The Influence of Lactic Acid Bacteria Fermentation on the Bioactivity of Crayfish (Faxonius limosus) Meat
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
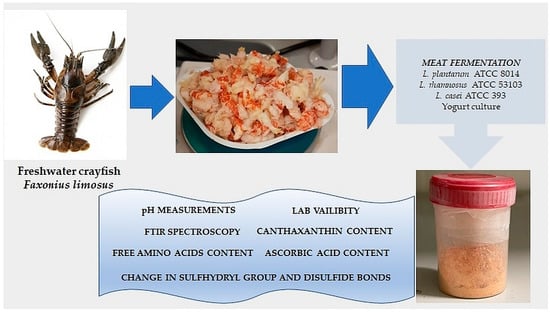
2.2. Fermentation and Preparation of the Samples
2.3. Microbiological Analyses, Determination of pH, Titratable Acidity (TA) and Total Solids Content (TSC)
2.4. Preparation of Extracts
2.5. Determination of Reducing Sugars Content (RSC) and Total Free Amino Acids Level (TFAAL)
2.6. Determination of Reducing Rower, DPPH, FRAP, ABTS, and Ascorbic Acid Content
2.7. Determination of Canthaxanthin
2.8. FTIR Analyses
2.9. Determination of Sulfhydryl Groups (–SH) and Disulfide Bonds (–S–S–) Contents
2.10. Color Measurements
2.11. Statistical Analysis
3. Results and Discussion
3.1. The Changes in pH, TA and TSC
3.2. Changes in LAB Content
3.3. The Changes in Reducing Sugars Content and Total Free Amino Acids Level
3.4. The Changes in Sulfhydryl Groups (–SH), Disulfide Bonds (–S–S–), Cantahxanthin and Functional Groups
3.5. Color Changes
3.6. The Changes in Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patoka, J.; Kocánová, B.; Kalous, L. Crayfish in Czech cultural space: The longest documented relationship between humans and crayfish in Europe. Knowl. Manag. Aquat. Ecosyst. 2016, 417, 5. [Google Scholar] [CrossRef]
- Śmietana, P. Uwarunkowania rozmieszczenia i mechanizmy konkurencji międzygatunkowej raka szlachetnego (Astacus astacus L.) i raka pręgowatego (Orconectes limosus Raf.). Habilitation Thesis, Uniwersytet Szczeciński, Szczecin, Poland, 2013. (In Polish). [Google Scholar]
- Kaldre, K.; Paaver, T.; Hurt, M.; Gross, R. Continuing expansion of non-indigenous crayfish species in Northern Europe: First established spiny-cheek crayfish Faxonius limosus (Rafinesque, 1817) population in Estonia. Bioinvasions Rec. 2020, 9, 127–132. [Google Scholar] [CrossRef]
- Śmietana, N.; Panicz, R.; Sobczak, M.; Śmietana, P.; Nędzarek, A. Spiny-cheek crayfish, Faxonius limosus (Rafinesque, 1817), as an alternative food source. Animals 2021, 11, 59. [Google Scholar] [CrossRef]
- Śmietana, N.; Panicz, R.; Sobczak, M.; Nędzarek, A.; Śmietana, P. Variability of elements and nutritional value of spiny-cheek crayfish Faxonius limosus, Rafinesque, 1817. J. Food Compos. Anal. 2020, 94, 103656. [Google Scholar] [CrossRef]
- Bora, B. The role of Lactic Acid Bacteria in the production of fermented meat products. Vigyan. Varta. 2022, 3, 14–15. [Google Scholar]
- Hu, Y.; Zhang, L.; Liu, Q.; Wang, Y.; Chen, Q.; Kong, B. The potential correlation between bacterial diversity and the characteristic volatile flavor of traditional dry sausages from Northeast China. Food Microbiol. 2020, 91, 103505. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, Y.; Wang, Y.; Huang, Y.; Li, P.; Li, P. Lactobacillus plantarum LPL-1, a bacteriocin producing strain, changed the bacterial community composition and improved the safety of low-salt fermented sausages. LWT 2020, 128, 109385. [Google Scholar] [CrossRef]
