The Effects of Selenium on Rumen Fermentation Parameters and Microbial Metagenome in Goats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selenium Yeast
2.2. Animals, Diets, and Experimental Design
2.3. Chemical Composition
2.4. Rumen Fermentation Parameters
2.5. Shotgun Metagenome
2.6. Statistical Analysis
3. Results
3.1. Rumen Fermentation Parameters
3.2. Macrogenome Sequencing Results
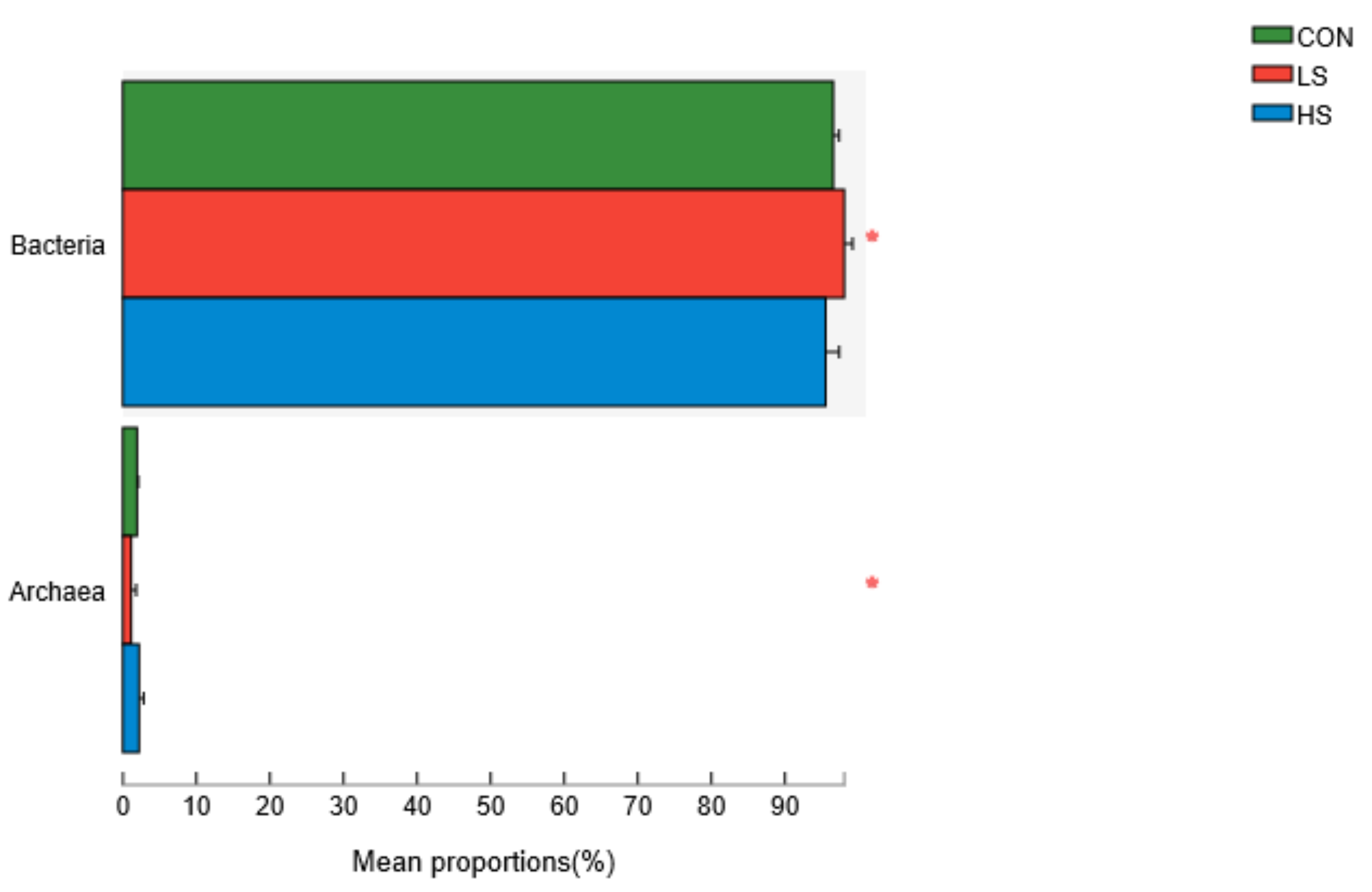
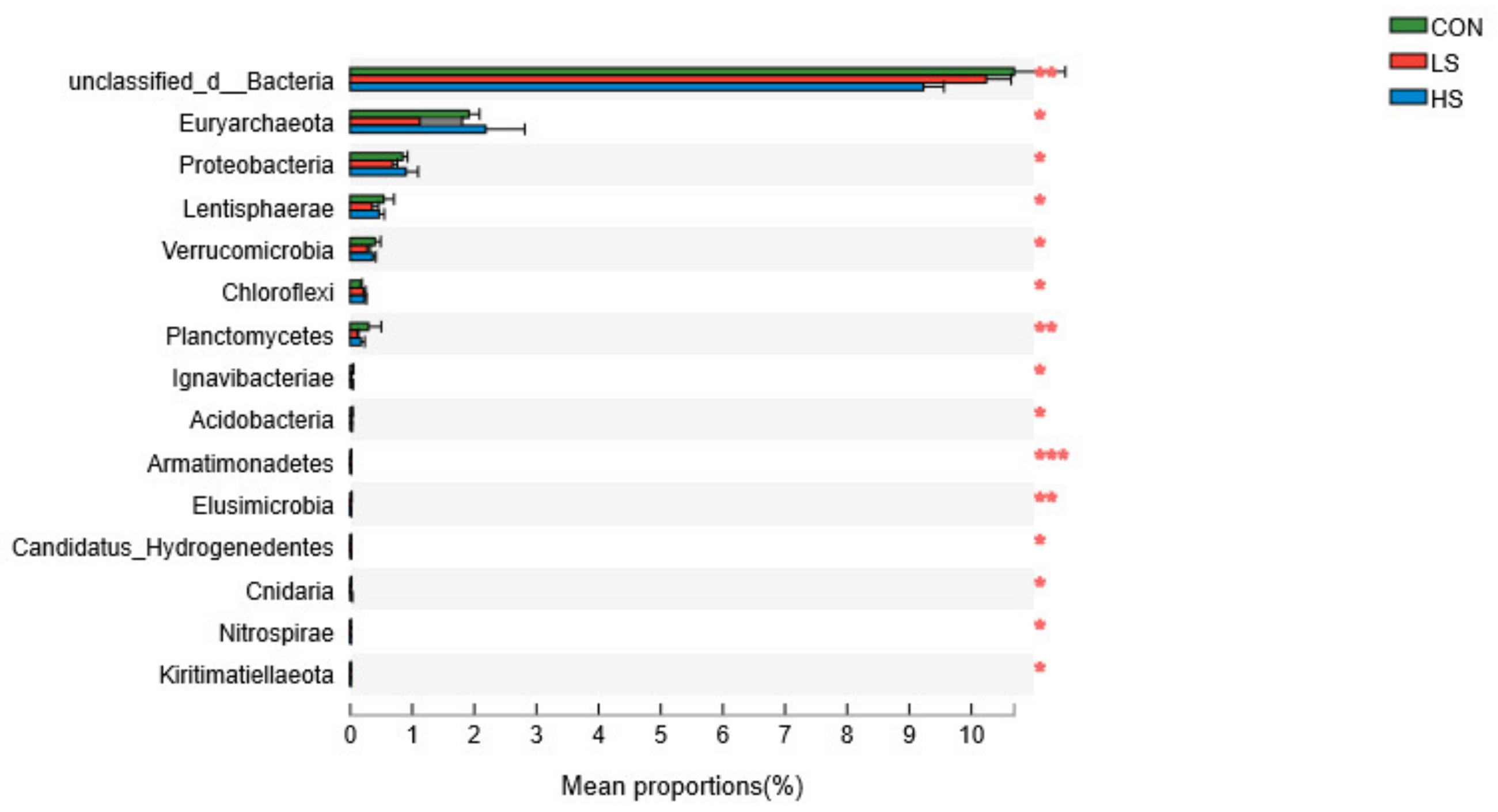
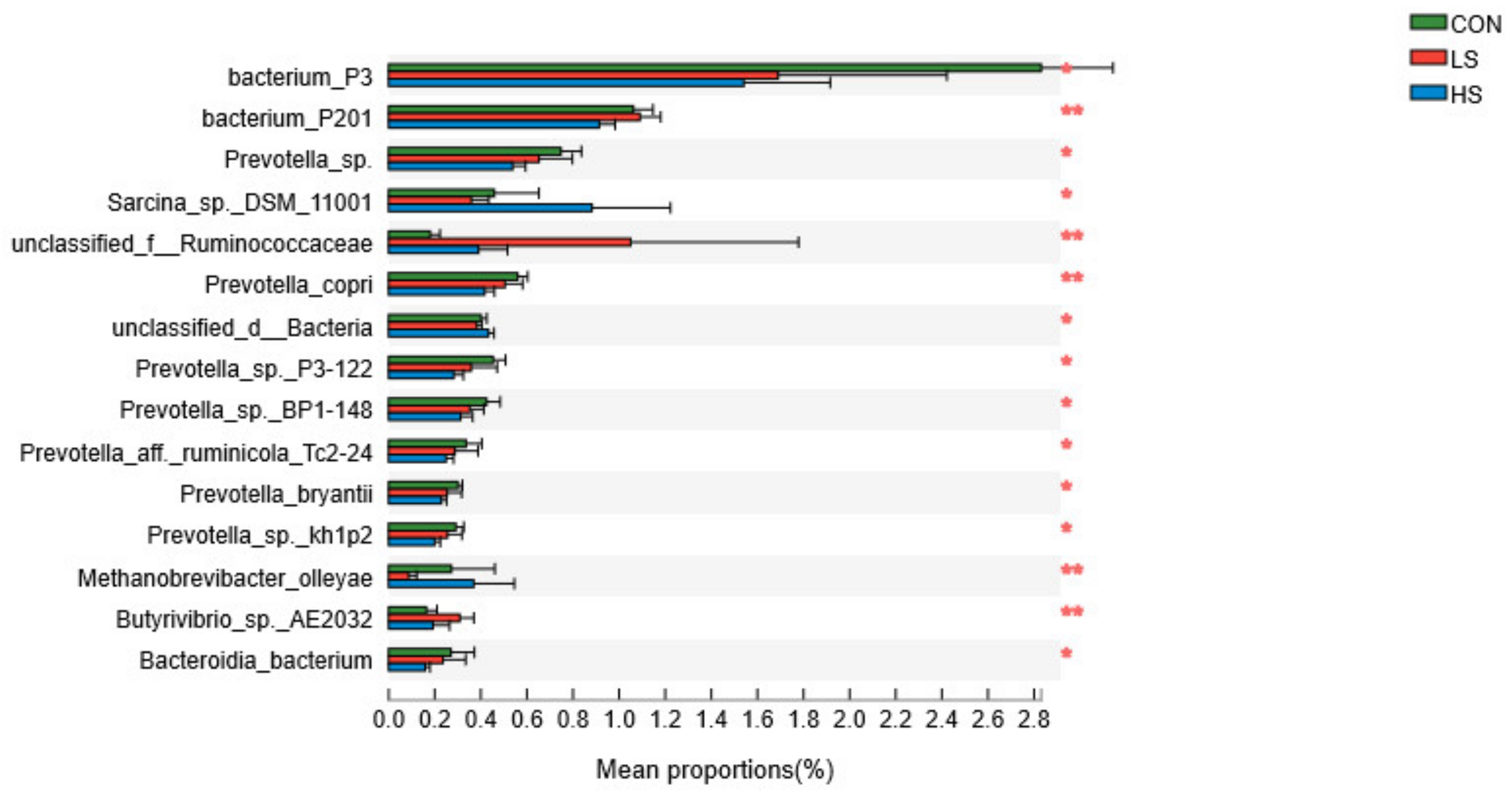
3.3. Relative Abundances of Rumen Microorganisms
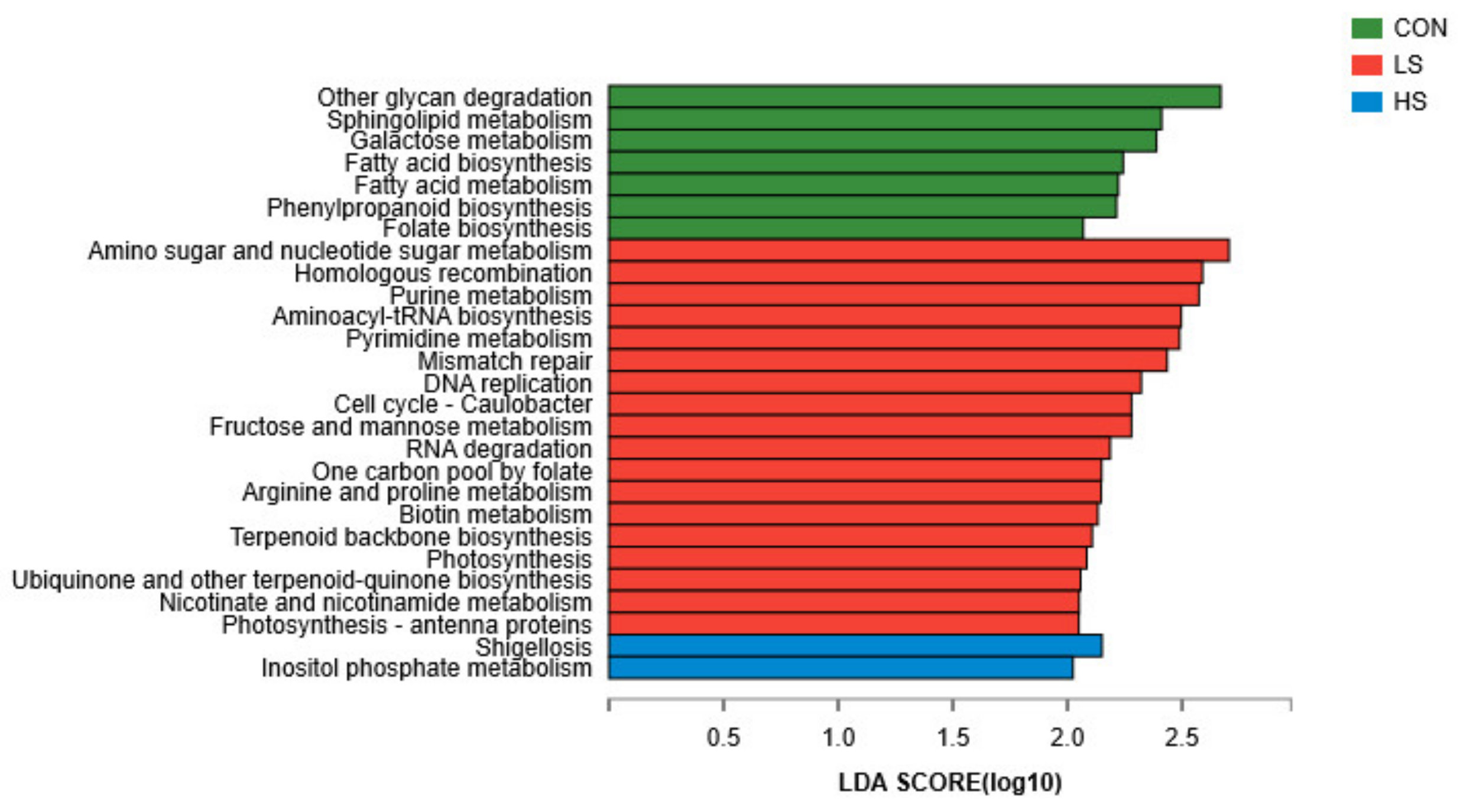
3.4. Relative Abundances of KEGG Pathways
3.5. Relative Abundance of CAZy Enzymes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tapio, I.; Snelling, T.J.; Strozzi, F.; Wallace, R.J. The ruminal microbiome associated with methane emissions from ruminant livestock. J. Anim. Sci. Biotechnol. 2017, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Ebeid, H.M.; Yang, C.; Hassan, F.U. Phytogenic additives can modulate rumen microbiome to mediate fermentation kinetics and methanogenesis through exploiting diet-microbe interaction. Front. Vet. Sci. 2020, 7, 575801. [Google Scholar]
- Henderson, G.; Cox, F.; Ganesh, S.; Jonker, A.; Young, W.; Janssen, P.H. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci. Rep. 2015, 5, 14567. [Google Scholar] [CrossRef] [PubMed]
- Ryman, V.E.; Packiriswamy, N.; Sordillo, L. Role of endothelial cells in bovine mammary gland health and disease. Anim. Health Res. Rev. 2015, 16, 135–149. [Google Scholar] [CrossRef]
- Tian, X.Z.; Lu, Q.; Paengkoum, P.; Paengkoum, S. Short communication: Effect of purple corn pigment on change of anthocyanin composition and unsaturated fatty acids during milk storage. J. Dairy Sci. 2020, 103, 7808–7812. [Google Scholar] [CrossRef]
- Tian, X.Z.; Wang, X.; Ban, C.; Luo, Q.Y.; Li, J.X.; Lu, Q. Effect of purple corn anthocyanin on antioxidant activity, volatile compound and sensory property in milk during storage and light prevention. Front. Nutr. 2022, 9, 862689. [Google Scholar] [CrossRef]
