Bioactive Properties of Fermented Beverages: Wine and Beer
Abstract
1. Introduction
2. Winemaking and Brewing Process
2.1. Wine
2.1.1. Must Grape Composition
- -
- After water, which constitutes approximately 80–85% of the must, serving as the solvent for all other components [21], the most abundant substances in grapes at ripeness are sugars, in the form of fructose and glucose. These are found in almost equimolar amounts since they arise from hydrolysis of the disaccharide sucrose, produced through leaf photosynthesis. These fermentable sugars provide the primary substrate for yeast during fermentation [22]. Small amounts of pentoses and other sugars are also present alongside the primary sugars. Related to sugars are polysaccharides, which are long chains of sugar molecules that come from the cell walls of grape berries. These carbohydrate polymers are naturally present in the grape must. During winemaking, certain enzymatic treatments can hydrolyze some of these polysaccharides, causing them to be released into the must and ultimately into the wine. It can affect its texture, mouthfeel, and overall quality by making it feel fuller or smoother [23].
- -
- Organic acids: Organic acids are a crucial group of compounds found in grape juices and wines. They play a key role in influencing the wine’s chemical and microbiological stability, impacting factors like appearance, pH, and titratable acidity. Additionally, these acids contribute significantly to the sensory characteristics of wine, such as its flavor [24,25,26]. Tartaric acid is typically the dominant acid in grape juice and serves as an important indicator of its composition. It plays a crucial role in preserving the chemical stability of wine, influencing factors like color and flavor [27]. Tartaric acid is produced during the early stages of berry cell development and continues to form as the berries mature [26]. Malic acid is produced early in the growing season, but its behavior during grape maturation and winemaking differs from that of tartaric acid. Initially, malic acid levels are gradually broken down as the berries ripen. The concentration of malic acid tends to be lower in warmer climates and in fully ripened grapes compared to cooler regions and less ripe grapes, depending on the specific growing conditions [25,26]. Malic acid will also contribute substantially to acidity and can be metabolized by lactic acid bacteria during malolactic fermentation, softening the wine’s taste. Minor acids in grapes include citric and ascorbic, although the former will also be produced through yeast metabolism, and traces of other acids have also been documented [28]. These acids are naturally found in grape juices. Other organic acids, such as succinic, lactic, and acetic acids, are also present in juice or wine, but they are primarily by-products of the winemaking process, including fermentation and microbial activity [25]. Succinic acid is produced early in the fermentation process, with its formation influenced by the yeast strain used and the specific nitrogen source available [26]. Citric acid plays a key role in metabolic processes, such as the Krebs cycle, and serves as an acidifying agent in food and beverages. In wine, an elevated concentration of citric acid may indicate adulteration [27]. Lactic acid bacteria can partially or completely metabolize citric acid, with acetic acid as a potential product. Additionally, citric acid metabolism contributes to the formation of diacetyl, acetoin, and 2,3-butanediol. The buttery aroma present in some wines arises from the diacetyl pathway. Higher acetic acid levels in wine typically result from spoilage by lactic acid or acetic acid bacteria [26]. Table 1 contains a summary of the main organic acids, their location, and their benefits.
- -
- Phenolic compounds in grapes must play a crucial role in the flavor, color, mouthfeel, and overall quality of wine. These compounds are primarily derived from grape skins, seeds, and stems, and they can be extracted into the must during crushing and maceration. Phenolic compounds are naturally occurring substances consisting of one or more hydroxyl groups bonded to aromatic or benzene rings. Based on their benzene ring structure, they are classified as either flavonoids or nonflavonoids [29]. In wine, polyphenols play a crucial role in shaping various sensory characteristics, including appearance, color, astringency, bitterness, and flavor, while also contributing to the wine’s stability during oxidative processes [30]. These compounds originate from various parts of the grape: (1) the skins, which are rich in anthocyanins, flavan-3-ols, flavonols, dihydroflavonols, hydroxycinnamoyl tartaric acids, hydroxybenzoic acids, and hydroxystilbenes; (2) the seeds, where flavan-3-ols and gallic acid predominate; and (3) the juice, which primarily contains hydroxycinnamoyl tartaric acids [31]. The phenolic profile of a wine is shaped by the composition of the grape, the extent to which these compounds are extracted during juice processing, and the chemical reactions that occur during vinification, post-fermentation treatments, and aging [32].
- -
- Nitrogen compounds, especially amino acids and ammonium, are the major nitrogenous compounds present in grapes and are critical for yeast growth and fermentation. In grape must, nitrogen compounds play a key role in fermentation. About half of the nitrogen compounds are α-amino acids, which are forms of nitrogen that yeast can use during fermentation [23]. These are referred to as yeast assimilable nitrogen (YAN), which is crucial because it helps yeast grow and complete fermentation properly. The remainder is proline and cannot be utilized. Environmental factors as well as grape variety can influence the amino acid content of the must [33]. That is why when the levels of NFA are low, the must is usually supplemented with nitrogen, such as di-ammonium phosphate, to avoid fermentation problems.
- -
- The mineral content that can be present in must and wine refers to cations and their elements. These minerals are classified according to their electrical charge, as well as their abundance, into abundant cations (plant macronutrients): K+, Ca2+, Mg2+, Na+, and Si4+, and less abundant cations (micronutrients): Fe3+, Mn2+, Zn2+, Al3+, Cu2+, Ni2+, Li+, Mo4+, Co2+, and V3+. And in more abundant anions: PO43, SO42−, Cl−, and less abundant: Br−, I− [22].
- -
- The vitamin content of grapes is usually used up by yeasts during the fermentation process, but due to yeast metabolism, we find vitamins in the wine at levels similar to the beginning [34]. Vitamins generally found in fresh grapes are ascorbic acid, niacin, vitamin B6, riboflavin, thiamine, folate, and vitamin A (USDA nutritional analysis of fresh grapes) [35].
- -
- Aromatic compounds: The precursors of C13-norisoprenoid aromatic compounds are carotenoids, which, although found in low concentrations in grapes, are present in all plants. On the other hand, isoprenoids will also contribute to the aroma of wine, and among these are monoterpenoids, sesquiterpenoids, and C13-norisoprenoids. The floral aroma of Muscat grapes is due on the one hand to the C13-norisoprenoids (they exist as non-volatile glycosides) and on the other hand to a free fraction of monoterpenoids [36]. Pyrazines are nitrogenous compounds derived from the metabolism of amino acids [37]. They are associated with typical vegetal aromas related to herbaceous character and are characteristic of Sauvignon Blanc [38,39] and Cabernet Franc varieties. They are mostly found in the skins and seeds, with a smaller proportion in the pulp [40]. Three main pyrazines have been identified in grapes: 3-isobutyl-2-methoxypyrazine (IBMP), 3-sec-butyl-2-methoxypyrazine (SBMP), and 3-isopropyl-2-methoxypyrazine (IPMP) [41].
2.1.2. Winemaking
2.1.3. Ecology During Wine Fermentation
2.2. Beer
2.2.1. Wort Composition
2.2.2. Brewing Process
3. Bioactive Compounds in Raw Materials
3.1. Wine
Grapes
3.2. Beer
3.2.1. Hop and Barley
3.2.2. Botanicals
3.2.3. Microorganisms
Functional Yeasts
Functional LAB
Elements
4. Nutritional Composition and Bioactive Compounds in the Fermentation Process
4.1. Nutritional Composition
4.1.1. Protein
4.1.2. Carbohydrates
4.1.3. Energy Value
4.2. Bioactive Compounds in Fermented Beverages
4.2.1. Antioxidant Capacity
4.2.2. Polyphenols
4.3. Melatonin Production
5. Bio Healthy Beverages
5.1. Low-Alcohol and Alcohol-Free Beer and Wine
5.2. Gluten-Free Beer
5.3. Healthier Beer and Wine
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Radonjić, S.; Maraš, V.; Raičević, J.; Košmerl, T. Wine or Beer? Comparison, Changes and Improvement of Polyphenolic Compounds during Technological Phases. Molecules 2020, 25, 4960. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.; Lahlou, R.A.; Pires, P.; Salgado, M.; Silva, L.R. Natural Functional Beverages as an Approach to Manage Diabetes. Int. J. Mol. Sci. 2023, 24, 16977. [Google Scholar] [CrossRef] [PubMed]
- Giovinazzo, G.; Grieco, F. Functional Properties of Grape and Wine Polyphenols. Plant Foods Hum. Nutr. 2015, 70, 454–462. [Google Scholar] [CrossRef] [PubMed]
- El Rayess, Y.; Nehme, N.; Azzi-Achkouty, S.; Julien, S.G. Wine Phenolic Compounds: Chemistry, Functionality and Health Benefits. Antioxidants 2024, 13, 1312. [Google Scholar] [CrossRef]
- Esteban-Fernández, A.; Zorraquín-Peña, I.; González de Llano, D.; Bartolomé, B.; Moreno-Arribas, M.V. The Role of Wine and Food Polyphenols in Oral Health. Trends Food Sci. Technol. 2017, 69, 118–130. [Google Scholar] [CrossRef]
- Salanță, L.C.; Coldea, T.E.; Ignat, M.V.; Pop, C.R.; Tofană, M.; Mudura, E.; Borșa, A.; Pasqualone, A.; Anjos, O.; Zhao, H. Functionality of Special Beer Processes and Potential Health Benefits. Processes 2020, 8, 1613. [Google Scholar] [CrossRef]
- Paiva, R.A.M.; Mutz, Y.S.; Conte-Junior, C.A. A Review on the Obtaining of Functional Beers by Addition of Non-Cereal Adjuncts Rich in Antioxidant Compounds. Antioxidants 2021, 10, 1332. [Google Scholar] [CrossRef]
- Cardinali, D.P.; Esquifino, A.I.; Srinivasan, V.; Pandi-Perumal, S.R. Melatonin and the Immune System in Aging. Neuroimmunomodulation 2008, 15, 272–278. [Google Scholar] [CrossRef]
- Garcia-Parrilla, M.C.; Cantos, E.; Troncoso, A.M. Analysis of Melatonin in Foods. J. Food Compos. Anal. 2009, 22, 177–183. [Google Scholar] [CrossRef]
- Ballester, P.; Zafrilla, P.; Arcusa, R.; Galindo, A.; Cerdá, B.; Marhuenda, J. Food as a Dietary Source of Melatonin and Its Role in Human Health: Present and Future Perspectives. In Current Topics in Functional Food; Shiomi, N., Savitskaya, A., Eds.; IntechOpen: London, UK, 2022; p. 16. [Google Scholar]
- Yang, F.; Chen, C.; Ni, D.; Yang, Y.; Tian, J.; Li, Y.; Chen, S.; Ye, X.; Wang, L. Effects of Fermentation on Bioactivity and the Composition of Polyphenols Contained in Polyphenol-Rich Foods: A Review. Foods 2023, 12, 3315. [Google Scholar] [CrossRef]
- Canonico, L.; Agarbati, A.; Comitini, F.; Ciani, M. Torulaspora delbrueckii in the Brewing Process: A New Approach to Enhance Bioflavour and to Reduce Ethanol Content. Food Microbiol. 2016, 56, 45–51. [Google Scholar] [CrossRef] [PubMed]
- García, M.; Esteve-Zarzoso, B.; Cabellos, J.M.; Arroyo, T. Sequential Non-Saccharomyces and Saccharomyces Cerevisiae Fermentations to Reduce the Alcohol Content in Wine. Fermentation 2020, 6, 60. [Google Scholar] [CrossRef]
- Postigo, V.; Sánchez, A.; Cabellos, J.M.; Arroyo, T. New Approaches for the Fermentation of Beer: Non-Saccharomyces Yeasts from Wine. Fermentation 2022, 8, 280. [Google Scholar] [CrossRef]
- Fernández-Pacheco, P.; Pintado, C.; Pérez, A.B.; Arévalo-Villena, M. Potential Probiotic Strains of Saccharomyces and Non-Saccharomyces: Functional and Biotechnological Characteristics. J. Fungi 2021, 7, 177. [Google Scholar] [CrossRef] [PubMed]
- Diguță, C.F.; Mihai, C.; Toma, R.C.; Cîmpeanu, C.; Matei, F. In Vitro Assessment of Yeasts Strains with Probiotic Attributes for Aquaculture Use. Foods 2023, 12, 124. [Google Scholar] [CrossRef]
- Marsico, A.D.; Velenosi, M.; Perniola, R.; Bergamini, C.; Sinonin, S.; David-vaizant, V.; Maggiolini, F.A.M.; Hervè, A.; Cardone, M.F.; Ventura, M. Native Vineyard Non-Saccharomyces Yeasts Used for Biological Control of Botrytis Cinerea in Stored Table Grape. Microorganisms 2021, 9, 457. [Google Scholar] [CrossRef]
- Agarbati, A.; Canonico, L.; Pecci, T.; Romanazzi, G.; Ciani, M.; Comitini, F. Biocontrol of Non-Saccharomyces Yeasts in Vineyard against the Gray Mold Disease Agent Botrytis Cinerea. Microorganisms 2022, 10, 200. [Google Scholar] [CrossRef]
- Gomomo, Z.; Fanadzo, M.; Mewa-Ngongang, M.; Chidi, B.S.; Hoff, J.W.; van der Rijst, M.; Mokwena, L.; Setati, M.E.; du Plessis, H.W. The Use of Specific Non-Saccharomyces Yeasts as Sustainable Biocontrol Solutions Against Botrytis Cinerea on Apples and Strawberries. J. Fungi 2025, 11, 26. [Google Scholar] [CrossRef]
- Viana, F.; Gil, J.V.; Vallés, S.; Manzanares, P. Increasing the Levels of 2-Phenylethyl Acetate in Wine through the Use of a Mixed Culture of Hanseniaspora Osmophila and Saccharomyces Cerevisiae. Int. J. Food Microbiol. 2009, 135, 68–74. [Google Scholar] [CrossRef]
- Jackson, R.S. Wine Science: Principles and Applications, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 9780123814685. [Google Scholar]
- Moreno, J.; Peinado, R. Composition of Grape Must. In Enological Chemistry; Academic Press: Amsterdam, The Netherlands, 2012; pp. 13–22. ISBN 9780123884381. [Google Scholar]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Grape Must Composition Overview. In Understanding Wine Chemistry; Wiley, Ed.; John Wiley & Sons Ltd.: London, UK, 2024; pp. 200–206. [Google Scholar]
- Coelho, E.M.; da Silva Padilha, C.V.; Miskinis, G.A.; de Sá, A.G.B.; Pereira, G.E.; de Azevêdo, L.C.; dos Santos Lima, M. Simultaneous Analysis of Sugars and Organic Acids in Wine and Grape Juices by HPLC: Method Validation and Characterization of Products from Northeast Brazil. J. Food Compos. Anal. 2018, 66, 160–167. [Google Scholar] [CrossRef]
- Robles, A.; Fabjanowicz, M.; Chmiel, T.; Płotka-Wasylka, J. Determination and Identification of Organic Acids in Wine Samples. Problems and Challenges. TrAC-Trends Anal. Chem. 2019, 120, 115630. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry. In Understanding Wine Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 19–33. ISBN 9781118730720. [Google Scholar]
- Ivanova-Petropulos, V.; Petruševa, D.; Mitrev, S. Rapid and Simple Method for Determination of Target Organic Acids in Wine Using HPLC-DAD Analysis. Food Anal. Methods 2020, 13, 1078–1087. [Google Scholar] [CrossRef]
- Kliewer, W.M. Sugars and Organic Acids of Vitis Vinifera. Plant Physiol. 1966, 41, 923–931. [Google Scholar] [CrossRef]
- Gawel, R.; Smith, P.A.; Cicerale, S.; Keast, R. The Mouthfeel of White Wine. Crit. Rev. Food Sci. Nutr. 2018, 58, 2939–2956. [Google Scholar] [CrossRef]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine Polyphenol Content and Its Influence on Wine Quality and Properties: A Review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef]
- Ivanova-Petropulos, V.; Durakova, S.; Ricci, A.; Parpinello, G.P.; Versari, A. Extraction and Evaluation of Natural Occurring Bioactive Compounds and Change in Antioxidant Activity during Red Winemaking. J. Food Sci. Technol. 2016, 53, 2634–2643. [Google Scholar] [CrossRef]
- Ivanova, V.; Vojnoski, B.; Stefova, M. Effect of Winemaking Treatment and Wine Aging on Phenolic Content in Vranec Wines. J. Food Sci. Technol. 2012, 49, 161–172. [Google Scholar] [CrossRef]
- Huang, Z.; Ough, C.S. Amino Acid Profiles of Commercial Grape Juices and Wines. Am. J. Enol. Vitic. 1991, 42, 261–267. [Google Scholar] [CrossRef]
- Ough, C.S.; Amerine, M.A. Methods Analysis of Musts and Wines; Wiley, Ed.; John Wiley & Sons Ltd.: New York, NY, USA, 1988; ISBN 9780471627579. [Google Scholar]
- U.S. Department of Agriculture; Agricultural Research Service. Composition of Foods Raw, Processed, Prepared USDA National Nutrient Database for Standard Reference, Release 21; U.S. Department of Agriculture; Agricultural Research Service: Beltsville, MD, USA, 2011.
