Abstract
Anaerobically digested digestate is mostly used as organic fertilizer, but there is still a potential risk of heavy metal pollution. Biochar and bio-organic fertilizer through passivation can effectively reduce the mobility of heavy metal ions in soil, with the strong adsorption capacity of heavy metals, and are widely used in soil remediation. In this study, digestate as raw material supplemented with biochar was applied to simulated heavy metal-contaminated soil, and its effects on heavy metal content and the transformation of forms in soil and crop systems were systematically investigated. The application of biochar-based organic fertilizer to simulated heavy metal-contaminated soils resulted in large differences in the morphological distribution of heavy metals, which was reflected in a significant decrease in the content of heavy metals in the weakly acid-extractable state and an increase in the content of heavy metals in the residue state, and promoted the transformation of soil heavy metals from the weakly acid-extractable state to the residue state. There were differences in the accumulation of heavy metals in the above ground and below ground parts of cabbage, and Cu, Zn, Cd, and Pb were mainly accumulated in the below ground parts of the plants. The present study offers an effective methodology for the remediation of soil and plant contamination by a range of heavy metals (Cu, Zn, Cd, and Pb), from the weak acid extraction stage to the residue stage. This approach is of particular significance for the advancement of sustainable agriculture and environmental remediation.
1. Introduction
With the development of large-scale animal husbandry, a variety of elemental additives have been more and more widely used, especially in feed to add additives rich in Cu, Zn, Cd, Pb, etc. [1]. The addition of these elements can inhibit the growth of pathogenic microorganisms, promote the growth of animals, improve the appearance and color of livestock products, etc. [2]. While beneficial, heavy metal-rich additives are also harmful. Heavy metal elements are ingested by the animal with the feed, and some of these elements are absorbed through the digestive tract and accumulate in the body, which can cause the poisoning of animals in the long term [3]. The other part of the animal cannot be absorbed and is excreted. Excreta in the soil, water, and crops are a threat to the ecological environment [4]. Biogas fermentation is one of the most effective ways to achieve the harmlessness, reduction, and resourcefulness of pig manure [5]. Anaerobic fermentation has a number of raw material pre-treatment methods to mitigate environmental hazards [6]. Livestock manure undergoing biogas fermentation is mainly dominated by C-H conversion, and heavy metals will directly enter into the biogas fertilizer, which will lead to soil heavy metal pollution if applied directly without treatment [7]. Heavy metals are highly toxic and have residual properties. Long-term application will lead to a series of soil pollution problems, including soil fertility decline. In the short term, it is difficult to repair these problems naturally, which in turn affects the growth of plants in the soil [8]. The detrimental effects of heavy metal elements are largely contingent upon their mobility and bioavailability, which are in turn influenced by their morphology. Consequently, the alteration of heavy metal morphology during biogas fermentation represents a pivotal strategy for mitigating their deleterious impact [9].
The aerobic composting of digestate is a more effective way to solve the problem of the comprehensive utilization of biogas fermentation residues and prevent their secondary pollution [10]. Biogas residue contains cellulose and other difficult-to-degrade organic matter. The efficiency of aerobic composting alone is poor. Through the addition of livestock and poultry manure, conditioning agents and additives to the compost raw materials can effectively improve the effect of the aerobic composting of biogas residue decomposition [11]. It has been shown that the aerobic composting of digestate applied to the soil as an organic fertilizer improves soil porosity and organic carbon content. Organic fertilizers are therefore a superior option to chemical fertilizers and help to improve the soil environment. It is well documented that the aerobic composting of waste residues as an organic fertilizer has the effect of improving soil porosity and organic carbon content. This makes it a superior option to chemical fertilizers in terms of mitigating their negative impact. Biochar has a wide range of physico-chemical properties, such as pH, particle size distribution, structure, specific surface area, etc., which enable it to have different effects in various applications [12]. Now, the application of biochar in agriculture is mainly focused on soil improvement to increase agricultural productivity, climate change mitigation, and energy production [13]. The utilization of biomass for the reduction in heavy metals in order to facilitate the removal of pollutants from soil has recently become a prominent area of interest due to the low cost and extensive availability of this material. The by-product of corncob waste is typically employed as a food source for livestock, with any surplus incinerated. It is estimated that millions of tons of agricultural by-products, including corn cob and rice straw, are burned annually in open fields, resulting in significant environmental contamination, including air, soil, and water pollution. To enhance their utility, corncob can be employed in the production of biochar, which can then be utilized for the removal of heavy metals from soil. Biochar can be used as an ideal additive in the composting process. Biochar, with its unique physico-chemical properties, affects the temperature, EC, pH, C/N, and nutrients during aerobic composting, thus changing the micro-environmental conditions of composting, accelerating the composting process, and improving the quality of the finished compost [14]. Heavy metal passivation is an environmental remediation technology that reduces the bioavailability and mobility of heavy metals by converting them to an insoluble residue state through the addition of biochar-based organic fertilizers. The addition of biochar to compost can effectively promote the transformation of heavy metals to the oxidation and residue states, achieve the passivation of heavy metals, and achieve the fixation of heavy metals and reduce their bioavailability [15,16]. The purpose of fixing heavy metals and reducing their bioavailability is achieved. Therefore, the addition of biochar to digestate can effectively reduce the environmental risk of digestate application.
Heavy metal enrichment and the accumulation of polycyclic aromatic hydrocarbons (PAHs) in large quantities of biogas applied over a long period of time can lead to soil contamination and thus adversely affect crop quality and yield [17]. Heavy metals can be exchanged with colloidal ions and adsorbed and resolved with soil particles after entering the soil [18]. In general, heavy metals have low mobility with aqueous solutions in soil and are difficult to degrade by microorganisms. Some heavy metals are also converted to the more toxic methylated form, which enters the body along the food chain and poses a health hazard [19].