- Tian, X.Z.; Paengkoum, P.; Paengkoum, S.; Thongpea, S.; Ban, C. Comparison of forage yield, silage fermentative quality, anthocyanin stability, antioxidant activity, and in vitro rumen fermentation of anthocyanin-rich purple corn (Zea mays L.) stover and sticky corn stover. J. Integr. Agr. 2018, 17, 2082–2095. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, S.S.; Celi, P.; Ponnampalam, E.N.; Leury, B.J.; Liu, F.; Dunshea, F.R. Antioxidant dynamics in the live animal and implications for ruminant health and product (meat/milk) quality: Role of vitamin E and selenium. Anim. Prod. Sci. 2014, 54, 1525–1536. [Google Scholar] [CrossRef]
- Silveira, R.M.F.; Silva, B.E.B.E.; Vasconcelos, A.M.D.; Façanha, D.A.E.; Martins, T.P.; Rogério, M.C.P.M.; Ferreira, J. Does organic selenium supplement affect the thermoregulatory responses of dairy goats? Biol. Rhythm. Res. 2021, 52, 1–13. [Google Scholar] [CrossRef]
- Ceballos, A.; Sanchez, J.; Stryhn, H.; Montgomery, J.B.; Barkema, H.W.; Wichtel, J.J. Meta-analysis of the effect of oral selenium supplementation on milk selenium concentration in cattle. J. Dairy Sci. 2009, 92, 324–342. [Google Scholar] [CrossRef] [Green Version]
- Juniper, D.T.; Phipps, R.H.; Givens, D.I.; Jones, A.K.; Green, C.; Bertin, G. Tolerance of ruminant animals to high dose in-feed administration of a selenium-enriched yeast. J. Anim. Sci. 2008, 86, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Mainville, A.M.; Odongo, N.E.; Bettger, W.J.; Mcbride, B.W.; Osborne, V.R. Selenium uptake by ruminal microorganisms from organic and inorganic sources in dairy cows. Can. J. Anim. Sci. 2009, 89, 105–110. [Google Scholar] [CrossRef]
- Milewski, S.; Sobiech, P.; Baejak-Grabowska, J.; Wójcik, R.; Zbek, K. The efficacy of a long-acting injectable selenium preparation administered to pregnant ewes and lambs. Animals 2021, 11, 1076. [Google Scholar] [CrossRef] [PubMed]
- Koenig, K.M.; Rode, L.M.; Cohen, R.; Buckley, W.T. Effects of diet and chemical form of selenium on selenium metabolism in sheep. J. Anim. Sci. 1997, 75, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Kišidayová, S.; Mihaliková, K.; Siroka, P.; Čobanová, K.; Váradyová, Z. Effects of inorganic and organic selenium on the fatty acid composition of rumen contents of sheep and the rumen bacteria and ciliated protozoa. Anim. Feed Sci. Technol. 2014, 193, 51–57. [Google Scholar] [CrossRef]
