Eco-Friendly Biosurfactant: Tackling Oil Pollution in Terrestrial and Aquatic Ecosystems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microorganism and Preparation of Inoculum
2.3. The Production of the Biosurfactant
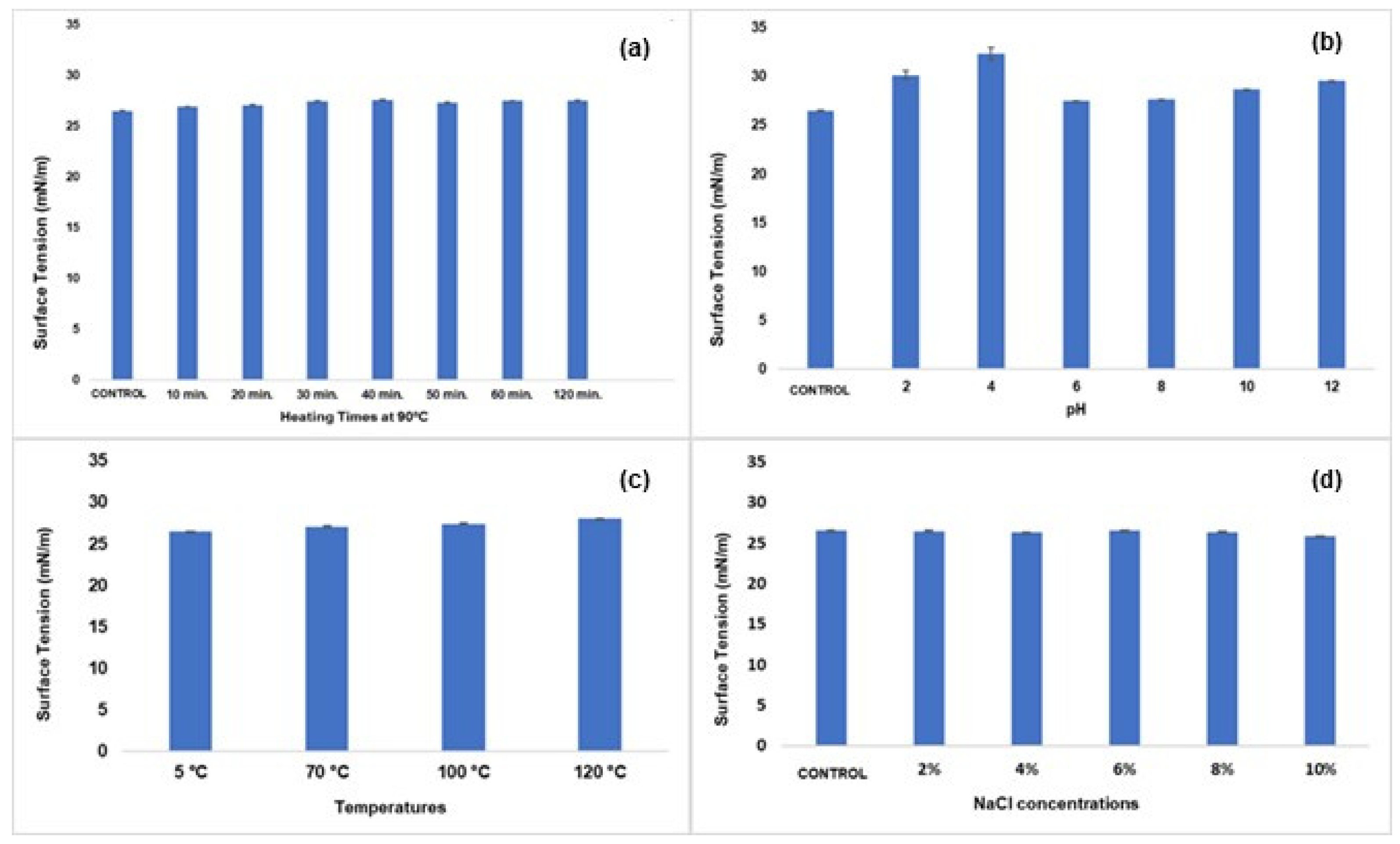
2.4. Determination of Surface Tension
2.5. Emulsification Activity Assessment
2.6. Assessment of Biosurfactant Stability
2.7. Extraction and Isolation of Biosurfactant
2.8. Determination of Critical Micelle Concentration
2.9. Composition of Biosurfactant
2.10. Analysis of Ecotoxicity
2.11. Dispersion of Hydrophobic Compounds in Seawater
2.12. Removal of Petroleum Derivative Adsorbed to Sand and Soils Using Biosurfactant in Flasks—Kinetic Assay
2.13. Removal of Petroleum Derivative Adsorbed to Sand and Soils Using Biosurfactant in Packed Columns—Static Assay
2.14. Cleaning of Oily Surface
2.15. Destabilization of OCB1 and Diesel Oil on Smooth Surface
2.16. Application of Biosurfactant in Treatment of Oily Industrial Effluent
2.17. Statistical Analysis of Results
3. Results and Discussion
3.1. Selection of Biosurfactant Production Medium
3.2. Emulsification Activity
3.3. Physicochemical Properties of the Biosurfactant
3.4. Critical Micelle Concentration of Biosurfactant
3.5. Ecotoxicity with Seeds
3.6. Toxicity with Artemia salina
3.7. Chemical Composition of Biosurfactant
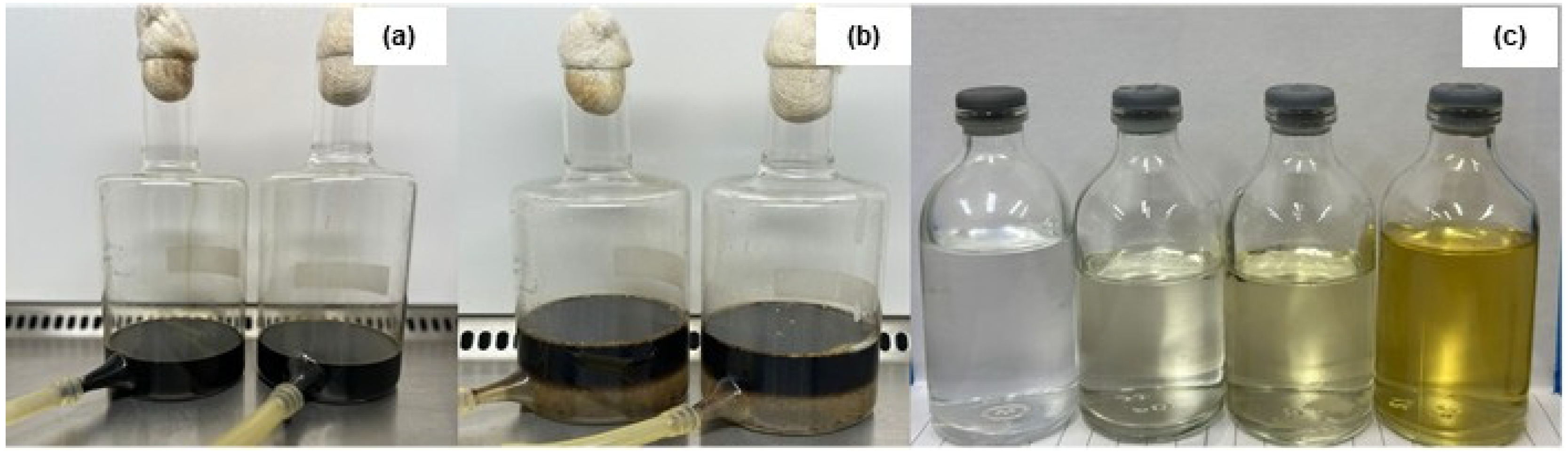
3.8. Dispersion of Hydrophobic Compounds in Seawater
3.9. Removal of Petroleum Derivative Adsorbed to Sand and Soils by Biosurfactant—Kinetic Assay
3.10. Removal of Petroleum Derivative Adsorbed to Sand and Soils by Biosurfactant—Static Assay
3.11. Cleaning of Petroleum Derivative on Oily Surface by Biosurfactant
3.12. Destabilization of Petroleum Derivative
3.13. Treatment of Oily Industrial Effluent by Biosurfactant
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aryan, V.; Kraft, A. The crude tall oil value chain: Global availability and the influence of regional energy policies. J. Clean. Prod. 2021, 280, 124616. [Google Scholar] [CrossRef]
- Griffiths, S.; Sovacool, B.K.; Kim, J.; Bazilian, M.; Uratani, J.M. Decarbonizing the oil refining industry: A systematic review of sociotechnical systems, technological innovations, and policy options. Energy Res. Soc. Sci. 2022, 89, 102542. [Google Scholar] [CrossRef]
- Shaikhah, D.; Loise, V.; Angelico, R.; Porto, M.; Calandra, P.; Abe, A.A.; Caputo, P. New trends in biosurfactants: From renewable origin to green enhanced oil recovery applications. Molecules 2024, 29, 301. [Google Scholar] [CrossRef] [PubMed]
- Płaza, G.; Achal, V. Biosurfactants: Eco-friendly and innovative biocides against biocorrosion. Int. J. Biol. Macromol. 2020, 21, 2152. [Google Scholar] [CrossRef]
- Purohit, B.K.; Tewari, S.; Prasad, K.S.N.V.; Talari, V.K.; Pandey, N.; Choudhury, P.; Panda, S.S. Marine oil spill clean-up: A review on technologies with recent trends and challenges. Reg. Stud. Mar. Sci. 2024, 80, 103876. [Google Scholar] [CrossRef]
- Zacharias, D.C.; Lemos, A.T.; Keramea, P.; Dantas, R.C.; Da Rocha, R.P.; Crespo, N.M.; Sylaios, G.; Jovane, L.; Da Silva Santos, I.G.; Montone, R.C.; et al. Offshore oil spills in Brazil: An extensive review and further development. Mar. Pollut. Bull. 2024, 205, 116663. [Google Scholar] [CrossRef]
