Optimization of Bacterial Cellulose Production from Waste Figs by Komagataeibacter xylinus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism and Culture Conditions
2.2. Waste Fig Extract Production
2.3. Fermentation Experiments
2.4. Experimental Design for BC Production
2.4.1. Plackett–Burman Design
2.4.2. Face-Centered Central Composite Design
2.5. Analytical Methods
2.6. Structural Characterization of Bacterial Cellulose Samples
2.6.1. Attenuated Total Reflection–Fourier Transform Infrared Spectrophotometry (ATR-FTIR)
2.6.2. Scanning Electron Microscope (SEM)
2.6.3. X-ray Diffraction (XRD)
3. Results and Discussion
3.1. Screening of Nutrients for Cellulose Production Using Plackett–Burman Design
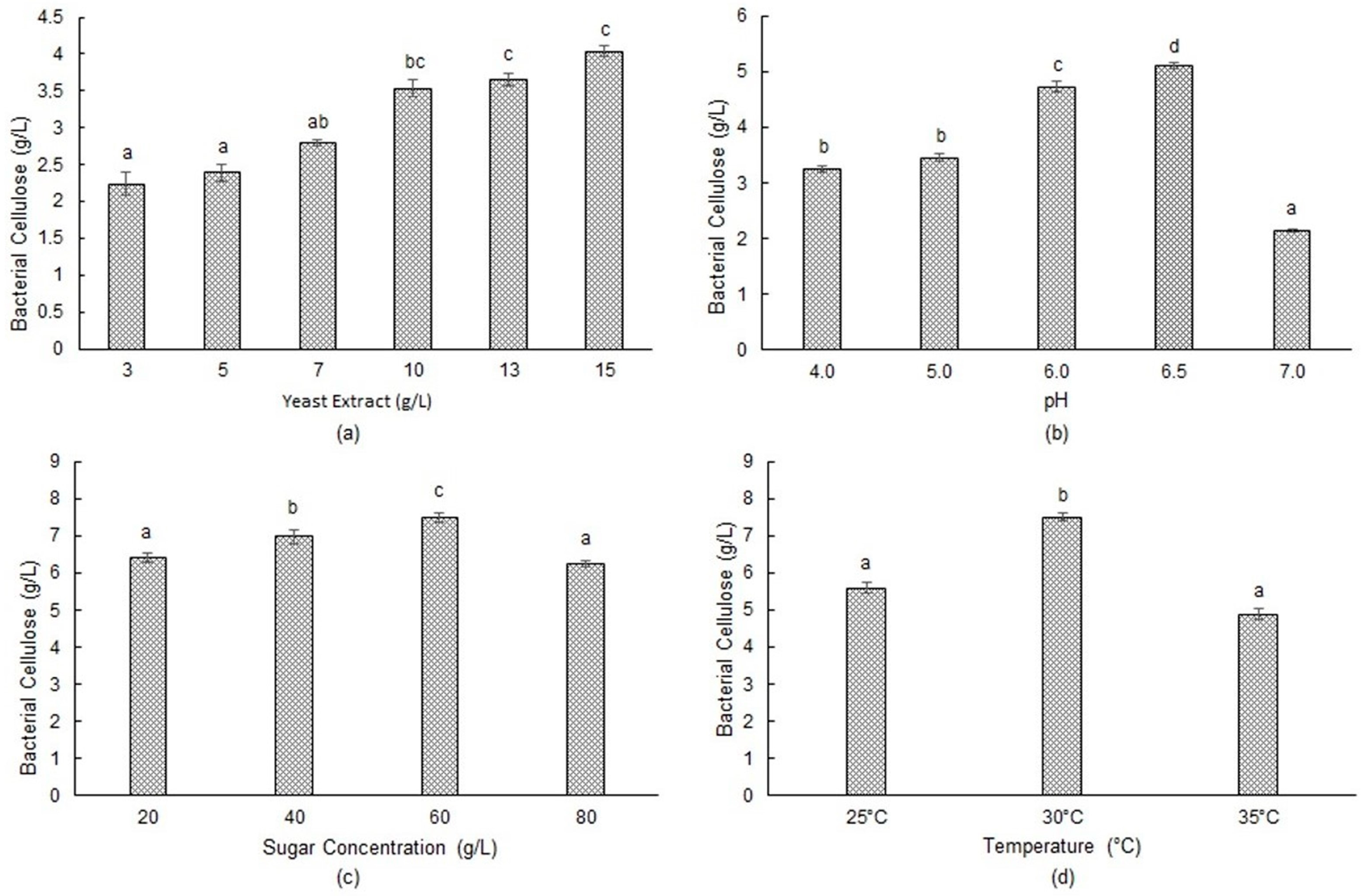
3.2. Effect of Yeast Extract Concentration
3.3. Effect of Initial pH
3.4. Effect of Initial Sugar Concentration
3.5. Effect of Temperature
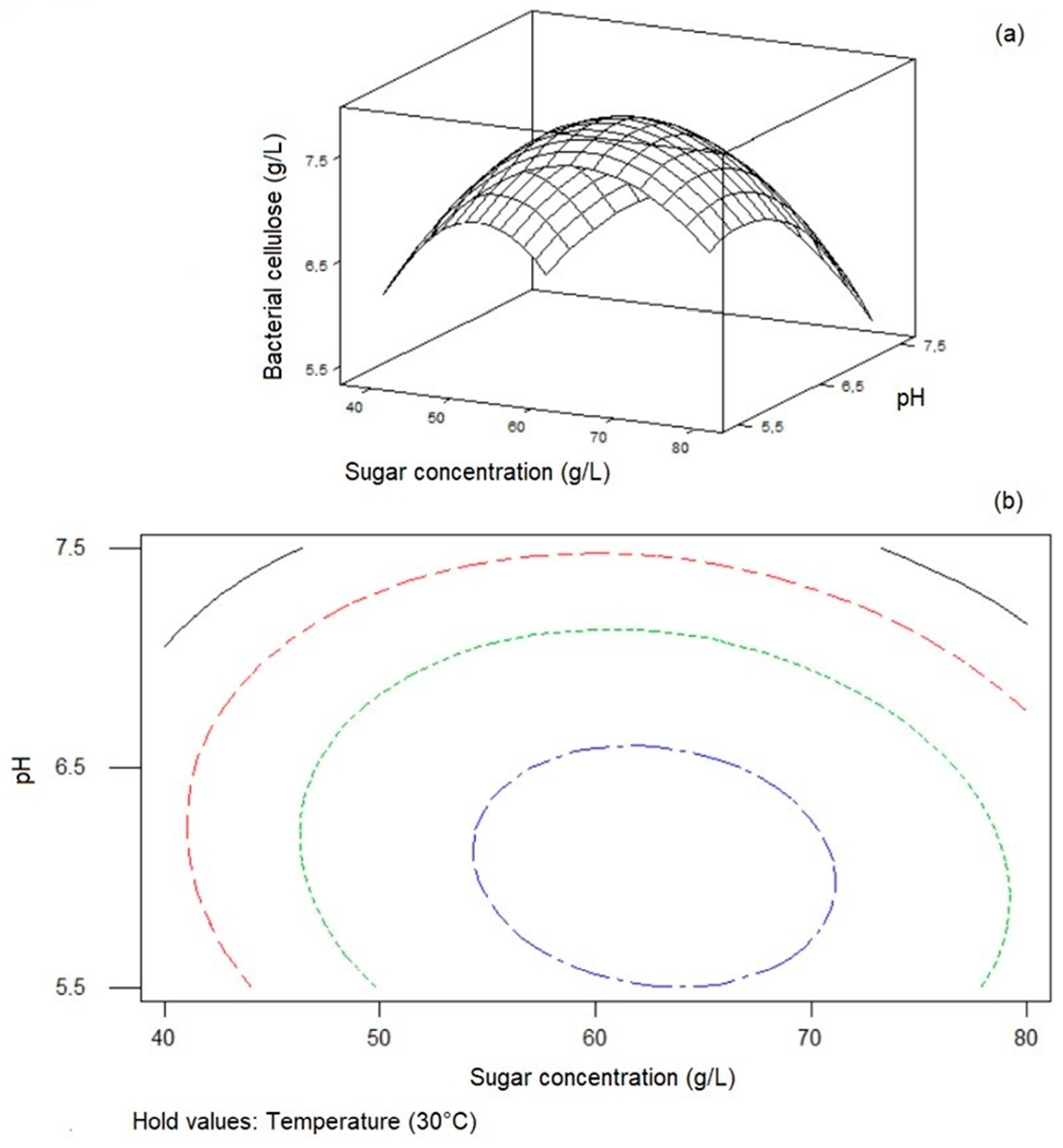
3.6. Optimization of BC Production
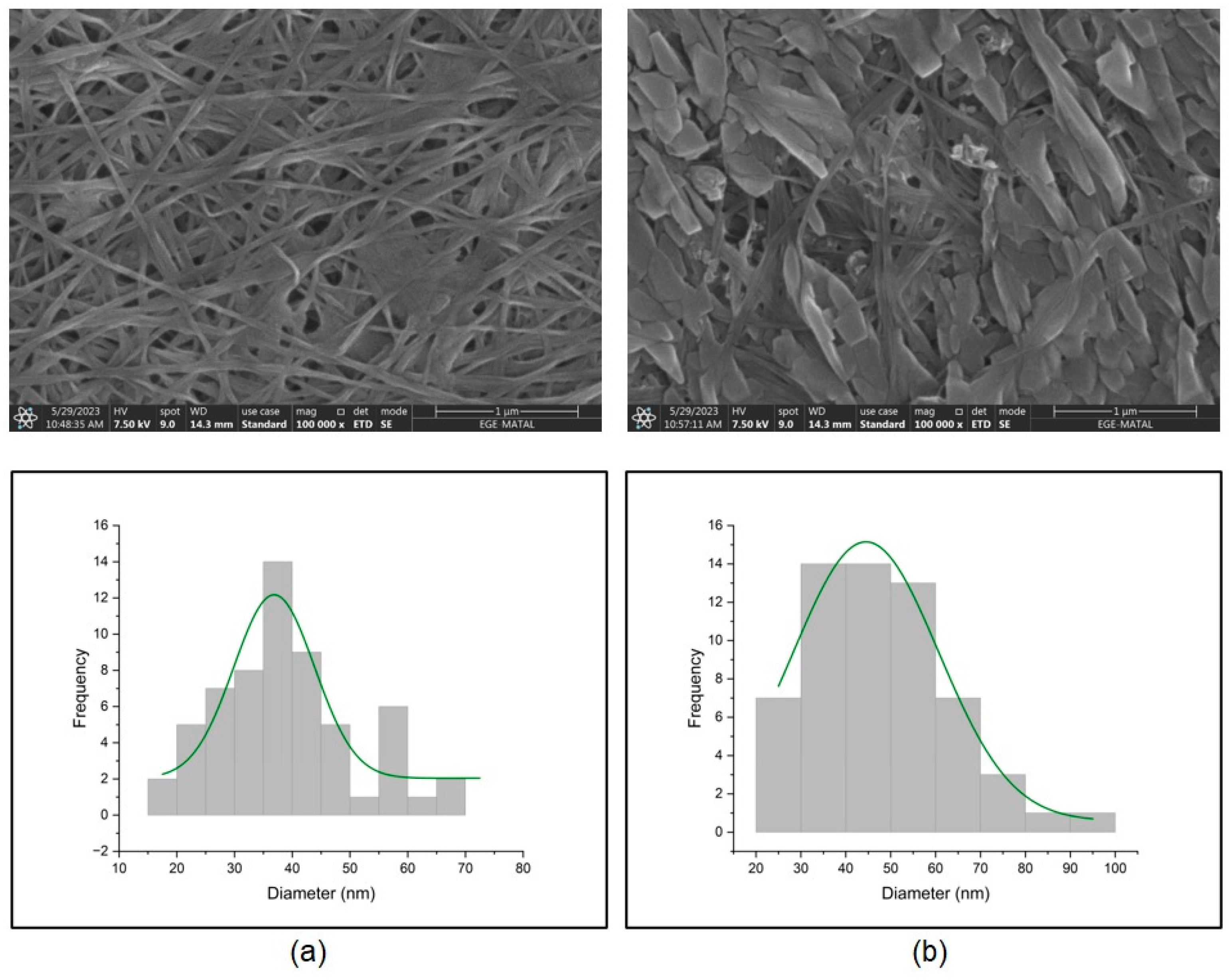
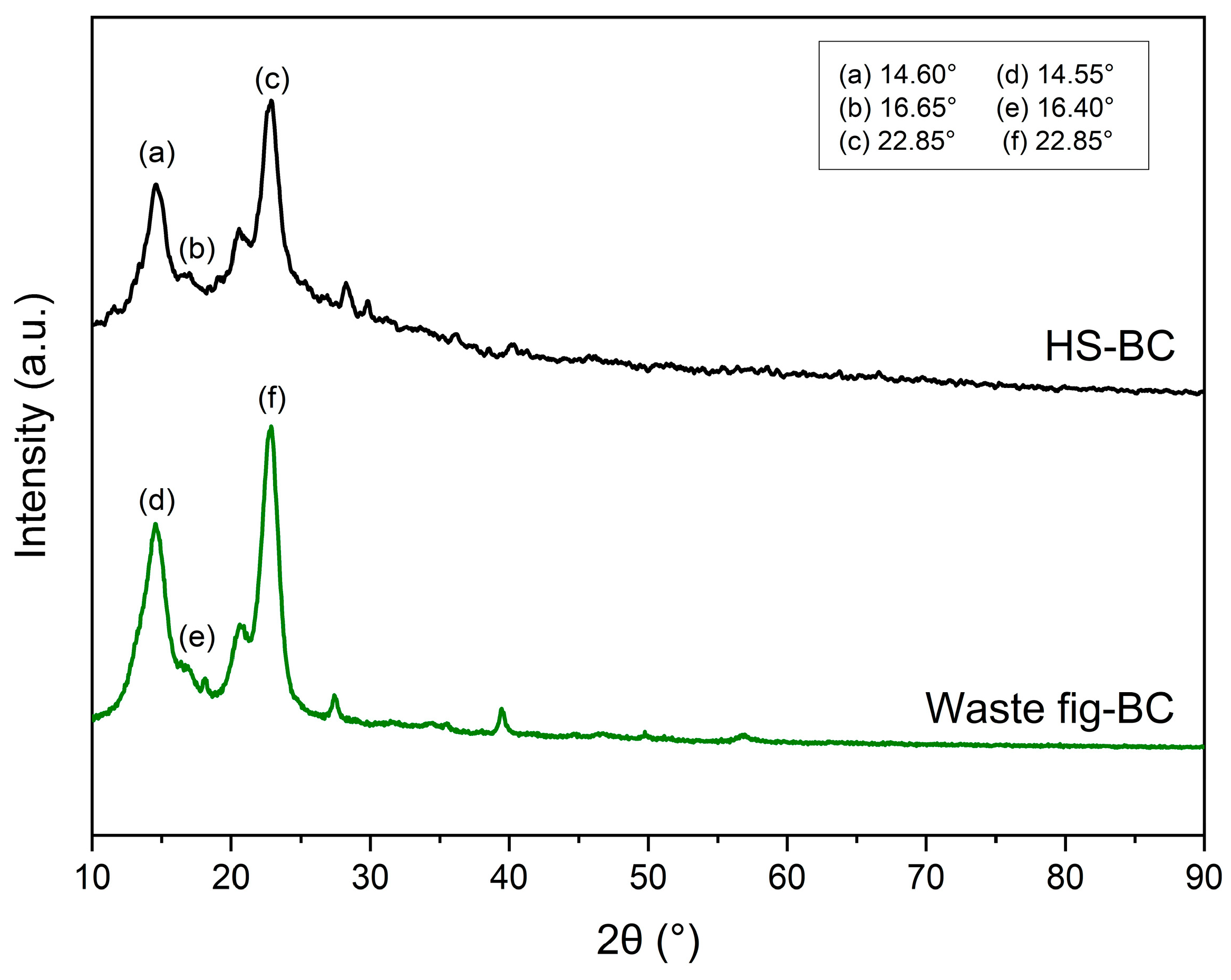
3.7. Characterization of Bacterial Cellulose
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Çakar, F.; Özer, I.; Aytekin, A.Ö.; Şahin, F. Improvement production of bacterial cellulose by semi-continuous process in molasses medium. Carbohydr. Polym. 2014, 106, 7–13. [Google Scholar] [CrossRef]
- El-Gendi, H.; Taha, T.H.; Ray, J.B.; Saleh, A.K. Recent advances in bacterial cellulose: A low-cost effective production media, optimization strategies and applications. Cellulose 2022, 29, 7495–7533. [Google Scholar] [CrossRef]
- Günerhan, Ü.; Us, E.; Dumlu, L.; Yılmaz, V.; Carrère, H.; Perendeci, A.N. Impacts of chemical-assisted thermal pretreatments on methane production from fruit and vegetable harvesting wastes: Process optimization. Molecules 2020, 25, 500. [Google Scholar] [CrossRef] [PubMed]
- Mehri, D.; Perendeci, N.A.; Goksungur, Y. Utilization of whey for red pigment production by Monascus purpureus in submerged fermentation. Fermentation 2021, 7, 75. [Google Scholar] [CrossRef]
- Chen, L.; Hong, F.; Yang, X.X.; Han, S.F. Biotransformation of wheat straw to bacterial cellulose and its mechanism. Bioresour. Technol. 2013, 135, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Cavka, A.; Jönsson, L.J.; Hong, F. Comparison of methods for detoxification of spruce hydrolysate for bacterial cellulose production. Microb. Cell Fact. 2013, 12, 93. [Google Scholar] [CrossRef] [PubMed]