- Pino, F.; Heinrichs, A.J. Effect of trace minerals and starch on digestibility and rumen fermentation in diets for dairy heifers. J. Dairy Sci. 2016, 99, 2797–2810. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Wang, Z.; Tan, Y.; Chang, S.; Zheng, H.; Wang, H.; Yan, T.; Guru, T.; Hou, F. Selenium yeast dietary supplement affects rumen bacterial population dynamics and fermentation parameters of Tibetan sheep (Ovis aries) in alpine meadow. Front. Microbiol. 2021, 12, 663945. [Google Scholar] [CrossRef]
- Arshad, M.A.; Ebeid, H.M.; Hassan, F. Revisiting the effects of different dietary sources of selenium on the health and performance of dairy animals: A review. Biol. Trace Elem. Res. 2021, 199, 3319–3337. [Google Scholar] [CrossRef]
- Tian, X.Z.; Lu, Q.; Zhao, S.G.; Li, J.X.; Luo, Q.Y.; Wang, X.; Zhang, Y.; Zheng, N. Purple corn anthocyanin affects lipid mechanism, flavor compound profiles, and related gene expression of longissimus thoracis et lumborum muscle in goats. Animals 2021, 11, 2407. [Google Scholar] [CrossRef]
- Tian, X.Z.; Li, J.X.; Luo, Q.Y.; Wang, X.; Xiao, M.M.; Zhou, D.; Lu, Q.; Chen, Q. Effect of supplementation with selenium-yeast on muscle antioxidant activity, meat quality, fatty acids and amino acids in goats. Front. Vet. Sci. 2022, 8, 813672. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Goats: Angora, Dairy, and Meat Goats in Temperate and Tropical Countries; The National Academies Press: Washington, DC, USA, 1981. [Google Scholar]
- Association of Official Analytical Chemists. Association of Official Analytical Chemists Official Methods of Analysis, 18th ed.; AOAC: Washington, DC, USA, 2005. [Google Scholar]
- National Research Council. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids, 1st ed.; National Academy Press: Washington, DC, USA, 2007. [Google Scholar]
- Van Soest, P.V.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Bremner, J.M.; Keeney, D.R. Steam distillation methods for determination of ammonium, nitrate and nitrite. Anal. Chim. Acta 1965, 32, 485–495. [Google Scholar] [CrossRef]
