Enhancing the Nutritional Quality of Defatted Cottonseed Meal by Solid-State Fermentation with Probiotic Microbes
Abstract
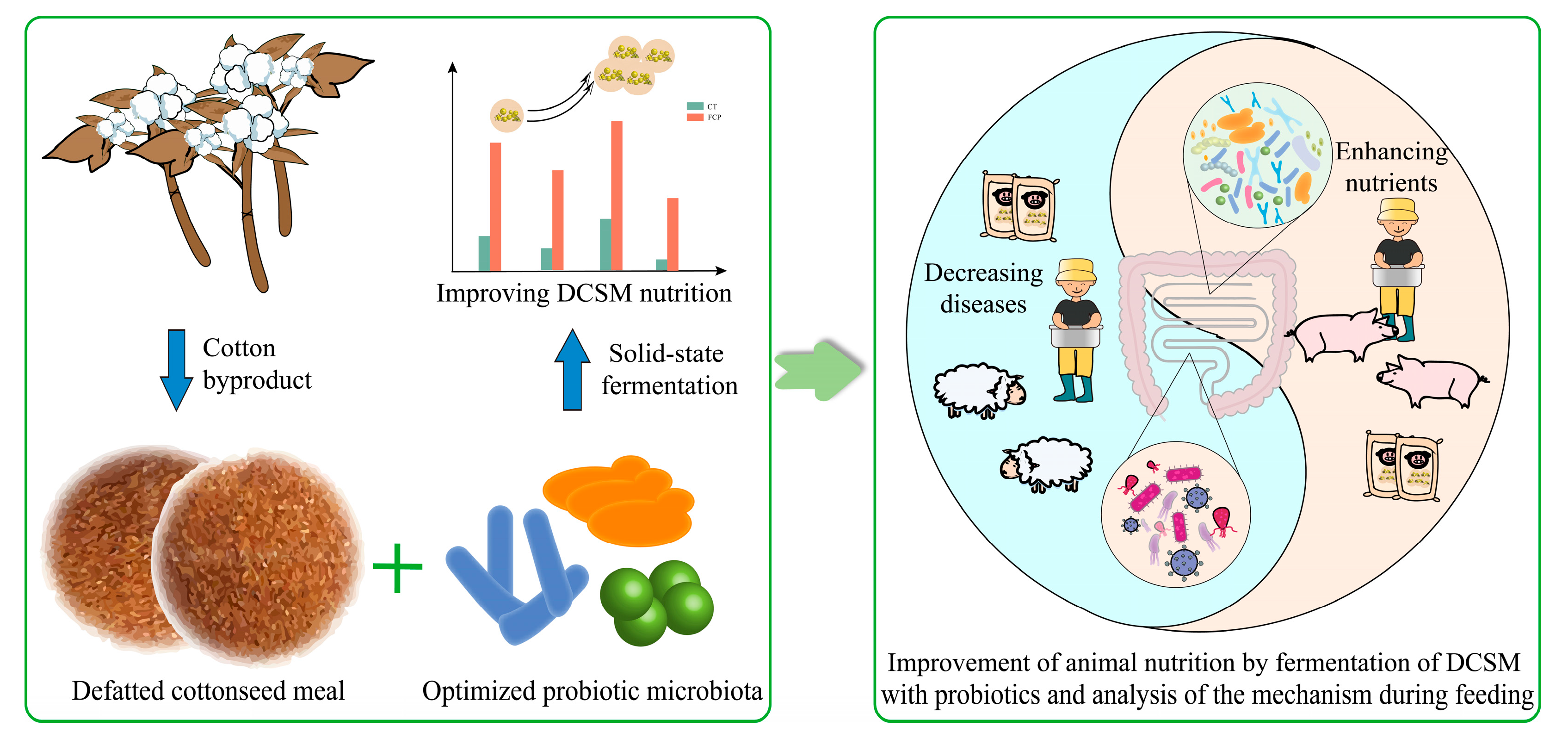
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Isolation and Identification of Strains
2.3. Strains and DCSM Solid-State Fermentation
2.4. Chemical Composition Analysis of DCSM
2.5. Analysis of Changes in Fermented DCSM Surface Features
2.6. Statistical Analysis
3. Results
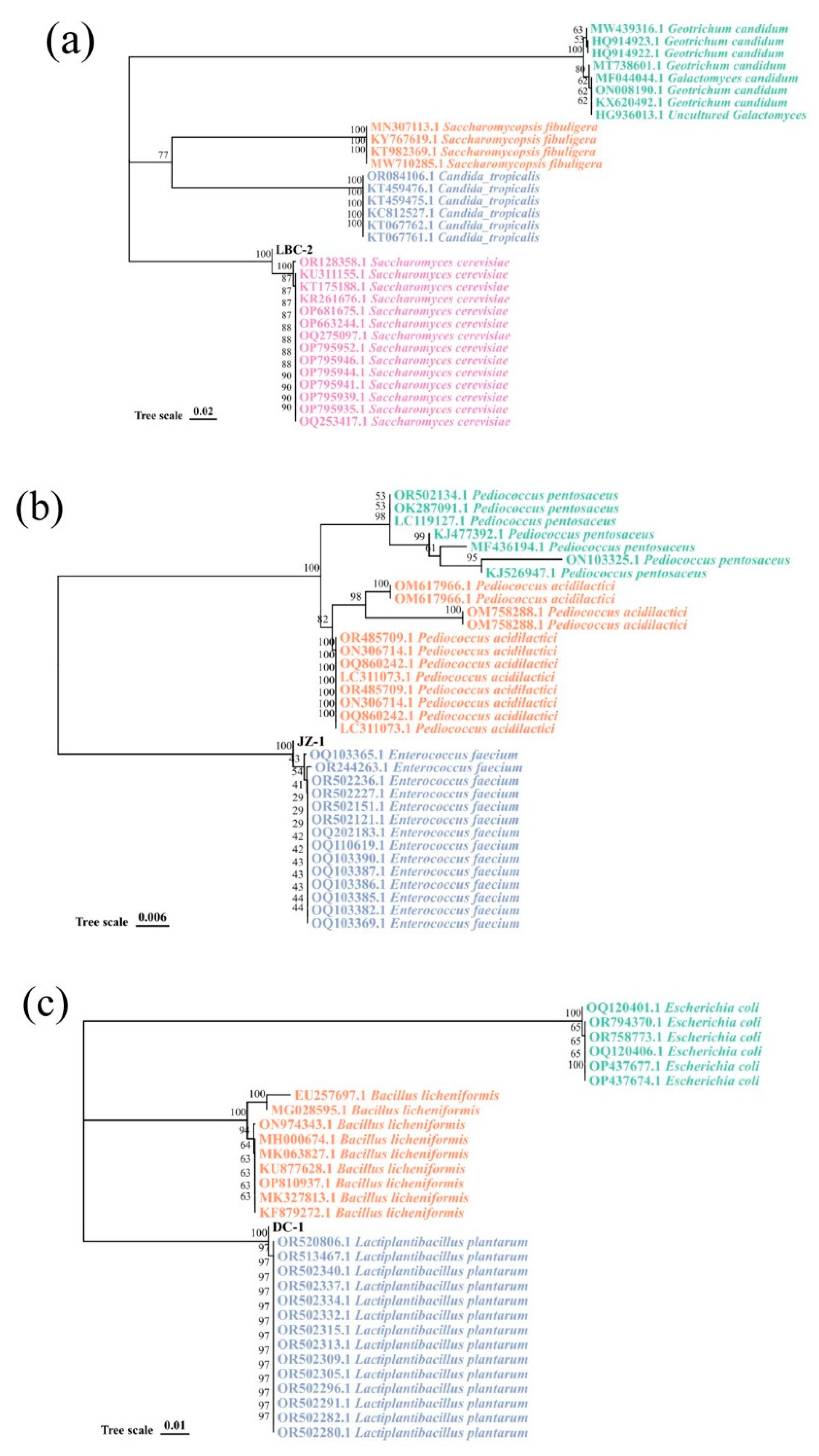
3.1. Isolation of Microbial Strains Used for DCSM Fermentation
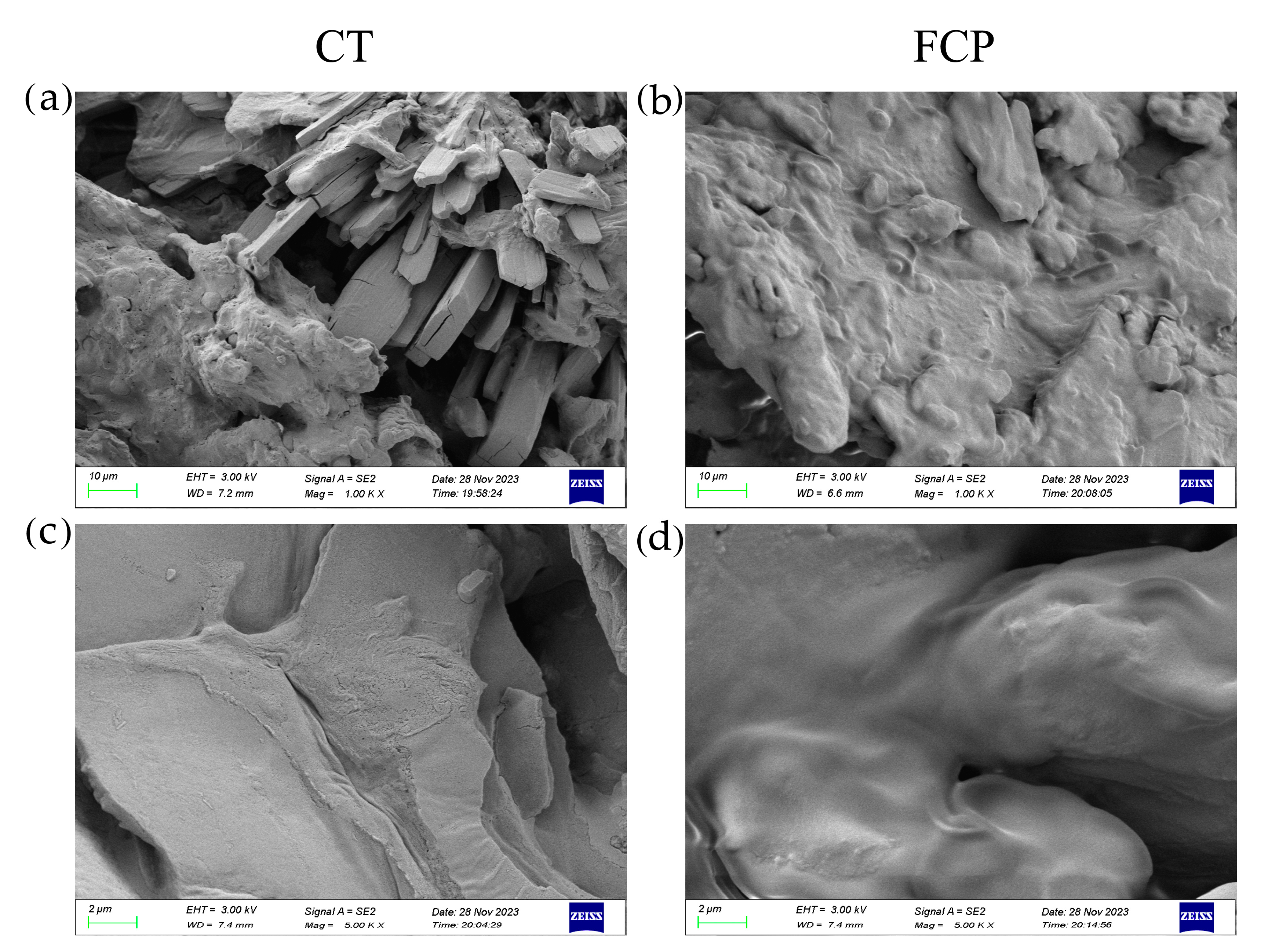
3.2. Changes in DCSM Surface Features
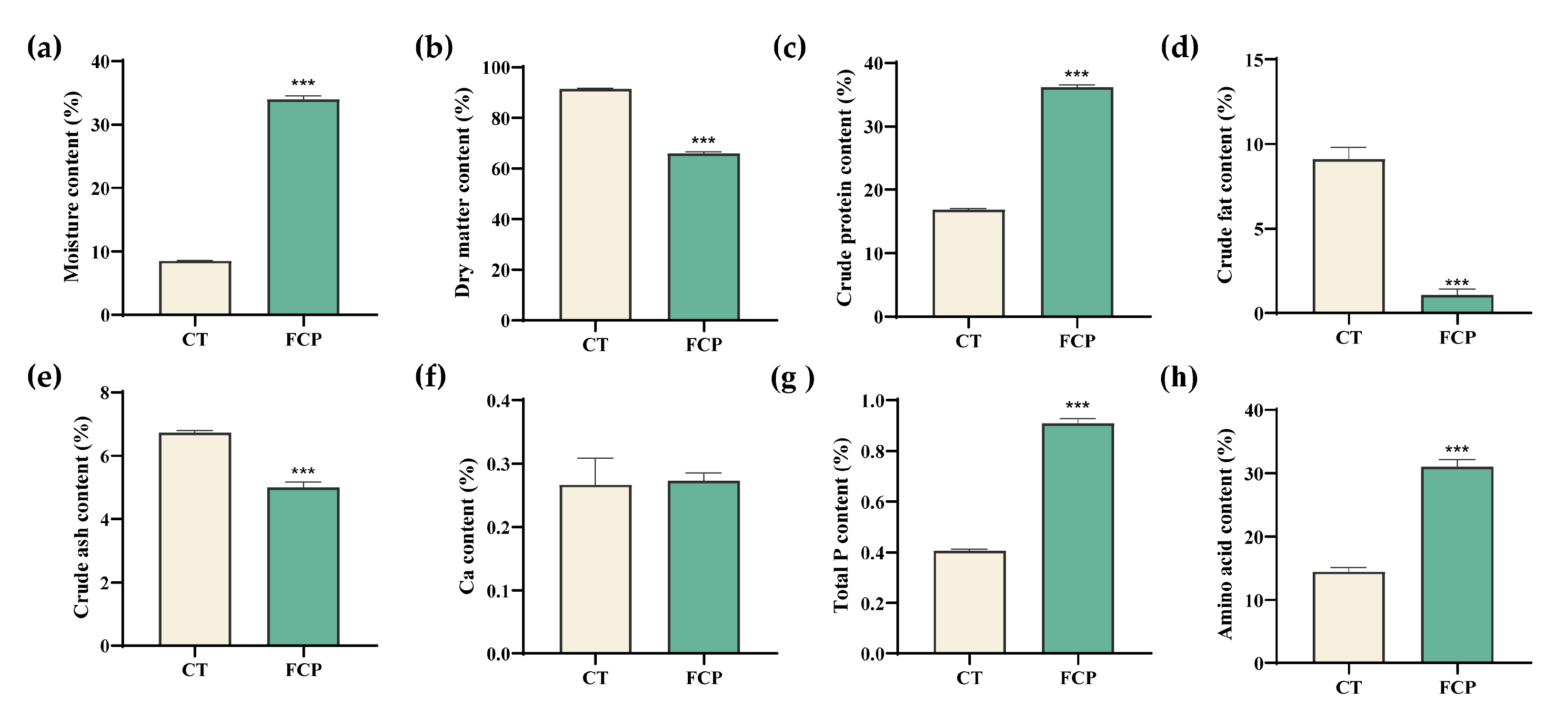
3.3. Changes in DCSM Characteristics
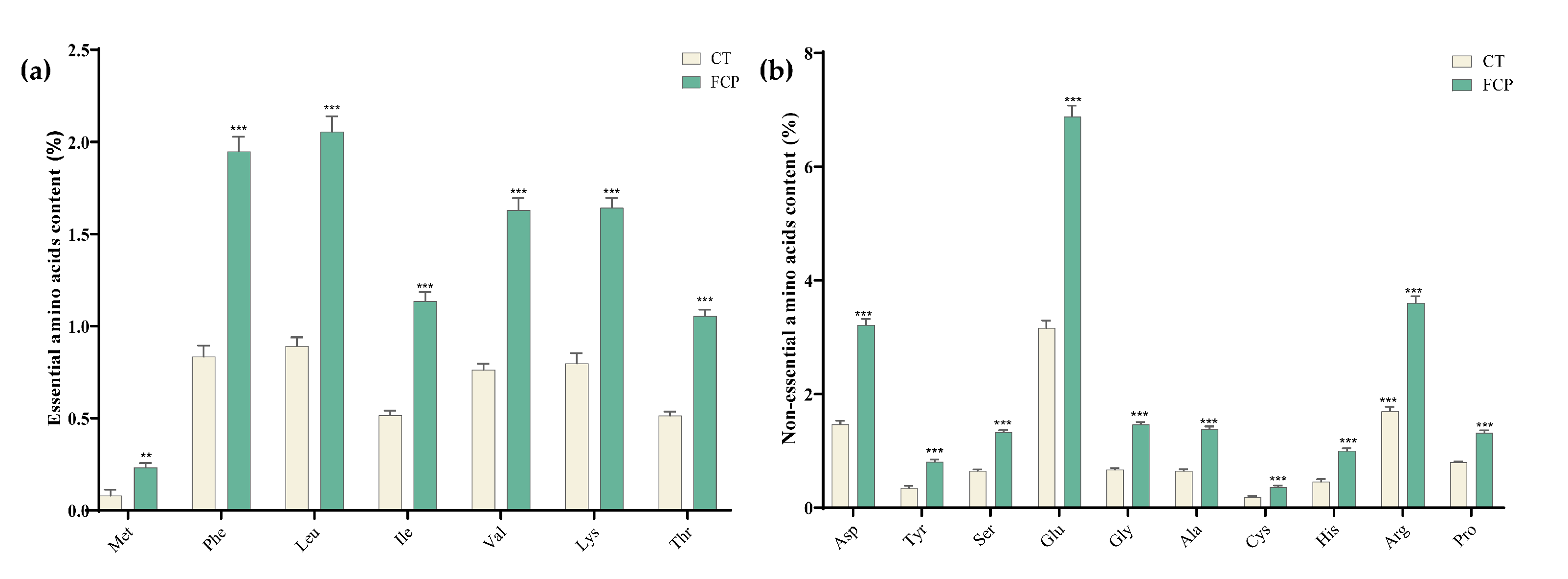
3.4. Changes in Amino Acids in FCP
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prawirodigdo, S.; Gannon, N.J.; Leury, B.J.; Dunshea, F.R. Basal diet and indigestible marker influence apparent digestibilities of nitrogen and amino acids of cottonseed meal and soybean meal in pigs. Anim. Nutr. 2019, 5, 234–240. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, H.; Olk, D.C. Chemical Composition of Defatted Cottonseed and Soy Meal Products. PLoS ONE 2015, 10, e0129933. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.A.; Yang, H.M.; Yang, Z.; Wang, Z.Y. Effect of heating time of cottonseed meal on nutrient and mineral element digestibility in chicken (based on cottonseed meal replaced with all soybean meal). Animals-Basel 2022, 12, 883. [Google Scholar] [CrossRef] [PubMed]
- Olukomaiya, O.; Fernando, C.; Mereddy, R.; Li, X.; Sultanbawa, Y. Solid-state fermented plant protein sources in the diets of broiler chickens: A review. Anim. Nutr. 2019, 5, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, H.A.; Rehemujiang, H.; Ma, T.; Piao, M.; Huo, R.; Tu, Y. Fermented total mixed ration with cottonseed meal or rapeseed meal improved growth performance and meat quality of Hu lamb compared to total mixed ration with soybean meal. Fermentation 2022, 8, 576. [Google Scholar] [CrossRef]
