Bacteriocin Production by Lactiplantibacillus plantarum LD1 in Solid-State Fermentation Using Lignocellulosic Substrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials, Growth Conditions, and Bacterial Strains
2.2. Growth and Production of Bacteriocin
2.3. Bioprocess Development Using Plackett–Burman Design
2.4. Bioprocess Development Using Response Surface Methodology
2.5. Scale-Up of Growth and Bacteriocin Production
2.6. Statistical Analysis
3. Results and Discussion
3.1. Lignocellulosic Substrates for the Production of Bacteriocin in SSF
3.2. Plackett–Burman Design
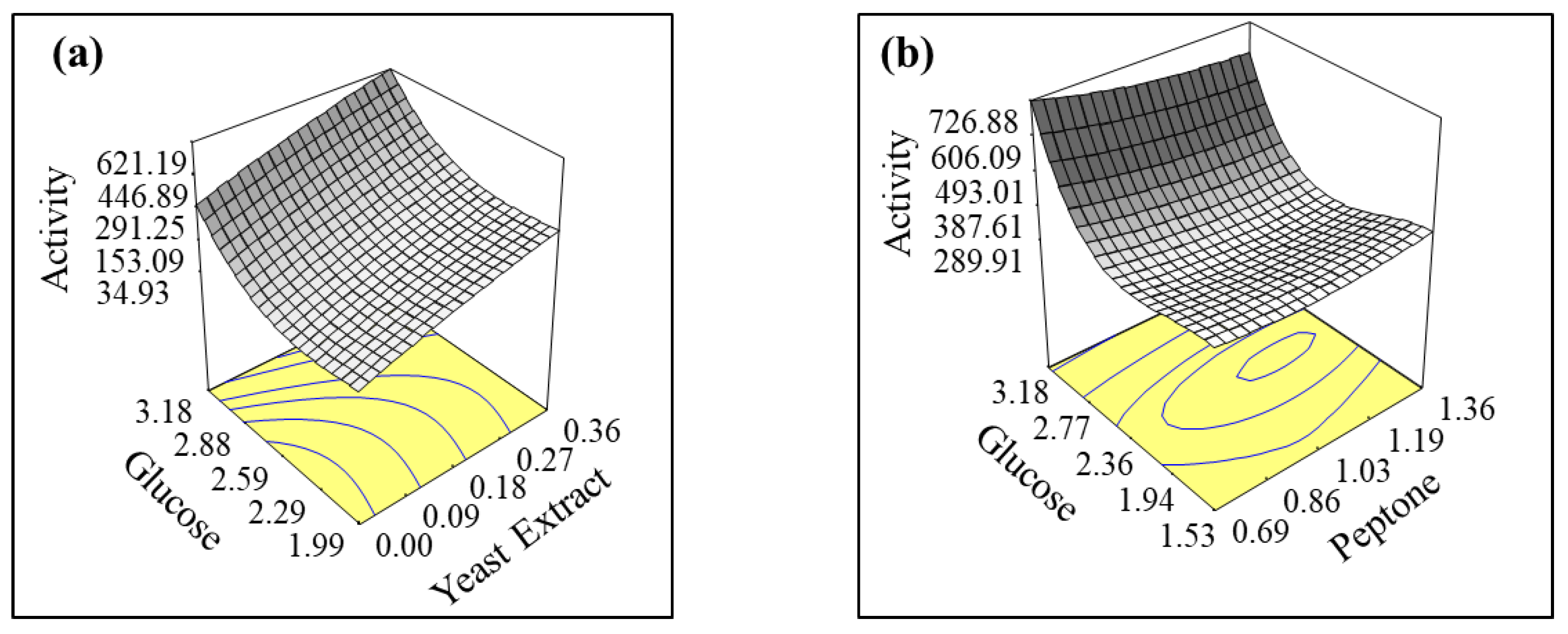
3.3. Response Surface Methodology
189.79D2 + 1.19B3 + 1.60C3 + 225.00D3 − 0.91AB − 0.64AC + 1.71AD − 2.83BC −
21.94BD − 10.17CD + 11.89BCD.
