Abstract
The ethanolic (EE) and hydroalcoholic (HE) extracts of Urochloa brizantha concentrations were developed with the aim of evaluating their effect on rumen fermentation using an in vitro gas production technique. The EE and HE presented 3.62 and 5.38 mg protodioscin/mL, respectively. Ten treatments were evaluated in a completely randomized factorial arrangement (2 × 4 + 2), where the main effects were two extracts (EE and HE) and four levels (50, 100, 150, and 200 mL of extract/kg of DM) plus two controls: one positive (25 ppm of monensin–MON) and one (with no additives–CTL). The extract treatments (EXT, EE, and HE) reduced colonization time by 33.59% compared to the MON. IVDMD (p < 0.001) and IVOMD (p < 0.0001) were negatively affected by EXT addition when compared to CTL. Additionally, EXT reduced the proportion of propionic acid and increased the proportion of butyric acid in relation to CTL and MON treatments. Both EE and HE extracts of U. brizantha were able to alter rumen fermentation kinetic, with HE showing a higher concentration of protodioscin. Further research is needed to optimize extraction methodologies, comprehensively profile secondary compounds, and conduct trials with varying doses to effectively assess the viability of U. brizantha extract as an additive.
1. Introduction
The rumen’s unique evolutionary advantage, through its symbiosis with microorganisms, allows ruminants to utilize lignocellulosic material and convert non-protein nitrogen into microbial protein [1]. Animal nutritionists frequently employ rumen manipulation to enhance fibrous feed degradability and reduce nitrogen excretion by ruminants, thereby improving their performance [2]. Consequently, various nutritional alternatives have been employed, including ionophore antibiotics, lipids, probiotics, and plant extracts.
Among these, ionophore antibiotics, particularly monensin, have been the most widely used in ruminant production in recent years [3]. However, the international community is increasingly demanding environmental measures, especially concerning the use of additives in ruminant feed. This adds to the complexity of the issue, especially given the restrictions on synthetic drugs [4]. Therefore, plant extracts are particularly promising as an alternative to synthetic drugs due to their secondary compounds—such as saponins, tannins, flavonoids, and essential oils, which exhibit various biological activities [5].
These compounds can modulate ruminal microorganisms, improve animal performance, reduce energy loss, and mitigate GHG emissions [6,7]. Among them, saponins are notable bioactive compounds abundantly present in the plant kingdom [8]. They are classified into steroidal saponins, which are found in monocots, and triterpenoid saponins, which are found in eudicots [9]. However, the use of saponins from various sources as additives has led to inconsistencies in research results [10,11,12,13]. These inconsistencies may arise from the significant variability in both the type and concentration of extracts and the nature of the secondary metabolites present [14].
In addition, the most researched extracts have significant human applications, either for use in food or as herbal medicines, which can lead to competition and increased production costs [15]. However, the plant kingdom offers a vast array of species, providing numerous options [16,17], including forage species [18]. Forage is a crucial component of ruminant nutrition, with species such as Urochloa, which is economically important and contains saponins like protodioscin [19].
Although some studies have examined the secondary compounds in the Urochloa genus, their primary focus has been on identifying saponins responsible for allelopathic effects [20,21] and inducing photosensitization in ruminants [22,23]. Consequently, several significant gaps remain. These include the development of saponin-rich extracts, the choice of extraction method and solvent, the optimal concentrations of the extract, and the selection of forages with higher nutritional value to maximize the positive impact of the additive on animal nutrition.
Among the Urochloa species, Urochloa brizantha is the most widely distributed in Brazil due to its excellent digestibility and palatability [24]. We hypothesize that ethanolic and hydroalcoholic extracts of Urochloa brizantha containing saponins, when used as natural additives, can modulate the fermentation profile by affecting microbial activity in the rumen. Therefore, our study aimed to investigate the effects of concentrations of extracts from U. brizantha cv. Marandu on in vitro fermentation kinetics.
2. Materials and Methods
The study was approved by the ethics committee of the College of Animal Science and Food Engineering, University of São Paulo, Pirassununga, Brazil (Protocol number CEUA: 1344101121).
2.1. Inoculum Donors and Substrate
Ruminal fluid was collected through the rumen cannula from eight male Santa Inês x Dorper sheep (41.11 ± 8.08 kg) fed an experimental diet consisting of 70% corn silage and 30% concentrate, with free access to freshwater and mineral supplements. On the day of incubation, rumen content was collected before the morning feeding. The solid phase was collected manually from the dorsal sac through the cannula, squeezed, placed in plastic bags, and stored in heated boxes at 39 °C. The liquid phase was collected from the ventral sac using a stainless-steel probe (2.5 mm screen) attached to a 500 mL graduated pipette and stored in pre-warmed thermal bottles, which had been previously flushed with CO2. Both fluids and solids were transported immediately to the laboratory.
Four inocula were prepared, each from two donors. The solid and liquid phases were homogenized in a 1:1 ratio in a blender for 10 s and filtered through two layers of cotton cloth following the method described by Bueno et al. [25]. The inocula was transferred to anaerobic jars, continuously saturated with CO2, and maintained in a Daisy Incubator (Ankom Technology, Macedon, NY, USA) at 39 °C until use.
The diet was dried and ground using a Willey mill with a 1 mm sieve as a substrate for gas production bioassay. The substrate analysis revealed the following composition: 436.1 g of dry matter (DM; ID 930.15), 55.6 g of mineral matter (ash; ID 942.05), 114.9 g of crude protein (CP; ID 954.01), 29.1 g of ether extract (EE; ID 920.39), 438.6 g of neutral detergent fiber (NDF; ID 973.18), and 245.1 g of acid detergent fiber (ADF; ID 973.18), according to AOAC [26].
2.2. Urochloa Brizantha Extract Preparation
U. brizantha cv. Marandu, in a period of growth, was harvested from a pasture established in the experimental area of the Department of Animal Science at FZEA-USP, Pirassununga, São Paulo (21°59′ S latitude, 47°26′ W longitude, and 634 m altitude) The local climate, according to Koppen’s classification, is of the Cwa type, characterized by an average annual temperature of over 22 °C, annual rainfall of 1.238 mm, and a relative humidity of 73%. The soil in the experimental area was classified as dystrophic red latosol, gentle to moderately undulating [27].
