Lactic Fermentation of Broccoli (Brassica oleracea var. italica) to Enhance the Antioxidant and Antiproliferative Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Starter Culture Preparation
2.3. Fermentation Process
2.4. Enumeration of Microorganisms
2.5. Chemical Composition
2.5.1. Determination of pH and Total Titratable Acidity
2.5.2. Proximal Analysis
2.6. Extraction Procedures
2.7. Glucosinolate Analysis (HPLC/ESI)
2.8. Identification and Quantification of Phenolic Compounds
2.8.1. Total Hydrolyzable Polyphenols
2.8.2. Total Condensed Polyphenols
2.8.3. HPLC-ESI-MS for Qualitative Analysis of Polyphenols
2.9. Antioxidant Activity of Broccoli Extracts
2.9.1. DPPH Radical Scavenging Activity
2.9.2. FRAP Assay
2.9.3. ABTS Assay
2.10. Cellular Antioxidant Activity Assay
2.11. Proliferation (SRB) Assay on Cancer Cell Lines
2.12. IL-8 Immunomodulation
2.13. Statistical Analysis
3. Results and Discussion
3.1. Growth of Bacteria during Fermentation
3.2. Changes in pH and Titratable Acidity during Fermentation
3.3. Chemical Composition
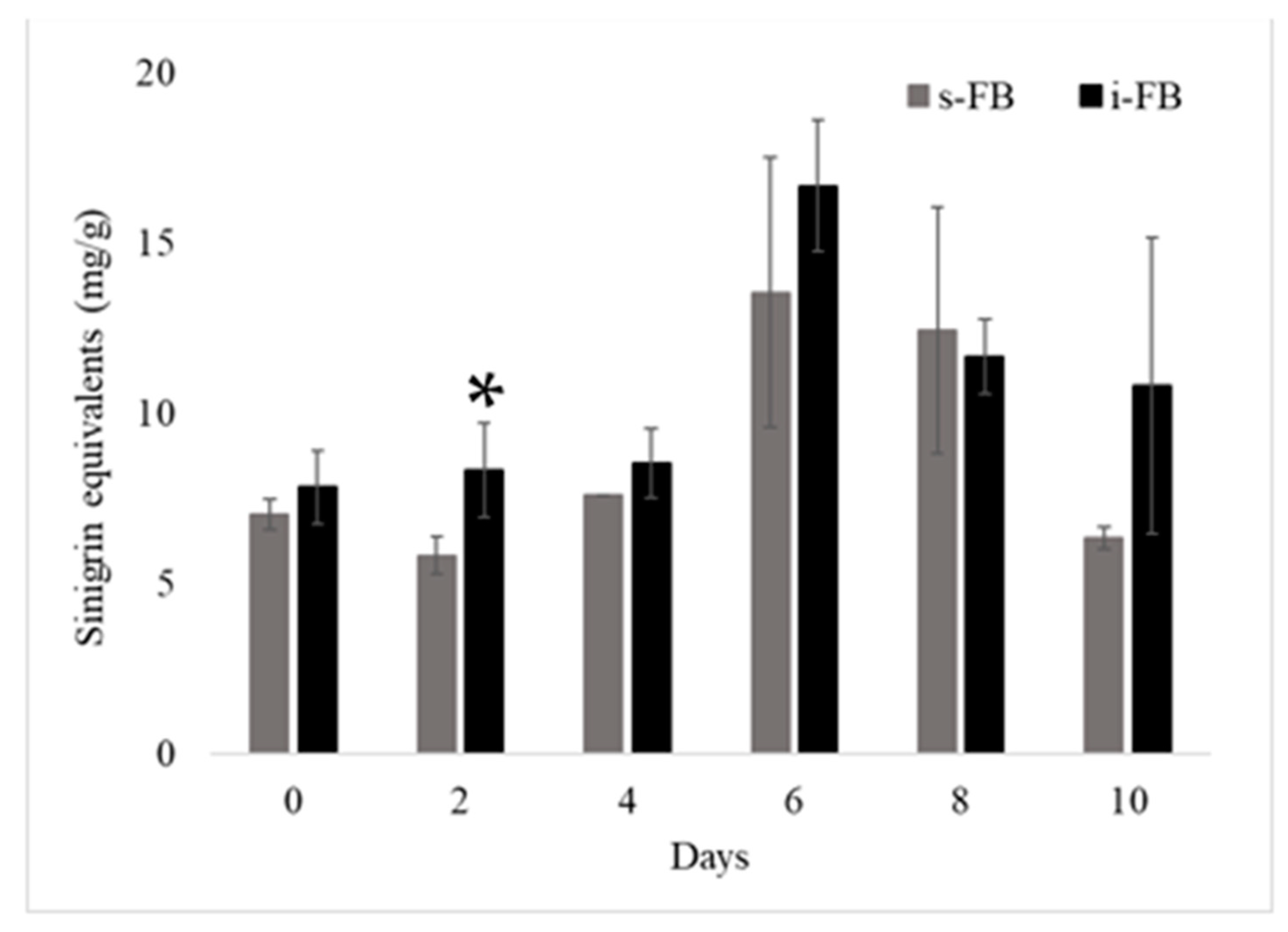
3.4. Glucosinolate Content Changes
3.5. Effect of Fermentation and Starter Culture on Total Phenolic Content and Antioxidant Activity of Broccoli
3.6. Identification of Phenolic Compounds
3.7. Cellular Antioxidant Activity (CAA) Assay Results from Caco2 Cells
3.8. Proliferation Assay
3.9. IL-8 Immunomodulation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Howlader, N.; Noone, A.; Krapcho, M. SEER Cancer Statistics Review, 1975–2011; National Cancer Institute, Bethesda: Rockville, MD, USA, 2014. [Google Scholar]
- Benarba, B.; Meddah, B.; Hamdani, H. Cancer incidence in North West Algeria (Mascara) 2000–2010: Results from a population-based cancer registry. EXCLI J. 2014, 13, 709–723. [Google Scholar] [PubMed]
- Brenner, H.; Kloor, M.; Pox, C.P. Colorectal cancer. Lancet 2014, 383, 1490–1502. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.M.; Wei, C.; Ensor, J.E.; Smolenski, D.J.; Amos, C.I.; Levin, B.; Berry, D.A. Meta-analyses of colorectal cancer risk factors. Cancer Causes Control. 2013, 24, 1207–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Cancer Society. Treatment of Colon Cancer, by Stage. 2016. Available online: https://www.cancer.org/cancer/colon-rectal-cancer/treating/by-stage-colon.html# (accessed on 23 October 2022).
- Divisi, D.; Di Tommaso, S.; Salvemini, S.; Garramone, M.; Crisci, R. Diet and cancer. Acta Biomed. 2006, 77, 118–123. [Google Scholar] [PubMed]