- Ma, M.; Ren, Y.; Xie, W.; Zhou, D.; Tang, S.; Kuang, M.; Wang, Y.; Du, S.K. Physicochemical and functional properties of protein isolate obtained from cottonseed meal. Food Chem. 2018, 240, 856–862. [Google Scholar] [CrossRef]
- Zendeboodi, F.; Khorshidian, N.; Mortazavian, A.M.; da Cruz, A.G. Probiotic: Conceptualization from a new approach. Curr. Opin. Food. Sci. 2020, 32, 103–123. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, G.; Ali, S.A. Dairy-Based Probiotic-Fermented Functional Foods: An Update on Their Health-Promoting Properties. Fermentation 2022, 8, 425. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, D.; Liu, L.; Chang, Z.; Peng, N. Effective gossypol removal from cottonseed meal through optimized solid-state fermentation by Bacillus coagulans. Microb. Cell Fact. 2022, 21, 252. [Google Scholar] [CrossRef]
- Mitri, S.; Salameh, S.-J.; Khelfa, A.; Leonard, E.; Maroun, R.G.; Louka, N.; Koubaa, M. Valorization of Brewers’ Spent Grains: Pretreatments and Fermentation, a Review. Fermentation 2022, 8, 50. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Su, W.; Jiang, Z.; He, H.; Gong, T.; Kai, L.; Xu, H.; Wang, Y.; Lu, Z. Co-fermented yellow wine lees by Bacillus subtilis and Enterococcus faecium regulates growth performance and gut microbiota in finishing pigs. Front. Microbiol. 2022, 13, 1003498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Miao, Q.; Pan, C.; Yin, J.; Wang, L.; Qu, L.; Yin, Y.; Wei, Y. Research advances in probiotic fermentation of Chinese herbal medicines. iMeta 2023, e93. [Google Scholar] [CrossRef]
- Zou, G.; Nielsen, J.B.; Wei, Y.J. Harnessing synthetic biology for mushroom farming. Trends Biotechnol. 2023, 41, 480–483. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, B.; Wen, S.; Wang, Y.; Pan, C.; Qu, L.; Yin, Y.; Wei, Y. Advancements in the Biotransformation and Biosynthesis of the Primary Active Flavonoids Derived from Epimedium. Molecules 2023, 28, 7173. [Google Scholar] [CrossRef]