An initial cut was performed on 10 December 2020, leaving a residue height of 15 cm, followed by fertilization on 18 December to standardize growth between the paddocks. The plots were regularly monitored to assess vegetation growth and control invasive plants. Irrigation was not required due to abundant rainfall during this period. On 15 February 2021, a uniformization cut was performed on all plots to maintain consistent growing conditions. At this time, the plants were 65 days old and fully grown. Each plot was allocated 8 m2. After the uniformization cut, maintenance fertilization was applied with nitrogen (N, 25 g of NPK/m2) and phosphorus (200 g of P2O5/m2) to enhance secondary metabolite production [28]. When the plants reached 30 cm in height, they were cut on March 26, 2021, using a tractor mower, leaving a residue height of 15 cm. The collected material was dried in a shaded greenhouse at a controlled temperature (below 40 °C to avoid inactivating secondary metabolites) for 14 days, then ground in a disintegrator (Nogueira, model DPM-4, São João da Boa Vista, SP, Brazil) with 2 mm mesh sieves.
To produce U. brizantha extract, the pressurized liquid extraction (PLE) technique on a pilot scale was utilized with 99.5% ethanol (pure) and a hydroalcoholic solution (70% ethanol). A sample of dried and crushed Marandu grass (±400 g) was placed in a 2 L cell. The solvent (ethanolic—99.5% of ethanol and hydroalcoholic—70% ethanol) was pumped into the cell, and the intermittent process began once temperature (80 °C) and pressure (10.35 MPa) were adjusted. Each rinse cycle used 480 mL of solvent. At the end of the process, the purge valve was opened, and nitrogen gas (N2) was used to transfer all remaining extract from the cell to the collection vessel [29].
The concentrations of saponins in the extracts were determined and quantified as protodioscin by the high-performance liquid chromatographic (HPLC) method described by Lee et al. [30]. The saponin contents in the EE and HE extracts were 3.62 and 5.38 mg protodioscin/mL, respectively.
2.3. Treatments
Ten treatments were tested, involving two extracts: ethanolic (EE) and hydroalcoholic (HE); four levels: 50, 100, 150, and 200 mL of extract/kg of DM; and two controls: one positive (MON, 25 ppm of monensin) and one negative control (CTL, no additives). The four levels used corresponded to dosages of 0.37, 0.75, 1.12, and 1.50 mg of saponin (protodioscin; g/mL of extract).
2.4. In Vitro Gas Production Assay
To evaluate the effect of extracts on fermentation kinetics, the semi-automatic in vitro gas production technique described by Theodorou et al. [31], modified by Mauricio et al. [32], was used. A total of 132 glass bottles, each with a capacity of 160 mL, were prepared for the study, which included four inocula and eleven treatments (ten tested plus a blank), with each treatment tested in triplicates. In the negative control, a hydroalcoholic solution was used to ensure that any observed effects in the experimental treatments could be attributed specifically to the additives rather than the solvent. Additionally, a hydroalcoholic solution was employed to dilute the monensin.
In each bottle we added 1.0 g of substrate (70% silage and 30% concentrate) in filter bags (TNT, 100 g/m2), 10 mL of rumen inoculum, and 90 mL of nutrient solution. The nutrient solution was composed of micromineral solutions (CaCl2·2H2O; MnCl2·2H2O; CoCl2·6H2O and FeCl3·6H2O), macrominerals (Na2HPO4; KH2PO4 and MgSO4·7H2O), buffer (NH4CO3 and NaHCO3), reducing agent (cysteine-HCl; 1 M NaOH and Na2S·9H2O), and indicator (resazurin), prepared according to the method described by Menke et al. [33]. Blanks were used for each inoculum to measure the fraction of total gas production due to substrate in the inocula; these values were subtracted from the total to obtain net gas production.
The bottles were sealed with butyl rubber stoppers, shaken, and incubated in an oven with forced-air circulation at 39 °C for 96 h. All treatments were incubated simultaneously.
The headspace pressure was measured at 4, 8, 12, 16, 20, 24, 30, 36, 42, 48, 60, 72, and 96 h of incubation using a transducer (Pressure Press Data 800; Piracicaba, SP, Brazil). After each measurement, the accumulated gas was released from all bottles. The total gas volume produced in each bottle was estimated according to the equation V = (4.6788 ∗ P), where V = volume of gases (mL) and P = measured pressure (psi) [34]. This equation is validated for the in vitro experimental conditions for specific fermentation kinetic bioassays at the Ruminal Fermentability Laboratory. Cumulative gas production was determined by summing the gas volumes obtained from each pressure measurement after 96 h of fermentation.
Fermentation kinetics were analyzed using gas production data based on the following model by France et al. [35]:
where Y = cumulative production of gases (mL/g DM) in incubation time t (h), A = potential gas production (mL/g DM), L = colonization time (lag time), b and c are mathematical constants of the mode, T1/2 = time to half-asymptote (h), and µ = fractional rate of gas production (/h). The kinetic parameters (A, L, µ, and T½) were compared in the statistical analysis.
At the end of the 96-h incubation, the bottles were placed in ice containers to stop the fermentation process. Two mL samples of ruminal fluid from each bottle were collected to determine short-chain fatty acid (SCFA) and ammonia nitrogen (NH3-N).
The filter bags for substrate incubation were washed in running water and dried in an oven with forced-air circulation (105 °C). The in vitro dry matter degradability (IVDMD) was determined by the difference between the weights before and after dry incubation in an oven at 105 °C to determine the residue. The in vitro organic matter degradability (IVOMD) was estimated by the difference in residues (ash) after burning in a muffle furnace at 550 °C for four hours. The partitioning factor (PF), calculated by relating dry matter degradation to total gas production (mL) over 96 h, was used to compare microbial efficiency [36].
2.5. Short-Chain Fatty Acid (SCFA) and Ammonia Nitrogen (NH3-N) Determination
A short-chain fatty acid (SCFA) profile in ruminal fluid was analyzed using gas chromatography (GC–2014; Shimadzu) as described by Bueno et al. [37].
Ammonia nitrogen (NH3-N) concentrations in the rumen fluid were determined by colorimetry, according to the methodology of Kulasek [38] and adapted by Foldager et al. [39].
