Improved Production of α-Amylase by Aspergillus terreus in Presence of Oxygen-Vector
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bioreactor and Operating Parameters
2.2. Strain and Medium
2.3. Measurement and Analysis Methods
3. Results
3.1. The Oxygen-Vector Effect on the Fermentation Broth pH
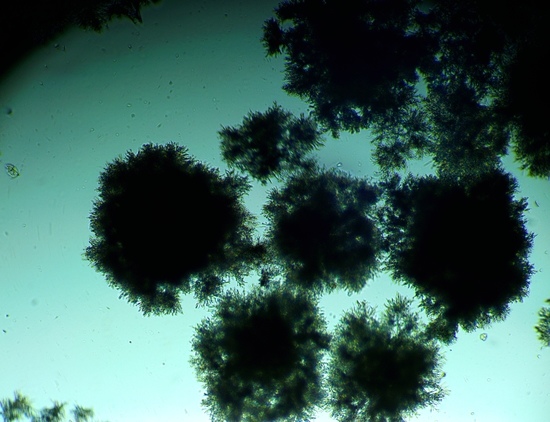
3.2. The Effect of the Oxygen-Vector on A. terreus Morphology
3.3. The Effect of the Oxygen-Vector on the Biomass Accumulation and α-Amylase Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saranraj, P.; Stella, D. Fungal Amylase—A Review. Int. J. Microbiol. Res. 2013, 4, 203–211. [Google Scholar] [CrossRef]
- Rajagopalan, G.; Krishnan, C. α-Amylase production from catabolite derepressed Bacillus subtilis KCC103 utilizing sugarcane bagasse hydrolysate. Bioresour. Technol. 2008, 99, 3044–3050. [Google Scholar] [CrossRef] [PubMed]
- Oguro, Y.; Nakamura, A.; Kurahashi, A. Effect of temperature on saccharification and oligosaccharide production efficiency in koji amazake. J. Biosci. Bioeng. 2019, 127, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Putri, A.Z.; Nakagawa, T. Microbial α-Amylases in the Industrial Extremozymes. Rev. Agric. Sci. 2020, 8, 158–169. [Google Scholar] [CrossRef]
- Gopinath, S.C.B.; Anbu, P.; Arshad, M.K.M.; Lakshmipriya, T.; Voon, C.H.; Hashim, U.; Chinni, S.V. Biotechnological Processes in Microbial Amylase Production. BioMed Res. Int. 2017, 2017, 1272193. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Gigras, P.; Mohapatra, H.; Goswami, V.K.; Chauhan, B. Microbial α-amylases: A biotechnological perspective. Process Biochem. 2003, 38, 1599–1616. [Google Scholar] [CrossRef]
- Kunamneni, A.; Permaul, K.; Singh, S. Amylase production in solid state fermentation by the thermophilic fungus Thermomyces lanuginosus. J. Biosci. Bioeng. 2005, 100, 168–171. [Google Scholar] [CrossRef]
- Sundarramand, A.; Murthy, T.P.K. α-Amylase production and applications: A review. Appl. Environ. Microbiol. 2014, 2, 166–175. [Google Scholar] [CrossRef]
- Sethi, B.K.; Jana, A.; Nanda, P.K.; Das Mohapatra, P.K.; Sahoo, S.L.; Patra, J.K. Production of α-Amylase by Aspergillus terreus NCFT 4269.10 using pearl millet and its structural characterization. Front. Plant Sci. 2016, 7, 639. [Google Scholar] [CrossRef] [Green Version]
- Zaferanloo, B.; Bhattacharjee, S.; Ghorbani, M.M.; Mahon, P.J.; Palombo, E.A. Amylase production by Preussia minima, a fungus of endophytic origin: Optimization of fermentation conditions and analysis of fungal secretome. BMC Microbiol. 2014, 14, 55. [Google Scholar] [CrossRef] [Green Version]
- Galaction, A.I.; Cascaval, D.; Oniscu, C.; Turnea, M. Evaluation and modelling of the aerobic stirred bioreactor performances for fungus broths. Chem. Biochem. Eng. Q. 2005, 19, 87–97. [Google Scholar]
- Galaction, A.I.; Tucaliuc, A.; Ciobanu, C.; Caşcaval, D. Fumaric acid production by Rhyzopus oryzae in presence of n-dodecane as oxygen-vector. Biochem. Eng. J. 2020, 164, 107795. [Google Scholar] [CrossRef]
- Dumont, E.; Andres, Y.; Le Cloirec, P. Effect of organic solvents on oxygen mass transfer in multiphase systems: Application to bioreactors in environmental protection. Biochem. Eng. J. 2006, 30, 245–252. [Google Scholar] [CrossRef]
- Caşcaval, D.; Galaction, A.I.; Folescu, E.; Turnea, M. Comparative study on the effects of n-dodecane addition on oxygen transfer in stirred bioreactors for simulated, bacterial and yeasts broths. Biochem. Eng. J. 2006, 31, 56–66. [Google Scholar] [CrossRef]
- Blaga, A.C.; Ciobanu, C.; Cașcaval, D.; Galaction, A.I. Enhancement of ergosterol production by Saccharomyces cerevisiae in batch and fed-batch fermentation processes using n-dodecane as oxygen-vector. Biochem. Eng. J. 2018, 131, 70–76. [Google Scholar] [CrossRef]