- Huang, S.; Shao, L.; Chen, M.; Wang, L.; Liu, J.; Zhang, W.; Huang, W. Biotransformation differences of ginsenoside compound K mediated by the gut microbiota from diabetic patients and healthy subjects. Chin. J. Nat. Med. 2023, 21, 723–729. [Google Scholar] [CrossRef]
- Brandt, B.A.; García-Aparicio, M.D.P.; Görgens, J.F.; van Zyl, W.H. Rational engineering of Saccharomyces cerevisiae towards improved tolerance to multiple inhibitors in lignocellulose fermentations. Biotechnol. Biofuels 2021, 14, 173. [Google Scholar] [CrossRef] [PubMed]
- Schlabitz, C.; Lehn, D.N.; de Souza, C.F.V. A review of Saccharomyces cerevisiae and the applications of its byproducts in dairy cattle feed: Trends in the use of residual brewer’s yeast. J. Clean. Prod. 2022, 332, 130059. [Google Scholar] [CrossRef]
- Peng, J.J.; Wang, L.; Wang, M.G.; Du, R.; Qin, S.S.; Jin, C.Y.; Wei, Y.J. Yeast synthetic biology for the production of Lycium barbarum polysaccharides. Molecules 2021, 26, 1641. [Google Scholar] [CrossRef]
- Wei, Y.J.; Ji, B.Y.; Ledesma-Amaro, R.; Chen, T.; Ji, X.J. Editorial: Engineering yeast to produce plant natural products. Front. Bioeng. Biotechnol. 2021, 9, 798097. [Google Scholar] [CrossRef]
- Lu, C.J.; Du, R.; Fu, H.; Zhang, J.Z.; Zhao, M.; Wei, Y.J.; Lin, W. Heterologous biosynthesis of medicarpin using engineered Saccharomyces cerevisiae. Syn. Syst. Biotechno. 2023, 8, 749–756. [Google Scholar] [CrossRef]
- Wei, Y.J.; Ji, B.Y.; Siewers, V.; Xu, D.Y.; Halkier, B.A.; Nielsen, J. Identification of genes involved in shea butter biosynthesis from Vitellaria paradoxa fruits through transcriptomics and functional heterologous expression. Appl. Microbiol. Biot. 2019, 103, 3727–3736. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.G.; Wei, Y.J.; Ji, B.Y.; Nielsen, J. Advances in metabolic engineering of Saccharomyces cerevisiae for cocoa butter equivalent production. Front. Bioeng. Biotech. 