Turmeric-Enriched Yogurt: Increased Antioxidant and Phenolic Contents
Abstract
1. Introduction
2. Materials and Methods
2.1. Production of Yogurt
2.2. Physicochemical Properties
2.3. Extraction of Bioactive Components
2.4. Antioxidant Activity Assay
2.5. Total Phenolic Content Analysis
2.6. Evaluation of Gastric pH and Bile Salt Tolerance
2.7. Antibacterial Activity
2.8. Enumeration of Yogurt Bacteria
2.9. Sensory Evaluation
2.10. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of Yogurt Samples
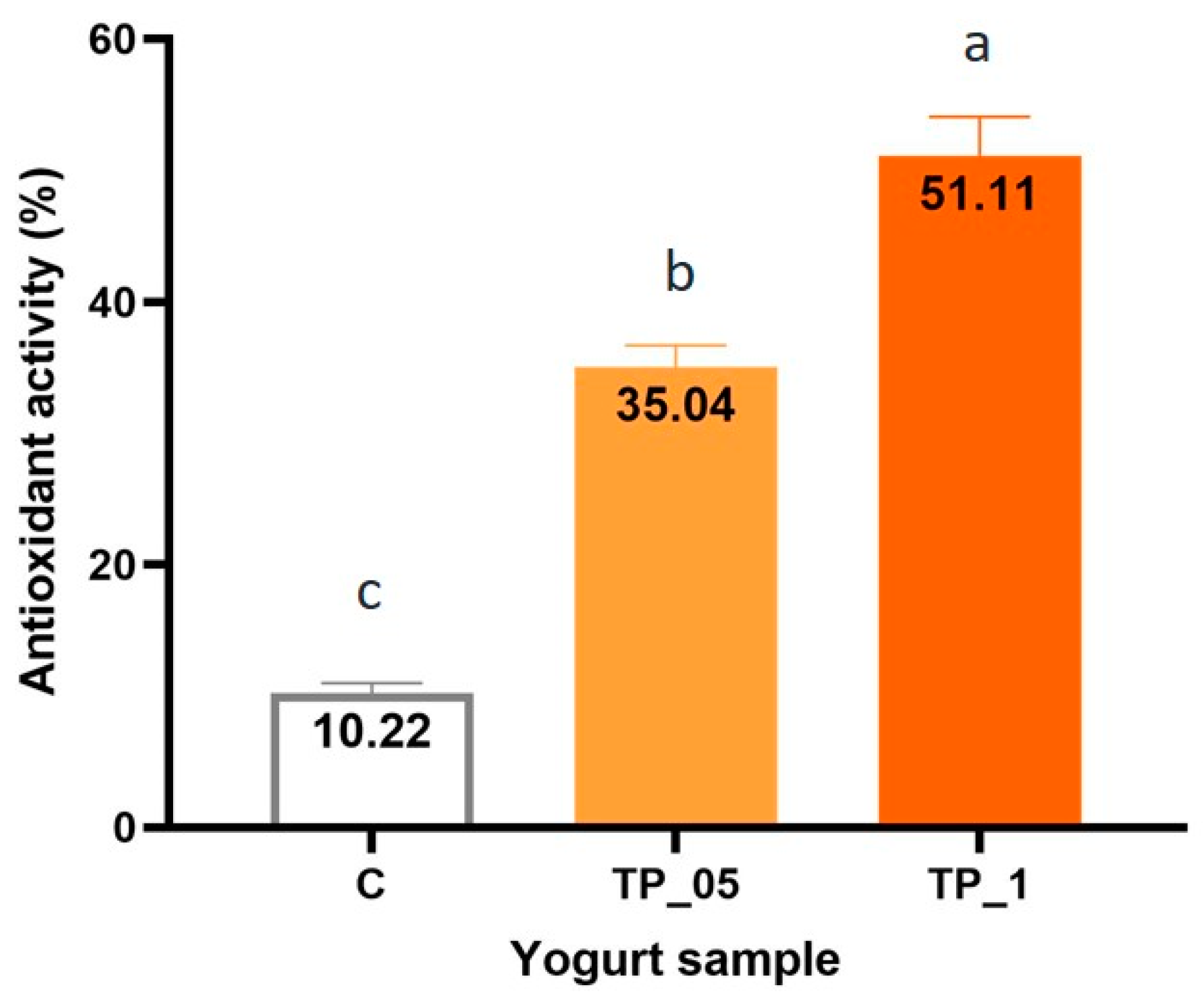
3.2. Antioxidant Activity of Yogurt Samples
3.3. Total Phenolic Content of Yogurt Samples
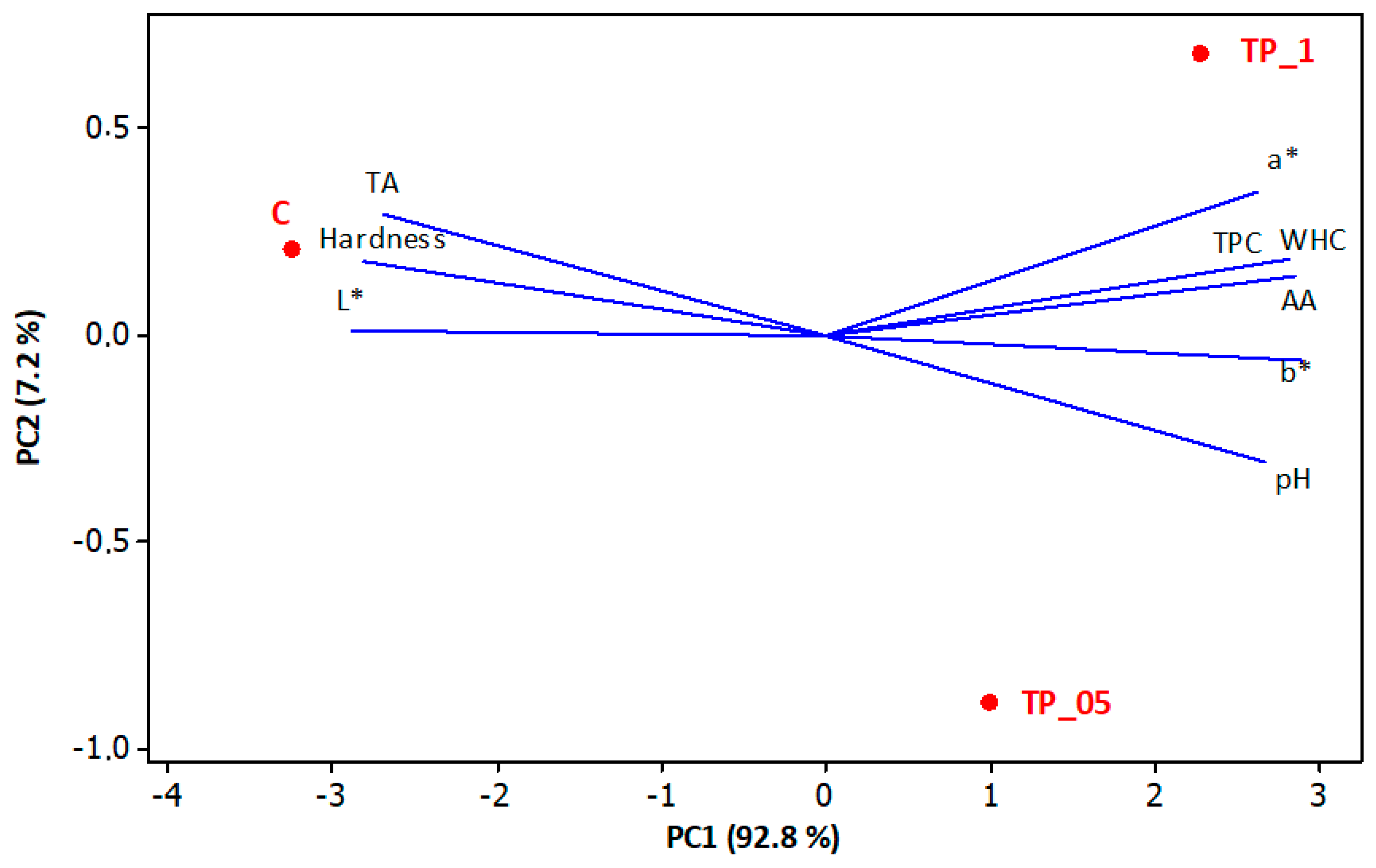
3.4. Principle Component Analysis (PCA) of Some Physicochemical and Bioactive Properties of Yogurt
3.5. Gastric pH and Bile Salt Tolerance of Bioactive Compounds
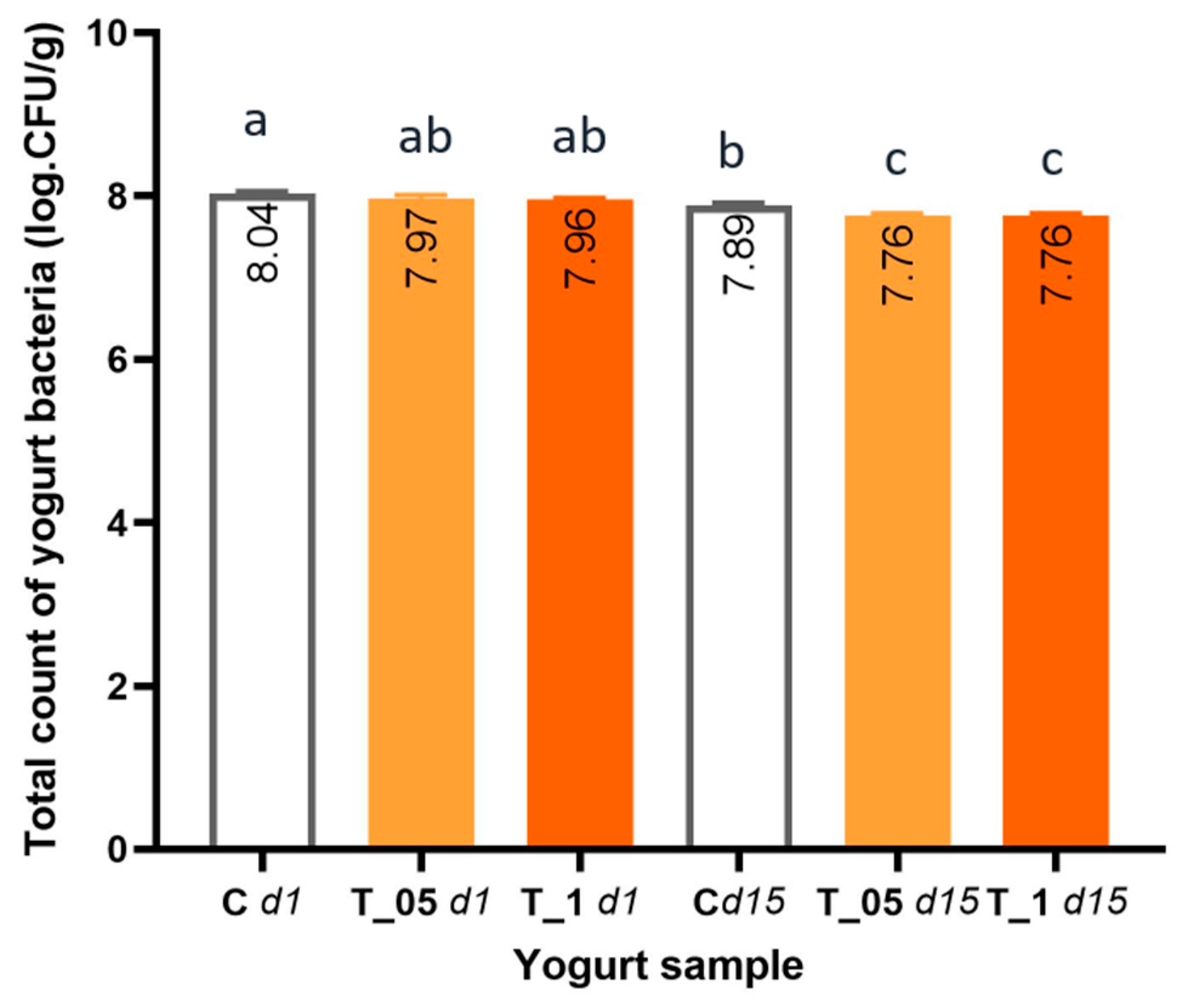
3.6. Antibacterial Activity and Yogurt Bacteria Counts of Samples
3.7. Sensory Attributes
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kok, C.R.; Hutkins, R. Yogurt and Other Fermented Foods as Sources of Health-Promoting Bacteria. Nutr. Rev. 2018, 76, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Adolfsson, O.; Meydani, S.N.; Russell, R.M. Yogurt and Gut Function. Am. J. Clin. Nutr. 2004, 80, 245–256. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Oniszczuk, T.; Gancarz, M.; Szymańska, J. Role of Gut Microbiota, Probiotics and Prebiotics in the Cardiovascular Diseases. Molecules 2021, 26, 1172. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Sun, D. Consumption of Yogurt and the Incident Risk of Cardiovascular Disease: A Meta-Analysis of Nine Cohort Studies. Nutrients 2017, 9, 315. [Google Scholar] [CrossRef] [PubMed]