- Hjelmeland, A.K.; Ebeler, S.E. Glycosidically Bound Volatile Aroma Compounds in Grapes and Wine: A Review. Am. J. Enol. Vitic. 2015, 66, 1–11. [Google Scholar] [CrossRef]
- Conde, C.; Silva, P.; Fontes, N.; Dias, A.C.P.; Tavares, R.M.; Sousa, M.J.; Agasse, A.; Delrot, S.; Gerós, H. Biochemical Changes throughout Grape Berry Development and Fruit and Wine Quality. Food 2007, 1, 1–22. [Google Scholar]
- Allen, M.; Lacey, M.; Harris, R.; Brown, W.V. Contribution of Methoxypyrazines to Sauvignon Blanc Wine Aroma. Am. J. Enol. Vitic. 1991, 42, 109–112. [Google Scholar] [CrossRef]
- Lacey, M.J.; Allen, M.S.; Harris, R.L.N.; Brown, W.V. Methoxypyrazines in Sauvignon Blanc Grapes and Wines. Am. J. Enol. Vitic. 1991, 42, 103–108. [Google Scholar] [CrossRef]
- Roujou de Boubee, D. Research on 2-Methoxy-3-Isobutylpyrazine in Grapes and Wine; Academy Amorim Bordx: Napa, CA, USA, 2003. [Google Scholar]
- Allen, M.S.; Lacey, M.J.; Boyd, S.J. Methoxypyrazines in Red Wines: Occurrence of 2-Methoxy-3-(1-Methylethyl) Pyrazine. J. Agric. Food Chem. 1995, 43, 769–772. [Google Scholar] [CrossRef]
- Reynolds, A.G. The Grapevine, Viticulture, and Winemaking: A Brief Introduction. In Grapevine Viruses: Molecular Biology, Diagnostics and Management; Meng, B., Martelli, G.P., Golino, D.A., Fuchs, M., Eds.; Springer: New York, NY, USA, 2017; pp. 3–29. [Google Scholar]
- Alba-Lois, L.; Segal-kischinevzky, C. Yeast Fermentation and the Making of Beer and Wine The History of Beer and Wine Production. Nat. Educ. 2010, 3, 17. [Google Scholar]
- Rice, S.; Tursumbayeva, M.; Clark, M.; Greenlee, D.; Dharmadhikari, M.; Fennell, A.; Koziel, J.A. Effects of Harvest Time on the Aroma of White Wines Made from Cold-Hardy Brianna and Frontenac Gris Grapes Using Headspace Solid-Phase Microextraction and Gas Chromatography-Mass Spectrometry-Olfactometry. Foods 2019, 8, 29. [Google Scholar] [CrossRef]
- Ribereau-Gayon, P. Part II: Vinification. Conditions of Yeast Development. In Handbook of Enology: Volume 1, The Microbiology of Wine and Vinifications; Wiley, Ed.; John Wiley & Sons Ltd.: London, UK, 2006; pp. 79–106. [Google Scholar]
- Unterkofler, J.; Muhlack, R.A.; Jeffery, D.W. Processes and Purposes of Extraction of Grape Components during Winemaking: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2020, 104, 4737–4755. [Google Scholar] [CrossRef]
- Cordero-Bueso, G.; Ruiz-Muñoz, M.; Florido-Barba, A.; Manuel Cantoral Fernández, J. Update on the Role of Saccharomyces Cerevisiae in Sherry Wines. In New Advances in Saccharomyces; Morata, A., González, C., Loira, I., Escott, C., Eds.; Intechopen: London, UK, 2023. [Google Scholar]
- Maicas, S. The Role of Yeasts in Fermentation Processes. Microorganisms 2020, 8, 1142. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, J.; Song, Y.; Zang, X.; Wang, G.; Pei, Y.; Song, Y.; Qin, Y.; Liu, Y. Yeast Diversity during Spontaneous Fermentations and Oenological Characterisation of Indigenous Saccharomyces Cerevisiae for Potential as Wine Starter Cultures. Microorganisms 2022, 10, 1455. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Chen, J.; Philipp, C.; Zhao, X.; Wang, J.; Liu, Y.; Suo, R. Effect of Commercial Yeast Starter Cultures on Cabernet Sauvignon Wine Aroma Compounds and Microbiota. Foods 2022, 11, 1725. [Google Scholar] [CrossRef]
- Ilieva, F.; Petrov, K.; Veličkovska, S.K.; Gunova, N.; Dimovska, V.; Rocha, J.M.F.; Esatbeyoglu, T. Influence of Autochthonous and Commercial Yeast Strains on Fermentation and Quality of Wines Produced from Vranec and Cabernet Sauvignon Grape Varieties from Tikveš Wine-Growing Region, Republic of North Macedonia. Appl. Sci. 2021, 11, 6135. [Google Scholar] [CrossRef]
- Mills, D.A.; Phister, T.; Neeley, E.; Johannsen, E. Wine Fermentation. In Molecular Techniques in the Microbial Ecology of Fermented Foods; Luca Cocolin, D.E., Ed.; Springer: New York, NY, USA, 2008; pp. 162–192. [Google Scholar]
- Vázquez, J.; Mislata, A.M.; Vendrell, V.; Moro, C.; de Lamo, S.; Ferrer-Gallego, R.; Andorrà, I. Enological Suitability of Indigenous Yeast Strains for ‘Verdejo’ Wine Production. Foods 2023, 12, 1888. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, K.L.; Bisson, L.F.; Adams, D.O. A Review of the Effect of Winemaking Techniques on Phenolic Extraction in Red Wines. Am. J. Enol. Vitic. 2005, 56, 197–206. [Google Scholar] [CrossRef]
- Molina, A.M.; Swiegers, J.H.; Varela, C.; Pretorius, I.S.; Agosin, E. Influence of Wine Fermentation Temperature on the Synthesis of Yeast-Derived Volatile Aroma Compounds. Appl. Microbiol. Biotechnol. 2007, 77, 675–687. [Google Scholar] [CrossRef]
- Lasik, M. The Application of Malolactic Fermentation Process to Create Good-Quality Grape Wine Produced in Cool-Climate Countries: A Review. Eur. Food Res. Technol. 2013, 237, 843–850. [Google Scholar] [CrossRef]
- Cerdán, T.G.; Ancín-Azpilicueta, C. Effect of Oak Barrel Type on the Volatile Composition of Wine: Storage Time Optimization. LWT 2006, 39, 199–205. [Google Scholar] [CrossRef]
- Pérez-Prieto, L.J.; López-Roca, J.M.; Martínez-Cutillas, A.; Pardo Mínguez, F.; Gómez-Plaza, E. Maturing Wines in Oak Barrels. Effects of Origin, Volume, and Age of the Barrel on the Wine Volatile Composition. J. Agric. Food Chem. 2002, 50, 3272–3276. [Google Scholar] [CrossRef]
- Bartkovský, M.; Semjon, B.; Marcinčák, S.; Turek, P.; Baričičová, V. Effect of Wine Maturing on the Colour and Chemical Properties of Chardonnay Wine. Czech J. Food Sci. 2020, 38, 223–228. [Google Scholar] [CrossRef]
- Boulton, R.B.; Singleton, V.L.; Bisson, L.F.; Kunkee, R.E. The Maturation and Aging of Wines. In Principles and Practices of Winemaking; Springer Science + Business Media: New York, NY, USA, 1999; pp. 382–424. [Google Scholar]
- Bordet, F.; Joran, A.; Klein, G.; Roullier-Gall, C.; Alexandre, H. Yeast-Yeast Interactions: Mechanisms, Methodologies and Impact on Composition. Microorganisms 2020, 8, 600. [Google Scholar] [CrossRef]
- Englezos, V.; Jolly, N.P.; Di Gianvito, P.; Rantsiou, K.; Cocolin, L. Microbial Interactions in Winemaking: Ecological Aspects and Effect on Wine Quality. Trends Food Sci. Technol. 2022, 127, 99–113. [Google Scholar] [CrossRef]
- Ciani, M.; Capece, A.; Comitini, F.; Canonico, L.; Siesto, G.; Romano, P. Yeast Interactions in Inoculated Wine Fermentation. Front. Microbiol. 2016, 7, 555. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Thorngate, J.H.; Richardson, P.M.; Mills, D.A. Microbial Biogeography of Wine Grapes Is Conditioned by Cultivar, Vintage, and Climate. Proc. Natl. Acad. Sci. USA 2014, 111, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Martins, G.; Casini, C.; Da Costa, J.P.; Geny, L.; Lonvaud, A.; Masneuf-Pomarède, I. Correlation between Water Activity (Aw) and Microbial Epiphytic Communities Associated with Grapes Berries. Oeno One 2020, 54, 49–61. [Google Scholar] [CrossRef]
- Wei, R.T.; Chen, N.; Ding, Y.T.; Wang, L.; Gao, F.F.; Zhang, L.; Liu, Y.H.; Li, H.; Wang, H. Diversity and Dynamics of Epidermal Microbes During Grape Development of Cabernet Sauvignon (Vitis vinifera L.) in the Ecological Viticulture Model in Wuhai, China. Front. Microbiol. 2022, 13, 935647. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Fleet, G.H. The Effects of Temperature and PH on the Ethanol Tolerance of the Wine Yeasts, Saccharomyces Cerevisiae, Candida Stellata and Kloeckera Apiculata. J. Appl. Bacteriol. 1988, 65, 405–409. [Google Scholar] [CrossRef]
- Jussier, D.; Morneau, A.D.; De Orduña, R.M. Effect of Simultaneous Inoculation with Yeast and Bacteria on Fermentation Kinetics and Key Wine Parameters of Cool-Climate Chardonnay. Appl. Environ. Microbiol. 2006, 72, 221–227. [Google Scholar] [CrossRef]
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and Future of Non-Saccharomyces Yeasts: From Spoilage Microorganisms to Biotechnological Tools for Improving Wine Aroma Complexity. Front. Microbiol. 2016, 7, 411. [Google Scholar] [CrossRef]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not Your Ordinary Yeast: Non-Saccharomyces Yeasts in Wine Production Uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef]
- Alonso-del-Real, J.; Lairón-Peris, M.; Barrio, E.; Querol, A. Effect of Temperature on the Prevalence of Saccharomyces Non Cerevisiae Species against a S. Cerevisiae Wine Strain in Wine Fermentation: Competition, Physiological Fitness, and Influence in Final Wine Composition. Front. Microbiol. 2017, 8, 150. [Google Scholar] [CrossRef]
- Morrison-Whittle, P.; Goddard, M.R. From Vineyard to Winery: A Source Map of Microbial Diversity Driving Wine Fermentation. Environ. Microbiol. 2018, 20, 75–84. [Google Scholar] [CrossRef]
- Albertin, W.; Zimmer, A.; Miot-Sertier, C.; Bernard, M.; Coulon, J.; Moine, V.; Colonna-Ceccaldi, B.; Bely, M.; Marullo, P.; Masneuf-Pomarede, I. Combined Effect of the Saccharomyces Cerevisiae Lag Phase and the Non-Saccharomyces Consortium to Enhance Wine Fruitiness and Complexity. Appl. Microbiol. Biotechnol. 2017, 101, 7603–7620. [Google Scholar] [CrossRef]
- Benito, S. The Impact of Torulaspora Delbrueckii Yeast in Winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 3081–3094. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Rousseaux, S.; Tourdot-Maréchal, R.; Sadoudi, M.; Gougeon, R.; Schmitt-Kopplin, P.; Alexandre, H. Wine Microbiome: A Dynamic World of Microbial Interactions. Crit. Rev. Food Sci. Nutr. 2017, 57, 856–873. [Google Scholar] [CrossRef] [PubMed]
- Fleet, G.H. Yeast Interactions and Wine Flavour. Int. J. Food Microbiol. 2003, 86, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Bartowsky, E.J.; Costello, P.J.; Chambers, P.J. Emerging Trends in the Application of Malolactic Fermentation. Aust. J. Grape Wine Res. 2015, 21, 663–669. [Google Scholar] [CrossRef]
- Berbegal, C.; Fragasso, M.; Russo, P.; Bimbo, F.; Grieco, F.; Spano, G.; Capozzi, V. Climate Changes and Food Quality: The Potential of Microbial Activities as Mitigating Strategies in the Wine Sector. Fermentation 2019, 5, 85. [Google Scholar] [CrossRef]
- Berbegal, C.; Peña, N.; Russo, P.; Grieco, F.; Pardo, I.; Ferrer, S.; Spano, G.; Capozzi, V. Technological Properties of Lactobacillus Plantarum Strains Isolated from Grape Must Fermentation. Food Microbiol. 2016, 57, 187–194. [Google Scholar] [CrossRef]
- Virdis, C.; Sumby, K.; Bartowsky, E.; Jiranek, V. Lactic Acid Bacteria in Wine: Technological Advances and Evaluation of Their Functional Role. Front. Microbiol. 2021, 11, 16. [Google Scholar] [CrossRef]
- Valdés La Hens, D.; Bravo-Ferrada, B.M.; Delfederico, L.; Caballero, A.C.; Semorile, L.C. Prevalence of Lactobacillus Plantarum and Oenococcus Oeni during Spontaneous Malolactic Fermentation in Patagonian Red Wines Revealed by Polymerase Chain Reaction-Denaturing Gradient Gel Electrophoresis with Two Targeted Genes. Aust. J. Grape Wine Res. 2015, 21, 49–56. [Google Scholar] [CrossRef]
- Liszkowska, W.; Berlowska, J. Yeast Fermentation at Low Temperatures: Adaptation to Changing Environmental Conditions and Formation of Volatile Compounds. Molecules 2021, 26, 1035. [Google Scholar] [CrossRef]
- Bartowsky, E.J. Bacterial Spoilage of Wine and Approaches to Minimize It. Lett. Appl. Microbiol. 2009, 48, 149–156. [Google Scholar] [CrossRef]
- Bogdan, P.; Kordialik-Bogacka, E. Alternatives to Malt in Brewing. Trends Food Sci. Technol. 2017, 65, 1–9. [Google Scholar] [CrossRef]
- Nelson, M. The Barbarian’s Beverage; Routledge: Oxfordshire, UK, 2005; ISBN 9781134386727. [Google Scholar]
- Linko, M.; Haikara, A.; Ritala, A.; Penttilä, M. Recent Advances in the Malting and Brewing Industry1Based on a Lecture Held at the Symposium ‘Biotechnology in Advanced Food and Feed Processing’, at the 8th European Congress on Biotechnology (ECB8) in Budapest, Hungary, August 1997.1. J. Biotechnol. 1998, 65, 85–98. [Google Scholar] [CrossRef]
- Donadini, G.; Fumi, M.D.; Kordialik-Bogacka, E.; Maggi, L.; Lambri, M.; Sckokai, P. Consumer Interest in Specialty Beers in Three European Markets. Food Res. Int. 2016, 85, 301–314. [Google Scholar] [CrossRef]
- Yeo, H.Q.; Liu, S.-Q. An Overview of Selected Specialty Beers: Developments, Challenges and Prospects. Int. J. Food Sci. Technol. 2014, 49, 1607–1618. [Google Scholar] [CrossRef]
- Basso, R.F.; Alcarde, A.R.; Portugal, C.B. Could Non-Saccharomyces Yeasts Contribute on Innovative Brewing Fermentations? Food Res. Int. 2016, 86, 112–120. [Google Scholar] [CrossRef]
- Humia, B.V.; Santos, K.S.; Barbosa, A.M.; Sawata, M.; Mendonça, M.d.C.; Padilha, F.F. Beer Molecules and Its Sensory and Biological Properties: A Review. Molecules 2019, 24, 1568. [Google Scholar] [CrossRef]
- Rubio-Flores, M.; Serna-Saldivar, S.O. Technological and Engineering Trends for Production of Gluten-Free Beers. Food Eng. Rev. 2016, 8, 468–482. [Google Scholar] [CrossRef]
- Blanco, C.A.; Nimubona, D.; Fernández-Fernández, E.; Álvarez, I. Sensory Characterization of Commercial Lager Beers and Their Correlations with Iso-α-Acid Concentrations. J. Food Nutr. Res. 2015, 3, 1–8. [Google Scholar] [CrossRef]
- Sánchez-Muniz, F.J.; Macho-González, A.; Garcimartín, A.; Santos-López, J.A.; Benedí, J.; Bastida, S.; González-Muñoz, M.J. The Nutritional Components of Beer and Its Relationship with Neurodegeneration and Alzheimer’s Disease. Nutrients 2019, 11, 1558. [Google Scholar] [CrossRef]
- Habschied, K.; Živković, A.; Krstanović, V.; Mastanjević, K. Functional Beer—A Review on Possibilities. Beverages 2020, 6, 51. [Google Scholar] [CrossRef]
- Díaz Prieto, L.E.; Gómez-martínez, S.; Nova, E.; Marcos, A. Do We Know What Moderate Alcohol Consumption Is? The Particular Case of Beer. Nutr. Hosp. 2022, 39, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Quici, L. Craft Beer Mon Amour: An Exploration of Italian Craft Consumers. Br. Food J. 2020, 122, 2671–2687. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive Review of Antimicrobial Activities of Plant Flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Ndubisi, C.F.; Okafor, E.T.; Amadi, O.C.; Nwagu, T.N.; Okolo, B.N.; Moneke, A.N.; Odibo, F.J.C.; Okoro, P.M.; Agu, R.C. Effect of Malting Time, Mashing Temperature and Added Commercial Enzymes on Extract Recovery from a Nigerian Malted Yellow Sorghum Variety. J. Inst. Brew. 2016, 122, 156–161. [Google Scholar] [CrossRef]
- Agu, R.C.; Palmer, G.H. Evaluation of the Potentials of Millet, Sorghum and Barley with Similar Nitrogen Contents Malted at Their Optimum Germination Temperatures for Use in Brewing. J. Inst. Brew. 2013, 119, 258–264. [Google Scholar] [CrossRef]
- Yorke, J.; Cook, D.; Ford, R. Brewing with Unmalted Cereal Adjuncts: Sensory and Analytical Impacts on Beer Quality. Beverages 2021, 7, 4. [Google Scholar] [CrossRef]