2020, 8, 594081. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, B.; Wityk, P.; Galecka, M.; Michalik, M. The Many aaces of Enterococcus spp.-commensal, probiotic and opportunistic pathogen. Microorganisms 2021, 9, 1900. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Gao, M.; Zhang, R.; Sun, Z.; Wang, C.; Yang, F.; Huang, T.; Qu, S.; Zhao, L.; Li, Y.; et al. Effects of soybean meal fermented by L. plantarum, B. subtilis and S. cerevisieae on growth, immune function and intestinal morphology in weaned piglets. Microb. Cell Fact. 2017, 16, 191. [Google Scholar] [CrossRef] [PubMed]
- Crognale, S.; Russo, C.; Petruccioli, M.; D’Annibale, A. Chitosan Production by Fungi: Current State of Knowledge, Future Opportunities and Constraints. Fermentation 2022, 8, 76. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Pan, L.Q.; Fan, D.P.; He, J.J.; Su, C.; Gao, S.; Zhang, M.Y. Study of fermented feed by mixed strains and their effects on the survival, growth, digestive enzyme activity and intestinal flora of Penaeus vannamei. Aquaculture 2021, 530, 735703. [Google Scholar] [CrossRef]
- Xie, J.M.; Chen, Y.R.; Cai, G.J.; Cai, R.L.; Hu, Z.; Wang, H. Tree visualization by one table (tvBOT): A web application for visualizing, modifying and annotating phylogenetic trees. Nucleic Acids Res. 2023, 51, W587–W592. [Google Scholar] [CrossRef]
- Fu, M.; Jahan, M.S.; Tang, K.; Jiang, S.Z.; Guo, J.X.; Luo, S.W.; Luo, W.L.; Li, G.H. Comparative analysis of the medicinal and nutritional components of different varieties of Pueraria thomsonii and Pueraria lobata. Front. Plant Sci. 2023, 14, 1115782. [Google Scholar] [CrossRef]
- Sinkovic, L.; Dez, M.; Kopinc, R.; Meglic, V. Macro/microelements, nutrients and bioactive components in common and Tartary buckwheat (Fagopyrum spp.) grain and stone-milling fractions. LWT-Food Sci. Technol. 2022, 161, 113422. [Google Scholar] [CrossRef]