- Zahed, M.A.; Matinvafa, M.A.; Azari, A.; Mohajeri, L. Biosurfactant, a green and effective solution for bioremediation of petroleum hydrocarbons in the aquatic environment. Discov. Water 2022, 2, 5. [Google Scholar] [CrossRef]
- Duarah, P.; Haldar, D.; Patel, A.K.; Dong, C.D.; Singhania, R.R.; Purkait, M.K.A. Review on global perspectives of sustainable development in bioenergy generation. Bioresour. Technol. 2022, 348, 126791. [Google Scholar] [CrossRef]
- Zhao, F.; Cui, Q.; Su, H.; Li, C.; Dong, M. Biosurfactants are green and versatile enhancers for sustainable treatment of oily sludge: A review. J. Environ. Chem. Eng. 2025, 13, 115087. [Google Scholar] [CrossRef]
- Segovia-Hernández, J.G.; Hernández, S.; Cossío-Vargas, E.; Sánchez-Ramírez, E. Tackling sustainability challenges in latin america and caribbean from the chemical engineering perspective: A literature review in the last 25 years. Chem. Eng. Res. Des. Trans. Inst. Chem. Eng. 2022, 188, 483–527. [Google Scholar] [CrossRef]
- Silva, M.D.G.C.; Medeiros, A.O.; Converti, A.; Almeida, F.C.G.; Sarubbo, L.A. Biosurfactants: Promising biomolecules for agricultural applications. Sustainability 2024, 16, 449. [Google Scholar] [CrossRef]
- Buskey, E.J.; White, H.K.; Esbaugh, A.J. Impact of oil spills on marine life in the Gulf of Mexico: Effects on plankton, nekton, and deep-sea benthos. Oceanography 2016, 29, 174–181. [Google Scholar] [CrossRef]
- Nikolova, C.; Gutierrez, T. Biosurfactants and their applications in the oil and gas industry: Current state of knowledge and future perspectives. Front. Bioeng. Biotechnol. 2021, 9, 626639. [Google Scholar] [CrossRef] [PubMed]
- Nikolopoulou, M.; Pasadakis, N.; Norf, H.; Kalogerakis, N. Enhanced ex situ bioremediation of crude oil contaminated beach sand by supplementation with nutrients and rhamnolipids. Mar. Pollut. Bull. 2013, 77, 37–44. [Google Scholar] [CrossRef]
- Kugaji, M.; Ray, S.K.; Parvatikar, P.; Raghu, A.V. Biosurfactants: A review of different strategies for economical production, their applications and recent advancements. Adv. Colloid Interface Sci. 2025, 337, 103389. [Google Scholar] [CrossRef]
- Markam, S.S.; Raj, A.; Kumar, A.; Khan, M.L. Microbial biosurfactants: Green alternatives and sustainable solution for augmenting pesticide remediation and management of organic waste. Curr. Res. Microb. Sci. 2024, 7, 100266. [Google Scholar] [CrossRef]
- Guzmán, E.; Maestro, A.; Ortega, F.; Rubio, R.G. Association of oppositely charged polyelectrolyte and surfactant in solution: Equilibrium and nonequilibrium features. J. Condens. Matter Phys. 2023, 35, 323001. [Google Scholar] [CrossRef]
- Lavanya, M.; Machado, A.A. Surfactants as biodegradable sustainable inhibitors for corrosion control in diverse media and conditions: A comprehensive review. Sci. Total Environ. 2024, 908, 168407. [Google Scholar] [CrossRef]
- Sorhie, V.; Alemtoshi; Gogoi, B.; Walling, B.; Acharjee, S.A.; Bharali, P. Role of micellar nanoreactors in organic chemistry: Green and synthetic surfactant review. Sustain. Chem. Pharm. 2022, 30, 100875. [Google Scholar] [CrossRef]
- Al-Sakkaf, M.K.; Onaizi, S.A. Effects of emulsification factors on the characteristics of crude oil emulsions stabilized by chemical and biosurfactants: A review. Fuel 2024, 361, 130604. [Google Scholar] [CrossRef]
