Scaling Up Red Ginger Kombucha Fermentation: Insights into Its Chemical Profile and Health-Promoting Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Equipment
2.2. Optimization of Red Ginger Extraction and Development of Kombucha Ginger Inoculants
2.3. Fermentation of Red Ginger Kombucha
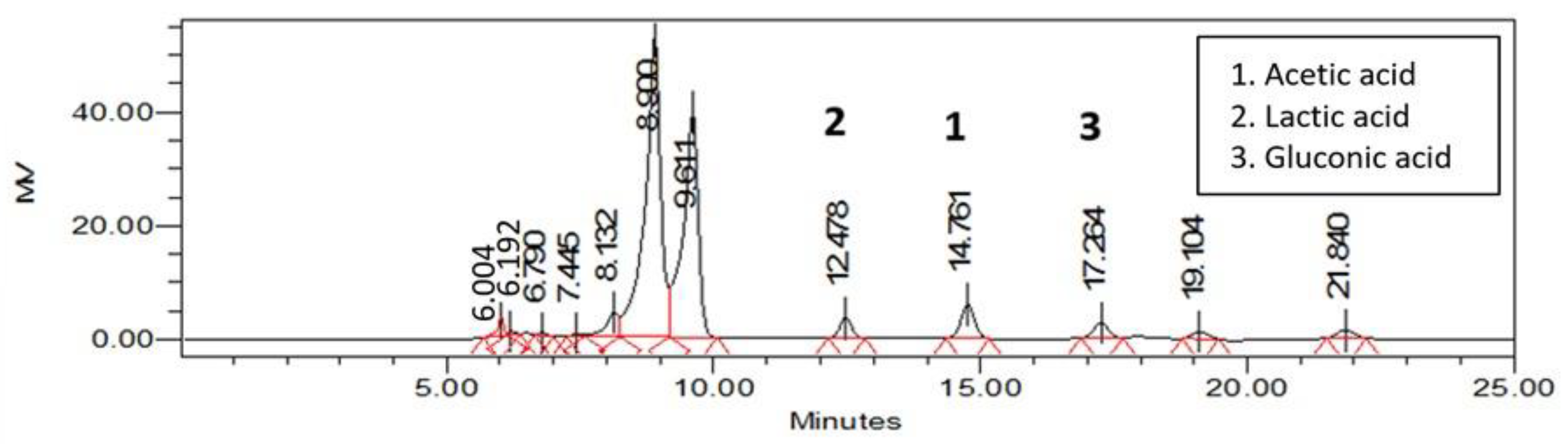
2.4. Analysis of Sugar and Organic Acid Contents Using HPLC
2.5. Analysis of Bioactive Compounds Using GC-MS
2.6. Determination of pH and Total Acidity
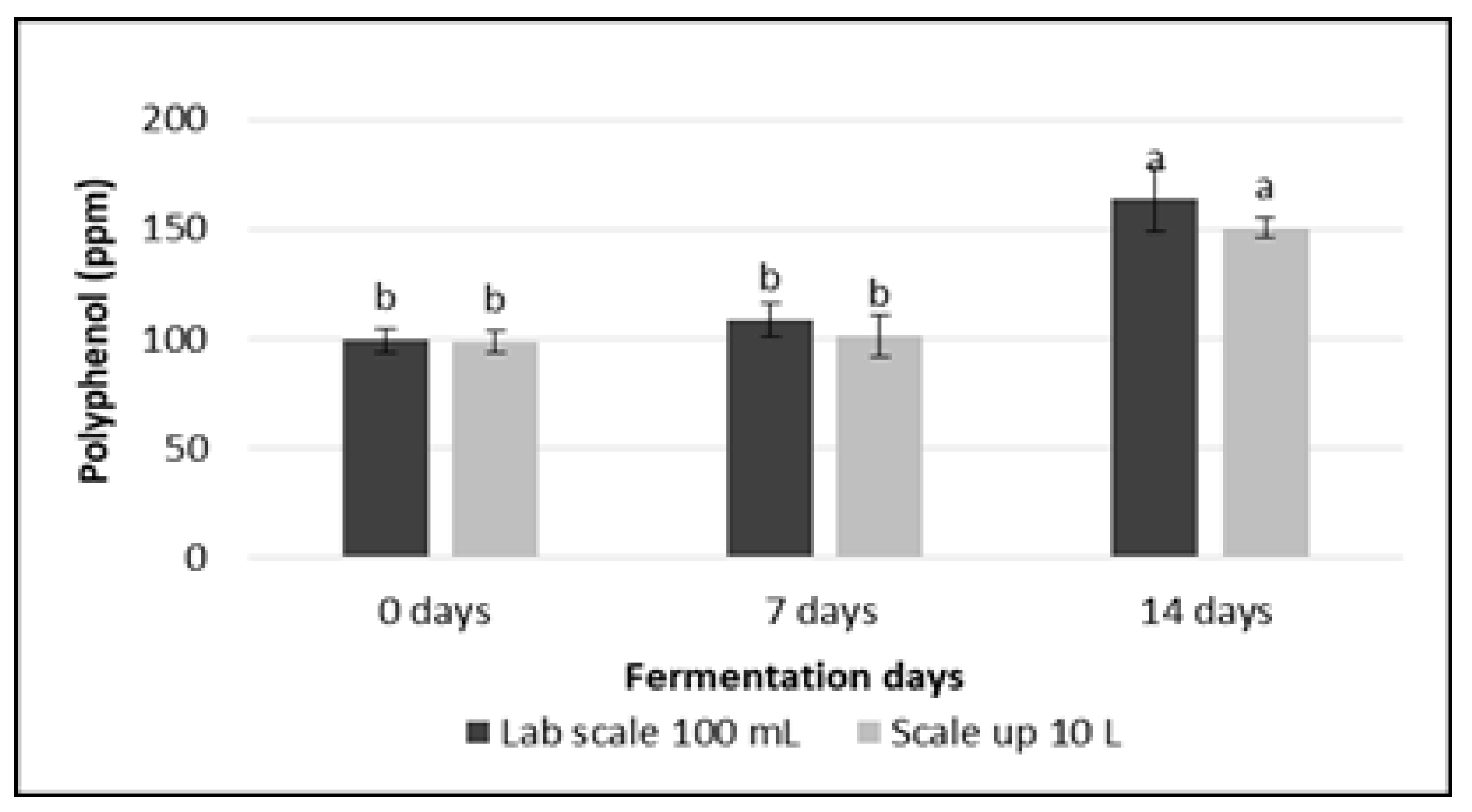
2.7. Analysis of Total Polyphenol Content
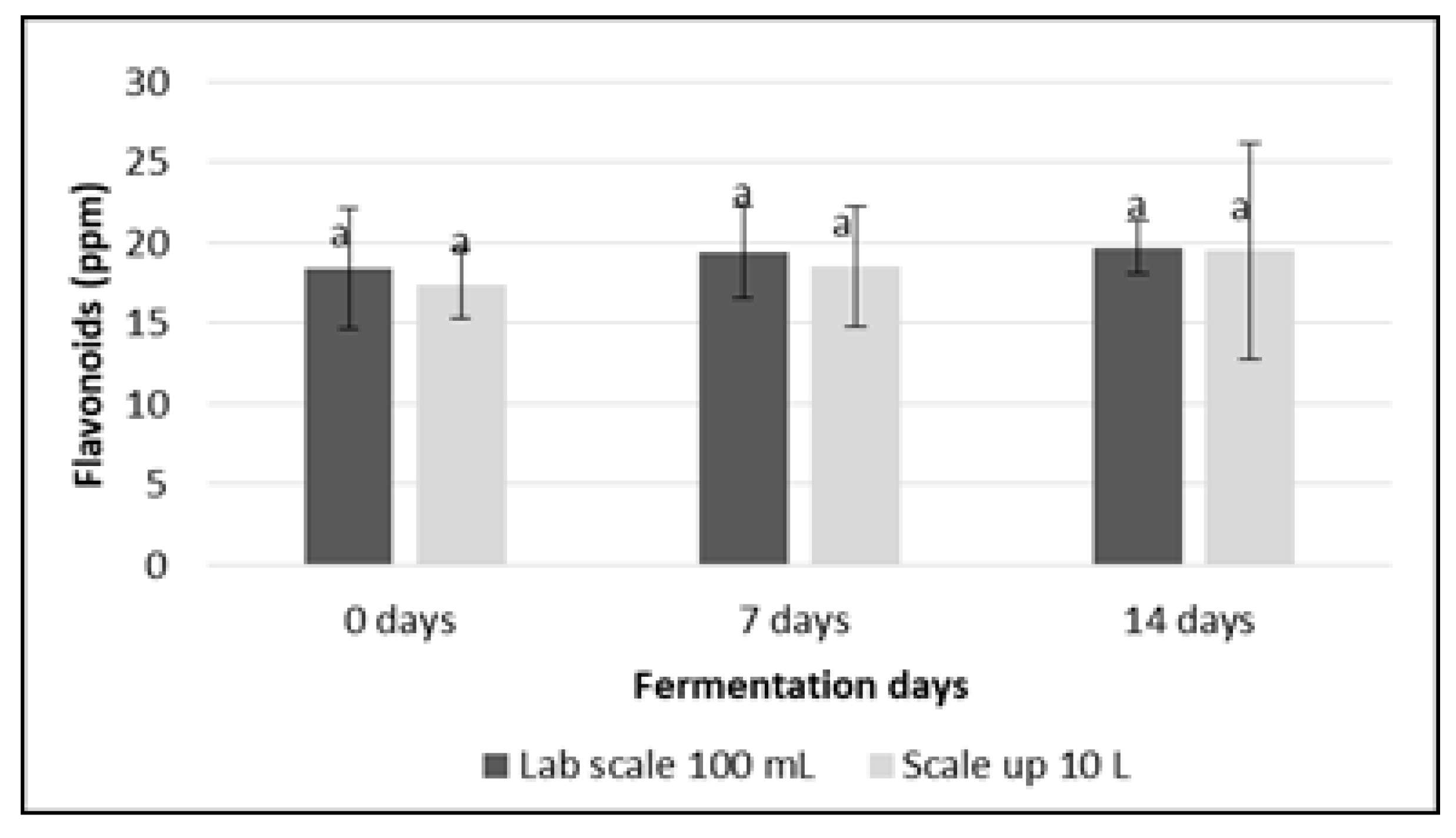
2.8. Analysis of Total Flavonoid Content
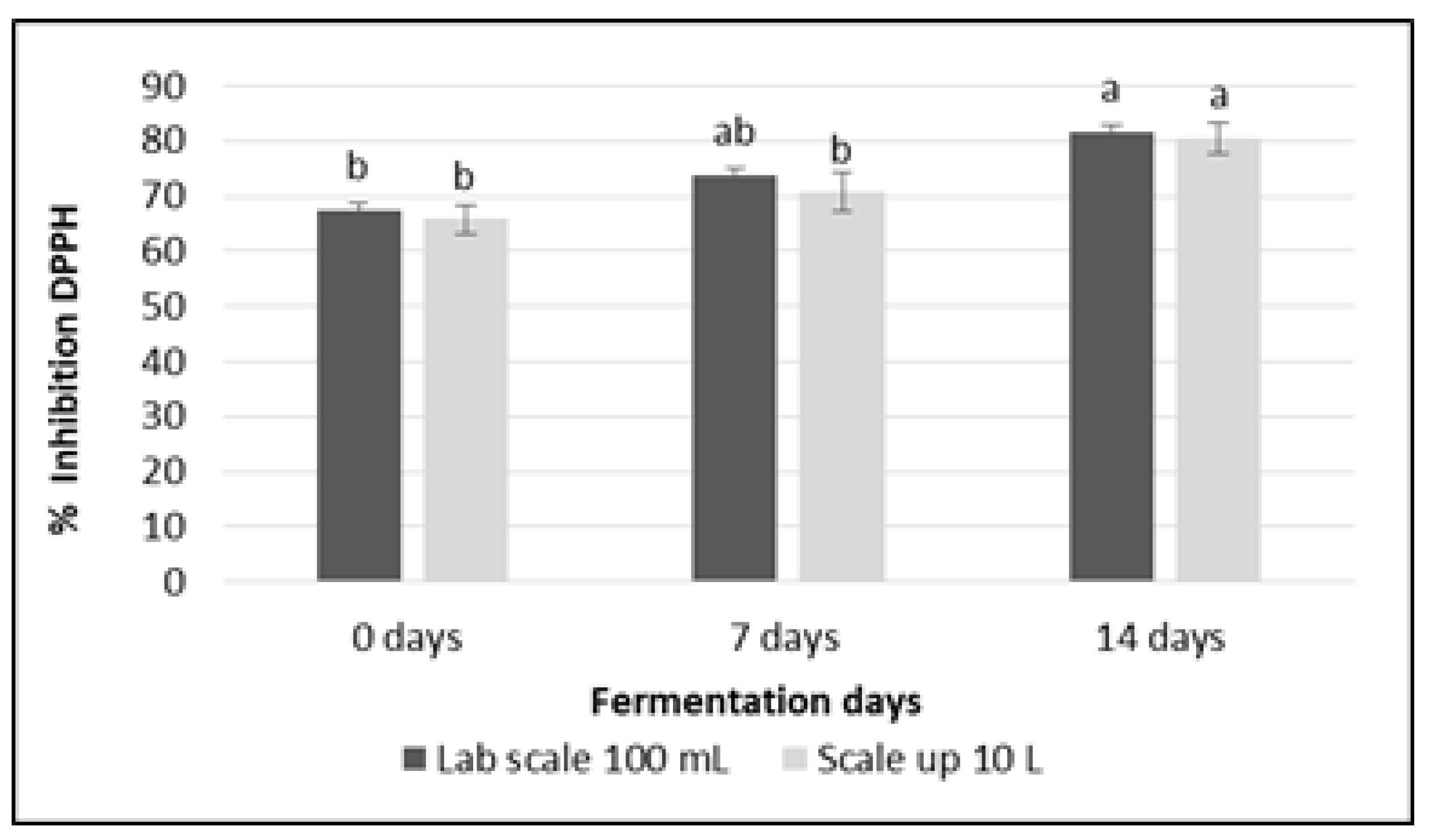
2.9. Measurement of DPPH Free Radical Scavenging Activity
2.10. α-Glucosidase Inhibition Assay
2.11. Statistical Analysis
3. Result and Discussions
3.1. Analysis of Initial Red Ginger Extract Before Fermentation
3.2. Sugar and Organic Acid Content
3.3. pH and Total Acid
3.4. Total Polyphenol Content
3.5. Total Flavonoid Content
3.6. Free Radical Inhibitory Activity
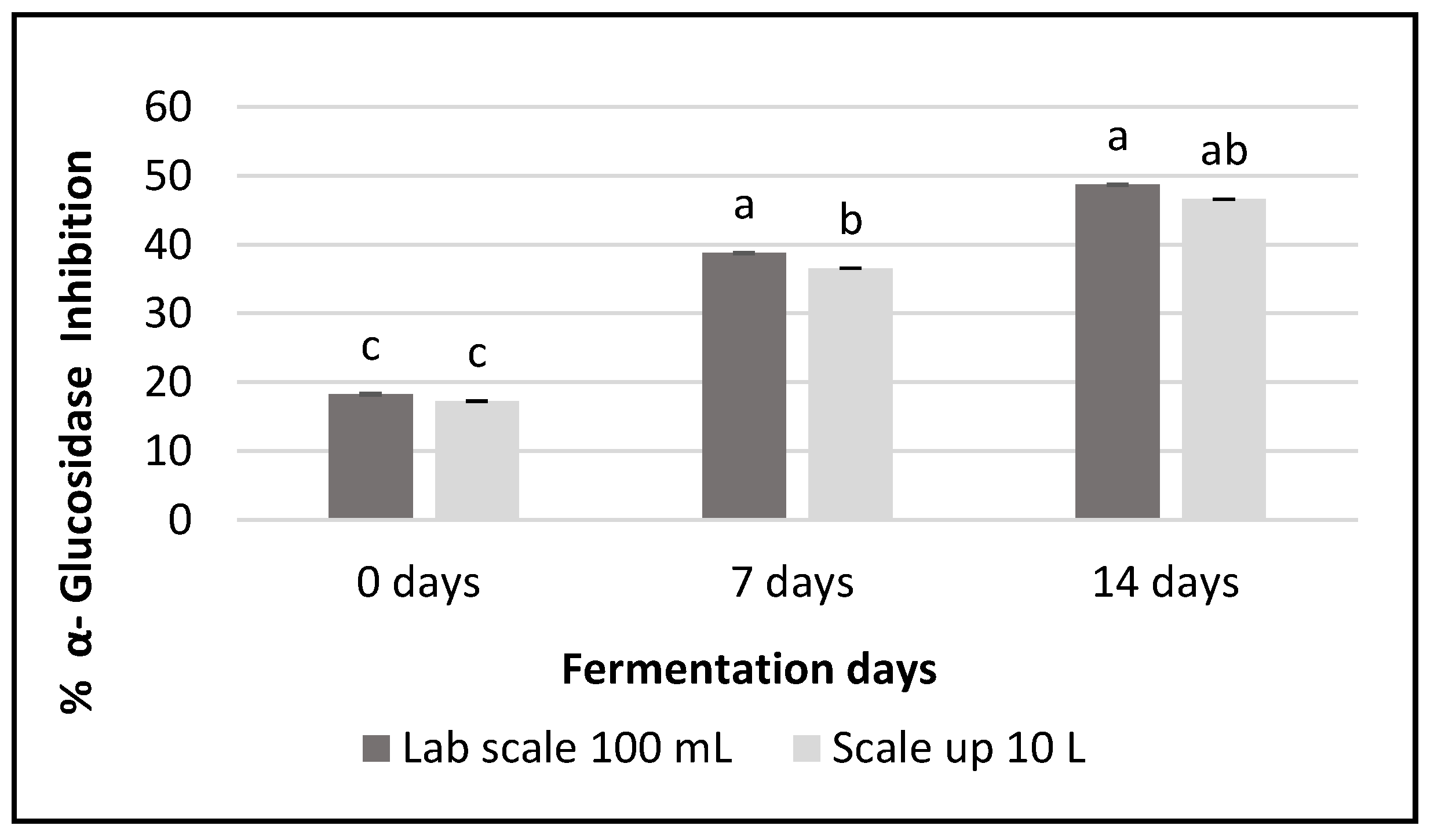
3.7. α-Glucosidase Inhibitory Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pratami, M.P.; Anggraeni, A.; Sujarwo, W. Ethnobotany of medicinal plants in Leuwiliang (Bogor), Indonesia. Ethnobot. Res. Appl. 2024, 27, 1–41. [Google Scholar] [CrossRef]
- Ali, K.; Flare, A.; Flinn, G. An Overview of the traditional and modern applications of ginger. JHSciRes 2024, 4, 10–16. [Google Scholar] [CrossRef]
- Zhang, S.; Kou, X.; Zhao, H.; Mak, K.-K.; Balijepalli, M.K.; Pichika, M.R. Zingiber officinale var. rubrum: Red ginger’s medicinal uses. Molecules 2022, 27, 775. [Google Scholar] [CrossRef]
