Effects of Different Yeasts on the Physicochemical Properties and Aroma Compounds of Fermented Sea Buckthorn Juice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Chemical and Reagents
2.3. Beverage Preparation and Fermentation
2.4. Determination of Physicochemical Properties
2.5. Phytochemical Concentration Assay
2.6. Antioxidant Activity Assay
2.7. HS-SPME-GC-MS Analysis
2.8. Odor Activity Value (OAV) Calculation
2.9. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Physicochemical Properties
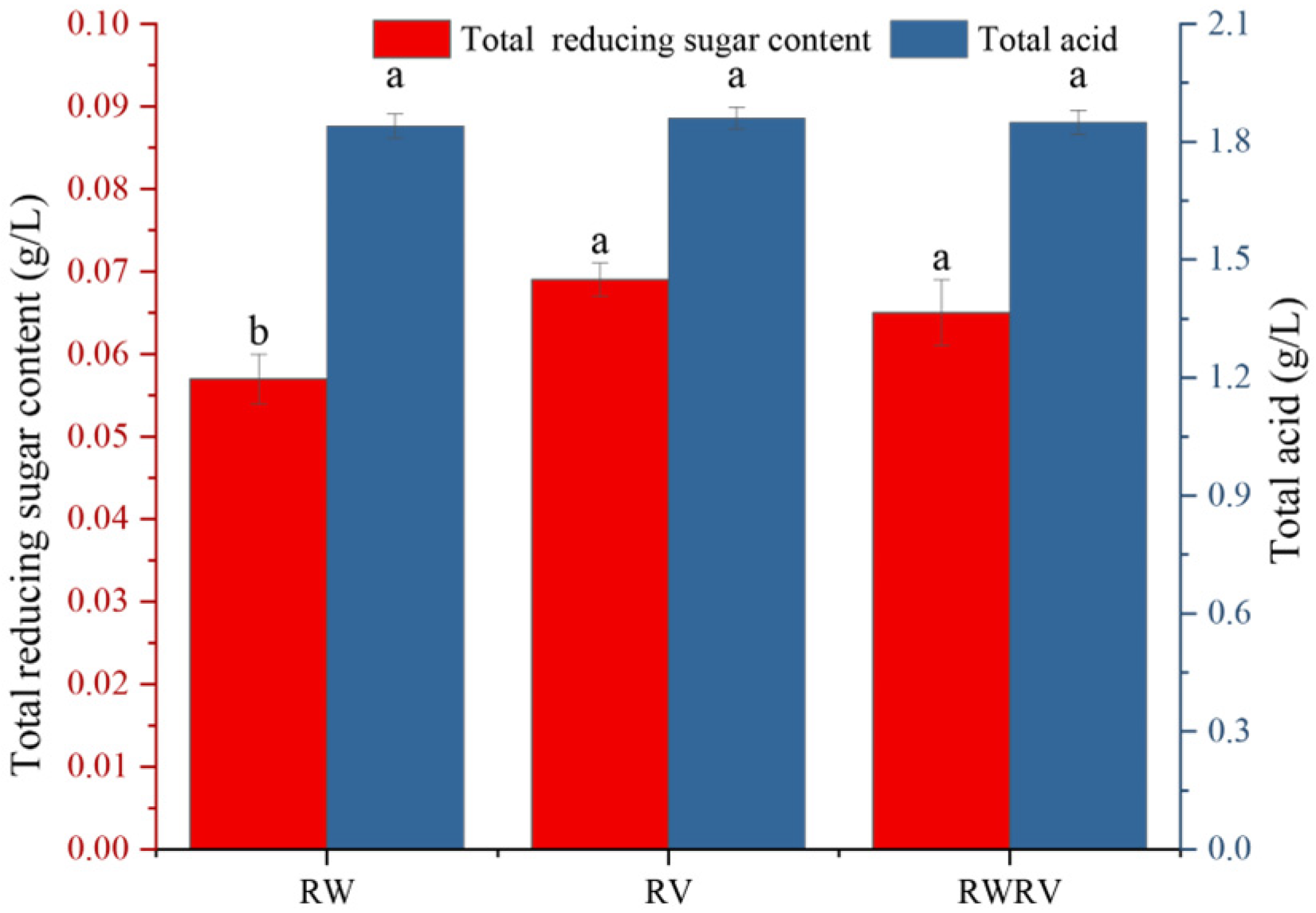
3.1.1. The Total Reducing Sugar and Acid Content in FSBJs
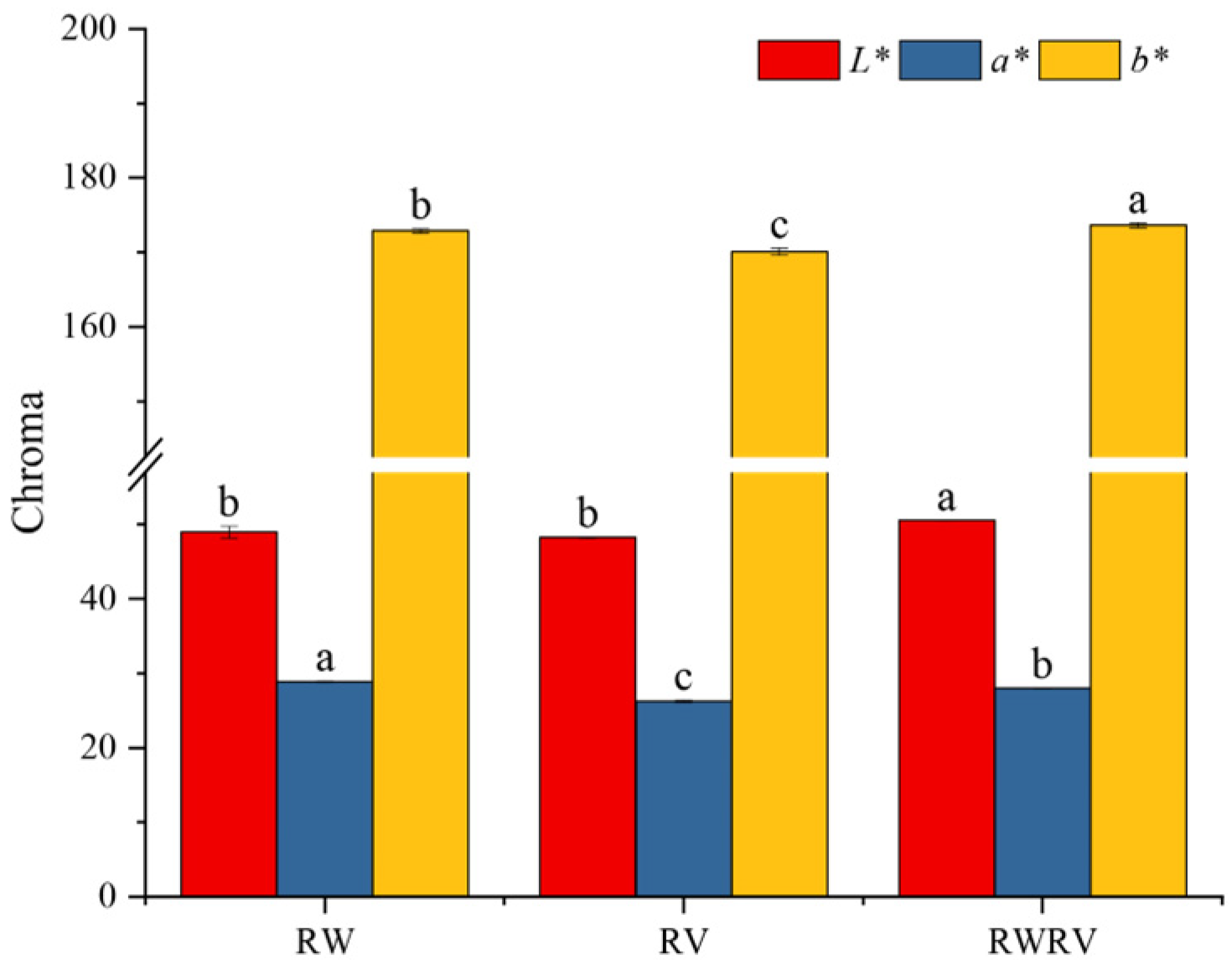
3.1.2. The Color Properties of FSBJs
3.1.3. The Content of Functional Components in FSBJs
3.1.4. The Antiradical Properties of FSBJs
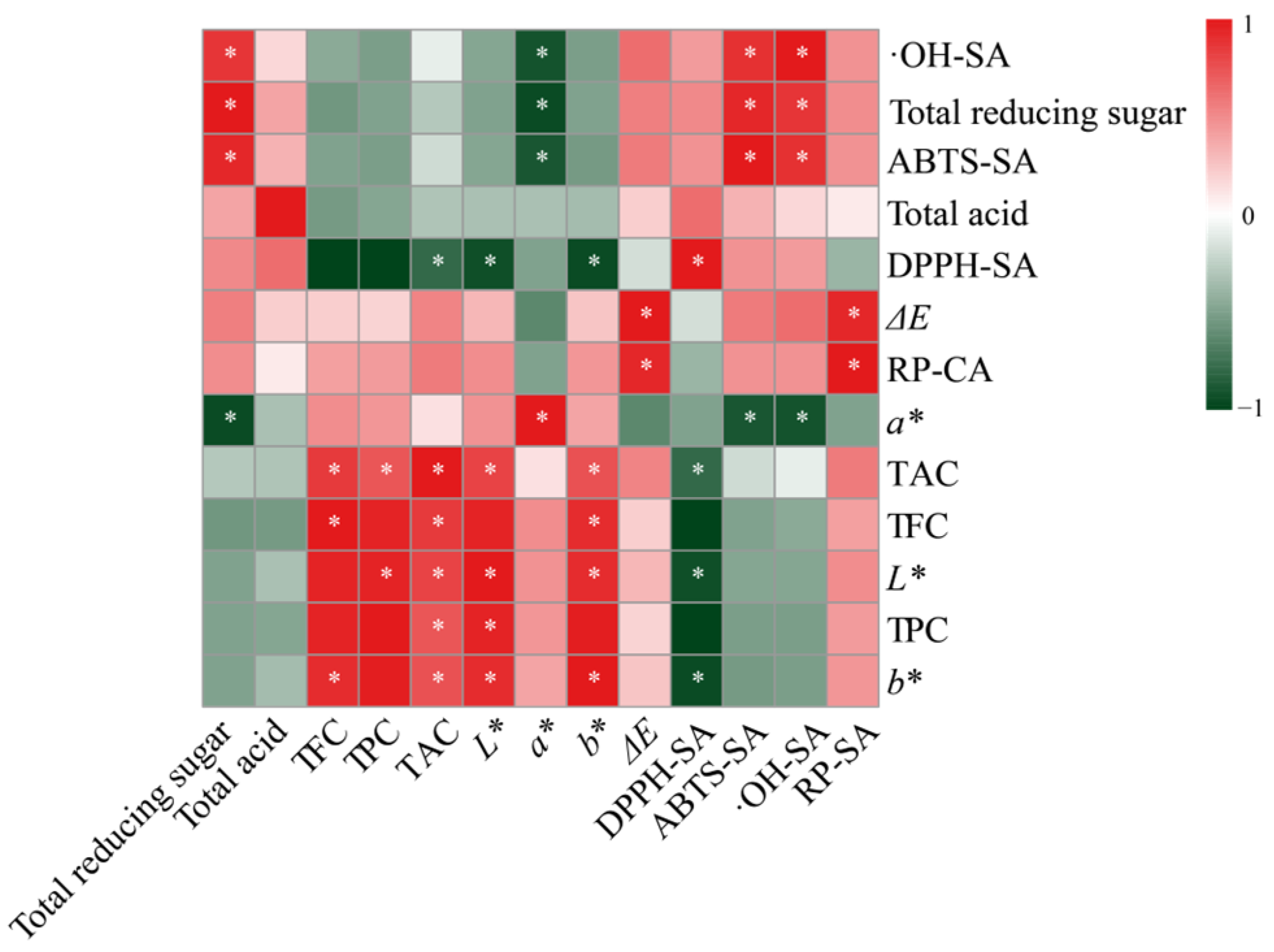
3.1.5. Correlation Analysis
3.2. HS-SPME-GC-MS Analysis of FSBJs
3.2.1. Esters
3.2.2. Alcohols
3.2.3. Acids, Ketones, Aldehydes, Hydrocarbons, and Other Compounds
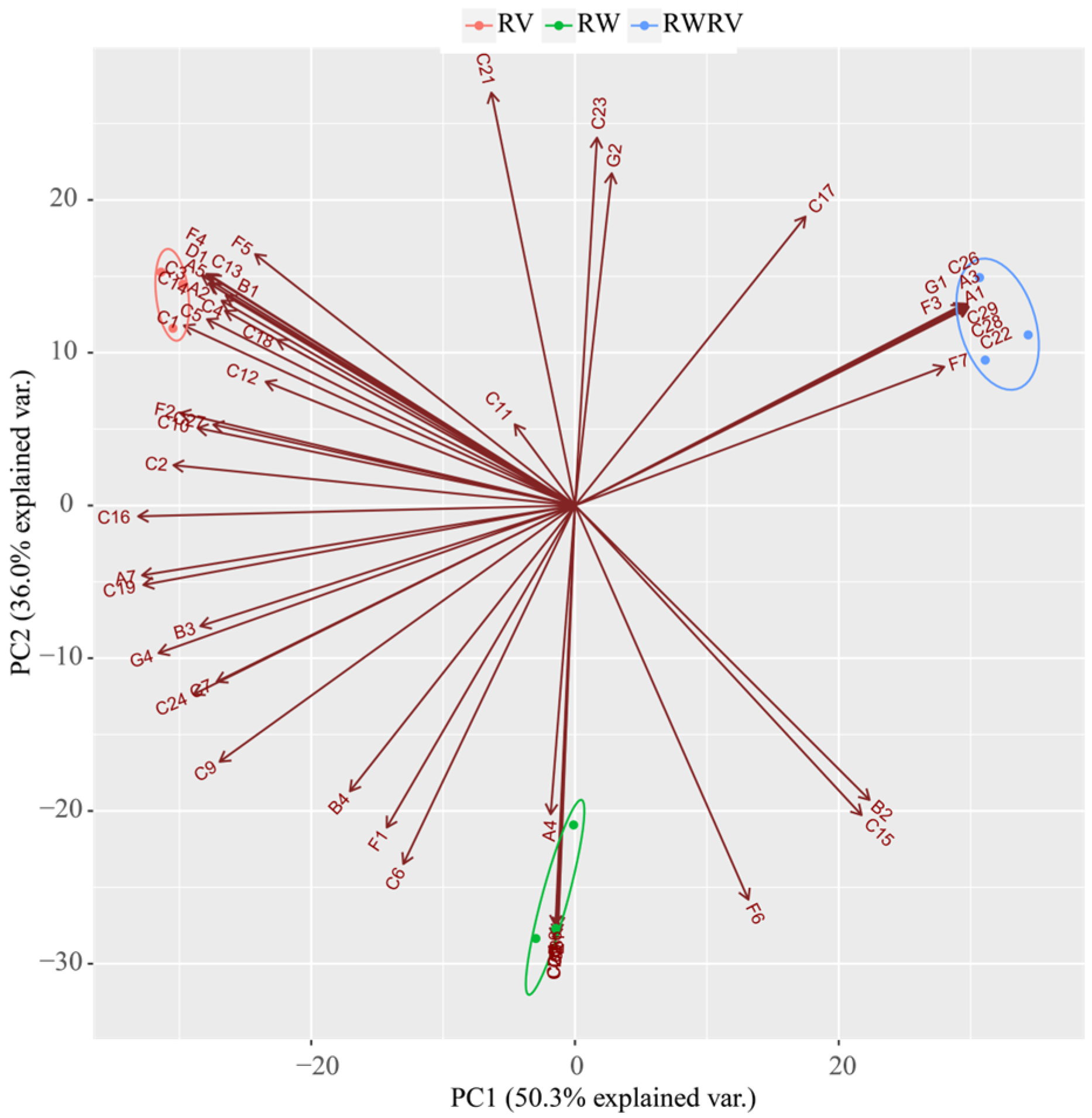
3.2.4. PCA Analysis of the FSBJs
3.2.5. OAV Analysis of the FSBJs
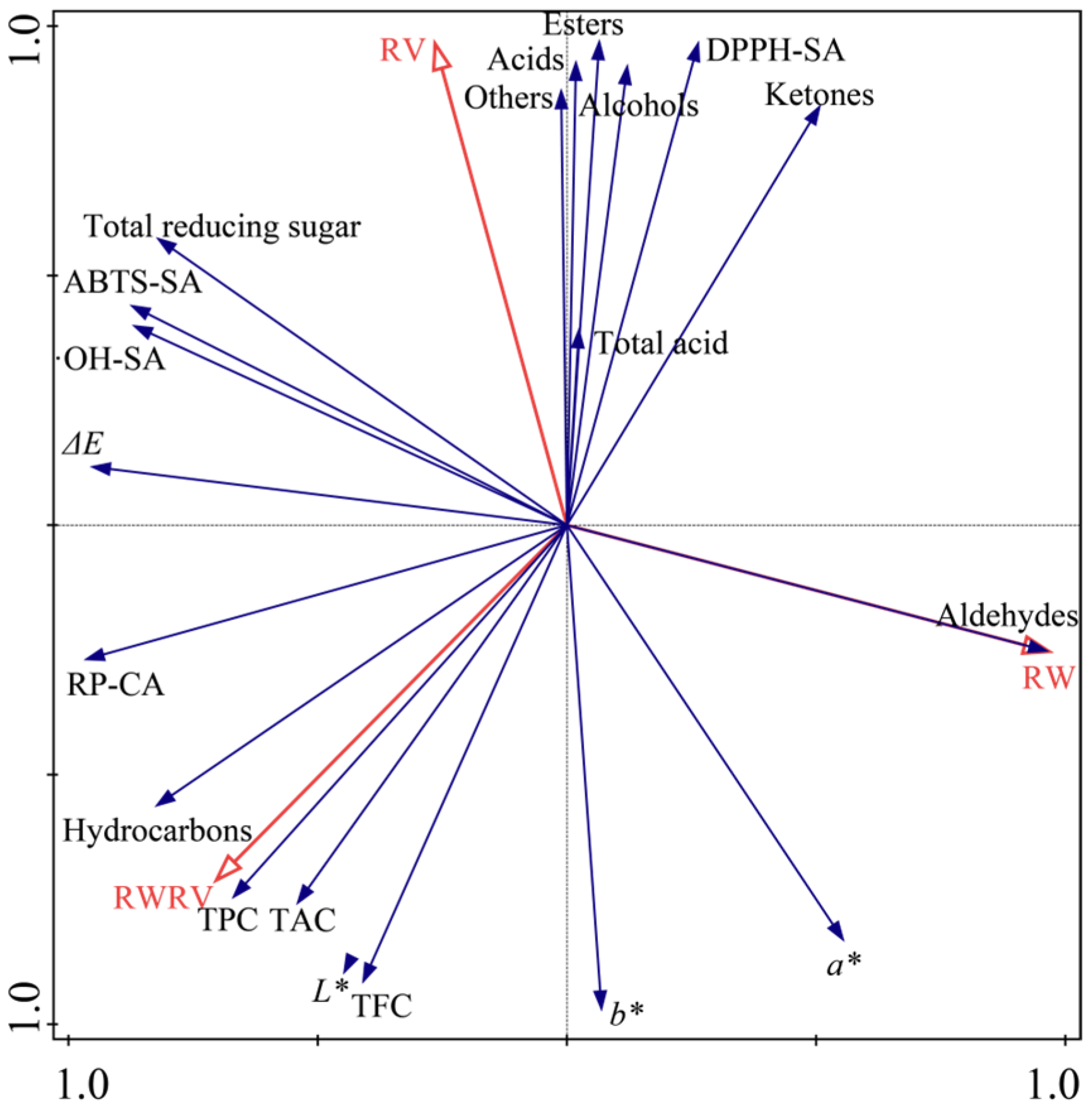