- Nandwani, S.K.; Saxena, N.; Kumar, A. Enhanced oil recovery potential analysis through simulation of a bio-based surfactant using CFD. J. Mol. Liq. 2024, 396, 124112. [Google Scholar] [CrossRef]
- Dini, S.; Bekhit, A.E.D.A.; Roohinejad, S.; Vale, J.M.; Agyei, D. The physicochemical and functional properties of biosurfactants: A review. Molecules 2024, 29, 2544. [Google Scholar] [CrossRef] [PubMed]
- Biktasheva, L.; Gordeev, A.; Usova, A.; Kirichenko, A.; Kuryntseva, P.; Selivanovskaya, S. Bioremediation of oil-contaminated soils using biosurfactants produced by bacteria of the genus Nocardiopsis sp. Microbiology 2024, 15, 2575–2592. [Google Scholar] [CrossRef]
- Raddadi, N.; Giacomucci, L.; Totaro, G.; Fava, F. Marinobacter sp. from marine sediments produce highly stable surface-active agents for combatting marine oil spills. Microb. Cell Factories 2017, 16, 186. [Google Scholar] [CrossRef]
- Guzmán, E.; Ortega, F.; Rubio, R.G. Exploring the world of rhamnolipids: A critical review of their production, interfacial properties, and potential application. Curr. Opin. Colloid Interface Sci. 2024, 69, 101780. [Google Scholar] [CrossRef]
- Kee, S.H.; Ganeson, K.; Rashid, N.F.M.; Yatim, A.F.M.; Vigneswari, S.; Amirul, A.A.A.; Ramakrishna, S.; Bhubalan, K. A review on biorefining of palm oil and sugar cane agro-industrial residues by bacteria into commercially viable bioplastics and biosurfactants. Fuel 2022, 321, 124039. [Google Scholar] [CrossRef]
- Thakur, V.; Baghmare, P.; Verma, A.; Verma, J.S.; Geed, S.R. Recent progress in microbial biosurfactants production strategies: Applications, technological bottlenecks, and future outlook. Bioresour. Technol. 2024, 408, 131211. [Google Scholar] [CrossRef]
- Abbot, V.; Paliwal, D.; Sharma, A.; Sharma, P. A review on the physicochemical and biological applications of biosurfactants in biotechnology and pharmaceuticals. Heliyon 2022, 8, 10149. [Google Scholar] [CrossRef]
- Vieira, I.M.M.; Santos, B.L.P.; Ruzene, D.S.; Silva, D.P. An overview of current research and developments in biosurfactants. J. Ind. Eng. Chem. 2021, 100, 1–18. [Google Scholar] [CrossRef]
- Vučurović, D.; Bajić, B.; Trivunović, Z.; Dodić, J.; Zeljko, M.; Jevtić-Mučibabić, R.; Dodić, S. Biotechnological utilization of agro-industrial residues and by-products—Sustainable production of biosurfactants. Foods 2024, 13, 711. [Google Scholar] [CrossRef]
- Verma, C.; Hussain, C.M.; Quraishi, M.A.; Alfantazi, A. Green surfactants for corrosion control: Design, performance and applications. Adv. Colloid Interface Sci. 2023, 311, 102822. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, L.; Irorere, V.U.; Marchant, R.; Banat, I.M. Marine derived biosurfactants: A vast potential future resource. Biotechnol. Lett. 2018, 40, 1441–1457. [Google Scholar] [CrossRef] [PubMed]
- Jimoh, A.A.; Lin, J. Biosurfactant: A new frontier for greener technology and environmental sustainability. Ecotoxicol. Environ. Saf. 2019, 184, 109607. [Google Scholar] [CrossRef] [PubMed]
- Elayaperumal, S.; Sivamani, Y.; Bhattacharya, D.; Lahiri, D.; Nag, M. Eco-friendly biosurfactant solutions for petroleum hydrocarbon cleanup in aquatic ecosystems. Sustain. Chem. Environ. 2025, 9, 2949–8392. [Google Scholar] [CrossRef]
- Castilho, L.V.A.; Duarte, A.M.; Pasqualino, I.P.; Sousa, J.S.; Nogueira, F.C.S.; Gomez, J.G.C.; Seldin, L.; Freire, D.M.G. Mono- and di-rhamnolipids mixtures from Pseudomonas aeruginosa for use in extreme conditions of pre- and post-salt oil reservoirs compared with synthetic surfactants. Colloids Surf. B Biointerfaces 2025, 245, 114311. [Google Scholar] [CrossRef]