- Fadda, S.; López, C.; Vignolo, G. Role of Lactic Acid Bacteria during meat conditioning and fermentation: Peptides generated as sensorial and hygienic biomarkers. Meat Sci. 2010, 86, 66–79. [Google Scholar] [CrossRef]
- Zang, J.; Xu, Y.; Xia, W.; Regenstein, J.M. Quality, functionality, and microbiology of fermented fish: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1228–1242. [Google Scholar] [CrossRef]
- Prapasuwannakul, N.; Suwannahong, K. Chemical composition and antioxidant activity of Klongkone shrimp paste. Procedia. Soc. Behav. Sci. 2015, 197, 1095–1100. [Google Scholar] [CrossRef]
- Kleekayai, T.; Saetae, D.; Wattanachaiyingyong, O.; Tachibana, S.; Yasuda, M.; Suntornsuk, W. Characterization and in vitro biological activities of Thai traditional fermented shrimp pastes. J. Food Sci. Technol. 2015, 52, 1839–1848. [Google Scholar] [CrossRef] [PubMed]
- Talon, R.; Leroy, S.; Lebert, I. Microbial ecosystems of traditional fermented meat products: The importance of indigenous starters. Meat Sci. 2007, 77, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Ciuciu Simion, A.M.; Vizireanu, C.; Alexe, P.; Franco, I.; Carballo, J. Effect of the use of selected starter cultures on some quality, safety and sensorial properties of Dacia sausage, a traditional Romanian dry-sausage variety. Food Control 2014, 35, 123–131. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Łopusiewicz, Ł.; Śmietana, N.; Paradowska, D.; Drozłowska, E. Black cumin (Nigella sativa L.) seed press cake as a novel material for the development of new non-dairy beverage fermented with kefir grains. Microorganisms 2022, 10, 300. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Waszkowiak, K.; Polanowska, K.; Mikołajczak, B.; Śmietana, N.; Hrebień-Filisińska, A.; Sadowska, J.; Mazurkiewicz-Zapałowicz, K.; Drozłowska, E. The effect of yogurt and kefir starter cultures on the bioactivity of fermented industrial by-product from Cannabis sativa production—Hemp press cake. Fermentation 2022, 8, 490. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Drozłowska, E.; Kwiatkowski, P. Production and characterization of yogurt–like fermented beverage based on Camelina (Camelina sativa L.) seed press cake. Appl. Sci. 2022, 12, 1085. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Drozłowska, E.; Trocer, P.; Kwiatkowski, P.; Bartkowiak, A.; Gefrom, A.; Sienkiewicz, M. The effect of fermentation with kefir grains on the physicochemical and antioxidant properties of beverages from blue lupin (Lupinus angustifolius L.) seeds. Molecules 2020, 25, 5791. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.J.; Shi, A.M.; Liu, H.Z.; Liu, L.; Hu, H.; Adhikari, B.; Wang, Q. Emulsifying properties and structure changes of spray and freeze-dried peanut protein isolate. J. Food Eng. 2016, 170, 33–40. [Google Scholar] [CrossRef]
- Hu, Y.; Xia, W.; Ge, C. Characterization of fermented silver carp sausages inoculated with a mixed starter culture. LWT 2008, 41, 730–738. [Google Scholar] [CrossRef]
- Nursyam, H. Fermented sausage processing using Lactobacillus plantarum starter culture against pH, total acid, N-total, and N-amino. J. Ilm. Perikan Kelaut 2011, 3, 221–228. [Google Scholar] [CrossRef]
