Exploring Microbial Dynamics: The Interaction between Yeasts and Acetic Acid Bacteria in Port Wine Vinegar and Its Implications on Chemical Composition and Sensory Acceptance
Abstract
1. Introduction
2. Historical Development and Traditional Practices
Evolution of Vinegar Production, Raw Materials, and European Legislation
3. Microbial Ecosystem and Chemical Evolution in Wine Vinegar Production
3.1. Diversity of Microorganisms and Their Roles
3.2. Functions of Yeasts in Vinegar Production
| Metabolite | Sensory Impact | Health Benefit |
|---|---|---|
| Saccharomyces | ||
| Acetaldehyde | Fruity, green apple notes | - |
| Acetic acid | Vinegar-like aroma and taste | Can improve digestion in small amounts |
| B-complex vitamins (e.g., thiamine) | Nutritional enhancement | Essential for energy metabolism; nerve function |
| Beta-glucans | Viscosity, mouthfeel | Immunomodulatory effects |
| Citric acid | Tartness | Antioxidant; may enhance nutrient absorption |
| Diacetyl | Buttery or butterscotch flavor | - |
| Esters (e.g., ethyl acetate) | Fruity aromas | - |
| Ethanol | Contributes to the alcohol content | Antiseptic properties |
| Glutathione | Antioxidant | Detoxification; supports immune function |
| Glycerol | Sweetness, fullness | Hydrating properties |
| Higher alcohols (e.g., isoamyl alcohol) | Solvent-like, fuel oils | - |
| Lactic acid | Tangy, sour taste | Metabolic acid; contributes to microbiota balance |
| Minerals (e.g., potassium, magnesium) | - | Essential for enzymatic reactions; muscle function |
| Phenolic compounds | Spicy, clove-like flavors | Antioxidant properties |
| Polysaccharides | Mouthfeel enhancement | Prebiotic effects |
| Succinic acid | Bitterness, acidity | May support cellular metabolism |
| Non-Saccharomyces | ||
| 4-Ethylguaiacol | Smoky, clove aroma | - |
| 4-Ethylphenol | Barnyard, medicinal aroma | - |
| Acetaldehyde | Green apple or ripe persimmon aroma | Acts as an antimicrobial agent |
| Acetoin | Butter-like aroma | Potential antioxidant properties |
| Carotenoids | Pigmentation, antioxidant properties | Antioxidant properties |
| Ergosterol | Contributes to vitamin D synthesis | Supports bone health |
| Ethanol | Contributes to alcohol content | Antiseptic properties |
| Ethyl acetate | Fruity, nail polish remover aroma | - |
| Ethyl butyrate | Pineapple aroma | - |
| Gluconic acid | Mild acidity | Supports mineral absorption |
| Isoamyl acetate | Banana aroma | - |
| Isoamyl alcohol | Solvent-like | - |
| Polyunsaturated fatty acids | Contributes to flavor and aroma | Supports cardiovascular health |
| Succinic acid | Bitterness, acidity, saltiness | It may support cellular metabolism |
| β-Galactosidase | Enhances sweetness from lactose | Potential probiotic effects |
3.3. Roles of Acetic Acid Bacteria in Vinegar Production
| Characteristic | Details |
|---|---|
| Size | Typically, 0.4 to 1.0 µm in width and 0.8 to 4.5 µm in length |
| Morphology | Gram-negative or gram-variable, they exist as single cells, filamentous or rod-shaped, with ellipsoidal to rod morphologies. They are mobile due to peritrichous or polar flagella. |
| Family and classification | The Acetobacteraceae family comprises 47 genera and 207 species; 20 genera and 108 species are classified under AAB. |
| Prominent genera in vinegar production | Acetobacter species (e.g., Acetobacter aceti, Acetobacter pasteurianus, Acetobacter xylinum) and Gluconobacter oxydans among Gluconobacter species |
| Enzymatic abilities | Catalase and oxidase positive; capable of degrading hydrogen peroxide and using oxygen as a terminal electron acceptor in respiration |
| Metabolic capabilities | Capable of nitrogen fixation; utilize nitrogen gas as a nitrogen source for growth |
| Optimal growth conditions | pH range of 4.0–6.0; temperature range of 25–30 °C |
| Substrate requirements | Require ethanol or other alcohols as substrates for growth and energy production. |
| Metabolic pathways | The primary pathway is oxidative fermentation (AAB oxidative fermentation–AOF), converting ethanol into acetic acid. |
| Supporting metabolic pathways | Utilize the pentose phosphate pathway (PPP) and Entner–Doudoroff (ED) pathways to provide energy and reduce power for ethanol oxidation. |
| Pentose phosphate pathway | Generates NADPH and pentose sugars essential for biosynthetic processes and redox balance |
| Entner–Doudoroff (ED) pathway | Produces pyruvate and NADPH through oxidation of glucose or related compounds, supporting energy production and redox homeostasis |
| Metabolites | Details |
|---|---|
| Acetaldehyde | Intermediate in acetic acid production, which also affects flavor |
| Acetic acid | The primary product of ethanol oxidation by AAB, responsible for vinegar’s acidity |
| Acetoin | Intermediate in butanediol fermentation pathway |
| Acetone | Ketone produced during fermentation |
| Bacteriocins | Antimicrobial peptides produced by LAB |
| Butyric acid | Short-chain fatty acids that may affect the flavor profile |
| Citric acid | Organic acid contributes to flavor complexity and freshness. |
| Diacetyl | A by-product that can impart a buttery flavor, contributing to the vinegar’s depth |
| Ethanol | Residual ethanol from incomplete oxidation |