- Erbas-Kiziltas, E.; Kiziltas, A.; Gardner, D.J. Synthesis of bacterial cellulose using hot water extracted wood sugars. Carbohydr. Polym. 2015, 124, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Algar, I.; Fernandes, S.C.M.; Mondragon, G.; Castro, C.; Garcia-Astari, C.; Gabilondo, N.; Retegi, A.; Eceiza, A. Pineapple agroindustrial residues for the production of high value bacterial cellulose with different morphologies. J. Appl. Polym. Sci. 2014, 132, 41237. [Google Scholar] [CrossRef]
- Kurosumi, A.; Sasaki, C.; Yamashita, Y.; Nakamura, Y. Utilization of various fruit juices as carbon source for production of bacterial cellulose by Acetobacter xylinum NBRC 13693. Carbohydr. Polym. 2009, 76, 333–335. [Google Scholar] [CrossRef]
- Jozala, A.F.; Pértile, R.A.N.; dos Santos, C.A.; de Carvalho Santos-Ebinuma, V.; Seckler, M.M.; Gama, F.M.; Pessoa, A. Bacterial cellulose production by Gluconacetobacter xylinus by employing alternative culture media. Appl. Microbiol. Biotechnol. 2014, 99, 1181–1190. [Google Scholar] [CrossRef]
- Hong, F.; Guo, X.; Zhang, S.; Han, S.; Yang, G.; Jönsson, L.J. Bacterial cellulose production from cotton-based waste textiles: Enzymatic saccharification enhanced by ionic liquid pretreatment. Bioresour. Technol. 2012, 104, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Carreira, P.; Mendes, J.A.S.; Trovatti, E.; Serafim, L.S.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Utilization of residues from agro-forest industries in the production of high value bacterial cellulose. Bioresour. Technol. 2011, 102, 7354–7360. [Google Scholar] [CrossRef]
- Suwanposri, A.; Yukphan, P.; Yamada, Y.; Ochaikul, D. Statistical optimisation of culture conditions for biocellulose production by Komagataeibacter sp. PAP1 using soya bean whey. Maejo Int. J. Sci. Technol. 2014, 8, 1–14. [Google Scholar] [CrossRef]
- Wu, J.M.; Liu, R.H. Thin stillage supplementation greatly enhances bacterial cellulose production by Gluconacetobacter xylinus. Carbohydr. Polym. 2012, 90, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, L.; Hua, J.; Jia, S.; Zhang, J.; Liu, H. Production of nano bacterial cellulose from waste water of candied jujube-processing industry using Acetobacter xylinum. Carbohydr. Polym. 2015, 120, 115–119. [Google Scholar] [CrossRef]
- Tsouko, E.; Kourmentza, C.; Ladakis, D.; Kopsahelis, N.; Mandala, I.; Papanikolaou, S.; Paloukis, F.; Alves, V.; Koutinas, A. Bacterial cellulose production from industrial waste and by-product streams. Int. J. Mol. Sci. 2015, 16, 14832–14849. [Google Scholar] [CrossRef]