- Selma, M.V.; Espin, J.C.; Tomas-Barberan, F.A. Interaction between phenolics and gut microbiota: Role in human health. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Ho, C.L.; Tan, H.Q.; Chua, K.J.; Kang, A.; Lim, K.H.; Ling, K.L.; Yew, W.S.; Lee, Y.S.; Thiery, J.P.; Chang, M.W. Engineered commensal microbes for diet-mediated colorectal-cancer chemoprevention. Nat. Biomed. Eng. 2018, 2, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Gómez-Jodar, I.; Moreno, D.A.; García-Viguera, C.; Periago, P.M. Broccoli and radish sprouts are safe and rich in bioactive phytochemicals. Postharvest Biol. Technol. 2017, 127, 60–67. [Google Scholar] [CrossRef]
- Lafarga, T.; Bobo, G.; Viñas, I.; Zudaire, L.; Simó, J.; Aguiló-Aguayo, I. Steaming and sous-vide: Effects on antioxidant activity, vitamin C, and total phenolic content of Brassica vegetables. Int. J. Gastron. Food Sci. 2018, 13, 134–139. [Google Scholar] [CrossRef] [Green Version]
- Sosińska, E.; Obiedziński, M.W. Effect of processing on the content of glucobrassicin and its degradation products in broccoli and cauliflower. Food Control. 2011, 22, 1348–1356. [Google Scholar] [CrossRef]
- Radošević, K.; Srček, V.G.; Bubalo, M.C.; Brnčić, S.R.; Takács, K.; Redovniković, I. Assessment of glucosinolates, antioxidative and antiproliferative activity of broccoli and collard extracts. J. Food Compos. Anal. 2017, 61, 59–66. [Google Scholar] [CrossRef]
- Palani, K.; Harbaum-Piayda, B.; Meske, D.; Keppler, J.K.; Bockelmann, W.; Heller, K.J.; Schwarz, K. Influence of fermentation on glucosinolates and glucobrassicin degradation products in sauerkraut. Food Chem. 2016, 190, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Peñas, E.; Pihlava, J.M.; Vidal-Valverde, C.; Frías, J. Influence of fermentation conditions of Brassica oleracea L. var. capitata on the volatile glucosinolate hydrolysis compounds of sauerkrauts. LWT—Food Sci. Technol. 2012, 48, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Filannino, P.; Bai, Y.; Di Cagno, R.; Gobbetti, M.; Gänzle, M.G. Metabolism of phenolic compounds by Lactobacillus spp. during fermentation of cherry juice and broccoli puree. Food Microbiol. 2015, 46, 272–279. [Google Scholar] [CrossRef]
- Xiong, T.; Li, X.; Guan, Q.; Peng, F.; Xie, M. Starter culture fermentation of Chinese sauerkraut: Growth, acidification and metabolic analyses. Food Control. 2014, 41, 122–127. [Google Scholar] [CrossRef]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 2013, 33, 10–1016. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Remize, F.; Poucheret, P. Fruits and vegetables, as a source of nutritional compounds and phytochemicals: Changes in bioactive compounds during lactic fermentation. Food Res. Int. 2018, 104, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Luang-In, V.; Albaser, A.A.; Nueno-Palop, C.; Bennett, M.H.; Narbad, A.; Rossiter, J.T. Glucosinolate and Desulfo-glucosinolate Metabolism by a Selection of Human Gut Bacteria. Curr. Microbiol. 