| Ethyl acetate | Ester that contributes to fruity and solvent-like aromas. |
| Formic acid | Minor by-products affecting flavor and acidity. |
| Gluconic acid | Organic acid formed from glucose oxidation |
| Lactic acid | Organic acid produced by LAB |
| Minerals | Micronutrients required for bacterial growth |
| Polyphenols | Extracted from Port wine, affecting flavor and color |
| Polysaccharides | Exopolysaccharides contribute to viscosity. |
| Propionic acid | Organic acid contributes to flavor. |
| Succinic acid | Depending on its concentration, dicarboxylic acid contributes to acidity, bitterness, and saltiness. |
| Trace elements | Essential for microbial enzyme function |
| Vitamins | Essential for bacterial metabolism |
| Water | Formed alongside acetic acid during oxidation |
3.4. Interaction between Yeasts and Acetic Acid Bacteria
3.5. Chemical Evolution during Fermentation during Port Wine Processing
4. Sensory Characteristics and Consumer Perception
4.1. Influence of Microbial Interactions on Sensory Properties and Chemical-Sensory Correlations
4.2. Consumer Preferences and Acceptance
5. Challenges, Future Directions, and Applications
5.1. Current Challenges and Research Opportunities in Port Wine Vinegar Production
5.2. Pratical Applications
6. Final Remarks
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Román-Camacho, J.J.; García-García, I.; Santos-Dueñas, I.M.; García-Martínez, T.; Mauricio, J.C. Latest Trends in Industrial Vinegar Production and the Role of Acetic Acid Bacteria: Classification, Metabolism, and Applications—A Comprehensive Review. Foods 2023, 12, 3705. [Google Scholar] [CrossRef]
- Tesfaye, W.; Morales, M.L.; García-Parrilla, M.C.; Troncoso, A.M. Wine Vinegar: Technology, Authenticity and Quality Evaluation. Trends Food Sci. Technol. 2002, 13, 12–21. [Google Scholar] [CrossRef]
- Bhat, S.V.; Akhtar, R.; Amin, T. An Overview on the Biological Production of Vinegar. Int. J. Fermented Foods 2014, 3, 139. [Google Scholar] [CrossRef]
- Melkis, K.; Jakubczyk, K. The Chemical Profiles and Antioxidant Properties of Live Fruit or Vegetable Vinegars Available on the Polish Food Market. Foods 2024, 13, 1488. [Google Scholar] [CrossRef]
- Chen, G.-L.; Zheng, F.-J.; Lin, B.; Yang, Y.-X.; Fang, X.-C.; Verma, K.K.; Yang, L.-F. Vinegar: A Potential Source of Healthy and Functional Food with Special Reference to Sugarcane Vinegar. Front. Nutr. 2023, 10, 1145862. [Google Scholar] [CrossRef]
- Budak, N.H.; Aykin, E.; Seydim, A.C.; Greene, A.K.; Guzel-Seydim, Z.B. Functional Properties of Vinegar. J. Food Sci. 2014, 79, R757–R764. [Google Scholar] [CrossRef]
- Mota, J.; Vilela, A. Aged to Perfection: The Scientific Symphony behind Port Wine, Vinegar, and Acetic Acid Bacteria. Fermentation 2024, 10, 200. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Erol, Z.; Rugji, J.; Taşçı, F.; Kahraman, H.A.; Toppi, V.; Musa, L.; Di Giacinto, G.; Bahmid, N.A.; Mehdizadeh, M.; et al. An Overview of Fermentation in the Food Industry—Looking Back from a New Perspective. Bioresour. Bioprocess. 2023, 10, 85. [Google Scholar] [CrossRef]
- Radman, S.; Brzović, P.; Radunić, M.; Rako, A.; Šarolić, M.; Ninčević Runjić, T.; Urlić, B.; Generalić Mekinić, I. Vinegar-Preserved Sea Fennel: Chemistry, Color, Texture, Aroma, and Taste. Foods 2023, 12, 3812. [Google Scholar] [CrossRef]
- Mas, A.; Torija, M.J.; García-Parrilla, M.D.C.; Troncoso, A.M. Acetic Acid Bacteria and the Production and Quality of Wine Vinegar. Sci. World J. 2014, 2014, 394671. [Google Scholar] [CrossRef]
- Ho, C.W.; Lazim, A.M.; Fazry, S.; Zaki, U.K.H.H.; Lim, S.J. Varieties, Production, Composition and Health Benefits of Vinegars: A Review. Food Chem. 2017, 221, 1621–1630. [Google Scholar] [CrossRef]
- Vilela, A. Microbial Dynamics in Sour–Sweet Wine Vinegar: Impacts on Chemical and Sensory Composition. Appl. Sci. 2023, 13, 7366. [Google Scholar] [CrossRef]
- Conner, H.A.; Allgeier, R.J. Vinegar: Its History and Development. Adv. Appl. Microbiol. 1976, 20, 81–133. [Google Scholar] [CrossRef]
- Harutyunyan, M.; Malfeito-Ferreira, M. Historical and Heritage Sustainability for the Revival of Ancient Wine-Making Techniques and Wine Styles. Beverages 2022, 8, 10. [Google Scholar] [CrossRef]
- de Almeida Costa, A.I.; Marano-Marcolini, C.; Malfeito-Ferreira, M.; Loureiro, V. Historical Wines of Portugal: The Classification, Consumer Associations and Marketing Implications. Foods 2021, 10, 979. [Google Scholar] [CrossRef]
- Bourgeois, J.; Barja, F. The History of Vinegar and of Its Acetification Systems. Arch. Sci. 2009, 62, 147–160. [Google Scholar]
- Vidra, A.; Németh, Á. Bio-Produced Acetic Acid: A Review. Period. Polytech. Chem. Eng. 2017, 62, 245–256. [Google Scholar] [CrossRef]
- Giudici, P.; Gullo, M.; Solieri, L.; Falcone, P.M. Technological and Microbiological Aspects of Traditional Balsamic Vinegar and Their Influence on Quality and Sensorial Properties. Adv. Food Nutr. Res. 2009, 58, 137–182. [Google Scholar] [CrossRef]
- Ministry of Agriculture, Rural Development and Fisheries. Decree Law No. 174/2007 of May 8. In Republic Diary, 1st ed.; Lisbon, Portugal, 2007; pp. 1–3. Available online: https://diariodarepublica.pt/dr/detalhe/decreto-lei/174-2007-520833 (accessed on 20 June 2024).