- Huang, C.; Yang, X.Y.; Xiong, L.; Guo, H.J.; Luo, J.; Wang, B.; Zhang, H.R.; Lin, X.Q.; Chen, X.D. Evaluating the possibility of using acetone-butanol-ethanol (ABE) fermentation wastewater for bacterial cellulose production by Gluconacetobacter xylinus. Lett. Appl. Microbiol. 2015, 60, 491–496. [Google Scholar] [CrossRef]
- Bilgi, E.; Bayir, E.; Sendemir-Urkmez, A.; Hames, E.E. Optimization of bacterial cellulose production by Gluconacetobacter xylinus using carob and haricot bean. Int. J. Biol. Macromol. 2016, 90, 2–10. [Google Scholar] [CrossRef]
- Bekatorou, A.; Plioni, I.; Sparou, K.; Maroutsiou, R.; Tsafrakidou, P.; Petsi, T.; Kordouli, E. Bacterial cellulose production using the corinthian currant finishing side-stream and cheese whey: Process optimization and textural characterization. Foods. 2019, 8, 193. [Google Scholar] [CrossRef]
- Zeng, X.; Small, D.P.; Wan, W. Statistical optimization of culture conditions for bacterial cellulose production by Acetobacter xylinum BPR 2001 from maple syrup. Carbohydr. Polym. 2011, 85, 506–513. [Google Scholar] [CrossRef]
- Kurt, A.S.; Cekmecelioglu, D. Bacterial cellulase production using grape pomace hydrolysate by shake-flask submerged fermentation. Biomass Convers. Biorefin. 2021, 2, 1–8. [Google Scholar] [CrossRef]
- Castro, C.; Zuluaga, R.; Putaux, J.L.; Caro, G.; Mondragon, I.; Gañán, P. Structural characterization of bacterial cellulose produced by Gluconacetobacter swingsii sp. from Colombian agroindustrial wastes. Carbohydr. Polym. 2011, 84, 96–102. [Google Scholar] [CrossRef]
- Ha, J.H.; Shehzad, O.; Khan, S.; Lee, S.Y.; Park, J.W.; Khan, T.; Park, J.K. Production of bacterial cellulose by a static cultivation using the waste from beer culture broth. Korean J. Chem. Eng. 2008, 25, 812–815. [Google Scholar] [CrossRef]
- Brugnoli, M.; La China, S.; Lasagni, F.; Romeo, F.V.; Pulvirenti, A.; Gullo, M. Acetic acid bacteria in agro-wastes: From cheese whey and olive mill wastewater to cellulose. Appl. Microbiol. Biotechnol. 2023, 107, 3729–3744. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zheng, S.; Zhang, Z.; Yang, F.; Ma, K.; Feng, Y.; Yang, X. Bacterial cellulose production by Acetobacter xylinum ATCC 23767 using tobacco waste extract as culture medium. Bioresour. Technol. 2019, 274, 518–524. [Google Scholar] [CrossRef]
- Ayuso, M.; Carpena, M.; Taofiq, O.; Albuquerque, T.G.; Simal-Gandara, J.; Oliveira, M.B.P.; Barros, L. Fig “Ficus carica L.” and its by-products: A decade evidence of their health-promoting benefits towards the development of novel food formulations. Trends Food Sci. Technol. 2022, 127, 1–13. [Google Scholar] [CrossRef]