2016, 73, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.T.; Duarte, R.O.; Bronze, M.R.; Duarte, C.M. Identification of bioactive response in traditional cherries from Portugal. Food Chem. 2011, 125, 318–325. [Google Scholar] [CrossRef]
- Salas-Millán, J.A.; Aznar, A.; Conesa, E.; Conesa-Bueno, A.; Aguayo, E. Functional food obtained from fermentation of broccoli by-products (stalk): Metagenomics profile and glucosinolate and phenolic compounds characterization by LC-ESI-QqQ-MS/MS. LWT 2022, 169, 113915. [Google Scholar] [CrossRef]
- Kellett, M.E.; Greenspan, P.; Pegg, R.B. Modification of the cellular antioxidant activity (CAA) assay to study phenolic antioxidants in a Caco-2 cell line. Food Chem. 2018, 125, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Orellana, E.A.; Kasinski, A.L. Sulforhodamine B (SRB) assay in cell culture to investigate cell proliferation. Bio-Protoc. 2016, 6, e1984. [Google Scholar] [CrossRef] [Green Version]
- Wong-Paz, J.E.; Muñiz-Márquez, D.B.; Aguilar-Zárate, P.; Rodríguez-Herrera, R.; Aguilar, C.N. Microplate Quantification of Total Phenolic Content from Plant Extracts Obtained by Conventional and Ultrasound Methods. Phytochem. Anal. 2014, 25, 439–444. [Google Scholar] [CrossRef]
- Shay, P.E.; Trofymow, J.A.; Constabel, C.P. An improved butanol-HCl assay for quantification of water-soluble, acetone:methanol-soluble, and insoluble proanthocyanidins (condensed tannins). Plant Methods 2017, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Ascacio-Valdés, J.A.; Aguilera-Carbó, A.F.; Buenrostro, J.J.; Prado-Barragán, A.; Rodríguez-Herrera, R.; Aguilar, C.N. The complete biodegradation pathway of ellagitannins by Aspergillus niger in solid-state fermentation. J. Basic Microbiol. 2016, 56, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Lin, K.Y.; Guarnieri, F.G.; Staveley-O’Carroll, K.F.; Levitsky, H.I.; August, J.T.; Pardoll, D.M.; Wu, T.C. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996, 56, 21–26. [Google Scholar]
- Kechaou, N.; Chain, F.; Gratadoux, J.-J.; Blugeon, S.; Bertho, N.; Chevalier, C.; Le Goffic, R.; Courau, S.; Molimard, P.; Marc Chatel, J. Identification of One Novel Candidate Probiotic Lactobacillus plantarum Strain Active against Influenza Virus Infection in Mice by a Large-Scale Screening. Appl. Environ. Microbiol. Am. Soc. Microbiol. 2013, 79, 1491–1499. [Google Scholar] [CrossRef] [Green Version]
