Abstract
An amylolytic industrial yeast strain named 1974-GA-temA, developed previously by our research team by coexpressing the α-amylase and glucoamylase genes, combines enzyme production, sweet potato residue (SPR) hydrolysis, and glucose fermentation into ethanol in a one-step process. This consolidated bioprocessing (CBP) method has great application potential in the commercial production of bioethanol from SPR, but important fermentation parameters should be optimized to further increase the ethanol concentration and yield. In this study, the effects of the initial fermentation pH, solid-to-liquid ratio, inoculation volume, addition of exogenous enzyme, and supplementation with metal ions were systemically investigated. Single-factor experiments revealed that the optimal pH was 4.0. In the solid-to-liquid ratio test, an increase in the solid-to-liquid ratio corresponded with a gradual increase in the ethanol concentration, peaking at 1:5. However, the ethanol yield gradually decreased, with the optimal solid-to-liquid ratio identified as 1:5. The ethanol concentration and yield reached 9.73 g/L and 5.84%, respectively. Additionally, an increase in the inoculum size resulted in increased ethanol concentration and yield, with the optimal inoculum level determined to be 10%. An ethanol concentration of 7.87 g/L was attained under these specified conditions, equating to an ethanol yield of 4.72%. Further analysis was conducted to assess the effects of exogenous cellulase, hemicellulase, and pectinase, both individually and in combination, on ethanol concentration and yield. The results indicated that pectinase had a particularly significant effect. The highest ethanol concentration was observed when all three enzymes were administered concurrently, yielding 27.27 g/L ethanol. Then, the role of metal ions in SPR fermentation was evaluated. The metal ions did not significantly affect the process, with the exception of copper ions. The addition of copper ions at a specific concentration of 0.2 g/100 g SPR increased the ethanol concentration. However, concentrations exceeding 0.2 g/100 g SPR inhibited yeast cell growth. Finally, orthogonal optimization was employed to determine the optimal combination of factors: pH, 4.0; solid-to-liquid ratio, 1:6; inoculation volume, 10%; cellulase and pectinase addition; and the absence of Cu2+ addition. Under these conditions, strain 1974-GA-temA produced 34.83 ± 0.62 g/L ethanol after 8 days of fermentation, corresponding to a 20.90% ± 0.37% ethanol yield. This value markedly exceeds the outcomes of all the conducted orthogonal experiments. The fermentation optimization experiments in this study are expected to increase ethanol production during the CBP fermentation of SPR.
1. Introduction
Bioethanol is a promising alternative energy source to fossil fuels, with lignocellulosic material-based bioethanol garnering particular attention for its perceived advantages [1,2]. However, this form of bioethanol still faces the following challenges: the development of cost-effective pretreatment technologies for lignocellulosic materials; ensuring the robustness of Saccharomyces cerevisiae strains; enabling the utilization of various sugars; and tolerating inhibitors found in the hydrolysate [3]. Starch ethanol remains the predominant biofuel currently being produced; corn is the preferred substrate for large-scale bioethanol production [4] because of the ease of long-term storage, nontoxicity, and high reactivity of corn starch [5]. The production of biofuels from corn starch has indeed reached technological maturity. However, corn-based feedstock represents one of the main costs involved in the production of biobased ethanol [6,7,8], and alternative low-cost feedstocks are needed [4]. Cheap and abundant starchy byproducts from industries such as food and agricultural processing are good candidates [9,10,11].
Sweet potato is globally recognized as the eighth most cultivated food crop and is valued for its sustainable production practices and high starch content, making it an ideal source for starch extraction and starchy food production [12,13]. China is the leading producer of sweet potatoes worldwide, yielding approximately 71 million tons annually, with sweet potatoes contributing significantly to industrial starch production in the country [14]. However, the starch extraction process generates a byproduct known as sweet potato residue (SPR), with more than 80% of the original sweet potato weight being lost during processing [15]. This accumulation of SPR poses challenges due to the short harvest duration and perishable nature of sweet potatoes, leading to significant resource waste and environmental pollution when the waste is primarily used as animal feed or discarded as agro-waste [16,17,18]. Environmentally sustainable and economically viable methods for recycling SPR are urgently needed to address these issues.
After starch extraction, significant amounts of starch granules remain in the SPR instrument. The starch content in SPR, ranging from 30.01% to 60.89% dry weight, is influenced by the degree of crushing of sweet potato mash and the efficiency of separation processing techniques [13,19]. This high starch content and abundance make SPR an attractive raw material for biorefinery applications. Early research on SPR conversion focused primarily on the production of ethanol, lactic acid, and succinic acid [20,21,22,23]. These studies have demonstrated the potential of using SPR for the bioconversion of valuable chemicals.
Nevertheless, in the process of utilizing microorganisms for fermenting SPR to generate the aforementioned products, SPR typically needs to be pretreated to facilitate the conversion of starch into glucose, which can be utilized by yeast strains [24]. These pretreatment processes are predominantly associated with conventional energy-intensive gelatinization or the addition of exogenous enzyme cocktails for the liquefaction and saccharification of raw starch [25,26]. The energy required for conventional gelatinization constitutes approximately 10% to 20% of the fuel value of ethanol produced in a standard refinery [27]. Additionally, the expense associated with enzymes represents approximately 8% of the overall processing cost [28,29]. The production of ethanol from starch can be enhanced by integrating these processes into a unified approach known as consolidated bioprocessing (CBP). This method necessitates the use of genetically modified ethanologenic yeast strains, such as the amylolytic strains of S. cerevisiae, which can produce raw starch-degrading enzymes. These enzymes facilitate the simultaneous hydrolysis of starch and the fermentation of the resultant sugars into ethanol [23,25,30].
A number of S. cerevisiae strains with raw starch CBP capabilities have been developed with varying degrees of success [26,31,32]. Much of the research focuses on the construction process of recombinant S. cerevisiae strains. For instance, in the selection of chassis cells, some researchers opt for laboratory strains, while others favor industrial strains [23,33]. Another key factor to consider is whether the gene encoding starch hydrolysis enzymes is expressed via a plasmid or is integrated into the genome; the former allows for easier manipulation, whereas the latter typically demonstrates enhanced genetic stability [23,33,34]. Furthermore, the expression levels of starch hydrolase genes from different species in S. cerevisiae vary significantly, which subsequently impacts the strain’s capacity to utilize starch and produce ethanol [30]. Additionally, the copy number of the starch hydrolase gene present in recombinant S. cerevisiae also influences the strain’s starch utilization ability, as a higher gene copy number is often associated with increased enzyme activity [33]. Cripwell et al. employed ethanol red, an industrial S. cerevisiae strain, as a host; they also incorporated seven copies of the codon-optimized glucoamylase-encoding gene from Talaromyces emersonii (temG_Opt) and four copies of the native α-amylase-encoding gene from T. emersonii (temA) into the yeast genome via δ-integration. The resulting recombinant amylolytic yeast strain produced 89.35 g/L ethanol from 200 g/L raw corn starch, which is the highest yield reported to date, in a single-step process after 192 h at 30 °C [33]. Recently, an amylolytic strain of S. cerevisiae 1974-GA-temA was developed by our research team to integrate eight copies of the α-amylase gene originating from T. emersonii and eight copies of the glucoamylase gene originating from Saccharomycopsis fibuligera, respectively. The recombinant strain 1974-GA-temA showed superior fermenting capabilities in the CBP fermentation of raw corn starch and SPR and displayed exceptional volumetric amylase activity [23]. Nevertheless, studies on the optimization of CBP fermentation conditions for the production of ethanol via SPR are lacking. Further investigation is required to optimize their fermentation capabilities on SPR substrates.
In addition to the strain construction aspects mentioned above, several studies have focused on optimizing the fermentation process when using a specific CBP strain. For example, the incorporation of nutrients such as yeast extract and peptone into the fermentation medium has been explored [35]. Furthermore, the addition of proteases may facilitate the release of free amino acids for yeast utilization, while the direct introduction of urea can enhance the nitrogen content of the medium [8]. The addition of α-amylase and/or glucoamylase is also common to improve the efficiency of starch hydrolysis [23,33]. However, very few studies have systematically examined the effects of conditions such as pH value, solid-to-liquid ratio, inoculation volume, exogenous enzyme addition, and metal ions on ethanol production from uncooked SPR material. In this study, the effects of the initial fermentation pH, solid-to-liquid ratio, inoculation volume, addition of exogenous enzymes, and supplementation with metal ions were systematically investigated. Additionally, a fast and reliable orthogonal optimization test was conducted to determine the optimal conditions for the CBP fermentation of SPR using the amylolytic strain 1974-GA-temA. These findings offer a convenient method to increase the ethanol concentration and yield from SPR CBP fermentation.
2. Materials and Methods
2.1. Strains and Growth Conditions
The recombinant α-amylase and glucoamylase coexpressing strain 1974-GA-temA was used in the SPR bioethanol fermentation process [23]. Strain 1974-GA-temA was routinely cultured in YPD media (10 g/L yeast extract, 20 g/L tryptone, and 20 g/L D-glucose). The solid agar plates were prepared by adding 1.5% agar.