- Nie, X.; Zhang, Q.; Lin, S. Biogenic amine accumulation in silver carp sausage inoculated with Lactobacillus plantarum plus Saccharomyces cerevisiae. Food Chem. 2014, 153, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.J.; Pan, C.L.; Jiang, S.T. Effect of lactic acid bacterial fermentation on the characteristics of minced mackerel. J. Food Sci. 2002, 67, 786–792. [Google Scholar] [CrossRef]
- Szparaga, A.; Tabor, S.; Kocira, S.; Czerwińska, E.; Kuboń, M.; Płóciennik, B.; Findura, P. Survivability of probiotic bacteria in model systems of non-fermented and fermented coconut and hemp milks. Sustainability 2019, 11, 6093. [Google Scholar] [CrossRef]
- Sakhare, P.Z.; Narasimha Rao, D. Microbial profiles during Lactic Fermentation of meat by combined starter cultures at high temperatures. Food Control 2003, 14, 1–5. [Google Scholar] [CrossRef]
- Riebroy, S.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Changes during fermentation and properties of Som-Fug produced from different marine fish. J. Food Process Preserv. 2007, 31, 751–770. [Google Scholar] [CrossRef]
- Li, X.; Lee, P.R.; Taniasuri, F.; Liu, S.Q. Effect of Lactic Acid Bacterial fermentation on amino acids and volatile compounds of pork trimming hydrolysate. Int. J. Food Sci. Technol. 2021, 56, 429–440. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lee, H.W.; Yang, J.H.; Hong, S.W.; Park, S.H.; Lee, M.A. Changes in quality properties of kimchi based on the nitrogen content of fermented anchovy sauce, myeolchi aekjeot, during fermentation. Food Sci. Biotechnol. 2018, 27, 1145–1155. [Google Scholar] [CrossRef]
- Limsuwan, S.; Visessanguan, W.; Kongkiattikajorn, J. The Effects of starter cultures on biogenic amine and free amino acid contents in Nham during fermentation. Kasetsart J. Nat. Sci. 2007, 41, 367–373. [Google Scholar]
- Stadnik, J.; Kęska, P.; Gazda, P.; Siłka, Ł.; Kołożyn-Krajewska, D. Influence of LAB fermentation on the color stability and oxidative changes in dry-cured meat. Appl. Sci. 2022, 12, 11736. [Google Scholar] [CrossRef]
- Cheng, J.; Xiang, R.; Tang, D.; Zhu, M.; Liu, X. Regulation of protein oxidation in cantonese sausages by rutin, quercetin and caffeic acid. Meat Sci. 2021, 175, 108422. [Google Scholar] [CrossRef]
- Chen, Q.; Kong, B.; Sun, Q.; Dong, F.; Liu, Q. Antioxidant potential of a unique LAB culture isolated from harbin dry sausage: In vitro and in a sausage model. Meat Sci. 2015, 110, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Vukotić, G.; Strahinić, I.; Begović, J.; Lukić, J.; Kojić, M.; Fira, D. Survey on proteolytic activity and diversity of proteinase genes in mesophilic Lactobacilli. Microbiology 2016, 85, 33–41. [Google Scholar] [CrossRef]
- Vaithiyanathan, S.; Naveena, B.M.; Muthukumar, M.; Girish, P.S.; Kondaiah, N. Effect of dipping in pomegranate (Punica granatum) fruit juice phenolic solution on the shelf life of chicken meat under refrigerated storage (4 °C). Meat Sci. 2011, 88, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Chen, S.; Liu, R.; Chen, L.; Yang, B.; Yu, H.; Wu, M.; Zhang, W.; Zhou, G. Effects of Lactobacillus plantarum NJAU-01 on the protein oxidation of fermented sausage. Food Chem. 2019, 295, 361–367. [Google Scholar] [CrossRef]