- Mann, B.; Athira, S.; Sharma, R.; Bajaj, R. Bioactive Peptides in Yogurt. In Yogurt in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2017; pp. 411–426. [Google Scholar]
- Guiné, R.P.F.; De Lemos, E.T. Development of New Dairy Products with Functional Ingredients. J. Culin. Sci. Technol. 2020, 18, 159–176. [Google Scholar] [CrossRef]
- Pădureţ, S.; Ghinea, C.; Prisacaru, A.E.; Leahu, A. Physicochemical, Textural, and Antioxidant Attributes of Yogurts Supplemented with Black Chokeberry: Fruit, Juice, and Pomace. Foods 2024, 13, 3231. [Google Scholar] [CrossRef]
- Silva, F.A.; Queiroga, R.C.R.D.E.; de Souza, E.L.; Voss, G.B.; Borges, G.D.S.C.; Lima, M.D.S.; Pintado, M.M.E.; Vasconcelos, M.A.D.S. Incorporation of Phenolic-Rich Ingredients from Integral Valorization of Isabel Grape Improves the Nutritional, Functional and Sensory Characteristics of Probiotic Goat Milk Yogurt. Food Chem. 2022, 369, 130957. [Google Scholar] [CrossRef]
- Paul, S.K.K.; Islam, M.N.; Dewan, M.F.; Alim, M.A.; Ahmmed, R. Functional Yogurt: An Approach to Enhance Yogurt Quality with Peanut Polyphenols. Food Biosci. 2024, 60, 104398. [Google Scholar] [CrossRef]
- Kocaadam, B.; Şanlier, N. Curcumin, an Active Component of Turmeric (Curcuma longa), and Its Effects on Health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef]