2.2. SPR Fermentation Media Preparation
SPR was provided by the Luoyang Feed Factory of Henan Dongfang Zhengda Co., Ltd., Luoyang, China. The SPR was ground, passed through a 60-mesh sieve, and stored at 4 °C. The dried SPR mixture was composed of 51.94% ± 0.55% starch, 18.22% ± 0.47% cellulose, 3.38% ± 0.02% hemicellulose, 1.66% ± 1.31% pectin, 2.92% ± 0.03% protein, and 2.23% ± 0.01% ash [24]. α-Amylase and glucoamylase were purchased from Beijing Aoboxing Biotechnology Co., Ltd, Beijing, China. Cellulase, hemicellulase, and pectinase were purchased from Shanghai Yuanye Biotechnology Co., Ltd, Shanghai, China. The enzyme activities of α-amylase, glucoamylase, cellulase, hemicellulose, and pectinase were 3700, 100,000, 50,000, 20,000, and 500,000 U/g, respectively. The S. cerevisiae strain 1974-GA-temA was maintained in the laboratory and grown on YPD media. All other chemicals were analytical-grade reagents.
During the preparation of the SPR-based medium, water was added to the SPR system to achieve the desired solid-to-liquid ratio. Mixed enzymes (cellulase, hemicellulase, and pectinase) or metal ions were subsequently added to the mash. The amounts of cellulase, hemicellulase, and pectinase added were 0.043 g, 0.107 g, and 0.011 g, respectively. The corresponding enzyme activities were 215 U/g SPR, 215 U/g SPR, and 550 U/g SPR. The range for the addition of metal ions was from 0 g/100 g SPR to 1 g/100 g SPR, in increments of 0.2 g. The pH was adjusted using 1 M HCl or 1 M NaOH. Finally, the SPR-based medium was transferred into a 250 mL screw-capped anaerobic bottle for bioethanol fermentation without sterilization. Strain 1974-GA-temA was inoculated into the SPR-based medium at the desired volume at 30 °C and 240 rpm and cultured for 7 days.
2.3. Single-Factor Experiments
Single-factor experiments were conducted to investigate the effects of five factors on SPR ethanol production and ethanol yield. In the study of a certain factor, the other factors were set to their corresponding fixed values: 5.0 for pH, 1:7 for the solid-to-liquid ratio, and an inoculation volume of 10%, or without the addition of mixed enzymes or metal ions. The factors and levels are shown in Table 1. Three independent replicates were performed. The SAS software package (version 9.2) was used for a one-way ANOVA to statistically analyze the results of the single-factor experiments.

Table 1.
Factors and levels used in the one-factor-at-a-time experiments.
2.4. Ethanol Production from the Optimized Fermentation Conditions
Single colonies of the 1974-GA-temA strain were obtained by streaking YPD plates from a frozen glycerol stock. The samples were inoculated into 3 mL of YPD medium in test tubes and grown overnight at 30 °C and 240 rpm. A total of 200 μL of the cultures was transferred to 25 mL of YPD medium in flasks to repeat the procedure. After aerobic growth, the cells were harvested via centrifugation at 12,000 rpm, washed two times with sterile water, and inoculated into the SPR medium in a 250 mL anaerobic flask at an inoculation volume of 10% (v/v).
During fermentation, samples were taken every day for ethanol and glucose analysis. The ethanol and glucose concentrations were determined via a refractive index detector on an LC-2030C HPLC with a Bio-Rad HPX-87H column. The column was eluted at 65 °C with 5 mM sulfuric acid at a flow rate of 0.6 mL/min [36]. Ethanol productivity, ethanol yield from SPR, and estimated ethanol yield from glucose were calculated as previously described [23,24,37].
3. Results and Discussion
3.1. Effect of Initial pH on the Fermentation of Uncooked SPR Material
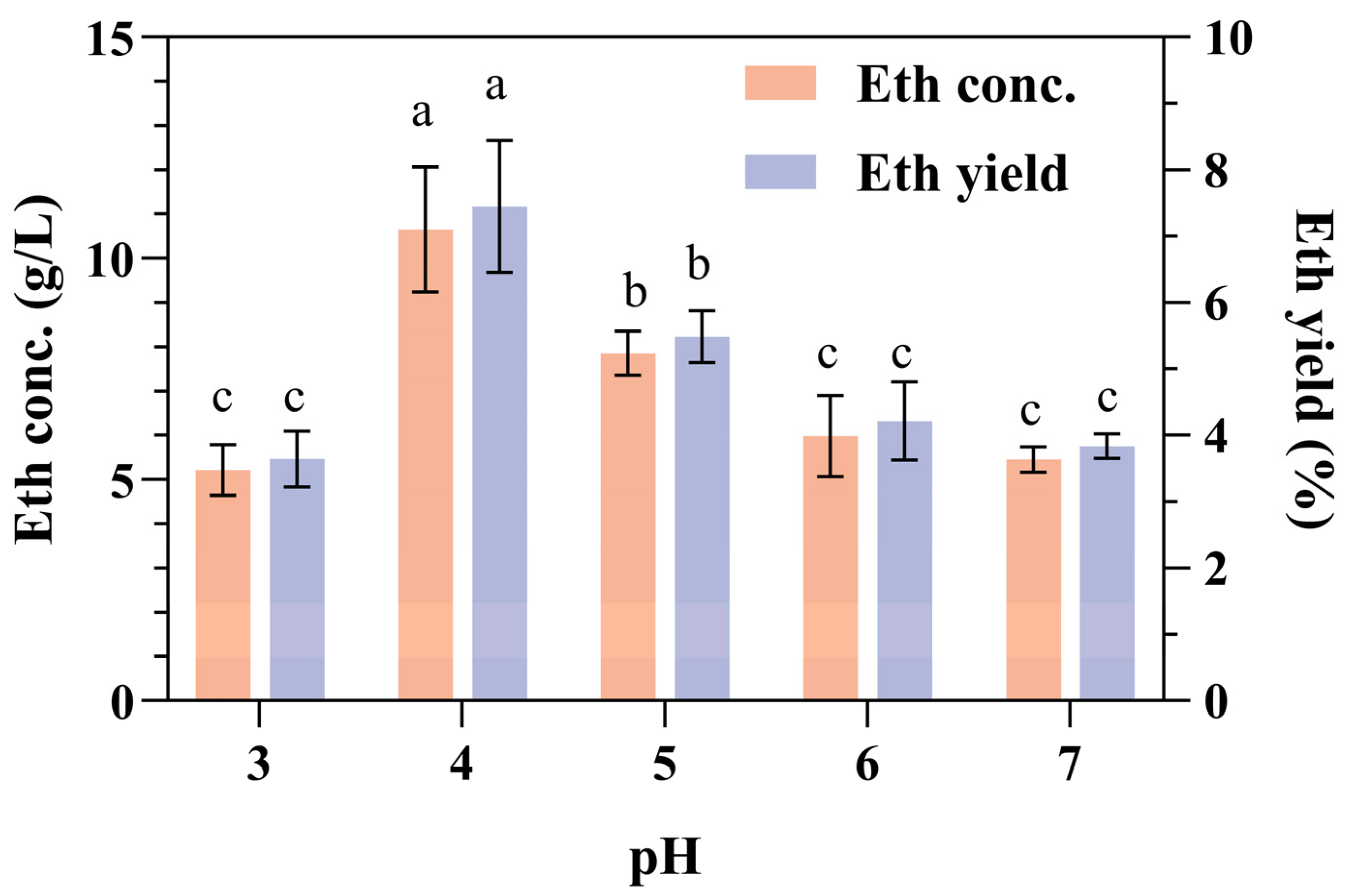
During bioethanol production, pH adjustment is typically needed, primarily because S. cerevisiae has an optimal growth pH. In general, brewer’s yeast can grow within a pH range of 3.0 to 7.5, with an optimal growth pH range of 4.0 to 5.0 [38]. We previously demonstrated that the optimal pH values for the α-amylase and glucoamylase were 4.0 and 5.0, respectively [23]. The highest ethanol concentration and yield were achieved at an initial pH of 4.0 (Figure 1). Thus, during the CBP fermentation of SPR with the recombinant amylolytic strain 1974-GA-temA, α-amylases play a more important role than glucoamylase.
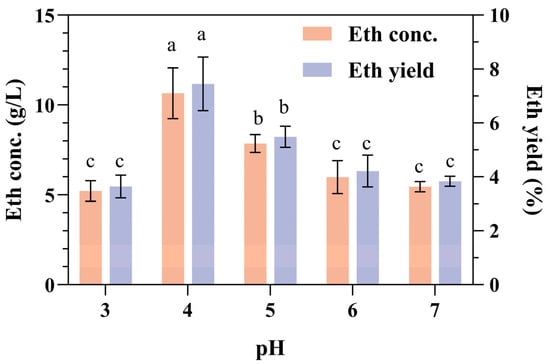
Figure 1.
Effect of initial pH on ethanol production and yield from SPR fermentation. Error bars represent the standard deviation from the mean of three replicates. Letters a–c: Significant difference in relation to ethanol concentration or ethanol yield in pH 4.0 (p < 0.01).