2.6. Experimental Design and Statistical Analysis
The experimental design was completely randomized in a factorial arrangement. 2 × 4 + 2, with two types of U. brizantha extracts (ethanolic—99% and hydroalcoholic—70%), four concentrations (50, 100, 150, and 200 mL/kg of DM), plus two additional treatments, one positive control (monensin, Elanco Brasil, Rumensin®, 25 mg/kg DM), and one negative control (no additive), with three replicates within each experimental unit.
The normality of the residuals was assessed using the Shapiro–Wilk test (PROC UNIVARIATE). Statistical analysis was carried out using the GLIMMIX procedure (SAS 9.4; SAS Institute, Cary, NC, USA) using orthogonal contrasts of the effects of grouped treatments:
- (1)
- Effect of monensin (MON) treatment against control (CTL);
- (2)
- Effect of extracts (EXT, the grouping of EE and HE extracts in all doses) concerning the positive control (MON);
- (3)
- Effect of extracts (EXT, the grouping of EE and HE extracts in all doses) concerning the negative control (CTL);
- (4)
- Effect of type of extract (ethanolic, EE, and hydroalcoholic, HE) at different doses: 50, 100, 150, and 200 mL/kg DM);
- (5)
- Level effect (LVL), comparing each level (50, 100, 150, and 200 mL/kg DM) against the other level of extracts;
- (6)
- EXT vs. LVL interaction effect.
The significance was set at p ≤ 0.05, and the p values obtained for the contrasts are reported.
3. Results
3.1. In Vitro Gas Production Assay
There was no significant difference (p > 0.05) between the treatments in maximum gas production potential (A), fractional rate of gas production (µ) at time to half-asymptote (T½), and T½ (Table 1). However, the colonization time (L, Lag time) was significant (p < 0.01) when comparing the EXT (6.13 h) and MON (9.29 h) treatments, representing a reduction of 33.59%, and there was also a difference (p = 0.02) between the EE (5.42 h) and EH (6.84 h) extracts.

Table 1.
Effect of ethanolic (EE) and hydroalcoholic (HE) extracts of Urochloa brizantha on kinetic parameters of gas production in vitro modeled according to France model, degradability and partitioning factor (FP).
IVDMD showed a level effect (NIV) (p = 0.048), indicating that increasing the level of inclusion of the extracts in the diet negatively impacted diet degradability. The CTL treatment (79.77%, p < 0.001) had the highest IVDMD, followed by MON (77.56%, p = 0.03) and EXT (73.94%). Among the extracts, HE (75.73%) had a higher IVDMD (p < 0.001) than EE (72.14%). There was also a significant effect (p < 0.0001) between EXT (72.69%) and CTL (79.38%) and between (p < 0.01) EE (70.82%) and HE (74.57%) extracts.
The partition factor (PF, mg DMD/mL) was significant (p < 0.01) in the contrast between MON (2.79 mg DMD/mL) and CTL (2.52 mg DMD/mL). It was also highly significant (p < 0.001) when comparing MON (2.79 mg DMD/mL) with EXT (2.40 mg DMD/mL).
The accumulated gas production (mL/g DM) curves after 96 h of fermentation for the treatments, the EE (Figure 1), and HE (Figure 2) extracts showed a similar fermentation pattern.
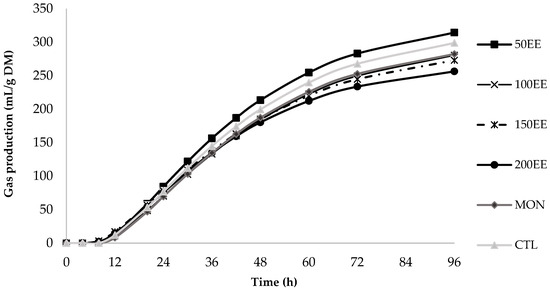
Figure 1.
In vitro cumulative gas production curve of ethanolic extract from Urochloa brizantha at 96 h according to the France model.
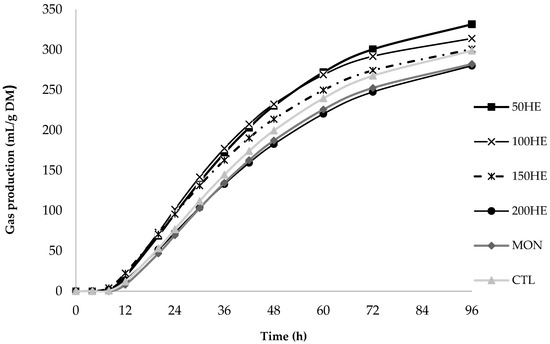
Figure 2.
In vitro cumulative gas production curve of hydroalcoholic extract from Urochloa brizantha at 96 h according to the France model.
3.2. Short-Chain Fatty Acids and Ammonia Nitrogen
The inclusion of extract (p > 0.05) in the diet did not affect the proportion of short-chain fatty acids (SCFAs) (Table 2).

Table 2.
Effect of ethanolic (EE) and hydroalcoholic (HE) extracts on the production of short-chain fatty acids (SCFAs) and ammoniacal nitrogen (NH3-N) at 96 h of incubation in vitro.
The inclusion of extracts (p > 0.05) in the diet in vitro did not affect the proportion of acetic acid (C2) compared to the control treatment (CTL) and monensin (MON) (Table 2). The addition of the EXT levels had a significant effect (p < 0.01) on concentrations, with higher levels at 50 mL/kg for EE (44.28) and at 100 mL/kg for EH (48.31).
The MON treatment (33.48%) provided a higher proportion of propionic acid (C3) than the CTL treatment (30.69%, p < 0.001) and EXT (23.34%, p < 0.001). The reduction of C3 with the addition of extracts resulted in an 18.13% increase in the C2:C3 ratio compared to the MON treatment.
Concerning butyric acid (C4), the MON (13.64%) treatment significantly reduced the proportion of C4 compared to the CTL treatments (p = 0.02) and EXT treatments (p = 0.01). Additionally, CTL (18.41%) was significantly lower (p < 0.01) than EXT (22.42%). Furthermore, the addition of EXT had a significant effect (p = 0.02), with concentrations reaching 24.93% at 100 mL/kg for EE and 22.27% at 150 mL/kg for EH.