- Buenrostro-Figueroa, J.J.; Velázquez, M.; Flores-Ortega, O.; Ascacio-Valdés, J.A.; Huerta-Ochoa, S.; Aguilar, C.N.; Prado-Barragán, L.A. Solid state fermentation of fig (Ficus carica L.) by-products using fungi to obtain phenolic compounds with antioxidant activity and qualitative evaluation of phenolics obtained. Process Biochem. 2017, 62, 16–23. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Smith, B.; Guo, Y. Ultrasound-microwave assisted extraction of pectin from fig (Ficus carica L.) skin: Optimization, characterization and bioactivity. Carbohydr. Polym. 2019, 222, 114992. [Google Scholar] [CrossRef]
- Abol-Fotouh, D.; Hassan, M.A.; Shokry, H.; Roig, A.; Azab, M.S.; Kashyout, A.E.H.B. Bacterial nanocellulose from agro-industrial wastes: Low-cost and enhanced production by Komagataeibacter saccharivorans MD1. Sci. Rep. 2020, 10, 3491. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.J.; Ida, E.I.; Spinosa, W.A. Nutritional supplementation with amino acids on bacterial cellulose production by Komagataeibacter intermedius: Effect analysis and application of response surface methodology. Appl. Biochem. Biotechnol. 2022, 194, 5017–5036. [Google Scholar] [CrossRef]
- Dagbagli, S.; Goksungur, Y. Optimization of β-galactosidase production in Stirred Tank Bioreactor using Kluyveromyces lactis NRRL Y-8279. Food Sci. Biotechnol. 2009, 18, 1342–1350. [Google Scholar] [CrossRef]
- Silbir, S.; Goksungur, Y. Natural red pigment production by Monascus purpureus in submerged fermentation systems using a food industry waste: Brewer’s spent grain. Foods. 2019, 8, 161. [Google Scholar] [CrossRef]
- Moosavi-Nasab, M.; Layegh, B.; Aminlari, L.; Hashemi, M.B. Microbial production of levan using date syrup and investigation of its properties. World Acad. Sci. Eng. Technol. 2010, 44, 1248–1254. [Google Scholar]
- Myers, R.H.; Montgomery, D.D. Response Surface Methodology: Process and Product Optimization Using Designed Experiments, 1st ed.; John Wiley & Sons: New York, NY, USA, 1995; pp. 1–67. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Kjeldahl, J.G.C. En ny Methode til Kvaelstofvestemmelse i organiske Stoffer. Z. Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Mikkelsen, D.; Flanagan, B.M.; Dykes, G.A.; Gidley, M.J. Influence of different carbon sources on bacterial cellulose production by Gluconacetobacter xylinus strain ATCC 53524. J. Appl. Microbiol. 2009, 107, 576–583. [Google Scholar] [CrossRef]