The enzymes α-amylase and glucoamylase function synergistically to facilitate the complete hydrolysis of starch into glucose units. α-Amylases (EC 3.2.1.1) are classified as endo-acting enzymes that randomly cleave α-1,4 glycosidic linkages within the starch granules, leading to rapid degradation of the starch structure and the subsequent release of nonreducing ends, which are then accessible for glucoamylase action. Conversely, glucoamylases (EC 3.2.1.3) are exo-acting enzymes that hydrolyze both the α-1,4 and α-1,6 glycosidic linkages from the nonreducing ends of starch chains, ultimately yielding glucose monomers [39]. The activity of α-amylases represents the rate-limiting step in the conversion of raw starch to oligosaccharides, which creates conditions favorable for the action of exo-acting glucoamylase [29]. In the future, the relative copy numbers of the two enzymes in the recombinant S. cerevisiae strain need to be considered. For instance, when the ratio of commercial α-amylase and glucoamylase activities was optimized, direct ethanol production from cassava starch was significantly improved [40,41].
3.2. Effects of the Solid-to-Liquid Ratio on the Fermentation of Uncooked SPR Material
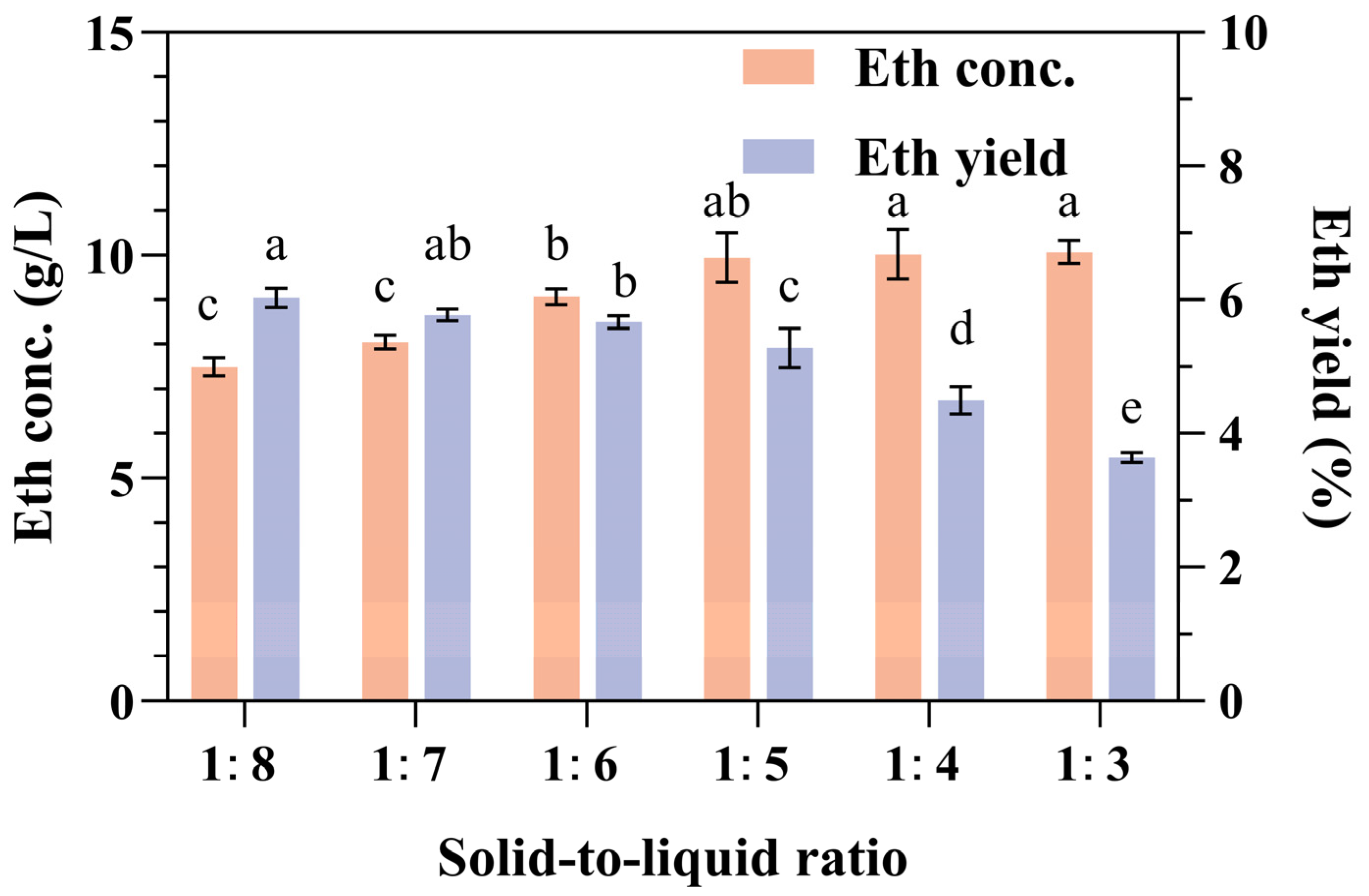
The optimal loading of biomass into fermentation media is crucial for maximizing the conversion efficiency and product yield [42]. To achieve elevated ethanol concentrations and yields, our research team manipulated the solid-to-liquid ratios of the relative SPR concentration with water, particularly at ratios of 1:8, 1:7, 1:6, 1:5, 1:4, and 1:3. The SPR concentration was positively correlated with the ethanol concentration, with the lowest ethanol concentration recorded at 7.01 ± 0.21 g/L for the 1:8 ratio and the highest at 9.7 ± 0.58 g/L for the 1:4 ratio (Figure 2). Conversely, the ethanol yield exhibited an inverse relationship with the SPR concentration. The lowest yield of 3.64% was observed at the highest loading ratio of 1:3, whereas the highest yield of 6.03% was observed at the lowest loading ratio of 1:8. There was no significant difference in ethanol concentrations at solid-to-liquid ratios greater than 1:5, such as at 1:4 and 1:3. However, the ethanol concentration at a ratio of 1:5 was significantly higher than that at a ratio of 1:6 (Figure 2). Upon further examination of the ethanol yield, there was no significant difference in ethanol yield among the 1:6, 1:7, and 1:8 ratios, but the yield at 1:5 was slightly lower (Figure 2).
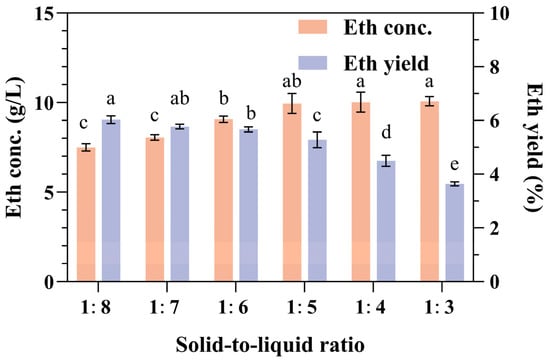
Figure 2.
Effects of the solid-to-liquid ratio on ethanol production and yield from SPR fermentation. Error bars represent the standard deviation from the mean of three replicates. Letters a–e: Significant difference in relation to ethanol concentration in solid-to-liquid ratio of 1:3 or ethanol yield in solid-to-liquid ratio of 1:8 (p < 0.01).
In general, increased biomass loading enhances substrate availability, which can potentially increase product yields. Consequently, research into enhancing biomass loading is often pursued to improve the economic viability of the fermentation process. However, excessive biomass loading can adversely affect the concentration of insoluble components and the rheological properties of the medium [43]. When the insoluble component concentration surpasses a certain threshold, fermentation efficiency is likely to decrease [44]. Observations of the media indicated a significant deficiency of free water at a solid-to-liquid ratio of 1:3. Considering both the ethanol concentration and yield, the optimal solid-to-liquid ratio for the fermentation of raw SPR should be 1:5 or 1:6.
3.3. Effects of Inoculation Volume on the Fermentation of Uncooked SPR Material
The amount of inoculum is an important parameter affecting the production of ethanol in SPR CBP fermentation. Insufficient inoculum can result in the sluggish proliferation of yeast, potentially leading to bacterial contamination of the fermentation broth. Conversely, an excessively high amount of inoculum may introduce an overabundance of aged cells into the fermentation broth, which is detrimental to the fermentation process [45].