This study employs a biochar-based organic fertilizer as a remediation material for heavy metal pollution in soil. It investigates the migration and transformation of heavy metals in simulated heavy metal-polluted soil by a biochar-based organic fertilizer, with the objective of reducing the content of heavy metals in the weakly acid-extracted state. The objective is to promote the transformation of the heavy metals from the weakly acid-extracted state to the residual state, thereby providing a theoretical basis for the reasonable and effective use of biochar-based organic fertilizer for the remediation of heavy metal-contaminated soil.
2. Materials and Methods
2.1. Experimental Materials
The biogas residue was taken from Lindian Siho Township Biogas Engineering Centre of Harbin Bonang Environmental Protection Science and Technology Co. (Harbin, China). Biochar was prepared from corn cobs at Northeast Agricultural University (Harbin, China). Corn cobs were collected from the experimental base of Xiangyang Farm of Northeast Agricultural University (Harbin, China), and the collected corn cobs were crushed through an 80-mesh sieve, washed with deionized water and anhydrous ethanol to remove the impurities, and put into an oven at 105 °C to dry until constant weight. The dried corn cobs were put into a tube furnace with N2 for heating and carbonation, with a heating rate of 10 °C/min and a carbonation temperature of 600 °C, and kept for 1 h to produce corn cob biochar. It was cleaned with ultrapure water, dried, and put into a drying dish for subsequent use. The specific surface area of the biochar was measured by the nitrogen adsorption method (BET) to be 120.24 m2/g, and the particle size ranged from 0.1 to 0.5 mm. The soil was collected from the 0–20 cm layer of Haoer Village, Wuxin Township, Dulbert Mongolian Autonomous County, Daqing City, Heilongjiang Province (45°55′38″ N, 126°55′34″ E). The soil water content was 20.06%. Five 1 m × 1 m sample squares were set up evenly in the maize field at the soil extraction site, the 0–20 cm surface soil was collected from each sample square after clearing the surface crop residues, the soil from the five sample squares was mixed thoroughly, and, then, some of the soil was taken back to the house in bags. Fresh soil, after removing debris such as crop leaves, roots, and stones, was sieved through a soil sieve with a pore size of 10 mesh and set aside. The air-dried soil samples and biochar-based organic fertilizer were ground through a 10-mesh sieve and set aside. The relatively brief growth cycle of cabbage, from seed to maturity, allows for the completion of this process in a few weeks. Cabbage is adaptable to the growth environment and suitable for control under laboratory conditions, facilitating researchers’ ability to accurately control temperature, moisture, light, and other variables to ensure the accuracy of the experiment. As a common vegetable, cabbage offers a relatively rich research material, providing theoretical support and an experimental basis for the experiment. Due to the aforementioned characteristics of cabbage, we selected it as the experimental subject. In the pot experiment, choose 50 cm long, 25 cm wide, and 20 cm high nursery pots for planting cabbage experiments in kind, as shown in Figure 1.

Figure 1.
Physical picture of pot experiment.
2.2. Experimental Design
The experiment was conducted in the Laboratory of Agricultural Waste Resource Utilization Processes and Equipment at Northeast Agricultural University. In this experiment, the digestate resulting from the solid–liquid separation of residue from the anaerobic fermentation of cow dung and corn stover was employed as the primary composting material, while biochar was utilized as a composting additive at an addition ratio of 0–10%. A modest quantity of crushed corn stover and pig manure was incorporated to calibrate the initial C/N ratio of the composting feedstock to 25:1 and the moisture content to the range of 60% to 65%. A total of five treatment groups were established for the experiment. Biochar organic fertilizer was applied to four simulated heavy metal-contaminated soils in five treatment groups as shown in Table 1:

Table 1.
Five groups of treatments designed according to different biochar additions.
In order to simulate soil contamination with heavy metals (Cu, Zn, Cd, and Pb), 1 L of a 5 g/L CuSO4-5H2O solution, 1 L of a 5 g/L ZnCl2 solution, 1 L of a 10 mg/L 3CdSO4-8H2O solution, and 1 L of a 5 g/L Pb(NO3)2 solution were added to every 10 kg of soil. The solutions were manually sprayed into the soil to achieve concentrations of 500, 500, 1, and 500 mg/kg for Cu, Zn, Cd, and Pb, respectively (by weighing method). The soil samples were then adjusted with deionized water to reach 70% of the field water holding capacity, which was maintained at room temperature and protected from light for 30 days.
Each pot contains 5 kg of air-dried soil and 6000 kg·hm−2 of biochar-based organic fertilizer (i.e., 75 g per pot) [20], and the basic physical and chemical properties of the test soil and biochar-based organic fertilizer are shown in Table 2. The mineral composition of the soil is predominantly composed of oxygen (48.5%), silicon (26.2%), aluminum (7.63%), and iron (4.77%), among other elements. The mixture of biochar-based organic fertilizer and air-dried soil was mixed thoroughly. The water content of the mixed soil samples was adjusted to 75% of the field holding capacity using demonized water and kept at room temperature for 10 d to bring the system to equilibrium. Subsequently, 20 cabbage seeds were sown in each pot, and three sets of parallel experiments were set up for each treatment. The room temperature and humidity were set at 19~21 °C and 55~60%, respectively, and the soil water holding capacity was adjusted by regular watering every day. Seedlings were inter-seeded after 1 week of emergence, and 5 well-grown seedlings were retained in each pot and managed according to the field cultivation measures; the seedlings were harvested 40 d after emergence, and the plants and soil samples were taken for analyses of different forms of heavy metal contents.

Table 2.
Basic physical and chemical properties of soil and biochar-based organic fertilizer.