- Lee, J.; Jo, N.Y.; Shim, S.Y.; Linh, L.T.Y.; Kim, S.R.; Lee, M.G.; Hwang, S.G. Effects of Hanwoo (Korean cattle) manure as organic fertilizer on plant growth, feed quality, and soil bacterial community. Front. Plant Sci. 2023, 14, 1135947. [Google Scholar] [CrossRef]
- Xu, D.M.; Ding, Z.T.; Bai, J.; Ke, W.C.; Zhang, Y.X.; Li, F.H.; Guo, X.S. Evaluation of the effect of feruloyl esterase -producing Lactobacillus plantarum and cellulase pretreatments on lignocellulosic degradation and cellulose conversion of co -ensiled corn stalk and potato pulp. Bioresource. Technol. 2020, 310, 123476. [Google Scholar] [CrossRef]
- Yusuf, H.A.; Piao, M.Y.; Ma, T.; Huo, R.Y.; Tu, Y. Effect of lactic acid bacteria and yeast supplementation on anti-nutritional factors and chemical composition of fermented total mixed ration containing cottonseed meal or rapeseed meal. Anim. Biosci. 2022, 35, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Lee, J.J.L.; Chen, W.N. Analysis of improved nutritional composition of potential functional food (Okara) after probiotic solid-state fermentation. J. Agric. Food Chem. 2018, 66, 5373–5381. [Google Scholar] [CrossRef]
- Guzmán, M. Fatty acids fuelling astroglia and beyond. Nat. Metab. 2023, 5, 1253–1254. [Google Scholar] [CrossRef]
- Naseem, S.; Bhat, S.U.; Gani, A.; Bhat, F.A. Perspectives on utilization of macrophytes as feed ingredient for fish in future aquaculture. Rev. Aquacult. 2021, 13, 282–300. [Google Scholar] [CrossRef]
- Prawirodigdo, S.; Gannon, N.J.; Leury, B.J.; Dunshea, F.R. Acid-insoluble ash is a better indigestible marker than chromic oxide to measure apparent total tract digestibility in pigs. Anim. Nutr. 2021, 7, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Rieger, H.; Ratert, C.; Wendt, M.; Schwennen, C.; Kamphues, J. Comparative study on the chemical composition of different bones/parts of bones in growing pigs differently supplied with