3.3. Redundancy Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, B.A.; Akhtar, N.; Mahmood, T. A comprehensive review of a magic plant, Hippophae rhamnoides. Pharmacogn. J. 2010, 2, 65–68. [Google Scholar] [CrossRef]
- Luntraru, C.M.; Apostol, L.; Oprea, O.B.; Neagu, M.; Popescu, A.F.; Tomescu, J.A.; Multescu, M.; Susman, I.E.; Gaceu, L. Reclaim and Valorization of Sea Buckthorn (Hippophae rhamnoides) By-Product: Antioxidant Activity and Chemical Characterization. Foods 2022, 11, 462. [Google Scholar] [CrossRef]
- Yadav, A.; Stobdan, T.; Chauhan, O.; Dwivedi, S.; Chaurasia, O. Sea buckthorn: A multipurpose medicinal plant from upper Himalayas. In Medicinal Plants: From Farm to Pharmacy; Springer Nature: London, UK, 2019; pp. 399–426. [Google Scholar]
- Wen, J.; Huang, R.J.; Li, S.; Jiang, L.; Shao, L.H.; Zhang, Q.; Shan, C.H. Polysaccharides from sea buckthorn—Ultrasound-assisted enzymatic extraction, purification, structural characterization, and antioxidant activity analysis. Food Chem.-X 2025, 26, 102265. [Google Scholar] [CrossRef]
- Zhang, G.K.; Liu, Y.F.; Liu, P. Active Components from Sea Buckthorn (Hippophae rhamnoides L.) Regulate Hepatic Stellate Cell Activation and Liver Fibrogenesis. J. Agric. Food Chem. 2018, 66, 12257–12264. [Google Scholar] [CrossRef]