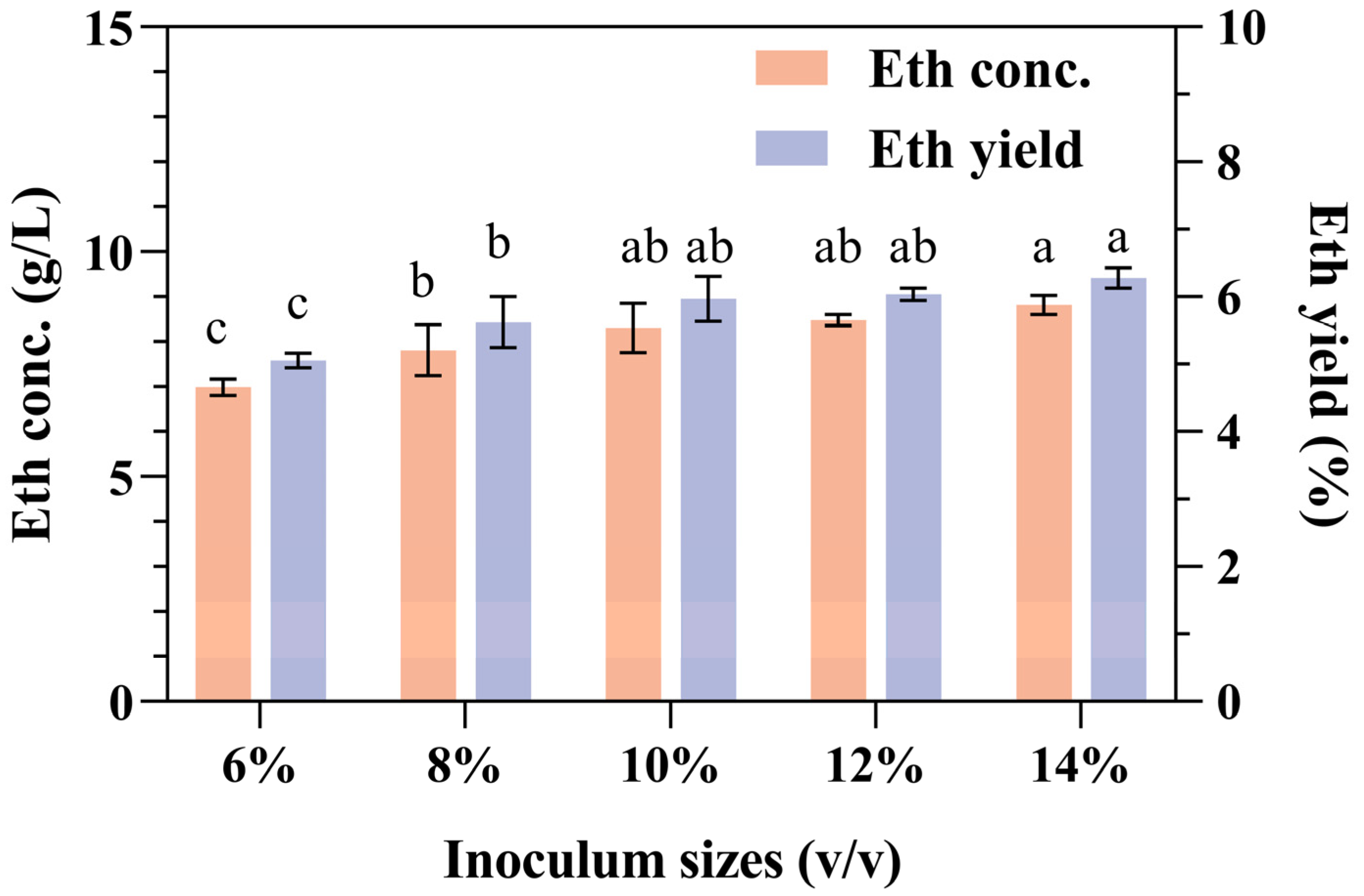
The effects of different inoculation volumes on ethanol concentration and yield are shown in Figure 3. The results of this research confirmed that the ethanol concentration and yield increased slightly with increasing inoculation volume, but an excessive inoculation volume (>10%) had no positive effect. Since larger inoculum quantities in industrial production result in increased production costs, the appropriate concentration of the strain culture seed that was added to the SPR fermentation mixture was determined to be 10%. In this case, the concentration of ethanol that accumulated was 7.9 ± 0.55 g/L.
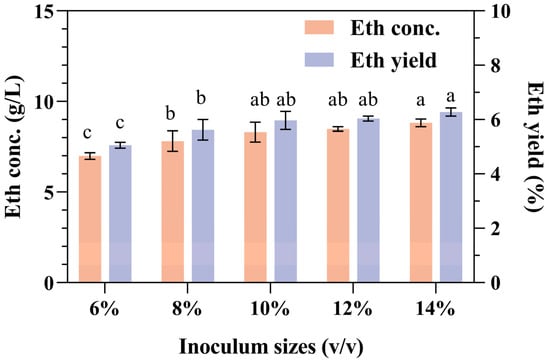
Figure 3.
Effects of inoculation volume on ethanol production and yield from SPR fermentation. Error bars represent the standard deviation from the mean of three replicates. Letters a–c: Significant difference in relation to ethanol concentration or ethanol yield in inoculum size of 10% (p < 0.01).
3.4. Effects of Exogenous Enzyme Addition on the Fermentation of Uncooked SPR Material
SPR is composed of various components, including starch, cellulose, hemicellulose, and pectin. Cellulose, hemicellulose, and pectin can cross-link with polymers and intertwine around starch to form a complex network that restricts the access of hydrolases to substrates [24]. This restricted access may ultimately affect the concentration and yield of ethanol fermentation via SPR.
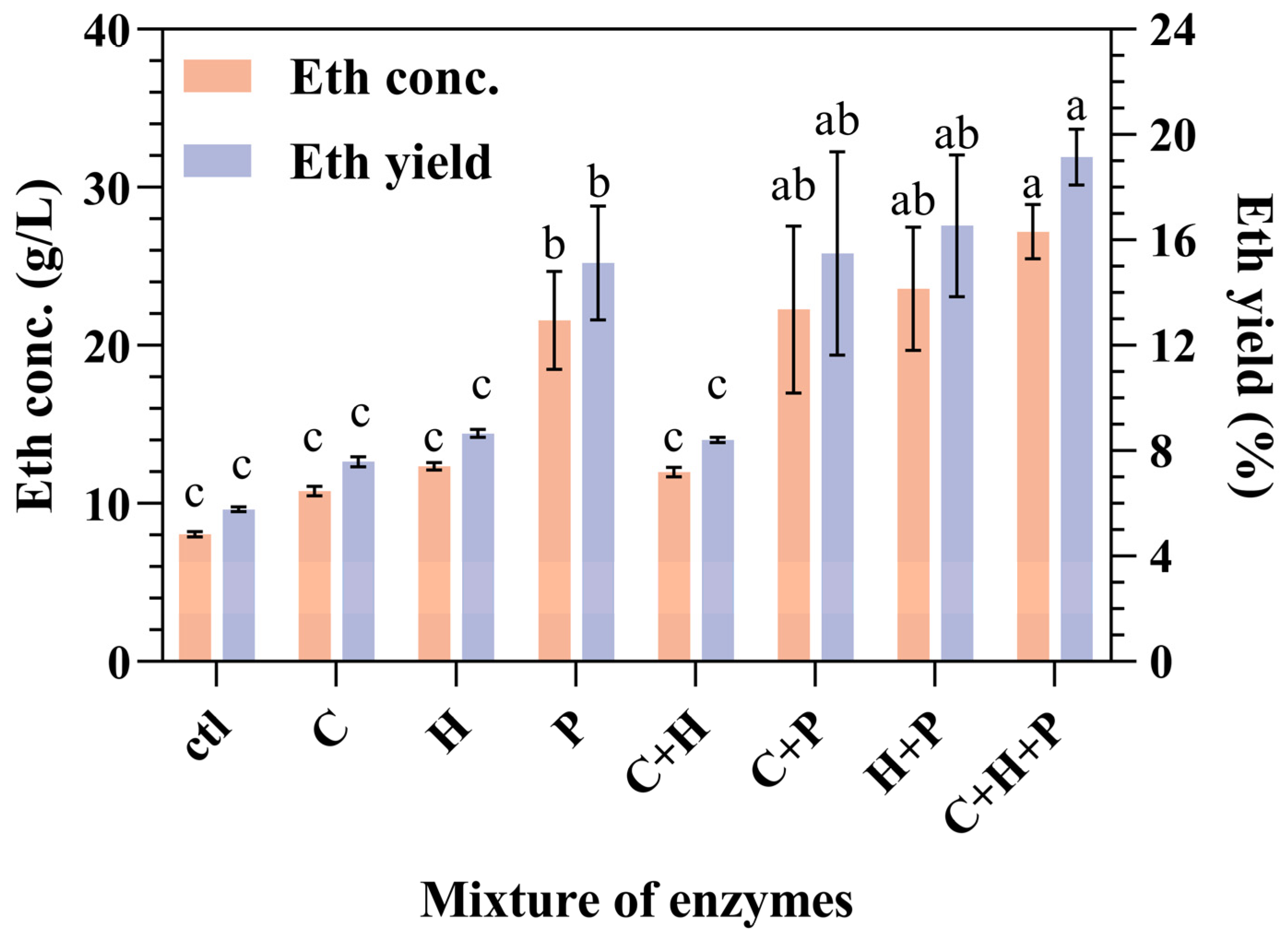
The impact of auxiliary enzymes, particularly cellulase, hemicellulase, and pectinase, on the CBP fermentation of SPR was examined (Figure 4). Compared with the control group, which did not incorporate auxiliary enzymes, the inclusion of enzymes increased both the ethanol concentration and the yield (Figure 4).
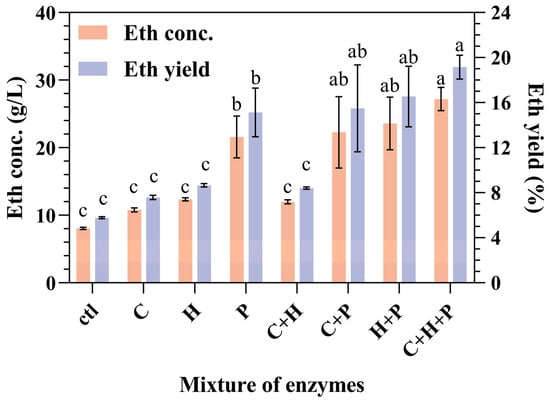
Figure 4.