2.3. Heavy Metal Measurement Indicators and Methods
Following the harvesting of the cabbage, the above ground portion was severed at the soil level within the pot, while the subterranean element was extracted and rinsed with deionized water until no residual foreign matter was evident on the surface. The plant samples were then placed within an oven set to 75 °C (WHL-25, Tianjin Tester Instrument Co., Tianjin, China) The plant samples were then dried at 70 °C for 24 h until reaching a constant weight. They were subsequently ground and subjected to acid digestion using a mixture of nitric and perchloric acids. The heavy metal contents were then determined by atomic absorption spectrophotometry [21]. In order to measure the concentration of heavy metals in soil, 70 g of soil were collected in a beaker and initially digested using the aqua regia–perchloric acid method. The resulting solution was then analyzed using atomic absorption spectrophotometry [22]. The content of soil heavy metals in each form was determined by the modified European Community Bureau of Reference (BCR) sequential extraction method, and the content of heavy metals Cu, Zn, Cd, and Pb were determined according to four forms: weak acid extraction state, reduced state, oxidized state, and residual state, respectively [23]. The specific determination steps were as follows:
(1) In the weak acid extraction state, 0.50 g of the dried sample was ground through a 100-mesh sieve, 20 mL of a 0.11 mol·L−1 acetic acid solution was added, and the sample was shaken at room temperature for 16 h, centrifuged for 20 min (3000 r·min−1 ), and then fixed by a 0.22 μm filtration membrane and left to be measured.
(2) In the reduced state, 20 mL of a 0.50 mol·L−1 hydroxylamine hydrochloride solution was added to the dried solid residue from the weak acid extraction state in the previous step, which was shaken, centrifuged, filtered, and calibrated for measurement.
(3) In the oxidation state, the solid residue obtained following the previous reduction step was added to a solution of 5 mL of 8.80 mol L−1 H2O2 and left for one hour. The solid residue was then heated in a water bath at 85 °C. The sample was then heated in a water bath (HWS-80B, Beijing Hengnuo Lixing Technology Co., Beijing, China) at 85 °C for one hour. Subsequently, 5 mL of 30% H2O2 was added, and the mixture was heated until the solid residue was dissolved. Once the liquid content of the container had diminished to a negligible quantity, 25 mL of a 1.00 mol L−1 ammonium acetate solution was introduced, followed by a thorough agitation, centrifugation, filtration, and subsequent volume determination.
(4) In the residue state (H4), 5 mL of a 16.00 mol·L−1 nitric acid solution and 1 mL of a 12.00 mol·L−1 hypochlorite solution were added to the dried solid residue from the previous step in the oxidation state, and the residual solids were eliminated and separated by filtration.
The liquids to be measured in the above four steps were used for the determination of the content of Cu, Zn, Cd, and Pb in each form using flame atomic absorption spectrometry (900 Hm, PerkinElmer, Waltham, MA, USA).
2.4. Data Processing
The experimental data were presented in the form of “mean ± standard error” and were compiled using Excel 2013 software and plotted using Origin 9.0 software. Redundancy analysis (RDA) between heavy metals and physicochemical property factors after composting was carried out using Canoco 5.
In order to evaluate the impact of finished compost products on the environmental capacity, the potential pollution risk of heavy metals from finished compost products was evaluated using the heavy metal ecological risk evaluation index, which is calculated as shown in Equations (1)–(3):
where represents the heavy metal contamination coefficient, is the heavy metal content in the exchangeable, reduced, and oxidized states in the compost or product, and is the heavy metal content in the residual state in the compost or product.
where represents the potential ecological risk coefficient for each type of heavy metal, and is the biotoxicity impact factor for each type of heavy metal (Cu = 5, Pb = 5, Cd = 30, and Zn = 1).
where represents the total potential ecological risk index for heavy metals.
The plant heavy metal enrichment factor (BF) and transfer factor (TF) were calculated as shown in Equations (4) and (5):
3. Results and Discussion
3.1. Effects on Heavy Metals Cu and Pb in Soil and Plants
3.1.1. Effect on Heavy Metal Cu in Soil and Plants
The effect of biochar-based organic fertilizer on the form of soil heavy metal Cu is shown in Figure 2a. The analysis shows that biochar-based organic fertilizer can decompose to generate organic acids after being applied to the soil, which promotes the mobility of soil Cu and complexes and cheaters with heavy metal Cu through its active groups, thus affecting the effectiveness of heavy metal Cu.
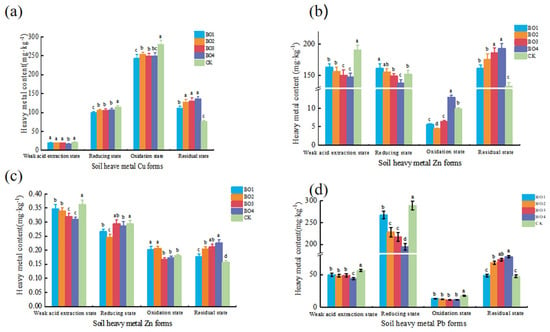
Figure 2.
(a) Effects of biochar-based organic fertilizer on heavy metal Cu speciation in soil. (b) Effects of biochar-based organic fertilizer on heavy metal Zn speciation in soil. (c) Effects of biochar-based organic fertilizer on heavy metal Cd speciation in soil. (d) Effects of biochar-based organic fertilizer on heavy metal Pb speciation in soil. The different letters denote significant differences (p > 0.05), while the same letters show no significant difference (p < 0.05) The data represent the mean ± standard deviation of three replicates.
The Cu content in the residual state decreased by 23.52% to 45.96% after the application of biochar-based organic fertilizer and increased with an increasing biochar addition. The reasons are twofold: on the one hand, the biochar in the biochar-based organic fertilizer changes the soil pH and organic matter components, which in turn promotes the tendency of the heavy metal Cu2+ to form the residue state; on the other hand, the humic acid contained in the biochar-based organic fertilizer complexed with Cu, and -COOH, -OH, and -C=O in the organic matter can promote Cu to the residue state to a certain extent through the complexion reaction. In general, the passivation mechanism of biochar-based organic fertilizer on heavy metal Cu-contaminated soil is mainly through the adsorption and buffering properties of biochar to change its morphology and bioaccumulation in soil and through the complexion reaction between wet acid and Cu2+, which is similar to that of organic materials in the transformation of heavy metals from the effective state to the ineffective state [24]. This is similar to the mechanism of organic materials in the transformation of active to inactive heavy metals, and the transformation effect was better at a 10.0% biochar addition.