- Okolo, B.N.; Amadi, O.C.; Moneke, A.N.; Nwagu, T.N.; Nnamchi, C.I. Influence of Malted Barley and Exogenous Enzymes on the Glucose/Maltose Balance of Worts with Sorghum or Barley as an Adjunct. J. Inst. Brew. 2020, 126, 46–52. [Google Scholar] [CrossRef]
- Mallett, J. Malt: A Practical Guide from Field to Brewhouse; Brewers Publications: Boulder, CO, USA, 2014; Volume 4, ISBN 978-1-938469-12-1. [Google Scholar]
- Coghe, S.; D’Hollander, H.; Verachtert, H.; Delvaux, F.R. Impact of Dark Specialty Malts on Extract Composition and Wort Fermentation. J. Inst. Brew. 2005, 111, 51–60. [Google Scholar] [CrossRef]
- Gąsior, J.; Kawa-Rygielska, J.; Kucharska, A.Z. Carbohydrates Profile, Polyphenols Content and Antioxidative Properties of Beer Worts Produced with Different Dark Malts Varieties or Roasted Barley Grains. Molecules 2020, 25, 3882. [Google Scholar] [CrossRef]
- Boulton, C.; Quain, D.; Boulton, C. Brewing Yeast and Fermentation; Boulton, C., Quain, D., Eds.; Blackwell Science Ltd.: Oxford, UK, 2006; ISBN 9780470999417. [Google Scholar]
- Briggs, D.; Boulton, C.; Brookes, P.; Stevens, R. Brewing; CRC Press: Boca Raton, FL, USA, 2004; Volume 1, ISBN 978-0-8493-2547-2. [Google Scholar]
- Verstrepen, K.J.; Derdelinckx, G.; Dufour, J.; Winderickx, J.; Thevelein, J.M.; Pretorius, I.S.; Delvaux, F.R. Flavor-Active Esters: Adding Fruitiness to Beer. J. Biosci. Bioeng. 2003, 96, 110–118. [Google Scholar] [CrossRef]
- Dekoninck, T.M.L.; Verbelen, P.J.; Delvaux, F.; Van Mulders, S.E.; Delvaux, F.R. The Importance of Wort Composition for Yeast Metabolism during Accelerated Brewery Fermentations. J. Am. Soc. Brew. Chem. 2012, 70, 195–204. [Google Scholar] [CrossRef]
- Hashimoto, T.; Maruhashi, T.; Yamaguchi, Y.; Hida, Y.; Oka, K. The Effect on Fermentation By-Products of the Amino Acids in Wort. In Proceedings of the World Brewing Congress, Portland, OR, USA, 28 July–1 August 2012. [Google Scholar]
- Saerens, S.M.G.; Verbelen, P.J.; Vanbeneden, N.; Thevelein, J.M.; Delvaux, F.R. Monitoring the Influence of High-Gravity Brewing and Fermentation Temperature on Flavour Formation by Analysis of Gene Expression Levels in Brewing Yeast. Appl. Microbiol. Biotechnol. 2008, 80, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Zhao, H.; Yu, Z.; Zhao, M. Effects of Wort Gravity and Nitrogen Level on Fermentation Performance of Brewer’s Yeast and the Formation of Flavor Volatiles. Appl. Biochem. Biotechnol. 2012, 166, 1562–1574. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Li, H.; Mo, F.; Zheng, L.; Zhao, H.; Zhao, M. Effects of Lys and His Supplementations on the Regulation of Nitrogen Metabolism in Lager Yeast. Appl. Microbiol. Biotechnol. 2013, 97, 8913–8921. [Google Scholar] [CrossRef]
- Stewart, G.G.; Hill, A.; Lekkas, C. Wort FAN–Its Characteristics and Importance during Fermentation. J. Am. Soc. Brew. Chem. 2013, 71, 179–185. [Google Scholar] [CrossRef]
- Kunz, T.; Müller, C.; Mato-Gonzales, D.; Methner, F.-J. The Influence of Unmalted Barley on the Oxidative Stability of Wort and Beer. J. Inst. Brew. 2012, 118, 32–39. [Google Scholar] [CrossRef]
- Gallardo, E.; De Schutter, D.P.; Zamora, R.; Derdelinckx, G.; Delvaux, F.R.; Hidalgo, F.J. Influence of Lipids in the Generation of Phenylacetaldehyde in Wort-Related Model Systems. J. Agric. Food Chem. 2008, 56, 3155–3159. [Google Scholar] [CrossRef]
- Saerens, S.; Thevelein, J.; Delvaux, F. Ethyl Ester Production during Brewery Fermentation, a Review. Cerevisia 2008, 33, 82–90. [Google Scholar]
- Saerens, S.M.G.; Delvaux, F.; Verstrepen, K.J.; Van Dijck, P.; Thevelein, J.M.; Delvaux, F.R. Parameters Affecting Ethyl Ester Production by Saccharomyces Cerevisiae during Fermentation. Appl. Environ. Microbiol. 2008, 74, 454–461. [Google Scholar] [CrossRef]
- Bravi, E.; Perretti, G.; Buzzini, P.; Della Sera, R.; Fantozzi, P. Technological Steps and Yeast Biomass as Factors Affecting the Lipid Content of Beer during the Brewing Process. J. Agric. Food Chem. 2009, 57, 6279–6284. [Google Scholar] [CrossRef]
- Walker, G.M. Role of Metal Ions in Brewing Yeast Fermentation Performance; Wiley-Blackwell: Hoboken, NJ, USA, 2000; Volume 10, pp. 86–91. [Google Scholar]
- Hucker, B.; Wakeling, L.; Vriesekoop, F. Investigations into the Thiamine and Riboflavin Content of Malt and the Effects of Malting and Roasting on Their Final Content. J. Cereal Sci. 2012, 56, 300–306. [Google Scholar] [CrossRef]
- Hucker, B.; Wakeling, L.; Vriesekoop, F. Vitamins in Brewing: The Impact of Wort Production on the Thiamine and Riboflavin Vitamer Content of Boiled Sweet Wort. J. Inst. Brew. 2014, 120, 164–173. [Google Scholar] [CrossRef]
- Viñas, P.; López-Erroz, C.; Balsalobre, N.; Hernández-Córdoba, M. Determination of Thiamine and Its Esters in Beers and Raw Materials Used for Their Manufacture by Liquid Chromatography with Postcolumn Derivatization. J. Agric. Food Chem. 2003, 51, 3222–3227. [Google Scholar] [CrossRef] [PubMed]
- Hucker, B.; Wakeling, L.; Vriesekoop, F. The Quantitative Analysis of Thiamin and Riboflavin and Their Respective Vitamers in Fermented Alcoholic Beverages. J. Agric. Food Chem. 2011, 59, 12278–12285. [Google Scholar] [CrossRef]
- Lewis, M.J.; Young, T.W. Brewing; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Mousia, Z.; Balkin, R.C.; Pandiella, S.S.; Webb, C. The Effect of Milling Parameters on Starch Hydrolysis of Milled Malt in the Brewing Process. Process Biochem. 2004, 39, 2213–2219. [Google Scholar] [CrossRef]
- Szwajgier, D. Dry and Wet Milling of Malt. A Preliminary Study Comparing Fermentable Sugar, Total Protein, Total Phenolics and the Ferulic Acid Content in Non-Hopped Worts. J. Inst. Brew. 2011, 117, 569–577. [Google Scholar] [CrossRef]
- Krottenthaler, M.; Back, W.; Zarnkow, M. Wort Production. In Handbook of Brewing Processes Technology Markets; Wiley: Hoboken, NJ, USA, 2009; pp. 165–202. [Google Scholar]
- Montanari, L.; Floridi, S.; Marconi, O.; Tironzelli, M.; Fantozzi, P. Effect of Mashing Procedures on Brewing. Eur. Food Res. Technol. 2005, 221, 175–179. [Google Scholar] [CrossRef]
- Parés Viader, R.; Yde, M.S.H.; Hartvig, J.W.; Pagenstecher, M.; Carlsen, J.B.; Christensen, T.B.; Andersen, M.L. Optimization of Beer Brewing by Monitoring α-Amylase and β-Amylase Activities during Mashing. Beverages 2021, 7, 13. [Google Scholar] [CrossRef]
- Mosher, M.; Trantham, K. Lautering and Sparging. In Brewing Science: A Multidisciplinary Approach; Springer International Publishing: Cham, Switzerland, 2021; pp. 199–240. [Google Scholar]
- Karabín, M.; Hanko, V.; Nešpor, J.; Jelínek, L.; Dostálek, P. Hop Tannin Extract: A Promising Tool for Acceleration of Lautering. J. Inst. Brew. 2018, 124, 374–380. [Google Scholar] [CrossRef]
- Willaert, R. The Beer Brewing Process: Wort Production and Beer Fermentation. In Handbook of Food Products Manufacturing; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; Volume 1, pp. 443–506. ISBN 9780470049648. [Google Scholar]
- Van Nierop, S.N.E.; Evans, D.E.; Axcell, B.C.; Cantrell, I.C.; Rautenbach, M. Impact of Different Wort Boiling Temperatures on the Beer Foam Stabilizing Properties of Lipid Transfer Protein 1. J. Agric. Food Chem. 2004, 52, 3120–3129. [Google Scholar] [CrossRef]
- Kirsop, B.H. Oxygen in Brewery Fermentation. J. Inst. Brew. 1974, 80, 252–259. [Google Scholar] [CrossRef]
- Kucharczyk, K.; Tuszyński, T. The Effect of Wort Aeration on Fermentation, Maturation and Volatile Components of Beer Produced on an Industrial Scale. J. Inst. Brew. 2017, 123, 31–38. [Google Scholar] [CrossRef]
- Eßlinger, H.M. Handbook of Brewing Processes Technology Markets-Wiley; Wiley: Weinheim, Germany, 2009; ISBN 9783527314065. [Google Scholar]
- Ullah, H.; Khan, A.; Riccioni, C.; Di Minno, A.; Tantipongpiradet, A.; Buccato, D.G.; De Lellis, L.F.; Khan, H.; Xiao, J.; Daglia, M. Polyphenols as Possible Alternative Agents in Chronic Fatigue: A Review. Phytochem. Rev. 2023, 22, 1637–1661. [Google Scholar] [CrossRef]
- Chou, Y.C.; Ho, C.T.; Pan, M.H. Stilbenes: Chemistry and Molecular Mechanisms of Anti-Obesity. Curr. Pharmacol. Rep. 2018, 4, 202–209. [Google Scholar] [CrossRef]
- Hasan, M.; Bae, H. An Overview of Stress-Induced Resveratrol Synthesis in Grapes: Perspectives for Resveratrol-Enriched Grape Products. Molecules 2017, 22, 294. [Google Scholar] [CrossRef]
- Toscano, G.; Riva, G.; Duca, D.; Pedretti, E.F.; Corinaldesi, F.; Rossini, G. Analysis of the Characteristics of the Residues of the Wine Production Chain Finalized to Their Industrial and Energy Recovery. Biomass Bioenergy 2013, 55, 260–267. [Google Scholar] [CrossRef]
- Choleva, M.; Boulougouri, V.; Panara, A.; Panagopoulou, E.; Chiou, A.; Thomaidis, N.S.; Antonopoulou, S.; Fragopoulou, E. Evaluation of Anti-Platelet Activity of Grape Pomace Extracts. Food Funct. 2019, 10, 8069–8080. [Google Scholar] [CrossRef]
- Karantonis, H.C.; Tsoupras, A.; Moran, D.; Zabetakis, I.; Nasopoulou, C. Olive, Apple, and Grape Pomaces with Antioxidant and Anti-Inflammatory Bioactivities for Functional Foods. In Functional Foods and Their Implications for Health Promotion; Elsevier: Amsterdam, The Netherlands, 2023; pp. 131–159. [Google Scholar]
- Sabra, A.; Netticadan, T.; Wijekoon, C. Grape Bioactive Molecules, and the Potential Health Benefits in Reducing the Risk of Heart Diseases. Food Chem. X 2021, 12, 100149. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Rodrigues, E.; Gonzaga, L.V.; Caliari, V.; Genovese, M.I.; Gonçalves, A.E.d.S.S.; Fett, R. Phenolic Compounds Content and Antioxidant Activity in Pomace from Selected Red Grapes (Vitis vinifera L. and Vitis labrusca L.) Widely Produced in Brazil. Food Chem. 2011, 127, 174–179. [Google Scholar] [CrossRef]
- Lingua, M.S.; Fabani, M.P.; Wunderlin, D.A.; Baroni, M. V From Grape to Wine: Changes in Phenolic Composition and Its Influence on Antioxidant Activity. Food Chem. 2016, 208, 228–238. [Google Scholar] [CrossRef]
- Szabó, É.; Marosvölgyi, T.; Szilágyi, G.; Kőrösi, L.; Schmidt, J.; Csepregi, K.; Márk, L.; Bóna, Á. Correlations between Total Antioxidant Capacity, Polyphenol and Fatty Acid Content of Native Grape Seed and Pomace of Four Different Grape Varieties in Hungary. Antioxidants 2021, 10, 1101. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Wang, Z.; Wei, C.; Amo, A.; Ahmed, B.; Yang, X.; Zhang, X. Phenylpropanoid Pathway Engineering: An Emerging Approach towards Plant Defense. Pathogens 2020, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- Matus, J.T. Transcriptomic and Metabolomic Networks in the Grape Berry Illustrate That It Takes More Than Flavonoids to Fight Against Ultraviolet Radiation. Front. Plant Sci. 2016, 7, 1337. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Mu, L.; Yan, G.L.; Liang, N.N.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Biosynthesis of Anthocyanins and Their Regulation in Colored Grapes. Molecules 2010, 15, 9057–9091. [Google Scholar] [CrossRef]
- Ramazzotti, S.; Filippetti, I.; Intrieri, C. Expression of Genes Associated with Anthocyanin Synthesis in Red-Purplish, Pink, Pinkish-Green and Green Grape Berries from Mutated ‘Sangiovese’Biotypes: A Case Study. Vitis 2008, 47, 147–151. [Google Scholar]
- Berli, F.J.; Fanzone, M.; Piccoli, P.; Bottini, R. Solar UV-B and ABA Are Involved in Phenol Metabolism of Vitis vinifera L. Increasing Biosynthesis of Berry Skin Polyphenols. J. Agric. Food Chem. 2011, 59, 4874–4884. [Google Scholar] [CrossRef]
- Ruiz-García, Y.; Romero-Cascales, I.; Gil-Muñoz, R.; Fernández-Fernández, J.I.; López-Roca, J.M.; Gómez-Plaza, E. Improving Grape Phenolic Content and Wine Chromatic Characteristics through the Use of Two Different Elicitors: Methyl Jasmonate versus Benzothiadiazole. J. Agric. Food Chem. 2012, 60, 1283–1290. [Google Scholar] [CrossRef]
- Iriti, M.; Vitalini, S.; DI Tommaso, G.; D’amico, S.; Borgo, M.; Faoro, F. New Chitosan Formulation Prevents Grapevine Powdery Mildew Infection and Improves Polyphenol Content and Free Radical Scavenging Activity of Grape and Wine. Aust. J. Grape Wine Res. 2011, 17, 263–269. [Google Scholar] [CrossRef]
- Ochoa-Villarreal, M.; Vargas-Arispuro, I.; Islas-Osuna, M.A.; González-Aguilar, G.; Martínez-Téllez, M.Á. Pectin-Derived Oligosaccharides Increase Color and Anthocyanin Content in Flame Seedless Grapes. J. Sci. Food Agric. 2011, 91, 1928–1930. [Google Scholar] [CrossRef]
- Gil, M.; Kontoudakis, N.; González, E.; Esteruelas, M.; Fort, F.; Canals, J.M.; Zamora, F. Influence of Grape Maturity and Maceration Length on Color, Polyphenolic Composition, and Polysaccharide Content of Cabernet Sauvignon and Tempranillo Wines. J. Agric. Food Chem. 2012, 60, 7988–8001. [Google Scholar] [CrossRef]
- Cheynier, V. Phenolic Compounds: From Plants to Foods. Phytochem. Rev. 2012, 11, 153–177. [Google Scholar] [CrossRef]
- Guaita, M.; Motta, S.; Messina, S.; Casini, F.; Bosso, A. Polyphenolic Profile and Antioxidant Activity of Green Extracts from Grape Pomace Skins and Seeds of Italian Cultivars. Foods 2023, 12, 3880. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.M.; Santos, L. A Potential Valorization Strategy of Wine Industry By-Products and Their Application in Cosmetics—Case Study: Grape Pomace and Grapeseed. Molecules 2022, 27, 969. [Google Scholar] [CrossRef]
- Gollucke, A.; Peres, R.; Jr, O.; Ribeiro, D. Polyphenols: A Nutraceutical Approach Against Diseases. Recent Pat. Food. Nutr. Agric. 2014, 5, 214–219. [Google Scholar] [CrossRef]
- Marina, G.C.; Samantha, S.C.; Josiane, D.V.; Carlos, A.B.C.S.; Marcelo, A.U.-G.; Bruna, A.S.M. Phytochemical Importance and Utilization Potential of Grape Residue from Wine Production. Afr. J. Biotechnol. 2017, 16, 179–192. [Google Scholar] [CrossRef]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef]
- Fernández-Mar, M.I.; Mateos, R.; García-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Bioactive Compounds in Wine: Resveratrol, Hydroxytyrosol and Melatonin: A Review. Food Chem. 2012, 130, 797–813. [Google Scholar] [CrossRef]
- Ahmad, I.; Song, X.; Hussein Ibrahim, M.E.; Jamal, Y.; Younas, M.U.; Zhu, G.; Zhou, G.; Adam Ali, A.Y. The Role of Melatonin in Plant Growth and Metabolism, and Its Interplay with Nitric Oxide and Auxin in Plants under Different Types of Abiotic Stress. Front. Plant Sci. 2023, 14, 1108507. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Plant Growth Regulator and/or Biostimulator during Stress? Trends Plant Sci. 2014, 19, 789–797. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Role of Melatonin to Enhance Phytoremediation Capacity. Appl. Sci. 2019, 9, 5293. [Google Scholar] [CrossRef]