- Mishra, S.; Palanivelu, K. The Effect of Curcumin (Turmeric) on Alzheimer′s Disease: An Overview. Ann. Indian. Acad. Neurol. 2008, 11, 13–19. [Google Scholar] [CrossRef]
- Unlu, A.; Nayir, E.; Kalenderoglu, M.D.; Kirca, O.; Ozdogan, M. Curcumin (Turmeric) and Cancer. JBUON 2016, 21, 1050–1060. [Google Scholar] [PubMed]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I Clinical Trial of Oral Curcumin. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef]
- Santos, J.; Wang, R.; Bhakta, V.; Driver, Z.; Vadim, Y.; Kiritoshi, T.; Ji, G.; Neugebauer, V.; Shen, C.-L. Turmeric Bioactive Compounds Alleviate Spinal Nerve Ligation-Induced Neuropathic Pain by Suppressing Glial Activation and Improving Mitochondrial Function in Spinal Cord and Amygdala. Nutrients 2023, 15, 4403. [Google Scholar] [CrossRef]
- Lahiff, C.; Moss, A.C. Curcumin for Clinical and Endoscopic Remission in Ulcerative Colitis. Inflamm. Bowel Dis. 2011, 17, E66. [Google Scholar] [CrossRef]
- Holt, P.R.; Katz, S.; Kirshoff, R. Curcumin Therapy in Inflammatory Bowel Disease: A Pilot Study. Dig. Dis. Sci. 2005, 50, 2191–2193. [Google Scholar] [CrossRef] [PubMed]
- Buniowska-Olejnik, M.; Urbański, J.; Mykhalevych, A.; Bieganowski, P.; Znamirowska-Piotrowska, A.; Kačániová, M.; Banach, M. The Influence of Curcumin Additives on the Viability of Probiotic Bacteria, Antibacterial Activity against Pathogenic Microorganisms, and Quality Indicators of Low-Fat Yogurt. Front. Nutr. 2023, 10, 1118752. [Google Scholar] [CrossRef]