- Li, K.; Yao, F.; Xue, Q.; Fan, H.; Yang, L.; Li, X.; Sun, L.; Liu, Y. Inhibitory effects against α-glucosidase and α-amylase of the flavonoids-rich extract from Scutellaria baicalensis shoots and interpretation of structure–activity relationship of its eight flavonoids by a refined assign-score method. Chem. Cent. J. 2018, 12, 82. [Google Scholar] [CrossRef]
- Sharma, S.; Shukla, M.K.; Sharma, K.C.; Tirath; Kumar, L.; Anal, J.M.H.; Upadhyay, S.K.; Bhattacharyya, S.; Kumar, D. Revisiting the therapeutic potential of gingerols against different pharmacological activities. Naunyn Schmiedebergs Arch. Pharmacol. 2022, 396, 633–647. [Google Scholar] [CrossRef]
- Nam, Y.H.; Hong, B.N.; Rodriguez, I.; Park, M.S.; Jeong, S.Y.; Lee, Y.-G.; Shim, J.H.; Yasmin, T.; Kim, N.W.; Koo, Y.T.; et al. Steamed ginger may enhance insulin secretion through KATP channel closure in pan-creatic β-cells potentially by increasing 1-dehydro-6-gingerdione content. Nutrients 2020, 12, 324. [Google Scholar] [CrossRef]
- Alharbi, K.S.; Nadeem, M.S.; Afzal, O.; Alzarea, S.; Altamimi, A.S.A.; Almalki, W.H.; Mubeen, B.; Iftikhar, S.; Shah, L.; Kazmi, I. Gingerol, a natural antioxidant, at-tenuates hyperglycemia and downstream complications. Metabolites 2022, 12, 1274. [Google Scholar] [CrossRef]
- William, J.; John, P.; Mumtaz, M.W.; Rashid, A.; Adnan, A.; Mukhtar, H.; Sharif, S.; Raza, S.A.; Akhtar, M.T. Antioxidant activity, α-glucosidase inhibition and phytochemical profiling of Hyophorbe lagenicaulis leaf extracts. PeerJ 2019, 7, e7022. [Google Scholar] [CrossRef]
- Salehi, B.; Ata, A.; Kumar, N.V.A.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Ayatollahi, S.A.; Fokou, P.V.T.; Kobarfard, F.; Zakaria, Z.A.; et al. Antidiabetic potential of medicinal plants and their active components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef]