- Antoniewicz, J.; Jakubczyk, K.; Kwiatkowski, P.; Maciejewska-Markiewicz, D.; Kochman, J.; Rębacz-Maron, E.; Janda-Milczarek, K. Analysis of Antioxidant Capacity and Antimicrobial Properties of Selected Polish Grape Vinegars Obtained by Spontaneous Fermentation. Molecules 2021, 26, 4727. [Google Scholar] [CrossRef]
- Martiniuk, J.T.; Hamilton, J.; Dodsworth, T.; Measday, V. Grape-Associated Fungal Community Patterns Persist from Berry to Wine on a Fine Geographical Scale. FEMS Yeast Res. 2023, 23, foac067. [Google Scholar] [CrossRef]
- Fleet, G.H. Wine Yeasts for the Future. FEMS Yeast Res. 2008, 8, 979–995. [Google Scholar] [CrossRef]
- Yassunaka Hata, N.N.; Surek, M.; Sartori, D.; Serrato, R.V.; Spinosa, W.A. Role of Acetic Acid Bacteria in Food and Beverages. Food Technol. Biotechnol. 2023, 61, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Zhang, X.; Xiao, Y.; Sheng, Q.; Tu, L.; Chen, F.; Yan, Y.; Zheng, Y.; Wang, M. Interaction of Acetic Acid Bacteria and Lactic Acid Bacteria in Multispecies Solid-State Fermentation of Traditional Chinese Cereal Vinegar. Front. Microbiol. 2022, 13, 964855. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hao, L.; Jin, Y.; Huang, J.; Zhou, R.; Wu, C. Functional Roles and Engineering Strategies to Improve the Industrial Functionalities of Lactic Acid Bacteria during Food Fermentation. Biotechnol. Adv. 2024, 74, 108397. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, F.; Dai, X.; Liu, Y.; Mu, J.; Wang, J.; Ma, Q.; Sun, J. Changes in Vinegar Quality and Microbial Dynamics during Fermentation Using a Self-Designed Drum-Type Bioreactor. Front. Nutr. 2023, 10, 1126562. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M. Lehninger Principles of Biochemistry, 8th ed.; Macmillan Learning: London, UK, 2021; ISBN 9781319381493. [Google Scholar]
- Durán-Soria, S.; Pott, D.M.; Osorio, S.; Vallarino, J.G. Sugar Signaling during Fruit Ripening. Front. Plant Sci. 2020, 11, 564917. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, T.; Ates, F.; Turan, M.; Hatterman-Valenti, H.; Kaya, O. Dynamics of Sugars, Organic Acids, Hormones, and Antioxidants in Grape Varieties ‘Italia’ and ‘Bronx Seedless’ during Berry Development and Ripening. Horticulturae 2024, 10, 229. [Google Scholar] [CrossRef]
- Knop, M. Yeast Cell Morphology and Sexual Reproduction—A Short Overview and Some Considerations. Comptes Rendus Biol. 2011, 334, 599–606. [Google Scholar] [CrossRef]
- Chavez, C.M.; Groenewald, M.; Hulfachor, A.B.; Kpurubu, G.; Huerta, R.; Hittinger, C.T.; Rokas, A. The Cell Morphological Diversity of Saccharomycotina Yeasts. FEMS Yeast Res. 2024, 24, foad055. [Google Scholar] [CrossRef]
- Gonzalez, R.; Morales, P. Truth in Wine Yeast. Microb. Biotechnol. 2022, 15, 1339–1356. [Google Scholar] [CrossRef]
- Lip, K.Y.F.; García-Ríos, E.; Costa, C.E.; Guillamón, J.M.; Domingues, L.; Teixeira, J.; van Gulik, W.M. Selection and Subsequent Physiological Characterization of Industrial Saccharomyces Cerevisiae Strains during Continuous Growth at Sub- and- Supra Optimal Temperatures. Biotechnol. Rep. 2020, 26, e00462. [Google Scholar] [CrossRef] [PubMed]
- Mencher, A.; Morales, P.; Tronchoni, J.; Gonzalez, R. Mechanisms Involved in Interspecific Communication between Wine Yeasts. Foods 2021, 10, 1734. [Google Scholar] [CrossRef] [PubMed]
- Labuschagne, P.; Divol, B. Thiamine: A Key Nutrient for Yeasts during Wine Alcoholic Fermentation. Appl. Microbiol. Biotechnol. 2021, 105, 953–973. [Google Scholar] [CrossRef] [PubMed]
- Concejero, B.; Hernandez-Orte, P.; Astrain, J.; Lacau, B.; Baron, C.; Ferreira, V. Evolution of Polyfunctional Mercaptans and Their Precursors during Merlot Alcoholic Fermentation. LWT—Food Sci. Technol. 2016, 65, 770–776. [Google Scholar] [CrossRef]
- Styger, G.; Jacobson, D.; Prior, B.A.; Bauer, F.F. Genetic Analysis of the Metabolic Pathways Responsible for Aroma Metabolite Production by Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2013, 97, 4429–4442. [Google Scholar] [CrossRef]
- Styger, G.; Prior, B.; Bauer, F.F. Wine Flavor and Aroma. J. Ind. Microbiol. Biotechnol. 2011, 38, 1145–1159. [Google Scholar] [CrossRef]
- Romano, P.; Braschi, G.; Siesto, G.; Patrignani, F.; Lanciotti, R. Role of Yeasts on the Sensory Component of Wines. Foods 2022, 11, 1921. [Google Scholar] [CrossRef]
- He, Y.; Wang, X.; Li, P.; Lv, Y.; Nan, H.; Wen, L.; Wang, Z. Research Progress of Wine Aroma Components: A Critical Review. Food Chem. 2023, 402, 134491. [Google Scholar] [CrossRef]
- Marques, C.; Dinis, L.-T.; Santos, M.J.; Mota, J.; Vilela, A. Beyond the Bottle: Exploring Health-Promoting Compounds in Wine and Wine-Related Products—Extraction, Detection, Quantification, Aroma Properties, and Terroir Effects. Foods 2023, 12, 4277. [Google Scholar] [CrossRef] [PubMed]