- Vega, G.R.; Stampino, P.G. Bio-Based surfactants and biosurfactants: An overview and main characteristics. Molecules 2025, 30, 863. [Google Scholar] [CrossRef]
- Qazi, M.A.; Wang, Q.; Dai, Z. Sophorolipids bioproduction in the yeast Starmerella bombicola: Current trends and perspectives. Bioresour. Technol. 2022, 346, 126593. [Google Scholar] [CrossRef]
- Eras-Muñoz, E.; Wongsirichot, P.; Ingham, B.; Winterburn, J.; Gea, T.; Font, X. Screening of alternative nitrogen sources for sophorolipid production through submerged fermentation using Starmerella bombicola. Waste Manag. 2024, 186, 23–34. [Google Scholar] [CrossRef]
- Cooper, D.G.; Goldenberg, B.G. Surface-active agents from two Bacillus species. Appl. Environ. Microbiol. 1987, 53, 224–229. [Google Scholar] [CrossRef]
- Samak, N.A.; Mahmoud, T.; Aboulrous, A.A.; Abdelhamid, M.M.; Xing, J. Enhanced biosurfactant production using developed fed-batch fermentation for effective heavy crude oil recovery. Energy Fuels 2020, 34, 14560–14572. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar]
- Manocha, M.S.; San-Blas, G.; Centeno, S. Lipid composition of Paracoccidioides brasiliensis: Possible correlation with virulence of different strains. Microbiology 1980, 117, 147–154. [Google Scholar]
- Tiquia, S.M.; Tam, N.F.Y.; Hodgkiss, I.J. Effects of composting on phytotocicity of spent pig-manure sawdust litter. Environ. Pollut. 1996, 93, 249–256. [Google Scholar] [CrossRef]
- Meyer, B.N.N.R.; Ferrigni, J.E.; Putnam, L.B.; Jacobsen, D.E.; Nichols, D.E.; Mclaughlin, J.L. Brine shrimp: A convenient general bioassay for active plant constituents. J. Med. Plant Res. 1982, 45, 31. [Google Scholar] [CrossRef]
- Saeki, H.; Sasaki, M.; Komatsu, K.; Miura, A.; Matsuda, H. Oil spill remediation by using the remediation agent JE1058BS that contains a biosurfactant produced by Gordonia sp. strain JE-1058. Bioresour. Technol. 2009, 100, 572–577. [Google Scholar] [CrossRef]
- Bezza, F.A.; Chirwa, E.M.N. Biosurfactant from Paenibacillus dendritiformis and its application in assisting polycyclic aromatic hydrocarbon (PAH) and motor oil sludge removal from contaminated soil and sand media. Process Saf. Environ. Prot. 2015, 98, 354–364. [Google Scholar] [CrossRef]
- Rufino, R.D.; Luna, J.M.; Marinho, P.H.C.; Farias, C.B.B.; Ferreira, S.R.M.; Sarubbo, L.A. Removal of petroleum derivative adsorbed to soil by biosurfactant Rufisan produced by Candida lipolytica. J. Petrol. Sci. Eng. 2013, 109, 117–122. [Google Scholar] [CrossRef]
- Pruthi, V.; Cameotra, S.S. Novel sucrose lipid produced by Serratia marcescens and its application in enhanced oil recovery. J. Surfactants Deterg. 2000, 3, 533–537. [Google Scholar]
- Sobrinho, H.B.S.; De Luna, J.M.; Rufino, R.D.; Figueiredo, A.L.; Sarubbo, P.A. Application of biosurfactant from Candida sphaerica UCP 0995 in removal of petroleum derivative from soil and sea water. J. Life Sci. 2013, 7, 559–569. [Google Scholar] [CrossRef]
- Soares Da Silva, R.D.C.F.; De Almeida, D.G.; Brasileiro, P.P.F.; Rufino, R.D.; De Luna, J.M.; Sarubbo, L.A. Production, formulation and cost estimation of a commercial biosurfactant. Biodegradation 2019, 30, 191–201. [Google Scholar] [CrossRef]