- Shokri, S.; Jegasothy, H.; Hliang, M.M.; Augustin, M.A.; Terefe, N.S. Thermosonication of Broccoli Florets Prior to Fermentation Increases Bioactive Components in Fermented Broccoli Puree. Fermentation 2022, 8, 236. [Google Scholar] [CrossRef]
- Chen, Y.S.; Liou, M.S.; Ji, S.H.; Yu, C.R.; Pan, S.F.; Yanagida, F. Isolation and characterization of lactic acid bacteria from yan-tsai-shin (fermented broccoli stems), a traditional fermented food in Taiwan. J. Appl. Microbiol. 2013, 115, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Kopermsub, P.; Yunchalard, S. Identification of lactic acid bacteria associated with the production of plaasom, a traditional fermented fish product of Thailand. Int. J. Food Microbiol. 2010, 138, 200–204. [Google Scholar] [CrossRef]
- Reis dos Ramos, L.C.; de Oliveira, V.R.; Hagen, M.E.; Jablonski, A.; Flôres, S.H.; de Oliveira Rios, A. Effect of cooking on the concentration of bioactive compounds in broccoli (Brassica oleracea var. Avenger) and cauliflower (Brassica oleracea var. Alphina F1) grown in an organic system. Food Chem. 2015, 172, 770–777. [Google Scholar] [CrossRef] [PubMed]
- National Database for Standard Reference. 2015. Available online: https://data.nal.usda.gov/dataset/usda-national-nutrient-database-standard-reference-legacy-release (accessed on 23 October 2022).
- Nuñez-Gastélum, J.A.; Moreno, C.R.; Lopez-cervantes, J. Biochemical composition and physicochemical properties of broccoli flours. Int. J. Food Sci. Nutr. 2009, 60, 163–173. [Google Scholar] [CrossRef]
- Bachiega, P.; Salgado, J.M.; de Carvalho, J.E.; Ruiz AL, T.; Schwarz, K.; Tezotto, T.; Morzelle, M.C. Antioxidant and antiproliferative activities in different maturation stages of broccoli (Brassica oleracea Italica) biofortified with selenium. Food Chem. 2016, 190, 771–776. [Google Scholar] [CrossRef] [Green Version]
- Marino, M.; Martini, D.; Venturi, S.; Tucci, M.; Porrini, M.; Riso, P.; Del Bo, C. An Overview of registered clinical trials on glucosinolates and human health: The current situation. Front. Nutr. 2021, 8, 798. [Google Scholar] [CrossRef] [PubMed]
- López-Chillón, M.T.; Carazo-Díaz, C.; Prieto-Merino, D.; Zafrilla, P.; Moreno, D.A.; Villaño, D. Effects of long-term consumption of broccoli sprouts on inflammatory markers in overweight subjects. Clin. Nutr. 2019, 38, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Dey, T.B.; Chakraborty, S.; Jain, K.K.; Sharma, A.; Kuhad, R.C. Antioxidant phenolics and their microbial production by submerged and solid state fermentation process: A review. Trends Food Sci. Technol. 2016, 53, 60–74. [Google Scholar] [CrossRef]