- Shalaby, S.M.; Amin, H.H. Red Cabbage and Turmeric Extracts as Potential Natural Colors and Antioxidants Additives in Stirred Yogurt. J. Probiotics Health 2018, 6, 1000206. [Google Scholar] [CrossRef]
- Li, Z.; Shi, M.; Li, N.; Xu, R. Application of Functional Biocompatible Nanomaterials to Improve Curcumin Bioavailability. Front. Chem. 2020, 8, 589957. [Google Scholar] [CrossRef]
- Papillo, V.A.; Arlorio, M.; Locatelli, M.; Fuso, L.; Pellegrini, N.; Fogliano, V. In Vitro Evaluation of Gastro-Intestinal Digestion and Colonic Biotransformation of Curcuminoids Considering Different Formulations and Food Matrices. J. Funct. Foods 2019, 59, 156–163. [Google Scholar] [CrossRef]
- Mimica, B.; Bučević Popović, V.; Banjari, I.; Jeličić Kadić, A.; Puljak, L. Methods Used for Enhancing the Bioavailability of Oral Curcumin in Randomized Controlled Trials: A Meta-Research Study. Pharmaceuticals 2022, 15, 939. [Google Scholar] [CrossRef]
- Cheng, A.; Hsu, C.; Lin, J.; Hsu, M.; Shen, T.; Ko, J.; Lin, J.; Lin, B.; Ming-Shiang, W.; Yu, S.; et al. Phase I Clinical Trial of Curcumin, a Chemopreventive Agent, in Patients with High-Risk or Pre-Malignant Lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar] [PubMed]
- Lao, C.D.; Ruffin, M.T.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose Escalation of a Curcuminoid Formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; Horwitz, W., Ed.; AOAC: Rockville, MD, USA, 2000. [Google Scholar]