inorganic phosphorus and phytase. J. Anim. Physiol. Anim. Nutr. 2021, 105, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Sieuwerts, S.; Bron, P.A.; Smid, E.J. Mutually stimulating interactions between lactic acid bacteria and Saccharomyces cerevisiae in sourdough fermentation. LWT-Food Sci. Technol. 2018, 90, 201–206. [Google Scholar] [CrossRef]
- Loh, L.X.; Ng, D.H.J.; Toh, M.Z.; Lu, Y.Y.; Liu, S.Q. Targeted and nontargeted metabolomics of amino acids and bioactive metabolites in probiotic-fermented unhopped beers using liquid chromatography high-resolution mass spectrometry. J. Agric. Food Chem. 2021, 69, 14024–14036. [Google Scholar] [CrossRef]
- Li, X.; Tian, J.; Zhang, Q.; Jiang, Y.; Hou, J.; Wu, Z.; Yu, Z. Effects of applying Lactobacillus plantarum and Chinese gallnut tannin on the dynamics of protein degradation and proteases activity in alfalfa silage. Grass Forage Sci. 2018, 73, 648–659. [Google Scholar] [CrossRef]
- Zhang, L.H.; Zha, M.M.; Li, S.F.; Zong, W. Changes on some quality characteristics of jujube juice with enzymatic hydrolysis prior to Lactobacillus plantarum fermentation. J. Food Meas. Charact. 2022, 16, 3196–3207. [Google Scholar] [CrossRef]
- Fonseca, L.D.; Lanferdini, E.; Moreira, R.H.R.; Chaves, R.F.; Perazolli, P.H.; Paula, Y.H.D.; Renn, L.N.; Garbossa, C.A.P.; Cantarelli, V.D.; De, L.T. Arginine supplementation in the feed of gestating sows. Livest. Sci. 2022, 263, 104999. [Google Scholar] [CrossRef]
- Qi, M.; Wang, J.; Tan, B.E.; Li, J.J.; Liao, S.M.; Liu, Y.H.; Yin, Y.L. Dietary glutamine, glutamate, and aspartate supplementation improves hepatic lipid metabolism in post-weaning piglets. Anim. Nutr. 2020, 6, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Zhang, X.; Wang, Y.; Li, X.; Wang, Z.; Tian, L.; Qu, L.; Wei, Y. Characterization of novel pectinolytic enzymes derived from the efficient lignocellulose degradation microbiota. Biomolecules 2022, 12, 1388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Miao, Q.; Tang, B.; Mijakovic, I.; Ji, X.