- Tekos, F.; Makri, S.; Skaperda, Z.-V.; Patouna, A.; Terizi, K.; Kyriazis, I.D.; Kotseridis, Y.; Mikropoulou, E.V.; Papaefstathiou, G.; Halabalaki, M.; et al. Assessment of Antioxidant and Antimutagenic Properties of Red and White Wine Extracts In Vitro. Metabolites 2021, 11, 436. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Knaze, V.; Luján-Barroso, L.; Romieu, I.; Scalbert, A.; Slimani, N.; Hjartåker, A.; Engeset, D.; Skeie, G.; Overvad, K.; et al. Differences in Dietary Intakes, Food Sources and Determinants of Total Flavonoids between Mediterranean and Non-Mediterranean Countries Participating in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Br. J. Nutr. 2013, 109, 1498–1507. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Mar, M.I.; Mateos, R.; García-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Bioactive Compounds in Wine: Resveratrol, Hydroxytyrosol and Melatonin: A Review. Food Chem. 2012, 130, 797–813. [Google Scholar] [CrossRef]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef] [PubMed]
- Sagonas, I.; Daoussis, D. Serotonin and Systemic Sclerosis. An Emerging Player in Pathogenesis. Jt. Bone Spine 2022, 89, 105309. [Google Scholar] [CrossRef] [PubMed]
- Marhuenda, J.; Villaño, D.; Arcusa, R.; Zafrilla, P. Melatonin in Wine and Beer: Beneficial Effects. Molecules 2021, 26, 343. [Google Scholar] [CrossRef] [PubMed]
- Ignacia Lambert-Royo, M.; Ubeda, C.; Del Barrio-Galán, R.; Sieczkowski, N.; Miquel Canals, J.; Peña-Neira, Á.; Gil i Cortiella, M. The Diversity of Effects of Yeast Derivatives during Sparkling Wine Aging. Food Chem. 2022, 390, 133174. [Google Scholar] [CrossRef] [PubMed]
- Nikolantonaki, M.; Julien, P.; Coelho, C.; Roullier-Gall, C.; Ballester, J.; Schmitt-Kopplin, P.; Gougeon, R.D. Impact of Glutathione on Wines Oxidative Stability: A Combined Sensory and Metabolomic Study. Front. Chem. 2018, 6, 182. [Google Scholar] [CrossRef]
- Binati, R.L.; Larini, I.; Salvetti, E.; Torriani, S. Glutathione Production by Non-Saccharomyces Yeasts and Its Impact on Winemaking: A Review. Food Res. Int. 2022, 156, 111333. [Google Scholar] [CrossRef]
- Vilela, A. Use of Nonconventional Yeasts for Modulating Wine Acidity. Fermentation 2019, 5, 27. [Google Scholar] [CrossRef]
- Fleet, G. Yeast Interactions and Wine Flavour. Int. J. Food Microbiol. 2003, 86, 11–22. [Google Scholar] [CrossRef]
- Jahangeer, M.; Riasat, A.; Mahmood, Z.; Numan, M.; Munir, N.; Ashiq, M.; Asad, M.; Ali, U.; Salman, M. Secondary Metabolites from Saccharomyces Cerevisiae Species with Anticancer Potential. In Saccharomyces; IntechOpen: London, UK, 2021. [Google Scholar]
- Santos, M.D.S.M.; Batistote, M.; Cardoso, C.A.L. The Production of Metabolites by Saccharomyces Cerevisiae and Its Application in Biotechnological Processes. Front. J. Soc. Technol. Environ. Sci. 2021, 10, 174–184. [Google Scholar] [CrossRef]
- Ellis, D.J.; Kerr, E.D.; Schenk, G.; Schulz, B.L. Metabolomics of Non-Saccharomyces Yeasts in Fermented Beverages. Beverages 2022, 8, 41. [Google Scholar] [CrossRef]
- Vilela, A. The Importance of Yeasts on Fermentation Quality and Human Health-Promoting Compounds. Fermentation 2019, 5, 46. [Google Scholar] [CrossRef]
- Aplin, J.J.; White, K.P.; Edwards, C.G. Growth and Metabolism of Non-Saccharomyces Yeasts Isolated from Washington State Vineyards in Media and High Sugar Grape Musts. Food Microbiol. 2019, 77, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Tufariello, M.; Fragasso, M.; Pico, J.; Panighel, A.; Castellarin, S.D.; Flamini, R.; Grieco, F. Influence of Non-Saccharomyces on Wine Chemistry: A Focus on Aroma-Related Compounds. Molecules 2021, 26, 644. [Google Scholar] [CrossRef]
- Zentou, H.; Zainal Abidin, Z.; Yunus, R.; Awang Biak, D.R.; Abdullah Issa, M.; Yahaya Pudza, M. A New Model of Alcoholic Fermentation under a Byproduct Inhibitory Effect. ACS Omega 2021, 6, 4137–4146. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, I.; Gullo, M.; Chen, F.; Garcia-Martinez, T. Editorial: Acetic Acid Bacteria. Front. Microbiol. 2023, 14, 1142659. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xie, Z.; Zhang, H.; Liebl, W.; Toyama, H.; Chen, F. Oxidative Fermentation of Acetic Acid Bacteria and Its Products. Front. Microbiol. 2022, 13, 879246. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Toyama, H.; Tonouchi, N.; Okamoto-Kainuma, A. Acetic Acid Bacteria; Springer: Tokyo, Japan, 2016; ISBN 978-4-431-55931-3. [Google Scholar]
- Qiu, X.; Zhang, Y.; Hong, H. Classification of Acetic Acid Bacteria and Their Acid Resistant Mechanism. AMB Express 2021, 11, 29. [Google Scholar] [CrossRef]
- Lynch, K.M.; Zannini, E.; Wilkinson, S.; Daenen, L.; Arendt, E.K. Physiology of Acetic Acid Bacteria and Their Role in Vinegar and Fermented Beverages. Compr. Rev. Food Sci. Food Saf. 2019, 18, 587–625. [Google Scholar] [CrossRef]
- Taban, B.M.; Saichana, N. Physiology and Biochemistry of Acetic Acid Bacteria. In Acetic Acid Bacteria: Fundamentals and Food Applications; Sengun, I.Y., Ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781315153490. [Google Scholar]
- Kourouma, M.C.; Mbengue, M.; Sarr, N.C.D.; Sarr, K.; Kane, C.T. Thermoresistant, Ethanol-Resistant and Acid-Resistant Properties of Acetic Acid Bacteria Isolated from Fermented Mango Alcohol. Adv. Microbiol. 2022, 12, 177–191. [Google Scholar] [CrossRef]
- Mamlouk, D.; Gullo, M. Acetic Acid Bacteria: Physiology and Carbon Sources Oxidation. Indian J. Microbiol. 2013, 53, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Chanivet, M.; Es-sbata, I.; Astola, A.; Durán-Guerrero, E.; Castro, R. Thermotolerant Acetic Acid Bacteria in the Production of a Red Wine Vinegar by Surface Culture at Different Temperatures: Volatile and Polyphenolic Composition. Eur. Food Res. Technol. 2024. [Google Scholar] [CrossRef]