- Gomes, E.R.; Barroso dos Anjos Pinto, C.; Stephani, R.; Fernandes de Carvalho, A.; Perrone, Í.T. Effect of Adding Different Types of Soluble Fibre to High-Protein Yoghurts on Water Holding Capacity, Particle Size Distribution, Apparent Viscosity, and Microstructure. Int. Dairy. J. 2023, 141, 105609. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant Activity and Phenolic Compounds in 32 Selected Herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Jeong, C.H.; Ryu, H.; Zhang, T.; Lee, C.H.; Seo, H.G.; Han, S.G. Green Tea Powder Supplementation Enhances Fermentation and Antioxidant Activity of Set-Type Yogurt. Food Sci. Biotechnol. 2018, 27, 1419–1427. [Google Scholar] [CrossRef]
- Sıçramaz, H.; Ayar, A. Investigation of the Effects of Different Processing Methods on the Selected Nutritional Properties of Pumpkin and Determining the Appropriate Process for Pumpkin Yogurt. Food Sci. Nutr. 2023, 11, 6878–6887. [Google Scholar] [CrossRef]
- Izzo, L.; Castaldo, L.; Lombardi, S.; Gaspari, A.; Grosso, M.; Ritieni, A. Bioaccessibility and Antioxidant Capacity of Bioactive Compounds From Various Typologies of Canned Tomatoes. Front. Nutr. 2022, 9, 849163. [Google Scholar] [CrossRef] [PubMed]
- Bonev, B.; Hooper, J.; Parisot, J. Principles of Assessing Bacterial Susceptibility to Antibiotics Using the Agar Diffusion Method. J. Antimicrob. Chemother. 2008, 61, 1295–1301. [Google Scholar] [CrossRef]
- International Standard ISO 7889 (IDF 117); Yogurt—Enumeration of Characteristic Microorganisms—Colony Count Technique at 37 °C. ISO: Geneva, Switzerland, 2003.