- Kapp, J.M.; Sumner, W. Kombucha: A systematic review of the empirical evidence of human health benefit. Ann. Epidemiol. 2019, 30, 66–70. [Google Scholar] [CrossRef]
- Morales, D. Biological activities of kombucha beverages: The need of clinical evidence. Trends Food Sci. Technol. 2020, 105, 323–333. [Google Scholar] [CrossRef]
- Kitwetcharoen, H.; Phung, L.T.; Klanrit, P.; Thanonkeo, S.; Tippayawat, P.; Yamada, M.; Thanonkeo, P. Kombucha healthy drink—Recent advances in production, chemical composition and health benefits. Fermentation 2023, 9, 48. [Google Scholar] [CrossRef]
- Laureys, D.; Britton, S.J.; De Clippeleer, J. Kombucha tea fermentation: A review. J. Am. Soc. Brew. Chem. 2020, 78, 165–174. [Google Scholar] [CrossRef]
- Selvaraj, S.; Gurumurthy, K. An overview of probiotic health booster-kombucha tea. Chin. Herb. Med. 2022, 15, 27–32. [Google Scholar] [CrossRef]
- Maryati, Y.; Melanie, H.; Handayani, W.; Yasman, Y. Bacterial cellulose production from fermented fruits and vegetables byproducts: A comprehensive study on chemical and morphological properties. Karbala Int. J. Mod. Sci. 2024, 10, 7. [Google Scholar] [CrossRef]
- Mulyani, H.; Artanti, N.; Filailla, E.; Budiari, S.; Maryati, Y.; Melanie, H.; Susilowati, A.; Yuniati, R.; Yasman, Y. Effect of fermented red ginger (Zingiber officinale var. rubrum) using kombucha culture toward free radical scavenging activity. AIP Conf. Proc. 2023, 2902, 060022. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Gachhui, R.; Sil, P.C. Effect of kombucha, a fermented black tea in attenuating oxidative stress mediated tissue damage in alloxan induced diabetic rats. Food Chem. Toxicol. 2013, 60, 328–340. [Google Scholar] [CrossRef]