Impact of both combined and individual additions of cellulase, hemicellulase, and pectinase on ethanol production and yield during the CBP fermentation of SPR by strain 1974-GA-temA. Error bars represent the standard deviation from the mean of three replicates. Letters a–c: Significant difference in relation to ethanol concentration or ethanol yield in condition of C + H + P (p < 0.01). ctl: without enzyme addition; C: cellulase; H: hemicellulase; P: pectinase.
In the single-enzyme addition experiments, the addition of pectinase demonstrated markedly greater efficacy than the addition of either cellulase or hemicellulose. Pectin and cellulose interact with starch and restrict the accessibility of α-amylase via SPR; therefore, pectinase acts as a “helper protein” to assist α-amylase in degrading starch during the hydrolysis of SPR [20]. Compared with the addition of either cellulase or hemicellulase alone, the inclusion of both cellulase and hemicellulase did not significantly affect the ethanol concentration or yield. This finding implies the absence of a synergistic effect between cellulase and hemicellulase.
The experimental group with the addition of cellulase, hemicellulase, and pectinase presented the highest ethanol yield of 27.40 ± 1. 56 g/L, followed by groups with the addition of either cellulase and pectinase or hemicellulase and pectinase. These results suggest that pectin plays a crucial role in limiting the effectiveness of strain-secreted starch hydrolase during SPR material fermentation. For future strain development, incorporating the genes responsible for pectinase expression alongside those for α-amylase and glucoamylase may be beneficial. This gene integration is expected to substantially increase both the concentration and the yield of ethanol produced from the fermentation of SPR.
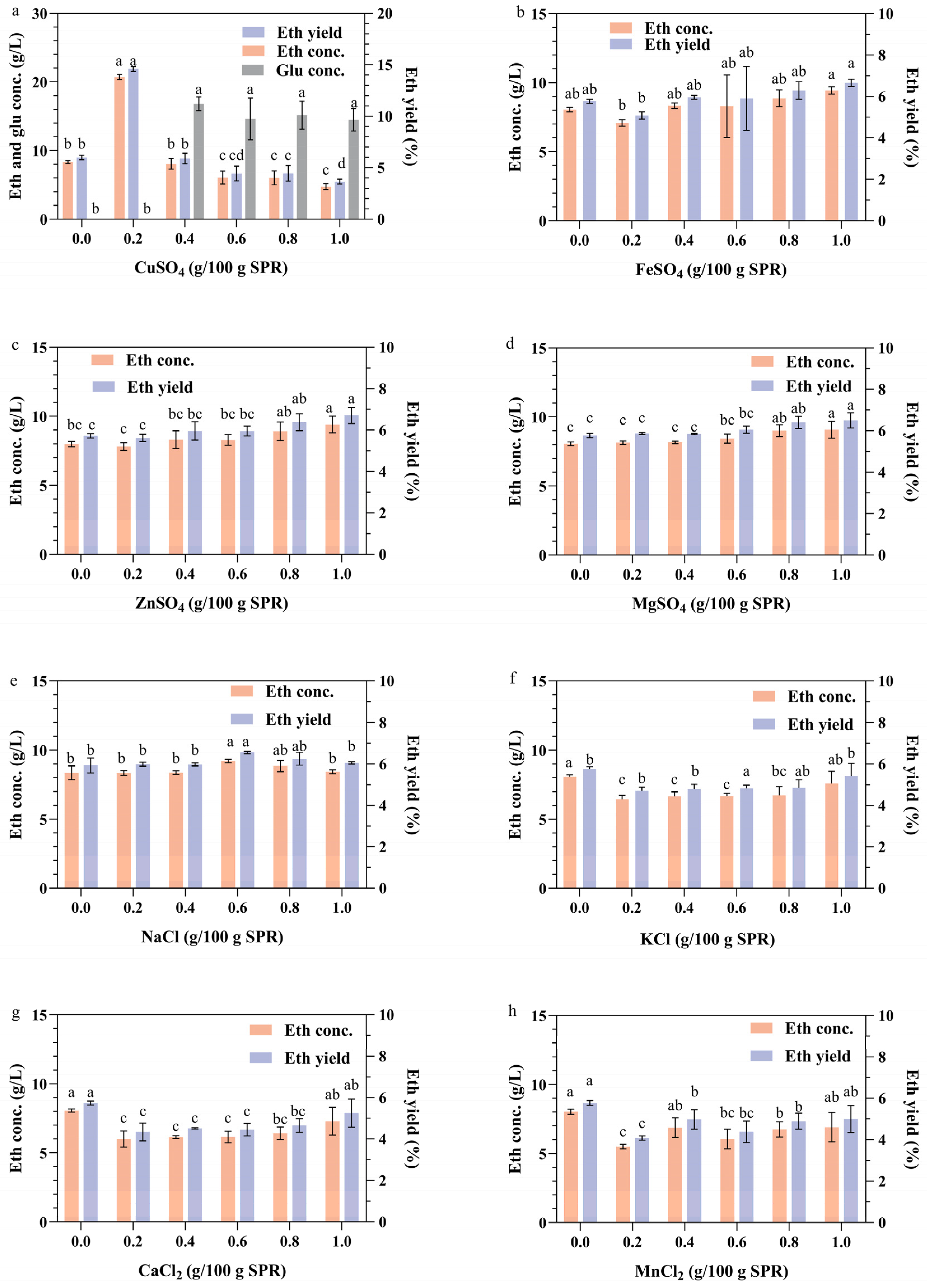
3.5. Effects of Metal Ions on the Fermentation of Uncooked SPR Material
Metal ions play crucial roles in the growth and metabolic processes of microorganisms. They are involved in the composition of cellular structures and can promote cellular metabolism, regulate cellular osmotic pressure, and maintain intracellular enzyme activities [46]. The impact of different metal ions on the fermentation of raw sweet potato pomace was investigated in this study (Figure 5). The results indicated that, except for Cu2+ (p < 0.01), the other metal ions had no significant effect (p > 0.05) on the fermentation of SPR in the given concentration range. Ethanol production increased with increasing copper ion concentration, ranging from 0 to 0.2 g/100 g SPR (Figure 5a). This increase may be attributed to the ability of low concentrations of copper ions to increase the permeability of cell membranes, facilitating the entry of small molecules, such as extracellular glucose, into the cells of S. cerevisiae or the secretion of α-amylase and glucoamylase outside the cells, consequently increasing the ethanol concentration and yield [47]. However, as the concentration of copper ions increased, the ethanol yield started to decrease, and the glucose could not be fully utilized. These findings suggest that increasing the copper ion concentration (≥ 0.4 g/100 g SPR) affects yeast sugar metabolism, thereby impeding the proliferation of brewer’s yeast cells. This interaction could result in prolonged fermentation time or even the premature termination of fermentation [48]. These findings indicate the importance of maintaining copper ion concentrations within an optimal range to facilitate fermentation of the strain.
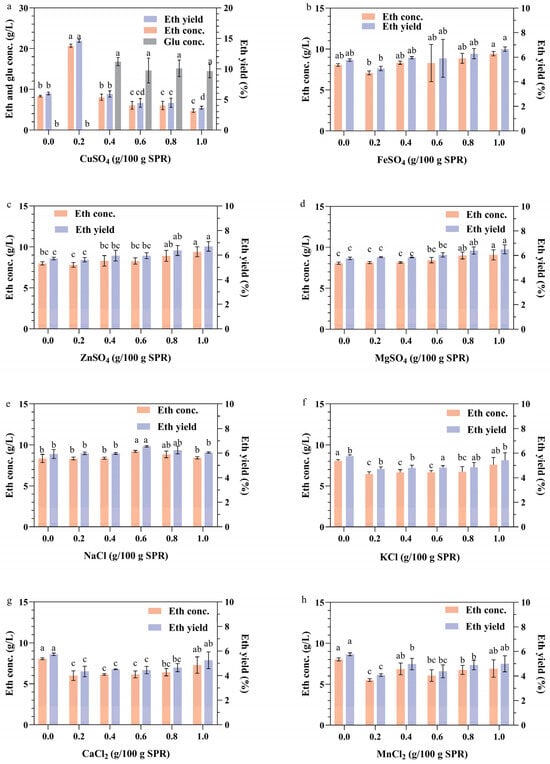
Figure 5.
Impact of metal ions on the production of ethanol from the CBP fermentation of SPR by strain 1974-GA-temA. The influence of CuSO4 (a), FeSO4 (b), ZnSO4 (c), MgSO4 (d), NaCl (e), KCl (f), CaCl2 (g), and MnCl2 (h) were shown. Error bars represent the standard deviation from the mean of three replicates. The letters on the bar graphs represent the results of significance analyses (p < 0.01).
Metal ions, including Zn2+, Mg2+, and Mn2+, have been identified as trace elements essential for yeast growth and ethanol fermentation [49]. Zn2+ and Mg2+ can enhance both heat and ethanol tolerance [50]. However, under standard conditions and within the tested concentration range, Zn2+ and Mg2+ did not significantly affect the ethanol production of the yeast strain (Figure 5c,d). Previous studies have indicated that NaCl and KCl inhibit glucose utilization at concentrations exceeding 0.1 M [51]. In this investigation, NaCl and KCl did not significantly affect the fermentation process of the strain within the specified concentration range (Figure 5e,f). Furthermore, Fe2+, Ca2+, and Mn2+ had no effect on the fermentation performance of the strain (Figure 5b,g,h).