- Monteiro, M.R.; Kugelmeier, C.L.; Pinheiro, R.S.; Batalha, M.O.; César, A.S. Glycerol from biodiesel production: Technological paths for sustainability. Renew. Sustain. Energy Rev. 2018, 88, 109–122. [Google Scholar] [CrossRef]
- Farias, C.B.B.; Almeida, F.C.; Silva, I.A.; Souza, T.C.; Meira, H.M.; Soares da Silva, R.C.F.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Production of green surfactants: Market prospects. Electron. J. Biotechnol. 2021, 51, 28–39. [Google Scholar] [CrossRef]
- Selva Filho, A.A.P.; Converti, A.; Soares Da Silva, R.D.C.F.; Sarubbo, L.A. Biosurfactants as multifunctional remediation agents of environmental pollutants generated by the petroleum industry. Energies 2023, 16, 1209. [Google Scholar] [CrossRef]
- Shaji, A.; Thamarai, P.; Deivayanai, V.C.; Saravanan, A.; Yaashikaa, P.R. Progress in sustainable remediation: Utilizing biosurfactants for eco-friendly contaminant cleanup. Bioresour. Technol. Rep. 2024, 27, 101901. [Google Scholar] [CrossRef]
- Maciel, C.D.C.S.; Da Silva Andrade, R.F.; De Gusmão, N.B.; De Campos Takaki, G.M. Caracterização de biossurfactante por Curvularia lunata-ufpeda885 para descontaminação de solos por óleo automotivo. Interfaces Cient.-Saúde E Ambiente 2024, 9, 7–23. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, J.; Wu, J.C.; Li, Q. Degradation of puffed feather to produce functional biosurfactants by Chitinophaga eiseniae 4 H. Process Biochem. 2025, 148, 168–175. [Google Scholar] [CrossRef]
- Ribeiro, B.G.; Leão, V.L.X.S.; Guerra, J.M.C.; Sarubbo, L.A. Cookies and muffins containing biosurfactant: Textural, physicochemical and sensory analyses. J. Food Sci. Technol. 2023, 60, 2180–2192. [Google Scholar] [CrossRef]
- da Silva, P.F.F.; da Silva, R.R.; Sarubbo, L.A.; Guerra, J.M.C. Production and optimization of biosurfactant properties using Candida mogii and Licuri oil (Syagrus coronata). Foods 2024, 13, 4029. [Google Scholar] [CrossRef]
- Poomalai, P.; Krishnan, J.; Ravichandran, A.; Sureshkumar, R. Biosurfactants: Sustainable alternative to synthetic surfactants and their applications. Int. J. Appl. Pharm. 2024, 16, 34–43. [Google Scholar] [CrossRef]
- Ferreira, I.N.S.; Rodríguez, D.M.; Campos-Takaki, G.M.; Da Silva Andrade, R.F. Biosurfactant and bioemulsifier as promising molecules produced by Mucor hiemalis isolated from Caatinga soil. Electron. J. Biotechnol. 2020, 47, 51–58. [Google Scholar] [CrossRef]
- Jaysree, R.C.; Basu, S.; Singh, P.P.; Ghosal, T.; Patra, P.A.; Keerthi, Y. Isolation of Biosurfactant Producing Bacteria from Environmental samples. Pharmacologyonline 2021, 63, 1427–1433. [Google Scholar]
- Hassanshahian, M. Isolation and characterization of biosurfactant producing bacteria from Persiangulf (bushehr provenance). Mar. Pollut. Bull. 2014, 86, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Barakat, K.M.; Hassan, S.W.M.; Darwesh, O.M. Biosurfactant production by haloalkaliphilic Bacillus strains isolated from Red Sea. Egypt. J. Aquat. Res. 2017, 43, 205–211. [Google Scholar] [CrossRef]
- Souza, K.S.T.; Gudiña, E.J.; Schwan, R.F.; Rodrigues, L.R.; Dias, D.R.; Teixeira, J.A. Improvement of biosurfactant production by Wickerhamomyces anomalus CCMA 0358 and its potential application in bioremediation. J. Hazard. Mater. 2018, 346, 152–158. [Google Scholar] [CrossRef]