3.4. Scale-Up of Growth and Bacteriocin Production in Optimized Condition
3.5. Cost-Effectiveness of the Bioprocess
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alokika; Singh, B. Enhanced Production of Bacterial Xylanase and its Utility in Saccharification of Sugarcane Bagasse. Bioprocess Biosyst. Eng. 2020, 43, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Pandey, L.K.; Singh, T.A.; Singh, R.; Passari, A.K.; Singh, T.; Pandey, P.K.; Khare, N. Utilization of Agro-Industrial based Polysaccharides Waste for Microbial Production of Prebiotics: A Review. Biol. Forum. 2023, 15, 955–961. [Google Scholar]
- Li, F.; Lv, B.; Zuo, J.; Nawaz, S.; Wang, Z.; Lian, L.; Yin, H.; Chen, S.; Han, X.; Wang, H. Effect of Solid-State Fermentation Products of Lactobacillus plantarum, Candida utilis, and Bacillus coagulans on Growth Performance of Broilers and Prevention of Avian Colibacillosis. Vet. Sci. 2024, 11, 468. [Google Scholar] [CrossRef] [PubMed]
- Salim, A.A.; Grbavčić, S.; Šekuljica, N.; Stefanović, A.; Tanasković, S.J.; Luković, N.; Knežević-Jugović, Z. Production of Enzymes by a Newly Isolated Bacillus sp. TMF-1 in Solid State Fermentation on Agricultural By-Products: The Evaluation of Substrate Pre-treatment methods. Biores. Technol. 2017, 228, 193–200. [Google Scholar] [CrossRef]
- Bartkiene, E.; Mozuriene, E.; Lele, V.; Zokaityte, E.; Gruzauskas, R.; Jakobsone, I.; Juodeikiene, G.; Ruibys, R.; Bartkevics, V. Changes of Bioactive Compounds in Barley Industry By-Products During Submerged and Solid-State Fermentation with Antimicrobial Pediococcu sacidilactici strain LUHS29. Food Sci. Nutri. 2020, 8, 340–350. [Google Scholar] [CrossRef]
- Onipe, O.O.; Jideani, A.I.; Beswa, D. Composition and Functionality of Wheat Bran and its Application in Some Cereal Food Products. Int. J. Food Sci. Technol. 2015, 50, 2509–2518. [Google Scholar] [CrossRef]
- Hemdane, S.; Langenaeken, N.A.; Jacobs, P.J.; Verspreet, J.; Delcour, J.A.; Courtin, C.M. Study of the Intrinsic Properties of Wheat Bran and Pearlings Obtained by Sequential Debranning and Their Role in Bran-Enriched Bread Making. J. Cereal Sci. 2016, 71, 78–85. [Google Scholar] [CrossRef]
- Coda, R.; Melama, L.; Rizzello, C.G.; Curiel, J.A.; Sibakov, J.; Holopainen, U.; Pulkkinen, M.; Sozer, N. Effect of Air Classification and Fermentation by Lactobacillus plantarum VTT E-133328 on Faba Bean (Vicia faba L.) Flour Nutritional Properties. Int. J. Food Microbiol. 2015, 193, 34–42. [Google Scholar] [CrossRef]
- Kumar, G.S.; Krishna, A.G. Studies on the Nutraceutical’s Composition of Wheat Derived Oils, Wheat Bran Oil and Wheat Germ Oil. J. Food Sci. Technol. 2015, 52, 1145–1151. [Google Scholar] [CrossRef]