- Mohamad, S.; Abdullah, L.C.; Jamari, S.S.; Al Edrus, S.S.O.; Aung, M.M.; Mohamad, S.F.S. Influence of drying method on the crystal structure and thermal property of oil palm frond juice-based bacterial cellulose. J. Mater. Sci. 2022, 57, 1462–1473. [Google Scholar] [CrossRef]
- Panesar, P.S.; Chavan, Y.; Chopra, H.K.; Kennedy, J.F. Production of microbial cellulose: Response surface methodology approach. Carbohydr. Polym. 2012, 87, 930–934. [Google Scholar] [CrossRef]
- Son, H.J.; Heo, M.S.; Kim, Y.G.; Lee, S.J. Optimization of fermentation conditions for the production of bacterial cellulose by a newly isolated Acetobacter sp. A9 in shaking cultures. Biotechnol. Appl. Biochem. 2001, 33, 1–5. [Google Scholar] [CrossRef]
- Hungund, B.S.; Gupta, S.G. Factors affecting production of bacterial cellulose from Gluconacetobacter xylinus. Asian J. Microbiol. Biotechnol. Environ. Sci. 2010, 12, 517–522. [Google Scholar]
- Rani, M.U.; Appaiah, A. Optimization of culture conditions for bacterial cellulose production from Gluconacetobacter hansenii UAC09. Ann. Microbiol. 2011, 61, 781–787. [Google Scholar] [CrossRef]
- Yang, H.J.; Take, L.; Kim, J.R.; Choi, Y.E.; Park, C. Improved production of bacterial cellulose from waste glycerol through investigation of inhibitory effects of crude glycerol-derived compounds by Gluconacetobacter xylinus. J. Ind. Eng. Chem. 2019, 75, 158–163. [Google Scholar] [CrossRef]
- Rangaswamy, B.E.; Vanitha, K.P.; Hungund, B.S. Microbial Cellulose Production from Bacteria Isolated from Rotten Fruit. Int. J. Polym. Sci. 2015, 2015, 280784. [Google Scholar] [CrossRef]
- Krystynowicz, A.; Czaja, W.K.; Wiktorowska-Jezierska, A.; Gonçalves-Mi’skiewicz, M.; Turkiewicz, M.; Bielecki, S. Factors affecting the yield and properties of bacterial cellulose. J. Ind. Microbiol. Biotechnol. 2002, 29, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Masaoka, S.; Ohe, T.; Sakota, N. Production of cellulose from glucose by Acetobacter xylinum. J. Ferment. Bioeng. 1993, 75, 18–22. [Google Scholar] [CrossRef]
- Santoso, S.P.; Lin, S.P.; Wang, T.Y.; Ting, Y.; Hsieh, C.W.; Yu, R.C.; Angkawijaya, A.E.; Soetaredjo, F.E.; Hsu, H.Y.; Cheng, K.C. Atmospheric cold plasma-assisted pineapple peel waste hydrolysate detoxification for the production of bacterial cellulose. Int. J. Biol. Macromol. 2021, 175, 526–534. [Google Scholar] [CrossRef]