3.6. Optimization of the Ethanol Production of the Strain via an Orthogonal Test
Various critical factors can enhance the fermentation process to effectively convert SPR into ethanol. Drawing upon the results of prior single-factor experiments, the solid-to-liquid ratio, pH, enzyme addition, and concentration of CuSO4 were selected in this study to design an L9 (34) orthogonal test and further optimize the CBP fermentation conditions of the 1974-GA-temA strain. The ethanol concentration was used as a response value to analyze the optimum conditions of the orthogonal experiment. The outcomes of the orthogonal design and the corresponding measurement results are shown in Table 2. Range analysis was conducted to clarify the important sequence of the solid-to-liquid ratio (factor A), pH (factor B), enzyme addition (factor C), and CuSO4 addition (factor D) for ethanol fermentation. The range analysis results of the L9 (34) orthogonal experiment for ethanol concentration revealed that factor D had the highest range (R), with a value of 19.37, followed by factors B (7.18), A (5.99), and C (3.03).

Table 2.
Orthogonal test design and results for the CBP fermentation of SPR using 1974-GA-temA.
A greater R-value represents a greater effect on the final ethanol concentration. Here, the order of influence was CuSO4 > pH > solid-to-liquid ratio > enzyme. Judging by the k values of different factors, the optimum fermentation condition for improving the ethanol concentration was A2B2C2D1, corresponding to a solid-to-liquid ratio of 1:6, a pH of 4.0, cellulase and pectinase addition, and the absence of Cu2+ addition. An ANOVA was used to confirm the order of the effects of the four parameters on the final ethanol concentration (Table 3). A model F value of 178.01 implied that the model was significant. All four factors significantly affected the ethanol concentration and yield (p < 0.01). The correlation between the predicted and actual ethanol concentrations had an R2 of 99.37%. These results confirmed an acceptable fit of the model to the data.

Table 3.
Analysis of variance for the orthogonal experiments.
3.7. Verification of the Orthogonal Test Optimization
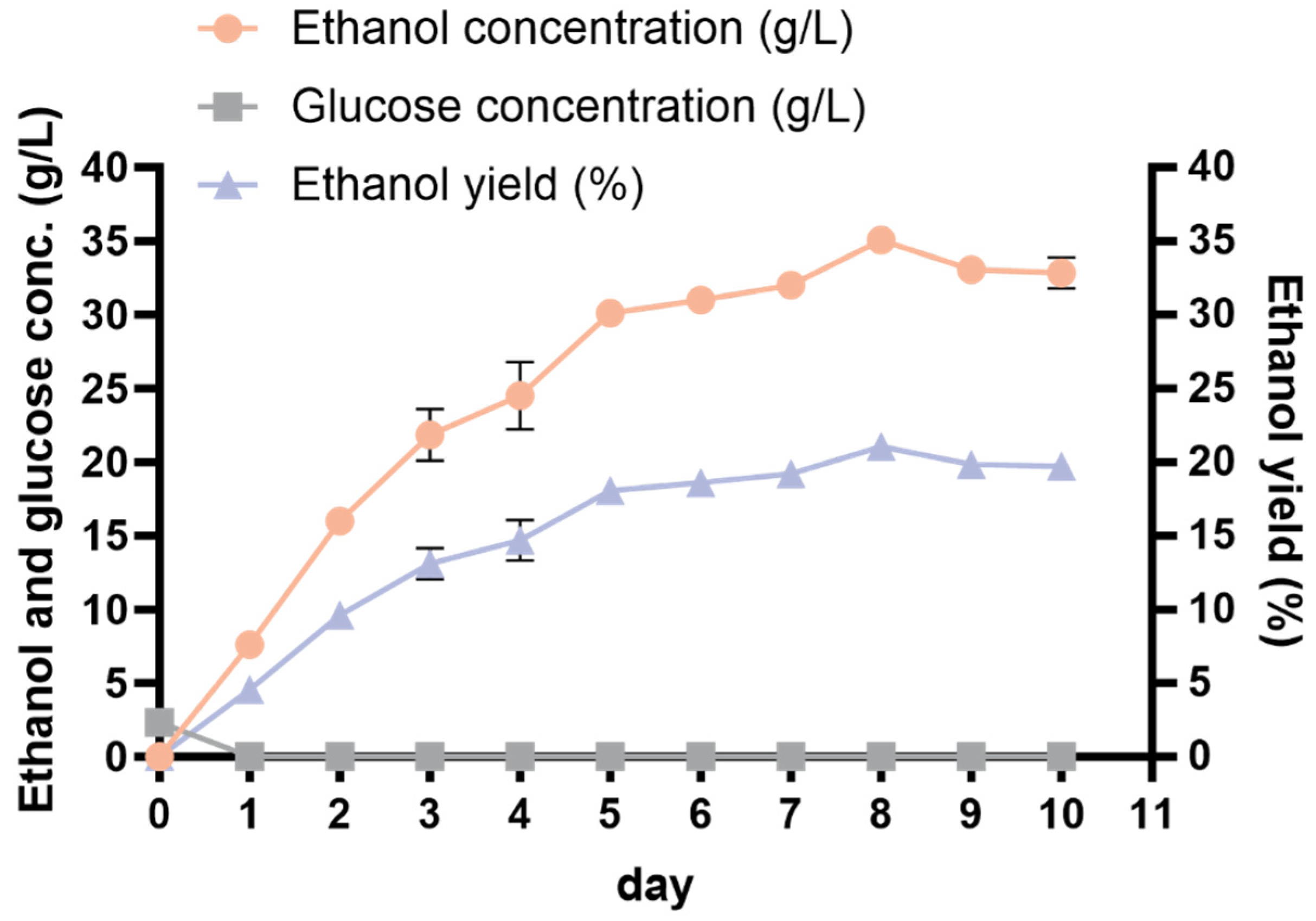
Given that the best combination deduced from the orthogonal optimization test was excluded from the existing trial, an additional experiment was conducted to verify the hypothesis. Samples were obtained every 24 h to measure the glucose and ethanol levels. The iodine–starch reaction was used to determine the completion of starch hydrolysis. The strain 1974-GA-temA produced 34.83 ± 0.62 g/L of ethanol after 8 days of fermentation (Figure 6, Table 4), corresponding to 20.90% ± 0.37% of the ethanol yield. This value markedly exceeded the outcomes of all the orthogonal experiments conducted, suggesting that the optimal fermentation process is reliable. Throughout the SPR fermentation cycle, the glucose content in the culture medium was extremely low (Figure 6), indicating that the strain was metabolically active and could quickly utilize the decomposed glucose.
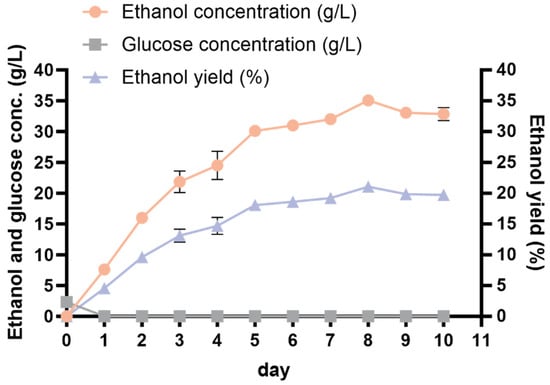
Figure 6.
Ethanol production from optimized SPR CBP fermentation by strain 1974-GA-temA. The data represent the means of three repeats with standard deviations.

Table 4.
CBP fermentation of SPR to produce ethanol using the strain 1974-GA-temA.
3.8. Economical Analysis of CBP Fermentation of SPR
The majority of bioethanol production from starchy substrates employs a four-step process. This process begins with the jet-cooking of starch at a temperature of 105 °C for a duration of several minutes, followed by a liquefaction phase conducted at temperatures ranging from 80 to 90 °C, using a thermostable α-amylase enzyme, and then saccharification occurs at 65 °C with the application of glucoamylase at a pH of 4.5, resulting in the generation of sugar streams that can be fermented into ethanol [52]. The energy demand for this conventional starch process represents 10–20% of the bioethanol price, which negatively affects the overall cost efficiency of bioethanol production [53].
The cost-effective conversion of SPR to bioethanol would benefit from the CBP fermentation method developed in this study. A comparative analysis of the economic benefits associated with ethanol production from CBP-fermented SPR versus conventional ethanol production methods is presented in Table 5. By leveraging the cost advantages of SPR as a raw material and the simplified CBP process, the production of ethanol from SPR demonstrates a markedly higher level of profitability compared to traditional corn-based ethanol production.

Table 5.
Forecast of economic benefits from ethanol-producing enterprises (calculated based on the production of one ton of ethanol, amounts in RMB yuan).