- Purwasena, I.A.; Fitri, D.K.; Putri, D.M.; Endro, H.; Zakaria, M.N. Lipopeptide biosurfactant as a potential root canal irrigation agent: Antimicrobial and anti-biofilm evaluation. J. Dent. 2024, 144, 104961. [Google Scholar] [CrossRef]
- Kumari, K.; Behera, H.T.; Nayak, P.P.; Sinha, A.; Nandi, A.; Ghosh, A.; Raina, V. Amelioration of lipopeptide biosurfactants for enhanced antibacterial and biocompatibility through molecular antioxidant property by methoxy and carboxyl moieties. Biomed. Pharmacother. 2023, 161, 114493. [Google Scholar] [CrossRef]
- Gaur, V.K.; Regar, R.K.; Dhiman, N.; Gautam, K.; Srivastava, J.K.; Patnaik, S.; Kamthan, M.; Manickam, N. Biosynthesis and characterization of sophorolipid biosurfactant by Candida spp.: Application as food emulsifier and antibacterial agent. Bioresour. Technol. 2019, 285, 121314. [Google Scholar] [CrossRef]
- Xia, M.; Wang, S.; Chen, B.; Qiu, R.; Fan, G. Enhanced solubilization and biodegradation of HMW-PAHs in water with a Pseudomonas mosselii-released biosurfactant. Polymers 2023, 15, 4571. [Google Scholar] [CrossRef]
- Sharma, R.; Singh, J.; Verma, N. Production, characterization and environmental applications of biosurfactants from Bacillus amyloliquefaciens and Bacillus subtilis. Biocatal. Agric. Biotechnol. 2018, 16, 132–139. [Google Scholar] [CrossRef]
- Silva, J.R.M.; De Oliveira Barros, V.P.; Terceiro, P.S.; De Oliveira, I.N.; Da Silva Moura, O.F.; De Freitas, J.D.; Landell, M.F. Brazilian mangrove sediments as a source of biosurfactant-producing yeast Pichia pseudolambica for bioremediation. Chemosphere 2024, 365, 143285. [Google Scholar] [CrossRef]
- Werrie, P.Y.; Durenne, B.; Delaplace, P.; Fauconnier, M.L. Phytotoxicity of essential oils: Opportunities and constraints for the development of biopesticides. Foods 2020, 9, 1291. [Google Scholar] [CrossRef] [PubMed]
- Umar, A.; Zafar, A.; Wali, H.; Siddique, M.P.; Malik, Z.A.; Ahmed, S. Surfactin-like biosurfactant production and optimization by Bacillus subtilis SNW3: Product characterization and its influence on seed development and plant growth. Res. Sq. 2021, 550205. [Google Scholar] [CrossRef]
- Lima, B.G.; Santos, J.C.; Silva, R.R.; Caldas, M.C.F.; Meira, H.M.; Rufino, R.D.; Luna, J.M. Sustainable production of biosurfactant grown in medium with industrial waste and use for removal of oil from Soil and Seawater. Surfaces 2024, 7, 537–549. [Google Scholar] [CrossRef]
- Da Silva, L.M.; Da Silva, F.J. Bioassay with Artemia salina L.: A gateway to understanding the toxicity of medicinal plant extracts. In Botânica, Ecologia e Sustentabilidade: Uma Perspectiva Multidisciplinar; Editora Científica Digital: Guarujá, Brazil, 2023; Volume 1, pp. 50–69. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Liang, S.; Song, D. Characterization of sophorolipids from the yeast Starmerella bombicola O-13-1 using waste fried oil and cane molasses as substrates. Desalination Water Treat. 2018, 119, 267–275. [Google Scholar] [CrossRef]
- To, M.H.; Wang, H.; Miao, Y.; Kaur, G.; Roelants, S.L.K.W.; Lin, C.S.K. Optimal preparation of food waste to increase its utility for sophorolipid production by Starmerella bombicola. Bioresour. Technol. 2023, 379, 128993. [Google Scholar] [CrossRef]