- Chakravorty, S.; Bhattacharya, S.; Chatzinotas, A.; Chakraborty, W.; Bhattacharya, D.; Gachhui, R. Kombucha tea fermentation: Microbial and biochemical dynamics. Int. J. Food Microbiol. 2016, 220, 63–72. [Google Scholar] [CrossRef]
- Martínez-Leal, J.; Ponce-García, N.; Es-calante-Aburto, A. Recent evidence of the beneficial effects associated with glucuronic acid contained in kombucha beverages. Curr. Nutr. Rep. 2020, 9, 163–170. [Google Scholar] [CrossRef]
- Zahn, J.A. Scale-up and optimization of natural product fermentation processes using mass-guided metabolite fingerprinting. Adv. Biotech. Micro 2017, 3, 555614. [Google Scholar] [CrossRef]
- Schneider, A.; Gerbi, V.; Redoglia, M. A rapid HPLC method for separation and determination of major organic acids in grape musts and wines. Am. J. Enol. Vitic. 1987, 38, 151–155. [Google Scholar] [CrossRef]
- Andreson, M.; Kazantseva, J.; Kuldjarv, R.; Malv, E.; Vaikma, H.; Kaleda, A.; Kütt, M.; Vilu, R. Characterisation of chemical, microbial and sensory profiles of commer-cial kombuchas. Int. J. Food Microbiol. 2022, 373, 109715. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.F.; Hikal, M.S.; Abou-Taleb, K.A. Biological, chemical and antioxidant activities of different types kombucha. Ann. Agric. Sci. 2020, 65, 35–41. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- Sukweenadhi, J.; Yunita, O.; Setiawan, F.; Kartini, K.; Siagian, M.T.; Danduru, A.P.; Avanti, C. Antioxidant activity screening of seven Indonesian herbal extract. Biodiversitas 2020, 21, 2062–2067. [Google Scholar] [CrossRef]
- Kim, Y.M.; Wang, M.H.; Rhee, H.I. A novel α-glucosidase inhibitor from pine bark. Carbohyd Res. 2004, 339, 715–717. [Google Scholar] [CrossRef]