- Spaggiari, M.; Ricci, A.; Calani, L.; Bresciani, L.; Neviani, E.; Dall’Asta, C.; Lazzi, C.; Galaverna, G. Solid State Lactic Acid Fermentation: A Strategy to Improve Wheat Bran Functionality. LWT 2020, 118, 108668. [Google Scholar] [CrossRef]
- O’Connor, P.M.; Kuniyoshi, T.M.; Oliveira, R.P.; Hill, C.; Ross, R.P.; Cotter, P.D. Antimicrobials for Food and Feed: A Bacteriocin Perspective. Curr. Opin. Biotechnol. 2020, 61, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Reuben, R.C.; Roy, P.C.; Sarkar, S.L.; Rubayet UI Alam, A.S.M.; Jahid, I.K. Characterization and Evaluation of Lactic Acid Bacteria from Indigenous Raw Milk for Potential Probiotic Properties. J. Dairy Sci. 2020, 103, 1223–1237. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, L.; Ross, R.P.; Hill, C. Potential of Bacteriocin-Producing Lactic Acid Bacteria for Improvements in Food Safety and Quality. Biochimie 2002, 84, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P. Recent Approaches in Food Bio-Preservation-A Review. Open Vet. J. 2018, 8, 104–111. [Google Scholar] [CrossRef]
- Cotter, P.D. An ‘Upp’-turn in Bacteriocin Receptor Identification. Mol. Microbiol. 2014, 92, 1159–1163. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An Overview of Natural Antimicrobials Role in Food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef]
- Zhang, J.; Bu, Y.; Zhang, C.; Yi, H.; Liu, D.; Jiao, J. Development of a Low-cost and High-efficiency Culture Medium for Bacteriocin Lac-B23 Production by Lactobacillus plantarum J23. Biology 2020, 9, 171. [Google Scholar] [CrossRef]
- Gupta, A.; Tiwari, S.K. Probiotic Potential of Lactobacillus plantarum LD1 Isolated from Batter of Dosa, a South Indian Fermented Food. Probiotics Antimicro. Proteins 2014, 6, 73–81. [Google Scholar] [CrossRef]
- Gupta, A.; Tiwari, S.K. Plantaricin LD1: A Bacteriocin Produced by Food Isolate of Lactobacillus plantarum LD1. Appl. Biochem. Biotechnol. 2014, 172, 3354–3362. [Google Scholar] [CrossRef]
- Cotârleț, M.; Stănciuc, N.; Bahrim, G.E. Yarrowialipolytica and Lactobacillus paracasei Solid State Fermentation as a Valuable Biotechnological Tool for the Pork Lard and Okara’s Biotransformation. Microorganisms 2020, 8, 1098. [Google Scholar] [CrossRef]