- Dai, H.; Sun, Y.; Xia, W.; Ma, L.; Li, L.; Wang, Q.; Zhang, Y. Effect of phospholipids on the physicochemical properties of myofibrillar proteins solution mediated by NaCl concentration. LWT 2021, 141, 110895. [Google Scholar] [CrossRef]
- Piao, Y.Z.; Bibat, M.A.D.; Hwang, S.J.; Eun, J.B. Protein degradation and texture properties of Skate (Raja kenojei) muscle during fermentation. J. Food Sci. Technol. 2022, 59, 4713–4722. [Google Scholar] [CrossRef]
- Shi, L.; Xiong, G.; Yin, T.; Ding, A.; Li, X.; Wu, W.; Qiao, Y.; Liao, L.; Jiao, C.; Wang, L. Effects of ultra-high pressure treatment on the protein denaturation and water properties of red swamp crayfish (Procambarus clarkii). LWT 2020, 133, 110124. [Google Scholar] [CrossRef]
- Velazquez, G.; Méndez-Montealvo, M.G.; Welti-Chanes, J.; Ramírez, J.A.; Martínez-Maldonado, M.A. Effect of high-pressure processing and heat treatment on the gelation properties of blue crab meat proteins. LWT 2021, 146, 111389. [Google Scholar] [CrossRef]
- Yan, B.; Jiao, X.; Zhu, H.; Wang, Q.; Huang, J.; Zhao, J.; Cao, H.; Zhou, W.; Zhang, W.; Ye, W.; et al. Chemical interactions involved in microwave heat-induced surimi gel fortified with fish oil and its formation mechanism. Food Hydrocoll. 2020, 105, 105779. [Google Scholar] [CrossRef]
- Shao, J.H.; Zou, Y.F.; Xu, X.L.; Wu, J.Q.; Zhou, G.H. Evaluation of structural changes in raw and heated meat batters prepared with different lipids using Raman spectroscopy. Int. Food Res. J. 2011, 44, 2955–2961. [Google Scholar] [CrossRef]
- Han, K.; Feng, X.; Yang, Y.; Wei, S.; Tang, X.; Li, S.; Chen, Y. Effects of Camellia oil on the properties and molecular forces of myofibrillar protein gel induced by microwave heating. Int. J. Food Sci. Technol. 2021, 56, 5708–5716. [Google Scholar] [CrossRef]
- Candoğan, K.; Altuntas, E.G.; İğci, N. Authentication and quality assessment of meat products by Fourier-Transform Infrared (FTIR) spectroscopy. Food Eng. Rev. 2020, 1, 66–91. [Google Scholar] [CrossRef]
- Scott, K.J. Detection and Measurement of Carotenoids by UV/VIS spectrophotometry. Curr. Protoc. Food Anal. Chem. 2001, 1, F2.2.1–F2.2.10. [Google Scholar] [CrossRef]
- Czeczuga, B.; Czeczuga-Semeniuk, E. Comparative studies of carotenoids in four species of crayfish. Crustaceana-Int. J. Crustacean Res. 1999, 73, 693–700. [Google Scholar]
- Cantrell, A.; McGarvey, D.J.; Truscott, T.G.; Rancan, F.; Böhm, F. Singlet oxygen quenching by dietary carotenoids in a model membrane environment. Arch. Biochem. Biophys 2003, 412, 47–54. [Google Scholar] [CrossRef]
- Rebelo, B.A.; Farrona, S.; Rita Ventura, M.; Abranches, R. Canthaxanthin, a red-hot carotenoid: Applications, synthesis, and biosynthetic evolution. Plants 2020, 9, 1039. [Google Scholar] [CrossRef]
- Kun, S.; Rezessy-Szabó, J.M.; Nguyen, Q.D.; Hoschke, Á. Changes of microbial population and some components in carrot juice during fermentation with selected Bifidobacterium strains. Process Biochem. 2008, 43, 816–821. [Google Scholar] [CrossRef]