The treatments had no significant effect on the proportions of isobutyric (IC4), valeric (C5), and isovaleric (IC5) acids.
The ammoniacal nitrogen (NH3-N) had a significant effect (p = 0.04) when comparing the MON treatment (44.77 mg/mL) with CTL (41.51 mg/mL). The concentration of NH3-N was different (p < 0.01) between EE (41.34 mg/mL) and HE (43.72 mg/mL).
4. Discussion
4.1. In Vitro Gas Production Assay
While some studies have evaluated the effects of plant extracts containing saponins on in vitro rumen fermentation kinetics, none have specifically assessed the effect of Urochloa brizantha extracts on degradability, fermentability, and microbial efficiency.
According to Seo et al. [40], the degradation of fiber by rumen microorganisms is a time-dependent process, with the colonization time corresponding to the time between adhesion and colonization at the start of incubation and the degradation of the substrate [41].
In this study, the inclusion of EXT reduced colonization time by 33.59% compared to the MON. This finding is consistent with the results of Freitas et al. [42], who observed that adding 75, 150, and 250 g/L m of ethanolic extracts of Urochloa humidicola decreased colonization time.
Similarly, Jiménez-Peralta et al. [43], observed a reduction in colonization time with Leucaena (Leucaena leucocephala) and Weeping Willow (Salix babylonica) extracts when using 1.8 mL/g DM. Additionally, they noted an improvement in the cumulative gas profile after 72 h. In contrast, Abarghuei, Rouzbeha, and Salem [44] found no significant effect of various pomegranate peel extracts (PEH: water; PEM: methanol, ethanol, and water) on cumulative gas production after 120 h. However, they did observe a decrease in the fractional rate of gas production with increasing doses of extracts.
These findings suggest that the extract may have reduced the colonization time, thereby improving the fermentation efficiency of the substrate. According to Zhou et al. [45], the addition of Neolamarckia cadamba leaves, which contain saponins, enhanced the digestibility of the diet, suggesting a similar potential for improving fermentation efficiency. This enhancement may be attributed to the extraction process, which removed the soluble fraction from the intracellular content, making it readily available to the microorganisms.
In our study, we observed a decrease in IVDMD and IVOMD with extract addition. This finding corroborates with the results of Hu et al. [46], who observed a reduction in IVOMD by adding 0.8 mg/mL of Tea saponin. In contrast, Abarghuei, Rouzbeha, and Salem [44] observed that the addition of PEH and PEM does not affect the IVOMD. However, Jiménez-Peralta et al. [43] observed that the two highest doses of extracts of Leucaena and Weeping Willow (1.2 and 1.8 mL/g DM) increased IVOMD values. Therefore, these variations can be attributed to the chemical nature, activities, and concentrations of the active compounds present in the extracts [47].
Furthermore, the type of solvent influences the composition of the secondary compounds. In this study, it was possible observe a greater reduction in degradability with the addition of EE than HE, which may have been due to the type of secondary compound extracted. Only the saponin protodioscin was quantified in our study, but different secondary compounds may be present, as the combination of ethanol and water enhances extraction efficiency [48].
Patra et al. [2] tested plant extracts using different solvents (methanol, ethanol, and water) and found that all extracts reduced feed degradability by 6 to 7%. The authors attributed this reduction to the impact of certain secondary compounds on the rumen microbiota. There may be some concern that the alcohol in alcoholic extracts could negatively affect the degradability of the substrate and mask the possible effects of the secondary compounds. However, Raun and Kristensen [49] suggest that microorganisms in the rumen can metabolize ethanol.
According to Leal et al. [19], protodioscin present in U. brizantha negatively correlates with the degradability of grasses by microorganisms, thereby adversely affecting IVDMD and IVOMD and prolonging bacterial colonization time. However, in our study, we did not observe an extended colonization time despite the decreased degradability following the addition of the extracts. This discrepancy may be because we used U. brizantha extract rather than the whole plant, as the whole plant retains its fibrous carbohydrate structure and has a more diluted concentration of secondary compounds compared to the extract.
4.2. Short-Chain Fatty Acids and Ammonia Nitrogen
SCFA production in the rumen is highly dependent on the degree and rate of fermentation [50], and saponins’ effects on the production of SCFA are directly associated with the diet provided [5]. This effect tends to be more pronounced in forage-based diets, as the mechanism of action of saponins is like that of ionophores, which have a greater impact on gram-positive bacteria than on gram-negative bacteria [51].
This effect of saponin on SCFA production is further supported by a meta-analysis which showed reductions in acetate and increases in propionate proportions [52]. Corroborating the findings of Kang et al. [53], they tested Momordica charantia saponins using the in vitro gas production technique and observed, after 48 h of incubation, an increase in the molar proportion of propionate and a reduction in the proportion of acetate, resulting in a lower C2:C3 ratio. According to Wina et al. [54], saponin inclusion reduces butyric acid (C4) because they are the main products of protozoan fermentation, while propionic acid (C3) competes with methanogenic archaea for the use of H2. However, despite expectations, in the present study, the addition of extracts resulted in an increase in C4 and a reduction in C3. Similarly, Patra and Yu [55] observed that butyrate increased linearly with increasing doses of vanillin. Therefore, some extracts may inhibit microorganisms that compete with butyrate-producing bacteria, thereby reducing the production of other short-chain fatty acids, such as propionic acid, and redirecting more substrates towards butyrate production.
The observed changes in SCFA production can also be influenced by the dietary characteristics of feeds, which influence rumen pH, rumen microorganisms, and, consequently, the concentration of ammonia nitrogen and the proportions of SCFA [56]. According to Hino and Russell [57], the deamination and decarboxylation of amino acids in the rumen produce branched-chain fatty acids. Branched-chain amino acids are crucial elements in protein synthesis [58]. However, when they are deaminated into branched-chain fatty acids, they are used by cellulolytic microorganisms to increase the activity of fiber-degrading enzymes, thereby improving the degradation of dry matter, neutral detergent fiber, and acid detergent fiber in bulk in vitro experiments [59]. This demonstrates that the MON treatment did not promote the deamination of amino acids, which allows other bacteria to utilize them, leading to an increased protein concentration in the rumen fluid [60].