- Tong, Y.; Guo, H.N.; Abbas, Z.; Zhang, J.; Wang, J.; Cheng, Q.; Peng, S.; Yang, T.; Bai, T.; Zhou, Y.; et al. Optimizing Postbiotic Production through Solid-State Fermentation with Bacillus amyloliquefaciens J and Lactiplantibacillus plantarum SN4 enhances Antibacterial, Antioxidant, and Anti-inflammatory Activities. Front. Microbiol. 2023, 14, 1229952. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.N.; Devadas, S.M.; Murugan, S.; Krishnan, S.G.; Thayumanavan, T. Production and Characterization of Bacteriocin by Lactic Acid Bacterium-Pediococcuspentosaceus NKSM1 Isolated from Fermented ‘appam’ batter. J. Pure Appl. Microbiol. 2018, 12, 1315–1330. [Google Scholar] [CrossRef]
- Yoo, H.; Rheem, I.; Rheem, S.; Oh, S. Optimizing Medium Components for the Maximum Growth of Lactobacillus plantarum JNU 2116 using Response Surface Methodology. Food Sci. Anim. Resour. 2018, 38, 240–250. [Google Scholar]
- Choi, G.H.; Lee, N.K.; Paik, H.D. Optimization of Medium Composition for Biomass Production of Lactobacillus plantarum 200655 using Response Surface Methodology. J. Microbiol. Biotechnol. 2021, 31, 717–725. [Google Scholar] [CrossRef]
- Natarajan, K.; Rajendran, A. Evaluation and Optimization of Food-Grade Tannin Acyl Hydrolase Production by a Probiotic Lactobacillus plantarum Strain in Submerged and Solid-State Fermentation. Food Bioprod. Process. 2012, 90, 780–792. [Google Scholar] [CrossRef]
- Costa-Trigo, I.; Paz, A.; Otero-Penedo, P.; Outeiriño, D.; Guerra, N.P.; Domínguez, J.M. Enhancing the saccharification of pretreated chestnut burrs to produce bacteriocins. J. Biotechnol. 2021, 329, 13–20. [Google Scholar] [CrossRef]
- Coghetto, C.C.; Vasconcelos, C.B.; Brinques, G.B.; Ayub, M.A.Z. Lactobacillus plantarum BL011 Cultivation in Industrial Isolated Soybean Protein Acid Residue. Braz. J. Microbiol. 2016, 47, 941–948. [Google Scholar] [CrossRef]
- Kuniyoshi, T.M.; Mendonça, C.M.N.; Vieira, V.B.; Robl, D.; de Melo Franco, B.D.G.; Todorov, S.D.; Tome, E.; O’Connor, P.M.; Converti, A.; Araujo, W.L.; et al. Pediocin PA-1 production by Pediococcus pentosaceus ET34 using non-detoxified hemicellulose hydrolysate obtained from hydrothermal pretreatment of sugarcane bagasse. Biores. Technol. 2021, 338, 125565. [Google Scholar] [CrossRef]
- Salman, M.; Shahid, M.; Sahar, T.; Naheed, S.; Arif, M.; Iqbal, M.; Nazir, A. Development of regression model for bacteriocin production from local isolate of Lactobacillus acidophilus MS1 using Box-Behnken design. Biocatal. Agric. Biotechnol. 2020, 24, 101542. [Google Scholar] [CrossRef]
- Gökmen, G.G.; Sarıyıldız, S.; Cholakov, R.; Nalbantsoy, A.; Baler, B.; Aslan, E.; Duzel, A.; Sargin, S.; Goksungur, Y.; Kışla, D. A novel Lactiplantibacillus plantarum strain: Probiotic properties and optimization of the growth conditions by response surface methodology. World J. Microbiol Biotechnol. 2024, 40, 66. [Google Scholar] [CrossRef]