- Essghaier, B.; Mallat, N.; Khwaldia, K.; Mottola, F.; Rocco, L.; Hannachi, H. Production and characterization of new biosurfactants/bioemulsifiers from Pantoea alhagi and their antioxidant, antimicrobial and anti-biofilm potentiality evaluations. Molecules 2023, 28, 1912. [Google Scholar] [CrossRef]
- Ren, H.; Zhou, S.; Wang, B.; Peng, L.; Li, X. Treatment mechanism of sludge containing highly viscous heavy oil using biosurfactant. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124117. [Google Scholar] [CrossRef]
- Vu, K.A.; Mulligan, C.N. An overview on the treatment of oil pollutants in soil using synthetic and biological surfactant foam and nanoparticles. Int. J. Mol. Sci. 2023, 24, 1916. [Google Scholar] [CrossRef]
- Galitskaya, P.; Gordeev, A.; Ezhkin, N.; Biktasheva, L.; Kuryntseva, P.; Selivanovskaya, S. Bacterial cultural media containing lipopeptides for heavy oil recovery enhancement: The results of sand-packed column experiment. Processes 2023, 11, 3203. [Google Scholar] [CrossRef]
- Barata, M.I.C.; Cavalcanti, M.H.C.; Rufino, R.D.; De Almeida, F.C.G.; Sarubbo, L.A. Optimized production and properties of biosurfactant from Bacillus invictae UCP1617 and its performance in a detergent formulation for environmental applications. J. Surfactants Deterg. 2024, 28, 155–169. [Google Scholar] [CrossRef]
- Soares da Silva, R.D.C.F.; Barbosa Farias, C.B.; Gomes de Almeida, F.C.; da Silva, I.A.; Sarubbo, L.A. Comparative Evaluation Between a Non-Toxic Biodetergent and a Commercial Degreaser for Industrial Use. CET J.-Chem. Eng. Trans. 2024, 110, 391–396. [Google Scholar] [CrossRef]
- Noll, P.; Solarte-Toro, J.C.; Restrepo-Serna, D.L.; Treinen, C.; Poveda-Giraldo, J.A.; Henkel, M.; Hausmann, R. Limits for sustainable biosurfactant production: Techno-economic and environmental assessment of a rhamnolipid production process. Bioresour. Technol. Rep. 2024, 25, 101767. [Google Scholar] [CrossRef]
- Volpato, C.P.T.; Heck, M.C.; Gigliolli, A.A.S.; Yoshimoto-Higaki, M.; Godoy, M.A.F.; Magnoni, D.M.; Vicentini, V.E.P. Utilization of glycerol as substrate in the production of biosurfactant. Res. Soc. Dev. 2022, 11, 16. [Google Scholar] [CrossRef]
- Freitas, B.G.; Brito, J.G.; Brasileiro, P.P.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Formulation of a commercial biosurfactant for application as a dispersant of petroleum and by-products spilled in oceans. Front. Microbiol. 2016, 7, 1646. [Google Scholar] [CrossRef]








| Media Tested | Culture Conditions |
|---|---|
| 5.0% glycerol + 2.0% glucose | pH 6.0 |
| 1.0% N-hexadecane + 2.0% glucose | 200 rpm |
| 3.0% sucrose + 0.1% yeast extract (*) | 96, 120 and 144 h |
| 2.0% sugarcane molasses + 3.0% corn steep liquor (*) | 28 °C |
| Concentration of Isolated Biosurfactant | Germination Index (%) | |
|---|---|---|
| Tomato (Solanum lycopersicum) | Cabbage (Brassica oleracea) | |
| ½ CMC | 90.5 ± 0.18 | 80.5 ± 0.11 |
| CMC | 86.0 ± 0.13 | 72.2 ± 0.14 |
| 2 CMC | 80.8 ± 0.15 | 65.0 ± 0.12 |
| Type of Soil | Biosurfactant | Contact Time (h) | Removal Rate (%) |
|---|---|---|---|
| Standard sand | Crude biosurfactant | 0.5 | 16.96 ± 0.21 |
| 1.0 | 27.39 ± 0.05 | ||
| 2.0 | 53.51 ± 0.81 | ||
| 24.0 | 55.11 ± 0.22 | ||
| Isolated biosurfactant at ½ CMC | 0.5 | 48.89 ± 0.16 | |
| 1.0 | 65.66 ± 0.92 | ||
| 2.0 | 72.26 ± 0.11 | ||
| 24.0 | 75.75 ± 0.14 | ||
| Isolated biosurfactant at CMC | 0.5 | 56.05 ± 0.16 | |
| 1.0 | 69.51 ± 0.18 | ||
| 2.0 | 73.62 ± 0.23 | ||