Heavy metal uptake by plants is mainly through the absorption of soluble heavy metal complexes by the root system and their transport to the above ground part of the plant. The BF indicates the ability of plants to enrich heavy metals, and a larger coefficient indicates that plants are more likely to take up heavy metals from the soil, which also reflects the higher mobility of heavy metals in the plant [25]. The effects of biochar-based organic fertilizer on heavy metal Cu content and correlation coefficients in plants are shown in Table 3. Combining the results of the above ground and below ground parts, it can be seen that the adsorption and passivation of Cu in the soil by biochar reduces the effectiveness of heavy metal Cu, thus reducing the accumulation of heavy metal Cu in cabbage; biochar-based organic fertilizer is more obvious for reducing the Cu content of the above ground part of the plant, which may be due to the plant’s rhizosphere soil environment is more stable than the above ground part of the heavy metals in deposition. The above ground BF was much lower than the below ground BF in all treatments, and the difference between the treatments with biochar-based organic fertilizer and CK reached a significant level, indicating that the enrichment capacity of Cu in the stems and leaves of Cu was lower than that of the root system, mainly due to the fact that the stems and leaves mainly played the role of transporter in the process of absorbing Cu but not the role of storage and enrichment. In addition, the TF of all treatments with biochar-based organic fertilizer was significantly lower than that of CK, indicating that biochar-based organic fertilizer can effectively inhibit the transfer of heavy metal Cu from roots to above ground.

Table 3.
Effect of biochar-based organic fertilizer on heavy metal Cu content and correlation coefficient of plant.
The correlation between different forms of heavy metal Cu in soil and Cu content in plants was analyzed as shown in Figure 3a. The Cu content in plants showed a highly significant positive correlation with the Cu content in the weak acid extraction state in soil, and a significant negative correlation with the Cu content in the residue state, which indicated that lowering the Cu content in the weak acid extraction state in soil as well as increasing the Cu content in the residue state could effectively reduce the accumulation of heavy metal Cu in plants.
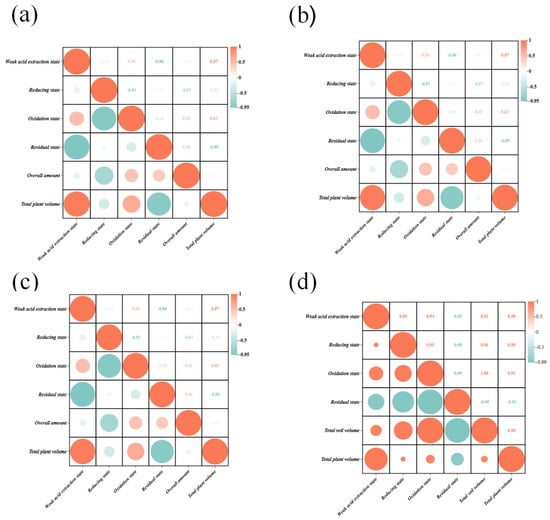
Figure 3.
(a) Correlation analysis of Cu speciation in soil and Cu content in plant. (b) Correlation analysis of Cu speciation in soil and Zn content in plant. (c) Correlation analysis of Cu speciation in soil and Zn content in plant. (d) Correlation analysis of Pb speciation in soil and Pb content in plant.
3.1.2. Effect on Heavy Metal Pb in Soil and Plants
The effects of biochar-based organic fertilizer on the form of soil heavy metal Pb are shown in Figure 2d. With the increase in the biochar addition in biochar-based organic fertilizer, the content of reduced and oxidized Pb gradually decreased in all treatments, while the content of residual Pb gradually increased. As an accumulative pollutant in soil, most of the Pb was partially adsorbed by soil colloids and particles after entering the soil, and a small part was combined with organic–inorganic compounds to form complexes [26]. Pb is an accumulated contaminant in soil. According to the relevant literature, Pb-containing compounds have low degradation freedom and solubility in soil and relatively weak migration capacity [27]; meanwhile, the results of this study showed that biochar treatment was beneficial in converting Pb to the residue state and had a significant effect on reducing the activity and effectiveness of Pb in soil. The analysis shows that the passivation of heavy metal Pb by biochar-based organic fertilizer can be attributed to the electrostatic interaction between the negative charge on the fertilizer surface and the positive charge of Pb, which is facilitated by the dissociation of some acidic functional groups in biochar-based organic fertilizer. Meanwhile, the biochar surface contains PO3–4 functional groups with which Pb2+ interacts to form the less soluble Pb5 (PO4)3 OH. In addition, after the application of biochar-based organic fertilizer to the soil, the humus of the mixed system can be adsorbed on the surface of biochar, which increases the adsorption capacity of biochar for Pb by affecting its surface charge properties. Results similar to a previous study conducted by Jiang et al. were obtained [28].
The effects of biochar-based organic fertilizer on heavy metal Pb content and correlation coefficients in plants are shown in Table 4. It can be analyzed that the addition of biochar in biochar-based organic fertilizer can help the conversion of Pb from the active state to the bound state, which not only reduces the bioavailability of Pb but also effectively inhibits the uptake of Pb from the soil by plants. The aboveground and belowground BF of all treatments showed the same trend of change, i.e., CK > BO1 > BO2 > BO3 > BO4, and the differences between the treatments of BO1 and BO2 and between the treatments of BO3 and BO4 were not significant. The differences between the treatments of BO1 and BO2 and between the treatments of BO3 and BO4 were not significant.

Table 4.
Effect of biochar-based organic fertilizer on heavy metal Pb content and correlation coefficient of plant.