- Panda, S.H.; Ray, R.C. Lactic Acid Fermentation of β-Carotene rich sweet potato (Ipomoea batatas L.) into Lacto-juice. Plant Foods Hum. Nutr. 2007, 62, 65–70. [Google Scholar] [CrossRef]
- Venugopalan, V.; Tripathi, S.K.; Nahar, P.; Saradhi, P.P.; Das, R.H.; Gautam, H.K. Characterization of canthaxanthin isomers isolated from a new soil Dietzia sp. and their antioxidant activities. J. Microbiol. Biotechnol. 2013, 23, 237–245. [Google Scholar] [CrossRef]
- Chaijan, M.; Panpipat, W. Darkening Prevention of fermented shrimp paste by pre-soaking whole shrimp with pyrophosphate. As. J. Food Ag. Ind. 2012, 5, 163–171. [Google Scholar]
- Jaswir, I.; Noviendri, D.; Hasrini, R.F.; Octavianti, F. Carotenoids: Sources, medicinal properties and their application in food and nutraceutical industry. J. Med. Plant Res. 2011, 5, 7119–7131. [Google Scholar] [CrossRef]
- Khairina, R.; Utami, T.; Raharjo, S.; Cahyanto, M.N. Changes in sensory, physicochemical and microbiological properties of Ronto during fermentation. Pak. J. Nutr. 2017, 16, 629–637. [Google Scholar] [CrossRef]
- Pongsetkul, J.; Benjakul, S.; Sampavapol, P.; Osako, K.; Faithong, N. Chemical composition and physical properties of salted shrimp paste (Kapi) produced in Thailand. Int. Aquat. Res. 2014, 6, 155–166. [Google Scholar] [CrossRef]
- Cai, L.; Wang, Q.; Dong, Z.; Liu, S.; Zhang, C.; Li, J. Biochemical, nutritional, and sensory quality of the low salt fermented shrimp paste. J. Aquat. Food Prod. Technol. 2017, 26, 706–718. [Google Scholar] [CrossRef]
- Faithong, N.; Benjakul, S. Changes in antioxidant activities and physicochemical properties of Kapi, a fermented shrimp paste, during fermentation. J. Food Sci. Technol. 2014, 51, 2463–2471. [Google Scholar] [CrossRef]
- Majumdar, R.K.; Roy, D.; Bejjanki, S.; Bhaskar, N. Chemical, and microbial properties of shidal, a traditional fermented fish of Northeast India. J. Food Sci. Technol. 2016, 53, 401–410. [Google Scholar] [CrossRef]
- Geeta; Yadav, A.S. Antioxidant and antimicrobial profile of chicken sausages prepared after fermentation of minced chicken meat with Lactobacillus plantarum and with additional dextrose and starch. LWT 2017, 77, 249–258. [Google Scholar] [CrossRef]
- Jemil, I.; Jridi, M.; Nasri, R.; Ktari, N.; ben Slama-Ben Salem, R.; Mehiri, M.; Hajji, M.; Nasri, M. Functional, antioxidant and antibacterial properties of protein hydrolysates prepared from fish meat fermented by Bacillus subtilis A26. Process Biochem. 2014, 49, 963–972. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, B.; Zhang, T.; Mu, W.; Liu, J. Antioxidant and free radical-scavenging activities of Chickpea Protein Hydrolysate (CPH). Food Chem. 2008, 106, 444–450. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, P.; Lou, L.; Zhan, J.; Fan, M.; Li, D.; Liao, Q. Antioxidant activities of Lactic Acid Bacteria for quality improvement of fermented sausage. J. Food Sci. 2017, 82, 2960–2967. [Google Scholar] [CrossRef]