| 24.0 | 82.48 ± 0.12 | ||
| Isolated biosurfactant at 2 × CMC | 0.5 | 55.85 ± 0.17 | |
| 1.0 | 69.82 ± 1.14 | ||
| 2.0 | 74.02 ± 0.27 | ||
| 24.0 | 82.01 ± 0.22 | ||
| Clayey soil | Crude biosurfactant | 0.5 | 62.40 ± 2.65 |
| 1.0 | 77.43 ± 0.05 | ||
| 2.0 | 77.92 ± 2.75 | ||
| 24.0 | 88.00 ± 0.24 | ||
| Isolated biosurfactant at ½ CMC | 0.5 | 76.87 ± 0.48 | |
| 1.0 | 91.00 ± 0.08 | ||
| 2.0 | 91.40 ± 1.08 | ||
| 24.0 | 98.68 ± 0.01 | ||
| Isolated biosurfactant at CMC | 0.5 | 81.07 ± 0.06 | |
| 1.0 | 91.45 ± 0.17 | ||
| 2.0 | 91.72 ± 2.23 | ||
| 24.0 | 98.81 ± 0.01 | ||
| Isolated biosurfactant at 2 × CMC | 0.5 | 85.79 ± 1.58 | |
| 1.0 | 92.66 ± 0.17 | ||
| 2.0 | 93.87 ± 0.63 | ||
| 24.0 | 99.01 ± 0.02 | ||
| Silty soil | Crude biosurfactant | 0.5 | 50.31 ± 0.45 |
| 1.0 | 82.94 ± 0.05 | ||
| 2.0 | 85.38 ± 0.04 | ||
| 24.0 | 89.80 ± 0.01 | ||
| Isolated biosurfactant at ½ CMC | 0.5 | 62.57 ± 1.57 | |
| 1.0 | 96.00 ± 0.00 | ||
| 2.0 | 96.20 ± 0.78 | ||
| 24.0 | 97.01 ± 0.03 | ||
| Isolated biosurfactant at CMC | 0.5 | 68.50 ± 0.85 | |
| 1.0 | 96.25 ± 0.01 | ||
| 2.0 | 97.00 ± 0.11 | ||
| 24.0 | 97.21 ± 0.01 | ||
| Isolated biosurfactant at 2 × CMC | 0.5 | 71.00 ± 2.18 | |
| 1.0 | 97.04 ± 0.08 | ||
| 2.0 | 97.22 ± 0.63 | ||
| 24.0 | 97.61 ± 0.00 |
| Soil | Removal Rate (%) | ||||
|---|---|---|---|---|---|
| Water (Control) | Crude Biosurfactant | Isolated Biosurfactant | |||
| ½ CMC | CMC | 2 × CMC | |||
| Standard sand | 10.52 ± 2.3% | 49.35 ± 1.08% | 52.00 ± 0.60% | 63.70 ± 0.59% | 72.05 ± 0.80% |
| Silty soil | 5.67 ± 0.64% | 85.48 ± 0.34% | 92. 81 ± 0.21% | 93.95 ± 0.43% | 96.87 ± 0.30% |
| Clayey soil | 8.12 ± 1.59% | 81.40 ± 0.27% | 97.15 ± 0.11% | 99.20 ± 0.08% | 99. 50 ± 0.03% |
| Hydrophobic Compound | Concentration of Biosurfactant | ||||
|---|---|---|---|---|---|
| Water (Control) | Isolated Biosurfactant | ||||
| ½ CMC | CMC | 2 × CMC | 5 × CMC | ||
| Motor oil | 25.38 ± 4.21 | 72.15 ± 0.69 | 80.83 ± 8.10 | 83.34 ± 2.54 | 85.58 ± 0.71 |
| OCB1 oil | 5.94 ± 2.21 | 58.93 ± 8.93 | 72.76 ± 0.57 | 86.87 ± 1.36 | 88.49 ± 3.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, K.W.; Selva Filho, A.A.P.; Faccioli, Y.E.S.; Araújo, G.P.; Converti, A.; Soares da Silva, R.d.C.F.; Sarubbo, L.A. Eco-Friendly Biosurfactant: Tackling Oil Pollution in Terrestrial and Aquatic Ecosystems. Fermentation 2025, 11, 199. https://doi.org/10.3390/fermentation11040199
Oliveira KW, Selva Filho AAP, Faccioli YES, Araújo GP, Converti A, Soares da Silva RdCF, Sarubbo LA. Eco-Friendly Biosurfactant: Tackling Oil Pollution in Terrestrial and Aquatic Ecosystems. Fermentation. 2025; 11(4):199. https://doi.org/10.3390/fermentation11040199
Chicago/Turabian StyleOliveira, Kaio Wêdann, Alexandre Augusto P. Selva Filho, Yslla Emanuelly S. Faccioli, Gleice Paula Araújo, Attilio Converti, Rita de Cássia F. Soares da Silva, and Leonie A. Sarubbo. 2025. "Eco-Friendly Biosurfactant: Tackling Oil Pollution in Terrestrial and Aquatic Ecosystems" Fermentation 11, no. 4: 199. https://doi.org/10.3390/fermentation11040199
APA StyleOliveira, K. W., Selva Filho, A. A. P., Faccioli, Y. E. S., Araújo, G. P., Converti, A., Soares da Silva, R. d. C. F., & Sarubbo, L. A. (2025). Eco-Friendly Biosurfactant: Tackling Oil Pollution in Terrestrial and Aquatic Ecosystems. Fermentation, 11(4), 199. https://doi.org/10.3390/fermentation11040199