In addition to these effects on fermentation, protodioscin has been studied for its various biological activities, including anti-inflammatory, neuroprotective, and antitumor effects [61,62,63]. Furthermore, the diverse methods for extracting and determining saponins, as well as a wide range of saponin structures, can have different effects on rumen microorganisms, complicating the comparison of studies [64]. However, to date, no work has produced the extract in pressurized liquid to test its effect on rumen fermentation, so we cannot infer whether it harms or improves ruminant production.
Therefore, developing a new additive from U. brizantha is a complex challenge involving the selection of the plant, the compound of interest, the solvent, and the extraction technique used. However, gaps remain that warrant future research, mainly optimizing the extraction process of protodioscin from U. brizantha. New extraction approaches should be explored, especially concerning the timing of plant harvest and the extraction conditions used. Additionally, it is crucial to analyze the composition, quantify the extract’s secondary metabolites, and concentrate on the desired active ingredient to understand the effectiveness of U. brizantha extract as an additive.
5. Conclusions
The addition of 100, 150, and 200 mL/kg DM of Urochloa brizantha extracts in vitro decreased IVDMD, IVOMD, and propionate production while increasing butyric acid production. Both ethanolic and hydroalcoholic extracts were able to modulate rumen fermentation. Further research is required to optimize the extraction methodologies, comprehensively profile the secondary compounds, and conduct additional trials with different doses to effectively assess the viability of U. brizantha extracts as an additive.
Author Contributions
Conceptualization, I.C.d.S.B., J.S.d.S. and R.S.X.d.F.; methodology, R.S.X.d.F.; software, J.S.d.S. and R.S.X.d.F.; validation, I.C.d.S.B. and R.S.X.d.F.; formal analysis, J.S.d.S. and R.S.X.d.F.; investigation, R.S.X.d.F. and J.S.d.S.; resources, A.L.d.O., F.P.J. and A.J.F.; data curation, R.S.X.d.F. and J.S.d.S.; writing—original draft preparation, R.S.X.d.F. and J.S.d.S.; writing—review and editing, J.S.d.S., R.S.X.d.F., F.P.J. and A.J.F.; visualization, J.S.d.S. and R.S.X.d.F.; supervision, J.S.d.S. and A.L.d.O.; project administration, J.S.d.S. and I.C.d.S.B.; funding acquisition, I.C.d.S.B. and J.S.d.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful to the Fundação de Amparo à Pesquisa do Estado de São Paulo–FAPESP (Process nº 2021/14739-4) for their financial support of this research and the Conselho Nacional de Desenvolvimento Científico e Tecnológic–CNPq (Grant nº 152217/2022-3).
Institutional Review Board Statement
The animal study protocol was approved by Ethics Committee of the Department of Animal Science, College of Animal Science and Food Engineering, University of São Paulo (Protocol number CEUA: 1344101121 approved in January of 2022).
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
We would like to thank Bernardo Dias Ribeiro, from the Chemistry Department at UFRJ, for his availability and assistance in his laboratory.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Newbold, C.J.; Ramos-Morales, E. Review: Ruminal Microbiome and Microbial Metabolome: Effects of Diet and Ruminant Host. Animal 2020, 14, S78–S86. [Google Scholar] [CrossRef]
- Patra, A.K.; Kamra, D.N.; Agarwal, N. Effect of Plant Extracts on in Vitro Methanogenesis, Enzyme Activities and Fermentation of Feed in Rumen Liquor of Buffalo. Anim. Feed Sci. Technol. 2006, 128, 276–291. [Google Scholar] [CrossRef]
- e Silva, S.N.S.; Chabrillat, T.; Kerros, S.; Guillaume, S.; Gandra, J.R.; de Carvalho, G.G.P.; Silva, F.F.d.; Mesquita, L.G.; Gordiano, L.A.; Camargo, G.M.F.; et al. Effects of Plant Extract Supplementations or Monensin on Nutrient Intake, Digestibility, Ruminal Fermentation and Metabolism in Dairy Cows. Anim. Feed Sci. Technol. 2021, 275, 114886. [Google Scholar] [CrossRef]
- da Silva, C.S.; de Souza, E.J.O.; Pereira, G.F.C.; Cavalcante, E.O.; de Lima, E.I.M.; Torres, T.R.; da Silva, J.R.C.; da Silva, D.C. Plant Extracts as Phytogenic Additives Considering Intake, Digestibility, and Feeding Behavior of Sheep. Trop. Anim. Health Prod. 2017, 49, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Jayanegara, A.; Yogianto, Y.; Wina, E.; Sudarman, A.; Kondo, M.; Obitsu, T.; Kreuzer, M. Combination Effects of Plant Extracts Rich in Tannins and Saponins as Feed Additives for Mitigating in Vitro Ruminal Methane and Ammonia Formation. Animals 2020, 10, 1531. [Google Scholar] [CrossRef]
- Dhanasekaran, D.K.; Dias-Silva, T.P.; Abdalla Filho, A.L.; Sakita, G.Z.; Abdalla, A.L.; Louvandini, H.; Elghandour, M.M.M.Y. Plants Extract and Bioactive Compounds on Rumen Methanogenesis. Agrofor. Syst. 2020, 94, 1541–1553. [Google Scholar] [CrossRef]