- El-Gendi, H.; Salama, A.; El-Fakharany, E.M.; Saleh, A.K. Optimization of bacterial cellulose production from prickly pear peels and its ex situ impregnation with fruit byproducts for antimicrobial and strawberry packaging applications. Carbohydr. Polym. 2023, 302, 120383. [Google Scholar] [CrossRef]
- Lin, S.P.; Huang, S.H.; Ting, Y.; Hsu, H.Y.; Cheng, K.C. Evaluation of detoxified sugarcane bagasse hydrolysate by atmospheric cold plasma for bacterial cellulose production. Int. J. Biol. Macromol. 2022, 204, 136–143. [Google Scholar] [CrossRef]
- Dubey, S.; Singh, J.; Singh, R.P. Biotransformation of sweet lime pulp waste into high-quality nanocellulose with an excellent productivity using Komagataeibacter europaeus SGP37 under static intermittent fed-batch cultivation. Bioresour Technol. 2018, 247, 73–80. [Google Scholar] [CrossRef]
- Guo, X.; Chen, L.; Tang, J.; Jönsson, L.J.; Hong, F.F. Production of bacterial nanocellulose and enzyme from [AMIM]Cl-pretreated waste cotton fabrics: Effects of dyes on enzymatic saccharification and nanocellulose production. J. Chem. Technol. Biothnol. 2015, 91, 1413–1421. [Google Scholar] [CrossRef]
- Ullah, M.W.; Ul-Islam, M.; Khan, S.; Kim, Y.; Park, J.K. Innovative production of bio-cellulose using a cell-free system derived from a single cell line. Carbohydr. Polym. 2015, 132, 286–294. [Google Scholar] [CrossRef]
- Volova, T.G.; Prudnikova, S.V.; Sukovatyi, A.G.; Shishatskaya, E.I. Production and properties of bacterial cellulose by the strain Komagataeibacter xylinus B-12068. Appl. Microbiol. Biotechnol. 2018, 102, 7417–7428. [Google Scholar] [CrossRef]
- Vazquez, A.; Foresti, M.L.; Cerrutti, P.; Galvagno, M. Bacterial cellulose from simple and low cost production media by Gluconacetobacter xylinus. J. Polym. Environ. 2012, 21, 545–554. [Google Scholar] [CrossRef]
- Cheng, Z.; Yang, R.; Liu, X. Production of bacterial cellulose by Acetobacter xylinum through utilizing acetic acid hydrolysate of bagasse as low-cost carbon source. BioResources 2017, 12, 1190–1200. [Google Scholar] [CrossRef]
- Jittaut, P.; Hongsachart, P.; Audtarat, S.; Dasri, T. Production and characterization ofc bacterial cellulose produced by Gluconacetobacter xylinus BNKC 19 using agricultural waste products as nutrient source. Arab. J. Basic Appl. Sci. 2023, 30, 221–230. [Google Scholar] [CrossRef]
- Atykyan, N.; Revin, V.; Shutova, V. Raman and FT-IR Spectroscopy investigation the cellulose structural differences from bacteria Gluconacetobacter sucrofermentans during the different regimes of cultivation on a molasses media. AMB Express 2020, 10, 84. [Google Scholar] [CrossRef]