- Gowdhaman, D.; Sugumaran, K.R.; Ponnusami, V. Optimization of Lactic Acid Production from Tea Waste by Lactobacillus plantarum MTCC 6161 in Solid State Fermentation by Central Composite Design. Int. J. Chemtech. Res. 2012, 4, 143–148. [Google Scholar]
- Prema, P.; Ali, D.; Nguyen, V.H.; Pradeep, B.V.; Veeramanikandan, V.; Daglia, M.; Arciola, C.R.; Balaji, P. A Response Surface Methodological Approach for Large-Scale Production of Antibacterials from Lactiplantibacillus plantarum with Potential Utility against Foodborne and Orthopedic Infections. Antibiotics 2024, 13, 437. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.H.; Pan, T.M. Characterization of an Antimicrobial Substance Produced by Lactobacillus plantarum NTU 102. J. Microbiol. Immunol. Infect. 2019, 52, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Trinetta, V.; Rollini, M.; Manzoni, M. Development of a Low-cost Culture Medium for SakacinA Production by L. sakei. Process Biochem. 2008, 43, 1275–1280. [Google Scholar] [CrossRef]

| S. No. | Substrates | Growth (log10 CFU/mL) * | Final pH * | Bacteriocin Production (AU/mL) * |
|---|---|---|---|---|
| 1 | Wheat bran | 8.15 ± 0.20 | 4.64 ± 0.07 | 391.69 ± 12.58 |
| 2 | Rice straw | 7.66 ± 0.05 | 5.17 ± 0.06 | 219.80 ± 8.58 |
| 4 | Mustard oil cake | 7.43 ± 0.05 | 4.84 ± 0.04 | 362.50 ± 22.50 |
| 5 | Sugarcane bagasse | 8.43 ± 0.13 | 4.92 ± 0.20 | 284.56 ± 17.50 |
| 6 | Corn cobs | 7.92 ± 0.03 | 4.68 ± 0.01 | 10.89 ± 0.21 |
| 7 | Congress Grass | 7.69 ± 0.37 | 5.15 ± 0.03 | 4.68 ± 0.12 |
| 8 | Rice bran | 8.09 ± 0.09 | 4.78 ± 0.19 | 380.73 ± 18.29 |
| 9 | Oat bran | 7.74 ± 0.24 | 5.54 ± 0.12 | 20.54 ± 0.20 |
| 10 | Chickpea bran | 6.16 ± 0.09 | 5.72 ± 0.12 | 17.12 ± 0.13 |
| Run | Peptone (%) | Meat Extract (%) | Yeast Extract (%) | Glucose (%) | Sodium Acetate (%) | Tri-Ammonium Citrate (%) | Di-potassium Phosphate (%) | Manganese Sulphate (%) | Magnesium Sulphate (%) | Tween 80 (%) | Substrate | Growth (log10 CFU/mL) * | Final pH * | Bacteriocin Production (AU/mL) * |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 (−) | 0 (−) | 0.5 (+) | 2 (+) | 0.5 (+) | 0 (−) | 0.2 (+) | 0.02 (+) | 0 (−) | 0.1 (+) | WB | 6.31 ± 0.27 | 4.82 ± 0.16 | 1.89 ± 0.01 |
| 2 | 1 (+) | 0 (−) | 0 (−) | 0 (−) | 0.5 (+) | 0.2 (+) | 0.2 (+) | 0 (−) | 0.05 (+) | 0.1 (+) | WB | 7.95 ± 0.38 | 5.30 ± 0.03 | 11.22 ± 0.12 |
| 3 | 1 (+) | 1 (+) | 0 (−) | 2 (+) | 0.5 (+) | 0 (−) | 0.2 (+) | 0 (−) | 0 (−) | 0 (−) | WB + RS | 7.66 ± 0.29 | 4.56 ± 0.18 | 22.13 ± 0.10 |
| 4 | 0 (−) | 1 (+) | 0 (−) | 0 (−) | 0 (−) | 0.2 (+) | 0.2 (+) | 0.02 (+) | 0 (−) | 0.1 (+) | WB + RS | 7.66 ± 0.25 | 5.17 ± 0.09 | 31.42 ± 1.18 |