- Tian, X.Z.; Li, J.X.; Luo, Q.Y.; Zhou, D.; Long, Q.M.; Wang, X.; Lu, Q.; Wen, G.L. Effects of purple corn anthocyanin on blood biochemical indexes, ruminal fluid fermentation, and rumen microbiota in goats. Front. Vet. Sci. 2021, 8, 715710. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.Z.; Xin, H.L.; Paengkoum, P.; Paengkoum, S.; Ban, C.; Sorasak, T. Effects of anthocyanin-rich purple corn (Zea mays L.) stover silage on nutrient utilization, rumen fermentation, plasma antioxidant capacity, and mammary gland gene expression in dairy goats. J. Anim. Sci. 2019, 97, 1384–1397. [Google Scholar] [CrossRef] [PubMed]
- Xun, W.; Shi, L.; Yue, W.; Zhang, C.; Ren, Y.; Qiang, L. Effect of high-dose nano-selenium and selenium-yeast on feed digestibility, rumen fermentation, and purine derivatives in sheep. Biol. Trace Elem. Res. 2012, 150, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Lemosquet, S.; Delamaire, E.; Lapierre, H.; Blum, J.W.; Peyraud, J.L. Effects of glucose, propionic acid, and nonessential amino acids on glucose metabolism and milk yield in Holstein dairy cows. J. Dairy Sci. 2009, 92, 3244–3257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suriyapha, C.; Cherdthong, A.; Suntara, C.; Polyorach, S. Utilization of yeast waste fermented citric waste as a protein source to replace soybean meal and various roughage to concentrate ratios on in vitro rumen fermentation, gas kinetic, and feed digestion. Fermentation 2021, 7, 120. [Google Scholar] [CrossRef]
- Hendawy, A.O.; Sugimura, S.; Sato, K.; Mansour, M.M.; Abd El-Aziz, A.H.; Samir, H.; Islam, M.A.; Bostami, A.B.M.R.; Mandour, A.S.; Elfadadny, A.; et al. Effects of selenium supplementation on rumen microbiota, rumen fermentation, and apparent nutrient digestibility of ruminant animals: A review. Fermentation 2022, 8, 4. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, Y.; Lou, S.; Wanapat, M.; Wang, Z.; Zhu, W.; Hou, F. Selenium supplementation improves nutrient intake and digestibility, and mitigates CH4 emissions from sheep grazed on the mixed pasture of alfalfa and tall fescue. J. Anim. Physiol. Anim. Nutr. 2021, 105, 611–620. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Q.; Yang, W.Z.; Dong, Q.; Yang, X.M.; He, D.C.; Zhang, P.; Dong, K.H.; Huang, Y.X. Effects of selenium yeast on rumen fermentation, lactation performance and feed digestibilities in lactating dairy cows. Livest. Sci. 2009, 126, 239–244. [Google Scholar] [CrossRef]
- Stewart, R.D.; Auffret, M.D.; Warr, A.; Walker, A.W.; Watson, M. Compendium of 4941 rumen metagenome-assembled genomes for rumen microbiome biology and enzyme discovery. Nat. Biotechnol. 2019, 37, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, K.; Tian, X.; Ma, Z.; Wu, W. Feeding a negative dietary cation–anion difference to female goats is feasible, as indicated by the non-deleterious effect on rumen fermentation and rumen microbial population and increased plasma calcium level. Animals 2021, 11, 664. [Google Scholar] [CrossRef] [PubMed]
- Chloe, M.; Fiona, C.; Eva, L.; Michael, R.; O’Toole, W.P.; Cotter, P.D. The rumen microbiome: A crucial consideration when optimising milk and meat production and nitrogen utilisation efficiency. Gut Microbes 2018, 10, 115–132. [Google Scholar]
- Mihaliková, K.; Grešáková, L.; Boldižárová, K.; Faix, Š.; Leng, L.; Kišidayová, S. The effects of organic selenium supplementation on the rumen ciliate population in sheep. Folia Microbiol. 2005, 50, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Lenártová, V.; Holovská, K.; Javorský, P. The influence on the antioxidant enzyme activity of rumen bacteria Streptococcus bovis and Selenomonas ruminantium. FEMS Microbiol. Ecol. 1998, 27, 319–325. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.D.; Wang, C.; Du, H.S.; Liu, Q.; Guo, G.; Huo, W.J.; Zhang, J.; Zhang, Y.L.; Pei, C.X.; Zhang, S.L. Effects of sodium selenite and coated sodium selenite on lactation performance, total tract nutrient digestion and rumen fermentation in Holstein dairy cows. Anim. Int. J. Anim. Biosci. 2020, 14, 2091–2099. [Google Scholar] [CrossRef]
- Minoru, K.V.; Miho, F.; Mao, T.; Yoko, S.; Kanae, M. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar]