Luo et al. [29] showed that the accumulation of Pb in the plant tended to be positively correlated with the soil Pb content, and the Pb accumulated by the plant increased with the increase in Pb content in the soil within a certain concentration range. The correlation analysis between different forms of soil heavy metal Pb and Pb content in plants (Figure 3d) showed that the Pb content in plants was positively correlated with the total amount of Pb in soil. In addition, there was a highly significant positive correlation between the Pb content in plants and the reduced Pb content in soil, and a highly significant negative correlation with the residual Pb content, indicating that lowering the reduced Pb content in soil and increasing the residual Pb content can effectively reduce the accumulation of heavy metal Cd in plants.
3.2. Effect on Heavy Metal Zn in Soil and Plants
The effects of biochar-based organic fertilizer on the form of soil heavy metal Zn are shown in Figure 2b. The effect of a biochar-based organic fertilizer application on the distribution of heavy metal Zn morphology in soil was specifically reflected in two aspects: on the one hand, there was a significant reduction in Zn content in the weak acid extraction state, which was reduced by 14.79% to 22.98% compared with CK; on the other hand, there was a significant increase in Zn content in the residue state, which was increased by 10.07% to 31.76% compared with CK, and the influence effect was enhanced with the proportion of the increased biochar addition. The analysis showed that biochar-based organic fertilizer could promote the transformation of soil heavy metal Zn from weak acid extraction state to residue state, thus reducing its effectiveness.
Studies have shown that the migration of Zn in soil mainly depends on the pH. When the soil is under acidic conditions, the heavy metal Zn adsorbed by clay minerals is prone to desorption, and Zn(OH)2 can be converted to Zn by the action of H2+ [25,30]. In this study, biochar-based organic fertilizer not only facilitates the conversion of various forms of Zn to the residue state but also plays a role in the fixation of heavy metal Zn and reduces the environmental risks caused by heavy metals by promoting the migration of Zn from the soil to the surface of biochar. The immobilization mechanism mainly focuses on the effect of biochar on soil pH, increasing its value ion exchange and surface complexion. In addition, Karami et al. [31] showed that the co-application of organic materials with biochar significantly improved the fixation of heavy metals in soil, while biochar alone was less effective, and biochar-based organic fertilizer composted with biochar and digestate was effective in reducing the mobility of Zn in soil in this study. In conclusion, the effect of biochar-based organic fertilizer on heavy metal Zn in soil is a combined effect of physical, chemical, and biological actions.
Excessive Zn can injure the root system of the plant and inhibit root growth. The effects of biochar-based organic fertilizer on heavy metal Zn content and the correlation coefficients of plants are shown in Table 5. With the increase in the proportion of the biochar addition, the total Zn in plants showed a decreasing trend, which was reduced by 14.76%, 34.34%, 43.85%, and 53.49% in each treatment, respectively. At the same time, the application of biochar-based organic fertilizer reduced the Zn content in both above ground and below ground parts of Brassica napus, mainly because the addition of biochar made the biochar-based organic fertilizer reduces the content of Zn in the effective state in the soil by adsorption, ion exchange, complexion, and fixation after being applied to the soil and then reduced the accumulation of Zn in the plants.

Table 5.
Effect of biochar-based organic fertilizer on heavy metal Zn content and correlation coefficient of plant.
Although biochar-based organic fertilizer can reduce the content of Cu and Zn in plants, the content of Zn is higher than that of Cu. This is due to the fact that the weak acid exchange state of the soil heavy metal Zn accounts for more than that of Cu and is easily absorbed by plants when Zn is dominated in the soil by Zn2+; moreover, plants and soil organisms can aggregate Zn from the deeper soil to the surface layer under the biochemical action. The enrichment coefficients of Zn in all parts of Brassica napus after the application of biochar-based organic fertilizer showed a gradual decrease with the addition of biochar, indicating that the application of biochar-based organic fertilizer in the presence of biochar can significantly reduce the enrichment of Zn in the plant. Compared with CK, the TFs of BO1~BO4 were reduced by 9.87%, 13.04%, 9.25%, and 9.40%, respectively, indicating that the application of biochar-based organic fertilizer could inhibit the transfer of soil Zn from the roots to the stems and leaves of Brassica napus, which may be due to the downward migration of Zn leaching from the surface layer of the soil and the return of Zn to the surface layer of the soil under the action of biochar and soil organisms and plant focus.
The correlation analysis between various forms of heavy metal Zn in soil and Zn content in plants is shown in Figure 3b. The Zn content in plants showed a significant positive correlation with the Zn content in the weak acid-extracted state in soil and a highly significant negative correlation with the Zn content in the residue state, indicating that increasing the Zn content in the residue state and decreasing the Zn content in the weak acid-extracted state in soil can effectively reduce the accumulation of heavy metal Zn in plants.
3.3. Effect on Heavy Metal Cd in Soil and Plants
The effects of biochar-based organic fertilizer on the form of soil heavy metal Cd are shown in Figure 2c. Overall, there were differences in the morphological distribution of Cd in the soil with different biochar additions, mainly dominated by the weak acid extractive state and the reduced state. The weak acid extractive state Cd contents of the treatments with the biochar addition were all significantly lower than that of CK and decreased more and more with the increase in the addition amount, indicating that the biochar-based organic fertilizer can effectively reduce the environmental hazards of Cd and its activity after being applied to the soil. Compared with CK, the content of reduced Cd was reduced in all treatments, with the greatest reduction in BO2. The residual state Cd content increased by 9.26%, 26.54%, 30.86%, and 39.51% in all treatments with the addition of biochar, respectively, and all of them reached the level of significant difference with CK. Differences in the nature of functional groups on the surface of biochar (oxygen content and volatile organic matter) are the main factors determining the adsorption and sequestration of the heavy metal Cd2+, and the results of previous studies have shown that Cd was significantly immobilized by the application of biochar [32,33].