- Xu, L.; Xiang, G.; Sun, Q.; Ni, Y.; Jin, Z.; Gao, S.; Yao, Y. Melatonin Enhances Salt Tolerance by Promoting MYB108A-Mediated Ethylene Biosynthesis in Grapevines. Hortic. Res. 2019, 6, 114. [Google Scholar] [CrossRef] [PubMed]
- Iriti, M.; Rossoni, M.; Faoro, F. Melatonin Content in Grape: Myth or Panacea? J. Sci. Food Agric. 2006, 86, 1432–1438. [Google Scholar] [CrossRef]
- Stege, P.W.; Sombra, L.L.; Messina, G.; Martinez, L.D.; Silva, M.F. Determination of Melatonin in Wine and Plant Extracts by Capillary Electrochromatography with Immobilized Carboxylic Multi-Walled Carbon Nanotubes as Stationary Phase. Electrophoresis 2010, 31, 2242–2248. [Google Scholar] [CrossRef]
- Vitalini, S.; Gardana, C.; Zanzotto, A.; Simonetti, P.; Faoro, F.; Fico, G.; Iriti, M. The Presence of Melatonin in Grapevine (Vitis vinifera L.) Berry Tissues. J. Pineal Res. 2011, 51, 331–337. [Google Scholar] [CrossRef]
- Murch, S.J.; Hall, B.A.; Le, C.H.; Saxena, P.K. Changes in the Levels of Indoleamine Phytochemicals during Véraison and Ripening of Wine Grapes. J. Pineal Res. 2010, 49, 95–100. [Google Scholar] [CrossRef]
- Mercolini, L.; Mandrioli, R.; Raggi, M.A. Content of Melatonin and Other Antioxidants in Grape-Related Foodstuffs: Measurement Using a MEPS-HPLC-F Method. J. Pineal Res. 2012, 53, 21–28. [Google Scholar] [CrossRef]
- De Simone, N.; Russo, P.; Tufariello, M.; Fragasso, M.; Solimando, M.; Capozzi, V.; Grieco, F.; Spano, G. Autochthonous Biological Resources for the Production of Regional Craft Beers: Exploring Possible Contributions of Cereals, Hops, Microbes, and Other Ingredients. Foods 2021, 10, 1831. [Google Scholar] [CrossRef]
- Statista. Protein Content in Selected Types of Beer Worldwide 2016; Statista: Hamburg, Germany, 2016. [Google Scholar]
- Zeng, Y.; Ahmed, H.G.M.-D.; Li, X.; Yang, L.; Pu, X.; Yang, X.; Yang, T.; Yang, J. Physiological Mechanisms by Which the Functional Ingredients in Beer Impact Human Health. Molecules 2024, 29, 3110. [Google Scholar] [CrossRef]
- Bamforth, C.W. Nutritional Aspects of Beer; The Royal Society of Chemistry: London, UK, 2007; Volume 22, pp. 98–119. [Google Scholar] [CrossRef]
- Leskošek-Čukalović, I.J. Beer as an Integral Part of Healthy Diets: Current Knowledge and Perspective. Emerg. Tradit. Technol. Safe Healthy Qual. Food 2015, 17, 111–144. [Google Scholar]
- Nardini, M. An Overview of Bioactive Phenolic Molecules and Antioxidant Properties of Beer: Emerging Trends. Molecules 2023, 28, 3221. [Google Scholar] [CrossRef]
- Gribkova, I.N.; Kharlamova, L.N.; Lazareva, I.V.; Zakharov, M.A.; Zakharova, V.A.; Kozlov, V.I. The Influence of Hop Phenolic Compounds on Dry Hopping Beer Quality. Molecules 2022, 27, 740. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Pu, X.; Du, J.; Yang, X.; Li, X.; Mandal, M.S.N.; Yang, T.; Yang, J. Molecular Mechanism of Functional Ingredients in Barley to Combat Human Chronic Diseases. Oxid. Med. Cell. Longev. 2020, 2020, 3836172. [Google Scholar] [CrossRef] [PubMed]
- Idehen, E.; Tang, Y.; Sang, S. Bioactive Phytochemicals in Barley. J. Food Drug Anal. 2017, 25, 148–161. [Google Scholar] [CrossRef]
- Zhao, H.; Li, H.; Sun, G.; Yang, B.; Zhao, M. Assessment of Endogenous Antioxidative Compounds and Antioxidant Activities of Lager Beers. J. Sci. Food Agric. 2013, 93, 910–917. [Google Scholar] [CrossRef]
- Girisa, S.; Saikia, Q.; Bordoloi, D.; Banik, K.; Monisha, J.; Daimary, U.D.; Verma, E.; Ahn, K.S.; Kunnumakkara, A.B. Xanthohumol from Hop: Hope for Cancer Prevention and Treatment. IUBMB Life 2021, 73, 1016–1044. [Google Scholar] [CrossRef]
- Djordjevic, S.; Popovic, D.; Despotovic, S.; Veljovic, M.; Atanackovic, M.; Cvejic, J.; Nedovic, V.; Leskosek-Cukalovic, I. Extracts of Medicinal Plants as Functional Beer Additives. Chem. Ind. Chem. Eng. Q. 2016, 22, 301–308. [Google Scholar] [CrossRef]
- Nardini, M.; Garaguso, I. Characterization of Bioactive Compounds and Antioxidant Activity of Fruit Beers. Food Chem. 2020, 305, 125437. [Google Scholar] [CrossRef]
- Pirrone, A.; Prestianni, R.; Naselli, V.; Todaro, A.; Farina, V.; Tinebra, I.; Raffaele, G.; Badalamenti, N.; Maggio, A.; Gaglio, R.; et al. Influence of Indigenous Hanseniaspora uvarum and Saccharomyces cerevisiae from Sugar-Rich Substrates on the Aromatic Composition of Loquat Beer. Int. J. Food Microbiol. 2022, 379, 109868. [Google Scholar] [CrossRef]
- Veljovíc, M. Chemical, Functional and Sensory Properties of Beer Enriched with Biologically Active Compounds of Grape; University of Belgrade: Belgrade, Serbia, 2016. [Google Scholar]
- Stewart, G. Saccharomyces Species in the Production of Beer. Beverages 2016, 2, 34. [Google Scholar] [CrossRef]
- Shakibazadeh, S.; Saad, C.R.; Christianus, A.; Kamarudin, M.S.; Sijam, K.; Sinaian, P. Assessment of Possible Human Risk of Probiotic Application in Shrimp Farming. Int. Food Res. J. 2011, 18, 125–135. [Google Scholar]
- Moslehi-Jenabian, S.; Lindegaard, L.; Jespersen, L. Beneficial Effects of Probiotic and Food Borne Yeasts on Human Health. Nutrients 2010, 2, 449–473. [Google Scholar] [CrossRef] [PubMed]
- Perricone, M.; Bevilacqua, A.; Corbo, M.R.; Sinigaglia, M. Technological Characterization and Probiotic Traits of Yeasts Isolated from Altamura Sourdough to Select Promising Microorganisms as Functional Starter Cultures for Cereal-Based Products. Food Microbiol. 2014, 38, 26–35. [Google Scholar] [CrossRef]
- Hatoum, R.; Labrie, S.; Fliss, I. Antimicrobial and Probiotic Properties of Yeasts: From Fundamental to Novel Applications. Front. Microbiol. 2012, 3, 421. [Google Scholar] [CrossRef]
- Regon, P.; Chowra, U.; Awasthi, J.P.; Borgohain, P.; Panda, S.K. Genome-Wide Analysis of Magnesium Transporter Genes in Solanum Lycopersicum. Comput. Biol. Chem. 2019, 80, 498–511. [Google Scholar] [CrossRef]
- Arroyo-López, F.N.; Bautista-Gallego, J.; Domínguez-Manzano, J.; Romero-Gil, V.; Rodriguez-Gómez, F.; García-García, P.; Garrido-Fernández, A.; Jiménez-Díaz, R. Formation of Lactic Acid Bacteria–Yeasts Communities on the Olive Surface during Spanish-Style Manzanilla Fermentations. Food Microbiol. 2012, 32, 295–301. [Google Scholar] [CrossRef]
- Mumy, K.L.; Chen, X.; Kelly, C.P.; McCormick, B.A. Saccharomyces boulardii Interferes with Shigella Pathogenesis by Postinvasion Signaling Events. Am. J. Physiol. Liver Physiol. 2008, 294, G599–G609. [Google Scholar] [CrossRef]
- Mulero-Cerezo, J.; Briz-Redón, Á.; Serrano-Aroca, Á. Saccharomyces cerevisiae Var. Boulardii: Valuable Probiotic Starter for Craft Beer Production. Appl. Sci. 2019, 9, 3250. [Google Scholar] [CrossRef]
- Capece, A.; Romaniello, R.; Pietrafesa, A.; Siesto, G.; Pietrafesa, R.; Zambuto, M.; Romano, P. Use of Saccharomyces cerevisiae var. boulardii in Co-Fermentations with S. cerevisiae for the Production of Craft Beers with Potential Healthy Value-Added. Int. J. Food Microbiol. 2018, 284, 22–30. [Google Scholar] [CrossRef]
- Reitenbach, A.F.; Iwassa, I.J.; Barros, B.C.B. Production of Functional Beer with the Addition of Probiotic: Saccharomyces boulardii. Res. Soc. Dev. 2021, 10, e5010212211. [Google Scholar] [CrossRef]
- Canonico, L.; Zannini, E.; Ciani, M.; Comitini, F. Assessment of Non-Conventional Yeasts with Potential Probiotic for Protein-Fortified Craft Beer Production. LWT 2021, 145, 111361. [Google Scholar] [CrossRef]
- Vinicius De Melo Pereira, G.; De Carvalho Neto, D.P.; Junqueira, A.C.D.O.; Karp, S.G.; Letti, L.A.J.; Magalhães Júnior, A.I.; Soccol, C.R. A Review of Selection Criteria for Starter Culture Development in the Food Fermentation Industry. Food Rev. Int. 2020, 36, 135–167. [Google Scholar] [CrossRef]
- Guyot, J. Cereal-based Fermented Foods in Developing Countries: Ancient Foods for Modern Research. Int. J. Food Sci. Technol. 2012, 47, 1109–1114. [Google Scholar] [CrossRef]
- Peyer, L.C.; Zannini, E.; Arendt, E.K. Lactic Acid Bacteria as Sensory Biomodulators for Fermented Cereal-Based Beverages. Trends Food Sci. Technol. 2016, 54, 17–25. [Google Scholar] [CrossRef]
- Greffeuille, V.; Polycarpe Kayodé, A.P.; Icard-Vernière, C.; Gnimadi, M.; Rochette, I.; Mouquet-Rivier, C. Changes in Iron, Zinc and Chelating Agents during Traditional African Processing of Maize: Effect of Iron Contamination on Bioaccessibility. Food Chem. 2011, 126, 1800–1807. [Google Scholar] [CrossRef]
- Gupta, M.; Abu-Ghannam, N.; Gallaghar, E. Barley for Brewing: Characteristic Changes during Malting, Brewing and Applications of Its By-Products. Compr. Rev. Food Sci. Food Saf. 2010, 9, 318–328. [Google Scholar] [CrossRef]
- Peyer, L.C.; Zannini, E.; Jacob, F.; Arendt, E.K. Growth Study, Metabolite Development, and Organoleptic Profile of a Malt-Based Substrate Fermented by Lactic Acid Bacteria. J. Am. Soc. Brew. Chem. 2015, 73, 303–313. [Google Scholar] [CrossRef]
- Waters, D.M.; Mauch, A.; Coffey, A.; Arendt, E.K.; Zannini, E. Lactic Acid Bacteria as a Cell Factory for the Delivery of Functional Biomolecules and Ingredients in Cereal-Based Beverages: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 503–520. [Google Scholar] [CrossRef]
- Vriesekoop, F.; Krahl, M.; Hucker, B.; Menz, G. 125 Th Anniversary Review: Bacteria in Brewing: The Good, the Bad and the Ugly. J. Inst. Brew. 2012, 118, 335–345. [Google Scholar] [CrossRef]
- Zheng, F.; Wang, T.; Niu, C.; Jia, Y.; Zheng, R.; Liu, C.; Wang, J.; Li, Q. Proteomic Analysis of Hop Bitter Compound Iso-α-Acid Tolerance in Beer Spoilage Lactobacillus Casei 2-9-5. J. Am. Soc. Brew. Chem. 2021, 79, 347–355. [Google Scholar] [CrossRef]
- Hinojosa-Avila, C.R.; García-Cayuela, T. Enhancing Probiotic Viability in Beer Fermentation: Selection of Stress-Resistant Lactic Acid Bacteria and Alternative Approaches. ACS Food Sci. Technol. 2024, 4, 2575–2584. [Google Scholar] [CrossRef]
- Chan, M.Z.A.; Toh, M.; Liu, S.-Q. Beer With Probiotics and Prebiotics. In Probiotics and Prebiotics in Foods; Elsevier: Amsterdam, The Netherlands, 2021; pp. 179–199. [Google Scholar]
- Silva, L.C.; de Souza Lago, H.; Rocha, M.O.T.; de Oliveira, V.S.; Laureano-Melo, R.; Stutz, E.T.G.; de Paula, B.P.; Martins, J.F.P.; Luchese, R.H.; Guerra, A.F.; et al. Craft Beers Fermented by Potential Probiotic Yeast or Lacticaseibacilli Strains Promote Antidepressant-Like Behavior in Swiss Webster Mice. Probiotics Antimicrob. Proteins 2021, 13, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium and Human Health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in Human Health and Disease. Antioxid. Redox Signal. 2011, 14, 1337–1383. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, J.; Yang, K.; Liu, M.; Qi, Y.; Zhang, T.; Fan, M.; Wei, X. Antibacterial Activity of Selenium-Enriched Lactic Acid Bacteria against Common Food-Borne Pathogens in Vitro. J. Dairy Sci. 2018, 101, 1930–1942. [Google Scholar] [CrossRef]
- Martínez, F.G.; Moreno-Martin, G.; Pescuma, M.; Madrid-Albarrán, Y.; Mozzi, F. Biotransformation of Selenium by Lactic Acid Bacteria: Formation of Seleno-Nanoparticles and Seleno-Amino Acids. Front. Bioeng. Biotechnol. 2020, 8, 506. [Google Scholar] [CrossRef]
- Gibson, C.; Park, Y.H.; Myoung, K.H.; Suh, M.K.; McArthur, T.; Lyons, G.; Stewart, D. The Bio-Fortification of Barley with Selenium Catherine; Institute of Brewery & Distillating, Asia-Pacific Section, Carventron: Hobart, Australia, 2006; pp. 19–24. [Google Scholar]
- Rodrigo, S.; Santamaria, O.; Chen, Y.; McGrath, S.P.; Poblaciones, M.J. Selenium Speciation in Malt, Wort, and Beer Made from Selenium-Biofortified Two-Rowed Barley Grain. J. Agric. Food Chem. 2014, 62, 5948–5953. [Google Scholar] [CrossRef]
- Revenco, D.; Vomáčková, M.; Jelínek, L.; Mestek, O.; Koplík, R. Selenium Species in Selenium-Enriched Malt. Kvas. Prum. 2019, 65, 134–141. [Google Scholar] [CrossRef]
- Gorinstein, S.; Zemser, M.; Vargas-Albores, F.; Ochoa, J.-L.; Paredes-Lopez, O.; Scheler, C.; Salnikow, J.; Martin-Belloso, O.; Trakhtenberg, S. Proteins and Amino Acids in Beers, Their Contents and Relationships with Other Analytical Data. Food Chem. 1999, 67, 71–78. [Google Scholar] [CrossRef]
- Decloedt, A.; Van Landschoot, A.; Watson, H.; Vanderputten, D.; Vanhaecke, L. Plant-Based Beverages as Good Sources of Free and Glycosidic Plant Sterols. Nutrients 2017, 10, 21. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Piçarra-Pereira, M.A.; Monteiro, S.; Loureiro, V.B.; Teixeira, A.R. The Wine Proteins. Trends Food Sci. Technol. 2001, 12, 230–239. [Google Scholar] [CrossRef]
- Díaz-Rubio, M.E.; Saura-Calixto, F. Dietary Fiber Complex in Beer. J. Am. Soc. Brew. Chem. 2009, 67, 38–43. [Google Scholar] [CrossRef]
- Cibrario, A.; Perello, M.C.; Miot-Sertier, C.; Riquier, L.; de Revel, G.; Ballestra, P.; Dols-Lafargue, M. Carbohydrate Composition of Red Wines during Early Aging and Incidence on Spoilage by Brettanomyces bruxellensis. Food Microbiol. 2020, 92, 103577. [Google Scholar] [CrossRef] [PubMed]
- Riou, V.; Vernhet, A.; Doco, T.; Moutounet, M. Aggregation of Grape Seed Tannins in Model Wine-Effect of Wine Polysaccharides. Food Hydrocoll. 2002, 16, 17–23. [Google Scholar] [CrossRef]
- Poncet-Legrand, C.; Doco, T.; Williams, P.; Vernhet, A. Inhibition of Grape Seed Tannin Aggregation by Wine Mannoproteins: Effect of Polysaccharide Molecular Weight. Am. J. Enol. Vitic. 2007, 58, 87–91. [Google Scholar] [CrossRef]
- Vidal, S.; Courcoux, P.; Francis, L.; Kwiatkowski, M.; Gawel, R.; Williams, P.; Waters, E.; Cheynier, V. Use of an Experimental Design Approach for Evaluation of Key Wine Components on Mouth-Feel Perception. Food Qual. Prefer. 2004, 15, 209–217. [Google Scholar] [CrossRef]
- Chalier, P.; Angot, B.; Delteil, D.; Doco, T.; Gunata, Z. Interactions between Aroma Compounds and Whole Mannoprotein Isolated from Saccharomyces cerevisiae Strains. Food Chem. 2007, 100, 22–30. [Google Scholar] [CrossRef]
- Lomolino, G.; Curioni, A. Protein Haze Formation in White Wines: Effect of Saccharomyces cerevisiae Cell Wall Components Prepared with Different Procedures. J. Agric. Food Chem. 2007, 55, 8737–8744. [Google Scholar] [CrossRef]
- Schmidt, S.A.; Tan, E.L.; Brown, S.; Nasution, U.J.; Pettolino, F.; Macintyre, O.J.; de Barros Lopes, M.; Waters, E.J.; Anderson, P.A. Hpf2 Glycan Structure Is Critical for Protection against Protein Haze Formation in White Wine. J. Agric. Food Chem. 2009, 57, 3308–3315. [Google Scholar] [CrossRef]
- Abdallah, Z.; Aguié-Béghin, V.; Abou-Saleh, K.; Douillard, R.; Bliard, C. Isolation and Analysis of Macromolecular Fractions Responsible for the Surface Properties in Native Champagne Wines. Food Res. Int. 2010, 43, 982–987. [Google Scholar] [CrossRef]