- Lee, Y.H.; Jang, H.J.; Park, K.H.; Kim, S.H.; Kim, J.K.; Kim, J.C.; Jang, T.S.; Kim, K.H. Phytochemical Analysis of the Fruits of Sea Buckthorn (Hippophae rhamnoides): Identification of Organic Acid Derivatives. Plants 2021, 10, 860. [Google Scholar] [CrossRef]
- Laaksonen, O.; Knaapila, A.; Niva, T.; Deegan, K.C.; Sandell, M. Sensory properties and consumer characteristics contributing to liking of berries. Food Qual. Prefer. 2016, 53, 117–126. [Google Scholar] [CrossRef]
- Pellerin, G.; Bazinet, L.; Grenier, D. Effect of cranberry juice deacidification on its antibacterial activity against periodontal pathogens and its anti-inflammatory properties in an oral epithelial cell model. Food Funct. 2021, 12, 10470–10483. [Google Scholar] [CrossRef]
- Tian, T.T.; Wu, D.H.; Ng, C.T.; Yang, H.; Sun, J.Y.; Liu, J.M.; Lu, J. A multiple-step strategy for screening Saccharomyces cerevisiae strains with improved acid tolerance and aroma profiles. Appl. Microbiol. Biotechnol. 2020, 104, 3097–3107. [Google Scholar] [CrossRef]