In this study, biochar-based organic fertilizer can significantly reduce the bioefficacy of Cd in soil, and the higher the biochar addition, the better the reduction effect. The reason is that the surface of biochar has a certain number of acidic (-OH and -COOH) and basic (e.g., π = π and -NH2) functional groups, which are positively or negatively charged after release with the change in soil pH. The pH of the test soil is 6.48, which is lower than the zero electric point of biochar to make it positively charged. Most of the Cd in the soil is concentrated in the surface layer of the soil, and Cd2+ and CdCl+ are the main forms of Cd in the soil solution. The biochar-based organic fertilizer will repel the above cations due to the inclusion of positively charged biochar, which explains its role in improving the residual state of Cd in the soil. Comprehensive analysis shows that the passivation mechanism of Cd by biochar-based organic fertilizer is mainly reflected in the formation of hydroxide precipitates or salts from soil Cd2+ to increase the active sites on the soil surface and the electrostatic interaction between the surface charge of biochar and Cd in the soil. In addition, Cd forms specific complexes with the surface functional groups of biochar-based organic fertilizer, which reduces the bioefficacy of biochar-based organic fertilizer by passivating Cd.
The effects of biochar-based organic fertilizer on heavy metal Cd content and correlation coefficients in plants are shown in Table 6. Compared with CK, the above ground Cd content of cabbage in BO1~BO4 treatments was reduced in the range of 8.89~35.17%, the below ground Cd content was reduced in the range of 7.31~54.13%, and the lowering effect was more significant with the increase in the biochar addition. The reason is that the absorption and accumulation of Cd in plants are mainly affected by the content, form, and activity of Cd in soil, the form of Cd determines the basis of its effectiveness to plants, and the water-soluble and ionic states of Cd are easily absorbed by the roots of plants and produce the accumulation of Cd in various parts of plants [34]. The previous results showed that the addition of biochar significantly reduced the concentration of Cd in the weakly acid-extractable state in the soil, and, therefore, the uptake of Cd by the plant showed a decreasing trend. All biochar-based organic fertilizer treatments had a significant effect on reducing the above ground and below ground enrichment coefficients, and the most significant reduction was achieved when the biochar addition was 10.0%, which was 35.32% and 54.09%, respectively, compared with CK.

Table 6.
Effect of biochar-based organic fertilizer on heavy metal Cd content and correlation coefficient of plant.
The correlation between different forms of heavy metal Cd in soil and Cd content in plants was analyzed as shown in Figure 3c, and the Cd content in plants showed a highly significant positive correlation with the Cd content in the weak acid extraction state in soil and a significant negative correlation with the Cd content in the residue state, which indicated that lowering the Cd content in the weak acid extraction state in soil as well as increasing the Cd content in the residue state could effectively reduce the accumulation of heavy metal Cd in plants.
3.4. Environmental Risk Assessment for Heavy Metals
Using the Potential Ecological Hazard Index Method, the evaluation of environmental risks of heavy metals after a biochar-based organic fertilizer application is shown in Table 7. The individual potential ecological risk coefficients as well as potential ecological risk indices of Cu, Zn, Cd, and Pb were significantly lower in the biochar-based organic fertilizer treatment compared with CK, and the respective coefficients and indices showed a gradual decreasing trend with the increase in the biochar addition. The results of the potential ecological risk assessment took into account the general transport and transformation of heavy metal toxicity in soil sediment and the sensitivity of heavy metal pollution to the evaluation area, and the analyses showed that the addition of biochar could significantly reduce the degree of potential hazards of heavy metals to the surface soil.

Table 7.
Potential ecological risk assessment of heavy metals after application of biochar-based organic fertilizer.
4. Conclusions
The objective of this study was to investigate the transformation pattern and passivation mechanism of biochar on various forms of heavy metals in soil following heavy metal pollution. To this end, potting experiments were conducted with different additive ratios of biochar in biochar-based organic fertilizers. The following conclusions were drawn from this study:
Compared with CK, the application of biochar-based organic fertilizer could significantly reduce the content of Cu, Zn, Cd, and Pb in the weak acid extraction state of the soil and promote its transformation from the weak acid extraction state to the residue state, and the higher the proportion of biochar added, the more obvious reduction in the bioefficacy of the heavy metal ions. The BO1~BO4 treatments significantly reduced the uptake of heavy metals in all parts of Brassica napus, and the different amounts of biochar added were significant for the effect of uptake. The differences were significant. The results of the correlation analysis showed that the contents of heavy metals in the weakly acid-extractive and reducing states in the soil were significantly and positively correlated with the accumulation of heavy metals in the plants. The potential ecological risk assessment coefficients of heavy metals gradually decreased with the increase in the biochar addition.
Overall, biochar-based organic fertilizer treatments significantly reduced the uptake of Cu, Zn, Cd, and Pb in the above ground and below ground parts of Brassica napus, and there were significant differences in the reduction effects of different biochar additions. The plant transfer coefficients of the four heavy metals were roughly Cd > Zn > Cu > Pb. The results obtained in this study were analogous to those reported in Qian’s study [35].
Biochar-based organic fertilizers could reduce the accumulation of the four heavy metals in the plant by reducing the weakly acid-extractable states of Cd, Zn, and Cu, and the reduced state of Pb, which were all present in the soil state. In other words, biochar-based organic fertilizer can effectively reduce the bio-efficacy of heavy metals in heavy metal-contaminated soil.
This study provides a theoretical basis for the rational and effective use of biochar-based organic fertilizer for the remediation of heavy metal-contaminated soils and the reduction in the risk of heavy metals in soils entering the human body along the food chain. The biochar-based organic fertilizer in this study was demonstrated to enhance the abundance of functional soil microorganisms and the multifunctionality of agro-ecosystems [36]. The biochar-based organic fertilizer proposed in this paper has the potential for widespread applications in soil improvement. As this study employs a pot experiment in a laboratory setting to simulate heavy metal contamination, it may not be sufficiently comprehensive. Therefore, future studies should be conducted in a field environment over an extended period of time.