-J.; Qu, L.; Wei, Y. Discovery of novel alkaline-tolerant xylanases from fecal microbiota of dairy cows. Biotechnol. Biof. Biop. 2023, 16, 182. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.X.; Yu, Y.X.; Li, Z.; Yan, B.W.; Pei, W.H.; Wu, H. The preparation technology and application of xylo-oligosaccharide as prebiotics in different fields: A review. Front. Nutr. 2022, 9, 996811. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liang, J.W.; Li, Y.H.; Tian, L.M.; Wei, Y.J. Characterization of efficient xylanases from industrial-scale pulp and paper wastewater treatment microbiota. Amb Express 2021, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Li, R.; Tang, W.; Zheng, Y.; Meng, X. Improve Enzymatic Hydrolysis of Lignocellulosic Biomass by Modifying Lignin Structure via Sulfite Pretreatment and Using Lignin Blockers. Fermentation 2022, 8, 558. [Google Scholar] [CrossRef]
- Liu, S.Q.; Du, M.; Tu, Y.; You, W.J.; Chen, W.T.; Liu, G.L.; Li, J.Y.; Wang, Y.Z.; Lu, Z.Q.; Wang, T.H.; et al. Fermented mixed feed alters growth performance, carcass traits, meat quality and muscle fatty acid and amino acid profiles in finishing pigs. Anim. Nutr. 2023, 12, 87–95. [Google Scholar] [CrossRef]
- Wang, W.L.; Zijlstra, R.T.; Gänzle, M.G. Feeding Limosilactobacillus fermentum K9–2 and Lacticaseibacillus casei K9–1, or Limosilactobacillus reuteri TMW1.656 Reduces Pathogen Load in Weanling Pigs. Front. Microbiol. 2020, 11, 608293. [Google Scholar] [CrossRef]
- Gao, X.; Zheng, T.; Yuan, X.L.; Li, Z.K.; Wei, Y.J. Draft genome sequence of an epiphytic strain, Bacillus sp. strain WL1, isolated from the surface of Nostoc flagelliforme Colonies in Yinchuan, Ningxia, China. Microbiol. Resour. Announc. 2021, 10, e0038221. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.; Li, B.; Wang, Y.; Yin, X.; Gong, M.; Shang, J.J.; Wei, Y.J.; Li, X.L.; Bao, D.P. Efficient conversion of spent mushroom substrate into a high value-added anticancer drug pentostatin with engineered Cordyceps militaris. Green Chem. 2021, 23, 10030–10038. [Google Scholar] [CrossRef]