- Lai, L.S.T.; Tsai, T.H.; Wang, T.C. Application of oxygen vectors to Aspergillus terreus cultivation. J. Biosci. Bioeng. 2002, 94, 453–459. [Google Scholar] [CrossRef]
- Zhang, S.; Song, P.; Li, S. Application of n-dodecane as an oxygen vector to enhance the activity of fumarase in recombinant Escherichia coli: Role of intracellular microenvironment. Braz. J. Microbiol. 2018, 49, 662–667. [Google Scholar] [CrossRef]
- Xu, P.; Pan, C.; Cui, G.; Wei, C.Y.; Wang, L.; Li, Y.; Li, X.; Huang, S. Enhancement of l-amino acid oxidase production by Bacillus subtilis HLZ-68 with oxygen-vector and asymmetric degradation of dl-arginine to d-arginine. Biotechnol. Biotechnol. Equip. 2020, 34, 1273–1279. [Google Scholar] [CrossRef]
- Amaral, P.F.; Freire, M.G.; Rocha-Leão, M.H.; Marrucho, I.M.; Coutinho, J.A.; Coelho, M.A. Optimization of oxygen mass transfer in a multiphase bioreactor with perfluorodecalin as a second liquid phase. Biotechnol. Bioeng. 2008, 99, 588–598. [Google Scholar] [CrossRef]
- Keharom, S.; Mahachai, R.; Chanthai, S. The optimization study of α-amylase activity based on central composite design-response surface methodology by dinitrosalicylic acid method. Int. Food Res. J. 2016, 23, 10–17. [Google Scholar]
- Porfiri, M.C.; Farruggia, B.M.; Romanini, D. Bioseparation of alpha-amylase by forming insoluble complexes with polyacrylate from a culture of Aspergillus oryzae grown in agricultural wastes. Sep. Purif. Technol. 2012, 92, 11–16. [Google Scholar] [CrossRef]
- Songserm, P.; Karnchanatat, A.; Thitiprasert, S.; Tanasupawat, S.; Assabumrungrat, S.; Yang, S.T.; Thongchul, N. Metabolic responses of Aspergillus terreus under low dissolved oxygen and pH levels. Ann. Microbiol. 2018, 68, 195–205. [Google Scholar] [CrossRef]
- Saha, B.C. Emerging biotechnologies for production of itaconic acid and its applications as a platform chemical. J. Ind. Microbiol. Biotechnol. 2017, 44, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Harvey, L.M.; McNeil, B. Oxidative stress in submerged cultures of fungi. Crit. Rev. Biotechnol. 2003, 23, 267–302. [Google Scholar] [CrossRef]
- Dwiarti, L.; Otsuka, M.; Miura, S.; Yaguchi, M.; Okabe, M. Itaconic acid production using sago starch hydrolysate by Aspergillus terreus TN484-M1. Bioresour. Technol. 2007, 98, 3329–3337. [Google Scholar] [CrossRef]
- Galitskaya, P.; Biktasheva, L.; Blagodatsky, S.; Selivanovskaya, S. Response of bacterial and fungal communities to high petroleum pollution in different soils. Sci. Rep. 2021, 11, 164. [Google Scholar] [CrossRef]
- Coelho, M.A.Z.; Belo, I.; Pinheiro, R.; Amaral, A.L.; Mota, M.; Cutinho, J.A.P.; Ferreira, E.C. Effect of hyperbaric stress on yeast morphology: Study by automated image analysis. Appl. Microbiol. Biotechnol. 2004, 66, 318–324. [Google Scholar] [CrossRef] [Green Version]
- Lopes, M.; Gomes, N.; Gonçalves, C.; Coelho, M.A.; Mota, M.; Belo, I. Yarrowia lipolytica lipase production enhanced by increased air pressure. Lett. Appl. Microbiol. 2008, 46, 255–260. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blaga, A.C.; Caşcaval, D.; Galaction, A.I. Improved Production of α-Amylase by Aspergillus terreus in Presence of Oxygen-Vector. Fermentation 2022, 8, 271. https://doi.org/10.3390/fermentation8060271
Blaga AC, Caşcaval D, Galaction AI. Improved Production of α-Amylase by Aspergillus terreus in Presence of Oxygen-Vector. Fermentation. 2022; 8(6):271. https://doi.org/10.3390/fermentation8060271
Chicago/Turabian StyleBlaga, Alexandra Cristina, Dan Caşcaval, and Anca Irina Galaction. 2022. "Improved Production of α-Amylase by Aspergillus terreus in Presence of Oxygen-Vector" Fermentation 8, no. 6: 271. https://doi.org/10.3390/fermentation8060271
APA StyleBlaga, A. C., Caşcaval, D., & Galaction, A. I. (2022). Improved Production of α-Amylase by Aspergillus terreus in Presence of Oxygen-Vector. Fermentation, 8(6), 271. https://doi.org/10.3390/fermentation8060271