- Saeki, A.; Taniguchi, M.; Matsushita, K.; Toyama, H.; Theeragool, G.; Lotong, N.; Adachi, O. Microbiological Aspects of Acetate Oxidation by Acetic Acid Bacteria, Unfavorable Phenomena in Vinegar Fermentation. Biosci. Biotechnol. Biochem. 1997, 61, 317–323. [Google Scholar] [CrossRef]
- Shin, M.; Kim, J.-W.; Gu, B.; Kim, S.; Kim, H.; Kim, W.-C.; Lee, M.-R.; Kim, S.-R. Comparative Metabolite Profiling of Traditional and Commercial Vinegars in Korea. Metabolites 2021, 11, 478. [Google Scholar] [CrossRef]
- Rusu, A.V.; Trif, M.; Rocha, J.M. Microbial Secondary Metabolites via Fermentation Approaches for Dietary Supplementation Formulations. Molecules 2023, 28, 6020. [Google Scholar] [CrossRef]
- Li, W.-L.; Tong, S.-G.; Yang, Z.-Y.; Xiao, Y.-Q.; Lv, X.-C.; Weng, Q.; Yu, K.; Liu, G.-R.; Luo, X.-Q.; Wei, T.; et al. The Dynamics of Microbial Community and Flavor Metabolites during the Acetic Acid Fermentation of Hongqu Aromatic Vinegar. Curr. Res. Food Sci. 2022, 5, 1720–1731. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, Y.; Phillips, G.O.; Yang, G. Utilization of Bacterial Cellulose in Food. Food Hydrocoll. 2014, 35, 539–545. [Google Scholar] [CrossRef]
- Mizzi, J.; Gaggìa, F.; Bozzi Cionci, N.; Di Gioia, D.; Attard, E. Selection of Acetic Acid Bacterial Strains and Vinegar Production From Local Maltese Food Sources. Front. Microbiol. 2022, 13, 897825. [Google Scholar] [CrossRef]
- Solieri, L.; Giudici, P. Vinegars of the World; Springer Milan: Milano, Italy, 2009; ISBN 978-88-470-0865-6. [Google Scholar]
- Maicas, S. The Role of Yeasts in Fermentation Processes. Microorganisms 2020, 8, 1142. [Google Scholar] [CrossRef]
- Timmermans, E.; Bautil, A.; Brijs, K.; Scheirlinck, I.; Van der Meulen, R.; Courtin, C.M. Sugar Levels Determine Fermentation Dynamics during Yeast Pastry Making and Its Impact on Dough and Product Characteristics. Foods 2022, 11, 1388. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Grandvalet, C.; Verdier, F.; Martin, A.; Alexandre, H.; Tourdot-Maréchal, R. Microbial Dynamics between Yeasts and Acetic Acid Bacteria in Kombucha: Impacts on the Chemical Composition of the Beverage. Foods 2020, 9, 963. [Google Scholar] [CrossRef] [PubMed]
- Younes, B.; Cilindre, C.; Villaume, S.; Parmentier, M.; Jeandet, P.; Vasserot, Y. Evidence for an Extracellular Acid Proteolytic Activity Secreted by Living Cells of Saccharomyces cerevisiae PlR1: Impact on Grape Proteins. J. Agric. Food Chem. 2011, 59, 6239–6246. [Google Scholar] [CrossRef]
- Gomes, R.J.; Borges, M.D.F.; de Freitas Rosa, M.; Castro-Gómez, R.J.H.; Spinosa, W.A. Acetic Acid Bacteria in the Food Industry: Systematics, Characteristics and Applications. Food Technol. Biotechnol. 2018, 56, 139–151. [Google Scholar] [CrossRef]
- Hernández-González, J.C.; Martínez-Tapia, A.; Lazcano-Hernández, G.; García-Pérez, B.E.; Castrejón-Jiménez, N.S. Bacteriocins from Lactic Acid Bacteria. A Powerful Alternative as Antimicrobials, Probiotics, and Immunomodulators in Veterinary Medicine. Animals 2021, 11, 979. [Google Scholar] [CrossRef]
- Darbandi, A.; Asadi, A.; Mahdizade Ari, M.; Ohadi, E.; Talebi, M.; Halaj Zadeh, M.; Darb Emamie, A.; Ghanavati, R.; Kakanj, M. Bacteriocins: Properties and Potential Use as Antimicrobials. J. Clin. Lab. Anal. 2022, 36, 24093. [Google Scholar] [CrossRef]
- Huang, W.-C.; Tang, I.-C. Bacterial and Yeast Cultures—Process Characteristics, Products, and Applications. In Bioprocessing for Value-Added Products from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2007; pp. 185–223. [Google Scholar]
- Postaru, M.; Tucaliuc, A.; Cascaval, D.; Galaction, A.-I. Cellular Stress Impact on Yeast Activity in Biotechnological Processes—A Short Overview. Microorganisms 2023, 11, 2522. [Google Scholar] [CrossRef]
- Farrugia, G.; Balzan, R. Oxidative Stress and Programmed Cell Death in Yeast. Front. Oncol. 2012, 2, 64. [Google Scholar] [CrossRef]
- Tonoli, F.C. Adaptação de Leveduras Para Fermentação Com Alto Teor Alcoólico. Master’s Thesis, University of São Paulo, São Paulo, Brazil, 2017. [Google Scholar]
- Douradinho, R.; Sica, P.; Tonoli, F.; Mattos, E.; Oliveira, M.; Pinto, A.; Mota, L.; Faria, T.; Costa, V.F.; Leite, G.; et al. Osmotic Stress Alleviation in Saccharomyces cerevisiae for High Ethanol Fermentations with Different Wort Substrates. Stresses 2023, 3, 813–826. [Google Scholar] [CrossRef]
- Liang, C.; Liu, L.-X.; Liu, J.; Aihaiti, A.; Tang, X.-J.; Liu, Y.-G. New Insights on Low-Temperature Fermentation for Food. Fermentation 2023, 9, 477. [Google Scholar] [CrossRef]
- Palma, M.; Guerreiro, J.F.; Sá-Correia, I. Adaptive Response and Tolerance to Acetic Acid in Saccharomyces Cerevisiae and Zygosaccharomyces Bailii: A Physiological Genomics Perspective. Front. Microbiol. 2018, 9, 274. [Google Scholar] [CrossRef]
- Gullo, M.; Verzelloni, E.; Canonico, M. Aerobic Submerged Fermentation by Acetic Acid Bacteria for Vinegar Production: Process and Biotechnological Aspects. Process Biochem. 2014, 49, 1571–1579. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, Y.; Li, D.; Wang, C.; Xu, N.; Wu, S.; He, S.; Hu, Y. Correlation between Ethanol Resistance and Characteristics of PQQ-Dependent ADH in Acetic Acid Bacteria. Eur. Food Res. Technol. 2016, 242, 837–847. [Google Scholar] [CrossRef]