- Han, X.; Peng, Q.; Yang, H.; Hu, B.; Shen, C.; Tian, R. Influence of different carbohydrate sources on physicochemical properties and metabolites of fermented greengage (Prunus mume) wines. Lwt-Food Sci. Technol. 2020, 121, 108929. [Google Scholar] [CrossRef]
- Peng, B.; Hao, X.S.; Zhang, Q.; Cai, W.C.; Zhao, X.X.; Tang, F.X.; Shan, C.H. Effects of low-temperature storage on the aroma composition and quality of Golden Empress Hami melons: A transcriptomic and metabolomic analysis. Food Biosci. 2024, 57, 103422. [Google Scholar] [CrossRef]
- Ma, J.; Ma, Y.H.; Zhang, H.Z.; Chen, Z.L.; Wen, B.; Wang, Y.; Huang, W.Y. The quality change of fig wine fermented by RV171 yeast during the six-month aging process. Lwt-Food Sci. Technol. 2022, 166, 113789. [Google Scholar] [CrossRef]
- Wang, X.J.; Liu, B.C.; Xie, Q. Analysis of genetic diversity in the free-pollinated F1 population of Hippophae rhamnoides L. subsp. mongolica ‘Shenqiuhong’ via simple sequence repeat markers. In Genetic Resources and Crop Evolution; Springer Nature: London, UK, 2024. [Google Scholar] [CrossRef]
- GB 5009.225–2016; Determination of Ethanol Concentration in National Standard Liquor for Food Safety. Chinese Standard Press: Beijing, China, 2016.