4. Conclusions
In this study, an orthogonal experiment was designed and conducted to optimize ethanol production from the CBP fermentation of SPR by using a recombinant amylolytic strain, 1974-GA-temA. The effects of fermentation, initial pH, solid-to-liquid ratio, inoculation volume, exogenous enzyme addition, and metal ion supplementation were systemically investigated. Single-factor experiments indicated that the optimal pH was 4.0. An increase in the solid-to-liquid ratio corresponded with a gradual increase in the ethanol concentration. Conversely, the ethanol yield gradually decreased, with an optimal solid-to-liquid ratio of 1:5. Additionally, an increase in the inoculum size resulted in increased ethanol concentration and yield, and the optimal inoculum level was 10%. According to the results of the exogenous enzyme addition experiments, pectinase had a particularly significant effect on the ethanol concentration and yield. The highest ethanol concentration was observed when all three enzymes (cellulase, hemicellulase, and pectinase) were added simultaneously, yielding 27.27 g/L ethanol. Metal ion supplementation experiments subsequently revealed that metal ions did not significantly affect the process, except for copper ions, which increased the ethanol concentration at a specific addition amount of 0.2 g/100 g SPR. Finally, orthogonal optimization was utilized to determine the optimal combination of parameters: pH of 4.0, solid-to-liquid ratio of 1:6, and inoculation volume of 10%, along with the addition of cellulase and pectinase, while excluding Cu2+. Under these specified conditions, the strain 1974-GA-temA achieved ethanol production of 34.83 ± 0.62 g/L after a fermentation period of 8 days, corresponding to an ethanol yield of 20.90% ± 0.37%. This result significantly surpasses the outcomes observed in all previously conducted orthogonal experiments. Overall, the findings from the fermentation optimization studies of this study are anticipated to facilitate an increase in ethanol production during the CBP fermentation of SPR.
Author Contributions
Conceptualization, X.W.; Methodology, C.G., H.Z. and Y.L.; Formal analysis, C.G. and H.Z.; Investigation, X.W.; Writing—original draft, C.G.; Writing—review & editing, X.W.; Visualization, N.G.; Project administration, X.W. and J.H.; Funding acquisition, A.L., N.L., H.T. and J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key Research and Development Project of Henan Province (grant number 241111111200), the Open Competition Research Projects of Xuchang University (grant number 2022JBGS06), the Key Research and Development Project of Henan Province (grant number 231111310700), the Major Science and Technology Projects in Henan Province (grant number 231100110300), the Open Project Program of the National Engineering Research Center of Wheat and Corn Further Processing, Henan University of Technology (grant number NL2022015), and the Science and Technology Foundation of Henan Province (grant number 232102310302).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hahn-Hägerdal, B.; Galbe, M.; Gorwa-Grauslund, M.-F.; Lidén, G.; Zacchi, G. Bio-ethanol–the fuel of tomorrow from the residues of today. Trends Biotechnol. 2006, 24, 549–556. [Google Scholar] [CrossRef]
- Peplow, M. Cellulosic ethanol fights for life. Nature 2014, 507, 152–153. [Google Scholar] [CrossRef]
- Jansen, M.L.A.; Bracher, J.M.; Papapetridis, I.; Verhoeven, M.D.; de Bruijn, H.; de Waal, P.P.; van Maris, A.J.A.; Klaassen, P.; Pronk, J.T. Saccharomyces cerevisiae strains for second-generation ethanol production: From academic exploration to industrial implementation. Fems Yeast Res. 2017, 17, fox044. [Google Scholar] [CrossRef]
- Niphadkar, S.; Bagade, P.; Ahmed, S. Bioethanol production: Insight into past, present and future perspectives. Biofuels 2018, 9, 229–238. [Google Scholar] [CrossRef]
- Zabed, H.; Sahu, J.N.; Suely, A.; Boyce, A.N.; Faruq, G. Bioethanol production from renewable sources: Current perspectives and technological progress. Renew. Sustain. Energy Rev. 2017, 71, 475–501. [Google Scholar] [CrossRef]
- Favaro, L.; Jooste, T.; Basaglia, M.; Rose, S.H.; Saayman, M.; Görgens, J.F.; Casella, S.; van Zyl, W.H. Designing industrial yeasts for the consolidated bioprocessing of starchy biomass to ethanol. Bioengineered 2013, 4, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.M.; Xu, Z.X.; Liu, L.; Chen, S.T.; Wang, S.W.; Jin, M.J. Process integration for ethanol production from corn and corn stover as mixed substrates. Bioresour. Technol. 2019, 279, 10–16. [Google Scholar] [CrossRef]
- Wang, X.; Liao, B.; Li, Z.J.; Liu, G.X.; Diao, L.Y.; Qian, F.H.; Yang, J.J.; Jiang, Y.; Zhao, S.M.; Li, Y.G.; et al. Reducing glucoamylase usage for commercial-scale ethanol production from starch using glucoamylase expressing Saccharomyces cerevisiae. Bioresour. Bioprocess. 2021, 8, 20. [Google Scholar] [CrossRef]
- Atitallah, I.B.; Antonopoulou, G.; Ntaikou, I.; Alexandropoulou, M.; Nasri, M.; Mechichi, T.; Lyberatos, G. On the evaluation of different saccharification schemes for enhanced bioethanol production from potato peels waste via a newly isolated yeast strain of Wickerhamomyces anomalus. Bioresour. Technol. 2019, 289, 121614. [Google Scholar] [CrossRef]
- Nizami, A.S.; Rehan, M.; Waqas, M.; Naqvi, M.; Ouda, O.K.M.; Shahzad, K.; Miandad, R.; Khan, M.Z.; Syamsiro, M.; Ismail, I.M.I.; et al. Waste biorefineries: Enabling circular economies in developing countries. Bioresour. Technol. 2017, 241, 1101–1117. [Google Scholar] [CrossRef]
- Ntaikou, I.; Menis, N.; Alexandropoulou, M.; Antonopoulou, G.; Lyberatos, G. Valorization of kitchen biowaste for ethanol production via simultaneous saccharification and fermentation using co-cultures of the yeasts Saccharomyces cerevisiae and Pichia stipitis. Bioresour. Technol. 2018, 263, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Lyu, R.Q.; Ahmed, S.; Fan, W.J.; Yang, J.; Wu, X.Y.; Zhou, W.Z.; Zhang, P.; Yuan, L.; Wang, H.X. Engineering properties of sweet potato starch for industrial applications by biotechnological techniques including genome Editing. Int. J. Mol. Sci. 2021, 22, 9533. [Google Scholar] [CrossRef]
- Xia, J.; Shu, J.; Yao, K.; Xu, J.; Yu, X.; Xue, X.; Ma, D.; Lin, X. Synergism of cellulase, pectinase and xylanase on hydrolyzing differently pretreated sweet potato residues. Prep. Biochem. Biotechnol. 2020, 50, 181–190. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.Z.; Zhou, S.M.; Wang, T.T.Y.; Zhou, S.H.; Yang, K.L.; Li, Y.X.; Tian, J.; Wang, J. Dietary fiber isolated from sweet potato residues promotes a healthy gut microbiome profile. Food Funct. 2020, 11, 689–699. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, S.H.; Li, Y.X.; Tian, J.; Zhang, C.L. Structure, physicochemical properties and effects on nutrients digestion of modified soluble dietary fiber extracted from sweet potato residue. Food Res. Int. 2021, 150, 110761. [Google Scholar] [CrossRef] [PubMed]
- Arachchige, M.P.M.; Mu, T.; Ma, M. Structural, physicochemical and emulsifying properties of sweet potato pectin treated by high hydrostatic pressure and/or pectinase: A comparative study. J. Sci. Food Agric. 2020, 100, 4911–4920. [Google Scholar] [CrossRef] [PubMed]
- Arachchige, M.P.M.; Mu, T.H.; Ma, M.M. Effect of high hydrostatic pressure-assisted pectinase modification on the Pb2+ adsorption capacity of pectin isolated from sweet potato residue. Chemosphere 2021, 262, 128102. [Google Scholar] [CrossRef]
- Chen, X.F.; Peng, X.W.; Ma, X.Q.; Wang, J.J. Investigation of mannich reaction during co-liquefaction of microalgae and sweet potato waste. Bioresour. Technol. 2019, 284, 286–292. [Google Scholar] [CrossRef]
- Mei, X.; Mu, T.H.; Han, J.J. Composition and physicochemical properties of dietary fiber extracted from residues of 10 varieties of sweet potato by a sieving method. J. Agric. Food Chem. 2010, 58, 7305–7310. [Google Scholar] [CrossRef]
- Wang, F.Z.; Jiang, Y.; Guo, W.; Niu, K.L.; Zhang, R.Q.; Hou, S.L.; Wang, M.Y.; Yi, Y.; Zhu, C.X.; Jia, C.J.; et al. An environmentally friendly and productive process for bioethanol production from potato waste. Biotechnol. Biofuels 2016, 9, 50. [Google Scholar] [CrossRef]