- Shareef, H.K.; Muhammed, H.J.; Hussein, H.M.; Hameed, I.H. Antibacterial effect of ginger (Zingiber officinale) roscoe and bioactive chemical analysis using gas chromatography mass spectrum. Orient. J. Chem. 2016, 32, 817–837. [Google Scholar] [CrossRef]
- Mao, Q.-Q.; Xu, X.-Y.; Cao, S.-Y.; Gan, R.-Y.; Corke, H.; Beta, T.; Li, H.-B. Bioactive compounds and bioactivities of ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Wang, Y.; Wang, S.; Zou, Y.; Sun, Z.; Yuan, L. Changes on physiochemical properties and volatile compounds of Chinese kombucha during fermentation. Food Biosci. 2023, 55, 103029. [Google Scholar] [CrossRef]
- Aung, T.; Eun, J.B. Production and characterization of a novel beverage from laver (Porphyra dentata) through fermentation with kombucha consortium. Food Chem. 2021, 350, 129274. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, Y.; Gao, J.; Li, T.; Li, H.; Mastroyannis, A.; He, S.; Rahaman, A.; Chang, K. Effect of fermentation time on physiochemical properties of kombucha produced from different teas and fruits: Comparative study. J. Food Qual. 2022, 2022, 2342954. [Google Scholar] [CrossRef]
- Yang, L.; Lübeck, M.; Souroullas, K.; Lübeck, P.S. Co-consumption of glucose and xylose for organic acid production by Aspergillus carbonarius cultivated in wheat straw hydrolysate. World J. Microbiol. Biotechnol. 2016, 32, 57. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, B.; Zhang, X.; Wang, C.; Chen, W. Production of gluconic acid and its derivatives by microbial fermentation: Process improvement based on integrated routes. Front. Bioeng. Biotechnol. 2022, 10, 864787. [Google Scholar] [CrossRef] [PubMed]
- Ardheniati, M.; Andriani, M.A.M.; Amanto, B.S. Fermentation kinetics in kombucha tea with tea kind variation based on its processing. J. Biofarmasi 2009, 7, 48–55. [Google Scholar] [CrossRef]