- Hassan, A.; Abu Hafsa, S.H.; Elghandour, M.M.Y.; Kanth Reddy, P.R.; Salem, M.Z.M.; Anele, U.Y.; Ranga Reddy, P.P.; Salem, A.Z.M. Influence of Corymbia Citriodora Leaf Extract on Growth Performance, Ruminal Fermentation, Nutrient Digestibility, Plasma Antioxidant Activity and Faecal Bacteria in Young Calves. Anim. Feed Sci. Technol. 2020, 261, 114394. [Google Scholar] [CrossRef]
- Ashour, A.S.; El Aziz, M.M.A.; Gomha Melad, A.S. A Review on Saponins from Medicinal Plants: Chemistry, Isolation, and Determination. J. Nanomed. Res. 2019, 7, 282–288. [Google Scholar] [CrossRef]
- Chiocchio, I.; Mandrone, M.; Tomasi, P.; Marincich, L.; Poli, F. Plant Secondary Metabolites: An Opportunity for Circular Economy. Molecules 2021, 26, 495. [Google Scholar] [CrossRef]
- Śliwiński, B.J.; Kreuzer, M.; Wettstein, H.R.; Machmüller, A. Rumen Fermentation and Nitrogen Balance of Lambs Fed Diets Containing Plant Extracts Rich in Tannins and Saponins, and Associated Emissions of Nitrogen and Methane. Arch. Anim. Nutr./Arch. Fur Tierernahr. 2002, 56, 379–392. [Google Scholar] [CrossRef]
- Zhang, F.; Li, B.; Ban, Z.; Liang, H.; Li, L.; Zhao, W.; Yan, X. Evaluation of Origanum Oil, Hydrolysable Tannins and Tea Saponin in Mitigating Ruminant Methane: In Vitro and In Vivo Methods. journal Anim. Physiol. Anim. Nutr. 2021, 105, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Trotta, R.J.; Kreikemeier, K.K.; Foote, S.; McLeod, K.R.; Harmon, D.L. Influence of Anti-Coccidial Compounds and Phytogenic Saponin Extracts on In Vitro and In Vivo Ruminal Fermentation and Methane Production of Cattle. Animals 2023, 13, 2308. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Xu, Z.; Yang, H.E.; McAllister, T.A.; Acharya, S.; Wang, Y. In Vitro Ruminal Fermentation of Fenugreek (Trigonella Foenum-Graecum, L.) Produced Less Methane than That of Alfalfa (Medicago sativa). Anim. Biosci. 2021, 34, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Preez, D.A.D.; Akanmu, A.M.; Adejoro, F.A.; Hassen, A. The Effect of Monensin vs. Neem, and Moringa Extracts on Nutrient Digestibility, Growth Performance, Methane, and Blood Profile of Merino Lambs. Animals 2023, 13, 3514. [Google Scholar] [CrossRef]
- Freitas, R.S.X.; Nepomuceno, D.d.D.; Modesto, E.C.; de Oliveira, D.R.; Pereira, T.P.; de Morais, L.F.; de Carvalho Almeida, J.C.; de Carvalho, M.G. Caracterização Do Extrato Metanólico de Urochloa Humidicola Como Aditivo Fitogênico Para Ruminantes. Biosci. J. 2017, 33, 1586–1591. [Google Scholar] [CrossRef]
- Gupta, A.; Naraniwal, M.; Kothari, V. Modern Extraction Methods for Preparation of Bioactive Plant Extracts. Int. J. Appl. Nat. Sci. 2012, 1, 8–26. [Google Scholar]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Niderkorn, V.; Jayanegara, A. Opportunities Offered by Plant Bioactive Compounds to Improve Silage Quality, Animal Health and Product Quality for Sustainable Ruminant Production: A Review. Agronomy 2021, 11, 86. [Google Scholar] [CrossRef]
- Leal, E.S.; Ítavo, L.C.V.; Do Valle, C.B.; Ítavo, C.C.B.F.; Dias, A.M.; Dos Santos Difante, G.; Barbosa-Ferreira, M.; Nonato, L.M.; De Melo, G.K.A.; Gurgel, A.L.C. Influence of Protodioscin Content on Digestibility and in Vitro Degradation Kinetics in Urochloa Brizantha Cultivars. Crop Pasture Sci. 2020, 71, 278–284. [Google Scholar] [CrossRef]
- Feitoza, R.B.B.; Varela, R.M.; Torres, A.; Molinillo, J.M.G.; Lima, H.R.P.; Moraes, L.F.D.; Da Cunha, M.; Macías, F.A. Evaluation of the Phytotoxicity of Urochloa Humidicola Roots by Bioassays and Microscopic Analysis. Characterization of New Compounds. J. Agric. Food Chem. 2020, 68, 4851–4864. [Google Scholar] [CrossRef]
- de Oliveira, D.R.; Nepomuceno, D.D.; Castro, R.N.; Braz Filho, R.; Carvalho, M.G.d. Special Metabolites Isolated from Urochloa Humidicola (Poaceae). An. Da Acad. Bras. De Ciências 2017, 89, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Brum, K.B.; Haraguchi, M.; Lemos, R.A.A.; Riet-Correa, F.; Fioravanti, M.C.S. Crystal-Ystal-Associated Cholangiopathy in Sheep Grazing Brachiaria Decumbens Containing the Saponin Protodioscin Protodioscin. Pesqui. Vet. Bras. 2007, 27, 39–42. [Google Scholar] [CrossRef]
- da Costa, M.C.M.; Ítavo, L.C.V.; Ítavo, C.C.B.F.; Dias, A.M.; dos Santos Difante, G.; Buschinelli de Goes, R.H. de T.; de Souza Leal, E.; Nonato, L.M.; Kozerski, N.D.; de Moraes, G.J.; et al. Natural Intoxication Caused by Protodioscin in Lambs Kept in Brachiaria Pastures. Trop. Anim. Health Prod. 2021, 53, 1–9. [Google Scholar] [CrossRef]
- Oliveira, M.W.; Lopes Goretti, A.; Lana, R.d.P.; Camacho Rodrigues, T. DRY MATTER AND PROTEIN ACCUMULATION AS A FUNCTION OF NITROGEN FERTILIZATION IN Brachiaria Brizantha CV. MARANDU (Urochloa Brizantha). Rev. Bras. De Agropecuária Sustentável 2022, 12, 10–18. [Google Scholar] [CrossRef]
- Bueno, I.C.S.; Cabral Filho, S.L.S.; Gobbo, S.P.; Louvandini, H.; Vitti, D.M.S.S.; Abdalla, A.L. Influence of Inoculum Source in a Gas Production Method. Anim. Feed Sci. Technol. 2005, 123–124 Pt 1, 95–105. [Google Scholar] [CrossRef]