- Akther, F.; Le, B.; Chung, G.; Yang, S.H. Optimizing the fermentation condition of low salted squid jeotgal by Lactic Acid Bacteria with enhanced antioxidant activity. J. Appl. Biol. Chem. 2017, 60, 391–402. [Google Scholar] [CrossRef]
- Giri, A.; Osako, K.; Okamoto, A.; Okazaki, E.; Ohshima, T. Antioxidative properties of aqueous and aroma extracts of squid miso prepared with Aspergillus oryzae—Inoculated Koji. Int. Food Res. J. 2011, 44, 317–325. [Google Scholar] [CrossRef]
- Silva, M.M.; Lidon, F.C. An overview on applications and side effects of antioxidant food additives. Emir. J. Food Agric. 2016, 28, 823–832. [Google Scholar] [CrossRef]
- Kusznierewicz, B.; Śmiechowska, A.; Bartoszek, A.; Namieśnik, J. The effect of heating and fermenting on antioxidant properties of white cabbage. Food Chem. 2008, 108, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Adetuyi, F.O.; Ibrahim, T.A. Effect of fermentation time on the phenolic, flavonoid and vitamin C contents and antioxidant activities of okra (Abelmoschus esculentus) seeds. Niger. Food J. 2014, 32, 128–137. [Google Scholar] [CrossRef]
- Rompies, R.; Mayulu, N.; Nurkolis, F.; Faradila, F.; Kepel, B.J.; Natanael, H. Antioxidant capacity of snack cookies made from mango and pineapple fermentation. Food Res. 2021, 5, 145–148. [Google Scholar] [CrossRef]
- Li, H.; Huang, J.; Wang, Y.; Wang, X.; Ren, Y.; Yue, T.; Wang, Z.; Gao, Z. Study on the nutritional characteristics and antioxidant activity of dealcoholized sequentially fermented apple juice with Saccharomyces cerevisiae and Lactobacillus plantarum fermentation. Food Chem. 2021, 363, 130351. [Google Scholar] [CrossRef]



| 0 | 1 | |
|---|---|---|
| TSC (%) | ||
| Control | 19.59 ± 0.58 a | |
| Lactiplantibacillus plantarum ATCC 8014 | 16.81 ± 0.25 b | |
| Lactobacillus casei ATCC 393 | 16.66 ± 0.15 b | |
| Lacticaseibacillus rhamnosus ATCC 53103 | 16.74 ± 0.21 b | |
| Yogurt culture | 19.26 ± 0.16 a | |
| pH | ||
| Control | 8.00 ± 0.01 a | |
| Lactiplantibacillus plantarum ATCC 8014 | 7.35 ± 0.02 d | |
| Lactobacillus casei ATCC 393 | 7.01 ± 0.03 b | |
| Lacticaseibacillus rhamnosus ATCC 53103 | 7.11 ± 0.02 c | |
| Yogurt culture | 6.94 ± 0.01 b | |
| TA (mg/g) | ||
| Control | 0.18 ± 0.23 a | |
| Lactiplantibacillus plantarum ATCC 8014 | 0.40 ± 0.02 ab | |
| Lactobacillus casei ATCC 393 | 0.93 ± 0.25 c | |
| Lacticaseibacillus rhamnosus ATCC 53103 | 0.52 ± 0.06 b | |
| Yogurt culture | 0.62 ± 0.04 b |
| RSC (mg/g) | |
|---|---|
| Control | 12.25 ± 0.06 a |
| Lactiplantibacillus plantarum ATCC 8014 | 11.74 ± 0.22 c |
| Lactobacillus casei ATCC 393 | 11.39 ± 0.22 bc |
| Lacticaseibacillus rhamnosus ATCC 53103 | 11.10 ± 0.51 b |
| Yogurt culture | 11.49 ± 0.06 bc |
| TFAAL (mg/g) | |
| Control | 7.89 ± 0.12 a |
| Lactiplantibacillus plantarum ATCC 8014 | 4.68 ± 0.03 b |
| Lactobacillus casei ATCC 393 | 5.15 ± 0.37 b |
| Lacticaseibacillus rhamnosus ATCC 53103 | 9.15 ± 0.54 a |
| Yogurt culture | 6.01 ± 0.32 b |
| –SH (µmol/g) | –S–S– (µmol/g) | |
|---|---|---|
| Control | 37.56 ± 0.91 a | 13.64 ± 0.49 a |
| Lactiplantibacillus plantarum ATCC 8014 | 53.33 ± 0.57 b | 17.83 ± 0.32 b |