- Pratiwi, A.; Elfita, E.; Aryawati, R. Pengaruh waktu fermentasi terhadap sifat fisik dan kimia pada pembuatan minuman kombucha dari rumput laut Sargasssum sp. Maspari J. Mar. Sci. Res. 2012, 4, 131–136. [Google Scholar]
- Bhattacharya, S.; Prasenjit, M.; Gachhui, R.; Sil, P.C. Protective effect of kombucha tea against tertiary butyl hydrperoxide induced cytotoxicity and cell death in murine hepatocytes. Indian J. Exp. Biol. 2011, 49, 511–524. [Google Scholar]
- Santamaría, L.; Reverón, I.; de Felipe, F.L.; de las Rivas, B.; Muñoz, R. Ethylphenol formation by Lactobacillus plantarum: Identification of the enzyme involved in the reduction of vinylphenols. Appl. Env. Microbiol. 2018, 84, e01064-18. [Google Scholar] [CrossRef]
- Sinir, G.Ö.; Tamer, C.E.; Suna, S. 10—Kombucha: A Promising Fermented Functional Beverage. Fermented Beverages, Volume 5: The Science of Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 401–432. [Google Scholar] [CrossRef]
- Sova, M.; Saso, L. Natural sources, pharmacokinetics, biological activities and health benefits of hydroxycinnamic acids and their metabolites. Nutrients 2020, 12, 2190. [Google Scholar] [CrossRef]
- Cardoso, R.R.; Neto, R.O.; dos Santos D’Al-meida, C.T.; do Nascimento, T.P.; Pressete, C.G.; Azevedo, L.; Martino, H.S.D.; Cameron, L.C.; Ferreira, M.S.L.; de Barros, F.A.R. Kombuchas from green and black teas have different phenolic profile, which impacts their antioxidant capacities, antibacterial and antiproliferative activities. Food Res. Int. 2020, 128, 108782. [Google Scholar] [CrossRef]
- Simanjuntak, D.H.; Herpandi, H.; Lestari, S.D. Chemical characteristics and antioxidant activity of water lettuce (Pistia straiotes) leaves kombucha during fermentation. J. Teknol. Has. Perikan. 2016, 5, 123–133. [Google Scholar] [CrossRef]