- AOAC. Association of Official Analytical Chemists (AOAC), 18th ed.; AOAC International, Ed.; AOAC: Gaithersburg, MD, USA, 2011. [Google Scholar]
- dos Santos, H.G.; Jacomine, P.K.T.; dos Anjos, L.H.C.; de Oliveira, V.Á.; Lumbreras, J.F.; Coelho, M.R.; de Almeida, J.A.; de Araújo Filho, J.C.; de Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa, Ed.; Embrapa: Brasília, Brasil, 2018; ISBN 978-85-7035-800-4. [Google Scholar]
- Omer, E.A.; Hussein, E.A.A.; Hendawy, S.F.; El-din, A.A.E.; El-Gendy, A.G. Effect of Nitrogen and Potassium Fertilizers on Growth, Yield, Essential Oil and Artemisinin of Artemisia Annua L Plant. Int. Res. J. Hortic. 2014, 2, 11. [Google Scholar] [CrossRef]
- Rodrigues, L.d.C.; Bodini, R.B.; Caneppele, F. de L.; Dacanal, G.C.; Crevelin, E.J.; de Moraes, L.A.B.; de Oliveira, A.L. Pressurized Liquid Extraction (PLE) in an Intermittent Process as an Alternative for Obtaining Passion Fruit (Passiflora edulis) Leaf Hydroalcoholic Extract (Tincture). Processes 2023, 11, 2308. [Google Scholar] [CrossRef]
- Lee, S.T.; Mitchell, R.B.; Wang, Z.; Heiss, C.; Gardner, D.R.; Azadi, P. Isolation, Characterization, and Quantification of Steroidal Saponins in Switchgrass ( Panicum virgatum, L.). J. Agric. Food Chem. 2009, 57, 2599–2604. [Google Scholar] [CrossRef]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; McAllan, A.B.; France, J. A Simple Gas Production Method Using a Pressure Transducer to Determine the Fermentation Kinetics of Ruminant Feeds. Anim. Feed Sci. Technol. 1994, 48, 185–197. [Google Scholar] [CrossRef]
- Mauricio, R.M.; Mould, F.L.; Dhanoa, M.S.; Owen, E.; Channa, K.S.; Theodorou, M.K. A Semi-Automated In Vitro Gas Production Technique for Ruminant Feedstuff Evaluation. Anim. Feed Sci. Technol. 1999, 79, 321–330. [Google Scholar] [CrossRef]
- Menke, K.H.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The Estimation of the Digestibility and Metabolizable Energy Content of Ruminant Feedingstuffs from the Gas Production When They Are Incubated with Rumen Liquor In Vitro. J. Agric. Sci. 1979, 93, 217–222. [Google Scholar] [CrossRef]
- Fagundes, G.M.; Benetel, G.; Santos, K.C.; Welter, K.C.; Melo, F.A.; Muir, J.P.; Bueno, I.C.S. Tannin-Rich Plants as Natural Manipulators of Rumen Fermentation in the Livestock Industry. Molecules 2020, 25, 2943. [Google Scholar] [CrossRef]
- France, J.; Dhanoa, M.S.; Theodorou, M.K.; Lister, S.J.; Davies, D.R.; Isac, D. A Model to Interpret Gas Accumulation Profile Associated with In Vitro Degradation of Ruminant Feeds. J. Theor. Biol. 1993, 163, 99–111. [Google Scholar] [CrossRef]
- Blummel, B.Y.M.; Becker, K. The Degradability Characteristics of Fifty-Four Roughages and Roughage Neutral-Detergent Fibres as Described by In Vitro Gas Production and Thies Relationship to Voluntary Feed Intake. Br. J. Nutr. 1997, 77, 757–768. [Google Scholar] [CrossRef]
- Bueno, I.C.S.; Brandi, R.A.; Fagundes, G.M.; Benetel, G.; Muir, J.P. The Role of Condensed Tannins in the In Vitro Rumen Fermentation Kinetics in Ruminant Species: Feeding Type Involved? Animals 2020, 10, 635. [Google Scholar] [CrossRef] [PubMed]
- Kulasek, G. A Micromethod for Determining Urea in Blood Plasma, Whole Blood and Blood Corpuscles with the Use of Urease and Phenol Reagent. Pol. Arch. Weter. 1972, 15, 801–810. [Google Scholar]
- Foldager, J. Protein Requirement and Non-Protein Nitrogen for High Producing Cows in Early Lactation. Ph.D. Thesis, Michigan State University, East Lansing, MI, USA, 1977. [Google Scholar]
- Seo, S.; Lee, S.C.; Lee, S.Y.; Seo, J.G.; Ha, J.K. Degradation Kinetics of Carbohydrate Fractions of Ruminant Feeds Using Automated Gas Production Technique. Asian-Australas. J. Anim. Sci. 2009, 22, 356–364. [Google Scholar] [CrossRef]
- de Oliveira, J.S.; Zanine, A.D.M.; Santos, E.M. Diversidade Microbiana No Ecossitema Ruminal. Rev. Electrónica De Vet. 2007, 8, 1–12. [Google Scholar]
- de Freitas, R.S.X.; Nepomuceno, D.D.D.; Modesto, E.C.; Pereira, T.P.; Almeida, J.C.d.C.; de Morais, L.F.; Pereira, L.G.R.; Machado, F.S.; Campos, M.M.; Tomich, T.R. Methanolic Extract of Urochloa Humidicola on In Vitro Rumen Fermentation. Pesqui. Agropecuária Bras. 2018, 53, 504–513. [Google Scholar] [CrossRef]
- Jiménez-Peralta, F.S.; Salem, A.Z.M.; Mejia-Hernández, P.; González-Ronquillo, M.; Albarrán-Portillo, B.; Rojo-Rubio, R.; Tinoco-Jaramillo, J.L. Influence of Individual and Mixed Extracts of Two Tree Species on In Vitro Gas Production Kinetics of a High Concentrate Diet Fed to Growing Lambs. Livest. Sci. 2011, 136, 192–200. [Google Scholar] [CrossRef]
- Abarghuei, M.J.; Rouzbehan, Y.; Salem, A.F. The Influence of Pomegranate-Peel Extracts on In Vitro Gas Production Kinetics of Rumen Inoculum of Sheep. Turk. J. Vet. Anim. Sci. 2014, 38, 212–219. [Google Scholar] [CrossRef]