| 5 | 0 (−) | 1 (+) | 0.5 (+) | 2 (+) | 0 (−) | 0.2 (+) | 0.2 (+) | 0 (−) | 0.05 (+) | 0 (−) | WB | 8.73 ± 0.20 | 4.32 ± 0.16 | 451.44 ± 20.58 |
| 6 | 1 (+) | 0 (−) | 0.5 (+) | 0 (−) | 0 (−) | 0 (−) | 0.2 (+) | 0.02 (+) | 0.05 (+) | 0 (−) | WB + RS | 7.37 ± 0.24 | 5.17 ± 0.16 | 30.52 ± 1.01 |
| 7 | 1 (+) | 1 (+) | 0 (−) | 2 (+) | 0 (−) | 0 (−) | 0 (−) | 0.02 (+) | 0.05 (+) | 0.1 (+) | WB | 8.45 ± 0.10 | 4.37 ± 0.20 | 667.25 ± 28.08 |
| 8 | 0 (−) | 0 (−) | 0 (−) | 2 (+) | 0.5 (+) | 0.2 (+) | 0 (−) | 0.02 (+) | 0.05 (+) | 0 (−) | WB + RS | 6.93 ± 0.25 | 4.53 ± 0.20 | 18.67 ± 1.32 |
| 9 | 0 (−) | 1 (+) | 0.5 (+) | 0 (−) | 0.5 (+) | 0 (−) | 0 (−) | 0 (−) | 0.05 (+) | 0.1 (+) | WB + RS | 7.24 ± 0.07 | 5.27 ± 0.08 | 22.19 ± 2.01 |
| 10 | 0 (−) | 0 (−) | 0 (−) | 0 (−) | 0 (−) | 0 (−) | 0 (−) | 0 (−) | 0 (−) | 0 (−) | WB | 7.57 ± 0.46 | 5.31 ± 0.19 | 29.81 ± 1.41 |
| 11 | 1 (+) | 0 (−) | 0.5 (+) | 2 (+) | 0 (−) | 0.2 (+) | 0 (−) | 0 (−) | 0 (−) | 0.1 (+) | WB + RS | 8.63 ± 0.20 | 4.15 ± 0.07 | 353.25 ± 21.20 |
| 12 | 1 (+) | 1 (+) | 0.5 (+) | 0 (−) | 0.5 (+) | 0.2 (+) | 0 (−) | 0.02 (+) | 0 (−) | 0 (−) | WB | 7.69 ± 0.16 | 5.15 ± 0.04 | 29.09 ± 1.11 |
| S. No. | Variables (%) | Actual Value of Each Coded Level | ||||
|---|---|---|---|---|---|---|
| −∞ | −1 | 0 | +1 | +∞ | ||
| 1 | Glucose | 0.50 | 1.13 | 1.75 | 2.38 | 3.00 |
| 2 | Peptone | 0.25 | 0.69 | 1.13 | 1.56 | 2.00 |
| 3 | Yeast Extract | 0.25 | 0.69 | 1.13 | 1.56 | 2.00 |
| 4 | Tri-ammonium citrate | 0.10 | 0.20 | 0.30 | 0.40 | 0.50 |
| Run | Peptone (A) | Yeast Extract (B) | Glucose (C) | Tri-Ammonium Citrate (D) | Growth (log10 CFU/mL) * | Final pH * | Bacteriocin Production (AU/mL) * |
|---|---|---|---|---|---|---|---|
| 1 | +1 | +1 | −1 | −1 | 8.80 ± 0.18 | 4.69 ± 0.22 | 502.40 ± 22.05 |
| 2 | 0 | 0 | 0 | 0 | 8.46 ± 0.40 | 4.51 ± 0.33 | 377.49 ± 18.29 |
| 3 | 0 | 0 | 0 | 0 | 8.29 ± 0.31 | 4.51 ± 0.33 | 377.49 ± 27.29 |
| 4 | +1 | +1 | +1 | +1 | 8.46 ± 0.34 | 4.54 ± 0.30 | 529.32 ± 19.05 |
| 5 | +1 | +1 | +1 | −1 | 8.41 ± 0.24 | 4.52 ± 0.33 | 307.48 ± 20.02 |
| 6 | 0 | 0 | −∞ | 0 | 8.47 ± 0.38 | 4.68 ± 0.21 | 01.89 ± 0.12 |
| 7 | 0 | 0 | 0 | 0 | 8.29 ± 0.31 | 4.51 ± 0.33 | 329.46 ± 28.58 |
| 8 | 0 | 0 | 0 | 0 | 8.40 ± 0.38 | 4.51 ± 0.33 | 307.48 ± 23.20 |
| 9 | −1 | −1 | +1 | +1 | 8.23 ± 0.23 | 4.53 ± 0.30 | 241.38 ± 12.10 |
| 10 | −1 | −1 | +1 | −1 | 8.64 ± 0.20 | 4.52 ± 0.32 | 353.25 ± 24.15 |
| 11 | +1 | −1 | +1 | +1 | 8.86 ± 0.05 | 4.58 ± 0.25 | 241.38 ± 11.07 |
| 12 | −1 | −1 | +1 | −1 | 8.54 ± 0.09 | 4.54 ± 0.30 | 307.48 ± 22.13 |
| 13 | +1 | −1 | −∞ | −1 | 8.63 ± 0.18 | 4.62 ± 0.33 | 262.10 ± 25.02 |