- Sahraeian, S.; Rashidinejad, A.; Golmakani, M.-T. Recent advances in the conjugation approaches for enhancing the bioavailability of polyphenols. Food Hydrocoll. 2024, 146, 109221. [Google Scholar] [CrossRef]
- de Oliveira, P.V.; da Silva Júnior, A.H.; de Oliveira, C.R.S.; Assumpção, C.F.; Ogeda, C.H. Kombucha benefits, risks and regulatory frameworks: A review. Food Chem. Adv. 2023, 2, 100288. [Google Scholar] [CrossRef]
- Thummala, S.; Khrisna, M.K.; Natarajan, A.; Uppala., S. Antihyperglycaemic efficacy of kombucha in streptozotocin-induced rats. J. Funct. Foods 2013, 5, 1794–1802. [Google Scholar] [CrossRef]
- Maryam, G.-D.; Hossein, A.-K.; Zahra, L.; Mahmoud, R.-K. Oxidative stress and antioxidants in diabetes mellitus. Asian Pac. J. Trop. Med. 2020, 13, 431–438. [Google Scholar] [CrossRef]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef]
- Mendelson, C.; Sparkes, S.; Merenstein, D.J.; Christensen, C.; Sharma, V.; Desale, S.; Auchtung, J.M.; Kok, C.R.; Hallen-Adams, H.E.; Hutkins, R. Kombucha tea as an anti-hyperglycemic agent in humans with diabetes—A randomized controlled pilot investigation. Front. Nutr. 2023, 10, 1190248. [Google Scholar] [CrossRef]
- Cvetković, D.; Markov, S.; Djurić, M.; Savić, M.; Velićanski, A. Specific interfacial area as a key variable in scaling-up Kombucha fermentation. J. Food Eng. 2008, 85, 387–392. [Google Scholar] [CrossRef]







| Peaks | Retention Time (Minute) | Area Under the Peak (%) | Formula | Molecular Weight | Compound Name | Similarity (%) |
|---|---|---|---|---|---|---|
| 1 | 16.716 | 46.21 | C11H14O3 | 194 | Butan-2-one-4-(3-hydroxy-2-methoxyphenyl) | 98 |
| 2 | 22.443 | 1.01 | C17H24O3 | 276 | (6)-Isoshogaol | 97 |