- Karagül-Yüceer, Y.; Drake, M. Sensory Analysis of Yogurt. In Manufacturing Yogurt and Fermented Milks; Wiley: New York, NY, USA, 2006; pp. 265–278. [Google Scholar]
- Rahmatalla, S.A.; Aazeem, L.A.; Abdallah, M. Quality Evaluation of Set Yoghurt Supplemented with Turmeric Powder (Curcoma longa L.) during Storage Period. In Proceedings of the Tropentag, International Research on Food Security, Natural Resource Management and Rural Development, Prague, Czech Republic, 17–19 September 2014; Eric, T., Ed.; p. 399. [Google Scholar]
- Kim, D.-H.; Kim, S. Effect of Turmeric on the Physicochemical Characteristics of ‘Doenjang’ during Fermentation. Korean J. Food Preserv. 2020, 27, 7–16. [Google Scholar] [CrossRef][Green Version]
- Sugiharto, S.; Yudiarti, T. The Effect of Using Acidified Turmeric on Some Productive Parameters and Intestinal Bacterial Counts in Broilers at High Stocking Density Pens. J. Adv. Vet. Anim. Res. 2022, 9, 87. [Google Scholar] [CrossRef]
- Wang, X.; Kristo, E.; LaPointe, G. The Effect of Apple Pomace on the Texture, Rheology and Microstructure of Set Type Yogurt. Food Hydrocoll. 2019, 91, 83–91. [Google Scholar] [CrossRef]
- Sheikh, S.; Siddique, F.; Ameer, K.; Ahmad, R.S.; Hameed, A.; Ebad, A.; Mohamed Ahmed, I.A.; Shibli, S. Effects of White Mulberry Powder Fortification on Antioxidant Activity, Physicochemical, Microbial and Sensorial Properties of Yogurt Produced from Buffalo Milk. Food Sci. Nutr. 2023, 11, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Vénica, C.I.; Spotti, M.J.; Pavón, Y.L.; Molli, J.S.; Perotti, M.C. Influence of Carrot Fibre Powder Addition on Rheological, Microstructure and Sensory Characteristics of Stirred-type Yogurt. Int. J. Food Sci. Technol. 2020, 55, 1916–1923. [Google Scholar] [CrossRef]
- Korkmaz, I.O.; Bilici, C.; Korkmaz, S. Sensory, PH, Syneresis, Water-Holding Capacity, and Microbiological Changes in Homemade Yogurt Prepared with Maca (Lepidium Meyenii) Powder and Propolis Extract. Int. J. Gastron. Food Sci. 2021, 23, 100291. [Google Scholar] [CrossRef]
- Wulandari, A.; Sunarti, T.C.; Fahma, F.; Enomae, T. The Potential of Bioactives as Biosensors for Detection of PH. IOP Conf. Ser. Earth Environ. Sci. 2020, 460, 012034. [Google Scholar] [CrossRef]
- de Oliveira Filho, J.G.; de Almeida, M.J.; Sousa, T.L.; dos Santos, D.C.; Egea, M.B. Bioactive Compounds of Turmeric (Curcuma longa L.). In Bioactive Compounds in Underutilized Vegetables and Legumes. Reference Series in Phytochemistry; Murthy, H.N., Paek, K.Y., Eds.; Springer: Cham, Switzerland, 2021; pp. 297–318. ISBN 978-3-030-57415-4. [Google Scholar]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 1021. [Google Scholar] [CrossRef]
- Tanvir, E.M.; Hossen, M.S.; Hossain, M.F.; Afroz, R.; Gan, S.H.; Khalil, M.I.; Karim, N. Antioxidant Properties of Popular Turmeric (Curcuma longa) Varieties from Bangladesh. J. Food Qual. 2017, 2017, 8471785. [Google Scholar] [CrossRef]
- Cho, W.-Y.; Hwa, S.-H.; Yang, F.; Lee, C.-H. Quality Characteristics and Antioxidant Activity of Yogurt Containing Raw Omija and Sugared Omija during Storage. J. Chem. 2020, 2020, 1274591. [Google Scholar] [CrossRef]
- Nguyen, L.; Hwang, E.-S. Quality Characteristics and Antioxidant Activity of Yogurt Supplemented with Aronia (Aronia Melanocarpa) Juice. Prev. Nutr. Food Sci. 2016, 21, 330–337. [Google Scholar] [CrossRef]