| 14 | 0 | 0 | −1 | −∞ | 8.63 ± 0.11 | 4.61 ± 0.36 | 284.56 ± 28.01 |
| 15 | +1 | +1 | 0 | +1 | 8.66 ± 0.22 | 4.61 ± 0.34 | 179.21 ± 17.02 |
| 16 | −1 | +1 | 0 | +1 | 8.82 ± 0.07 | 4.57 ± 0.28 | 529.32 ± 31.06 |
| 17 | −∞ | 0 | −1 | 0 | 8.69 ± 0.19 | 4.63 ± 0.32 | 425.86 ± 38.01 |
| 18 | +1 | −1 | −∞ | −1 | 8.49 ± 0.25 | 4.37 ± 0.41 | 377.50 ± 21.05 |
| 19 | +∞ | 0 | −1 | 0 | 8.67 ± 0.19 | 4.59 ± 0.32 | 475.94 ± 27.02 |
| 20 | 0 | 0 | −1 | 0 | 8.23 ± 0.23 | 4.36 ± 0.34 | 284.56 ± 20.12 |
| 21 | −1 | +1 | −1 | −1 | 8.72 ± 0.16 | 4.62 ± 0.33 | 425.86 ± 36.13 |
| 22 | −1 | −1 | −1 | +1 | 8.72 ± 0.24 | 4.50 ± 0.23 | 241.38 ± 12.10 |
| 23 | 0 | +∞ | 0 | 0 | 8.58 ± 0.50 | 4.56 ± 0.29 | 502.40 ± 33.04 |
| 24 | +1 | −1 | −1 | +1 | 8.72 ± 0.24 | 4.59 ± 0.18 | 329.46 ± 19.17 |
| 25 | −1 | +1 | +1 | −1 | 8.85 ± 0.08 | 4.53 ± 0.24 | 451.44 ± 27.03 |
| 26 | 0 | 0 | +∞ | 0 | 8.83 ± 0.06 | 4.59 ± 0.28 | 475.94 ± 36.01 |
| 27 | 0 | −∞ | 0 | 0 | 8.37 ± 0.35 | 4.44 ± 0.28 | 158.96 ± 06.01 |
| 28 | 0 | 0 | 0 | 0 | 8.26 ± 0.27 | 4.35 ± 0.35 | 377.50 ± 18.06 |
| 29 | 0 | 0 | 0 | +∞ | 8.56 ± 0.42 | 4.30 ± 0.45 | 582.86 ± 28.15 |
| 30 | −1 | +1 | −1 | +1 | 8.22 ± 0.20 | 4.47 ± 0.26 | 377.50 ± 07.26 |
| S. No. | Culture Volume (g) | Growth (log10 CFU/mL) | Final pH | Bacteriocin Activity (AU/mL) |
|---|---|---|---|---|
| 1 | 5 | 8.56 ± 0.42 | 4.30 ± 0.45 | 582.86 ± 32.87 |
| 2 | 10 | 8.60 ± 0.38 | 4.31 ± 0.30 | 630.23 ± 23.76 |
| 3 | 50 | 8.63 ± 0.26 | 4.32 ± 0.23 | 601.13 ± 18.17 |
| 4 | 100 | 8.64 ± 0.05 | 4.32 ± 0.57 | 591.89 ± 33.04 |
| 5 | 150 | 8.64 ± 0.34 | 4.31 ± 0.34 | 592.04 ± 12.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rani, P.; Singh, B.; Tiwari, S.K. Bacteriocin Production by Lactiplantibacillus plantarum LD1 in Solid-State Fermentation Using Lignocellulosic Substrates. Fermentation 2025, 11, 233. https://doi.org/10.3390/fermentation11040233
Rani P, Singh B, Tiwari SK. Bacteriocin Production by Lactiplantibacillus plantarum LD1 in Solid-State Fermentation Using Lignocellulosic Substrates. Fermentation. 2025; 11(4):233. https://doi.org/10.3390/fermentation11040233
Chicago/Turabian StyleRani, Pushpa, Bijender Singh, and Santosh Kumar Tiwari. 2025. "Bacteriocin Production by Lactiplantibacillus plantarum LD1 in Solid-State Fermentation Using Lignocellulosic Substrates" Fermentation 11, no. 4: 233. https://doi.org/10.3390/fermentation11040233
APA StyleRani, P., Singh, B., & Tiwari, S. K. (2025). Bacteriocin Production by Lactiplantibacillus plantarum LD1 in Solid-State Fermentation Using Lignocellulosic Substrates. Fermentation, 11(4), 233. https://doi.org/10.3390/fermentation11040233