- Zhou, W.; Pian, R.; Yang, F.; Chen, X.; Zhang, Q. The Sustainable Mitigation of Ruminal Methane and Carbon Dioxide Emissions by Co-Ensiling Corn Stalk with Neolamarckia Cadamba Leaves for Cleaner Livestock Production. J. Clean. Prod. 2021, 311, 127680. [Google Scholar] [CrossRef]
- Hu, W.; Liu, J.; Wu, Y.; Guo, Y.; Ye, J. Effects of Tea Saponins on In Vitro Ruminal Fermentation and Growth Performance in Growing Boer Goat. Arch. Anim. Nutr. 2006, 60, 89–97. [Google Scholar] [CrossRef]
- Hart, K.J.; Yáñez-Ruiz, D.R.; Duval, S.M.; McEwan, N.R.; Newbold, C.J. Plant Extracts to Manipulate Rumen Fermentation. Anim. Feed Sci. Technol. 2008, 147, 8–35. [Google Scholar] [CrossRef]
- Yusoff, I.M.; Mat Taher, Z.; Rahmat, Z.; Chua, L.S. A Review of Ultrasound-Assisted Extraction for Plant Bioactive Compounds: Phenolics, Flavonoids, Thymols, Saponins and Proteins. Food Res. Int. 2022, 157, 111268. [Google Scholar] [CrossRef] [PubMed]
- Raun, B.M.L.; Kristensen, N.B. Metabolic Effects of Feeding Ethanol or Propanol to Postpartum Transition Holstein Cows. J. Dairy Sci. 2011, 94, 2566–2580. [Google Scholar] [CrossRef]
- Cone, J.W.; Becker, P.M. Fermentation Kinetics and Production of Volatile Fatty Acids and Microbial Protein by Starchy Feedstuffs. Anim. Feed Sci. Technol. 2012, 172, 34–41. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. The Effect and Mode of Action of Saponins on the Microbial Populations and Fermentation in the Rumen and Ruminant Production. Nutr. Res. Rev. 2009, 22, 204–219. [Google Scholar] [CrossRef]
- Jayanegara, A.; Wina, E.; Takahashi, J. Meta-Analysis on Methane Mitigating Properties of Saponin-Rich Sources in the Rumen: Influence of Addition Levels and Plant Sources. Asian-Australas. J. Anim. Sci. 2014, 27, 1426–1435. [Google Scholar] [CrossRef]
- Kang, J.; Zeng, B.; Tang, S.; Wang, M.; Han, X.; Zhou, C.; Yan, Q.; Liu, J.; Tan, Z. Effects of Momordica Charantia Polysaccharide on In Vitro Ruminal Fermentation and Cellulolytic Bacteria. Ital. J. Anim. Sci. 2017, 16, 226–233. [Google Scholar] [CrossRef]
- Wina, E.; Muetzel, S.; Becker, K. The Impact of Saponins or Saponin-Containing Plant Materials on Ruminant Production—A Review. J. Agric. Food Chem. 2005, 53, 8093–8105. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K.; Yu, Z. Effects of Vanillin, Quillaja Saponin, and Essential Oils on In Vitro Fermentation and Protein-Degrading Microorganisms of the Rumen. Appl. Microbiol. Biotechnol. 2014, 98, 897–905. [Google Scholar] [CrossRef]
- Faniyi, T.O.; Adegbeye, M.J.; Elghandour, M.M.M.Y.; Pilego, A.B.; Salem, A.Z.M.; Olaniyi, T.A.; Adediran, O.; Adewumi, M.K. Role of Diverse Fermentative Factors towards Microbial Community Shift in Ruminants. J. Appl. Microbiol. 2019, 127, 2–11. [Google Scholar] [CrossRef]
- Hino, T.; Russell, J.B. Effect of Reducing-Equivalent Disposal and NADH/NAD on Deamination of Amino Acids by Intact Rumen Microorganisms and Their Cell Extracts. Appl. Environ. Microbiol. 1985, 50, 1368–1374. [Google Scholar] [CrossRef]
- An, J.; He, H.; Lan, X.; Liu, L.; Wang, Z.; Ge, Y.; Shen, W.; Cheng, A.; Wan, F. Branched-Chain Amino Acids in Ruminant Nutrition: Function Effects and Summary of Recent Advances. Anim. Feed Sci. Technol. 2024, 312, 1–15. [Google Scholar] [CrossRef]
- Roman-Garcia, Y.; Denton, B.L.; Mitchell, K.E.; Lee, C.; Socha, M.T.; Firkins, J.L. Conditions Stimulating Neutral Detergent Fiber Degradation by Dosing Branched-Chain Volatile Fatty Acids. I: Comparison with Branched-Chain Amino Acids and Forage Source in Ruminal Batch Cultures. J. Dairy Sci. 2021, 104, 6739–6755. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Russell, J.B. The Effect of Monensin Supplementation on Ruminal Ammonia Accumulation In Vivo and the Numbers of Amino Acid-Fermenting Bacteria. J. Anim. Sci. 1993, 71, 3470–3476. [Google Scholar] [CrossRef]
- Liu, J.Y.; Hou, Y.L.; Cao, R.; Qiu, H.X.; Cheng, G.H.; Tu, R.; Wang, L.; Zhang, J.L.; Liu, D. Protodioscin Ameliorates Oxidative Stress, Inflammation and Histology Outcome in Complete Freund’s Adjuvant Induced Arthritis Rats. Apoptosis 2017, 22, 1454–1460. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Liu, P.; Shi, H.; Fan, W.; Feng, X.; Chen, J.; Jing, S.; Wang, L.; Zheng, Y.; Zhang, D.; et al. Identification of Anti-Inflammatory Components in Dioscorea Nipponica Makino Based on HPLC-MS/MS, Quantitative Analysis of Multiple Components by Single Marker and Chemometric Methods. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2022, 1213, 123531. [Google Scholar] [CrossRef]
- Huang, C.F.; Hsieh, Y.H.; Yang, S.F.; Kuo, C.H.; Wang, P.H.; Liu, C.J.; Lin, R.C. Mitophagy Effects of Protodioscin on Human Osteosarcoma Cells by Inhibition of P38MAPK Targeting NIX/LC3 Axis. Cells 2023, 12, 395. [Google Scholar] [CrossRef]
- Belanche, A.; Pinloche, E.; Preskett, D.; Newbold, C.J. Effects and Mode of Action of Chitosan and Ivy Fruit Saponins on the Microbiome, Fermentation and Methanogenesis in the Rumen Simulation Technique. FEMS Microbiol. Ecol. 2016, 92, fiv160. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).