- Kanchanarach, W.; Theeragool, G.; Yakushi, T.; Toyama, H.; Adachi, O.; Matsushita, K. Characterization of Thermotolerant Acetobacter Pasteurianus Strains and Their Quinoprotein Alcohol Dehydrogenases. Appl. Microbiol. Biotechnol. 2010, 85, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.G.; Martins, C.; Tavares, T.; Rudnitskaya, A.; Alves, F.; Rocha, S.M. Volatile Composition of Fortification Grape Spirit and Port Wine: Where Do We Stand? Foods 2023, 12, 2432. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.; Kim, G.-R.; Yeo, S.-H.; Jeong, Y.-J.; Noh, B.S.; Kwon, J.-H. Analysis of Aroma Compounds of Commercial Cider Vinegars with Different Acidities Using SPME/GC-MS, Electronic Nose, and Sensory Evaluation. Food Sci. Biotechnol. 2013, 22, 1559–1565. [Google Scholar] [CrossRef]
- Callejón, R.M.; Morales, M.L.; Ferreira, A.C.S.; Troncoso, A.M. Defining the Typical Aroma of Sherry Vinegar: Sensory and Chemical Approach. J. Agric. Food Chem. 2008, 56, 8086–8095. [Google Scholar] [CrossRef]
- Plata, C.; Millán, C.; Mauricio, J.C.; Ortega, J.M. Formation of Ethyl Acetate and Isoamyl Acetate by Various Species of Wine Yeasts. Food Microbiol. 2003, 20, 217–224. [Google Scholar] [CrossRef]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative Determination of the Odorants of Young Red Wines from Different Grape Varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Arias-Pérez, I.; Sáenz-Navajas, M.P.; de-la-Fuente-Blanco, A.; Ferreira, V.; Escudero, A. Insights on the Role of Acetaldehyde and Other Aldehydes in the Odour and Tactile Nasal Perception of Red Wine. Food Chem. 2021, 361, 130081. [Google Scholar] [CrossRef]
- Mina, M.; Tsaltas, D. Contribution of Yeast in Wine Aroma and Flavour. In Yeast—Industrial Applications; InTech: London, UK, 2017. [Google Scholar] [CrossRef]
- Parapouli, M.; Vasileiadi, A.; Afendra, A.-S.; Hatziloukas, E. Saccharomyces Cerevisiae and Its Industrial Applications. AIMS Microbiol. 2020, 6, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Durán-Guerrero, E.; Castro, R. Novel Analysis on Aroma Compounds of Wine, Vinegar and Derived Products. Foods 2021, 10, 1245. [Google Scholar] [CrossRef] [PubMed]
- Calugar, P.C.; Coldea, T.E.; Salanță, L.C.; Pop, C.R.; Pasqualone, A.; Burja-Udrea, C.; Zhao, H.; Mudura, E. An Overview of the Factors Influencing Apple Cider Sensory and Microbial Quality from Raw Materials to Emerging Processing Technologies. Processes 2021, 9, 502. [Google Scholar] [CrossRef]
- Xie, Z.; Koysomboon, C.; Zhang, H.; Lu, Z.; Zhang, X.; Chen, F. Vinegar Volatile Organic Compounds: Analytical Methods, Constituents, and Formation Processes. Front. Microbiol. 2022, 13, 907883. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, W.; Morales, M.L.; Garcia-Parrilla, M.C.; Troncoso, A.M. Improvement of Wine Vinegar Elaboration and Quality Analysis: Instrumental and Human Sensory Evaluation. Food Rev. Int. 2009, 25, 142–156. [Google Scholar] [CrossRef]
- Marques, C.; Correia, E.; Dinis, L.T.; Vilela, A. An Overview of Sensory Characterization Techniques: From Classical Descriptive Analysis to the Emergence of Novel Profiling Methods. Foods 2022, 11, 255. [Google Scholar] [CrossRef]
- Starowicz, M. Analysis of Volatiles in Food Products. Separations 2021, 8, 157. [Google Scholar] [CrossRef]
- Rabehi, A.; Helal, H.; Zappa, D.; Comini, E. Advancements and Prospects of Electronic Nose in Various Applications: A Comprehensive Review. Appl. Sci. 2024, 14, 4506. [Google Scholar] [CrossRef]
- Wilson, A.D.; Baietto, M. Applications and Advances in Electronic-Nose Technologies. Sensors 2009, 9, 5099–5148. [Google Scholar] [CrossRef]
- Liu, R.-C.; Li, R.; Wang, Y.; Jiang, Z.-T. Analysis of Volatile Odor Compounds and Aroma Properties of European Vinegar by the Ultra-Fast Gas Chromatographic Electronic Nose. J. Food Compos. Anal. 2022, 112, 104673. [Google Scholar] [CrossRef]
- Liang, S.; Liu, Y.; Yuan, S.; Liu, Y.; Zhu, B.; Zhang, M. Study of Consumer Liking of Six Chinese Vinegar Products and the Correlation between These Likings and the Volatile Profile. Foods 2022, 11, 2224. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, C.; Silva, P.; Câmara, J. Establishment of the Volatile Signature of Wine-Based Aromatic Vinegars Subjected to Maceration. Molecules 2018, 23, 499. [Google Scholar] [CrossRef]
- Ríos-Reina, R.; Segura-Borrego, M.P.; García-González, D.L.; Morales, M.L.; Callejón, R.M. A Comparative Study of the Volatile Profile of Wine Vinegars with Protected Designation of Origin by Headspace Stir Bar Sorptive Extraction. Food Res. Int. 2019, 123, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Gawel, R.; Smith, P.A.; Cicerale, S.; Keast, R. The Mouthfeel of White Wine. Crit. Rev. Food Sci. Nutr. 2018, 58, 2939–2956. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, P.; McDaniel, M.R. Flavor Characteristics of Lactic, Malic, Citric, and Acetic Acids at Various PH Levels. J. Food Sci. 1995, 60, 384–388. [Google Scholar] [CrossRef]
- Nurgel, C.; Pickering, G. Contribution of Glycerol, Ethanol and Sugar to the Perception of Viscosity and Density Elicited by Model White Wines. J. Texture Stud. 2005, 36, 303–323. [Google Scholar] [CrossRef]