- Martínez-Lapuente, L.; Guadalupe, Z.; Ayestarán, B.; Ortega-Heras, M.; Pérez-Magariño, S. Changes in Polysaccharide Composition during Sparkling Wine Making and Aging. J. Agric. Food Chem. 2013, 61, 12362–12373. [Google Scholar] [CrossRef]
- Johnson, S.; Soprano, S.; Wickham, L.; Fitzgerald, N.; Edwards, J. Nuclear Magnetic Resonance and Headspace Solid-Phase Microextraction Gas Chromatography as Complementary Methods for the Analysis of Beer Samples. Beverages 2017, 3, 21. [Google Scholar] [CrossRef]
- Olšovská, J.; Štěrba, K.; Pavlovič, M.; Čejka, P. Determination of the Energy Value of Beer. J. Am. Soc. Brew. Chem. 2015, 73, 165–169. [Google Scholar] [CrossRef]
- Osorio-Paz, I.; Brunauer, R.; Alavez, S. Beer and Its Non-Alcoholic Compounds in Health and Disease. Crit. Rev. Food Sci. Nutr. 2020, 60, 3492–3505. [Google Scholar] [CrossRef]
- Ramos-Peralonso, M.J. European Food Safety Authority (EFSA) Scientific Opinion on Dietary Reference Values for Energy. EFSA J. 2013, 11, 20. [Google Scholar]
- Saranraj, P.; Sivasakthivelan, P.; Naveen, M. Fermentation of Fruit Wine and Its Quality Analysis: A Review. Aust. J. Sci. Technol. 2017, 1, 85–97. [Google Scholar]
- Hornedo-Ortega, R.; Cerezo, A.B.; Troncoso, A.M.; Garcia-Parrilla, M.C.; Mas, A. Melatonin and Other Tryptophan Metabolites Produced by Yeasts: Implications in Cardiovascular and Neurodegenerative Diseases. Front. Microbiol. 2016, 6, 1565. [Google Scholar] [CrossRef]
- Vilela, A. The Importance of Yeasts on Fermentation Quality and Human Health-Promoting Compounds. Fermentation 2019, 5, 46. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Bartosz, G. Total antioxldant capacity. Adv. Clin. Chem. 2003, 37, 219–289. [Google Scholar]
- di Pietro, M.B.; Bamforth, C.W. A Comparison of the Antioxidant Potential of Wine and Beer. J. Inst. Brew. 2011, 117, 547–555. [Google Scholar] [CrossRef]
- de Quirós, A.R.B.; Lage-Yusty, M.A.; López-Hernández, J. HPLC-Analysis of Polyphenolic Compounds in Spanish White Wines and Determination of Their Antioxidant Activity by Radical Scavenging Assay. Food Res. Int. 2009, 42, 1018–1022. [Google Scholar] [CrossRef]
- Marques, C.; Dinis, L.T.; Santos, M.J.; Mota, J.; Vilela, A. Beyond the bottle: Exploring health-promoting compounds in wine and wine-related products—Extraction, detection, quantification, aroma properties, and terroir effects. Foods 2023, 12, 4277. [Google Scholar] [CrossRef]
- Souza, L.P.; Calegari, F.; Zarbin, A.J.G.; Marcolino-Júnior, L.H.; Bergamini, M.F. Voltammetric Determination of the Antioxidant Capacity in Wine Samples Using a Carbon Nanotube Modified Electrode. J. Agric. Food Chem. 2011, 59, 7620–7625. [Google Scholar] [CrossRef]
- Mavric-Scholze, E.; Simijonović, D.; Avdović, E.; Milenković, D.; Šaćirović, S.; Ćirić, A.; Marković, Z. Comparative Analysis of Antioxidant Activity and Content of (Poly)Phenolic Compounds in Cabernet Sauvignon and Merlot Wines of Slovenian and Serbian Vineyards. Food Chem. X 2025, 25, 102108. [Google Scholar] [CrossRef]
- Elejalde, E.; Villarán, M.C.; Lopez-De-armentia, I.; Ramón, D.; Murillo, R.; Alonso, R.M. Study of Unpicked Grapes Valorization: A Natural Source of Polyphenolic Compounds and Evaluation of Their Antioxidant Capacity. Resources 2022, 11, 33. [Google Scholar] [CrossRef]
- Yang, J.; Martinson, T.E.; Liu, R.H. Phytochemical Profiles and Antioxidant Activities of Wine Grapes. Food Chem. 2009, 116, 332–339. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, G.Y.; Zhao, C.N.; Feng, X.L.; Xu, X.Y.; Cao, S.Y.; Meng, X.; Li, S.; Gan, R.Y.; Li, H. Bin Comparison of Antioxidant Activities of Different Grape Varieties. Molecules 2018, 23, 2432. [Google Scholar] [CrossRef]
- Oh, G.; La, I.; Lee, D.; Chae, J.; Im, J.; Park, S.W.; Fu, X.; Lim, J.; Kim, M.; Seong, Y.; et al. Assessment of Bioactive Compounds and Antioxidant Activity of Barley Sprouts. Separations 2025, 12, 68. [Google Scholar] [CrossRef]
- Wang, R.; Yang, B.; Jia, S.; Dai, Y.; Lin, X.; Ji, C.; Chen, Y. The Antioxidant Capacity and Flavor Diversity of Strawberry Wine Are Improved Through Fermentation with the Indigenous Non-Saccharomyces Yeasts Hanseniaspora Uvarum and Kurtzmaniella Quercitrusa. Foods 2025, 14, 886. [Google Scholar] [CrossRef]
- Binati, R.L.; Lemos Junior, W.J.F.; Torriani, S. Contribution of Non-Saccharomyces Yeasts to Increase Glutathione Concentration in Wine. Aust. J. Grape Wine Res. 2021, 27, 290–294. [Google Scholar] [CrossRef]
- Lemos Junior, W.J.F.; Binati, R.L.; Bersani, N.; Torriani, S. Investigating the Glutathione Accumulation by Non-Conventional Wine Yeasts in Optimized Growth Conditions and Multi-Starter Fermentations. LWT-Food Sci. Technol. 2021, 142, 110990. [Google Scholar] [CrossRef]
- Voce, S.; Iacumin, L.; Comuzzo, P. Production of Polysaccharides and Antioxidant Compounds by Non-Saccharomyces Yeast Strains after Growth and Induced Lysis. In Proceedings of the Infowine-Enoforum Web Scientists, Virtual, 13 March 2023; pp. 1–8. [Google Scholar]
- Postigo, V.; García, M.; Cabellos, J.M.; Arroyo, T. Wine Saccharomyces Yeasts for Beer Fermentation. Fermentation 2021, 7, 290. [Google Scholar] [CrossRef]
- Granato, D.; Branco, G.F.; Faria, J.D.A.F.; Cruz, A.G. Characterization of Brazilian Lager and Brown Ale Beers Based on Color, Phenolic Compounds, and Antioxidant Activity Using Chemometrics. J. Sci. Food Agric. 2011, 91, 563–571. [Google Scholar] [CrossRef]
- Chailapakul, O.; Siangproh, W.; Jampasa, S.; Chaiyo, S.; Teengam, P.; Yakoh, A.; Pinyorospathum, C. Paper-Based Sensors for the Application of Biological Compound Detection, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2020; Volume 89. [Google Scholar]
- Tekos, F.; Makri, S.; Skaperda, Z.V.; Patouna, A.; Terizi, K.; Kyriazis, I.D.; Kotseridis, Y.; Mikropoulou, E.V.; Papaefstathiou, G.; Halabalaki, M.; et al. Assessment of Antioxidant and Antimutagenic Properties of Red and White Wine Extracts in Vitro. Metabolites 2021, 11, 436. [Google Scholar] [CrossRef]
- Rodrigo, R.; Rivera, G.; Orellana, M.; Araya, J.; Bosco, C. Rat Kidney Antioxidant Response to Long-Term Exposure to Flavonol Rich Red Wine. Life Sci. 2002, 71, 2881–2895. [Google Scholar] [CrossRef]
- Giriwono, P.E.; Hashimoto, T.; Ohsaki, Y.; Shirakawa, H.; Hokazono, H.; Komai, M. Extract of Fermented Barley Attenuates Chronic Alcohol Induced Liver Damage by Increasing Antioxidative Activities. Food Res. Int. 2010, 43, 118–124. [Google Scholar] [CrossRef]
- Osorio-Valencia, A.I.; de Jesús Franco-Mejía, J.; Hoyos-Arbeláez, J.A.; Blandón-Naranjo, L.; Vega-Castro, O.A.; del Carmen Contreras-Calderón, J. Evaluation of Antioxidant Capacity in Different Food Matrices through Differential Pulse Voltammetry and Its Correlation with Spectrophotometric Methods. J. Appl. Electrochem. 2023, 53, 2495–2505. [Google Scholar] [CrossRef]
- Haque, M.A.; Morozova, K.; Ferrentino, G.; Scampicchio, M. Electrochemical Methods to Evaluate the Antioxidant Activity and Capacity of Foods: A Review. Electroanalysis 2021, 33, 1419–1435. [Google Scholar] [CrossRef]
- Buenaventura, T.M.A.; Catangay, C.J.L.; Dolendo, C.D.C.; Soriano, A.N.; Lardizabal, D.D.; Rubi, R.V.C. The Role of Voltammetric Analysis in the Wine Industry. Eng. Proc. 2023, 56, 152. [Google Scholar] [CrossRef]
- Vilas-Boas, Â.; Valderrama, P.; Fontes, N.; Geraldo, D.; Bento, F. Evaluation of Total Polyphenol Content of Wines by Means of Voltammetric Techniques: Cyclic Voltammetry vs Differential Pulse Voltammetry. Food Chem. 2019, 276, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Bernal, Ó.A.; Vazquez-Flores, A.A.; de la Rosa, L.A.; Rodrigo-García, J.; Martínez-Ruiz, N.R.; Alvarez-Parrilla, E. Enriched Red Wine: Phenolic Profile, Sensory Evaluation and In Vitro Bioaccessibility of Phenolic Compounds. Foods 2023, 12, 1194. [Google Scholar] [CrossRef] [PubMed]
- Esparza, I.; Martínez-Inda, B.; Cimminelli, M.J.; Jimeno-Mendoza, M.C.; Moler, J.A.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C. Reducing SO2 Doses in Red Wines by Using Grape Stem Extracts as Antioxidants. Biomolecules 2020, 10, 1369. [Google Scholar] [CrossRef]
- Ma, Y.; Yu, K.; Chen, X.; Wu, H.; Xiao, X.; Xie, L.; Wei, Z.; Xiong, R.; Zhou, X. Effects of Plant-Derived Polyphenols on the Antioxidant Activity and Aroma of Sulfur-Dioxide-Free Red Wine. Molecules 2023, 28, 5255. [Google Scholar] [CrossRef]
- Lippolis, T.; Cofano, M.; Caponio, G.R.; De Nunzio, V.; Notarnicola, M. Bioaccessibility and Bioavailability of Diet Polyphenols and Their Modulation of Gut Microbiota. Int. J. Mol. Sci. 2023, 24, 3813. [Google Scholar] [CrossRef]
- Caponio, G.; Cofano, M.; Lippolis, T.; Gigante, I.; De Nunzio, V.; Difonzo, G.; Noviello, M.; Tarricone, L.; Gambacorta, G.; Giannelli, G.; et al. Anti-Proliferative and Pro-Apoptotic Effects of Digested Aglianico Grape Pomace Extract in Human Colorectal Cancer Cells. Molecules 2022, 27, 6791. [Google Scholar] [CrossRef]
- Elbling, L.; Weiss, R.-M.; Teufelhofer, O.; Uhl, M.; Knasmueller, S.; Schulte-Hermann, R.; Berger, W.; Micksche, M. Green Tea Extract and (−)-epigallocatechin-3-gallate, the Major Tea Catechin, Exert Oxidant but Lack Antioxidant Activities. FASEB J. 2005, 19, 807–809. [Google Scholar] [CrossRef]
- Lambert, J.D.; Hong, J.; Yang, G.; Liao, J.; Yang, C.S. Inhibition of Carcinogenesis by Polyphenols: Evidence from Laboratory Investigations. Am. J. Clin. Nutr. 2005, 81, 284S–291S. [Google Scholar] [CrossRef]
- Castro-Barquero, S.; Lamuela-Raventós, R.M.; Doménech, M.; Estruch, R. Relationship between Mediterranean Dietary Polyphenol Intake and Obesity. Nutrients 2018, 10, 1523. [Google Scholar] [CrossRef]
- Godos, J.; Marventano, S.; Mistretta, A.; Galvano, F.; Grosso, G. Dietary Sources of Polyphenols in the Mediterranean Healthy Eating, Aging and Lifestyle (MEAL) Study Cohort. Int. J. Food Sci. Nutr. 2017, 68, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Knaze, V.; Rothwell, J.A.; Hémon, B.; Moskal, A.; Overvad, K.; Tjønneland, A.; Kyrø, C.; Fagherazzi, G.; Boutron-Ruault, M.-C.; et al. Dietary Polyphenol Intake in Europe: The European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Eur. J. Nutr. 2016, 55, 1359–1375. [Google Scholar] [CrossRef] [PubMed]
- Tresserra-Rimbau, A.; Medina-Remón, A.; Pérez-Jiménez, J.; Martínez-González, M.A.; Covas, M.I.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; Arós, F.; et al. Dietary Intake and Major Food Sources of Polyphenols in a Spanish Population at High Cardiovascular Risk: The PREDIMED Study. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 953–959. [Google Scholar] [CrossRef]
- Probst, Y.; Guan, V.; Kent, K. A Systematic Review of Food Composition Tools Used for Determining Dietary Polyphenol Intake in Estimated Intake Studies. Food Chem. 2018, 238, 146–152. [Google Scholar] [CrossRef]
- Di Stefano, R.; Flamini, R. High Performance Liquid Chromatography Analysis of Grape and Wine Polyphenols. In Hyphenated Techniques in Grape and Wine Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 33–79. [Google Scholar]
- Ivanova, V.; Stefova, M.; Vojnoski, B.; Dörnyei, Á.; Márk, L.; Dimovska, V.; Stafilov, T.; Kilár, F. Identification of Polyphenolic Compounds in Red and White Grape Varieties Grown in R. Macedonia and Changes of Their Content during Ripening. Food Res. Int. 2011, 44, 2851–2860. [Google Scholar] [CrossRef]
- Koundouras, S. Environmental and Viticultural Effects on Grape Composition and Wine Sensory Properties. Elements 2018, 14, 173–178. [Google Scholar] [CrossRef]
- Gris, E.F.; Burin, V.M.; Brighenti, E.; Vieira, H.; Luiz, M.B. Phenology and Ripening of Vitis vinifera L. Grape Varieties in São Joaquim, Southern Brazil: A New South American Wine Growing Region. Cienc. Investig. Agrar. 2010, 37, 61–75. [Google Scholar] [CrossRef]
- Cheng, G.; He, Y.-N.; Yue, T.-X.; Wang, J.; Zhang, Z.-W. Effects of Climatic Conditions and Soil Properties on Cabernet Sauvignon Berry Growth and Anthocyanin Profiles. Molecules 2014, 19, 13683–13703. [Google Scholar] [CrossRef]
- Ubalde, J.M.; Sort, X.; Zayas, A.; Poch, R.M. Effects of Soil and Climatic Conditions on Grape Ripening and Wine Quality of Cabernet Sauvignon. J. Wine Res. 2010, 21, 1–17. [Google Scholar] [CrossRef]
- Chavarria, G.; Santos, H.P.; Sônego, O.R.; Marodin, G.A.; Bergamaschi, H.; Cardoso, L.S. Incidencia de Doencas e Necessidade de Controle Em Cultivo Protegido de Videira. Rev. Bras. Frutic. 2007, 29, 477–482. [Google Scholar] [CrossRef]
- Jones, G.V.; Davis, R.E. Climate Influences on Grapevine Phenology, Grape Composition, and Wine Production and Quality for Bordeaux, France. Am. J. Enol. Vitic. 2000, 51, 249–261. [Google Scholar] [CrossRef]
- Buffara, C.R.; Angelotti, F.; Vieira, R.A.; Bogo, A.; Tessmann, D.J.; Bem, B.P. Elaboration and Validation of a Diagrammatic Scale to Assess Downy Mildew Severity in Grapevine. Cienc. Rural 2014, 44, 1384–1391. [Google Scholar] [CrossRef]
- Dai, Z.W.; Ollat, N.; Gomès, E.; Decroocq, S.; Tandonnet, J.-P.; Bordenave, L.; Pieri, P.; Hilbert, G.; Kappel, C.; van Leeuwen, C.; et al. Ecophysiological, Genetic, and Molecular Causes of Variation in Grape Berry Weight and Composition: A Review. Am. J. Enol. Vitic. 2011, 62, 413–425. [Google Scholar] [CrossRef]
- Mota, R.V.; Regina, M.D.; Amorim, D.A.; Fávero, A.C. Fatores que afetam a maturação e a qualidade da uva para vinificação. Inf. Agrop. 2006, 27, 56–64. [Google Scholar]
- Downey, M.O.; Dokoozlian, N.K.; Krstic, M.P. Cultural Practice and Environmental Impacts on the Flavonoid Composition of Grapes and Wine: A Review of Recent Research. Am. J. Enol. Vitic. 2006, 57, 257–268. [Google Scholar] [CrossRef]
- Brighenti, E.; Casagrande, K.; Cardoso, P.Z.; da Silveira Pasa, M.; Ciotta, M.N.; Brighenti, A.F. Total Polyphenols Contents in Different Grapevine Varieties in Highlands of Southern Brazil. BIO Web Conf. 2017, 9, 01024. [Google Scholar] [CrossRef]
- Coletta, A.; Berto, S.; Crupi, P.; Cravero, M.C.; Tamborra, P.; Antonacci, D.; Daniele, P.G.; Prenesti, E. Effect of Viticulture Practices on Concentration of Polyphenolic Compounds and Total Antioxidant Capacity of Southern Italy Red Wines. Food Chem. 2014, 152, 467–474. [Google Scholar] [CrossRef]
- Abreu, T.; Luís, C.; Câmara, J.S.; Teixeira, J.; Perestrelo, R. Unveiling Potential Functional Applications of Grape Pomace Extracts Based on Their Phenolic Profiling, Bioactivities, and Circular Bioeconomy. Biomass Convers. Biorefin. 2025. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, C.; Silva, P.; Câmara, J.S. Rapid Spectrophotometric Methods as a Tool to Assess the Total Phenolics and Antioxidant Potential over Grape Ripening: A Case Study of Madeira Grapes. J. Food Meas. Charact. 2018, 12, 1754–1762. [Google Scholar] [CrossRef]