- Spiridon, I.; Teaca, C.; Bodirlau, R. Structural changes evidenced by FTIR spectroscopy in cellulosic materials after pre-treatment with ionic liquid and enzymatic hydrolysis. BioResources 2011, 6, 400–413. [Google Scholar] [CrossRef]
- Amorim, L.F.A.; Li, L.; Gomes, A.P.; Fangueiro, R.; Gouveia, I.C. Sustainable bacterial cellulose production by low cost feedstock: Evaluation of apple and tea by-products as alternative sources of nutrients. Cellulose 2023, 30, 5589–5606. [Google Scholar] [CrossRef]
- Apriyana, A.Y.; Andriani, D.; Karina, M. Production of bacterial cellulose from tofu liquid waste and rice-washed water: Morphological property and its functional groups analysis. IOP Conf. Ser. Earth Environ. Sci. 2020, 483, 012005. [Google Scholar] [CrossRef]
- Hasanin, M.S.; Abdelraof, M.; Hashem, A.H.; El Saied, H. Sustainable bacterial cellulose production by Achromobacter using mango peel waste. Microb. Cell Fact. 2023, 22, 24. [Google Scholar] [CrossRef]
- Rusdi, R.A.A.; Halim, N.A.; Norizan, M.N.; Abidin, Z.H.Z.; Abdullah, N.; Ros, F.C.; Ahmad, N.; Azmi, A.F.M. Pre-treatment effect on the structure of bacterial cellulose from Nata de Coco (Acetobacter xylinum). Polimery 2022, 67, 110–118. [Google Scholar] [CrossRef]



| Code | Variable | Low Level (−) | High Level (+) | # | A | B | C | D | Bacterial Cellulose Production (g/L) |
|---|---|---|---|---|---|---|---|---|---|
| A | Yeast extract | 0.5 | 5.0 | 1 | − | − | + | − | 0.64 |
| B | Peptone | 0.5 | 5.0 | 2 | − | − | − | − | 0.42 |
| C | Citric acid | 0.115 | 1.15 | 3 | + | + | − | − | 2.45 |
| D | Na2HPO4 | 0.27 | 2.7 | 4 | + | − | + | + | 2.15 |
| 5 | + | − | − | + | 2.38 | ||||
| 6 | − | + | − | + | 1.1 | ||||
| 7 | − | + | + | + | 1.17 | ||||
| 8 | + | + | + | − | 2.17 |
| Factor Run | X1 Initial Sugar Concentration (g/L) Level −1 0 +1 40 60 80 Experimental Level for X1 | X2 Initial pH Level −1 0 +1 5.5 6.5 7.5 Experimental Level for X2 | X3 Temperature (°C) Level −1 0 +1 25 30 35 Experimental Level for X3 | Bacterial Cellulose (g/L) |
|---|---|---|---|---|
| 1 | 60 | 7.5 | 30 | 6.35 |
| 2 | 80 | 7.5 | 35 | 3.41 |
| 3 | 80 | 6.5 | 30 | 6.67 |
| 4 | 60 | 6.5 | 35 | 5.07 |
| 5 | 40 | 7.5 | 25 | 4.01 |
| 6 | 80 | 5.5 | 25 | 4.19 |
| 7 | 60 | 6.5 | 30 | 7.5 |
| 8 | 60 | 5.5 | 30 | 7.69 |
| 9 | 40 | 6.5 | 30 | 6.52 |
| 10 | 60 | 6.5 | 25 | 5.79 |
| 11 | 60 | 6.5 | 30 | 7.62 |
| 12 | 40 | 7.5 | 35 | 2.8 |