- Podrażka, M.; Bączyńska, E.; Kundys, M.; Jeleń, P.; Witkowska Nery, E. Electronic Tongue—A Tool for All Tastes? Biosensors 2017, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Moazzem, M.d.S. Recent Applications of Potentiometric Electronic Tongue and Electronic Nose in Sensory Evaluation. Prev. Nutr. Food Sci. 2022, 27, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Seddaoui, N.; Amine, A. Recent Advances in Sensor and Biosensor Technologies for Adulteration Detection. In Advanced Sensor Technology; Elsevier: Amsterdam, The Netherlands, 2023; pp. 699–739. [Google Scholar]
- Coskun, O. Separation Techniques: Chromatography. North. Clin. Istanb. 2016, 3, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Zacharioudaki, D.-E.; Fitilis, I.; Kotti, M. Review of Fluorescence Spectroscopy in Environmental Quality Applications. Molecules 2022, 27, 4801. [Google Scholar] [CrossRef]
- Marcus, J.B. A Taste Primer. In Aging, Nutrition and Taste; Elsevier: Amsterdam, The Netherlands, 2019; pp. 105–140. [Google Scholar]
- Agorastos, G. Review of Mouthfeel Classification. A New Perspective of Food Perception. J. Food Sci. Nutr. 2020, 1–10. [Google Scholar] [CrossRef]
- Byrne, D.V. Current Trends in Multidisciplinary Approaches to Understanding Consumer Preference and Acceptance of Food Products. Foods 2020, 9, 1380. [Google Scholar] [CrossRef]
- Rai, S.; Wai, P.P.; Koirala, P.; Bromage, S.; Nirmal, N.P.; Pandiselvam, R.; Nor-Khaizura, M.A.R.; Mehta, N.K. Food Product Quality, Environmental and Personal Characteristics Affecting Consumer Perception toward Food. Front. Sustain. Food Syst. 2023, 7, 1222760. [Google Scholar] [CrossRef]
- Drake, M.A.; Watson, M.E.; Liu, Y. Sensory Analysis and Consumer Preference: Best Practices. Annu. Rev. Food Sci. Technol. 2023, 14, 427–448. [Google Scholar] [CrossRef]
- Talukder, M.B.; Kumar, S.; Das, I.R. Perspectives of Digital Marketing for the Restaurant Industry; IGI Global: Hershey, PA, USA, 2024; pp. 118–134. [Google Scholar]
- Vitsentzatou, E.; Tsoulfas, G.T.; Mihiotis, A.N. The Digital Transformation of the Marketing Mix in the Food and Beverage Service Supply Chain: A Grey DEMATEL Approach. Sustainability 2022, 14, 15228. [Google Scholar] [CrossRef]
- Sgroi, F.; Sciortino, C.; Baviera-Puig, A.; Modica, F. Analyzing Consumer Trends in Functional Foods: A Cluster Analysis Approach. J. Agric. Food Res. 2024, 15, 101041. [Google Scholar] [CrossRef]
- Baker, M.T.; Lu, P.; Parrella, J.A.; Leggette, H.R. Consumer Acceptance toward Functional Foods: A Scoping Review. Int. J. Environ. Res. Public. Health 2022, 19, 1217. [Google Scholar] [CrossRef]
- Taktakishvili, T.; Sachaleli, N. Sustainable Marketing as a Buyer’s Motivating Factor in the Retail Business: Example of Georgia. Eur. Sci. J. ESJ 2024, 20, 211. [Google Scholar] [CrossRef]
- Ray, S.; Nayak, L. Marketing Sustainable Fashion: Trends and Future Directions. Sustainability 2023, 15, 6202. [Google Scholar] [CrossRef]
- Quintela, J.A.; Albuquerque, H.; Freitas, I. Port Wine and Wine Tourism: The Touristic Dimension of Douro’s Landscape. Sustainability 2023, 15, 11718. [Google Scholar] [CrossRef]
- Rivaroli, S.; Baldi, B.; Spadoni, R. Consumers’ Perception of Food Product Craftsmanship: A Review of Evidence. Food Qual. Prefer. 2020, 79, 103796. [Google Scholar] [CrossRef]
- Wang, Q.J.; Mielby, L.A.; Junge, J.Y.; Bertelsen, A.S.; Kidmose, U.; Spence, C.; Byrne, D.V. The Role of Intrinsic and Extrinsic Sensory Factors in Sweetness Perception of Food and Beverages: A Review. Foods 2019, 8, 211. [Google Scholar] [CrossRef] [PubMed]
- Ker, J.-K.; Lee, C.-S.; Chen, Y.-C.; Chiang, M.-C. Exploring Taiwanese Consumer Dietary Preferences for Various Vinegar Condiments: Novel Dietary Patterns across Diverse Cultural Contexts. Nutrients 2023, 15, 3845. [Google Scholar] [CrossRef] [PubMed]
- Piqueras-Fiszman, B.; Spence, C. Sensory Expectations Based on Product-Extrinsic Food Cues: An Interdisciplinary Review of the Empirical Evidence and Theoretical Accounts. Food Qual. Prefer. 2015, 40, 165–179. [Google Scholar] [CrossRef]
- Schulte-Holierhoek, A.; Verastegui-Tena, L.; Goedegebure, R.P.G.; Piqueras Fiszman, B.; Smeets, P.A.M. Sensory Expectation, Perception, and Autonomic Nervous System Responses to Package Colours and Product Popularity. Food Qual. Prefer. 2017, 62, 60–70. [Google Scholar] [CrossRef]
- Deliza, R.; MacFie, H.J.H. The Generation of Sensory Expectation by External Cues and Its Effect on Sensory Perception and Hedonic Ratings: A Review. J. Sens. Stud. 1996, 11, 103–128. [Google Scholar] [CrossRef]
- Cancellieri, U.G.; Petruccelli, I.; Cicero, L.; Milani, A.; Bonaiuto, F.; Bonaiuto, M. Reputation and Emotion: How the Mind Drives Our Food Preferences and Choices. Food Qual. Prefer. 2022, 101, 104637. [Google Scholar] [CrossRef]
- Piqueras-Fiszman, B.; Jaeger, S.R. The Incidental Influence of Memories of Past Eating Occasions on Consumers’ Emotional Responses to Food and Food-Related Behaviors. Front. Psychol. 2016, 7, 943. [Google Scholar] [CrossRef]
- María Luzón-Quintana, L.; Castro, R.; Durán-Guerrero, E. Biotechnological Processes in Fruit Vinegar Production. Foods 2021, 10, 945. [Google Scholar] [CrossRef]