- Negro, C.; Aprile, A.; Luvisi, A.; De Bellis, L.; Miceli, A. Antioxidant Activity and Polyphenols Characterization of Four Monovarietal Grape Pomaces from Salento (Apulia, Italy). Antioxidants 2021, 10, 1406. [Google Scholar] [CrossRef]
- Carbone, K.; Giannini, B.; Cecchini, F. Confronto della variabilita’ fenotipica, polifenolica ed antiossidante tra le cultivar Malvasia del Lazio e Cabernet Sauvignon.Comparison of phenotypic, polyphenolic and antioxidant variability among Malvasia del Lazio and Cabernet Sauvignon cvs. Riv. Vitic. Enol. 2011, 64, 47–56. [Google Scholar]
- Du, B.; He, B.J.; Shi, P.B.; Li, F.Y.; Li, J.; Zhu, F.M. Phenolic Content and Antioxidant Activity of Wine Grapes and Table Grapes. J. Med. Plants Res. 2012, 6, 3381–3387. [Google Scholar] [CrossRef]
- Feliciano, R.P.; Bravo, M.N.; Pires, M.M.; Serra, A.T.; Duarte, C.M.; Boas, L.V.; Bronze, M.R. Phenolic Content and Antioxidant Activity of Moscatel Dessert Wines from the Setúbal Region in Portugal. Food Anal. Methods 2009, 2, 149–161. [Google Scholar] [CrossRef]
- Lachman, J.; Šulc, M.; Hejtmánková, A.; Pivec, V.; Orsák, M. Content of Polyphenolic Antioxidants and Trans-Resveratrol in Grapes of Different Varieties of Grapevine (Vitis vinifera L.). Zahrad. Hortic. Sci. 2004, 31, 63–69. [Google Scholar] [CrossRef]
- Deng, Q.; Penner, M.H.; Zhao, Y. Chemical Composition of Dietary Fiber and Polyphenols of Five Different Varieties of Wine Grape Pomace Skins. Food Res. Int. 2011, 44, 2712–2720. [Google Scholar] [CrossRef]
- Zou, H.; Kilmartin, P.A.; Inglis, M.J.; Frost, A. Extraction of Phenolic Compounds during Vinification of Pinot Noir Wine Examined by HPLC and Cyclic Voltammetry. Aust. J. Grape Wine Res. 2002, 8, 163–174. [Google Scholar] [CrossRef]
- Krasteva, D.; Ivanov, Y.; Chengolova, Z.; Godjevargova, T. Antimicrobial Potential, Antioxidant Activity, and Phenolic Content of Grape Seed Extracts from Four Grape Varieties. Microorganisms 2023, 11, 395. [Google Scholar] [CrossRef]
- Lorrain, B.; Chira, K.; Teissedre, P.-L. Phenolic Composition of Merlot and Cabernet-Sauvignon Grapes from Bordeaux Vineyard for the 2009-Vintage: Comparison to 2006, 2007 and 2008 Vintages. Food Chem. 2011, 126, 1991–1999. [Google Scholar] [CrossRef]
- Panceri, C.P.; Gomes, T.M.; De Gois, J.S.; Borges, D.L.G.; Bordignon-Luiz, M.T. Effect of Dehydration Process on Mineral Content, Phenolic Compounds and Antioxidant Activity of Cabernet Sauvignon and Merlot Grapes. Food Res. Int. 2013, 54, 1343–1350. [Google Scholar] [CrossRef]
- Obreque-Slier, E.; López-Solís, R.; Castro-Ulloa, L.; Romero-Díaz, C.; Peña-Neira, Á. Phenolic Composition and Physicochemical Parameters of Carménère, Cabernet Sauvignon, Merlot and Cabernet Franc Grape Seeds (Vitis vinifera L.) during Ripening. LWT-Food Sci. Technol. 2012, 48, 134–141. [Google Scholar] [CrossRef]
- Benbouguerra, N.; Richard, T.; Saucier, C.; Garcia, F. Voltammetric Behavior, Flavanol and Anthocyanin Contents, and Antioxidant Capacity of Grape Skins and Seeds during Ripening (Vitis Vinifera Var. Merlot, Tannat, and Syrah). Antioxidants 2020, 9, 800. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.K.; Hakimuddin, F.; Paliyath, G.; Fisher, H. Antioxidant and Antiproliferative Activity of Polyphenols in Novel High-Polyphenol Grape Lines. Food Res. Int. 2008, 41, 419–428. [Google Scholar] [CrossRef]
- Bozan, B.; Tosun, G.; Özcan, D. Study of Polyphenol Content in the Seeds of Red Grape (Vitis vinifera L.) Varieties Cultivated in Turkey and Their Antiradical Activity. Food Chem. 2008, 109, 426–430. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; González-San José, M.L. Polyphenols and Colour Variability of Red Wines Made from Grapes Harvested at Different Ripeness Grade. Food Chem. 2006, 96, 197–208. [Google Scholar] [CrossRef]
- Lachman, J.; Hejtmánková, A.; Hejtmánková, K.; Horníčková, Š.; Pivec, V.; Skala, O.; Dědina, M.; Přibyl, J. Towards Complex Utilisation of Winemaking Residues: Characterisation of Grape Seeds by Total Phenols, Tocols and Essential Elements Content as a by-Product of Winemaking. Ind. Crops Prod. 2013, 49, 445–453. [Google Scholar] [CrossRef]
- Ghanem, C.; Taillandier, P.; Rizk, Z.; Nehme, N.; Souchard, J.P.; El Rayess, Y. Evolution of Polyphenols during Syrah Grapes Maceration: Time versus Temperature Effect. Molecules 2019, 24, 2845. [Google Scholar] [CrossRef]
- Ky, I.; Crozier, A.; Cros, G.; Teissedre, P. Polyphenols Composition of Wine and Grape Sub-Products and Potential Effects on Chronic Diseases. Nutr. Aging 2014, 2, 165–177. [Google Scholar] [CrossRef]
- Ky, I.; Teissedre, P.L. Characterisation of Mediterranean Grape Pomace Seed and Skin Extracts: Polyphenolic Content and Antioxidant Activity. Molecules 2015, 20, 2190–2207. [Google Scholar] [CrossRef]
- Oliveira Neto, J.R.; Lopes de Macêdo, I.Y.; Lopes de Oliveira, N.R.; de Queiroz Ferreira, R.; de Souza Gil, E. Antioxidant Capacity and Total Phenol Content in Hop and Malt Commercial Samples. Electroanalysis 2017, 29, 2788–2792. [Google Scholar] [CrossRef]
- Mikyška, A.; Hrabák, M.; Hašková, D.; Šrogl, J. The Role of Malt and Hop Polyphenols in Beer Quality, Flavour and Haze Stability. J. Inst. Brew. 2002, 108, 78–85. [Google Scholar] [CrossRef]
- Koistinen, V.M.; Tuomainen, M.; Lehtinen, P.; Peltola, P.; Auriola, S.; Jonsson, K.; Hanhineva, K. Side-Stream Products of Malting: A Neglected Source of Phytochemicals. npj Sci. Food 2020, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Knez Hrnčič, M.; Španinger, E.; Košir, I.J.; Knez, Ž.; Bren, U. Hop Compounds: Extraction Techniques, Chemical Analyses, Antioxidative, Antimicrobial, and Anticarcinogenic Effects. Nutrients 2019, 11, 257. [Google Scholar] [CrossRef]
- Gerhäuser, C. Beer Constituents as Potential Cancer Chemopreventive Agents. Eur. J. Cancer 2005, 41, 1941–1954. [Google Scholar] [CrossRef]
- Habschied, K.; Košir, I.J.; Krstanović, V.; Kumrić, G.; Mastanjević, K. Beer Polyphenols—Bitterness, Astringency, and Off-Flavors. Beverages 2021, 7, 38. [Google Scholar] [CrossRef]
- Shopska, V.; Denkova-Kostova, R.; Dzhivoderova-Zarcheva, M.; Teneva, D.; Denev, P.; Kostov, G. Comparative Study on Phenolic Content and Antioxidant Activity of Different Malt Types. Antioxidants 2021, 10, 1124. [Google Scholar] [CrossRef]
- Bagatini, L.; Veras, F.F.; Azevedo, G.D.; Sant Anna, V.; Brandelli, A. Pilsen, Red Ale and Stout Brewer’s Spent Grain: Polyphenols, Antioxidant and Antihypertensive Activities, and Physicochemical Properties. Bol. Cent. Pesqui. Process. Aliment. 2021, 37. [Google Scholar] [CrossRef]
- Ambra, R.; Pastore, G.; Lucchetti, S. The Role of Bioactive Phenolic Compounds on the Impact of Beer on Health. Molecules 2021, 26, 486. [Google Scholar] [CrossRef]
- Jurić, A.; Ćorić, N.; Odak, A.; Herceg, Z.; Tišma, M. Analysis of Total Polyphenols, Bitterness and Haze in Pale and Dark Lager Beers Produced under Different Mashing and Boiling Conditions. J. Inst. Brew. 2015, 121, 541–547. [Google Scholar] [CrossRef]
- Qingming, Y.; Xianhui, P.; Weibao, K.; Hong, Y.; Yidan, S.; Li, Z.; Yanan, Z.; Yuling, Y.; Lan, D.; Guoan, L. Antioxidant Activities of Malt Extract from Barley (Hordeum vulgare L.) toward Various Oxidative Stress in Vitro and in Vivo. Food Chem. 2010, 118, 84–89. [Google Scholar] [CrossRef]
- Hughes, P.S.; Simpson, W.J. Production and Composition of Hop Products. MBAA Tech. Q. 1993, 30, 146–154. [Google Scholar]
- Preedy, V.R. Beer in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2009; ISBN 978-0-12-373891-2. [Google Scholar]
- Krofta, K.; Mikyška, A.; Hašková, D. Antioxidant Characteristics of Hops and Hop Products. J. Inst. Brew. 2008, 114, 160–166. [Google Scholar] [CrossRef]
- Chiancone, B.; Guarrasi, V.; Leto, L.; Del Vecchio, L.; Calani, L.; Ganino, T.; Galaverni, M.; Cirlini, M. Vitro-Derived Hop (Humulus lupulus L.) Leaves and Roots as Source of Bioactive Compounds: Antioxidant Activity and Polyphenolic Profile. Plant Cell Tissue Organ. Cult. 2023, 153, 295–306. [Google Scholar] [CrossRef]
- Afonso, S.; Dias, M.I.; Ferreira, I.C.F.R.; Arrobas, M.; Cunha, M.; Barros, L.; Rodrigues, M.Â. The Phenolic Composition of Hops (Humulus lupulus L.) Was Highly Influenced by Cultivar and Year and Little by Soil Liming or Foliar Spray Rich in Nutrients or Algae. Horticulturae 2022, 8, 385. [Google Scholar] [CrossRef]
- Lermusieau, G.; Liégeois, C.; Collin, S. Reducing Power of Hop Cultivars and Beer Ageing. Food Chem. 2001, 72, 413–418. [Google Scholar] [CrossRef]
- Willett, W.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean Diet Pyramid: A Cultural Model for Healthy Eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S. [Google Scholar] [CrossRef]
- Lindberg, M.L.; Amsterdam, E.A. Alcohol, Wine, and Cardiovascular Health. Clin. Cardiol. 2008, 31, 347–351. [Google Scholar] [CrossRef]
- Friedman, L.A.; Kimball, A.W. Coronary Heart Disease Mortality and Alcohol Consumption in Framingham. Am. J. Epidemiol. 1986, 124, 481–489. [Google Scholar] [CrossRef]
- Muntwyler, J.; Hennekens, C.H.; Buring, J.E.; Gaziano, J.M. Mortality and Light to Moderate Alcohol Consumption after Myocardial Infarction. Lancet 1998, 352, 1882–1885. [Google Scholar] [CrossRef]
- Vinson, J.A.; Mandarano, M.; Hirst, M.; Trevithick, J.R.; Bose, P. Phenol Antioxidant Quantity and Quality in Foods: Beers and the Effect of Two Types of Beer on an Animal Model of Atherosclerosis. J. Agric. Food Chem. 2003, 51, 5528–5533. [Google Scholar] [CrossRef]
- Ramos, S. Cancer Chemoprevention and Chemotherapy: Dietary Polyphenols and Signalling Pathways. Mol. Nutr. Food Res. 2008, 52, 507–526. [Google Scholar] [CrossRef]
- Palmieri, D.; Pane, B.; Barisione, C.; Spinella, G.; Garibaldi, S.; Ghigliotti, G.; Brunelli, C.; Fulcheri, E.; Palombo, D. Resveratrol Counteracts Systemic and Local Inflammation Involved in Early Abdominal Aortic Aneurysm Development. J. Surg. Res. 2011, 171, e237–e246. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.R.; Lokhandwala, M.F.; Banday, A.A. Resveratrol Prevents Endothelial Nitric Oxide Synthase Uncoupling and Attenuates Development of Hypertension in Spontaneously Hypertensive Rats. Eur. J. Pharmacol. 2011, 667, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Crescente, M.; Jessen, G.; Momi, S.; Höltje, H.-D.; Gresele, P.; Cerletti, C.; de Gaetano, G. Interactions of Gallic Acid, Resveratrol, Quercetin and Aspirin at the Platelet Cyclooxygenase-1 Level Functional and Modelling Studies. Thromb. Haemost. 2009, 102, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Arranz, S.; Chiva-Blanch, G.; Valderas-Martínez, P.; Medina-Remón, A.; Lamuela-Raventós, R.M.; Estruch, R. Wine, Beer, Alcohol and Polyphenols on Cardiovascular Disease and Cancer. Nutrients 2012, 4, 759–781. [Google Scholar] [CrossRef]
- Vinson, J.A.; Jang, J.; Dabbagh, Y.A.; Serry, M.M.; Cai, S. Plant Polyphenols Exhibit Lipoprotein-Bound Antioxidant Activity Using an in Vitro Oxidation Model for Heart Disease. J. Agric. Food Chem. 1995, 43, 2798–2799. [Google Scholar] [CrossRef]
- Poklar Ulrih, N.; Opara, R.; Skrt, M.; Košmerl, T.; Wondra, M.; Abram, V. Part I. Polyphenols Composition and Antioxidant Potential during ‘Blaufränkisch’ Grape Maceration and Red Wine Maturation, and the Effects of Trans-Resveratrol Addition. Food Chem. Toxicol. 2020, 137, 111122. [Google Scholar] [CrossRef]
- Antonopoulou, S. Lipid Minor Constituents in Wines. A Biochemical Approach in the French Paradox. Int. J. Wine Res. 2009, 1, 131. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Antonopoulou, S.; Demopoulos, C.A. Biologically Active Lipids with Antiatherogenic Properties from White Wine and Must. J. Agric. Food Chem. 2002, 50, 2684–2694. [Google Scholar] [CrossRef]
- Xanthopoulou, M.N.; Kalathara, K.; Melachroinou, S.; Arampatzi-Menenakou, K.; Antonopoulou, S.; Yannakoulia, M.; Fragopoulou, E. Wine Consumption Reduced Postprandial Platelet Sensitivity against Platelet Activating Factor in Healthy Men. Eur. J. Nutr. 2017, 56, 1485–1492. [Google Scholar] [CrossRef]
- Basalekou, M.; Kallithraka, S.; Kyraleou, M. Wine Bioactive Compounds. In Functional Foods and Their Implications for Health Promotion; Elsevier: Amsterdam, The Netherlands, 2023; pp. 341–363. [Google Scholar]
- Gallone, B.; Steensels, J.; Prahl, T.; Soriaga, L.; Saels, V.; Herrera-Malaver, B.; Merlevede, A.; Roncoroni, M.; Voordeckers, K.; Miraglia, L.; et al. Domestication and Divergence of Saccharomyces cerevisiae Beer Yeasts. Cell 2016, 166, 1397–1410. [Google Scholar] [CrossRef]
- Garrido, J.; Borges, F. Wine and Grape Polyphenols—A Chemical Perspective. Food Res. Int. 2013, 54, 1844–1858. [Google Scholar] [CrossRef]
- Brown, L.; Kroon, P.A.; Das, D.K.; Das, S.; Tosaki, A.; Chan, V.; Singer, M.V.; Feick, P. The Biological Responses to Resveratrol and Other Polyphenols From Alcoholic Beverages. Alcohol. Clin. Exp. Res. 2009, 33, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Nassiri-Asl, M.; Hosseinzadeh, H. Review of the Pharmacological Effects of Vitis Vinifera (Grape) and Its Bioactive Compounds. Phyther. Res. 2009, 23, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- WATERHOUSE, A.L. Wine Phenolics. Ann. N. Y. Acad. Sci. 2002, 957, 21–36. [Google Scholar] [CrossRef]
- Fumi, M.D.; Galli, R.; Lambri, M.; Donadini, G.; De Faveri, D.M. Effect of Full-Scale Brewing Process on Polyphenols in Italian All-Malt and Maize Adjunct Lager Beers. J. Food Compos. Anal. 2011, 24, 568–573. [Google Scholar] [CrossRef]
- Di Lorenzo, A.; Bloise, N.; Meneghini, S.; Sureda, A.; Tenore, G.; Visai, L.; Arciola, C.; Daglia, M. Effect of Winemaking on the Composition of Red Wine as a Source of Polyphenols for Anti-Infective Biomaterials. Materials 2016, 9, 316. [Google Scholar] [CrossRef]
- Kühbeck, F.; Schütz, M.; Thiele, F.; Krottenthaler, M.; Back, W. Influence of Lauter Turbidity and Hot Trub on Wort Composition, Fermentation, and Beer Quality. J. Am. Soc. Brew. Chem. 2006, 64, 16–28. [Google Scholar] [CrossRef]
- Pascoe, H.M.; Ames, J.M.; Chandra, S. Critical Stages of the Brewing Process for Changes in Antioxidant Activity and Levels of Phenolic Compounds in Ale. J. Am. Soc. Brew. Chem. 2003, 61, 203–209. [Google Scholar] [CrossRef]
- Coghe, S.; Benoot, K.; Delvaux, F.; Vanderhaegen, B.; Delvaux, F.R. Ferulic Acid Release and 4-Vinylguaiacol Formation during Brewing and Fermentation: Indications for Feruloyl Esterase Activity in Saccharomyces Cerevisiae. J. Agric. Food Chem. 2004, 52, 602–608. [Google Scholar] [CrossRef]
- Boronat, A.; Soldevila-Domenech, N.; Rodríguez-Morató, J.; Martínez-Huélamo, M.; Lamuela-Raventós, R.M.; de la Torre, R. Beer Phenolic Composition of Simple Phenols, Prenylated Flavonoids and Alkylresorcinols. Molecules 2020, 25, 2582. [Google Scholar] [CrossRef]
