglutaryl–Coenzyme A Reductase and Application of Statins as A Novel Effective Therapeutic Approach Against Acanthamoeba Infections. Antimicrob. Agents Chemother. 2013, 57, 375–381. [Google Scholar] [CrossRef]
| Ingredient | g/kg DM |
|---|---|
| Maize silage | 490 |
| Oat straw | 210 |
| Ground corn | 210 |
| Soybean meal | 90 |
| Chemical composition | |
| Crude protein | 113.6 |
| Ether extract | 42.2 |
| Ash | 71.9 |
| Neutral detergent fiber | 461.8 |
| Acid detergent fiber | 262.9 |
| Item | Sheep | Goats | Cows | SEM | p Values | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Con | L | H | Con | L | H | Con | L | H | I | D | I × D | ||
| mV, mL/g | 277.7 | 274.5 | 251.0 | 263.6 | 246.3 | 250.2 | 284.1 | 290.4 | 274.3 | 28.37 | 0.181 | 0.557 | 0.926 |
| S, mL/h | 0.036 | 0.034 | 0.031 | 0.034 | 0.036 | 0.033 | 0.034 | 0.035 | 0.032 | 0.002 | 0.896 | 0.117 | 0.553 |
| L, h | 1.95 | 1.49 | 1.16 | 1.71 | 1.90 | 2.1 | 1.95 | 1.61 | 1.36 | 0.71 | 0.593 | 0.709 | 0.735 |
| CH4, mL/g DM | 44.9 a | 31.2 ab | 20.1 bcd | 27.4 b | 19.2 d | 16.7 cd | 30.2 ab | 18.8 bcd | 23.72 bc | 4.40 | 0.0002 | <0.0001 | 0.011 |
| CO2, mL/g DM | 216.4 b | 242.6 ab | 205.4 b | 233 c | 225.5 c | 236 c | 270.6 ab | 290.9 a | 267.9 ab | 19.44 | <0.0001 | 0.077 | 0.765 |
| GWI, CO2e | 1249 a | 961 ab | 575 cde | 724 bc | 537 e | 425 de | 965 ab | 723 bcd | 813 bc | 97.75 | <0.0001 | <0.0001 | 0.007 |
| Item | Sheep | Goats | Cows | SEM | p Values | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Con | L | H | Con | L | H | Con | L | H | I | D | I × D | ||
| RFF (mg/g) | 123.1 ab | 124.9 ab | 110.2 a | 187.32 b | 197.8 bc | 197.9 bc | 214.0 c | 198.49 bc | 195.01 bc | 38 | 0.0004 | 0.917 | 0.955 |
| MFF (mg/g) | 200.5 | 186.9 | 165.84 | 184.74 | 183.36 | 175.81 | 199.93 | 193.10 | 179.74 | 22 | 0.666 | 0.153 | 0.915 |
| SFF (mg/g) | 243.3 ab | 268.1 a | 275.4 a | 212.9 ab | 171.16 b | 199.8 ab | 199.4 ab | 211.45 ab | 213.15 ab | 29 | 0.0003 | 0.615 | 0.392 |
| TFF (mg/g) | 567.98 | 579.16 | 551.20 | 585.02 | 552.40 | 573.54 | 613.39 | 602.92 | 588.71 | 58 | 0.382 | 0.817 | 0.948 |
| IVNFD (g/kg) | 541 a | 433 bc | 477 bc | 483 abc | 420 bc | 403 c | 548 a | 499 ab | 410 c | 24 | 0.005 | 0.001 | 0.020 |
| Item | Sheep | Goats | Cows | SEM | p Values | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Con | L | H | Con | L | H | Con | L | H | I | D | I × D | ||
| VFA production (mmol/24 h) | |||||||||||||
| Total VFA | 3.3 b | 5.2 bc | 5.8 bc | 8.8 ab | 4.9 b | 9.4 ab | 2.8 c | 3.9 bc | 3.7 bc | 0.83 | 0.0134 | 0.1499 | 0.2030 |
| Acetate | 2.1 ab | 3.3 ab | 3.8 ab | 6.8 ab | 3.4 ab | 7.1 a | 1.8 b | 2.7 ab | 2.5 ab | 1.34 | 0.0079 | 0.1477 | 0.1472 |
| Propionate | 0.79 | 1.6 | 1.8 | 1.2 | 1.1 | 2.5 | 0.67 | 0.87 | 0.82 | 0.89 | 0.1922 | 0.1863 | 0.5485 |
| Butyrate | 0.43 | 0.20 | 0.19 | 0.63 | 0.52 | 0.37 | 0.15 | 0.25 | 0.26 | 0.14 | 0.0725 | 0.5498 | 0.5148 |
| Isovalerate | ND | ND | ND | 0.10 | 0.15 | 0.09 | 0.03 | 0.04 | 0.01 | 0.04 | 0.1445 | 0.3354 | 0.8795 |
| Valerate | 0.03 b | 0.05 b | 0.02 b | 0.06 b | 0.34 a | 0.10 b | 0.03 b | 0.04 b | 0.00 b | 0.024 | 0.0033 | 0.0050 | 0.0171 |
| A/P | 2.6 | 2.1 | 2.2 | 5.5 | 3.0 | 2.9 | 2.7 | 3.6 | 3.2 | 1.34 | 0.3651 | 0.6845 | 0.6858 |
| NH3-N (mg/dL) | 1.1 ab | 0.6 b | 0.6 b | 2.2 a | 0.9 ab | 1.3 ab | 0.3 b | 0.6 b | 0.7 ab | 0.47 | 0.003 | 0.1541 | 0.1986 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ábrego-García, A.; Medina-Mendoza, G.G.; Miranda-Romero, L.A. The Anti-Methanogenic Activity of Lovastatin in Batch Cultures Using Rumen Inoculum from Sheep, Goats, and Cows. Fermentation 2024, 10, 393. https://doi.org/10.3390/fermentation10080393
Ábrego-García A, Medina-Mendoza GG, Miranda-Romero LA. The Anti-Methanogenic Activity of Lovastatin in Batch Cultures Using Rumen Inoculum from Sheep, Goats, and Cows. Fermentation. 2024; 10(8):393. https://doi.org/10.3390/fermentation10080393
Chicago/Turabian StyleÁbrego-García, Amaury, Gustavo Gerardo Medina-Mendoza, and Luis Alberto Miranda-Romero. 2024. "The Anti-Methanogenic Activity of Lovastatin in Batch Cultures Using Rumen Inoculum from Sheep, Goats, and Cows" Fermentation 10, no. 8: 393. https://doi.org/10.3390/fermentation10080393
APA StyleÁbrego-García, A., Medina-Mendoza, G. G., & Miranda-Romero, L. A. (2024). The Anti-Methanogenic Activity of Lovastatin in Batch Cultures Using Rumen Inoculum from Sheep, Goats, and Cows. Fermentation, 10(8), 393. https://doi.org/10.3390/fermentation10080393