- Boasiako, T.A.; Boateng, I.D.; Ekumah, J.-N.; Johnson, N.A.N.; Appiagyei, J.; Murtaza, M.S.; Mubeen, B.; Ma, Y. Advancing Sustainable Innovations in Mulberry Vinegar Production: A Critical Review on Non-Thermal Pre-Processing Technologies. Sustainability 2024, 16, 1185. [Google Scholar] [CrossRef]
- Sala, S.; Anton, A.; McLaren, S.J.; Notarnicola, B.; Saouter, E.; Sonesson, U. In Quest of Reducing the Environmental Impacts of Food Production and Consumption. J. Clean. Prod. 2017, 140, 387–398. [Google Scholar] [CrossRef]
- Elroi, H.; Zbigniew, G.; Agnieszka, W.-C.; Piotr, S. Enhancing Waste Resource Efficiency: Circular Economy for Sustainability and Energy Conversion. Front. Environ. Sci. 2023, 11, 1303792. [Google Scholar] [CrossRef]
- Toni, M. Conceptualization of Circular Economy and Sustainability at the Business Level. Circular Economy and Sustainable Development. Int. J. Empir. Res. Methods 2023, 1, 81–89. [Google Scholar] [CrossRef]
- Li, C.; Gao, J.; Ge, L.; Hu, W.; Ban, Q. Do Geographical Indication Products Promote the Growth of the Agricultural Economy? An Empirical Study Based on Meta-Analysis. Sustainability 2023, 15, 14428. [Google Scholar] [CrossRef]
- Hadi, A.; Pourmasoumi, M.; Najafgholizadeh, A.; Clark, C.C.T.; Esmaillzadeh, A. The Effect of Apple Cider Vinegar on Lipid Profiles and Glycemic Parameters: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. BMC Complement. Med. Ther. 2021, 21, 179. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Henry, C.J.; Haldar, S. Vinegar as a Functional Ingredient to Improve Postprandial Glycemic Control—Human Intervention Findings and Molecular Mechanisms. Mol. Nutr. Food Res. 2016, 60, 1837–1849. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Zhang, B.; Duan, W.; Zhang, J.; Wang, M. Nutrients and Bioactive Components from Vinegar: A Fermented and Functional Food. J. Funct. Foods 2020, 64, 103681. [Google Scholar] [CrossRef]
- Samad, A.; Azlan, A.; Ismail, A. Therapeutic Effects of Vinegar: A Review. Curr. Opin. Food Sci. 2016, 8, 56–61. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Zhang, S. Inhibition of Propionibacterium Acnes by Refined Bamboo Vinegar and Preparation of the Slow-Release System with Bamboo Charcoal as the Carrier. J. Dermatol. Sci. Cosmet. Technol. 2024, 1, 100016. [Google Scholar] [CrossRef]
- McMullen, R.L.; Dell’Acqua, G. History of Natural Ingredients in Cosmetics. Cosmetics 2023, 10, 71. [Google Scholar] [CrossRef]
- Visconti, F.; López, R.; Olego, M.Á. The Health of Vineyard Soils: Towards a Sustainable Viticulture. Horticulturae 2024, 10, 154. [Google Scholar] [CrossRef]
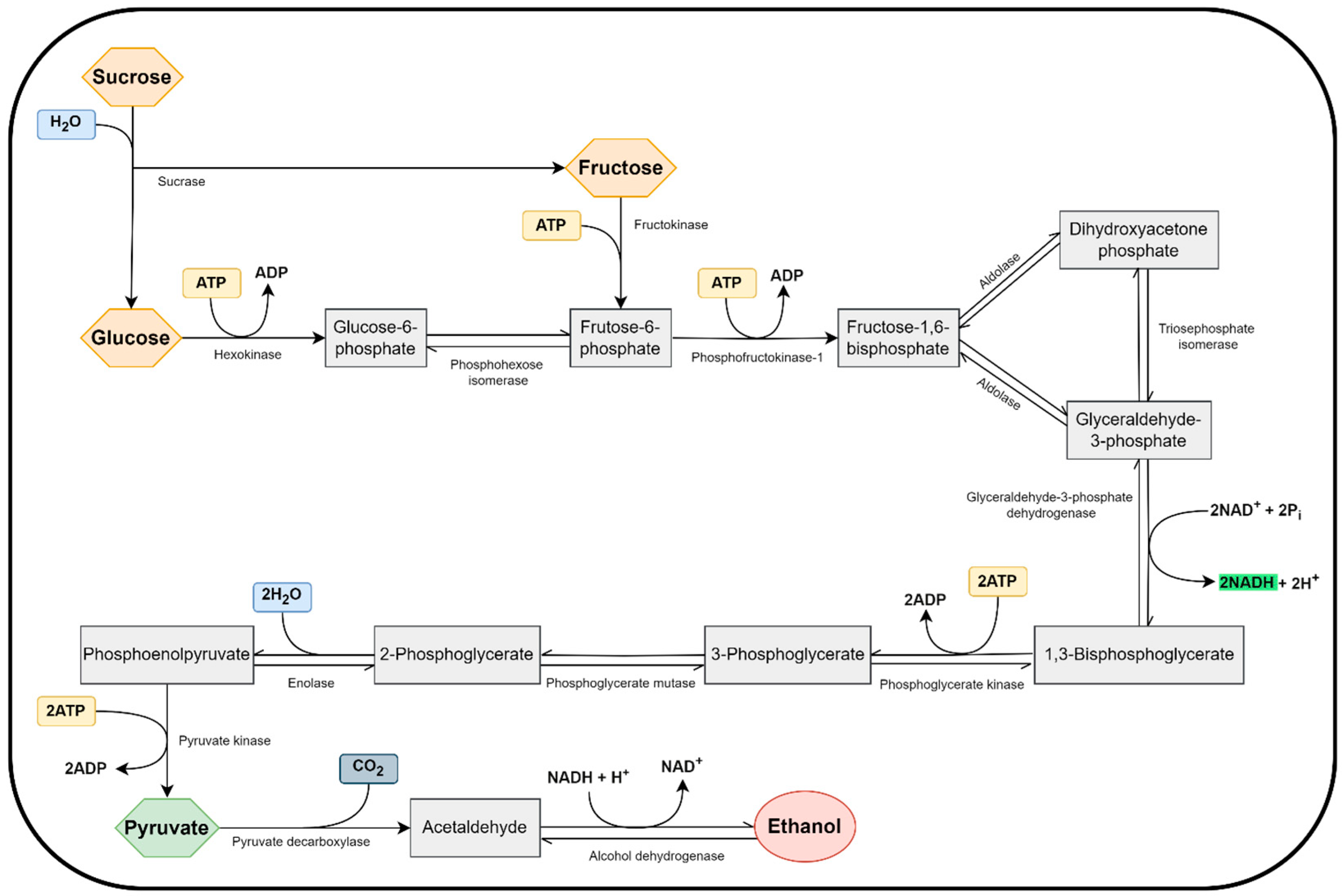
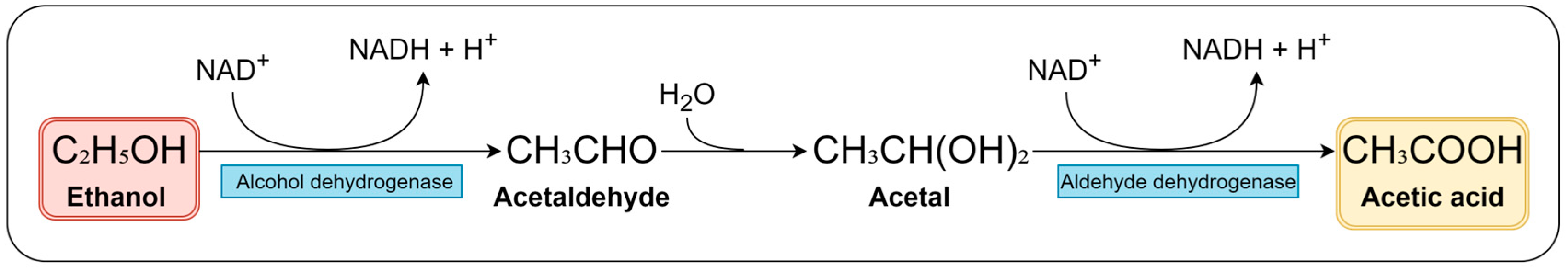


| Classification | Description |
|---|---|
| Wine vinegar | Exclusively produced from wine through the biological process of acetic fermentation. |
| Fruit and berry vinegar | Obtained from fruit or berries through the biological process of alcoholic and acetic fermentation. |
| Cider vinegar | Obtained from cider through the biological process of acetic fermentation. |
| Spirit vinegar | Obtained from distilled agricultural alcohol through the biological process of acetic fermentation. |
| Cereal vinegar | Obtained, without intermediate distillation, through the biological process of dual fermentation (alcoholic and acetic) from cereals whose starch has been converted to sugars by malted barley diastase or another method. |
| Malt vinegar | It is obtained without intermediate distillation through the biological process of dual fermentation (alcoholic and acetic) from malted barley, with or without adding other cereals, where starch has been converted to sugars solely by malted barley diastase. |
| Distilled malt vinegar | It is obtained by distilling malt vinegar under reduced pressure, containing only the volatile constituents of the malt vinegar from which it is derived. |
| Other vinegars | Vinegar from other agricultural products through dual fermentation, including honey and beer, is not covered in the previous items. |
| Flavored and spiced vinegars | From the previous categories to which aromatic plants or parts thereof, spices and flavoring extracts have been added, perceptible organoleptically. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota, J.; Vilela, A. Exploring Microbial Dynamics: The Interaction between Yeasts and Acetic Acid Bacteria in Port Wine Vinegar and Its Implications on Chemical Composition and Sensory Acceptance. Fermentation 2024, 10, 421. https://doi.org/10.3390/fermentation10080421
Mota J, Vilela A. Exploring Microbial Dynamics: The Interaction between Yeasts and Acetic Acid Bacteria in Port Wine Vinegar and Its Implications on Chemical Composition and Sensory Acceptance. Fermentation. 2024; 10(8):421. https://doi.org/10.3390/fermentation10080421
Chicago/Turabian StyleMota, João, and Alice Vilela. 2024. "Exploring Microbial Dynamics: The Interaction between Yeasts and Acetic Acid Bacteria in Port Wine Vinegar and Its Implications on Chemical Composition and Sensory Acceptance" Fermentation 10, no. 8: 421. https://doi.org/10.3390/fermentation10080421
APA StyleMota, J., & Vilela, A. (2024). Exploring Microbial Dynamics: The Interaction between Yeasts and Acetic Acid Bacteria in Port Wine Vinegar and Its Implications on Chemical Composition and Sensory Acceptance. Fermentation, 10(8), 421. https://doi.org/10.3390/fermentation10080421