- Back, W.; Zürcher, A.; Wunderlich, S. Verfahren Zur Herstellung Eines Xanthohumolhaltigen Getränkes Aus Malz-Und/Oder Rohfruchtwürze Sowie Derart Hergestelltes Getränk. DE10256166A1, 24 June 2004. [Google Scholar]
- Kostadinović, S.; Wilkens, A.; Stefova, M.; Ivanova, V.; Vojnoski, B.; Mirhosseini, H.; Winterhalter, P. Stilbene Levels and Antioxidant Activity of Vranec and Merlot Wines from Macedonia: Effect of Variety and Enological Practices. Food Chem. 2012, 135, 3003–3009. [Google Scholar] [CrossRef] [PubMed]
- Grieco, F.; Carluccio, M.A.; Giovinazzo, G. Autochthonous Saccharomyces Cerevisiae Starter Cultures Enhance Polyphenols Content, Antioxidant Activity, and Anti-Inflammatory Response of Apulian Red Wines. Foods 2019, 8, 453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ma, W.; Meng, Y.; Zhang, Y.; Jin, G.; Fang, Z. Wine Phenolic Profile Altered by Yeast: Mechanisms and Influences. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3579–3619. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.E.; Romaní, A.; Møller-Hansen, I.; Teixeira, J.A.; Borodina, I.; Domingues, L. Valorisation of Wine Wastes by de Novo Biosynthesis of Resveratrol Using a Recombinant Xylose-Consuming Industrial Saccharomyces Cerevisiae Strain. Green Chem. 2022, 24, 9128–9142. [Google Scholar] [CrossRef]
- Escribano-Viana, R.; Portu, J.; Garijo, P.; López, R.; Santamaría, P.; López-Alfaro, I.; Gutiérrez, A.R.; González-Arenzana, L. Effect of the Sequential Inoculation of Non-Saccharomyces/Saccharomyces on the Anthocyans and Stilbenes Composition of Tempranillo Wines. Front. Microbiol. 2019, 10, 773. [Google Scholar] [CrossRef]
- Aliyu, S.; Bala, M. Brewer’s Spent Grain: A Review of Its Potentials and Applications. Afr. J. Biotechnol. 2011, 10, 324–331. [Google Scholar]
- Robertson, J.A.; I’Anson, K.J.A.; Treimo, J.; Faulds, C.B.; Brocklehurst, T.F.; Eijsink, V.G.H.; Waldron, K.W. Profiling Brewers’ Spent Grain for Composition and Microbial Ecology at the Site of Production. LWT-Food Sci. Technol. 2010, 43, 890–896. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, J.C.; del Carmen Esapadas Aldana, G.; Cruz, A.I.C.; Segura-Campos, M.R. Antioxidant Activity of Polyphenols Extracted From Hop Used in Craft Beer. In Biotechnological Progress and Beverage Consumption; Elsevier: Amsterdam, The Netherlands, 2020; pp. 283–310. [Google Scholar]
- Estruch, R.; Urpi-Sarda, M.; Chiva, G.; Romero, E.S.; Covas, M.I.; Salas-Salvadó, J.; Wärnberg, J.; Lamuela-Raventós, R.M. Cerveza, Dieta Mediterránea y Enfermedad Cardiovascular; Centro de Información Cerveza y: Madrid, Spain, 2011; pp. 1–81. [Google Scholar]
- Wannenmacher, J.; Cotterchio, C.; Schlumberger, M.; Reuber, V.; Gastl, M.; Becker, T. Technological Influence on Sensory Stability and Antioxidant Activity of Beers Measured by ORAC and FRAP. J. Sci. Food Agric. 2019, 99, 6628–6637. [Google Scholar] [CrossRef]
- Jandera, P.; Škeříková, V.; Řehová, L.; Hájek, T.; Baldriánová, L.; Škopová, G.; Kellner, V.; Horna, A. RP-HPLC Analysis of Phenolic Compounds and Flavonoids in Beverages and Plant Extracts Using a CoulArray Detector. J. Sep. Sci. 2005, 28, 1005–1022. [Google Scholar] [CrossRef]
- Neveu, V.; Perez-Jimenez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An Online Comprehensive Database on Polyphenol Contents in Foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef]
- Marova, I.; Parilova, K.; Friedl, Z.; Obruca, S.; Duronova, K. Analysis of Phenolic Compounds in Lager Beers of Different Origin: A Contribution to Potential Determination of the Authenticity of Czech Beer. Chromatographia 2011, 73, 83–95. [Google Scholar] [CrossRef]
- Kellner, V.; Jurková, M.; Čulík, J.; Horák, T.; Čejka, P. Some Phenolic Compounds in Czech Hops and Beer of Pilsner Type. Brew. Sci. 2007, 60, 31–37. [Google Scholar]
- Wendelin, R.P.S.; Eder, R. Phenolic Composition and Varietal Discrimination of Montenegrin Red Wines (Vitis vinifera Var. Vranac, Kratošija, and Cabernet Sauvignon). Eur. Food Res. Technol. 2018, 244, 2243–2254. [Google Scholar] [CrossRef]
- Habschied, K.; Lončarić, A.; Mastanjević, K. Screening of Polyphenols and Antioxidative Activity in Industrial Beers. Foods 2020, 9, 238. [Google Scholar] [CrossRef]
- Szwajgier, D. Content of Individual Phenolic Acids in Worts and Beers and Their Possible Contribution to the Antiradical Activity of Beer. J. Inst. Brew. 2009, 115, 243–252. [Google Scholar] [CrossRef]
- Socha, R.; Pająk, P.; Fortuna, T.; Buksa, K. Antioxidant Activity and the Most Abundant Phenolics in Commercial Dark Beers. Int. J. Food Prop. 2017, 20, S595–S609. [Google Scholar] [CrossRef]
- Piazzon, A.; Forte, M.; Nardini, M. Characterization of Phenolics Content and Antioxidant Activity of Different Beer Types. J. Agric. Food Chem. 2010, 58, 10677–10683. [Google Scholar] [CrossRef]
- Cui, Y.; Li, Q.; Liu, Z.; Geng, L.; Zhao, X.; Chen, X.; Bi, K. Simultaneous Determination of 20 Components in Red Wine by LC-MS: Application to Variations of Red Wine Components in Decanting. J. Sep. Sci. 2012, 35, 2884–2891. [Google Scholar] [CrossRef]
- Mcmurrough, I.; Roche, G.P.; Cleary, K.G. Phenolic Acids In Beers And Worts. J. Inst. Brew. 1984, 90, 181–187. [Google Scholar] [CrossRef]
- Cernîşev, S. Analysis of Lignin-Derived Phenolic Compounds and Their Transformations in Aged Wine Distillates. Food Control 2017, 73, 281–290. [Google Scholar] [CrossRef]
- de Souza Dias, F.; Silva, M.F.; David, J.M. Determination of Quercetin, Gallic Acid, Resveratrol, Catechin and Malvidin in Brazilian Wines Elaborated in the Vale Do São Francisco Using Liquid–Liquid Extraction Assisted by Ultrasound and GC-MS. Food Anal. Methods 2013, 6, 963–968. [Google Scholar] [CrossRef]
- Nettleton, J.A.; Harnack, L.J.; Scrafford, C.G.; Mink, P.J.; Barraj, L.M.; Jacobs, D.R. Dietary Flavonoids and Flavonoid-Rich Foods Are Not Associated with Risk of Type 2 Diabetes in Postmenopausal Women. J. Nutr. 2006, 136, 3039–3045. [Google Scholar] [CrossRef] [PubMed]
- Tzounis, X.; Vulevic, J.; Kuhnle, G.G.C.; George, T.; Leonczak, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P.E. Flavanol Monomer-Induced Changes to the Human Faecal Microflora. Br. J. Nutr. 2008, 99, 782–792. [Google Scholar] [CrossRef]
- Gerhäuser, C.; Becker, H. Phenolic Compounds in Beer. In Beer in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2009; Volume 539. [Google Scholar]
- Chen, W.; Becker, T.; Qian, F.; Ring, J. Beer and Beer Compounds: Physiological Effects on Skin Health. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 142–150. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Espín, J.C. Effect of Food Structure and Processing on (Poly)Phenol–Gut Microbiota Interactions and the Effects on Human Health. Annu. Rev. Food Sci. Technol. 2019, 10, 221–238. [Google Scholar] [CrossRef]
- Singh, M.; Kaur, M.; Silakari, O. Flavones: An Important Scaffold for Medicinal Chemistry. Eur. J. Med. Chem. 2014, 84, 206–239. [Google Scholar] [CrossRef]
- Oliver Chen, C.-Y.; Blumberg, J.B. Flavonoids in Beer and Their Potential Benefit on the Risk of Cardiovascular Disease. In Beer in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2009; pp. 831–841. [Google Scholar]
- Galarce-Bustos, O.; Novoa, L.; Pavon-Perez, J.; Henriquez-Aedo, K.; Aranda, M. Chemometric Optimization of QuEChERS Extraction Method for Polyphenol Determination in Beers by Liquid Chromatography with Ultraviolet Detection. Food Anal. Methods 2019, 12, 448–457. [Google Scholar] [CrossRef]
- Nardini, M.; Foddai, M.S. Phenolics Profile and Antioxidant Activity of Special Beers. Molecules 2020, 25, 2466. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Elrod, S.M. Xanthohumol and the Medicinal Benefits of Beer. In Polyphenols: Mechanisms of Action in Human Health and Disease; Elsevier: Amsterdam, The Netherlands, 2018; pp. 19–32. [Google Scholar]
- Andrés-Iglesias, C.; Blanco, C.A.; Blanco, J.; Montero, O. Mass Spectrometry-Based Metabolomics Approach to Determine Differential Metabolites between Regular and Non-Alcohol Beers. Food Chem. 2014, 157, 205–212. [Google Scholar] [CrossRef]
- Manchester, L.; Poeggler, B.; Alvares, F.; Ogden, G.; Reiter, R. Melatonin Immunoreactivity in the Photosynthetic Prokaryote Rhodospirillum Rubrum: Implications for an Ancient Antioxidant System. Cell. Biol. Res. 1995, 41, 391–395. [Google Scholar]
- Hardeland, R.; Fuhrberg, B. Ubiquitous Melatonin. Presence and Effects in Unicells, Plants and Animals. Trends Comp. Biochem. Physiol. Sci. Open 1996, 2, 25–45. [Google Scholar]
- Tilden, A.R.; Becker, M.A.; Amma, L.L.; Arciniega, J.; McGaw, A.K. Melatonin Production in an Aerobic Photosynthetic Bacterium: An Evolutionarily Early Association with Darkness. J. Pineal Res. 1997, 22, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, C.; Kocadaǧli, T.; Gökmen, V. Formation of Melatonin and Its Isomer during Bread Dough Fermentation and Effect of Baking. J. Agric. Food Chem. 2014, 62, 2900–2905. [Google Scholar] [CrossRef]
- Grao-Cruces, E.; Calvo, J.R.; Maldonado-Aibar, M.D.; Millan-Linares, M.d.C.; Montserrat-de la Paz, S. Mediterranean Diet and Melatonin: A Systematic Review. Antioxidants 2023, 12, 264. [Google Scholar] [CrossRef]
- Varoni, E.M.; Paroni, R.; Antognetti, J.; Lodi, G.; Sardella, A.; Carrassi, A.; Iriti, M. Effect of Red Wine Intake on Serum and Salivary Melatonin Levels: A Randomized, Placebo-Controlled Clinical Trial. Molecules 2018, 23, 2474. [Google Scholar] [CrossRef]
- Sprenger, J.; Hardeland, R.; Fuhrberg, B. Melatonin Presence and Other Concentrations Indoles and in Yeast: In High Dependence on Tryptophan Availability under Occlusion from Oxygen and of Presence of 5% Ethanol. None of These Other Treatments Led to Jected Cells to a Passage through Salt, Fo. Cytol. 1999, 64, 209–221. [Google Scholar] [CrossRef]
- Rodriguez-Naranjo, M.I.; Gil-Izquierdo, A.; Troncoso, A.M.; Cantos-Villar, E.; Garcia-Parrilla, M.C. Melatonin Is Synthesised by Yeast during Alcoholic Fermentation in Wines. Food Chem. 2011, 126, 1608–1613. [Google Scholar] [CrossRef]
- Rodriguez-Naranjo, M.I.; Torija, M.J.; Mas, A.; Cantos-Villar, E.; Garcia-Parrilla, M.D.C. Production of Melatonin by Saccharomyces Strains under Growth and Fermentation Conditions. J. Pineal Res. 2012, 53, 219–224. [Google Scholar] [CrossRef]
- Gomez, F.J.V.; Raba, J.; Cerutti, S.; Silva, M.F. Monitoring Melatonin and Its Isomer in Vitis Vinifera Cv. Malbec by UHPLC-MS/MS from Grape to Bottle. J. Pineal Res. 2012, 52, 349–355. [Google Scholar] [CrossRef]
- Mercolini, L.; Saracino, M.A.; Bugamelli, F.; Ferranti, A.; Malaguti, M.; Hrelia, S.; Raggi, M.A. HPLC-F Analysis of Melatonin and Resveratrol Isomers in Wine Using an SPE Procedure. J. Sep. Sci. 2008, 31, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Vitalini, S.; Gardana, C.; Zanzotto, A.; Faoro, F.; Simonetti, P.; Iriti, M. From Vineyard to Glass: Agrochemicals Enhance the Melatonin and Total Polyphenol Contents and Antiradical Activity of Red Wines. J. Pineal Res. 2011, 51, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Naranjo, M.I.; Gil-Izquierdo, A.; Troncoso, A.M.; Cantos, E.; Garcia-Parrilla, M.C. Melatonin: A New Bioactive Compound in Wine. J. Food Compos. Anal. 2011, 24, 603–608. [Google Scholar] [CrossRef]
- Vitalini, S.; Gardana, C.; Simonetti, P.; Fico, G.; Iriti, M. Melatonin, Melatonin Isomers and Stilbenes in Italian Traditional Grape Products and Their Antiradical Capacity. J. Pineal Res. 2013, 54, 322–333. [Google Scholar] [CrossRef]
- Fracassetti, D.; Vigentini, I.; Lo Faro, A.F.F.; De Nisi, P.; Foschino, R.; Tirelli, A.; Orioli, M.; Iriti, M. Assessment of Tryptophan, Tryptophan Ethylester, and Melatonin Derivatives in Red Wine by SPE-HPLC-FL and SPE-HPLC-MS Methods. Foods 2019, 8, 99. [Google Scholar] [CrossRef]
- Albu, C.; Radu, L.E.; Radu, G.L. Assessment of Melatonin and Its Precursors Content by a HPLC-MS/MS Method from Different Romanian Wines. ACS Omega 2020, 5, 27254–27260. [Google Scholar] [CrossRef]
- Xiang, G.; Jia, R.; Wang, F.; Wang, S.; Li, Y.; Yao, Y. Exogenous L-Tryptophan Treatment Increases the Concentrations of Melatonin and Aroma Compounds in Grape Berries and Wine. Food Qual. Saf. 2024, 8, fyad042. [Google Scholar] [CrossRef]
- Fernández-Cruz, E.; Álvarez-Fernández, M.A.; Valero, E.; Troncoso, A.M.; García-Parrilla, M.C. Melatonin and Derived L-Tryptophan Metabolites Produced during Alcoholic Fermentation by Different Wine Yeast Strains. Food Chem. 2017, 217, 431–437. [Google Scholar] [CrossRef]
- Valera, M.J.; Morcillo-Parra, M.Á.; Zagórska, I.; Mas, A.; Beltran, G.; Torija, M.J. Effects of Melatonin and Tryptophol Addition on Fermentations Carried out by Saccharomyces Cerevisiae and Non-Saccharomyces Yeast Species under Different Nitrogen Conditions. Int. J. Food Microbiol. 2019, 289, 174–181. [Google Scholar] [CrossRef]
- Scutarașu, E.C.; Niță, R.G.; Vlase, L.; Zamfir, C.I.; Cioroiu, B.I.; Colibaba, L.C.; Muntean, D.; Luchian, C.E.; Vlase, A.M.; Cotea, V. Maximizing Wine Antioxidants: Yeast’s Contribution to Melatonin Formation. Antioxidants 2024, 13, 916. [Google Scholar] [CrossRef]
- Postigo, V.; Sanz, P.; García, M.; Arroyo, T. Impact of Non-Saccharomyces Wine Yeast Strains on Improving Healthy Characteristics and the Sensory Profile of Beer in Sequential Fermentation. Foods 2022, 11, 2029. [Google Scholar] [CrossRef] [PubMed]
- Fracassetti, D.; Francesco Lo Faro, A.F.; Moiola, S.; Orioli, M.; Tirelli, A.; Iriti, M.; Vigentini, I.; Foschino, R. Production of Melatonin and Other Tryptophan Derivatives by Oenococcus Oeni under Winery and Laboratory Scale. Food Microbiol. 2020, 86, 103265. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Moreno, H.; Calvo, J.R.; Maldonado, M.D. High Levels of Melatonin Generated during the Brewing Process. J. Pineal Res. 2013, 55, 26–30. [Google Scholar] [CrossRef]
- Cheng, G.; Ma, T.; Deng, Z.; Gutiérrez-Gamboa, G.; Ge, Q.; Xu, P.; Zhang, Q.; Zhang, J.; Meng, J.; Reiter, R.J.; et al. Plant-Derived Melatonin from Food: A Gift of Nature. Food Funct. 2021, 12, 2829–2849. [Google Scholar] [CrossRef]
- Maldonado, M.D.; Moreno, H.; Calvo, J.R. Melatonin Present in Beer Contributes to Increase the Levels of Melatonin and Antioxidant Capacity of the Human Serum. Clin. Nutr. 2009, 28, 188–191. [Google Scholar] [CrossRef]
- Eelderink-Chen, Z.; Mazzotta, G.; Sturre, M.; Bosman, J.; Roenneberg, T.; Merrow, M. A Circadian Clock in Saccharomyces Cerevisiae. Proc. Natl. Acad. Sci. USA 2010, 107, 2043–2047. [Google Scholar] [CrossRef]