- Zhang, Y.K.; Zhang, X.X.; Li, F.D.; Li, C.; Li, G.Z.; Zhang, D.Y.; Song, Q.Z.; Li, X.L.; Zhao, Y.; Wang, W.M. Characterization of the rumen microbiota and its relationship with residual feed intake in sheep. Animals 2021, 15, 100161. [Google Scholar] [CrossRef]
- Xue, M.Y.; Sun, H.Z.; Wu, X.H.; Liu, J.X.; Guan, L.L. Multi-omics reveals that the rumen microbiome and its metabolome together with the host metabolome contribute to individualized dairy cow performance. Microbiome 2020, 8, 64. [Google Scholar] [CrossRef]
- Singh, K.M.; Reddy, B.; Patel, D.; Patel, A.K.; Joshi, C.G. High potential source for biomass degradation enzyme discovery and environmental aspects revealed through metagenomics of Indian buffalo rumen. Biomed. Res. Int. 2014, 5938, 267189. [Google Scholar] [CrossRef]
- Spears, J.W.; Weiss, W.P. Invited review: Mineral and vitamin nutrition in ruminants. Prof. Anim. Sci. 2014, 30, 180–191. [Google Scholar] [CrossRef]
- Yu, X.; Yang, G.; Yan, C.; Baylon, J.L.; Yan, N. Dimeric structure of the uracil:proton symporter UraA provides mechanistic insights into the SLC4/23/26 transporters. Cell Res. 2017, 27, 1020–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.S.; Yin, S.A. Effect of vitamin B6 status on selenium retention in the tissues in rats fed selenium from sodium selenate. J. Hyg. Res. 2005, 34, 422–424. [Google Scholar]
- Tian, X.Z.; Paengkoum, P.; Paengkoum, S.; Chumpawadee, S.; Ban, C.; Thongpea, S. Short communication: Purple corn (Zea mays L.) stover silage with abundant anthocyanins transferring anthocyanin composition to the milk and increasing antioxidant status of lactating dairy goats. J. Dairy Sci. 2019, 102, 413–418. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.; Li, J.; Luo, Q.; Wang, X.; Wang, T.; Zhou, D.; Xie, L.; Ban, C.; Lu, Q. Effects of purple corn anthocyanin on growth performance, meat quality, muscle antioxidant status, and fatty acid profiles in goats. Foods 2022, 11, 1255. [Google Scholar] [CrossRef]
- Shi, L.; Xun, W.; Yue, W.; Zhang, C.; Ren, Y.; Lei, S.; Wang, Q.; Yang, R.; Lei, F. Effect of sodium selenite, se-yeast and nano-elemental selenium on growth performance, se concentration and antioxidant status in growing male goats. Small Rumin. Res. 2011, 96, 49–52. [Google Scholar] [CrossRef]