- Zhao, X.X.; Xue, Y.; Tang, F.X.; Cai, W.C.; Hao, G.F.; Shan, C.H. Quality improvement of jujube wine through mixed fermentation with Saccharomyces cerevisiae and Bacillus licheniformis. Lwt-Food Sci. Technol. 2022, 164, 113444. [Google Scholar] [CrossRef]
- Dong, W.; Ni, Y.; Kokot, S. A Near-Infrared Reflectance Spectroscopy Method for Direct Analysis of Several Chemical Components and Properties of Fruit, for Example, Chinese Hawthorn. J. Agric. Food Chem. 2013, 61, 540–546. [Google Scholar] [CrossRef]
- Zhao, X.X.; Tang, F.X.; Cai, W.C.; Peng, B.; Zhang, P.L.; Shan, C.H. Effect of fermentation by lactic acid bacteria on the phenolic composition, antioxidant activity, and flavor substances of jujube-wolfberry composite juice. Lwt-Food Sci. Technol. 2023, 184, 114884. [Google Scholar] [CrossRef]
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M.T.; Wu, M.; Sackey, A.S.; Xiao, L.; Tahir, H.E.J.F.C. Effect of lactobacillus strains on phenolic profile, color attributes and antioxidant activities of lactic-acid-fermented mulberry juice. Food Chem. 2018, 250, 148–154. [Google Scholar] [CrossRef]
- Cai, W.; Tang, F.; Shan, C.; Yang, L.; Zhan, Q.; Ning, M. Changes in volatile compounds of fermented mixed drink using commercial yeast strain. Przem. Chem. 2018, 97, 1398–1405. [Google Scholar]
- Cai, W.; Tang, F.; Guo, Z.; Guo, X.; Shan, C.J.F.C. Effects of pretreatment methods and leaching methods on jujube wine quality detected by electronic senses and HS-SPME–GC–MS. Food Chem. 2020, 330, 127330. [Google Scholar] [CrossRef]
- van Wyk, N.; Grossmann, M.; Wendland, J.; von Wallbrunn, C.; Pretorius, I.S. The Whiff of Wine Yeast Innovation: Strategies for Enhancing Aroma Production by Yeast during Wine Fermentation. J. Agric. Food Chem. 2019, 67, 13496–13505. [Google Scholar] [CrossRef]
- Vitova, E.; Sukalova, K.; Mahdalova, M.; Butorova, L.; Melikantova, M. Comparison of selected aroma compounds in cultivars of sea buckthorn (Hippophae rhamnoides L.). Chem. Pap. 2015, 69, 881–888. [Google Scholar] [CrossRef]
- Ran, B.-B.; Li, W.-D. Research progress on chemical constituents and their differences between sea buckthorn berries and leaves. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China J. Chin. Mater. Medica 2019, 44, 1767–1773. [Google Scholar] [CrossRef]
- Živković, N.M.; Čakar, U.D.; Petrović, A.V. Effects of spontaneous and inoculated fermentation on the total phenolic content and antioxidant activity of Cabernet Sauvignon wines and fermented pomace. Food Feed. Res. 2024, 51, 119–129. [Google Scholar] [CrossRef]
- Wang, J.J.; Zhang, W.W.; Guan, Z.J.; Thakur, K.; Hu, F.; Zhang, J.G.; Wei, Z.J. Effect of fermentation methods on the quality and in vitro antioxidant properties of Lycium barbarum and Polygonatum cyrtonema compound wine. Food Chem. 2023, 409, 135277. [Google Scholar] [CrossRef] [PubMed]
- Rais, J.; Jafri, A.; Siddiqui, S.; Tripathi, M.; Arshad, M. Phytochemicals in the treatment of ovarian cancer. Front. Biosci. 2017, 9, 67–75. [Google Scholar]
- Shimomura-Shimizu, M.; Karube, I. Yeast Based Sensors. In Whole Cell Sensing Systems I: Reporter Cells and Devices; Belkin, S., Gu, M.B., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 117, pp. 1–19. [Google Scholar] [CrossRef]
- Van Gemert, L.; Liu, Q.; Mao, D.; Tang, E. Compendium of Compound Odor Thresholds, 2nd ed.; China Science Publishing & Media Ltd. (CSPM): Beijing, China, 2015; pp. 1–481. [Google Scholar]
- Van Gemert, L.J. Odour Thresholds: Compilations of Odour Threshold Values in Air, Water and Other Media (Edition 2011), 2nd ed.; Oliemans Punter & Partners BV: Zeist, The Netherlands, 2011. [Google Scholar]