| 3 | 22.524 | 1.65 | C17H26O3 | 278 | 6-Paradol | 96 |
| 4 | 23.081 | 18.62 | C17H24O3 | 276 | 6-Shogaol | 99 |
| 5 | 23.315 | 1.60 | C19H28O6 | 352 | (4)-Gingerdiol 3,5-diacetate | 95 |
| Peaks | Retention Time (Minute) | Area Under the Peak (%) | Formulae | Molecular Weight | Compound Name | Similarity (%) |
|---|---|---|---|---|---|---|
| 1 | 16.72 | 43.61 | C11H14O3 | 194 | 4-(3-Hydroxy-2-methoxyphenyl)butan-2-one | 98 |
| 2 | 4.39 | 22.35 | C6H12O | 100 | Hexanal | 95 |
| 3 | 23.08 | 16.97 | C17H24O3 | 276 | 6-Shogaol | 99 |
| 4 | 23.32 | 3.24 | C19H28O6 | 352 | [4]-Gingerdiol 3,5-diacetate | 98 |
| 5 | 13.02 | 2.04 | C10H16O2 | 168 | Geranic Acid | 94 |
| 6 | 22.53 | 1.99 | C17H26O3 | 278 | 6-Paradol | 98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulyani, H.; Artanti, N.; Yuniati, R.; Yasman, Y. Scaling Up Red Ginger Kombucha Fermentation: Insights into Its Chemical Profile and Health-Promoting Properties. Fermentation 2025, 11, 128. https://doi.org/10.3390/fermentation11030128
Mulyani H, Artanti N, Yuniati R, Yasman Y. Scaling Up Red Ginger Kombucha Fermentation: Insights into Its Chemical Profile and Health-Promoting Properties. Fermentation. 2025; 11(3):128. https://doi.org/10.3390/fermentation11030128
Chicago/Turabian StyleMulyani, Hani, Nina Artanti, Ratna Yuniati, and Yasman Yasman. 2025. "Scaling Up Red Ginger Kombucha Fermentation: Insights into Its Chemical Profile and Health-Promoting Properties" Fermentation 11, no. 3: 128. https://doi.org/10.3390/fermentation11030128
APA StyleMulyani, H., Artanti, N., Yuniati, R., & Yasman, Y. (2025). Scaling Up Red Ginger Kombucha Fermentation: Insights into Its Chemical Profile and Health-Promoting Properties. Fermentation, 11(3), 128. https://doi.org/10.3390/fermentation11030128