- Amirdivani, S.; Baba, A.S.H. Green Tea Yogurt: Major Phenolic Compounds and Microbial Growth. J. Food Sci. Technol. 2015, 52, 4652–4660. [Google Scholar] [CrossRef]
- Shori, A.B. Storage Quality and Antioxidant Properties of Yogurt Fortified with Polyphenol Extract from Nutmeg, Black Pepper, and White Pepper. Electron. J. Biotechnol. 2022, 57, 24–30. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Z.; Zhang, Y.; Gao, B.; Li, Y.; He, X.; Sun, J.; Choe, U.; Chen, P.; Blaustein, R.A.; et al. Chemical Composition of Turmeric (Curcuma longa L.) Ethanol Extract and Its Antimicrobial Activities and Free Radical Scavenging Capacities. Foods 2024, 13, 1550. [Google Scholar] [CrossRef]
- Karaaslan, M.; Ozden, M.; Vardin, H.; Turkoglu, H. Phenolic Fortification of Yogurt Using Grape and Callus Extracts. LWT-Food Sci. Technol. 2011, 44, 1065–1072. [Google Scholar] [CrossRef]
- Kulaitienė, J.; Vaitkevičienė, N.; Levickienė, D. Studies on Proximate Composition, Mineral and Total Phenolic Content of Yogurt Bites Enriched with Different Plant Raw Material. Fermentation 2021, 7, 301. [Google Scholar] [CrossRef]
- Akter, J.; Hossain, M.A.; Takara, K.; Islam, M.Z.; Hou, D.-X. Antioxidant Activity of Different Species and Varieties of Turmeric (Curcuma Spp): Isolation of Active Compounds. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 215, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Abdel Fattah, A.; Tawfik, M. Antioxidant Activities of Raw and Nano Turmeric (Curcuma longa L.) Powders. J. Food Dairy Sci. 2016, 7, 443–450. [Google Scholar] [CrossRef]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P. Influence of Piperine on the Pharmacokinetics of Curcumin in Animals and Human Volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Augustin, M.A.; Sanguansri, L.; Shen, Z.; Ng, K.; Ajlouni, S. Enhanced Bioaccessibility of Curcuminoids in Buttermilk Yogurt in Comparison to Curcuminoids in Aqueous Dispersions. J. Food Sci. 2016, 81, 13235. [Google Scholar] [CrossRef]
- Vuran, Ö.; Karagözlü, N.; Akpınar, A. The Antimicrobial Effects of Probiotic and Traditional Yoghurts Produced Using Commercial Starter Cultures on Some Foodborne Pathogens. Ege Üniversitesi Ziraat Fakültesi Derg. 2021, 58, 315–323. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Yuan, W.; Li, S.; Gupta, S.C. Curcumin-free Turmeric Exhibits Anti-inflammatory and Anticancer Activities: Identification of Novel Components of Turmeric. Mol. Nutr. Food Res. 2013, 57, 1529–1542. [Google Scholar] [CrossRef]
- Yin, M.; Weil, M.; Avallone, S.; Lebrun, M.; Conejero, G.; In, S.; Bohuon, P. Impact of Cooking and Drying Operations on Color, Curcuminoids, and Aroma of Curcuma longa L. J. Food Process Preserv. 2022, 46, 16643. [Google Scholar] [CrossRef]
- Komonsing, N.; Khuwijitjaru, P.; Nagle, M.; Müller, J.; Mahayothee, B. Effect of Drying Temperature Together with Light on Drying Characteristics and Bioactive Compounds in Turmeric Slice. J. Food Eng. 2022, 317, 110695. [Google Scholar] [CrossRef]
- Uthman, A.I.; Obafunmi, T.I.; Shitu, S.; Bello, N.U. Evaluation of Antibacterial Activity of Curcuma longa (Turmeric) Extract against Escherichia Coli and Staphylococcus Aureus. Res Sq. 2024. [Google Scholar] [CrossRef]
- CXS 243-2003; Standard for Fermented Milks—Codex Alimentarius International Food Standards. FAO: Rome, Italy, 2022.