Author Contributions
Conceptualization, Z.W.; Methodology, J.Q. and Y.C.; Validation, X.Q. and J.L.; Data curation, F.W. and S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (52206234), the China Postdoctoral Science Foundation (2023MD734138), the Natural Science Foundation of Heilongjiang Province of China for Excellent Youth Scholars (YQ2022E005), the Heilongjiang Province Postdoctoral Science Foundation (LBH-Z22006), the Technological Project of Heilongjiang Province “the open competition mechanism to select the best candidates” (2022ZXJ05C01-06), and the Academic Backbone Project of Northeast Agricultural University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, Y.; Xu, Y.; Yu, X.; Li, J.; Chen, G.; Wang, S.; Xu, Y.; Xu, R.; Zhang, B.; Zhang, H. Microbial metabolism and humic acid formation in response to enhanced copper and zinc passivation during composting of wine grape pomace and pig manure. Bioresour. Technol. 2023, 384, 129226. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, H.; Su, G.; Chen, J. Heavy metal distribution profiles in soil and groundwater near pig farms in China. Chemosphere 2022, 294, 133721. [Google Scholar] [CrossRef]
- Luo, M.; Zhou, D.-D.; Shang, A.; Gan, R.-Y.; Li, H.-B. Influences of food contaminants and additives on gut microbiota as well as protective effects of dietary bioactive compounds. Trends Food Sci. Technol. 2021, 113, 180–192. [Google Scholar] [CrossRef]
- Zuševica, A.; Adamovičs, A.; Dūmiņš, K.; Vendiņa, V.; Žīgure, S.; Lazdina, D. Soil Fertility Improvement with Mixtures of Wood Ash and Biogas Digestates Enhances Leaf Photosynthesis and Extends the Growth Period for Deciduous Trees. Plants 2023, 12, 1152. [Google Scholar] [CrossRef]
- Qu, Y.; Miao, X.; Chen, S.; Qin, N.; Li, J.; Che, Y.; Luo, L.; Sun, Y. Novel insights into the mechanism of dynamic changes in microstructure and physicochemical properties of corn straw pretreated by ball milling and feasibility analysis of anaerobic digestion. Sci. Total Environ. 2024, 945, 173943. [Google Scholar] [CrossRef]
- Qu, Y.; Lv, X.; Qin, N.; Zhang, K.; Ding, X.; Luo, L.; Qu, J.; Sun, Y. Mechanism of ball milling pretreatment to improve the anaerobic digestion performance and energy conversion efficiency of corn straw. Fuel 2024, 366, 131409. [Google Scholar] [CrossRef]
- Helaoui, S.; Boughattas, I.; Mkhinini, M.; Chebbi, L.; Elkribi-Boukhris, S.; Alphonse, V.; Livet, A.; Banni, M.; Bousserrhine, N. Biochar amendment alleviates heavy metal phytotoxicity of Medicago sativa grown in polymetallic contaminated soil: Evaluation of metal uptake, plant response and soil properties. Plant Stress 2023, 10, 100212. [Google Scholar] [CrossRef]
- Awad, M.; El-Sayed, M.M.; Li, X.; Liu, Z.; Mustafa, S.K.; Ditta, A.; Hessini, K. Diminishing Heavy Metal Hazards of Contaminated Soil via Biochar Supplementation. Sustainability 2021, 13, 12742. [Google Scholar] [CrossRef]
- Czekała, W.; Nowak, M.; Piechota, G. Sustainable management and recycling of anaerobic digestate solid fraction by composting: A review. Bioresour. Technol. 2023, 375, 128813. [Google Scholar] [CrossRef]
- Wang, J.; Pan, J.; Ma, X.; Li, S.; Chen, X.; Liu, T.; Wang, Q.; Wang, J.J.; Wei, D.; Zhang, Z.; et al. Solid digestate biochar amendment on pig manure composting: Nitrogen cycle and balance. Bioresour. Technol. 2022, 349, 126848. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, C.; Holm, P.E.; Hansen, H.C.B.; Brandt, K.K. Impacts of biochar materials on copper speciation, bioavailability, and toxicity in chromated copper arsenate polluted soil. J. Hazard. Mater. 2023, 459, 132067. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, H.; He, S.; Zhao, Q.; Wei, L. A review of biochar in anaerobic digestion to improve biogas production: Performances, mechanisms and economic assessments. Bioresour. Technol. 2021, 341, 125797. [Google Scholar] [CrossRef]
- Behera, S.; Samal, K. Sustainable approach to manage solid waste through biochar assisted composting. Energy Nexus 2022, 7, 100121. [Google Scholar] [CrossRef]
- Li, J.; Xia, C.; Cheng, R.; Lan, J.; Chen, F.; Li, X.; Li, S.; Chen, J.; Zeng, T.; Hou, H. Passivation of multiple heavy metals in lead–zinc tailings facilitated by straw biochar-loaded N-doped carbon aerogel nanoparticles: Mechanisms and microbial community evolution. Sci. Total Environ. 2022, 803, 149866. [Google Scholar] [CrossRef]
- Debode, J.; Ebrahimi, N.; D’Hose, T.; Cremelie, P.; Viaene, N.; Vandecasteele, B. Has compost with biochar added during the process added value over biochar or compost to increase disease suppression? Appl. Soil Ecol. 2020, 153, 103571. [Google Scholar] [CrossRef]
- Pastorelli, R.; Valboa, G.; Lagomarsino, A.; Fabiani, A.; Simoncini, S.; Zaghi, M.; Vignozzi, N. Recycling Biogas Digestate from Energy Crops: Effects on Soil Properties and Crop Productivity. Appl. Sci. 2021, 11, 750. [Google Scholar] [CrossRef]