- Shi, L.; Ren, Y.; Zhang, C.; Yue, W.; Lei, F. Effects of organic selenium (Se-enriched yeast) supplementation in gestation diet on antioxidant status, hormone profile and haemato-biochemical parameters in Taihang black goats. Anim. Feed. Sci. Tech. 2018, 238, 57–63. [Google Scholar] [CrossRef]
- Badgar, K.; Prokisch, J. The effects of selenium nanoparticles (SeNPs) on ruminant. Proc. Mong. Acad. Sci. 2020, 60, 1–8. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.H.; Chen, Y.X.; Cheng, Z.H.; Dong, H.M. Age-related response of rumen microbiota to mineral salt and effects of their interactions on enteric methane emissions in cattle. Microb. Ecol. 2016, 73, 1–12. [Google Scholar] [CrossRef]
- Geng, A.; Jin, M.; Li, N.; Zhu, D.; Xie, R.; Wang, Q.; Liu, H.; Sun, J. New insights into the co-occurrences of glycoside hydrolase genes among prokaryotic genomes through network analysis. Microorganisms 2021, 9, 427. [Google Scholar] [CrossRef]
- Wang, Y.; McAllister, T.A. Rumen microbes, enzymes and feed digestion—A review. Asian-Australas. J. Anim. Sci. 2002, 15, 1659–1676. [Google Scholar] [CrossRef]
- Lairson, L.L.; Henrissat, B.; Davies, G.J.; Withers, S.G. Glycosyltransferases: Structures, functions, and mechanisms. Annu. Rev. Biochem. 2008, 77, 521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Soest, P.; Combs, G.F. Studies on the effects of selenium on rumen microbial fermentation in vitro. Biol. Trace Elem. Res. 1997, 56, 203–213. [Google Scholar] [CrossRef]
- Waghorn, G.C.; Hegarty, R.S. Lowering ruminant methane emissions through improved feed conversion efficiency. Anim. Feed Sci. Technol. 2011, 166, 291–301. [Google Scholar] [CrossRef]


| Ingredients | Content |
|---|---|
| Peanut vines, % | 50.00 |
| White distiller’s grains, % | 10.00 |
| Soybean residues, % | 10.00 |
| Green hay, % | 9.30 |
| Corn, % | 16.00 |
| Soybean meal, % | 3.00 |
| Premix 2, % | 1.00 |
| Salt, % | 0.50 |
| Limestone, % | 0.20 |
| Total, % | 100.00 |
| Chemical composition 3 | |
| Dry matter, % | 90.15 |
| Metabolic energy 4, MJ/kg DM | 10.01 |
| Crude protein, % DM | 11.95 |