- Yuan, Y.Z.; Yang, Y.T.; Xiao, L.L.; Qu, L.B.; Zhang, X.L.; Wei, Y.J. Advancing insights into probiotics during vegetable fermentation. Foods 2023, 12, 3789. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, X.; Ji, B.; Qu, L. Recent advances on the recovery, modulation and synthetic biology of gut microbiota and hosts. Sci. Sin. Vitae 2022, 52, 249–265. [Google Scholar] [CrossRef]
- Lin, J.; Zou, G.; Liu, H.; Wei, Y. Application of CRISPR/Cas genome editing technology in the synthesis of secondary metabolites of filamentous fungi. Synth. Biol. J. 2023, 4, 738–755. [Google Scholar] [CrossRef]
- Liu, Y.F.; Li, X.; Yang, Y.D.; Liu, Y.; Wang, S.J.; Ji, B.Y.; Wei, Y.J. Exploring gut microbiota in patients with colorectal disease based on 16S rRNA gene amplicon and shallow metagenomic sequencing. Front. Mol. Biosci. 2021, 8, 703638. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.; Zhang, J.; Zou, G.; Zhang, X.; Shang, H.; Ji, B.; Bai, Y.; Qu, L.; Wei, Y. Enhancing the Nutritional Quality of Defatted Cottonseed Meal by Solid-State Fermentation with Probiotic Microbes. Fermentation 2024, 10, 429. https://doi.org/10.3390/fermentation10080429
Lin J, Zhang J, Zou G, Zhang X, Shang H, Ji B, Bai Y, Qu L, Wei Y. Enhancing the Nutritional Quality of Defatted Cottonseed Meal by Solid-State Fermentation with Probiotic Microbes. Fermentation. 2024; 10(8):429. https://doi.org/10.3390/fermentation10080429
Chicago/Turabian StyleLin, Jicong, Jingxian Zhang, Gen Zou, Xiaoling Zhang, Haihong Shang, Boyang Ji, Yueyu Bai, Lingbo Qu, and Yongjun Wei. 2024. "Enhancing the Nutritional Quality of Defatted Cottonseed Meal by Solid-State Fermentation with Probiotic Microbes" Fermentation 10, no. 8: 429. https://doi.org/10.3390/fermentation10080429