- Markkinen, N.; Laaksonen, O.; Yang, B.R. Impact of malolactic fermentation with Lactobacillus plantarum on volatile compounds of sea buckthorn juice. Eur. Food Res. Technol. 2021, 247, 719–736. [Google Scholar] [CrossRef]
- Wu, D.; Xia, Q.L.; Cheng, H.; Zhang, Q.C.; Wang, Y.B.; Ye, X.Q. Changes of Volatile Flavor Compounds in Sea Buckthorn Juice during Fermentation Based on Gas Chromatography-Ion Mobility Spectrometry. Foods 2022, 11, 3471. [Google Scholar] [CrossRef]
- Xia, Q.L.; Liu, C.X.; Cao, Y.; Zhao, Y.Q.; Lu, S.M.; Wu, D.; Aniya; Guan, R.F. Improving quality of sea buckthorn juice by high-pressure processing. Lwt-Food Sci. Technol. 2023, 185, 115149. [Google Scholar] [CrossRef]
- Castillo, M.V.; Brar, S.K.; Arriaga, S.; Blais, J.F.; Heitz, M.; Ramirez, A.A. Co-Fermentation of Agri-Food Residues Using a Co-Culture of Yeasts as a New Bioprocess to Produce 2-Phenylethanol. Molecules 2023, 28, 5536. [Google Scholar] [CrossRef]
- Dai, J.; Li, K.; Song, N.; Yao, W.T.; Xia, H.L.; Yang, Q.; Zhang, X.L.; Li, X.; Wang, Z.; Yao, L.; et al. Zygosaccharomyces rouxii, an Aromatic Yeast Isolated From Chili Sauce, Is Able to Biosynthesize 2-Phenylethanol via the Shikimate or Ehrlich Pathways. Front. Microbiol. 2020, 11, 597454. [Google Scholar] [CrossRef]
- Zhou, R.; Song, Q.Y.; Xia, H.L.; Song, N.; Yang, Q.; Zhang, X.L.; Yao, L.; Yang, S.H.; Dai, J.; Chen, X. Isolation and Identification of Non-Saccharomyces Yeast Producing 2-Phenylethanol and Study of the Ehrlich Pathway and Shikimate Pathway. J. Fungi 2023, 9, 878. [Google Scholar] [CrossRef]


| Sample | TFC (μg/mL) | TPC (μg/mL) | TAC (μg/mL) |
|---|---|---|---|
| RW | 139.60 ± 1.16 b | 143.70 ± 1.02 b | 0.02 ± 0.01 b |
| RV | 122.73 ± 0.33 c | 133.30 ± 1.12 c | 0.02 ± 0.002 b |
| RWRV | 176.09 ± 0.44 a | 157.90 ± 1.35 a | 0.04 ± 0.004 a |
| No. | Lable | RT | CAS | Concentrations (mg/L) | ||
|---|---|---|---|---|---|---|
| Alcohols | RW | RV | RWRV | |||
| A1 | Trans-2-dodecen-1-ol | 22.775 | 69064-37-5 | - | - | 0.68 ± 0.05 |
| A2 | Phenylethyl alcohol | 50.276 | 60-12-8 | 178.07 ± 19.30 b | 257.55 ± 2.99 a | 174.34 ± 24.97 b |
| A3 | Linalool | 28.211 | 78-70-6 | - | - | 0.77 ± 0.08 |
| A4 | D(+)-2-Octanol | 20.342 | 6169-06-8 | 2.35 ± 0.25 a | 1.91 ± 0.19 b | 1.88 ± 0.28 b |
| A5 | 1-Octanol-2, 7-dimethyl- | 18.854 | 15250-22-3 | - | 0.32 ± 0.06 | - |
| A6 | 1-Dodecanol | 24.264 | 112-53-8 | 0.79 ± 0.16 | - | - |
| A7 | 3-Methyl-1-butanol | 11.537 | 123-51-3 | 42.48 ± 2.09 | 61.93 ± 6.68 | - |
| Total concentrations | 223.69 ± 20.74 b | 321.71 ± 6.37 a | 177.67 ± 24.76 b | |||
| Acids | ||||||
| B1 | Octanoic acid | 56.435 | 124-07-2 | 48.59 ± 2.53 b | 71.14 ± 6.03 a | 47.68 ± 5.23 b |
| B2 | Nonanoic acid | 59.94 | 112-05-0 | 0.87 ± 0.13 | - | 0.66 ± 0.13 |
| B3 | Hexanoic acid | 48.131 | 142-62-1 | 26.96 ± 1.91 a | 28.67 ± 2.57 a | 21.02 ± 0.45 b |
| B4 | Heptanoic acid | 20.065 | 111-14-8 | 1.95 ± 0.20 a | 1.72 ± 0.26 ab | 1.35 ± 0.03 b |
| Total concentrations | 78.37 ± 0.90 b | 101.53 ± 7.80 a | 70.71 ± 5.26 b | |||
| Esters | ||||||
| C1 | Ethyl caprylate | 20.983 | 106-32-1 | 44.92 ± 4.10 b | 75.68 ± 5.92 a | 39.57 ± 2.48 b |
| C2 | Ethyl nonanoate | 26.959 | 123-29-5 | 1.13 ± 0.19 b | 1.58 ± 0.17 a | 0.75 ± 0.03 c |
| C3 | Hexanoic acid, propyl ester | 15.454 | 626-77-7 | - | 0.65 ± 0.12 | - |
| C4 | Ethyl hexanoate | 12.178 | 123-66-0 | 146.14 ± 12.50 b | 197.42 ± 14.46 a | 143.56 ± 7.44 b |
| C5 | Ethyl heptanoate | 16.181 | 106-30-9 | 3.07 ± 0.36 b | 4.7 ± 0.38 a | 2.85 ± 0.28 b |
| C6 | Ethyl trans-4-decenoate | 36.66 | 76649-16-6 | 13.27 ± 0.78 a | 10.68 ± 0.51 b | 9.11 ± 0.88 b |
| C7 | Ethyl 3-hydroxy-3-methylbutanoate | 19.739 | 18267-36-2 | 16.36 ± 1.65 a | 16.51 ± 0.37 a | 11.77 ± 1.23 b |