- Olsen, H.; Grimmer, S.; Aaby, K.; Saha, S.; Borge GI, A. Antiproliferative effects of fresh and thermal processed green and red cultivars of curly kale (Brassica oleracea L. convar. acephala var. sabellica). J. Agric. Food Chem. 2012, 60, 7375–7383. [Google Scholar] [CrossRef]
- Pandi, A.; Kalappan, M.V. Pharmacological and therapeutic applications of Sinapic acid—An updated review. Mol. Biol. Rep. 2021, 48, 3733–3745. [Google Scholar] [CrossRef]
- Thomas, M.; Badr, A.; Desjardins, Y.; Gosselin, A.; Angers, P. Characterization of industrial broccoli discards (Brassica oleracea var. italica) for their glucosinolate, polyphenol and flavonoid contents using UPLC MS/MS and spectrophotometric methods. Food Chem. 2018, 245, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Moreno, D.A.; Carvajal, M.; López-Berenguer, C.; García-Viguera, C. Chemical and biological characterisation of nutraceutical compounds of broccoli. J. Pharm. Biomed. 2006, 41, 1508–1522. [Google Scholar] [CrossRef] [PubMed]
- Moreno, D.A.; Carvajal, M.; López-Berenguer, C.; García-Viguera, C. Broccoli by-products improve the nutraceutical potential of gluten-free mini sponge cakes. Food Chem. 2017, 267, 170–177. [Google Scholar] [CrossRef]
- Lee, H.W.; Lee, C.G.; Rhee, D.K.; Um, S.H.; Pyo, S. Sinigrin inhibits production of inflammatory mediators by suppressing NF- κ B/MAPK pathways or NLRP3 in flammasome activation in macrophages. Int. Immunopharmacol. 2017, 45, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ming, C.; Zhang, W.; Okechukwu, P.N.; Morak-Młodawska, B.; Pluta, K.; Jeleń, M.; Akim, A.M.; Ang, K.P.; Ooi, K.K. 10H-3,6-Diazaphenothiazine induces G2/M phase cell cycle arrest and caspase-dependent apoptosis and inhibits cell invasion of A2780 ovarian carcinoma cells through the regulation of NF-κB and (BIRC6-XIAP) complexes. Drug Des. Devel Ther. 2017, 11, 3045–3063. [Google Scholar] [CrossRef] [Green Version]
- Navarro, S.L.; Schwarz, Y.; Song, X.; Wang, C.-Y.; Chen, C.; Trudo, S.P.; Kristal, A.R.; Kratz, M.; Eaton, D.L.; Lampe, J.W. Cruciferous Vegetables Have Variable Effects on Biomarkers of Systemic Inflammation in a Randomized Controlled Trial in Healthy Young Adults. J. Nutr. Nutr. Immunol. 2014, 144, 1850–1857. [Google Scholar] [CrossRef] [Green Version]






| Fermentation Day | Moisture (%) | Ash (g/100 g of Sample) | Protein (g/100 g of Sample) |
|---|---|---|---|
| s-FB | |||
| 0 | 90.47 ± 0.46 | 2.49 ± 0.18 | 19.91 ± 2.06 |
| 2 | 90.72 ± 0.24 | 2.42 ± 0.10 | 26.90 ± 3.29 |
| 4 | 90.17 ± 0.53 | 2.57 ± 0.11 | 23.36 ± 2.93 |
| 6 | 90.16 ± 1.00 | 2.58 ± 0.12 | 27.38 ± 1.46 |
| 8 | 90.85 ± 0.66 | 2.38 ± 0.14 | 25.55 ± 1.04 |