| Neutral detergent fiber, % DM | 42.74 |
| Acid detergent fiber, % DM | 28.61 |
| Ether extract, % DM | 2.35 |
| Ash, % DM | 8.72 |
| Item 2 | Group 3 | SEM | p-Value | ||
|---|---|---|---|---|---|
| CON | LS | HS | |||
| pH | 6.74 ± 0.38 | 6.80 ± 0.34 | 6.69 ± 0.17 | 0.1426 | 0.3851 |
| NH3-N, mg/dL | 11.18 ± 1.00 | 11.15 ± 0.68 | 10.94 ± 0.71 | 0.4673 | 0.9264 |
| Total VFAs, mM | 99.98 ± 12.99 | 104.61 ± 9.38 | 105.55 ± 5.48 | 3.9916 | 0.5829 |
| Individual VFA | |||||
| Acetic acid, % | 68.93 ± 2.98 | 68.39 ± 2.19 | 66.79 ± 1.90 | 0.9811 | 0.3051 |
| Propionic acid, % | 15.24 ± 1.55 b | 16.84 ± 0.84 a | 15.03 ± 0.81 b | 0.4574 | 0.0263 |
| Butyric acid, % | 12.17 ± 0.45 b | 10.28 ± 0.74 c | 14.89 ± 0.84 a | 0.2842 | <0.0001 |
| Valeric acid, % | 0.95 ± 0.17 b | 0.98 ± 0.06 b | 1.19 ± 0.08 a | 0.0467 | 0.0044 |
| Caproic acid, % | 0.06 ± 0.00 b | 0.11 ± 0.02 a | 0.13 ± 0.02 a | 0.0060 | <0.0001 |
| Isobutyric acid, % | 1.35 ± 0.10 b | 1.74 ± 0.29 a | 0.99 ± 0.10 c | 0.0767 | <0.0001 |
| Isovaleric acid, % | 1.30 ± 0.13 b | 1.67 ± 0.23 a | 0.98 ± 0.08 c | 0.0658 | <0.0001 |
| Acetate:propionate | 4.56 ± 0.51 a | 4.07 ± 0.19 b | 4.45 ± 0.19 ab | 0.1355 | 0.0405 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, X.; Wang, X.; Li, J.; Luo, Q.; Ban, C.; Lu, Q. The Effects of Selenium on Rumen Fermentation Parameters and Microbial Metagenome in Goats. Fermentation 2022, 8, 240. https://doi.org/10.3390/fermentation8050240
Tian X, Wang X, Li J, Luo Q, Ban C, Lu Q. The Effects of Selenium on Rumen Fermentation Parameters and Microbial Metagenome in Goats. Fermentation. 2022; 8(5):240. https://doi.org/10.3390/fermentation8050240
Chicago/Turabian StyleTian, Xingzhou, Xu Wang, Jiaxuan Li, Qingyuan Luo, Chao Ban, and Qi Lu. 2022. "The Effects of Selenium on Rumen Fermentation Parameters and Microbial Metagenome in Goats" Fermentation 8, no. 5: 240. https://doi.org/10.3390/fermentation8050240
APA StyleTian, X., Wang, X., Li, J., Luo, Q., Ban, C., & Lu, Q. (2022). The Effects of Selenium on Rumen Fermentation Parameters and Microbial Metagenome in Goats. Fermentation, 8(5), 240. https://doi.org/10.3390/fermentation8050240