| C8 | Ethyl laurate | 47.307 | 106-33-2 | 6.56 ± 0.83 | - | - |
| C9 | Diphosphoric acid, diisooctyl ester | 28.728 | 72101-07-6 | 4.69 ± 0.50 a | 3.98 ± 0.38 a | 0.04 ± 0.01 b |
| C10 | Benzyl isovalerate | 9.227 | 103-38-8 | 2.42 ± 0.24 b | 3.12 ± 0.32 a | 2.02 ± 0.28 b |
| C11 | Ethyl isovalerate | 6.356 | 108-64-5 | 49.43 ± 10.89 a | 52.98 ± 4.32 a | 50.42 ± 2.39 a |
| C12 | 3-Methylbutyl 3-methylbutanoate | 14.487 | 659-70-1 | 60.6 ± 7.10 ab | 77.2 ± 11.05 a | 55.36 ± 7.05 b |
| C13 | Ethyl 2-methylbutyrate | 5.962 | 7452-79-1 | - | 17.12 ± 2.97 | - |
| C14 | Butanoic acid, 2-methyl-3-methylbutyl ester | 13.64 | 27625-35-0 | - | 4.27 ± 0.68 | - |
| C15 | Boronic acid, ethyl-dimethyl ester | 15.154 | 7318-82-3 | 4.01 ± 0.75 | - | 2.94 ± 0.46 |
| C16 | Borinic acid, diethyl-methyl ester | 13.46 | 7397-46-8 | 0.98 ± 0.10 b | 1.7 ± 0.18 a | 0.07 ± 0.00 c |
| C17 | Benzoyl isothiocyanate | 25.767 | 532-55-8 | 3.05 ± 0.32 b | 3.56 ± 0.2 ab | 4.24 ± 0.45 a |
| C18 | Ethyl benzoate | 36.102 | 93-89-0 | 90.83 ± 11.76 a | 114.55 ± 8.05 a | 89.36 ± 9.66 a |
| C19 | Ethyl phenylacetate | 44.233 | 101-97-3 | 13.11 ± 1.56 | 18.44 ± 1.02 | - |
| C20 | Ethyl trans-4-octenoate | 22.952 | 78989-37-4 | 15.68 ± 1.34 | - | - |
| C21 | Ethyl (Z)-oct-4-enoate | 23.086 | 34495-71-1 | 4.84 ± 0.37 b | 18.99 ± 2.03 a | 15.53 ± 1.83 a |
| C22 | 2-Tetrahydrofurfuryl isothiocyanate | 36.301 | 36810-87-4 | - | - | 2.56 ± 0.09 |
| C23 | Ethyl trans-2-octenoate | 27.847 | 2351-90-8 | 0.39 ± 0.02 b | 0.56 ± 0.08 a | 0.56 ± 0.07 a |
| C24 | Ethyl hex-2-enoate | 16.65 | 1552-67-6 | 0.83 ± 0.16 | 0.83 ± 0.04 | - |
| C25 | Ethyl 3,3-dimethylacrylate | 11.807 | 638-10-8 | 5.16 ± 0.55 | - | - |
| C26 | Ethyl 2,4-hexadienate | 23.641 | 110318-09-7 | - | - | 0.35 ± 0.04 |
| C27 | Isoamyl benzoate | 49.923 | 94-46-2 | 76.69 ± 10.5 ab | 100.99 ± 12.94 a | 64.08 ± 5.17 b |
| C28 | Isoamyl acetate | 8.148 | 123-92-2 | - | - | 0.9 ± 0.07 |
| C29 | Diisobutyl phthalate | 69.458 | 84-69-5 | - | - | 0.46 ± 0.05 |
| Total concentrations | 564.17 ± 30.85 b | 725.53 ± 9.14 a | 496.5 ± 20.01 b | |||
| Ketones | ||||||
| D1 | 2-Cyclohexyl-1-tetrazol-2-yl-ethanone | 38.602 | 74897-66-8 | - | 0.51 ± 0.09 | - |
| D2 | 2, 4-Pentanedioneion (1-)lithium | 9.936 | 18115-70-3 | 0.36 ± 0.02 | - | - |
| Total concentrations | 0.36 ± 0.02 | 0.51 ± 0.09 | - | |||
| Aldehydes | ||||||
| E1 | Hexanal, 3-(hydroxymethyl)-4-methyl- | 18.633 | 56805-30-2 | 0.92 ± 0.11 | - | - |
| Total concentrations | 0.92 ± 0.11 | - | - | |||
| Hydrocarbons | ||||||
| F1 | Cyclohexane, 1-butenylidene- | 9.981 | 36144-40-8 | 10.23 ± 1.02 a | 8.97 ± 0.48 ab | 7.79 ± 0.58 b |
| F2 | Cyclohexane, ethenylidene- | 40.99 | 5664-20-0 | 2.83 ± 0.19 b | 3.83 ± 0.28 a | 2.34 ± 0.27 b |
| F3 | Butane, 1-chloro-3-methyl- | 11.331 | 107-84-6 | - | - | 43.54 ± 3.41 |
| F4 | Borane, dimethoxy- | 15.184 | 4542-61-4 | - | 5.06 ± 0.51 | - |
| F5 | Boranic acid dimethyl ester | 67.82 | 10468-64-1 | 0.63 ± 0.09 b | 1.15 ± 0.13 a | 0.69 ± 0.04 b |
| F6 | 2, 3-Heptadien-5-yne-2-4-dimethyl- | 16.02 | 41898-89-9 | 0.7 ± 0.07 | - | 0.3 ± 0.04 |
| F7 | 1-Silacyclo-2-4-hexadiene | 22.772 | 52023-17-3 | 0.46 ± 0.06 b | 0.43 ± 0.05 b | 0.64 ± 0.04 a |
| Total concentrations | 14.85 ± 0.84 b | 19.45 ± 0.63 b | 55.3 ± 4.15 a | |||
| Others | ||||||
| G1 | Morpholine, 4-methyl-4-oxide | 15.649 | 7529-22-8 | - | - | 0.5 ± 0.04 |
| G2 | 2,5-Dimethylbenzaldehyde | 44.758 | 5779-94-2 | 39.62 ± 2.52 b | 48.48 ± 5.54 ab | 49.5 ± 2.57 a |
| G3 | 3, 5-Methanocyclopentapyrazole-3a-6a-hexahydro-3a-4-trimethyl- | 11.218 | 87143-58-6 | 0.06 ± 0.00 | - | - |