| 10 | 90.92 ± 0.24 | 2.24 ± 0.18 | 25.15 ± 1.34 |
| i-FB | |||
| 0 | 90.20 ± 0.86 | 2.49 ± 0.01 | 24.56 ± 3.84 |
| 2 | 91.33 ± 0.38 | 2.57 ± 0.23 | 24.92 ± 1.21 |
| 4 | 91.36 ± 2.27 | 2.49 ± 0.20 | 27.71 ±7.86 |
| 6 | 91.15 ± 0.73 | 2.27 ± 0.09 | 26.49 ± 1.62 |
| 8 | 91.27 ± 0.54 | 2.32 ± 0.11 | 24.32 ± 3.50 |
| 10 | 91.03 ± 0.84 | 2.51 ± 0.04 | 28.32 ± 1.65 |
| Fermentation Day | DPPH (µM of Trolox Equivalent mg−1) | ABTS (µM of Trolox Equivalent mg−1) | FRAP (µM of Trolox Equivalent mg−1) | Condensed Phenols (mg of Catechin g−1) | Hydrolyzed Phenols (mg of Gallic Acid g−1) | |
|---|---|---|---|---|---|---|
| 0 | s-FB | 84.7 ± 5.32 | 148.54 ± 17.12 | 29.54 ± 7.43 | 21.75 ± 0.83 * | 23.88 ± 0.34 * |
| i-FB | 71.38 ± 7.99 | 166.66 ± 5.70 | 34.19 ± 3.25 | 26.33 ± 2.08 * | 24.90 ± 0 * | |
| 2 | s-FB | 74.04 ± 7.99 | 189.49 ± 5.7 | 35.81 ± 3.48 | 24.25 ± 0.83 | 24 ± 0.45 |
| i-FB | 68.72 ± 2.66 | 158.10 ± 19.97 | 31.63 ± 0.23 | 23.0 ± 0.41 | 23.43 ± 0.11 | |
| 4 | s-FB | 78.04 ± 6.65 | 183.78 ± 17.12 | 16.30 ± 4.41 * | 25.08 ± 2.5 | 24.45 ± 0.22 * |
| i-FB | 92.69 ± 13.31 | 158.10 ± 8.56 | 39.07 ± 6.73 * | 23.0 ± 1.25 | 23.54 ± 0.22 | |
| 6 | s-FB | 68.72 ± 0.0 | 212.32 ± 11.41 | 21.88 ± 3.01 * | 24.66 ± 0.41 * | 23.54 ± 0.22 |
| i-FB | 75.37 ± 14.64 | 186.64 ± 14.26 | 31.21 ± 4.64 * | 14.66 ± 4.58 * | 23.31 ± 0.22 | |
| 8 | s-FB | 84.70 ± 0.0 | 183.78 ± 17.12 | 27.92 ± 2.55 | 22.16 ± 1.25 | 23.65 ± 0.34 |
| i-FB | 72.71 ± 14.64 | 166.66 ± 11.41 | 25.59 ± 2.09 | 20.91 ± 0 | 23.20 ± 0.11 | |
| 10 | s-FB | 78.04 ± 9.32 | 146.68 ± 19.97 * | 17.0 ± 3.71 * | 24.25 ± 0.83 * | 23.72 ± 0.22 * |
| i-FB | 74.04 ± 2.66 | 209.47 ± 14.26 * | 32.61 ± 6.96 * | 22.16 ± 0.41 * | 23.20 ± 0.11 * | |
| Retention Time | Compound | Family | Molecular Mass [M-H]− |
|---|---|---|---|
| s-FB | |||
| 21.103 | 3-Caffeoylquinic acid | Hydroxycinnamic acids | 352.9 |
| 25.87 | 5-5′-Dehydrodiferulic acid | Methoxycinnamic acid dimers | 384.9 |
| 26.85 | Ferulic acid 4-O-glucoside | Methoxycinnamic acids | 354.8 |
| 27.403 | 5-8′-Dehydrodiferulic acid | Methoxycinnamic acid dimers | 384.8 |
| 27.33 | Feruloyl glucose | Methoxycinnamic acids | 354.9 |
| 27.586 | 5-5′-Dehydrodiferulic acid | Methoxycinnamic acid dimers | 384.9 |
| 30.646 | (+)-Gallocatechin | Catechins | 305.9 |
| 31.44 | (-)-Epigallocatechin | Catechins | 306 |
| 32.84 | Quercetin 3-O-xylosyl-glucuronide | Flavonols | 608.9 |
| 32.417 | Quercetin 3-O-galactoside 7-O-rhamnoside | Flavonols | 609 |
| 34.29 | Caffeic acid 4-O-glucoside | Hydroxycinnamic acids | 339.9 |
| 40.28 | d-viniferin | Stilbene dimers | 455.1 |
| 40.76 | 1,2,2'-Trisinapoylgentiobiose | Methoxycinnamic acids | 958.8 |