- Zhu, L.L.; Mu, T.H.; Ma, M.M.; Sun, H.N.; Zhao, G.H. Nutritional composition, antioxidant activity, volatile compounds, and stability properties of sweet potato residues fermented with selected lactic acid bacteria and bifidobacteria. Food Chem. 2022, 374, 131500. [Google Scholar] [CrossRef]
- Huang, M.H.; Cheng, J.; Chen, P.; Zheng, G.W.; Wang, D.; Hu, Y.L. Efficient production of succinic acid in engineered Escherichia coli strains controlled by anaerobically-induced nirB promoter using sweet potato waste hydrolysate. J. Environ. Manag. 2019, 237, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, N.; Hu, J.; Gou, C.; Xie, X.; Zheng, H.; Liao, A.; Huang, J.; Hui, M.; Liu, N. Construction of an amylolytic Saccharomyces cerevisiae strain with high copies of α-amylase and glucoamylase genes integration for bioethanol production from sweet potato residue. Front. Microbiol. 2024, 15, 1419293. [Google Scholar] [CrossRef] [PubMed]
- Gou, C.; Wang, X.; Yu, Y.; Huang, J.; Wang, X.; Hui, M. One-step enzymatic hydrolysis of sweet potato residue after gelatinization for bioethanol production by Saccharomyces cerevisiae. Biomass Convers. Biorefinery 2024, 14, 15853–15862. [Google Scholar] [CrossRef]
- Chandel, A.K.; Garlapati, V.K.; Singh, A.K.; Antunes, F.A.F.; da Silva, S.S. The path forward for lignocellulose biorefineries: Bottlenecks, solutions, and perspective on commercialization. Bioresour. Technol. 2018, 264, 370–381. [Google Scholar] [CrossRef]
- Sakwa, L.; Cripwell, R.A.; Rose, S.H.; Viljoen-Bloom, M. Consolidated bioprocessing of raw starch with Saccharomyces cerevisiae strains expressing fungal alpha-amylase and glucoamylase combinations. Fems Yeast Res. 2018, 18, foy085. [Google Scholar] [CrossRef]
- Meredith, J. Understanding energy use and energy users in contemporary ethanol plants. In The Alcohol Textbook, 4th ed.; Nottingham University Press: Nottingham, UK, 2003; pp. 355–361. [Google Scholar]
- Favaro, L.; Basaglia, M.; Saayman, M.; Rose, S.; Van Zyl, W.; Casella, S. Engineering amylolytic yeasts for industrial bioethanol production. In Chemical Engineering Transactions; AIDIC Servizi Srl.: Milano, Italy, 2010; pp. 97–102. [Google Scholar]
- Görgens, J.F.; Bressler, D.C.; van Rensburg, E. Engineering Saccharomyces cerevisiae for direct conversion of raw, uncooked or granular starch to ethanol. Crit. Rev. Biotechnol. 2015, 35, 369–391. [Google Scholar] [CrossRef]
- Cripwell, R.A.; Rose, S.H.; Viljoen-Bloom, M.; van Zyl, W.H. Improved raw starch amylase production by Saccharomyces cerevisiae using codon optimisation strategies. Fems Yeast Res. 2019, 19, foy127. [Google Scholar] [CrossRef]
- Viktor, M.J.; Rose, S.H.; van Zyl, W.H.; Viljoen-Bloom, M. Raw starch conversion by Saccharomyces cerevisiae expressing Aspergillus tubingensis amylases. Biotechnol. Biofuels 2013, 6, 167. [Google Scholar] [CrossRef]
- Favaro, L.; Viktor, M.J.; Rose, S.H.; Viljoen-Bloom, M.; van Zyl, W.H.; Basaglia, M.; Cagnin, L.; Casella, S. Consolidated bioprocessing of starchy substrates into ethanol by industrial Saccharomyces cerevisiae strains secreting fungal amylases. Biotechnol. Bioeng. 2015, 112, 1751–1760. [Google Scholar] [CrossRef]
- Cripwell, R.A.; Rose, S.H.; Favaro, L.; van Zyl, W.H. Construction of industrial Saccharomyces cerevisiae strains for the efficient consolidated bioprocessing of raw starch. Biotechnol. Biofuels 2019, 12, 201. [Google Scholar] [CrossRef] [PubMed]
- Apiwatanapiwat, W.; Murata, Y.; Kosugi, A.; Yamada, R.; Kondo, A.; Arai, T.; Rugthaworn, P.; Mori, Y. Direct ethanol production from cassava pulp using a surface-engineered yeast strain co-displaying two amylases, two cellulases, and β-glucosidase. Appl. Microbiol. Biotechnol. 2011, 90, 377–384. [Google Scholar] [CrossRef]
- Yamada, R.; Yamakawa, S.-i.; Tanaka, T.; Ogino, C.; Fukuda, H.; Kondo, A. Direct and efficient ethanol production from high-yielding rice using a Saccharomyces cerevisiae strain that express amylases. Enzym. Microb. Technol. 2011, 48, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, J.J.; Yang, S.; Jiang, Y. Unraveling the genetic basis of fast l-arabinose consumption on top of recombinant xylose-fermenting Saccharomyces cerevisiae. Biotechnol. Bioeng. 2019, 116, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Trancone, G.; Spasiano, D.; Race, M.; Luongo, V.; Petrella, A.; Pirozzi, F.; Fratino, U.; Piccinni, A. A combined system for asbestos-cement waste degradation by dark fermentation and resulting supernatant valorization in anaerobic digestion. Chemosphere 2022, 300, 134500. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Jia, B.; Sun, X.Y.; Ai, J.Y.; Wang, L.H.; Wang, C.; Zhao, F.; Zhan, J.C.; Huang, W.D. Effect of initial pH on growth characteristics and fermentation properties of Saccharomyces cerevisiae. J. Food Sci. 2015, 80, M800–M808. [Google Scholar] [CrossRef]
- Cripwell, R.A.; Favaro, L.; Viljoen-Bloom, M.; van Zyl, W.H. Consolidated bioprocessing of raw starch to ethanol by Saccharomyces cerevisiae: Achievements and challenges. Biotechnol. Adv. 2020, 42, 107579. [Google Scholar] [CrossRef]
- Zhang, B.; Dhital, S.; Gidley, M.J. Synergistic and antagonistic effects of α-amylase and amyloglucosidase on starch digestion. Biomacromolecules 2013, 14, 1945–1954. [Google Scholar] [CrossRef]
- Yingling, B.; Li, C.; Honglin, W.; Xiwen, Y.; Zongcheng, Y. Multi-objective optimization of bioethanol production during cold enzyme starch hydrolysis in very high gravity cassava mash. Bioresour. Technol. 2011, 102, 8077–8084. [Google Scholar] [CrossRef]
- Lai, F.; Jin, Y.L.; Tan, L.; He, K.Z.; Guo, L.; Tian, X.P.; Li, J.M.; Du, A.P.; Huang, Y.H.; Zhao, H.; et al. Bioconversion of wastewater-derived duckweed to lactic acid through fed-batch fermentation at high-biomass loading. Biomass Convers. Biorefinery 2021, 13, 2745–2756. [Google Scholar] [CrossRef]
- Cruz, A.G.; Scullin, C.; Mu, C.; Cheng, G.; Stavila, V.; Varanasi, P.; Xu, D.Y.; Mentel, J.; Chuang, Y.D.; Simmons, B.A.; et al. Impact of high biomass loading on ionic liquid pretreatment. Biotechnol. Biofuels 2013, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Modenbach, A.A.; Nokes, S.E. Enzymatic hydrolysis of biomass at high-solids loadings—A review. Biomass Bioenergy 2013, 56, 526–544. [Google Scholar] [CrossRef]
- Wanderley, A.d.A.; Soares, M.; Gouveia, E. Selection of inoculum size and Saccharomyces cerevisiae strain for ethanol production in simultaneous saccharification and fermentation (SSF) of sugar cane bagasse. Afr. J. Biotechnol. 2014, 13, 2762–2765. [Google Scholar]
- Udeh, H.; Kgatla, T.; Jideani, A. Effect of mineral ion addition on yeast performance during very high gravity wort fermentation. Int. J. Bioeng. Life Sci. 2014, 8, 1208–1216. [Google Scholar]
- Shi, H.; Jiang, Y.; Yang, Y.; Peng, Y.; Li, C. Copper metabolism in Saccharomyces cerevisiae: An update. Biometals 2021, 34, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Ruta, L.L.; Popa, C.V.; Nicolau, I.; Farcasanu, I.C. Calcium signaling and copper toxicity in Saccharomyces cerevisiae cells. Environ. Sci. Pollut. Res. 2016, 23, 24514–24526. [Google Scholar] [CrossRef]
- Walker, G.M. Yeast Physiology and Biotechnology; John Wiley & Sons: Hoboken, NJ, USA, 1998. [Google Scholar]
- Zhao, X.Q.; Xue, C.; Ge, X.M.; Yuan, W.J.; Wang, J.Y.; Bai, F.W. Impact of zinc supplementation on the improvement of ethanol tolerance and yield of self-flocculating yeast in continuous ethanol fermentation. J. Biotechnol. 2009, 139, 55–60. [Google Scholar] [CrossRef]
- Casey, E.; Mosier, N.S.; Adamec, J.; Stockdale, Z.; Ho, N.; Sedlak, M. Effect of salts on the Co-fermentation of glucose and xylose by a genetically engineered strain of Saccharomyces cerevisiae. Biotechnol. Biofuels 2013, 6, 83. [Google Scholar] [CrossRef]
- Bothast, R.; Schlicher, M. Biotechnological processes for conversion of corn into ethanol. Appl. Microbiol. Biotechnol. 2005, 67, 19–25. [Google Scholar] [CrossRef]
- Robertson, G.H.; Wong, D.W.; Lee, C.C.; Wagschal, K.; Smith, M.R.; Orts, W.J. Native or raw starch digestion: A key step in energy efficient biorefining of grain. J. Agric. Food Chem. 2006, 54, 353–365. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).