| 13 | 40 | 5.5 | 35 | 3.64 |
| 14 | 60 | 6.5 | 30 | 7.7 |
| 15 | 40 | 5.5 | 25 | 4.18 |
| 16 | 60 | 6.5 | 30 | 7.93 |
| 17 | 80 | 7.5 | 25 | 3.44 |
| 18 | 60 | 6.5 | 30 | 7.67 |
| 19 | 60 | 6.5 | 30 | 7.66 |
| 20 | 80 | 5.5 | 35 | 5.31 |
| Term | Effect | Coef | SE Coef | t | p |
|---|---|---|---|---|---|
| Constant | 3.76000 | 0.05995 | 62.72 | 0.000 | |
| (A) Yeast extract | 1.45500 | 0.72750 | 0.05995 | 12.14 | 0.001 |
| (B) Peptone | 0.32500 | 0.16250 | 0.05995 | 2.71 | 0.073 |
| (C) Citric acid | −0.05500 | −0.02750 | 0.05995 | −0.46 | 0.678 |
| (D) Na2HPO4 | 0.28000 | 0.14000 | 0.05995 | 2.34 | 0.102 |
| Source | DF a | Coefficient | Seq SS b | Adj SS c | Adj MS d | F | p |
|---|---|---|---|---|---|---|---|
| Regression | 9 | 60.1902 | 60.1902 | 6.6878 | 180.58 | <0.000 | |
| Constant | −108.011 | <0.000 | |||||
| Linear | 3 | 3.0401 | 3.0401 | 1.0134 | 27.36 | <0.000 | |
| Sugar conc. | 0.274520 | 0.012 | |||||
| pH | 9.17364 | <0.000 | |||||
| Temperature | 5.29151 | 0.047 | |||||
| Square | 3 | 55.3916 | 55.3916 | 18.4639 | 498.54 | <0.000 | |
| Sugar conc. × Sugar conc. | −0.00254205 | <0.000 | |||||
| pH × pH | −0.591818 | <0.000 | |||||
| Temperature × Temperature | −0.0872727 | <0.000 | |||||
| Interaction | 3 | 1.7584 | 1.7584 | 0.5861 | 15.83 | <0.000 | |
| Sugar conc. × pH | −0.0102500 | 0.013 | |||||
| Sugar conc. × Temperature | 0.00355000 | <0.000 | |||||
| pH × Temperature | −0.0455000 | 0.007 | |||||
| Residual error | 10 | 0.3704 | 0.3704 | 0.0370 | |||
| Lack of fit | 5 | 0.2710 | 0.2710 | 0.0542 | 2.73 | 0.148 | |
| Pure error | 5 | 0.0994 | 0.0994 | 0.0199 | |||
| Total | 19 | 60.5606 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yilmaz, M.; Goksungur, Y. Optimization of Bacterial Cellulose Production from Waste Figs by Komagataeibacter xylinus. Fermentation 2024, 10, 466. https://doi.org/10.3390/fermentation10090466
Yilmaz M, Goksungur Y. Optimization of Bacterial Cellulose Production from Waste Figs by Komagataeibacter xylinus. Fermentation. 2024; 10(9):466. https://doi.org/10.3390/fermentation10090466
Chicago/Turabian StyleYilmaz, Merve, and Yekta Goksungur. 2024. "Optimization of Bacterial Cellulose Production from Waste Figs by Komagataeibacter xylinus" Fermentation 10, no. 9: 466. https://doi.org/10.3390/fermentation10090466
APA StyleYilmaz, M., & Goksungur, Y. (2024). Optimization of Bacterial Cellulose Production from Waste Figs by Komagataeibacter xylinus. Fermentation, 10(9), 466. https://doi.org/10.3390/fermentation10090466