| 40.46 | p-Coumaric acid 4-O-glucoside | Hydroxycinnamic acids | 325 |
| 41.627 | Sesaminol | Lignans | 369.9 |
| 43.368 | Feruloyl tartaric acid (isomero) | Methoxycinnamic acids | 325.1 |
| 43.903 | Feruloyl tartaric acid (isomero) | Methoxycinnamic acids | 325 |
| 46.97 | Feruloyl tartaric acid | Methoxycinnamic acids | 325.1 |
| 47.18 | p-Coumaroyl tyrosine | Hydroxycinnamic acids | 327.1 |
| i-FB | |||
| 5.96 | Bisdemethoxycurcumin | Curcuminoids | 306.9 |
| 6.058 | Scopoletin | Hydroxycoumarins | 190.9 |
| 7.073 | Pyrogallol | Other polyphenols | 127.9 |
| 20.829 | 1-Caffeoylquinic acid | Hydroxycinnamic acids | 353 |
| 26.578 | 3-Caffeoylquinic acid | Hydroxycinnamic acids | 354.9 |
| 26.198 | 4-Caffeoylquinic acid | Hydroxycinnamic acids | 354.9 |
| 27.328 | Caffeic acid 3-sulfate | 258.9 | |
| 27.693 | Lariciresinol | Lignans | 358.9 |
| 31.339 | Apigenin galactoside-arabinoside | Flavones | 563 |
| 32.876 | Caffeic acid 4-O-glucoside | Hydroxycinnamic acids | 339.9 |
| 32.923 | Quercetin 3-O-xylosyl-glucuronide | Flavonols | 609 |
| 35.532 | Pedunculagin II | 784.8 | |
| 39.934 | d-Viniferin | Stilbene dimers | 455.2 |
| 40.209 | 1,2,2'-Trisinapoylgentiobiose | Methoxycinnamic acids | 958.9 |
| 40.426 | 1,2,2'-Trisinapoylgentiobiose (isomero) | Methoxycinnamic acids | 958.8 |
| 40.698 | 1,2'-Disinapoyl-2-feruloylgentiobiose | Methoxycinnamic acids | 928.9 |
| 40.872 | Sesaminol | Lignans | 369.9 |
| 46.18 | p-Coumaroyl tyrosine | Hydroxycinnamic acids | 327.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iga-Buitrón, D.; Torres-Maravilla, E.; Bermúdez-Humaran, L.G.; Ascacio-Valdes, J.A.; Rodríguez-Herrera, R.; Aguilar, C.N.; Flores-Gallegos, A.C. Lactic Fermentation of Broccoli (Brassica oleracea var. italica) to Enhance the Antioxidant and Antiproliferative Activities. Fermentation 2023, 9, 122. https://doi.org/10.3390/fermentation9020122
Iga-Buitrón D, Torres-Maravilla E, Bermúdez-Humaran LG, Ascacio-Valdes JA, Rodríguez-Herrera R, Aguilar CN, Flores-Gallegos AC. Lactic Fermentation of Broccoli (Brassica oleracea var. italica) to Enhance the Antioxidant and Antiproliferative Activities. Fermentation. 2023; 9(2):122. https://doi.org/10.3390/fermentation9020122
Chicago/Turabian StyleIga-Buitrón, Daniela, Edgar Torres-Maravilla, Luis G. Bermúdez-Humaran, Juan A. Ascacio-Valdes, Raúl Rodríguez-Herrera, Cristóbal N. Aguilar, and Adriana C. Flores-Gallegos. 2023. "Lactic Fermentation of Broccoli (Brassica oleracea var. italica) to Enhance the Antioxidant and Antiproliferative Activities" Fermentation 9, no. 2: 122. https://doi.org/10.3390/fermentation9020122