- Liang, W.; Wang, G.; Peng, C.; Tan, J.; Wan, J.; Sun, P.; Li, Q.; Ji, X.; Zhang, Q.; Wu, Y.; et al. Recent advances of carbon-based nano zero valent iron for heavy metals remediation in soil and water: A critical review. J. Hazard. Mater. 2022, 426, 127993. [Google Scholar] [CrossRef] [PubMed]
- Bat, L.; Yardım, Ö.; Öztekin, A.; Arıcı, E. Assessment of heavy metal concentrations in Scophthalmus maximus (Linnaeus, 1758) from the Black Sea coast: Implications for food safety and human health. J. Hazard. Mater. Adv. 2023, 12, 100384. [Google Scholar] [CrossRef]
- Anițaș, S.; Coman, M.; Cioruța, V.B. Some Considerations Regarding the Presence of Heavy Metals in Soil and the Human Body. Asian Soil Res. J. 2020, 39–46. [Google Scholar] [CrossRef]
- Li, K.; Yang, H.; Yuan, X.; Zhang, M. Recent developments of heavy metals detection in traditional Chinese medicine by atomic spectrometry. Microchem. J. 2021, 160, 105726. [Google Scholar] [CrossRef]
- Custodio, M.; Peñaloza, R.; Cuadrado, W.; Ochoa, S.; Álvarez, D.; Chanamé, F. Data on the detection of essential and toxic metals in soil and corn and barley grains by atomic absorption spectrophotometry and their effect on human health. Chem. Data Collect. 2021, 32, 100650. [Google Scholar] [CrossRef]
- Yuan, X.; Xiong, T.; Wang, H.; Wu, Z.; Jiang, L.; Zeng, G.; Li, Y. Immobilization of heavy metals in two contaminated soils using a modified magnesium silicate stabilizer. Environ. Sci. Pollut. Res. 2018, 25, 32562–32571. [Google Scholar] [CrossRef]
- Gonzaga, M.I.S.; Mackowiak, C.; de Almeida, A.Q.; Wisniewski, A.; de Souza, D.F.; Lima, I.d.S.; de Jesus, A.N. Assessing biochar applications and repeated Brassica juncea L. production cycles to remediate Cu contaminated soil. Chemosphere 2018, 201, 278–285. [Google Scholar] [CrossRef]
- Ahmad, K.; Khan, Z.I.; Yasmin, S.; Ashfaq, A.; Noorka, I.R.; Akram, N.A.; Shad, H.A.; Hussain, A.; Arshad, F.; Sher, M.; et al. Contamination of soil and carrots irrigated with different sources of water in Punjab, Pakistan. Environ. Earth Sci. 2016, 75, 426. [Google Scholar] [CrossRef]
- Twining, J.R.; Payne, T.E.; Itakura, T. Soil-water distribution coefficients and plant transfer factors for 134Cs, 85Sr and 65Zn under field conditions in tropical Australia. J. Environ. Radioact. 2004, 71, 71–87. [Google Scholar] [CrossRef]
- Lu, H.; Yang, Y.; Huang, K.; Huang, G.; Hu, S.; Pan, D.; Liu, T.; Li, X. Transformation kinetics of exogenous lead in an acidic soil during anoxic-oxic alteration: Important roles of phosphorus and organic matter. Environ. Pollut. 2023, 335, 122271. [Google Scholar] [CrossRef] [PubMed]
- Li, X.X.; Huang, S.; McBride, M.B. Rhizosphere effect on Pb solubility and phytoavailability in Pb-Contaminated soils. Environ. Pollut. 2021, 268, 115840. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.-Y.; Jiang, J.; Xu, R.-K.; Li, Z. Adsorption of Pb(II) on variable charge soils amended with rice-straw derived biochar. Chemosphere 2012, 89, 249–256. [Google Scholar] [CrossRef]
- Luo, L.; Shen, Y.; Liu, J.; Zeng, Y. Investigation of Pb species in soils, celery and duckweed by synchrotron radiation X-ray absorption near-edge structure spectrometry. Spectrochim. Acta Part B-At. Spectrosc. 2016, 122, 40–45. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Z.; Yuan, X.; Browne, P.; Chen, L.; Ji, J. The influences of soil properties on Cu and Zn availability in soil and their transfer to wheat (Triticum aestivum L.) in the Yangtze River delta region, China. Geoderma 2013, 193, 131–139. [Google Scholar] [CrossRef]
- Karami, N.; Clemente, R.; Moreno-Jiménez, E.; Lepp, N.W.; Beesley, L. Efficiency of green waste compost and biochar soil amendments for reducing lead and copper mobility and uptake to ryegrass. J. Hazard. Mater. 2011, 191, 41–48. [Google Scholar] [CrossRef]
- Uchimiya, M.; Chang, S.; Klasson, K.T. Screening biochars for heavy metal retention in soil: Role of oxygen functional groups. J. Hazard. Mater. 2011, 190, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Li, L.; Zhang, A.; Pan, G.; Bao, D.; Chang, A. Biochar amendment greatly reduces rice Cd uptake in a contaminated paddy soil: A two-year field experiment. Bioresources 2011, 6, 2605–2618. [Google Scholar] [CrossRef]
- Rafique, M.; Ortas, I.; Rizwan, M.; Sultan, T.; Chaudhary, H.J.; Işik, M.; Aydin, O. Effects of Rhizophagus clarus and biochar on growth, photosynthesis, nutrients, and cadmium (Cd) concentration of maize (Zea mays) grown in Cd-spiked soil. Environ. Sci. Pollut. Res. 2019, 26, 20689–20700. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Mei, C.; Li, T.; Luo, W.; Liu, W.; Chen, M.; Yang, X.; Li, X.; Cheng, B.; Ma, H. A versatile biochar fertilizer used for adsorption of heavy metals and enhancement of plant growth in metal contaminated soil. Environ. Technol. Innov. 2024, 36, 103743. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, Y.; Rong, X.; Zhou, X.; Fei, J.; Peng, J.; Luo, G. Biochar and organic fertilizer applications enhance soil functional microbial abundance and agroecosystem multifunctionality. Biochar 2024, 6, 3. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).