- Tu, B.P. Ultradian Metabolic Cycles in Yeast, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2010; Volume 470. [Google Scholar]
- Roussel, M.R.; Lloyd, D. Observation of a Chaotic Multioscillatory Metabolic Attractor by Real-Time Monitoring of a Yeast Continuous Culture. FEBS J. 2007, 274, 1011–1018. [Google Scholar] [CrossRef]
- Kembro, J.M.; Flesia, A.G.; Nieto, P.S.; Caliva, J.M.; Lloyd, D.; Cortassa, S.; Aon, M.A. A Dynamically Coherent Pattern of Rhythms That Matches between Distant Species across the Evolutionary Scale. Sci. Rep. 2023, 13, 5326. [Google Scholar] [CrossRef]
- Vázquez, J.; Grillitsch, K.; Daum, G.; Mas, A.; Torija, M.J.; Beltran, G. Melatonin Minimizes the Impact of Oxidative Stress Induced by Hydrogen Peroxide in Saccharomyces and Non-Conventional Yeast. Front. Microbiol. 2018, 9, 1933. [Google Scholar] [CrossRef]
- Muñiz-Calvo, S.; Bisquert, R.; Guillamón, J.M. Melatonin in Yeast and Fermented Beverages: Analytical Tools for Detection, Physiological Role and Biosynthesis. Melatonin Res. 2020, 3, 144–160. [Google Scholar] [CrossRef]
- Sunyer-Figueres, M.; Mas, A.; Beltran, G.; Torija, M.J. Protective Effects of Melatonin on Saccharomyces Cerevisiae under Ethanol Stress. Antioxidants 2021, 10, 1735. [Google Scholar] [CrossRef] [PubMed]
- Bisquert, R.; Muñiz-Calvo, S.; Guillamón, J.M. Protective Role of Intracellular Melatonin against Oxidative Stress and UV Radiation in Saccharomyces Cerevisiae. Front. Microbiol. 2018, 9, 318. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cruz, E.; Cerezo, A.B.; Cantos-Villar, E.; Troncoso, A.M.; García-Parrilla, M.D.C. Time Course of L-Tryptophan Metabolites When Fermenting Natural Grape Musts: Effect of Inoculation Treatments and Cultivar on the Occurrence of Melatonin and Related Indolic Compounds. Aust. J. Grape Wine Res. 2019, 25, 92–100. [Google Scholar] [CrossRef]
- Morcillo-Parra, M.Á.; Valera, M.J.; Beltran, G.; Mas, A.; Torija, M.J. Glycolytic Proteins Interact with Intracellular Melatonin in Saccharomyces Cerevisiae. Front. Microbiol. 2019, 10, 2424. [Google Scholar] [CrossRef]
- Hardeland, R.; Pandi-Perumal, S.R. Melatonin, a Potent Agent in Antioxidative Defense: Actions as a Natural Food Constituent, Gastrointestinal Factor, Drug and Prodrug. Nutr. Metab. 2005, 2, 22. [Google Scholar] [CrossRef]
- Tan, D.; Manchester, L.C.; Esteban-zubero, E.; Zhou, Z. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef]
- Riviere, E.; Rossi, S.P.; Tavalieri, Y.E.; Muñoz de Toro, M.M.; Ponzio, R.; Puigdomenech, E.; Levalle, O.; Martinez, G.; Terradas, C.; Calandra, R.S.; et al. Melatonin Daily Oral Supplementation Attenuates Inflammation and Oxidative Stress in Testes of Men with Altered Spermatogenesis of Unknown Aetiology. Mol. Cell. Endocrinol. 2020, 515, 110889. [Google Scholar] [CrossRef]
- Scheer, F.A.J.L.; Van Montfrans, G.A.; Van Someren, E.J.W.; Mairuhu, G.; Buijs, R.M. Daily Nighttime Melatonin Reduces Blood Pressure in Male Patients with Essential Hypertension. Hypertension 2004, 43, 192–197. [Google Scholar] [CrossRef]
- Karbownik, M.; Lewinski, A.; Reiter, R.J. Anticarcinogenic Actions of Melatonin Which Involve Antioxidative Processes: Comparison with Other Antioxidants. Int. J. Biochem. Cell Biol. 2001, 33, 735–753. [Google Scholar] [CrossRef]
- Cardinali, D.P. Melatonin: Clinical Perspectives in Neurodegeneration. Front. Endocrinol. 2019, 10, 480. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Choi, W.S. Use of Melatonin in Cancer Treatment: Where Are We ? Int. J. Mol. Sci. 2022, 23, 3779. [Google Scholar] [CrossRef] [PubMed]
- Bonnefont-rousselot, D.; Collin, F. Melatonin: Action as Antioxidant and Potential Applications in Human Disease and Aging. Toxicology 2010, 278, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Iriti, E.; Varoni, E.; Vitalini, S. Melatonin in Traditional Mediterranean Diets. J. Pineal Res. 2010, 49, 101–105. [Google Scholar] [CrossRef]
- Anderson, K. The Emergence of Lower-Alcohol Beverages: The Case of Beer. J. Wine Econ. 2023, 18, 66–86. [Google Scholar] [CrossRef]
- Liu, J.; Vaag, P.; Delgado, A.; Ramsey, I.; Chatzinikolaou, C.; Harholt, J.; Liu, Q.; Oladokun, O. The Impact of Ethanol on the Sensory Perception and Aroma Release of Alcohol-Free Beers. Brew. Sci. 2024, 77, 102. [Google Scholar] [CrossRef]
- Goold, H.D.; Kroukamp, H.; Williams, T.C.; Paulsen, I.T.; Varela, C.; Pretorius, I.S. Yeast’s Balancing Act between Ethanol and Glycerol Production in Low-alcohol Wines. Microb. Biotechnol. 2017, 10, 264–278. [Google Scholar] [CrossRef]
- Klimczak, K.; Cioch-Skoneczny, M.; Ciosek, A.; Poreda, A. Application of Non-Saccharomyces Yeast for the Production of Low-Alcohol Beer. Foods 2024, 13, 3214. [Google Scholar] [CrossRef]
- Marconi, O.; Sileoni, V.; Ceccaroni, D.; Perretti, G. The Use of Rice in Brewing. In Advances in International Rice Research; InTech: London, UK, 2017. [Google Scholar]
- Ledley, A.J.; Elias, R.J.; Cockburn, D.W. The Role of Starch Digestion in the Brewing of Gluten-Free Beers. Food Biosci. 2024, 61, 104949. [Google Scholar] [CrossRef]
- Cela, N.; Galgano, F.; Perretti, G.; Di Cairano, M.; Tolve, R.; Condelli, N. Assessment of Brewing Attitude of Unmalted Cereals and Pseudocereals for Gluten Free Beer Production. Food Chem. 2022, 384, 132621. [Google Scholar] [CrossRef]
- Watson, H.G.; Vanderputten, D.; Van Landschoot, A.; Decloedt, A.I. Applicability of Different Brewhouse Technologies and Gluten-Minimization Treatments for the Production of Gluten-Free (Barley) Malt Beers: Pilot- to Industrial-Scale. J. Food Eng. 2019, 245, 33–42. [Google Scholar] [CrossRef]
- Picard, F.; Kurtev, M.; Chung, N.; Topark-Ngarm, A.; Senawong, T.; Machado de Oliveira, R.; Leid, M.; McBurney, M.W.; Guarente, L. Sirt1 Promotes Fat Mobilization in White Adipocytes by Repressing PPAR-γ. Nature 2004, 429, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Tirado-Kulieva, V.A.; Hernández-Martínez, E.; Minchán-Velayarce, H.H.; Pasapera-Campos, S.E.; Luque-Vilca, O.M. A Comprehensive Review of the Benefits of Drinking Craft Beer: Role of Phenolic Content in Health and Possible Potential of the Alcoholic Fraction. Curr. Res. Food Sci. 2023, 6, 100477. [Google Scholar] [CrossRef] [PubMed]
- Cecarini, V.; Gogoi, O.; Bonfili, L.; Veneruso, I.; Pacinelli, G.; De Carlo, S.; Benvenuti, F.; D’Argenio, V.; Angeletti, M.; Cannella, N.; et al. Modulation of Gut Microbiota and Neuroprotective Effect of a Yeast-Enriched Beer. Nutrients 2022, 14, 2380. [Google Scholar] [CrossRef] [PubMed]
- Lao, E.J.; Dimoso, N.; Raymond, J.; Mbega, E.R. The Prebiotic Potential of Brewers’ Spent Grain on Livestock’s Health: A Review. Trop. Anim. Health Prod. 2020, 52, 461–472. [Google Scholar] [CrossRef]
- Kanyer, A.J.; Bornhorst, G.M.; Marco, M.L.; Bamforth, C.W. Is Beer a Source of Prebiotics? J. Inst. Brew. 2017, 123, 361–365. [Google Scholar] [CrossRef]
- Li, M.; Du, J.; Zheng, Y. Non-Starch Polysaccharides in Wheat Beers and Barley Malt Beers: A Comparative Study. Foods 2020, 9, 131. [Google Scholar] [CrossRef]
- Pastoriza, S.; Rufián-Henares, J.A. Contribution of Melanoidins to the Antioxidant Capacity of the Spanish Diet. Food Chem. 2014, 164, 438–445. [Google Scholar] [CrossRef]
- Alves, G.; Xavier, P.; Limoeiro, R.; Perrone, D. Contribution of Melanoidins from Heat-Processed Foods to the Phenolic Compound Intake and Antioxidant Capacity of the Brazilian Diet. J. Food Sci. Technol. 2020, 57, 3119–3131. [Google Scholar] [CrossRef]
- Schettino, R.; Verni, M.; Acin-Albiac, M.; Vincentini, O.; Krona, A.; Knaapila, A.; Di Cagno, R.; Gobbetti, M.; Rizzello, C.G.; Coda, R. Bioprocessed Brewers’ Spent Grain Improves Nutritional and Antioxidant Properties of Pasta. Antioxidants 2021, 10, 742. [Google Scholar] [CrossRef]
- Zugravu, C.-A.; Medar, C.; Manolescu, L.; Constantin, C. Beer and Microbiota: Pathways for a Positive and Healthy Interaction. Nutrients 2023, 15, 844. [Google Scholar] [CrossRef]
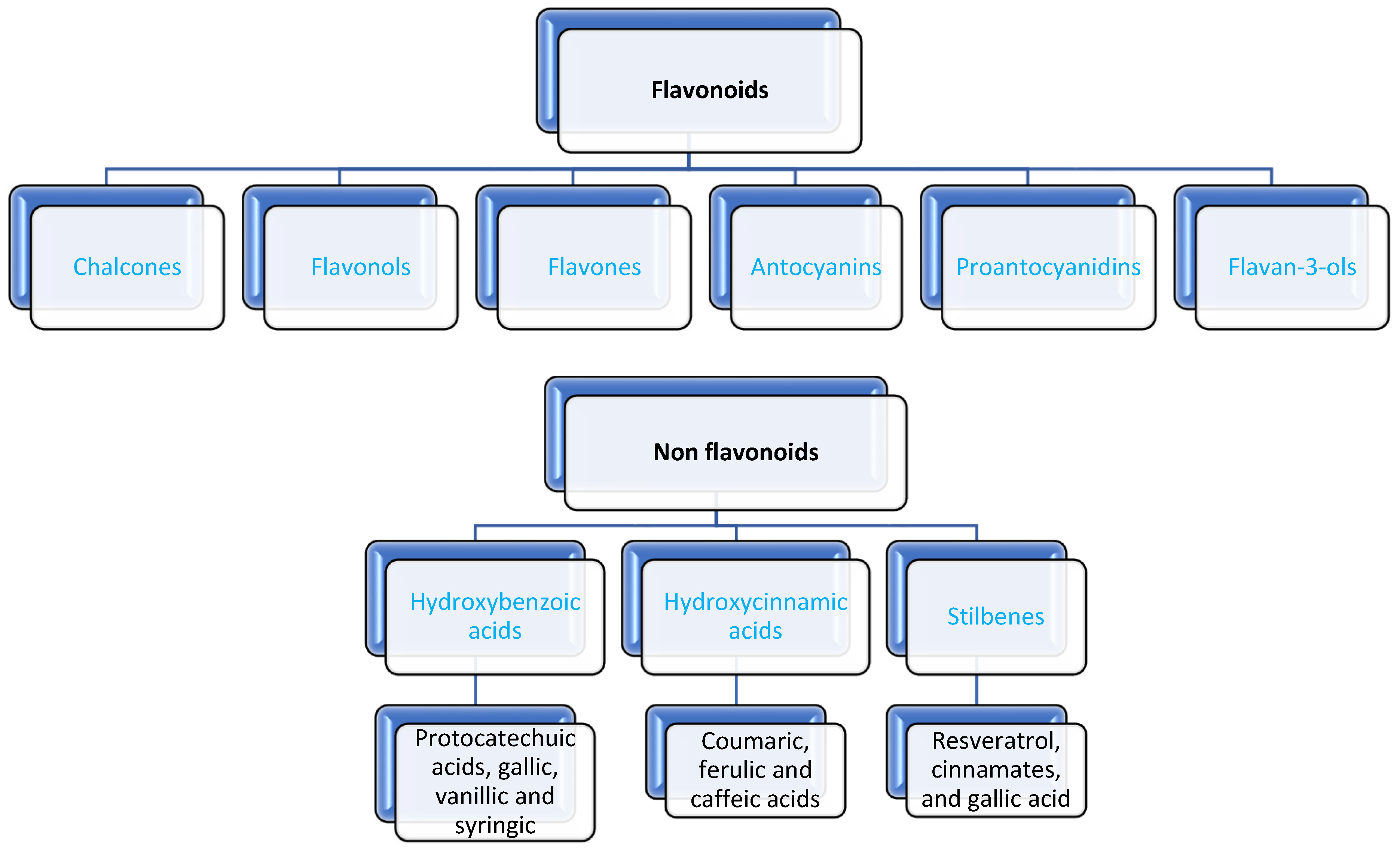
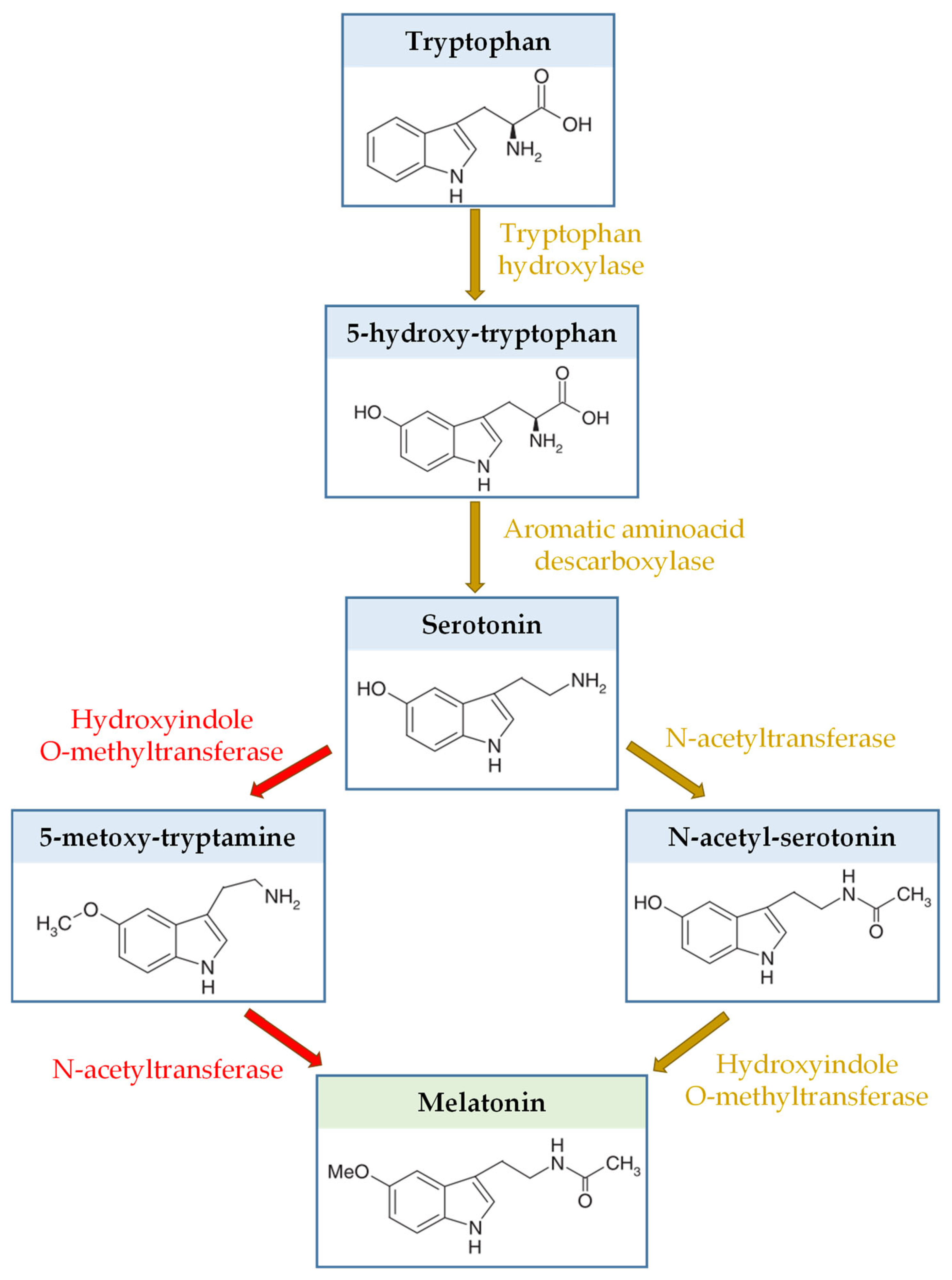
| Organic Acid | Presence/Location | Benefits |
|---|---|---|
| Tartaric Acid | Naturally occurring, unique to grapes | Stabilizes wine pH Adds freshness and structure Helps with preservation |
| Malic Acid | Natural, found in green grapes | Provides acidity and liveliness Adds freshness to young wines Converts to lactic acid during malolactic fermentation |
| Lactic Acid | Formed during malolactic fermentation | Softens acidity Adds creamy, rounded mouthfeel Enhances microbial stability |
| Acetic Acid | By-product of fermentation | In excess, causes vinegar-like off-flavors (a defect) |
| Citric Acid | Naturally present in small amounts in grapes | Increases acidity Enhances fruity flavor Acts as a natural antioxidant |
| Succinic Acid | Formed during alcoholic fermentation | Contributes to total acidity Adds pleasant salty and bitter notes in small doses |
| Nutritive Components | Range Value | Reference |
|---|---|---|
| Protein | 0.2–6.6 g/L | [94,173] |
| Flavonoids | 0.03–18.30 mg/L | [174] |
| Polyphenols | 34–426 (mg/L) | [6] |
| Vitamins | [174,175] | |
| Vitamin B1 | 0.0266 mg/L | |
| Vitamin B2 | 0.26–4.03 mg/L | |
| Vitamin B3 | 4.30–8.15 mg/L | |
| Vitamin B5 | 0.033–1.065 mg/L | |
| Vitamin B6 | 0.05–0.505 mg/L | |
| Vitamin B7 | 0.008–0.022 mg/L | |
| Vitamin B8 | 0–48,000 µg/L | |
| Vitamin B9 | 0–6000 µg/L | |
| Vitamin B12 | 23,000 µg/L | |
| Vitamin C | Up to 30 mg/L | |
| Elements | [94] | |
| Potassium | 200–600 mg/L | |
| Calcium | 20–160 mg/L | |
| Magnesium | 60–250 mg/L | |
| Zinc | 0.02–4.5 mg/L | |
| Copper | 0.02–0.4 mg/L | |
| Selenium | <0.007 mg/L | |
| Ethanol | 3–9 (% v/v) | [94] |
| Energy | 150–1100 kcal | [94,176] |
| Grape Varieties | Total Polyphenols (mg/L) | Seeds (mg/g) | Skins (mg/g) | References |
|---|---|---|---|---|
| White | ||||
| Verdelho | 319–2307 | - | - | [289,291] |
| Chardonnay | 649–2520 | 190 | 8.1 | [289] |
| Malvasia | 159–2958 | 63.9 | 9.1–27.6 | [289,290,291,292,293,294] |
| Muscat | 268–1321 | 102.2 | 10.4 | [295,296,297] |
| Red | ||||
| Pinot Nero | 21–693 | 38.7 | 21.4 | [289,298,299,300] |
| Merlot | 590–3560 | 13.5–867 | 30–280 | [289,295,301,302,303,304,305,306] |
| C. Sauvignon | 38.39–2195 | 10.2–103.7 | 29.5–33.2 | [289,295,301,303,306,307,308] |
| Tinto Fino | 1578–2530 | - | - | [307] |
| Syrah | 66–4380 | 33–215.9 | 20.8–146.5 | [309,310,311] |
| Varieties | Total Polyphenols (µg/mL) | References |
|---|---|---|
| Hop | ||
| Cascade | 2.8–19,600 | [312,326,327] |
| Columbus | 3.6–19,900 | [312,327] |
| Hallertau | 2.6–27,100 | [312,328] |
| Saaz | 229–37,900 | [325,328] |
| Nugget | 5200–16,700 | [327,328] |
| Malt | ||
| Chateau Cristal | 1.8 | [312] |
| Chateau Munich | 1 | [312] |
| Pilsen | 0.9 | [312] |
| Phenolic Compounds | Red Wine | Beer | Reference | Health Benefits |
|---|---|---|---|---|
| Flavonoids | ||||
| Flavanols | Reduces the risk of diabetes Improves the intestinal microbiota | |||
| Catechin | 6.98–91.99 | 0.03–5.4 | [358,365,366,367,368] | |
| Epicatechin | 8.07–37.8 | 0.19–3.3 | [358,365,367,368] | |
| Flavonols | Reduces the risk of diabetes Inhibit the release of histamine Block UV radiation Has an anti-aging effect Prevents weight gain Inhibits the growth of bacteria associated with an obesity-inducing die Treatment in neurodegenerative disorders Treatment in coronary heart disease Antioxidant, antitumor and anti-inflammatory activity | |||
| Kaempferol | 0.61–26.8 | 0.1–16.4 | [358,365,368] | |
| Quercetin | 1.27–65.9 | 0.06–10 | [358,365,368,369] | |
| Myricetin | 0.7–30.4 | 0.007–0.16 | [358,365,368,370] | |
| Non-Flavonoids | ||||
| Hydroxycinnamic acids | Antioxidant, anti-inflammatory, anti-allergic and anti-epileptic properties Anticarcinogenic activity Precursors of bioactive molecules that can be used in food | |||
| p-Coumaric acid | 0.02–8 | 0.003–55.8 | [358,365,366,368,371,372,373] | |
| Caffeic acid | 0.02–644.5 | 0.3–23.5 | [317,358,365,368,371,373,374,375] | |
| Ferulic acid | 0.05–10.43 | 0.01–6.5 | [358,365,368,371] | |
| Hydroxybenzoic acids | ||||
| Syringic acid | 0.27–2.7 | ≤0.5 | [365,368,375,376] | |
| Vanillic acid | 0.2–3.2 | 0.75–3.6 | [365,368,373,375,377,378] | |
| Gallic acid | 21.4–70 | 41.2–142.2 | [349,372,379] |
| Wine Variety | MEL Content (ng/mL) | Country | Reference |
|---|---|---|---|
| Sangiovese, Trebbiano, Albana | 0.4–0.6 | Italy | [171,402] |
| Chardonnay, Malbec, Cabernet Sauvignon | 0.16–0.32 | Argentina | [168] |
| Gropello, Merlot | 8.1 and 5.2 | Italy | [403] |
| Cabernet Sauvignon, Petit Verdot, Prieto Picudo, Syrah, Tempranillo | 5.1–129.5 | Spain | [404] |
| Cabernet Sauvignon, Merlot, Palomino Fino, Syrah, Tempranillo, Tintilla de Rota | 74.13–423.01 | Spain | [399] |
| Albana | 0.6 | Italy | [171] |
| Monovarietal red wines (Groppello DOC, Melag DOC, Nebbiolo IGT, Terre di Rubinoro DOCG and Syrah IGT) | 0.14–0.62 | Italy | [405] |
| Polyvaryetal red wines (Placido Rizzotto IGT and La Segreta IGT) | 0.05–0.31 | Italy | [405] |
| Monovarietal white wines (Chaudelune-Vin de glace DOC) | 0.11–0.31 | Italy | [405] |
| Nebbiolo | 0.57–0.63 | Italy | [406] |
| Monovarietal red wines (Cabernet Sauvignon, Merlot, Babeasca neagra, Seibel 1, Othello) | 1.0–66.6 | Romania | [407] |
| Polyvaryetal red wines (Cabernet Sauvignon and Merlot, Isabelle and Babeasca neagra) | 11.6–23.0 | Romania | [407] |
| Monovarietal rose wines (Cabernet Sauvignon, Lidia) | 1.4–82.6 | Romania | [407] |
| Polyvaryetal rose wines (Riesling and Chasselas) | 2.0 | Romania | [407] |
| Monovarietal white wines (Riesling, Noah) | 11.8–35.4 | Romania | [407] |
| Polyvaryetal rose wines (Riesling and Feteasca) | 1.0 | Romania | [407] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Postigo, V.; García, M.; Crespo, J.; Canonico, L.; Comitini, F.; Ciani, M. Bioactive Properties of Fermented Beverages: Wine and Beer. Fermentation 2025, 11, 234. https://doi.org/10.3390/fermentation11050234
Postigo V, García M, Crespo J, Canonico L, Comitini F, Ciani M. Bioactive Properties of Fermented Beverages: Wine and Beer. Fermentation. 2025; 11(5):234. https://doi.org/10.3390/fermentation11050234
Chicago/Turabian StylePostigo, Vanesa, Margarita García, Julia Crespo, Laura Canonico, Francesca Comitini, and Maurizio Ciani. 2025. "Bioactive Properties of Fermented Beverages: Wine and Beer" Fermentation 11, no. 5: 234. https://doi.org/10.3390/fermentation11050234
APA StylePostigo, V., García, M., Crespo, J., Canonico, L., Comitini, F., & Ciani, M. (2025). Bioactive Properties of Fermented Beverages: Wine and Beer. Fermentation, 11(5), 234. https://doi.org/10.3390/fermentation11050234