| Lactobacillus casei ATCC 393 | 97.69 ± 3.06 c | 28.20 ± 0.93 c |
| Lactobacillus rhamnosus ATCC 53103 | 91.52 ± 2.35 cd | 9.46 ± 1.29 d |
| Yogurt culture | 94.29 ± 3.21 d | 25.47 ± 1.75 e |
| 0 | ||||
|---|---|---|---|---|
| L* | a* | b* | ΔE | |
| Control | 58.90 ± 0.01 a | 18.48 ± 0.01 a | 21.81 ± 0.01 a | Used as a standard |
| 1 | ||||
| Lactiplantibacillus plantarum ATCC 8014 | 61.26 ± 0.01 b | 19.94 ± 0.00 b | 20.82 ± 0.01 b | 2.95 ± 0.01 a |
| Lactobacillus casei ATCC 393 | 59.92 ± 0.01 c | 19.99 ± 0.01 c | 21.28 ± 0.01 c | 1.90 ± 0.01 b |
| Lacticaseibacillus rhamnosus ATCC 53103 | 60.44 ± 0.01 d | 20.60 ± 0.01 d | 21.53 ± 0.01 d | 2.64 ± 0.00 c |
| Yogurt culture | 59.26 ± 0.01 e | 20.28 ± 0.01 e | 21.31 ± 0.01 e | 2.49 ± 0.01 d |
| FRAP (mg AAE/g) | |
|---|---|
| Control | 2.99 ± 0.04 a |
| Lactiplantibacillus plantarum ATCC 8014 | 3.60 ± 0.06 b |
| Lactobacillus casei ATCC 393 | 3.69 ± 0.02 b |
| Lactobacillus rhamnosus ATCC 53103 | 3.78 ± 0.01 bc |
| Yogurt culture | 4.06 ± 0.17 c |
| ABTS (μmol Trolox/g) | |
| Control | 2.15 ± 0.04 a |
| Lactiplantibacillus plantarum ATCC 8014 | 3.50 ± 0.20 b |
| Lactobacillus casei ATCC 393 | 3.05 ± 0.38 b |
| Lactobacillus rhamnosus ATCC 53103 | 2.85 ± 0.47 ab |
| Yogurt culture | 3.02 ± 0.04 b |
| DPPH (μmol Trolox/g) | |
| Control | 2.81 ± 0.64 a |
| Lactiplantibacillus plantarum ATCC 8014 | 4.01 ± 0.27 a |
| Lactobacillus casei ATCC 393 | 3.26 ± 0.55 a |
| Lactobacillus rhamnosus ATCC 53103 | 4.03 ± 0.39 a |
| Yogurt culture | 3.79 ± 1.05 a |
| RP(μmol Trolox/g) | |
| Control | 5.53 ± 0.05 a |
| Lactiplantibacillus plantarum ATCC 8014 | 9.91 ± 0.56 b |
| Lactobacillus casei ATCC 393 | 6.28 ± 0.41 a |
| Lactobacillus rhamnosus ATCC 53103 | 12.66 ± 0.48 c |
| Yogurt culture | 14.55 ± 0.25 d |
| AAC (mg/g) | |
| Control | 5.56 ± 0.02 a |
| Lactiplantibacillus plantarum ATCC 8014 | 8.24 ± 0.04 c |
| Lactobacillus casei ATCC 393 | 7.92 ± 0.06 b |
| Lactobacillus rhamnosus ATCC 53103 | 7.91 ± 0.13 b |
| Yogurt culture | 11.60 ± 0.17 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Śmietana, N.; Śmietana, P.; Drozłowska, E.; Łopusiewicz, Ł. The Influence of Lactic Acid Bacteria Fermentation on the Bioactivity of Crayfish (Faxonius limosus) Meat. Fermentation 2023, 9, 66. https://doi.org/10.3390/fermentation9010066
Śmietana N, Śmietana P, Drozłowska E, Łopusiewicz Ł. The Influence of Lactic Acid Bacteria Fermentation on the Bioactivity of Crayfish (Faxonius limosus) Meat. Fermentation. 2023; 9(1):66. https://doi.org/10.3390/fermentation9010066
Chicago/Turabian StyleŚmietana, Natalia, Przemysław Śmietana, Emilia Drozłowska, and Łukasz Łopusiewicz. 2023. "The Influence of Lactic Acid Bacteria Fermentation on the Bioactivity of Crayfish (Faxonius limosus) Meat" Fermentation 9, no. 1: 66. https://doi.org/10.3390/fermentation9010066
APA StyleŚmietana, N., Śmietana, P., Drozłowska, E., & Łopusiewicz, Ł. (2023). The Influence of Lactic Acid Bacteria Fermentation on the Bioactivity of Crayfish (Faxonius limosus) Meat. Fermentation, 9(1), 66. https://doi.org/10.3390/fermentation9010066