| G4 | 1H-1, 2, 3, 4-Tetrazol-5-ylmethanamine | 22.213 | 31602-63-8 | 13.76 ± 1.39 | 16.24 ± 0.63 | - |
| Total concentrations | 53.44 ± 2.38 b | 64.72 ± 5.43 a | 50.00 ± 2.57 b | |||
| No. | Odorants | Threshold (mg/kg) | OAVs | Odor Description | ||
|---|---|---|---|---|---|---|
| Alcohols | RW | RV | RWRV | |||
| A2 | Phenylethyl alcohol | 0.045 m | 3957 | 5723 | 3874 | Honey, spice, rose, lilac * |
| A3 | Linalool | 0.0015 m | / | / | 513 | Flower, lavender * |
| A6 | 1-Dodecanol | 0.066 m | 12 | / | / | Fat, wax * |
| A7 | 3-Methyl-1-butanol | 0.25 m | 169.9 | 247.72 | / | Alcoholic, banana, fruity, malt, whiskey ※ |
| Acids | ||||||
| B1 | Octanoic acid | 101 m | <1 | <1 | <1 | Sweat, cheese * |
| B2 | Nonanoic acid | 1.5 m | <1 | / | <1 | Green, fat * |
| B3 | Hexanoic acid | 80 m | <1 | <1 | <1 | Sweat * |
| B4 | Heptanoic acid | 10 m | <1 | <1 | <1 | Apricot, Floral, Sour ★ |
| Esters | ||||||
| C1 | Ethyl caprylate | 10 m | 4.5 | 7.6 | 4 | Apricot, banana, brandy, fat, fruit, pear, sweet ※ |
| C2 | Ethyl nonanoate | 1.2 m | <1 | 1.32 | <1 | Fruity, natural, rose, waxy, wine ※ |
| C4 | Ethyl hexanoate | 0.0005 m | 292,280 | 394,840 | 287,120 | Apple, peel, fruity * |
| C5 | Ethyl heptanoate | 0.17 m | 18.06 | 27.65 | 16.75 | Pineapple, cognac, rummy, winey ※ |
| C8 | Ethyl laurate | 0.33 m | 19.88 | / | / | clean, floral, leaf ※ |
| C10 | Benzyl isovalerate | 0.1 m | 24.2 | 31.2 | 20.2 | Sweet, fruity, apple, pineapple, herbal ★ |
| C11 | Ethyl isovalerate | 0.0002 m | 247,150 | 264,900 | 252,100 | Apple, fruit, pineapple, sweet ※ |
| C12 | 3-Methylbutyl 3-methylbutanoate | 0.046 n | 1317.4 | 1678.3 | 1203.5 | Apple, mango, fruity, jammy ※ |
| C13 | Ethyl 2-methylbutyrate | 0.00015 m | / | 114,133 | / | Apple, delicious * |
| C14 | Butanoic acid, 2-methyl-3-methylbutyl ester | 0.14 n | / | 30.5313 | / | Apple, blueberry, cherry, citrus ※ |
| C18 | Ethyl benzoate | 300 m | <1 | <1 | <1 | Camomile, flower, celery, fruit * |
| C19 | Ethyl phenylacetate | 0.1 m | 131.1 | 184.4 | / | Anise, balsam, bitter, chocolate * |
| C20 | Ethyl trans-4-octenoate | 0.05 n | 313.6 | / | / | Fruity, pear, citrus ★ |
| C25 | Ethyl 3,3-dimethylacrylate | 0.025 n | 20.64 | / | / | / |
| C27 | Isoamyl benzoate | 0.25 n | 306.76 | 403.96 | 256.32 | Balsamic, green, sweet ※ |
| C28 | Isoamyl acetate | 0.003 m | / | / | 300 | Banana, bitter, fruity ※ |
| others | ||||||
| G2 | 2,5-Dimethylbenzaldehyde | 0.2 | 198.1 | 242.4 | 247.5 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, B.; Fei, L.; Lu, Z.; Mao, Y.; Zhang, Q.; Zhao, X.; Tang, F.; Shan, C.; Zhang, D.; Cai, W. Effects of Different Yeasts on the Physicochemical Properties and Aroma Compounds of Fermented Sea Buckthorn Juice. Fermentation 2025, 11, 195. https://doi.org/10.3390/fermentation11040195
Peng B, Fei L, Lu Z, Mao Y, Zhang Q, Zhao X, Tang F, Shan C, Zhang D, Cai W. Effects of Different Yeasts on the Physicochemical Properties and Aroma Compounds of Fermented Sea Buckthorn Juice. Fermentation. 2025; 11(4):195. https://doi.org/10.3390/fermentation11040195
Chicago/Turabian StylePeng, Bo, Liyue Fei, Ziyi Lu, Yiwen Mao, Qin Zhang, Xinxin Zhao, Fengxian Tang, Chunhui Shan, Dongsheng Zhang, and Wenchao Cai. 2025. "Effects of Different Yeasts on the Physicochemical Properties and Aroma Compounds of Fermented Sea Buckthorn Juice" Fermentation 11, no. 4: 195. https://doi.org/10.3390/fermentation11040195
APA StylePeng, B., Fei, L., Lu, Z., Mao, Y., Zhang, Q., Zhao, X., Tang, F., Shan, C., Zhang, D., & Cai, W. (2025). Effects of Different Yeasts on the Physicochemical Properties and Aroma Compounds of Fermented Sea Buckthorn Juice. Fermentation, 11(4), 195. https://doi.org/10.3390/fermentation11040195