- Glušac, J.; Stijepić, M.; Đurđević-Milošević, D.; Milanović, S.; Kanurić, K.; Vukić, V. Growth and Viability of Lactobacillus Delbrueckii Subsp. Bulgaricus and Streptococcus Thermophilus in Traditional Yoghurt Enriched by Honey and Whey Protein Concentrate. Iran. J. Vet. Res. 2015, 16, 249. [Google Scholar] [PubMed]
- Rahmatalla, S.A.; Abd Alazeem, L.; Abdalla, M.O.M. Microbiological Quality of Set Yoghurt Supplemented with Turmeric Powder (Curcuma longa) during Storage. Asian J. Agric. Food Sci. 2017, 5, 1–6. [Google Scholar]
- Lučan Čolić, M.; Antunović, M.; Jukić, M.; Popović, I.; Lukinac, J. Sensory Acceptance and Characterisation of Turmeric- and Black-Pepper-Enriched Ice Cream. Appl. Sci. 2023, 13, 11802. [Google Scholar] [CrossRef]



| C | TP_05 | TP_1 | |
|---|---|---|---|
| pH | 4.10 ± 0.02 b | 4.19 ± 0.01 a | 4.17 ± 0.01 a |
| TA (LA%) | 1.25 ± 0.04 a | 1.06 ± 0.02 b | 1.09 ± 0.02 b |
| Hardness (g) | 18.4 ± 0.3 a | 18.0 ± 0.7 a | 18.0 ± 0.8 a |
| WHC (%) | 60.2 ± 0.6 c | 62.0 ± 0.3 b | 63.4 ± 0.6 a |
| L* | 84.8 ± 1.6 a | 78.6 ± 0.7 b | 76.9 ± 0.4 c |
| a* | −1.4 ± 0.0 b | −1.2 ± 0.3 b | −0.8 ± 0.2 a |
| b* | 5.9 ± 0.0 c | 57.9 ± 1.9 b | 67.8 ± 0.9 a |
| Gastric pH Tolerance (%) | Bile Salt Tolerance (%) | |
|---|---|---|
| Antioxidant capacity | ||
| C | 100 ± 1.4 a | 109.0 ± 1.4 a |
| TP_05 | 92.2 ± 3.2 a | 104.5 ± 1.0 a |
| TP_1 | 74.4 ± 0.2 b | 97.5 ± 2.1 b |
| Total phenolic compounds | ||
| C | 98.1 ± 2.0 a | 97.0 ± 4.1 a |
| TP_05 | 95.8 ± 1.9 ab | 95.5 ± 5.0 a |
| TP_1 | 88.9 ± 0.4 b | 87.5 ± 3.0 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sıçramaz, H. Turmeric-Enriched Yogurt: Increased Antioxidant and Phenolic Contents. Fermentation 2025, 11, 127. https://doi.org/10.3390/fermentation11030127
Sıçramaz H. Turmeric-Enriched Yogurt: Increased Antioxidant and Phenolic Contents. Fermentation. 2025; 11(3):127. https://doi.org/10.3390/fermentation11030127
Chicago/Turabian StyleSıçramaz, Hatice. 2025. "Turmeric-Enriched Yogurt: Increased Antioxidant and Phenolic Contents" Fermentation 11, no. 3: 127. https://doi.org/10.3390/fermentation11030127
APA StyleSıçramaz, H. (2025). Turmeric-Enriched Yogurt: Increased Antioxidant and Phenolic Contents. Fermentation, 11(3), 127. https://doi.org/10.3390/fermentation11030127





